Abstract
Background
The incidence of contrast-induced acute kidney injury (CI-AKI) in the general population ranges from 0.6 to 2.3%, whereas for specific high-risk patients, the incidence can reach more than 30–40%. Ultrasound measurements of the development of CI-AKI after contrast-enhanced imaging for diagnosis in the emergency department (ED) have yet to be adequately studied. Accordingly, we aimed to evaluate the usefulness of Doppler ultrasound measurements for predicting CI-AKI in patients with normal renal function.
Methods
This prospective, observational, single-center study was conducted in the ED of a tertiary teaching and research hospital between 1 January and 1 July 2024. All patients who presented to the tertiary training and research hospital ED, who were admitted to the hospital with a decision to undergo contrast-enhanced tomography for diagnosis, and who did not meet any exclusion criteria were included in the study. Patients included in the study were evaluated by ultrasonographic measurements (interlobar renal artery peak systolic velocity (PSV), interlobar renal artery end-diastolic velocity (EDV), inferior vena cava (IVC) collapsibility index, and renal resistive index (RRI)).
Results
The postcontrast RRI cutoff values were calculated to predict CI-AKI. The area under the curve (AUC) for the postcontrast RRI was 0.914, and the cutoff value for the postcontrast RRI was 0.70 (≥), exhibiting 72.7% sensitivity and 95.6% specificity.
Conclusion
Postcontrast RRI ultrasound measurements performed after diagnostic contrast imaging in the ED show high specificity in predicting CI-AKI development. Postcontrast ultrasound measurements may predict CI-AKI development, allowing further measures to be taken. Further studies are needed to confirm these findings.
Trial registration
Clinical trial number: not applicable.
Keywords: Contrast-induced acute kidney injury, Renal resistive index, Ultrasound
Introduction
Contrast-induced acute kidney injury (CI-AKI) is a major complication of exposure to iodinated contrast media utilized in diagnostic or interventional practices [1]. CI-AKI is the leading cause of hospital-acquired AKI [2]. The incidence of CI-AKI in the general population ranges from 0.6 to 2.3%, whereas for specific high-risk patients, the incidence can reach more than 30–40% [3, 4]. Generally, CI-AKI is defined as a 25% relative increase in serum creatinine or an absolute increase of 0.5 mg/dl within 48 h after contrast agent exposure [5–7]. Although there are many definitions of CI-AKI, the new KDIGO AKI definition applies to CI-AKI and allows us to use a common language [8]. Accordingly, The Acute Kidney Injury Network defines CI-AKI as the occurrence of at least 1 of 3 conditions within 48 h of contrast administration (absolute increase in serum creatinine ≥ 0.3 mg/dL (27 μmol/L) from baseline, relative increase in serum creatinine levels ≥ 50% from baseline, decrease in urine output ≤ 0.5 mL/kg/h for at least 6 h) [9]. CI-AKI usually causes transient and reversible acute renal failure [10]. Although most cases are brief and reversible, CI-AKI is associated with significant morbidity and mortality, including chronic renal failure and cardiovascular events [11, 12]. Although very few patients will require renal replacement therapy for renal function, approximately 25–30% of CI-AKI will progress to chronic renal failure [13, 14]. In addition, CI-AKI increases the average length of hospital stay. Therefore, CI-AKI is associated with more extended hospitalization and higher healthcare costs [15].
Given the importance of CI-AKI, several risk scoring systems have been developed to help predict the likelihood of CI-AKI occurring [16, 17]. Furthermore, there are studies in which CI-AKI can be predicted based on ultrasound measurements [18, 19]. In addition, it has also been suggested that it may predict the development of CI-AKI in patients with preserved kidney function [20].
Many studies have supported the use of medical history, physical examination, laboratory parameters, and ultrasound measurements to predict CI-AKI development. Most studies on ultrasound measurements have focused on the development of CI-AKI after coronary angiography [16, 17, 19, 20]. Ultrasound (US) measurements of the development of CI-AKI after contrast-enhanced imaging for diagnosis in the emergency department (ED) have yet to be adequately studied. Based on the hypothesis that ultrasound can rapidly predict CI-AKI that develops due to contrast-enhanced tomography imaging in the ED, we aimed to investigate the diagnostic value of bedside ultrasound with repeated renal Doppler measurements in predicting CI-AKI in contrast-received patients.
Methods
Study design and setting
This prospective, observational, single-center study was conducted in the ED of a tertiary teaching and research hospital in Turkey between 1 January and 1 July 2024. Local ethical committee approval was obtained before commencing the study (Decision No 2023/278).
