Abstract
During the stationary phase of growth, after 7 to 12 d in culture, the levels of phosphatidylinositol 4,5-bisphosphate (PtdInsP2) decreased by 75% in plasma membranes of the red alga Galdieria sulphuraria. Concomitant with the decrease in PtdInsP2 levels in plasma membranes, there was an increase in PtdInsP2 in microsomes, suggesting that the levels of plasma membrane PtdInsP2 are regulated differentially. The decline of PtdInsP2 in plasma membranes was accompanied by a 70% decrease in the specific activity of PtdInsP kinase and by reduced levels of protein cross-reacting with antisera against a conserved PtdInsP kinase domain. Upon osmotic stimulation, the loss of PtdInsP2from the plasma membrane increased from 10% in 7-d-old cells to 60% in 12-d-old cells, although the levels of inositol 1,4,5-trisphosphate (InsP3) produced in whole cells were roughly equal at both times. When cells with low plasma membrane PtdInsP2 levels were osmotically stimulated, a mild osmotic stress (12.5 mm KCl) activated PtdInsP kinase prior to InsP3 production, whereas in cells with high plasma membrane PtdInsP2, more severe stress (250 mm KCl) was required to induce an increase in PtdInsP kinase activity. The differential regulation of a plasma membrane signaling pool of PtdInsP2 is discussed with regard to the implications for understanding the responsive state of cells.
The unicellular thermo-acidophilic red alga Galdieria sulphuraria is found in volcanic areas where it occurs in hot sulfuric springs at temperatures of up to 55°C and at pH values between 0.5 and 4. It is one of only a few eukaryotic organisms adapted to such extreme environmental conditions (Gross et al., 1998). The algae are suspended in the springs and also grow endolithically, colonizing surrounding rocks (Smith and Brock, 1973; Merola et al., 1981; Gross et al., 1998). It has previously been shown that G. sulphuraria cells are sensitive to osmotic stress, and that dose-dependent transient increases in inositol 1,4,5-trisphosphate (InsP3) occur after hyperosmotic stimulation (Heilmann et al., 1999).
In G. sulphuraria, both the basal levels of phosphatidylinositol 4,5-bisphosphate (PtdInsP2) and the sequence of signaling events after stimulation change with the time in culture (Heilmann et al., 1999). Changes in phosphoinositide metabolism with the age of cell cultures or with senescence have previously been described in a number of plant systems (Heim and Wagner, 1986; Falkenau et al., 1987; Borochov et al., 1994; Heilmann et al., 1999). However, to our knowledge, the consequences of the age-related changes in phosphoinositide metabolism with regard to plasma membrane signaling have not been addressed in detail. The study of G. sulphuraria cells of different physiological status allows for the comparison of cells with differences in phosphoinositide metabolism.
Phosphoinositides and specific phosphoinositide kinases have been found in various cellular compartments of diverse eukaryotic organisms, in membranes and in soluble fractions, in the actin cytoskeleton, and also in the nucleus (for review, see Munnik et al., 1998; Drøbak et al., 1999; Stevenson et al., 2000). It is a key hypothesis of this work that the compartmentalization of phosphoinositides within eukaryotic cells aids in the orchestration of the various functions of phosphoinositides.
The phosphoinositides, and foremost PtdInsP2, can perform a multitude of cellular functions in addition to serving as the precursors of the second messenger, InsP3, and of diacylglycerol. PtdInsP2 can have regulatory effects on elements of the actin cytoskeleton, such as profilin or gelsolin (Drøbak et al., 1994; Janmey, 1994; Janmey et al., 1999), on ion channels and ATPases (Memon et al., 1989; Shyng and Nichols, 1998; Shyng et al., 2000), and on the formation and secretion of membrane vesicles (Hama et al., 1999, 2000; for review, see Toker, 1998; Anderson et al., 1999; Hinchliffe et al., 1998).
In this paper, we use the term “pool” to describe the entirety of phosphoinositides of a certain function within a cell, a membrane, or a compartment. In this context, a plasma membrane signaling pool of Ptd- InsP2 would be defined as the PtdInsP2 that can be hydrolyzed in the plasma membrane upon stimulation to generate InsP3 signals. Phosphoinositide pools are characterized by particular lipid-lipid and lipid-protein interactions. The immediate environment within which the phosphoinositides reside can be described as a “microdomain.” Different phosphoinositide microdomains can be part of the same phosphoinositide pool. For instance, plasma membrane PtdInsP2 can be associated with ion channels, and also be accessible for hydrolysis, and both microdomains may be involved in signaling, depending on the nature and intensity of the stimulus. Stimulation may affect certain combinations of microdomains within the signaling pool and would have a different physiological outcome according to the microdomains affected.
The transient and localized accumulation of Ptd- InsP2 in a microdomain could be conferred by localized increases in PtdInsP2 synthesis, e.g. through regulation of PtdInsP kinases. Activity and localization of PtdInsP kinases can be controlled by posttranslational modification; however, the exact regulatory mechanisms are as of now only partly understood. The activity of human type I and type II PtdInsP kinases in the plasma membrane (Vancurova et al., 1999) and in the endoplasmic reticulum (ER; Itoh et al., 1998) can be modulated by phosphorylation. Furthermore, in human platelets thrombin-induced increases in cytoskeleton-associated Ptd- InsP2 are mediated by the translocation of PtdInsP kinase (human PtdIns4P 5 kinase C) to the cytoskeleton (Hinchliffe et al., 1996). It has been suggested recently that the co-occurrance of type I and type II PtdInsP kinases in the nucleus of cultured human cells may facilitate multiple intranuclear functions of PtdInsP2 by allowing a distinction of functional pools within the same compartment (Ciruela et al., 2000). In addition, isoform-specific regulation of PtdInsP kinase has been demonstrated for the human PtdInsP kinase II α (Hinchliffe et al., 1999a), suggesting that cellular PtdInsP2 pools within the same membrane may be individually regulated.
Although it has been hypothesized that discrete pools of phosphoinositides are present in animals and plants (Drøbak et al., 1994; Staiger et al., 1997; Hinchliffe et al., 1998, 1999b; Heilmann et al., 1999; Kost et al., 1999), a biochemical characterization of phosphoinositide pools in distinct subcellular fractions has not been reported. The differential effects of phospholipase C inhibitors on short-term and long-term increases in InsP3 in response to gravistimulation in oat shoot pulvini (Perera et al., 2001) suggests the presence of distinct phosphoinositide pools in plants. Green fluorescent protein-pleckstrin homology domain fusion proteins have been employed to visualize PtdInsP2 in vivo to study phosphoinositide pools (Varnai and Balla, 1998; Lockyer et al., 1999; Varnai et al., 1999; Holz et al., 2000). In plants, Ptd- InsP2 microdomains visualized by this method have been implicated in a phosphoinositide pool controlling pollen tube growth (Kost et al., 1999).
Although yielding good results for many cell types studied, changes in the distribution of green fluorescent protein-pleckstrin homology fluorescence do not always reflect the turnover measured in [3H]inositol labeling studies (Varnai and Balla, 1998). Binding of the PH domain will depend on the properties of proteins in a PtdInsP2 microdomain. Also, the binding of PH domains to PtdInsP2 can interfere with normal cellular functions by itself (discussed in Balla et al., 2000). As another approach, fluorescent PtdInsP2 (Tuominen et al., 1999) may prove useful to study phosphoinositide pools. However, the use of fluorescent PtdInsP2 faces potential technical difficulties, such as whether the compound will be incorporated into the plasma membrane, and whether it will remain stable and not be hydrolyzed during development or under stress conditions. It was the aim of this study to present an alternative means of monitoring the cellular distribution of PtdInsP2 in plant cells.
