Abstract
Simultaneous measurements of CO2 (CER) and O2 (OER) exchange in roots and shoots of vegetative white lupin (Lupinus albus) were used to calculate the flow of reducing power to the synthesis of biomass that was more reduced per unit of carbon than carbohydrate. On a whole-plant basis, the diverted reductant utilization rate (DRUR which is: 4 × [CER + OER]) of shoot tissue was consistently higher than that of roots, and values obtained in the light were greater than those in the dark. An analysis of the biomass being synthesized over a 24-h period provided an estimate of whole-plant DRUR (3.5 mmol e− plant−1 d−1), which was similar to that measured by gas exchange (3.2 mmol e− plant−1 d−1). Given that nitrate reduction to ammonia makes up about 74% of whole-plant DRUR, root nitrate reduction in white lupin was estimated to account for less than 43% of whole-plant nitrate reduction. The approach developed here should offer a powerful tool for the noninvasive study of metabolic regulation in intact plants or plant organs.
Plants use CO2, H2O, mineral nutrients, and light energy to produce carbohydrate and other organic compounds during plant growth. Although the composition of biomass varies depending on species and environmental conditions, it is almost always more reduced than Glc per mole of carbon due to the presence of highly reduced molecules such as fatty acids, lignin, and reduced minerals such as nitrogen and sulphur. This is reflected in the fact that the heat of combustion of biomass (15–30 kJ g−1 dry weight; Griffin, 1994; Gary et al., 1995; Spencer et al., 1997; Eamus et al., 1999) is greater than that of Glc (15 kJ g−1 dry weight). In addition, the elemental composition of biomass (CH 1.3-1.8N 0.01-0.06O 0.5-0.6; McDermitt and Loomis, 1981; Williams et al., 1987; Walton and Fowke, 1995; Walton et al., 1999) has less oxygen and a higher relative carbon content than does Glc or other carbohydrates (CH2O).
In theory, the synthesis of biomass that is more reduced per unit of carbon than Glc should be reflected in a greater rate of gas production (CO2 in non-photosynthetic tissues, O2 in photosynthetic tissues) than gas uptake (O2 in non-photosynthetic tissues, CO2 in photosynthetic tissues), integrated over the growth of a plant. This is because the production of gases in plants is associated with the production of reducing power, whereas the uptake of gases are generally indicative of photosynthetic CO2 fixation or oxidative phosphorylation.
Therefore, quantification of the CO2 (CER) and O2 (OER) exchange rates of plant tissues (where production is positive and uptake is negative) can be used to obtain a noninvasive measure of the biosynthetic processes occurring within those tissues. A gas exchange ratio (GER) defined as:
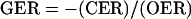 |
1A |
for tissues having net CO2 production (i.e. equivalent to respiratory quotient), or defined as:
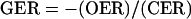 |
1B |
for tissues having net CO2 uptake (i.e. equivalent to photosynthetic quotient), would yield values greater than 1.0 when there is reductive biosynthesis, but less than 1.0 when the tissues that are synthesizing a more oxidized biomass.
Previous studies have reported large variations in the GER of plant tissues during growth. In barley, leaves reducing nitrate had GER values of 1.16 and 1.51 in the light and dark, respectively, compared with values for mutant leaves lacking nitrate reductase of 1.02 and 0.96 in the light and dark, respectively (Bloom et al., 1989). Cramer and Lewis (1993) reported low values for GER in roots of wheat and maize, although values measured in nitrate grown plants (1.0–1.1) were significantly higher than that those in NH 4+ grown plants (0. 5–0.6). Even larger nitrate-induced increases in GER have been reported in algae (Weger and Turpin, 1989), a finding consistent with the fact that algae have a much lower carbon:nitrogen ratio than herbaceous plants.