Historically, Wybraniec et al. reported that a renal resistive index (RRI) > 0.69 measured before the percutaneous coronary intervention (PCI) procedure had a sensitivity of 78% and a specificity of 81% for CI-AKI prediction [19]. Similarly, we hypothesized that the RRI measured before contrast-enhanced CT would have a sensitivity similar to or greater than that of CI-AKI prediction. Hence, to achieve a sensitivity of 90% via a 95% confidence interval (CI) and an expected incidence of approximately 10–40% (based on the expected prevalence in hospitalized and high-risk patients [3, 4]), we estimated the required sample size for the study to be 97 to 385, respectively. After calculating the estimated dropout ratio of 10%, the final sample size was set as 87 to 346.
Patient selection
All patients who presented to the tertiary training and research hospital ED, who decided to undergo contrast-enhanced tomography for diagnosis and did not meet any exclusion criteria, were included in the study. The study population was established after the inclusion and exclusion criteria were applied. The study population and design are shown in Fig. 1.
Fig. 1.
The study population and study design
Endpoints
The primary aim of this study was to evaluate the ability of ultrasonographic measurements to predict CI-AKI diagnosed by an absolute increase in serum creatinine ≥ 0.3 mg/dL (27 μmol/L) from baseline or a relative increase in serum creatinine ≥ 50% from baseline or a decrease in urine output ≤ 0.5 mL/kg/h for at least 6 h [9].
Study protocol and data collection
Initial patient evaluations in the ED were performed by an on-duty emergency medicine physician. All patients who presented to the tertiary training and research hospital ED and who were admitted to the hospital with a decision to undergo contrast-enhanced tomography for diagnosis were included in the study after informed consent was obtained. The emergency medicine physician who performed the initial evaluation recorded all the patients’ vital signs, physical examination findings, demographic characteristics, and comorbid conditions at the time of admission. In addition, the laboratory parameters (urea, creatine, and glomerular filtration rate (GFR)) of the patients on admission and the laboratory parameters (urea, creatine, and GFR) measured 48 h later were extracted from the hospital’s digital archive and recorded. Primary diagnoses and treatments were made and administered by the emergency medicine physicians who performed the initial evaluation.
One emergency medicine specialist performed US examinations. The entire ultrasonographic measurement protocol was performed by this emergency medicine specialist. This emergency medicine specialist had no responsibility for the patient’s primary care. This emergency medicine specialist who performed the US evaluation had participated in and successfully completed US courses (basic and advanced) that were certified by professional emergency medicine associations (five years of experience in POCUS with an average of 500 ultrasounds per year). Also, this emergency medicine specialist who conducted the US evaluations was blinded to the patient’s laboratory parameters, vital findings, diagnoses, and treatments. Ultrasound measurements were performed twice for each patient before and after contrast therapy (2 h after contrast therapy). The postcontrast measurement time was based on the half-life of the contrast medium (iohexol, 300 mg I/mL).
The same contrast medium (iohexol, 300 mg I/mL) was used for all patients. The emergency medicine physician determined the volume of the contrast medium according to the patient’s needs (usually at a dose of 1–3 mL/kg). Post-contrast hydration with intravenous 0.9% saline was started at 1 mL/kg/hour and continued until 6 h after the process. The MEHRAN score was calculated for all patients after determination of the amount of contrast medium to be administered. Mehran score, CI-AKI by Mehran et al., is a risk scoring system developed for prediction. According to the risk score, hypotension was 5 points, the use of an intra-aortic balloon pump was 5 points, congestive heart failure was 5 points, age > 75 years was 4 points, anemia was 3 points, diabetes was 3 points, every 100 cc3 contrast volume was 1 point, GFR 40–60 ml/min/1.73 m2 was 2 points, GFR 20–40 ml/min/1.73 m2 was 4 points, and GFR < 20 ml/min/1.73 m2 was 6 points. The risk of CI-AKI development is 7.5% if the risk score is ≤ 5, 14.0% if the risk score is 6–10, 26.1% if the risk score is 11–16, and 57.3% if the risk score is ≥ 16 [16].
Ultrasound protocol
Sonographic examinations were performed on a Fujifilm-SonoSite-FC1 (FUJIFILM SonoSite Inc., Bothell, WA, USA, 2015) model US device. Renal Doppler measurements were made with a 3.5-5 MHz convex (abdominal) probe, and inferior vena cava measurements were made with a 1–5 MHz sector (cardiac) probe. The study protocol included PW Doppler measurements for renal assessment in the coronal view and M-mode measurements of the inferior vena cava (IVC) in the sagittal view. Firstly, interlobar renal artery peak systolic velocity (PSV), interlobar renal artery end-diastolic velocity (EDV), and renal vein waveform measurements were evaluated by PW Doppler at the junction of the renal pelvis and renal cortex. Then, the IVC collapsibility index was measured using M-mode evaluation in a sagittal view. RRI was calculated using the following formula: (PSV-EDV)/PSV. The IVC collapsibility index was calculated via the ((Expiratory Diameter-Inspiratory Diameter/Expiratory Diameter) *100) process. Renal parenchymal vein waveforms were recorded as continuous, pulsatile, biphasic, or monophasic. Renal parenchymal vein Doppler pattern is usually a continuous flow. When the systolic component of the wave pattern decreases, the initially continuous flow pattern turns into a pulsatile pattern. If the systolic component of the wave continues to decrease, the pattern becomes biphasic (systolic/diastolic phases). If the flow in the systolic component of the wave disappears completely, the wave pattern becomes monophasic (Fig. 1). All these measurements are summarized in Fig. 2.