To overcome limitations of a single method, a combination of in vitro lipid phosphorylation assays and PtdInsP2 mass measurements was used with isolated plasma membranes and microsomes of G. sulphuraria to investigate and characterize phosphoinositide pools. Mass measurement of PtdIns(4,5)P2 employed in this study utilizes the high specificity of the bovine-brain Ins(1,4,5)P3 receptor for Ins(1,4,5)P3. Also, the levels of putative PtdInsP kinase protein were followed in plasma membranes and in microsomes along with the determination of PtdInsP kinase specific activity. G. sulphuraria was chosen as a model system for this study based on the indication that the phosphoinositide signaling pool may be regulated independently from other cellular phosphoinositide pools (Heilmann et al., 1999), thus offering the potential for a differential characterization of a plasma membrane phosphoinositide pool with signaling function. In this study, we show that during the stationary phase (between d 7 and 12) of the growth period of G. sulphuraria, the amount of PtdInsP2, the specific activity of PtdInsP kinase, and the levels of putative PtdInsP kinase protein in the plasma membrane gradually decreased. PtdInsP2 levels in the plasma membrane declined, whereas they antithetically increased in microsomes, suggesting that the plasma membrane PtdInsP2 represents a distinct phosphoinositide pool that is regulated differently from phosphoinositides in other cellular compartments.
RESULTS
PtdInsP2 Levels and Specific PtdInsP Kinase Activity Decrease in Plasma Membranes during the Stationary Phase
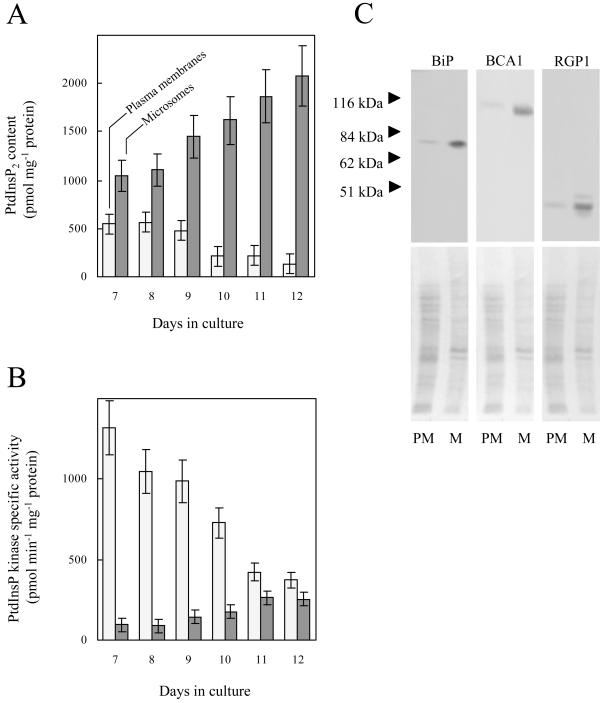
To characterize changes in the composition of plasma membranes during the stationary phase, plasma membranes from G. sulphuraria were enriched by aqueous two-phase partitioning and analyzed for PtdInsP2 content and specific activity of PtdInsP kinase. As indicated in Figure 1A, between d 7 and 12 the PtdInsP2 content in plasma membranes decreased from 549 ± 55 to 132 ± 95 pmol mg−1 protein. The in vitro formation of PtdInsP2 from exogenously supplied substrate (i.e. the specific activity of PtdInsP kinase) in plasma membranes concurrently decreased from 1,317 ± 145 to 374 ± 42 pmol min−1 mg−1 protein (Fig. 1B). In contrast, PtdInsP2 levels in microsomal membranes increased between d 7 and 12 from 1,050 ± 130 to 2,077 ± 265 pmol mg−1 protein (Fig. 1A), and the in vitro formation of PtdInsP2 by microsomal membranes from exogenously supplied substrate increased from 95 ± 32 to 254 ± 24 pmol min−1 mg−1 protein (Fig. 1B). In summary, PtdInsP2 and the specific activity of PtdInsP kinase in plasma membranes decreased during the stationary phase in contrast to a concurrent increase in microsomes.
Figure 1.
PtdInsP2 levels in the plasma membrane decrease during the stationary phase. The amounts of PtdInsP2 and the specific activity of PtdInsP kinase in plasma membranes and in microsomes were monitored over the period following the transition from logarithmic growth to the stationary phase. A, PtdInsP2 content in plasma membranes (light) and in microsomes (dark). B, Specific activity of PtdInsP kinase in plasma membranes (light) and in microsomes (dark). The results are averaged from two independent experiments, assayed in duplicate. C, Equal protein amounts (10 μg) of plasma membrane-enriched fractions (PM) and microsomes (M) were separated by SDS-PAGE and blotted to polyvinylidene difluoride (PVDF) membranes. Three sets of blots were prepared and independently subjected to immunodetection for BiP, an ER marker; BCA1, a vacuolar marker; and RGP1, a Golgi marker, as described in “Materials and Methods.”
The specific activity of the vanadate-sensitive ATPase was similar in plasma membrane-enriched fractions prepared from 7- and from 12-d-old cells (896 ± 100 and 943 ± 100 nmol min−1 mg−1 protein, respectively), indicating that distribution and activity of this plasma membrane enzyme did not significantly change in G. sulphuraria over the time period under investigation. Therefore, the decrease in PtdInsP2 and PtdInsP kinase activity in plasma membranes over the time period between d 7 and 12 and the concomitant increase in microsomes suggest that plasma membrane and microsomal PtdInsP2 pools are regulated differently. Marker proteins for endomembranes (the molecular chaperone binding protein, BiP, for the ER; BCA1 for the vacuole; and RGP1 for Golgi, as described in “Materials and Methods”) are significantly less abundant in the plasma membrane-enriched fraction compared with microsomes (Fig. 1C), indicating that the plasma membrane-enriched fraction has little endomembrane contamination.
The Levels of a Putative PtdInsP Kinase Decrease in Plasma Membranes during the Stationary Phase
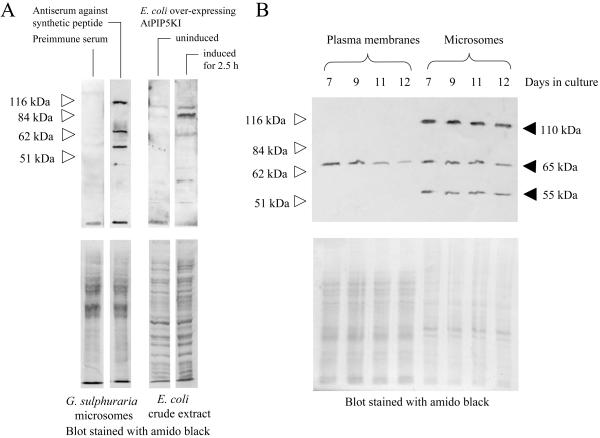
One way to regulate the intracellular distribution of PtdInsP2 is to alter the amount of PtdInsP kinase present. To test whether the levels of PtdInsP kinase protein changed, the enzyme was monitored by western-blot analysis of plasma membrane samples. Antisera against a conserved domain of an Arabidopsis PtdInsP kinase (AtPIP5KI), generated as described in “Materials and Methods,” cross-reacted with an active Arabidopsis PtdInsP 5 kinase expressed in Escherichia coli (Fig. 2A). The antiserum recognizes three putative PtdInsP kinase isoforms (55, 65, and 110 kD) in G. sulphuraria microsomes (Fig. 2A). Only the 65-kD protein was detected in plasma membranes from G. sulphuraria cells (Fig. 2B). The levels of this putative PtdInsP kinase protein decreased in plasma membranes between d 7 and 12 of the culture period, suggesting that the decrease in specific PtdInsP kinase activity and in PtdInsP2 levels in plasma membranes over this time period (compare Fig. 1) may have been due to a decreased level of PtdInsP kinase protein. No significant change in any of the immunopositive bands in microsomes was obvious over the time period recorded (Fig. 2B).