Fock et al. (1972) measured CER and OER in photosynthesizing leaves of 18 species and found average GER values of 0.79 for leaves at 400 μL L−1 CO2 in air. These low GER values for photosynthesizing leaves were challenged by Kaplan and Bjorkman (1980), who reported values of 1.04 to 1.14 in the C3 species Encelia californica and the C4 species Atriplex rosea. However, Tolbert et al. (1995) reported that GER values in tobacco leaves in the light decreased from about 1.0 at 21% (v/v) O2 and 350 μL L−1 CO2 to a value of 0 at 27% (v/v) O2 and 350 μL L−1 CO2. Moreover, higher pO2 and lower pCO2 were reported to result in the net uptake of CO2 and O2 (Tolbert et al., 1995).
These large variations in the GER may reflect the large biochemical diversity that exists in plant tissues or the inherent difficulties there are in measuring OER in an atmosphere that contains a high background O2 concentration (about 20.9% [v/v] or 209,000 μL L−1). For example, even if plant metabolism generates a 100 μL L−1 O2 changes in the composition of the air around the plant, that still represents an atmospheric pO2 change of only 0.05%.
This study uses a new differential oxygen analyzer capable of measuring less than 5 μL L−1 O2 differentials against a background of air (Willms et al., 1997, 1999). The instrument was incorporated into a whole-plant gas exchange system having separate shoot and root chambers so that continuous CER and OER measurements on a single plant could provide insights into the temporal and spatial variations that occur in reductive biosynthesis during the vegetative growth of a plant.
Nitrate-grown white lupins (Lupinus albus cv Manitoba) were chosen as the study organism since previous work (Atkins et al., 1979; Pate et al., 1979) has shown that nitrate reduction was predominantly in the roots. Roots have lower gas exchange rates than shoots, thereby making it easier to obtain precise measurements of OER, CER, and the differences between them. Plants were chosen at various times during vegetative growth (18–33 d after sowing) to test two hypotheses: first, that the reductive biosynthesis in nitrate-grown white lupins should be higher in the root than in the shoot, and second, that spatial (root versus shoot) and temporal (light versus dark period) variations in reductive biosynthesis should integrate to give net reductive biosynthesis (GER > 1.0) in the whole plant over a 24-h day.
RESULTS
Time Course of CO2 and O2 Gas Exchange in Shoots and Roots
The gas exchange system described here provided continuous measurements of CO2 and O2 exchange in the shoot and root of individual plants. Six replicate measurements, each made over a 2- to 4-d period, were carried out with white lupin plants that ranged in age from 18 to 33 d old and plant dry weight ranged from 1.2 to 4.5 g plant−1. Detailed results are presented for a single 24- to 25-d-old plant, whereas mean values (±se) for all six replicate plants are presented only for summary data.
The 24- to 25-d-old gas exchange plant was harvested on d 26, and its biomass was provided in Table I, along with the mean dry weights of six replicate plants from the same population harvested at d 23 and another six plants harvested at d 25. These plants were used to calculate the relative growth rate for roots, stems + petioles and leaves of 0.071, 0.069, and 0.079 Δg dry weight g−1 dry weight d−1, respectively (Table I).
Table I.
The dry wts and RGR of a population of white lupin plants (n = 6) from which was selected the experimental plant used for the gas analysis measurements reported in Figures 1, 2, and 4
| Items | Plant Dry Wt
|
RGRa
|
|
|---|---|---|---|
| 23 d | 25 d | Δg dry wt g−1 dry wt d−1 | |
| Leaves | 0.96 ± 0.05 | 1.12 ± 0.04 | 0.079 |
| Stems and petioles | 0.82 ± 0.06 | 0.94 ± 0.06 | 0.069 |
| Roots | 0.77 ± 0.05 | 0.89 ± 0.06 | 0.071 |
| Whole plant | 2.55 ± 0.10 | 2.95 ± 0.09 | 0.074 |
The experimental plant was harvested on d 26 and was found to have a total wt of 2.90 g dry wt including 1.18 g dry wt leaves, 0.89 g dry wt stems + petioles, and 0.83 g dry wt roots.
The exponential RGR was calculated according to Radford (1967) using the equation of RGR = (ln DW2 − ln dry wt)/(d2 − d1), where d is days and the subscripts “1” and “2” refer to values for d 23 and 25, respectively.