Fig. 2.
US measurements
Statistical analysis
All analyses were performed on Jamovi version 1.6 statistical software (The Jamovi Project (2021) Computer Software, version 1.6. Sidney, Australia). Categorical data are expressed as frequencies (n) and percentages. Normally distributed continuous variables are presented as the mean plus standard deviation (SD), and nonnormally distributed data are presented as the median and interquartile range (IQR). The normality of the distribution was evaluated using the Shapiro‒Wilk test. In comparing the continuous variables, groups with a normal distribution were compared with the t test, and those lacking such a distribution were compared with the Mann‒Whitney U test. The chi-squared test was used to compare categorical variables between groups. A receiver operating characteristic (ROC) curve was generated to determine the cutoff values for the postcontrast RRI, postcontrast EDV and MEHRAN score. In the ROC analysis, Youden’s index (maximum value) was used to select the cutoff value. Finally, sensitivity, specificity, likelihood ratios (+ LR and -LR), and positive and negative predictive values were calculated for the postcontrast RRI, postcontrast EDV and MEHRAN score. P values < 0.05 were considered significant for all analyses.
Results
During the study period, contrast-enhanced tomography was performed on 873 patients. Seven hundred seventy-one patients were excluded from the study, and the study population comprised 102 patients. In the study population, eleven patients developed CI-AKI, and 91 patients did not develop CI-AKI. Fifty-four (52.9%) of the patients enrolled were men, and 48 (47.1%) were women. The patients’ median age was 62 years, with an IQR of 48.5–76 years. Hypertension (HT) and diabetes were the most common comorbid diseases. According to the primary diagnoses, the most requested contrast-enhanced CT scans were abdominal and thorax-abdominal CT. Analysis of the differences between the CI-AKI and non-CI-AKI groups shows that the group that developed CI-AKI was statistically significantly older. In addition, the two groups had statistically significant differences in demographic data such as HT, Coronary Artery Disease (CAD), atrial fibrillation, and laboratory parameters such as urea, creatinine, and GFR. The patients’ demographic data, initial vital signs, baseline characteristics and statistics of these data are shown in Table 1.
Table 1.
The patients’ demographic data and baseline characteristics
| Characteristics | All Patients (N = 102) | CI-AKI (-) (N = 91) | CI-AKI (+) (N = 11) |
P Value |
|---|---|---|---|---|
| Gender | ||||
|
Male, n (%) Female, n (%) |
54 (52.9) 48 (47.1) |
47 (46.0) 44 (43.2) |
7 (6.9) 4 (3.9) |
0.452 |
| Age (years), median (IQR) | 62 (48.5–76) | 60 (47–74) | 77 (72–79) | 0.007 |
| BMI (kg/m2), mean ± sd | 27.3 ± 3.9 | 27.5 ± 4.0 | 25.8 ± 1.9 | 0.136 |
| Comorbidities | ||||
|
Hypertension, n (%) Diabetes, n (%) CAD, n (%) COPD, n (%) CHF, n (%) Atrial Fibrillation, n (%) Stroke, n (%) Dementia, n (%) Neoplasia, n (%) |
45 (44.1) 19 (18.6) 13 (12.7) 3 (2.9) 2 (2.0) 10 (9.8) 5 (4.9) 4 (3.9) 13 (12.7) |
35 (34.3) 15 (14.7) 9 (8.8) 3 (2.9) 2 (2.0) 7 (6.9) 5 (4.9) 3 (2.9) 10 (9.8) |