Figure 2.
A putative 65-kD PtdInsP kinase protein decreases in the plasma membrane during the stationary phase. Antisera were produced in rabbits against a synthetic 15-amino acid peptide representing a conserved PtdInsP 5 kinase domain from Arabidopsis. A, Equal protein amounts (20 μg) of microsomes from G. sulphuraria were separated by SDS-PAGE and blotted to PVDF membranes. Immunodetection with rabbit pre-immune serum, and with anti-PtdInsP kinase antiserum, as indicated. Soluble protein extracts from E. coli expressing AtPIP5KI were separated by SDS-PAGE and blotted to PVDF membranes. Immunodetection with anti-PtdInsP kinase antiserum with extract from uninduced cells, or from cells induced for 2.5 h with 1 mm IPTG, as indicated. Blots stained with amido black are shown (lower). B, Plasma membranes and microsomes were prepared from G. sulphuraria samples harvested over the period of time following the transition from logarithmic growth to the stationary phase. Equal amounts of protein (20 μg) were separated by SDS-PAGE and blotted to PVDF membranes. Upper, Immunodetection of PtdInsP kinase protein with the anti-PtdInsP kinase antiserum. Lower, Blot stained with amido black. White arrows indicate Mr markers.
Changes in PtdInsP2 Levels, Specific PtdInsP Kinase Activity, and InsP3 after Hyperosmotic Stimulation during the Stationary Phase
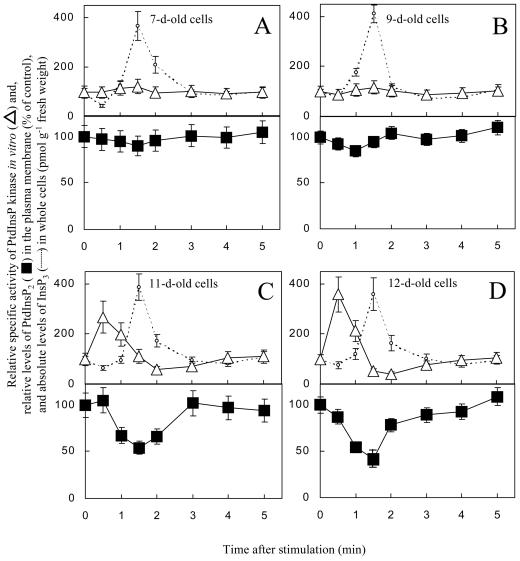
When G. sulphuraria cells were stimulated by the addition of 25 mm KCl in conditioned medium, the amount of PtdInsP2 lost from the plasma membrane increased from d 7 to 12 (Fig. 3). The timing of the PtdInsP2 loss in the plasma membrane correlated with the generation of InsP3 (dotted line; compare Heilmann et al., 1999), suggesting the hydrolysis of PtdInsP2 by phospholipase C. Because the analysis for PtdInsP2 was performed with isolated plasma membranes, whereas InsP3 levels have to be measured in whole cell extracts, no conclusion can be drawn as to whether the loss of plasma membrane PtdInsP2 accounts quantitatively for the amount of whole cell InsP3 generated.
Figure 3.
Changes in PtdInsP2 levels, specific activity of the PtdInsP kinase, and InsP3 levels after hyperosmotic stimulation. G. sulphuraria cultures were stimulated by the addition of 25 mm KCl in conditioned medium 7 (A), 9 (B), 11 (C), and 12 (D) d after transfer. Cells were harvested over the first few minutes after stimulation, and plasma membranes were isolated by aqueous two-phase partitioning. The plasma membranes were analyzed for PtdInsP2 content (squares), and PtdInsP kinase activity in vitro (triangles). Data are presented as the percentage of the conditioned medium control at each time point. Transient increases in InsP3 levels in the cells are represented by the dotted lines, given as pmol InsP3 g−1 fresh weight The results are averaged from two independent experiments, assayed in duplicate.
The absolute amounts of PtdInsP2 lost from plasma membranes of cells stimulated between d 7 and 12 were comparable. However, because the initial Ptd- InsP2 pool decreased over this time period, these amounts represent an increasing percentage of Ptd- InsP2 lost from the plasma membrane after stimulation (Fig. 3). To be specific, the hydrolysis of 55 ± 25 pmol PtdInsP2 mg−1 protein after stimulation of 7-d-old cells represented approximately 10% of the PtdInsP2 present in plasma membranes on d 7 (Fig. 3A). In contrast, a comparable loss of 78 ± 25 pmol PtdInsP2 mg−1 protein upon stimulation of 12-d-old cells represented approximately 60% of the PtdInsP2 present in the plasma membrane on d 12 (Fig. 3D).
Although in 7- and 9-d-old cells the specific activity of PtdInsP kinase in the plasma membranes did not change after stimulation, in 11- and 12-d-old cells PtdInsP kinase specific activity increased prior to changes in both InsP3 and in PtdInsP2. This suggests that the synthesis of PtdInsP2 prior to the generation of InsP3 was required only in cells with a reduced plasma membrane PtdInsP2 pool.
Changes in Specific PtdInsP Kinase Activity and in InsP3 after Severe Osmotic Stimulation during the Stationary Phase
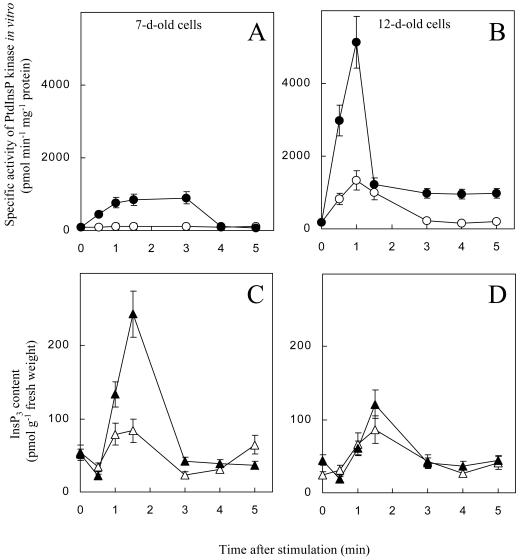
As seen in Figure 3, stimulation by 25 mm KCl in conditioned media did not activate PtdInsP kinase in 7-d-old cells. If the depletion of a plasma membrane PtdInsP2 pool dictates the demand for PtdInsP2 synthesis, then a more severe stimulus should deplete the plasma membrane PtdInsP2 pool and activate PtdInsP kinase in 7-d-old cells. To test this hypothesis, the effects of severe osmotic stimulation on the activation of PtdInsP kinase were studied in 7- and 12-d-old G. sulphuraria cells. Cultures were subjected to stimulation by 250 mm KCl in conditioned medium and to a milder stress by 12.5 mm KCl in conditioned medium (Fig. 4). In both 7- and 12-d-old cells, the osmotic stimulation caused transient increases in InsP3 with a maximum around 90 s (Fig. 4, C and D), consistent with our previous findings (Heilmann et al., 1999). Treatment with 12.5 mm KCl resulted in an approximately 2-fold increase in InsP3 with both 7- and 12-d-old cells, whereas stimulation by 250 mm KCl induced a 5-fold increase in 7-d-old cells, and only a 3-fold increase in 12-d-old cells. This result suggests that the signaling pool in 12-d-old cells had been drained by the severe osmotic stress and not been refilled. Of importance, stimulation by 250 mm KCl resulted in an increase in the specific activity of PtdInsP kinase in microsomes of both 7- and 12-d-old cells in addition to the InsP3 signal. In 7-d-old cells, the severe stimulus resulted in a less rapid increase and the activation was only about 12-fold (Fig. 4A). In contrast, PtdInsP kinase was activated by about 35-fold within 60 s in 12-d-old cells (Fig. 4B). The application of conditioned medium as a control produced a minor (approximately 30%), but reproducible, increase in the levels of InsP3 in 7- or 12-d-old cells (data not shown; compare Heilmann et al., 1999). It is important to notice that a mild stimulus (12.5 mm KCl) affected the PtdInsP kinase in the plasma membranes only in 12-d-old cells, resulting in a transient 8-fold increase in Ptd- InsP kinase activity (compare Fig. 4, A and B). Thus, the threshold of the osmostimulus necessary to activate PtdInsP kinase was higher for 7-d-old cells than for 12-d-old cells. As was reported before (Heilmann et al., 1999), stimulation with equivalent concentrations of methyl-Man and KCl induced very similar increases in PtdInsP kinase activity, suggesting that the observed changes were primarily the result of changes in osmotic potential.