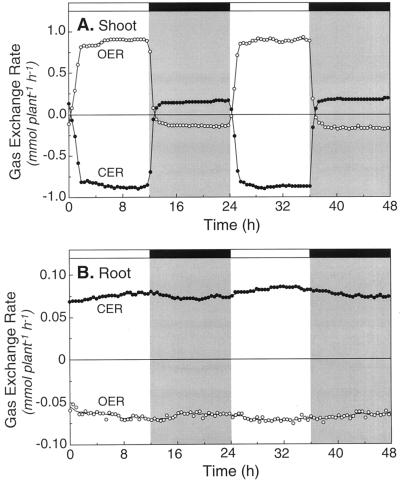
In the shoot of a typical 24- to 25-d-old plant, CER values were about −0.88 mmol CO2 plant−1 h−1 in the light, but in the dark, CER was in the opposite direction (0.15 mmol CO2 plant−1 h−1), and only 17% of the magnitude during the light (Fig. 1A). The OER values were of a similar magnitude (Fig. 1B), but were in the opposite direction of the CER values (Fig. 1A). Assuming that all of the daytime CO2 exchange was associated with leaves, the average leaf area-specific photosynthesis rate was 10 μmol CO2 m−2 s−1. These measurements were carried out when the temperature and relative humidity in the shoot chamber was 28°C and 86%, respectively, in the light, and 25°C and 75% in the dark. Root temperature was maintained at 24°C ± 0.6°C in the light and dark (data not shown).
Figure 1.
Continuous measurements of the CER (●) and OER (○) in the shoot (A) and root (B) of a 24- to 25-d-old white lupin plant. The black bar at the top of A and B, and the shading within A and B, denotes the 12-h night periods. The photoperiod, temperature, and humidity were 12 h/12 h, 28°C/25°C and 86%/75%, day/night, respectively, in the shoot chamber. The temperature was maintained at 24°C ± 0.6°C in root chamber.
Since roots only respire, CER values were always positive (production) and OER values were always negative (uptake; Fig. 1B). The magnitude of the CER and OER values ranged from ±50 to 80 μmol plant−1 h−1, and values obtained at dark were only 4% lower than those obtained during the photoperiod. Expressed per gram of dry weight root, CER values were about 98 μmol g−1 dry weight root h−1, a value similar to that reported previously for roots of white lupin (Pate et al., 1979). Since the shoot:root ratio of the plants was 2.3:1 (Table I), the average gram of shoot biomass had a specific gas-exchange rate six times that of root tissue during the light, but only 1.4 times that of roots at dark.
Daily Budget for CER and OER
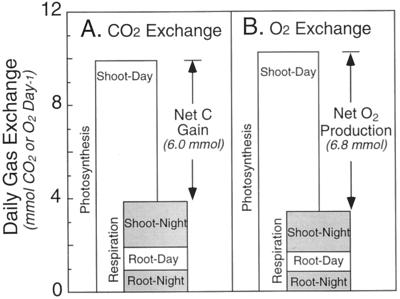
Measured values for CER and OER in a single plant (Fig. 1) were integrated over 24 h to calculate the daily CO2 and O2 interchanges within the whole plant (Fig. 2). About 60% of the net CO2 assimilation was incorporated into plant biomass production, whereas 40% was released again in plant respiration (Fig. 2A). Of this carbon loss, about one-half was attributed to shoot respiration in the dark, whereas root respiration in the light and dark accounted for the remainder (Fig. 2A).
Figure 2.
Net CO2 (A) and O2 (B) exchanges in the 24- to 25-d-old white lupin plant described in Figure 1. Shoot-day values are shown as photosynthetic CO2 uptake (A) or O2 production (B). Respiratory CO2 loss (A) or O2 uptake (B) for root-night, root-day, and shoot-night are presented in stacked bar graphs so that the difference between the sum of respiration and the photosynthetic values will illustrate the net carbon gain or the net O2 production of the plant over the 24-h period. Values are means of three diurnal measurements obtained sequentially using a single plant.