10 (9.8) 4 (3.9) 4 (3.9) 0 (0) 0 (0) 3 (2.9) 0 (0) 1 (1.0) 3 (2.9) |
0.001 0.110 0.013 0.541 0.619 0.039 0.425 0.350 0.126 |
| Vital Signs | ||||
|
SBP (mmHg), median (IQR) DBP (mmHg), median (IQR) Pulse (bpm), median (IQR) |
130 (120–140) 80 (70–80) 79 (75–85) |
130 (120–140) 80 (70–80) 78 (75–85) |
120 (105–140) 80 (70–90) 80 (75–86) |
0.196 0.402 0.543 |
| Admission Laboratory Indices | ||||
|
Urea (mg/dL), median (IQR) Creatine (mg/dL), median (IQR) GFR (ml/min/1.73m2), mean ± sd |
33.5 (27.0-45.8) 0.78 (0.61–0.91) 92.8 ± 18.2 |
32.0 (26.0-40.5) 0.76 (0.61–0.89) 95.5 ± 17.1 |
49.0 (45.0-56.5) 0.92 (0.89–1.02) 70.7 ± 11.8 |
0.001 0.001 0.001 |
| 48th Hour Laboratory Indices | ||||
|
Urea (mg/dL), median (IQR) Creatine (mg/dL), median (IQR) GFR (ml/min/1.73m2), mean ± sd |
31 (25.0-44.8) 0.80 (0.67–0.94) 88.8 ± 23.3 |
30.0 (23.5–38.5) 0.78 (0.66–0.86) 93.8 ± 19.0 |
67.0 (57.0–72.0) 1.30 (1.23–1.41) 47.4 ± 10.7 |
0.001 0.001 0.001 |
| Contrast volume (mL), mean ± sd | 118 ± 14.5 | 118 ± 15.0 | 115 ± 8.2 | 0.433 |
| Primary Diagnoses | ||||
|
Stroke, n Cerebral Venous Sinus Thrombosis, n Pulmonary Embolism, n Aortic Dissection, n Aortic Aneurysm Rupture, n Mesentery Ischaemia, n Acute Abdomen, n |
14 3 22 20 5 4 34 |
11 3 21 18 3 4 31 |
3 0 1 2 2 0 3 |
0.240 |
| Contrast-enhanced CT | ||||
|
Contrast-enhanced Brain-Neck CT, n Contrast-enhanced Thorax CT, n Contrast-enhanced Abdominal CT, n Contrast-enhanced Thorax-Abdominal CT, n |
17 22 38 25 |
14 21 35 21 |
3 1 3 4 |
0.430 |
IQR: Interquartile Range (25p, 75p), sd: standard deviation, CI-AKI: Contrast-Induced Acute Kidney Injury, BMI: Body Mass Index, CAD: Coronary Artery Disease, COPD: Chronic Obstructive Pulmonary Disease, CHF: Congestive Heart Failure, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure, GFR: Glomerular Filtration Rate, bpm: beats per minute
Analysis of precontrast measurements using PW Doppler in the renal artery coronal view revealed a median PSV of 27.4 cm/s, with an IQR of 25.3–32.7 cm/s, a median EDV of 9.4 cm/s, an IQR of 8.7–11.0 cm/s, a median RRI of 0.65, and an IQR of 0.63–0.68. Similarly, analysis of postcontrast measurements using PW Doppler in the renal artery coronal view revealed a median PSV of 28.1 cm/s, with an IQR of 25.9–31.1 cm/s, a median EDV of 9.4 cm/s, an IQR of 8.7–10.2 cm/s, and a median RRI of 0.66, with an IQR of 0.64–0.69. An evaluation of the MEHRAN score and ultrasound measurements and a statistical summary are shown in Table 2.
Table 2.
Statistical analysis of MEHRAN score and ultrasound measurements
| Pre-Contrast Ultrasound Measurements |
All Patients (N = 102) |
CI-AKI (-) (N = 91) |
CI-AKI (+) (N = 11) |
P Value |
|---|---|---|---|---|
| PSV (cm/s), median (IQR) | 27.4 (25.3–32.7) | 27.3 (25.3–30.9) | 28.7 (25.5–35.1) | 0.176 |
| EDV (cm/s), median (IQR) | 9.4 (8.7–11.0) | 9.4 (8.7–10.9) | 9.7 (9.1–10.9) | 0.503 |
| RRI (%), median (IQR) | 0.65 (0.63–0.68) | 0.65 (0.63–0.67) | 0.67 (0.66–0.68) | 0.086 |
| IVC-CI (%), median (IQR) | 0.25 (0.20–0.32) | 0.26 (0.20–0.32) | 0.21 (0.19–0.24) | 0.124 |
| RVP | ||||
| Continuous, n | 94 | 85 | 9 | |
| Pulsatile, n | 8 | 6 | 2 | 0.177 |
| Biphasic, n | 0 | 0 | 0 | |
| Monophasic, n | 0 | 0 | 0 |
| Post-Contrast (2nd Hour) Ultrasound Measurements |
All Patients (N = 102) |