Figure 4.
Changes in specific PtdInsP kinase activity and InsP3 after severe hyperosmotic stress. The specific activity of PtdInsP kinase in microsomes and the generation of InsP3 signals were monitored for the first few minutes after application 12.5 mm KCl (white symbols) or 250 mm KCl (black symbols). A and B, Specific activity of PtdInsP kinase after osmotic stimulation of 7-d-old cells (A) and 12-d-old cells (B). KCl (12.5 mm) affected PtdInsP kinase activity only in 12-d-old cells, not in 7-d-old cells. C and D, Levels of Ins(1,4,5)P3 after osmotic stimulation of 7-d-old cells (C) and 12-d-old cells (D). The results are averaged from two (PtdInsP kinase activity) or three (InsP3 levels) independent experiments, assayed in duplicate.
DISCUSSION
To characterize a signaling pool of PtdInsP2 in the plasma membrane of G. sulphuraria, the levels of PtdInsP2 and the specific activity of PtdInsP kinase were monitored in time course studies after osmotic stimulation, using cells from various time points of the growth cycle. By measuring the amounts of Ptd- InsP2 in plasma membranes from 7- and 12-d-old cells, we confirmed that plasma membranes of 7-d-old cells contained significantly more PtdInsP2 than those of 12-d-old cells. Between d 7 and 12, a gradual decrease in PtdInsP2 content and specific activity of PtdInsP kinase in plasma membranes contrasted with a concurrent increase in PtdInsP2 content and specific activity of PtdInsP kinase in microsomes, providing evidence for the presence of differentially regulated phosphoinositide pools within the cell.
There are several mechanisms by which phosphoinositide pools could be regulated. For example, the size of the plasma membrane PtdInsP2 pool may be regulated by increased transport of PtdIns or PtdInsP from the point of synthesis to the plasma membrane by lipid transfer proteins (Cunningham et al., 1995; Kauffmann-Zeh et al., 1995; Speed and Mitchell, 2000; for review, see Wirtz, 1997). In addition to altered lipid transfer, the distribution of enzymes involved in the synthesis of PtdInsP or PtdInsP2 could change with age. In plants, the specific activity of PtdIns 4-kinase is on average severalfold higher than that of PtdInsP kinase (Sommarin and Sandelius, 1988; Sandelius and Sommarin, 1990); therefore, the subsequent PtdInsP kinase reaction may be rate limiting and a candidate for a regulatory step in the synthesis of PtdInsP2. In G. sulphuraria, a decreasing amount of PtdInsP kinase protein in the plasma membrane could contribute to the decreases in PtdInsP kinase-specific activity and in the levels of PtdInsP2 between d 7 and 12.
Based on our previous observation that PtdInsP2 formation transiently increased in osmostimulated 12-d-old G. sulphuraria cells prior to the generation of InsP3 signals, we had hypothesized that PtdInsP kinase was activated to synthesize a PtdInsP2 pool for the generation of InsP3 signals (Heilmann et al., 1999). In this scenario, a stimulus-induced activation of PtdInsP kinase in the plasma membrane would serve as an indicator for a reduced signaling pool of PtdInsP2. The comparison of the osmotic stimulation of 7- and 12-d-old cells indicates that the stimulus intensity required to activate PtdInsP kinase was higher in 7-d-old cells (250 mm KCl or 460 mOsmol kg−1) than in 12-d-old cells (12.5 mm KCl or 28 mOsmol kg−1). The difference in the threshold for activation may be explained in part by the higher level of PtdInsP2 in the plasma membranes of 7-d-old cells. PtdInsP kinase activation after stimulation was sustained for a longer time in 7-d-old cells than in 12-d-old cells. In both 7- and 12-d-old cells, the need for the establishment of a signaling PtdInsP2 pool in plasma membranes ultimately was met by an increased activity of PtdInsP kinase upon osmotic stimulation. A similar activation of PtdInsP kinase preceding the generation of InsP3 in thrombin-stimulated platelets was reported by Lassing and Lindberg (1990).
The changes in PtdInsP kinase activity and Ptd- InsP2 levels during osmotic stress reported in this paper are transient within the first minutes of stimulation and localized to the plasma membrane. Einspahr et al. (1988), reported an increase in PtdIns(4,5)P2 after 4 min of hyperosmotic stress (3,280 mOsmol kg−1) in the halophilic green alga Dunaliella salina. Increases in whole plant cell PtdIns(3,5)P2 and PtdIns(4,5)P2 were reported by Meijer et al. (1999) and by Pical et al. (1999), respectively, after 5 min to 2 h of severe hyperosmotic stress (290–730 mOsmol kg−1). Increases in PtdIns(3,5)P2 were also reported for hyperosmotically stressed yeast (1,600 mOsmol kg−1; Dove et al., 1997). Meijer et al. (1999) suggested that the increases in PtdIns(3,5)P2 observed over time in osmotically stressed plants may be associated with intracellular membranes. Both PtdIns3P and PtdIns4P have been found in plant nuclei (Bunney et al., 2000), and the recent description of a possible function for PtdIns3P and PtdIns 3 kinase in the regulation of transcription in higher plants (Bunney et al., 2000) suggests a putative means of regulating transcription in response to stress. Thus, as cells adjust to hyperosmotic stress, after the initial stimulation, the increases in whole cell PtdIns(3,5)P2 and PtdIns(4,5)P2 could reflect a potential signaling cascade leading to changes in the patterns of both vesicle formation and gene expression (Hirayama et al., 1995; Mikami et al., 1998; Heilmann et al., 2000). Although it cannot be ruled out that PtdIns(3,5)P2 contributes to the total amount of phosphatidylinositol-bisphosphate observed in G. sulphuraria plasma membranes, neither phosphorylation of PtdIns to PtdIns3P nor phosphorylation of PtdIns3P to PtdIns(3,5)P2 were detected by in vitro assays of G. sulphuraria plasma membranes (data not shown).
As cells begin to adjust to osmotic stress to maintain cellular integrity, whole cell PtdInsP2 levels may be affected by changes in membrane biogenesis. Changes in cell volume in osmotically stressed mammalian cells have been shown to result in the rapid inhibition of transport processes from the ER to the Golgi apparatus, while leaving the retrograde traffic active (Lee and Linstedt, 1999). Consistent with these results, Kearns et al. (1998) proposed that changes in phosphorylation of the soybean phosphoinositide-specific lipid transfer protein Ssh1p during osmotic stress contribute to the accumulation of phosphoinositides in intracellular membranes. Increased accumulation of PtdIns(4,5)P2 could also result from increased de novo synthesis in the Golgi compartment (Jones et al., 2000).