In terms of O2 exchange, only 34% of the net O2 released in photosynthesis was offset by respiratory O2 consumption (Fig. 2B). Again, shoot respiration in the dark accounted for about one-half the O2 consumption, whereas root respiration in the light and dark consumed the remainder. As a result, the net daily production of O2 was about 13% higher than the net carbon gain. This was due to 2.6% more O2 production than CO2 fixation in photosynthesis, and 12% less O2 uptake than CO2 evolution in respiration (Fig. 2).
Gas Exchange Ratios and Diverted Reductant Utilization Rate (DRUR) in Shoots and Roots during the Day and Night
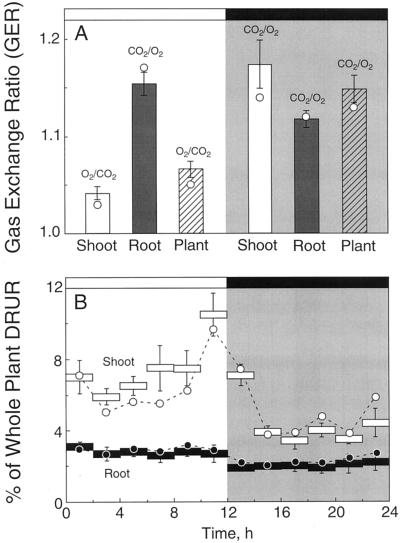
Integrated over the 12-h light or dark periods, and in all six replicate plants, the production of CO2 or O2 was of a greater magnitude than the corresponding uptake of O2 or CO2, respectively. Therefore, the GER values were consistently greater than 1.0 (Fig. 3A). During the light period, root GER was significantly greater than that in shoots, but since the total shoot gas exchange dwarfed that of roots (Fig. 1), whole-plant GER during the light was more a reflection of the shoot than the root (Fig. 3A).
Figure 3.
The imbalance between CO2 and O2 exchange in shoots and roots of white lupin plants quantified as either gas exchange (GER = [CO2 production]/[O2 uptake] or [O2 production]/[CO2 uptake]) (A) or as a percentage of whole-plant DRUR (4 × [CER + OER]) (B). In A, bar graphs denote GER values (±se, n = 6) for 18- to 33-d-old shoots (□), roots (▪), or whole plants (▧) during the light (white background) and dark (shaded background) periods. The circles represent the same values that were obtained for the 24- to 25-d-old experimental plant for which data is provided in Figures 1 and 2. In B, average DRUR values were calculated for each 2-h period of the day, and to minimize the effects of plant-to-plant variability, values were expressed (± se, n = 6) as a percent of the total DRUR measured for each plant (Average whole-plant DRUR = 3.5 ± 0.6 mmol e− plant−1 d−1). ○ and ● represented the same values for shoot and root, respectively, that were obtained for the 24- to 25-d-old experimental plants for which data was provided in Figures 1 and 2.
In the dark, GER values for roots and shoots were not significantly different, ranging from 1.12 ± 0.01 to 1.17 ± 0.03 (n = 6), although the average GER for the whole plant in the dark (1.15 ± 0.02) was significantly greater than the whole-plant GER during the light (1.07 ± 0.01). Values for the 24- to 25-d-old experimental plant described in Figures 1 and 2 (○, Fig. 3A) were similar to the mean values for the six replicate gas exchange plants.
To provide information on the relative contribution of shoots and roots to “reductive biosynthesis” during the light and dark, calculations were made of the DRUR (Eq. 2) for each 2-h interval over the 2-d study period. Plant-to-plant variation in DRUR was large, ranging from 2.0 to 5.5 mmol e− plant−1 d−1. To see through this variability and identify diurnal and organ-specific trends in DRUR, values were plotted as a percentage of whole-plant DRUR for the 24-h period (Fig. 3B).