CI-AKI (-) (N = 91) |
CI-AKI (+) (N = 11) |
P Value |
|---|---|---|---|---|
| PSV (cm/s), median (IQR) | 28.1 (25.9–31.1) | 28.0 (25.4–30.8) | 29.4 (28.5–31.6) | 0.103 |
| EDV (cm/s), median (IQR) | 9.4 (8.7–10.2) | 9.5 (8.8–10.5) | 9.0 (8.1–9.3) | 0.022 |
| RRI (%), median (IQR) | 0.66 (0.64–0.69) | 0.66 (0.64–0.68) | 0.71 (0.69–0.72) | 0.001 |
| IVC-CI (%), median (IQR) | 0.26 (0.21–0.31) | 0.27 (0.21–0.32) | 0.21 (0.21–0.22) | 0.103 |
| RVP | ||||
| Continuous, n | 96 | 87 | 9 | |
| Pulsatile, n | 6 | 4 | 2 | 0.066 |
| Biphasic, n | 0 | 0 | 0 | |
| Monophasic, n | 0 | 0 | 0 | |
| MEHRAN Score, median (IQR) | 1 (1–5) | 1 (1–4) | 5 (4–5) | 0.005 |
IQR: Interquartile Range (25p, 75p), CI-AKI: Contrast-Induced Acute Kidney Injury, PSV: Peak Sistolic Velocity, EDV: End Diastolic Velocity, RRI: Renal Resistive Index, IVC-CI: Inferior Vena Cava Collapsibility Index, RVP: Renal Venous Pattern
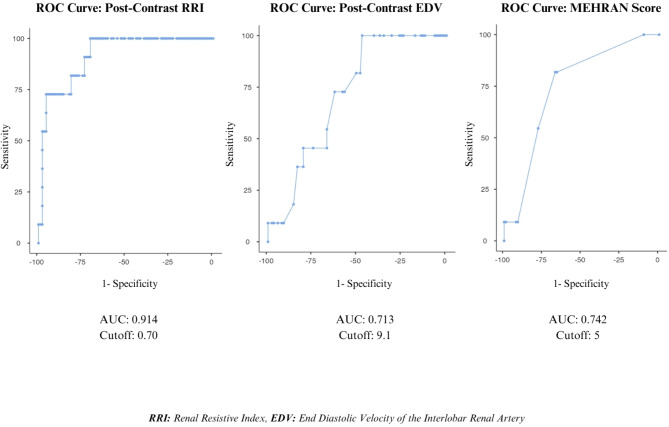
The postcontrast RRI, postcontrast EDV, and MEHRAN score cutoff values were calculated to predict CI-AKI. The area under the curve (AUC) for the postcontrast RRI was 0.914, and the cutoff value for the postcontrast RRI was 0.70 (≥), exhibiting 72.7% sensitivity and 95.6% specificity. The AUC for the postcontrast EDV was 0.713, and the cutoff value for postcontrast EDV was 9.1 (≤), exhibiting 72.7% sensitivity and 62.6% specificity. The AUC for the MEHRAN score was 0.742, and the cutoff value for the MEHRAN score was 5 (≥), exhibiting 54.6% sensitivity and 78.0% specificity. The postcontrast RRI, postcontrast EDV, and MEHRAN cutoff values for predicting CI-AKI according to the receiver operating characteristic (ROC) curve analysis are shown in Table 3; Fig. 3.
Table 3.
The cut-off values of Ultrasound measurements and MEHRAN score
| Post-Contrast RRI | Post-Contrast EDV | MEHRAN Score | |
|---|---|---|---|
|
AUC Cutoff* Sensitivity (%), (95% CI) Specificity (%), (95% CI) + LR, (95% CI) - LR, (95% CI) PPV (%), (95% CI) NPV (%), (95% CI) Accuracy (%), (95% CI) P Value |
0.914 0.70 72.7 (39.0–94.0) 95.6 (89.1–98.8) 16.6 (5.9–46.1) 0.3 (0.11–0.8) 66.7 (41.8–84.8) 96.7 (91.7–98.7) 93.1 (86.4–97.2) 0.001 |
0.713 9.1 72.7 (39.0-93.9) 62.6 (51.9–72.6) 1.95 (1.2–3.1) 0.4 (0.2–1.2) 19.1 (13.1–26.9) 95.0 (87.7–98.1) 63.7 (53.6–73.1) 0.003 |
0.742 5 54.6 (23.4–83.3) 78.0 (68.1–86.0) 2.5 (1.3–4.8) 0.6 (0.3–1.2) 23.1 (13.4–36.8) 93.4 (88.1–96.5) 75.5 (66.0-83.5) 0.004 |
*: Youden’s index (maximum value) was used to determine the cut-off value, AUC: Area Under the Curve, LR: Likelihood Ratio, PPV: Positive Predictive Value, NPV: Negative Predictive Value, CI: Confidence Interval, RRI: Renal Resistive Index, EDV: End Diastolic Velocity, Post-Contrast: 2nd Hour
Fig. 3.
Receiver operating curve (ROC) analysis
Discussion
In this prospective observational study of patients who presented to the ED, who were admitted to the hospital with a decision to undergo contrast-enhanced tomography for diagnosis, we demonstrated that renal ultrasonographic measurements performed by emergency medicine specialist have diagnostic accuracy in predicting the development of CI-AKI. Our results support that postcontrast RRI and postcontrast EDV can be used to predict the development of CI-AKI in the ED.