Because whole cell PtdInsP2 increases in aging cells (Heim and Wagner, 1986; Falkenau et al., 1987; Borochov et al., 1994; Heilmann et al., 1999), to meaningfully interpret changes in phosphoinositide levels in a cell, the cellular distribution of phosphoinositides must be considered when a particular system is studied. Phosphoinositide pools may be defined by the dynamic rearrangement of both lipids and proteins, and the status of the pools may be characteristic for a particular developmental stage. For instance, if restructuring of PtdInsP2 pools over the time in culture in G. sulphuraria reflects a change in the physiological state of the cells after they depleted the heterotrophic substrate around d 7 (Gross and Schnarrenberger, 1995), then this process could include a functional shift of PtdInsP2 from plasma membrane signaling to the regulation of vesicle trafficking and cytoskeletal structure (Drøbak et al., 1994; Shibasaki et al., 1997; Staiger et al., 1997).
In conclusion, the levels of PtdInsP2 in a cell are dynamic and change in both a spatial and a temporal dimension. The decrease in plasma membrane Ptd- InsP2 over time described here, as well as increases in whole cell PtdInsP2 previously described by this group and others, emphasize the need to identify the discrete subcellular PtdInsP2 pools of each system being studied, both with development and in response to stimulation.
MATERIAL AND METHODS
G. sulphuraria Cell Culture
G. sulphuraria strain 002 (type strain from the culture collection of the University of Naples) was grown in the dark at 37°C and pH 2.0 in 2-L flasks shaking at 100 rpm. Glc (50 mm) was added as heterotrophic substrate to the liquid culture medium according to Gross and Schnarrenberger (1995). Cell growth was monitored spectrophotometrically at 800 nm. The time of inoculation of a new culture was defined as time zero. Three days after inoculation, cells entered the phase of logarithmic growth. The stationary phase of growth was reached 7 d after inoculation. Cells from stationary cultures were retransferred to new media after 21 d. The terms 7- and 12-d-old cells refer to cells 7 and 12 d after inoculation of the culture, respectively.
Prior to stimulation, G. sulphuraria cells were kept overnight in 50 mL of culture medium in 200-mL culture flasks at 26°C in the dark with shaking (150 rpm). Immediately before an experiment, conditioned culture medium was obtained by centrifuging 100 mL of a cell culture at 2,000g for 10 min at room temperature and decanting the medium. KCl and methyl-Man solutions were prepared fresh in conditioned medium for each experiment and adjusted to 26°C before use. Methyl-Man was used for an osmotically active sugar derivative as a control because, unlike sorbitol or mannitol, it is not taken up and metabolized by G. sulphuraria (W. Gross, unpublished data). Stimulation with equivalent osmolar concentrations of methyl-Man or KCl in conditioned medium resulted in similar effects on the activity of PtdInsP kinase or the generation of InsP3, ruling out nonspecific salt effects (compare Heilmann et al., 1999). Cells were stimulated by the addition of 5 mL of KCl or methyl-Man stocks in conditioned culture medium to final concentrations as indicated in the results. Conditioned medium alone was used as a control.
Isolation of Microsomes
G. sulphuraria cells from 50-mL cultures (0.5–1 g fresh weight) were harvested by centrifugation at 2,500g for 30 s and homogenized in 20 mL of ice-cold buffer [250 mm Suc, 3 mm EDTA, 2 mm ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid, 14 mm β-mercaptoethanol, 2 mm dithiothreitol, and 50 mm Tris-HCl, pH 7.4] with 0.1 g of polyvinylpolypyrrolidone, using a blender (VirTis Co., Gardiner, NY) and glass beads. For time course experiments, the times indicated denote the initiation of homogenization. Microsomal membranes were prepared by centrifuging the homogenate at 2,500g for 15 min at room temperature and centrifuging the 2,500-g supernatant at 41,000g for 60 min at 4°C. The 41,000g pellets were resuspended in 30 mm Tris-HCl, pH 6.5, containing 15 mm MgCl2. Microsomes were solubilized by the addition of 1% (v/v) Triton X-100 with shaking for 1 h at 4°C. Soluble and insoluble compounds were separated by centrifugation at 10,000g for 10 min.
Isolation of a Plasma Membrane-Enriched Fraction
For enrichment of plasma membranes, microsomal pellets were resuspended in 1 mL of 30 mm Tris-HCl, pH 7.4, and 15 mm MgCl2, and layered on 5.9% (w/v) polyethylene glycol/dextran polymer two-phase gradients (CarbowaxPEG 3350, Fisher Scientific, Pittsburgh; Dextran T500, Amersham Pharmacia Biotech, Piscataway, NJ) to separate the plasma membranes according to Wheeler and Boss (1987). The gradient solutions and membranes were mixed by inversion (80 times) and centrifuged at 600g for 10 min at 4°C. The upper phase was diluted to 30 mL with 30 mm Tris-HCl, pH 7.4, and 15 mm MgCl2, and centrifuged at 41,000g for 1 h at 4°C. The pellet was washed twice in 30 mm Tris-HCl, pH 7.4, and 15 mm MgCl2, centrifuged at 41,000g for 45 min at 4°C, and resuspended in the same buffer.
Estimation of Protein Contents
Protein concentrations were estimated by using the Bradford assay (Bio-Rad, Hercules, CA) with bovine serum albumine as a standard.
Characterization of the Plasma Membrane-Enriched Fraction
To ensure a comparable integrity of the plasma membranes studied, the vanadate-sensitive ATPase activity of plasma membrane preparations from 7- and 12-d-old cells was determined as total phosphate released in vitro according to Taussky and Shorr (1953). Plasma membrane samples (10 μg protein per reaction) were assayed in quadruplets in a buffer of 200 mm Suc, 25 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-KOH, pH 7.0, 10 mm KCl, 3 mm MgSO4, 3 mm ATP, 1 mm dithiothreitol, and 1 μm CaCl2 in a final reaction volume of 200 μL. The reaction mixture was incubated at room temperature for 30 min. Reactions were stopped by the addition of 2 mL NH4/FeSO4 reagent containing 1% (w/v) (NH4)2MoO4, 1 n H2SO4 and 0.18 m FeSO4. The A660 was monitored to quantify the inorganic phosphate generated. Samples containing between 0 and 0.1 μmol KH2PO4 per assay were used as standards. Reactions were incubated with or without 200 μm vanadate, and the difference in phosphate release was calculated. To determine the degree of purity of plasma membrane-enriched fractions achieved by two-phase partitioning, immunoblots were performed to monitor endomembrane contaminants, using antisera against marker proteins for ER (the chaperone, BiP; Fontes et al., 1991), Golgi (the reversibly glycosylated polypeptide, RGP1; Dhugga et al., 1997), and vacuole (the vacuolar Ca2+-ATPase, BCA1; Askerlund, 1996). Equal protein amounts from plasma membrane-enriched fraction or microsomes were analyzed (Fig. 1C). The term “plasma membrane” is to be read as “plasma membrane-enriched fraction” throughout the manuscript.
Lipid Kinase Assays
PtdInsP kinase activity was assayed as described by Cho and Boss (1995), using 20 μg of microsomal or 2 μg of plasma membrane protein per assay in 50 μL of reaction mixture. The reaction mixture contained 30 mm Tris-HCl, pH 6.5, 7.5 mm MgCl2, 1 mm Na2MoO4, 0.01% (v/v) Triton X-100, and 0.9 mm [γ-32P]ATP (0.2 μCi nmol−1). Reactions were incubated for 10 min at room temperature with intermittant mixing. For assays containing exogenous substrate, PtdIns4P (Sigma, St. Louis) was presolubilized in 1% (v/v) Triton X-100, and added to give a final concentration of 25 μg of lipid in 0.1% (v/v) Triton X-100 per reaction. After incubation, inositol phospholipids were extracted according to Cho et al. (1992).