Roots, every 2-h period of the day, consumed about 3% (light) or 2% (dark) of the whole-plant DRUR for a 24-h period. In contrast, shoots contributed 6% to 10.5% of daily whole-plant DRUR for each 2-h period in the light, and about 4% for each 2-h period in the dark (Fig. 3B). Therefore, the shoot DRUR in the light period was about 1.8 times higher than that in the dark period, and the DRUR values of shoots were about 2.5 and 1.6 times higher than that of the root in the light and dark period, respectively (Fig. 3B). A similar pattern was obtained for the 24- to 25-d-old experimental plants described in Figures 1 and 2.
Relative Contribution of Shoot and Root to Reductive Biosynthesis
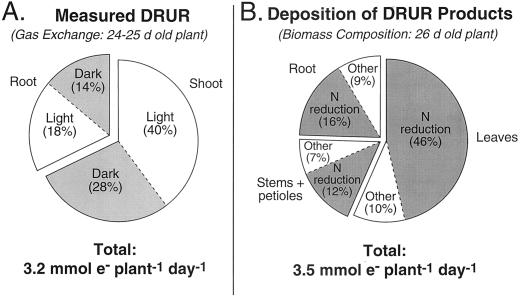
The results of Figure 3B for the 24- to 25-d-old experimental plant were integrated over a 24-h period and were used to calculate the relative contribution of shoots and roots in the light and dark to the daily whole-plant DRUR (Fig. 4A). The whole-plant DRUR was calculated as 3.2 μmol e− plant−1 d−1. A larger proportion (68%) of DRUR was found in the shoot compared with the root (32%) and within each organ, DRUR values were greater in the light than in the dark (Fig. 4A).
Figure 4.
The contribution of shoot and root tissues to the daily DRUR measured by gas exchange (A) or calculated from the growth of biomass (B) in white lupin plants. The measured values in A were obtained from the average data for the 24- to 25-d-old plant as reported in Figure 3B. The values for B were obtained from the results and calculations of Tables I and II, as described in the text.
However, shoot biomass in the study plants (2.07 g dry weight) was larger than root biomass (0.83 g dry weight), resulting in a shoot:root ratio (2.5) that was only slightly larger than the ratio of DRUR in shoots and roots (2.1). Since the shoots and roots had similar relative growth rates (RGR, Table I), if the DRUR values were normalized for biomass accumulation by plant organs, shoot and root values would be similar.
Deposition of DRUR Products in Plant Organs
Values of DRUR coefficient (βDRUR, units of μmole e− mg−1 element) were calculated from a knowledge of the net electron demand for nitrogen and sulfur reduction (items 1 and 2, Table II), or from the net reductant flow (per unit of carbon) associated with the synthesis of various organic matter constituents (items 4–10, Table II). Per milligram of nitrogen, sulfur, or organic matter product, the βDRUR was highest for nitrogen and sulfur reduction (571 and 250 μmol e− mg−1, respectively), but also high for the synthesis of lipids and lignin from carbohydrate (102 and 28 μmol e− mg−1, respectively). Organic acid, being more oxidized per carbon than carbohydrate, had a negative βDRUR (−36 μmol e− mg−1; Table II).
Table II.