In our study, the value of RRI in predicting CI-AKI was evaluated under two conditions: pre- and post-contrast. Median RRIs were not statistically different between the non-CI-AKI and CI-AKI groups before contrast. However, after contrast, median RRIs were statistically significantly different between the non-CI-AKI and CI-AKI groups. Furthermore, in the ROC analysis of RRI, the postcontrast cut-off value was 0.70 (≥), with a sensitivity of 72.7% and specificity of 95.6%. Previous studies have reported that the RRI from renal ultrasonographic measurements can predict the development of AKI with different sensitivities and specificities in various situations, such as in critically ill patients, patients with sepsis, patients with intraoperative conditions, and patients before PCI [21–24]. Our results suggest that postcontrast RRI shows high specificity for predicting the development of CI-AKI in the ED.
Wiersema et al. evaluated the diagnostic accuracy of the RRI for AKI in critically ill patients. The RRI was found to have low sensitivity (32%, 95% CI 24–41%) and moderate specificity (72%, 95% CI 66–78%) for detecting persistent AKI [21]. In a study by Song et al., the sensitivity and specificity of RRI ≥ 0.695 for predicting AKI due to sepsis were 52% and 87%, respectively (AUC = 0.811; 95% CI, 74–89) [22]. Kajel et al. aimed to evaluate the relationship between the preoperative RRI and AKI in patients undergoing coronary artery bypass grafting. They found that a preoperative RRI of 0.68 was the most accurate cutoff value for differentiating between non-AKI patients and AKI patients. This method has a sensitivity of 70% and a specificity of 67% [23]. Shayganfar et al. investigated whether the preprocedural RRI was sensitive enough to predict CI-AKI in patients receiving intra-arterial contrast. The sensitivity and specificity were 33.3% and 83.9%, respectively, using a precontrast RRI cutoff of 0.69 [24]. In our study, RRI was found to predict CI-AKI with a sensitivity similar to that of Kajel et al. [22] In contrast, RRI was more specific in its prediction of CI-AKI than other studies [21–24].
In this study, we also investigated the value of two parameters of RRI in predicting the development of CI-AKI. The value of PSV and EDV in predicting CI-AKI was evaluated under two conditions, pre- and post-contrast. Median PSV was not significantly different between the non-CI-AKI and CI-AKI groups both before and after contrast. However, median EDV was not significantly different between the non-CI-AKI and CI-AKI groups before contrast, whereas was significantly different between the non-CI-AKI and CI-AKI groups after contrast. In the ROC analysis of EDV, the postcontrast cut-off value was 9.1 (≤) with a sensitivity of 72.7% and specificity of 62.6%. Based on our findings, the diagnostic value of RRI in predicting CI-AKI may be primarily related to the reduction in EDV. Because the median PSV value did not create a statistically significant difference between the two groups, whereas the median EDV value created a statistical difference. Nevertheless, this is not the only explanatory circumstance. Because we found that although RRI and EDV showed similar sensitivity, EDV was less specific than RRI. This is an indication that the EDV alone is not an explanation for the significance of the RRI.
In the study conducted by Aykan et al., the predictive value of the MEHRAN score for CI-AKI in patients with ST-elevation myocardial infarction treated with primary PCI was evaluated. According to the ROC curve analysis at a cutoff value of 12.5, the MEHRAN score was found to have a sensitivity of 73.3% and a specificity of 88.9% [25]. A study by Zungur et al. evaluated the predictive value of the Mehran score for CI-AKI after transcatheter aortic valve implantation. The cutoff value of the MEHRAN score for predicting the development of CI-AKI was 13.0 (AUC 0.654; 95% CI, 0.495–0.758; sensitivity, 62%; specificity, 68%) [26]. In our study, the value of the MEHRAN score in predicting CI-AKI in the ED was evaluated. The mean MEHRAN score was statistically significantly different between the group without CI-AKI and those with CI-AKI. Furthermore, in the ROC analysis of the MEHRAN score, the cut-off point was set at 5 (≥) with a sensitivity of 54.6% and specificity of 78.0%. In many studies, the MEHRAN score shows high sensitivity for CI-AKI prediction at high scores. However, the MEHRAN score remained relatively low in our study because some factors evaluated in the scoring were excluded due to exclusion criteria. This may lead one to infer that a low MEHRAN score also effectively predicts CI-AKI. However, the relatively low score should not lead to the wrong inference. Furthermore, our findings suggest that post-contrast RRI may be more beneficial than MEHRAN score in low-risk patients.
When the predictive power of post-contrast RRI, post-contrast EDV, and MEHRAN score for CI-AKI is evaluated, it is observed that post-contrast RRI and EDV have similar sensitivity and are more sensitive than MEHRAN score. Again, postcontrast RRI is the most specific for predicting CI-AKI.
The literature suggests that many factors in the development of CI-AKI. Age, elevated urea, low GFR, a history of diabetes, and diuretic use are among the main factors [12, 27, 28]. In our study, age, HT, CAD, atrial fibrillation, urea, creatinine, and GFR were statistically different between CI-AKI and non-CI-AKI groups. Similar to the literature, our study suggests that some factors make a statistical difference in CI-AKI development. This occurred despite our meticulous patient selection, suggesting we cannot rule out this condition. The extent to which our results are clinically affected should be supported by more comprehensive studies.