Separation of Phospholipids
Lipids were separated by thin-layer chromatography on silica gel plates (type LK5D, Whatman, Clifton, NJ) by a solvent system containing CHCl3:methanol:NH4OH:H2O mixed in a ratio of respective volumes as 86:76:6:16 (Cho and Boss, 1995). The [32P]-labeled phospholipids were quantified using a radioimaging scanner (system 500, Bioscan, Inc., Washington, DC).
Quantification of Ins(1,4,5)P3 and PtdIns(4,5)P2 Content
For determination of Ins(1,4,5)P3 after various times of osmotic stimulation, G. sulphuraria cells were harvested by centrifugation at 2,000g for 10 to 30 s into preweighed tubes. The supernatant was discarded and cells were immediately frozen in liquid N2. The times indicated in the results denote the duration between the application of the stimulus and the freezing of the cells in liquid N2. The frozen cells were weighed and 500 μL of ice-cold 20% (v/v) perchloric acid (PCA) was added. After a 20-min incubation on ice, proteins were precipitated by centrifugation at 2,000g for 15 min at 4°C. For Ins(1,4,5)P3 assays, the supernatant was transferred to a new tube and adjusted to pH 7.5, using ice-cold 1.5 m KOH in 60 mm HEPES containing 5% (v/v) of universal pH indicator dye (Fisher Scientific). The neutralized samples were assayed for Ins(1,4,5)P3, using the [3H]Ins(1,4,5)P3 receptor-binding assay kit (Amersham Pharmacia Biotech). Assays were carried out along with controls for complete and nonspecific binding according to the manufacturer's instructions, using 50 μL of sample per assay in a total assay volume of 200 μL. The Ins(1,4,5)P3 content of each sample was determined by interpolation from a standard curve generated with commercial Ins(1,4,5)P3.
For the determination of PtdIns(4,5)P2, whole cell PCA precipitates were washed twice with ice-cold deionized water. Lipids were extracted from PCA precipitates or from microsomal or plasma membrane preparations according to Cho et al. (1992) and hydrolyzed by adding 1 mL of 1 m KOH and heating to 100°C for 15 min. After hydrolysis, samples were adjusted to pH 7.5 with 20% (v/v) PCA containing universal pH indicator dye. Fatty acids were removed by washing twice with 20% (v/v) petroleum ether in 1-butanol. A 500-μL aliquot of the aqueous phase from each sample was lyophilized, resuspended in 110 μL of deionized water, and assayed for Ins(1,4,5)P3.
Equal fresh weights of cells were used to compare how much PtdIns(4,5)P 2 was recovered from PCA precipitates of whole cells, microsomal pellets, or upper and lower phases of two-phase systems. Approximately 65% of the PtdIns(4,5)P2 measured in PCA-precipitates was recovered in microsomes, whereas 60% was recovered in the combined upper and lower phases of the two-phase systems. The soluble homogenate was not analyzed; therefore, lipid bound to soluble proteins cannot be taken into account.
To rule out the possibility that inositol phosphate metabolites other than Ins(1,4,5)P3 affected the displacement of [3H]Ins(1,4,5)P3 from the bovine Ins(1,4,5)P3 receptor in the assay, aliquots of the samples were pretreated with a recombinant human inositol polyphosphate 5′phosphatase I, as described by Perera et al. (1999). The content of Ins(1,4,5)P3 was determined before and after incubation with the phosphatase. The phosphatase pretreatment eliminated the Ins(1,4,5)P3 from G. sulphuraria samples. Heat-denatured phosphatase had no effect. These controls confirm the specificity of the assay for d-myo-inositol 1,4,5-trisphosphate. The human inositol polyphosphate 5′phosphatase I cDNA (Auethavekiat et al., 1997) was a gift from Dr. Philip Majerus (Washington University School of Medicine, St. Louis).
Design of a PtdInsP 5 Kinase Consensus Peptide and Production of Antisera
PtdInsP 5-kinases are characterized by four conserved domains common to all animal and plant PtdInsP 5-kinases. A consensus peptide was designed according to one of the conserved domains of seven Arabidopsis Ptd- InsP 5-kinase sequences. A BLAST (blastp) search against the nonredundant protein sequences using the 15-amino acid peptide DDRFMIKTVKKSEIK retrieved exclusively PtdInsP 5-kinase sequences of Arabidopsis. The template peptide was synthesized by Genosys Biotechnologies, Inc. (The Woodlands, TX). The synthetic peptide (200 μg in Complete Freund's Adjuvant) was injected into two rabbits (New Zealand white rabbit) to generate antisera against the synthetic peptide. Biweekly boost injections of the rabbits with 100 μg of the synthetic peptide in Incomplete Freund's Adjuvant were carried out three times before the first production bleed. ELISA experiments with first bleed antisera showed a several hundred-fold increase in reactivity with the syhthetic peptide compared with the preimmune sera. The third production bleed antisera used in this study cross-reacted with an active recombinant Ptd- InsP 5-kinase protein from Arabidopsis (AtPIP5KI, accession no. AF019380; Satterlee and Sussman, 1997) expressed in Escherichia coli (Fig. 2A). The synthetic peptide and antisera described are available upon request.
Affinity Purification of the Antipeptide Antibodies on Protein A Beads
To reduce nonspecific reactivity between the rabbit antisera and G. sulphuraria proteins in western blots, the antisera were purified on beads coated with protein A, specifically binding IgG antibodies. Protein A beads (Sigma) were washed twice in deionized water, filtered through a Büchner funnel, and rehydrated for 2 h in filtered 100 mm Tris-HCl, pH 8.0. The hydrated resin was equilibrated with 100 mm Tris-HCl, pH 8.0. Antiserum (2 mL) was adjusted to pH 8.0 by adding 200 μL of 1 m Tris-HCl, pH 8.0, and applied to the column. The column was washed sequentially with 100 mm Tris-HCl, pH 8.0, and 10 mm Tris-HCl, pH 8.0. The eluates of the loading and all washing steps were collected. Antibodies were eluted in 500-μL increments with 100 mm Gly, pH 3.0. Fractions of 500 μL were collected into microfuge tubes containing 50 μL 1 m Tris, pH 8.0, and the fractions were neutralized by gentle mixing.
Immunodetection (Western Blotting)
Proteins were separated by SDS-PAGE according to Laemmli (1970) in gels containing 8% (w/w) or 10% (w/w) polyacrylamide and transferred to PVDF membranes. Proteins were detected with affinity-purified rabbit antiserum against the synthetic PtdInsP 5-kinase peptide (diluted 1:5,000). Preimmune serum was used as a control. Primary antibodies were detected with goat anti-rabbit-IgG antisera conjugated to horseradish peroxidase. Horseradish peroxidase was detected using a chemiluminescence detection kit (Pierce, Rockford, IL) with subsequent exposure of X-Omat film (Eastman Kodak Co., Rochester, NY).
ACKNOWLEDGMENTS
The antisera against BiP, BCA1, and RGP1 were kindly provided by Dr. Rebecca Boston (North Carolina State University), Dr. Per Askerlund (Lund University, Sweden), and Dr. Kanwarpal Dhugga (Pioneer Hi-Bred International, Inc., Johnston, IA), respectively.
Footnotes
This work was supported by the National Science Foundation (grant no. MCB–9604285 to W.F.B.) and by a Deutscher Akademischer Austauschdienst fellowship (HSPIII to I.H.) financed by the German Federal Ministry of Education, Science, Research, and Technology.