Due to our study plan, we used a single type of contrast medium; therefore, the CI-AKI development rates of different contrast media could not be evaluated. The rate of CI-AKI due to iohexol was 10.8% (11/102).
Finally, the American College of Radiology and National Kidney Foundation consensus statement [29] states that the risk of developing CI-AKI in patients with reduced renal function following exposure to intravenous iodinated contrast media is overestimated. In contrast, our findings suggest that patient-based assessment with ultrasonography may also be appropriate to determine the need for prophylaxis in patients with normal GFR in the ED.
Limitations
There are several limitations to this study. The first is the small study population and the single-center nature of the research. Therefore, it is difficult to generalize our results to a wider population. Second, the exclusion criteria were too broad. This meant that selection bias could not be completely eliminated. Another limitation was the lack of long-term follow-up of patients who developed CI-AKI, making it impossible to assess whether the assessment predicted a persistent condition. Finally, one emergency specialist performed the ultrasonographic measurements in this study, making it impossible for another professional sonographer to evaluate the quality of the measurements. Considering that ultrasonographic measurements are person-dependent, the fact that PSV, EDV, and related RRI calculations, which include precise measurements, were performed by a single sonographer and not compared with another sonographer did not eliminate the person-dependency in our results. These factors may have affected our results. Accordingly, further studies are needed with a large population in which the understanding of image quality is evaluated, subgroup assessments are made, and exclusion criteria are minimized.
Conclusion
Postcontrast RRI and postcontrast EDV ultrasound measurements performed after diagnostic contrast imaging in the ED show high sensitivity and specificity in predicting CI-AKI development. In conclusion, postcontrast ultrasound measurements may predict CI-AKI development, allowing further measures to be taken. Further studies are needed to confirm these findings.
Acknowledgements
We would like to thank the Department of Emergency Medicine for their hard work on their help in data collection.
Author contributions
The initials of the contributing authors are listed in brackets after the relevant parts of the research: literature search (ÖY, MMY), study design (ÖB, ÖY, MMY), legislative applications (MMY, EH, NP, GA), data collection (MMY, NP, EH, GA), supervision and quality control (ÖY, ÖB), statistical data analysis (MMY, ÖY), data interpretation (MMY, ÖB), and drafting of the manuscript (MMY, ÖY). All authors were involved in the writing and critical revision of the manuscript and approved the final version. ÖY and MMY take responsibility for the entire paper.
Funding
This research has not received any specific support from any funding.
Data availability
Our study data is not recorded in a data repository to protect the confidentiality of its participants. Our data results are presented in text or in tables/figures.
Declarations
Ethical approval
This study was approved by the institutional review board and ethics committee (T.C. Recep Tayyip Erdoğan University Non-Interventional Clinical Research Ethics Committee, number: E-40465587-050.01.04-906 and ID: 2023/278). Informed consent was obtained from the patients before starting the study. The principles outlined in the Declaration of Helsinki and STARD guidelines have been followed.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chandiramani R, Cao D, Nicolas J, Mehran R. Contrast-induced acute kidney injury. Cardiovasc Interv Ther. 2020;35(3):209–17. 10.1007/s12928-020-00660-8 [DOI] [PubMed] [Google Scholar]
- 2.Pistolesi V, Regolisti G, Morabito S, et al. Contrast medium induced acute kidney injury: a narrative review. J Nephrol. 2018;31(6):797–812. 10.1007/s40620-018-0498-y [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen AL. Contrast-induced nephropathy: pathophysiology and preventive strategies. Crit Care Nurse. 2013;33(1):37–46. 10.4037/ccn2013680 [DOI] [PubMed] [Google Scholar]
- 4.Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33(16):2007–15. 10.1093/eurheartj/ehr494 [DOI] [PubMed] [Google Scholar]
- 5.Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol. 1999;9(8):1602–13. 10.1007/s003300050894 [DOI] [PubMed] [Google Scholar]
- 6.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20(3):672–9. 10.1681/ASN.2008070669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrin T, Descombes E, Cook S. Contrast-induced nephropathy in invasive cardiology. Swiss Med Wkly. 2012;142:w13608. 10.4414/smw.2012.13608 [DOI] [PubMed] [Google Scholar]
- 8.Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37(2):85–98. [PMC free article] [PubMed] [Google Scholar]
- 9.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 10.McCullough PA, Adam A, Becker CR, et al. CIN consensus working panel. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol. 2006;98(6A):5K-13K. 10.1016/j.amjcard.2006.01.019 [DOI] [PubMed]
- 11.Zheng-rong X, Jun C, Yuan-hui L, Yong L, Ning T. The predictive value of the renal resistive index for contrast-induced nephropathy in patients with acute coronary syndrome. BMC Cardiovasc Disord. 2019;19:36. 10.1186/s12872-019-1017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Wang J. Contrast-induced acute kidney injury: a review of definition, pathogenesis, risk factors, prevention and treatment. BMC Nephrol. 2024;25(1):140. 10.1186/s12882-024-03570-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moos SI, van Vemde DN, Stoker J, Bipat S. Contrast induced nephropathy in patients undergoing intravenous (IV) contrast enhanced computed tomography (CECT) and the relationship with risk factors: a meta-analysis. Eur J Radiol. 2013;82(9):e387–99. 10.1016/j.ejrad.2013.04.029 [DOI] [PubMed] [Google Scholar]
- 14.Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation. 2006;113(14):1799–806. 10.1161/CIRCULATIONAHA.105.595090 [DOI] [PubMed] [Google Scholar]
- 15.Azzalini L. The clinical significance and management implications of chronic total occlusion associated with surgical coronary artery revascularization. Can J Cardiol. 2016;32(11):1286–9. 10.1016/j.cjca.2016.02.072 [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–9. 10.1016/j.jacc.2004.06.068 [DOI] [PubMed] [Google Scholar]
- 17.Laskey WK, Jenkins C, Selzer F, et al. NHLBI dynamic registry investigators. Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50(7):584–90. 10.1016/j.jacc.2007.03.058 [DOI] [PubMed] [Google Scholar]
- 18.Schnell D, Deruddre S, Harrois A, et al. Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock. 2012;38(6):592–7. 10.1097/SHK.0b013e318271a39c [DOI] [PubMed] [Google Scholar]
- 19.Wybraniec MT, Bożentowicz-Wikarek M, Chudek J, Mizia-Stec K. Preprocedural renal resistive index accurately predicts contrast-induced acute kidney injury in patients with preserved renal function submitted to coronary angiography. Int J Cardiovasc Imaging. 2017;33(5):595–604. 10.1007/s10554-016-1039-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu ZR, Chen J, Liu YH, Liu Y, Tan N. The predictive value of the renal resistive index for contrast-induced nephropathy in patients with acute coronary syndrome. BMC Cardiovasc Disord. 2019;19(1):36. 10.1186/s12872-019-1017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiersema R, Kaufmann T, van der Veen HN, et al. Diagnostic accuracy of arterial and venous renal doppler assessment for acute kidney injury in critically ill patients: a prospective study. J Crit Care. 2020;59:57–62. 10.1016/j.jcrc.2020.05.012 [DOI] [PubMed] [Google Scholar]
- 22.Song J, Wu W, He Y, Lin S, Zhu D, Zhong M. Value of the combination of renal resistance index and central venous pressure in the early prediction of sepsis-induced acute kidney injury. J Crit Care. 2018;45:204–8. 10.1016/j.jcrc.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 23.Kajal K, Chauhan R, Negi SL, et al. Intraoperative evaluation of renal resistive index with transesophageal echocardiography for the assessment of acute renal injury in patients undergoing coronary artery bypass grafting surgery: a prospective observational study. Ann Card Anaesth. 2022;25(2):158–63. 10.4103/aca.aca_221_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shayganfar A, Moradi M, Moshiri R, Khosravi A, Ebrahimian S. Is high preprocedural renal resistive index sensitive enough to predict iodine contrast-induced nephropathy in patients receiving intra-arterial iodinate contrast? Curr Probl Diagn Radiol. 2021;50(3):328–31. 10.1067/j.cpradiol.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 25.Aykan AÇ, Gül I, Gökdeniz T, et al. Is coronary artery disease complexity valuable in the prediction of contrast induced nephropathy besides Mehran risk score, in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention? Heart Lung Circ. 2013;22(10):836–43. 10.1016/j.hlc.2013.03.085 [DOI] [PubMed] [Google Scholar]
- 26.Zungur M, Gul I, Tastan A, Damar E, Tavli T. Predictive value of the Mehran score for contrast-induced nephropathy after transcatheter aortic valve implantation in patients with aortic stenosis. Cardiorenal Med. 2016;6(4):279–88. 10.1159/000443936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Liang X, Xin S, et al. Risk factors for contrast-induced acute kidney injury (CI-AKI): protocol for systematic review and meta-analysis. BMJ Open. 2019;9(8):e030048. 10.1136/bmjopen-2019-030048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyuncuoğlu A, Avşar MG, Tarhan A, et al. Evaluation of contrast induced nephropathy related risk factors and long-term results. Ther Apher Dial. 2024;28(5):754–9. 10.1111/1744-9987.14133 [DOI] [PubMed] [Google Scholar]
- 29.Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294(3):660–8. 10.1148/radiol.2019192094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our study data is not recorded in a data repository to protect the confidentiality of its participants. Our data results are presented in text or in tables/figures.