LITERATURE CITED
- Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J Biol Chem. 1999;274:9907–9910. doi: 10.1074/jbc.274.15.9907. [DOI] [PubMed] [Google Scholar]
- Askerlund P. Modulation of an intracellular calmodulin-stimulated Ca2+-pumping ATPase in cauliflower by trypsin: the use of Calcium Green-5N to measure Ca2+transport in membrane vesicles. Plant Physiol. 1996;110:913–922. doi: 10.1104/pp.110.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auethavekiat V, Abrams CS, Majerus PW. Phosphorylation of platelet pleckstrin activates inositol polyphosphate 5-phosphatase I. J Biol Chem. 1997;272:1786–1790. doi: 10.1074/jbc.272.3.1786. [DOI] [PubMed] [Google Scholar]
- Balla T, Bondeva T, Varnai P. How accurately can we image inositol lipids in living cells? Trends Pharmacol Sci. 2000;21:238–241. doi: 10.1016/s0165-6147(00)01500-5. [DOI] [PubMed] [Google Scholar]
- Borochov A, Cho MH, Boss WF. Plasma membrane lipid metabolism of petunia petals during senescence. Phys Plant. 1994;90:279–284. [Google Scholar]
- Bunney TD, Watkins PA, Beven AF, Shaw PJ, Hernandez LE, Lomonossoff GP, Shanks M, Peart J, Drøbak BK. Association of phosphatidylinositol 3-kinase with nuclear transcription sites in higher plants. Plant Cell. 2000;12:1679–1688. doi: 10.1105/tpc.12.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MH, Boss WF. Transmembrane signaling and phosphoinositides. In: Galbraith DW, Bohnert HJ, Bourque DP, editors. Methods in Plant Cell Biology: Part A. New York: Academic Press; 1995. pp. 543–554. [DOI] [PubMed] [Google Scholar]
- Cho MH, Chen Q, Okpodu CM, Boss WF. Separation and quantification of [3H]inositol phospholipids using thin-layer chromatography and a computerized 3H imaging scanner. LC-GC. 1992;10:464–468. [Google Scholar]
- Ciruela A, Hinchliffe KA, Divecha N, Irvine RF. Nuclear targeting of the beta isoform of type II phosphatidylinositol phosphate kinase (phosphatidylinositol 5-phosphate 4-kinase) by its alpha-helix 7. Biochem J. 2000;346:587–591. [PMC free article] [PubMed] [Google Scholar]
- Cunningham E, Thomas GMH, Ball A, Hiles I, Cockroft S. Phosphatidylinositol transfer protein dictates the rate of inositol trisphosphate production by promoting the synthesis of PIP2. Curr Biol. 1995;5:775–783. doi: 10.1016/s0960-9822(95)00154-0. [DOI] [PubMed] [Google Scholar]
- Dhugga KS, Tiwari SC, Ray PM. A reversibly glycosylated polypeptide (RGP1) possibly involved in plant cell wall synthesis: purification, gene cloning, and trans-Golgi localization. Proc Natl Acad Sci USA. 1997;94:7679–7684. doi: 10.1073/pnas.94.14.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390:187–192. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- Drøbak BK, Dewey RE, Boss WF. Phosphoinositide kinases and the synthesis of polyphosphoinositides in higher plant cells. In: Jeon KW, editor. International Review of Cytology 189. San Diego: Academic Press; 1999. pp. 95–130. [DOI] [PubMed] [Google Scholar]
- Drøbak BK, Watkins PA, Valenta R, Dove SK, Lloyd CW, Staiger CJ. Inhibition of plant plasma membrane phosphoinositide phospholipase C by the actin binding protein, profilin. Plant J. 1994;6:389–400. [Google Scholar]
- Einspahr KJ, Maeda M, Thompson GA. Concurrent changes in Dunaliella salinaultrastructure and membrane phospholipid metabolism after hyperosmotic shock. J Cell Biol. 1988;107:529–538. doi: 10.1083/jcb.107.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenau C, Heim S, Wagner KG. Effect of cytokinins on the phospholipid phosphorylation of the suspension cultured Catharanthus roseuscells. Plant Sci. 1987;50:173–178. [Google Scholar]
- Fontes EBP, Shank BB, Wrobel RL, Moose SP, OBrian GR, Wurtzel ET, Boston RS. Characterization of an immunoglobulin binding protein homolog in the maize floury-2endosperm mutant. Plant Cell. 1991;3:483–496. doi: 10.1105/tpc.3.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross W, Küver J, Tischendorf G, Bouchaala N, Büsch W. Cryptoendolithic growth of the red alga Galdieria sulphurariain volcanic areas. Eur J Phycol. 1998;33:25–31. [Google Scholar]
- Gross W, Schnarrenberger C. Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol. 1995;36:633–638. [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34292–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Hama H, Takemoto JY, DeWald DB. Analysis of phosphoinositides in protein trafficking. Methods. 2000;20:465–473. doi: 10.1006/meth.2000.0959. [DOI] [PubMed] [Google Scholar]
- Heilmann I, Perera IY, Gross W, Boss WF. Changes in phosphoinositide metabolism with days in culture affect signal transduction pathways in Galdieria sulphuraria. Plant Physiol. 1999;119:1331–1339. doi: 10.1104/pp.119.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann I, Perera IY, Stevenson JM, Boss WF. Sense and sensibility: inositol phospholipids as mediators of abiotic stress responses. In: Cherry JH, Locy RD, Rychter A, editors. Plant Tolerance to Abiotic Stresses in Agriculture: Role of Genetic Engineering. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 285–296. [Google Scholar]
- Heim S, Wagner KG. Evidence of phosphorylated phosphatidylinositols in the growth cycle of suspension cultured plant cells. Biochem Biophys Res Comm. 1986;134:1175–1182. doi: 10.1016/0006-291x(86)90374-8. [DOI] [PubMed] [Google Scholar]
- Hinchliffe KA, Ciruela A, Irvine RF. PIPkins, their substrates and their products: new functions for old enzymes. Biochim Biophys Acta. 1998;1436:87–104. doi: 10.1016/s0005-2760(98)00140-4. [DOI] [PubMed] [Google Scholar]
- Hinchliffe KA, Ciruela A, Letcher AJ, Divecha N, Irvine RF. Regulation of type II alpha phosphatidylinositol phosphate kinase localization by the protein kinase CK2. Curr Biol. 1999a;9:983–986. doi: 10.1016/s0960-9822(99)80429-1. [DOI] [PubMed] [Google Scholar]
- Hinchliffe KA, Ciruela A, Morris JA, Divecha N, Irvine RF. The type II PIPkins (PtdIns5P 4-kinases): enzymes in search of a function? Biochem Soc Trans. 1999b;27:657–661. doi: 10.1042/bst0270657. [DOI] [PubMed] [Google Scholar]
- Hinchliffe KA, Irvine RF, Divecha N. Aggregation-dependent, integrin-mediated increases in cytoskeletally associated PtdInsP2(4,5) levels in human platelets are controlled by translocation of PtdIns 4-P 5-kinase C to the cytoskeleton. EMBO J. 1996;15:6516–6524. [PMC free article] [PubMed] [Google Scholar]
- Hirayama T, Ohto C, Mizoguchi T, Shinozaki K. A gene encoding a phosphatidylinositol-specific phospholipase C is induced by dehydration and salt stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1995;92:3903–3907. doi: 10.1073/pnas.92.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz RW, Hlubek MD, Sorensen SD, Fisher SK, Balla T, Ozaki S, Prestwich GD, Stuenkel EL, Bittner MA. A pleckstrin homology domain specific for phosphatidylinositol 4,5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2as being important in exocytosis. J Biol Chem. 2000;275:17878–17885. doi: 10.1074/jbc.M000925200. [DOI] [PubMed] [Google Scholar]
- Itoh T, Ijuin T, Takenawa T. A novel phosphatidylinositol-5-phosphate 4-kinase (phosphatidylinositol-phosphate kinase IIγ) is phosphorylated in the endoplasmic reticulum in response to mitogenic signals. J Biol Chem. 1998;273:20292–20299. doi: 10.1074/jbc.273.32.20292. [DOI] [PubMed] [Google Scholar]
- Janmey P. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Xian W, Flanagan LA. Controlling cytoskeleton structure by phosphoinositide-protein interactions: phosphoinositide binding protein domains and effects of lipid packing. Chem Phys Lipids. 1999;101:93–107. doi: 10.1016/s0009-3084(99)00058-4. [DOI] [PubMed] [Google Scholar]
- Jones DH, Morris JB, Morgan CP, Kondo H, Irvine RF, Cockroft S. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J Biol Chem. 2000;275:13962–13966. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Thomas GMH, Ball A, Prosser S, Cunningham E, Cockroft S, Hsuan JJ. Requirement for phosphatidylinositol transfer protein in epidermal growth factor signaling. Science. 1995;268:1188–1190. doi: 10.1126/science.7761838. [DOI] [PubMed] [Google Scholar]
- Kearns MA, Monks DE, Fang M, Rivas MP, Courtney PD, Chen J, Prestwich GD, Theibert AB, Dewey RE, Bankaitis VA. Novel developmentally regulated phosphoinositide binding proteins from soybean whose expression bypasses the requirement for an essential phosphatidylinositol transfer protein in yeast. EMBO J. 1998;17:4004–4017. doi: 10.1093/emboj/17.14.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua N-H. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lassing I, Lindberg U. Polyphosphoinositide synthesis in platelets stimulated with low concentrations of thrombin is enhanced before the activation of phospholipase C. FEBS Lett. 1990;262:231–233. doi: 10.1016/0014-5793(90)80197-q. [DOI] [PubMed] [Google Scholar]
- Lee TH, Linstedt AD. Osmotically induced cell volume changes alter anterograde and retrograde transport, Golgi structure, and COPI dissociation. Mol Biol Cell. 1999;10:1445–1462. doi: 10.1091/mbc.10.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer PJ, Wennstrom S, Kupzig S, Venkateswarlu K, Downward J, Cullen PJ. Identification of the ras GTPase-activating protein GAP1(m) as a phosphatidylinositol-3,4,5-trisphosphate-binding protein in vivo. Curr Biol. 1999;9:265–268. doi: 10.1016/s0960-9822(99)80116-x. [DOI] [PubMed] [Google Scholar]
- Meijer HJG, Divecha N, van den End H, Musgrave A, Munnik T. Hyperosmotic stress induces rapid synthesis of phosphatidyl-D-inositol 3,5-bisphosphate in plant cells. Planta. 1999;208:294–298. [Google Scholar]
- Memon AR, Chen Q, Boss WF. Inositol phospholipids activate plasma membrane ATPase in plants. Biochem Biophys Res Comm. 1989;162:1295–1301. doi: 10.1016/0006-291x(89)90814-0. [DOI] [PubMed] [Google Scholar]
- Merola A, Castaldo R, DeLuca P, Gambardella R, Musachio A, Taddei R. Revision of Cyanidium caldarium: three species of acidophilic algae. G Bot Ital. 1981;115:189–195. [Google Scholar]
- Mikami K, Katagiri T, Iuchi S, Yamaguchi-Shinozaki K, Shinozaki K. A gene encoding phosphatidylinositol-4-phosphate 5-kinase is induced by water stress and abscisic acid in Arabidopsis thaliana. Plant J. 1998;15:563–568. doi: 10.1046/j.1365-313x.1998.00227.x. [DOI] [PubMed] [Google Scholar]
- Munnik T, Irvine RF, Musgrave A. Phospholipid signaling in plants. Biochim Biophys Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Boss WF. Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA. 1999;96:5838–5834. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Chang SC, Boss WF, Kaufman PB. A role for inositol 1,4,5-trisphosphate in gravitropic signaling and the retention of cold-perceived gravistimulation of oat shoot pulvini. Plant Physiol. 2001;125:1499–1507. doi: 10.1104/pp.125.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pical C, Westergren T, Dove SK, Larsson C, Sommarin M. Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4,5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thalianacells. J Biol Chem. 1999;274:38232–38240. doi: 10.1074/jbc.274.53.38232. [DOI] [PubMed] [Google Scholar]
- Sandelius AS, Sommarin M. Membrane-localized reactions involved in polyphosphoinositide turnover in plants. In: Morré DJ, Boss WF, Loewus FA, editors. Inositol Metabolism in Plants. New York: Wiley-Liss; 1990. pp. 139–161. [Google Scholar]
- Satterlee JS, Sussman MR. An Arabidopsis phosphatidylinositol 4-phosphate 5-kinase homolog with seven novel repeats rich in aromatic and glycine residues (accession no. AF019380; PGR 97-150) Plant Physiol. 1997;115:864. [Google Scholar]
- Shibasaki Y, Ishihara H, Kizuki N, Asano T, Oka Y, Yazaki Y. Massive actin polymerization induced by phosphatidylinositol-4-phosphate 5-kinase in vivo. J Biol Chem. 1997;272:7578–7581. doi: 10.1074/jbc.272.12.7578. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Barbieri A, Gumusboga A, Cukras C, Pike L, Davis JN, Stahl PD, Nichols CG. Modulation of nucleotide sensitivity of ATP-sensitive potassium channels by phosphatidylinositol-4-phosphate 5-kinase. Proc Natl Acad Sci USA. 2000;97:937–941. doi: 10.1073/pnas.97.2.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATPchannels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Smith DW, Brock TD. The water relations of the alga Cyanidium caldariumin soil. J Gen Microbiol. 1973;79:219–231. [Google Scholar]
- Sommarin M, Sandelius AS. Phosphatidylinositol and phosphatidylinositolphosphate kinases in plant plasma membranes. Biochim Biophys Acta. 1988;958:268–278. [Google Scholar]
- Speed CJ, Mitchell CA. Sustained elevation of inositol 1,4,5-trisphosphate results in inhibition of phosphatidylinositol transfer protein activity and chronic depletion of the agonist-sensitive phosphoinositide pool. J Cell Sci. 2000;113:2631–2638. doi: 10.1242/jcs.113.14.2631. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Gibbon BC, Kovar DR, Zonia LE. Profilin and actin-depolymerizing factor: modulators of actin organization in plants. Trends Plant Sci. 1997;2:275–281. [Google Scholar]
- Stevenson JM, Perera IY, Heilmann I, Persson S, Boss WF. Inositol signaling and plant growth. Trends Plant Sci. 2000;5:252–258. doi: 10.1016/s1360-1385(00)01652-6. [DOI] [PubMed] [Google Scholar]
- Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- Toker A. The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr Cell Biol. 1998;10:254–261. doi: 10.1016/s0955-0674(98)80148-8. [DOI] [PubMed] [Google Scholar]
- Tuominen EK, Holopainen JM, Chen J, Prestwich GD, Bachiller PR, Kinnunen PK, Janmey PA. Fluorescent phosphoinositide derivatives reveal specific binding of gelsolin and other actin regulatory proteins to mixed lipid bilayers. Eur J Biochem. 1999;263:85–92. doi: 10.1046/j.1432-1327.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- Vancurova I, Choi JH, Lin H, Kuret J, Vancura A. Regulation of phosphatidylinositol 4-phosphate 5-kinase from Schizosaccharomyces pombeby casein kinase I. J Biol Chem. 1999;274:1147–1155. doi: 10.1074/jbc.274.2.1147. [DOI] [PubMed] [Google Scholar]
- Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J Biol Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- Wheeler JJ, Boss WF. Polyphosphoinositides are present in plasma membranes isolated from fusogenic carrot cells. Plant Physiol. 1987;85:389–392. doi: 10.1104/pp.85.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz KW. Phospholipid transfer proteins revisited. Biochem J. 1997;324:353–360. doi: 10.1042/bj3240353. [DOI] [PMC free article] [PubMed] [Google Scholar]