Calculation of the theoretical reductant demand (RD, units of mmol e−2 Δg−1 dry wt of each plant part) associated with the biosynthesis of various mineral and organic constituents of leaf, stem + petiole, or root tissues
| Item No. | Biosynthesis of Organic Compounds | Biomass Compositiona
|
βDRURb | RDb
|
||||
|---|---|---|---|---|---|---|---|---|
| Leaves | S + P | Roots | Leaves | S + P | Roots | |||
| mg Δg−1 dry wt | μmole e− mg−1 element or compound | mmole e− Δg−1 dry wt | ||||||
| Mineral Elements | ||||||||
| 1 | NO3− (N5+) → NH3 (N3−) | 33.0 | 12.8 | 18.0 | 571 | 18.9 | 7.30 | 10.3 |
| 2 | SO42− (S6+) → SH− (S2−) | 2.2 | 3.5 | 8.7 | 250 | 0.550 | 0.875 | 2.18 |
| 3 | Other minerals | 25.0 | 25.0 | 25.0 | 0 | 0 | 0 | 0 |
| Organic Matter | ||||||||
| 4 | (CH2O)n → Protein | 179.0 | 84.1 | 84.1 | 5.2 | 0.928 | 0.436 | 0.436 |
| 5 | (CH2O)n → Free amino acids | 20.6 | 9.4 | 9.4 | −5.1 | −0.106 | −0.048 | −0.048 |
| 6 | (CH2O)n → Nucleic acids | 6.2 | 2.9 | 2.9 | −23.0 | −0.142 | −0.067 | −0.067 |
| 7 | (CH2O)n → Lipids | 25.0 | 25.0 | 25.0 | 102 | 2.55 | 2.55 | 2.55 |
| 8 | (CH2O)n → Lignins | 80.0 | 80.0 | 80.0 | 28.4 | 2.27 | 2.27 | 2.27 |
| 9 | (CH2O)n → Organic acids | 50.0 | 50.0 | 50.0 | −36.1 | −1.81 | −1.81 | −1.81 |
| 10 | H2O + CO2 → (CH2O) | 579.0 | 707.3 | 696.9 | 0 | 0 | 0 | 0 |
| 11 | Total | 1,000 | 1,000 | 1,000 | – | 23.1 | 11.5 | 15.8 |
RD was calculated as the product of relative composition of leaves, stems + petioles (S + P), or roots and the DRUR coefficients (βDRUR, units of μmol e− imbalance between CER and OER per milligram of compound produced). βDRUR values were calculated from known biochemical pathways as described in the text, where the products of metabolism are more (positive value) or less (negative value) reduced per unit carbon than the substrates from which they are derived.
Values for nitrogen and sulphur composition were measured elemental contents in plant white lupin tissues. The nitrogen content was corrected for changes in the NO3− pool size in tissues. For other components, the organic composition of plant biomass was according to Penning De Vries et al. (1974).
Calculated from known biochemical pathways assuming Suc, NH3, and reduced sulphur were the starting material for synthesizing other organic compounds. The amino acid composition of protein was assumed to be the same as Rubisco (Ramshaw, 1982), and the free amino acid composition was as described by Chu et al. (1974). The theoretical DRUR production was obtained as the product of βDRUR and the corresponding biomass composition.
By combining the βDRUR values with measured or estimated values for biomass composition, an estimate was obtained for the RD (units of mmol e− Δg−1 dry weight) that would be associated with the synthesis of each biomass component (Table II). Nitrate reduction dominated the overall reductant demand associated with each tissue, accounting for 82% of leaves, 63% of stems + petioles, and 65% of roots.
When these values for reductant demand were applied to the white lupin plant that was harvested at 26 d following 2 d of gas analysis measurements (24–25 d), an estimate was obtained for the DRUR that should have been associated with 1 d biosynthesis of new leaves, stems + petioles, and roots (Fig. 4B). The whole-plant DRUR of 3.5 mmol e− plant−1 d−1 was predicted to be slightly greater in a 26-d-old plant than that which was measured in the same plant at 24 to 25 d old (3.2 mmol e− plant−1 d−1, Fig. 4A). The biosynthesis of new leaves was predicted to account for 56% of whole-plant DRUR (Fig. 4B), whereas values for stems + petioles and roots were 19% and 25%, respectively.
DISCUSSION
Whole-Plant CO2 and O2 Exchange
Previous studies have reported simultaneous measurements of CO2 and O2 exchange in plant tissues or organs (Myers, 1949; Fock et al., 1971, 1972; Kaplan and Bjorkman, 1980; Bloom et al., 1989; Weger and Turpin, 1989; Cramer and Lewis, 1993; Tolbert et al., 1995; Scheurwater et al., 1998; Van Der Westhuizen and Cramer, 1998; Willms et al., 1999). However, to our knowledge this is the first report of long-term, continuous, simultaneous measurements of CO2 and O2 exchange within a whole plant.
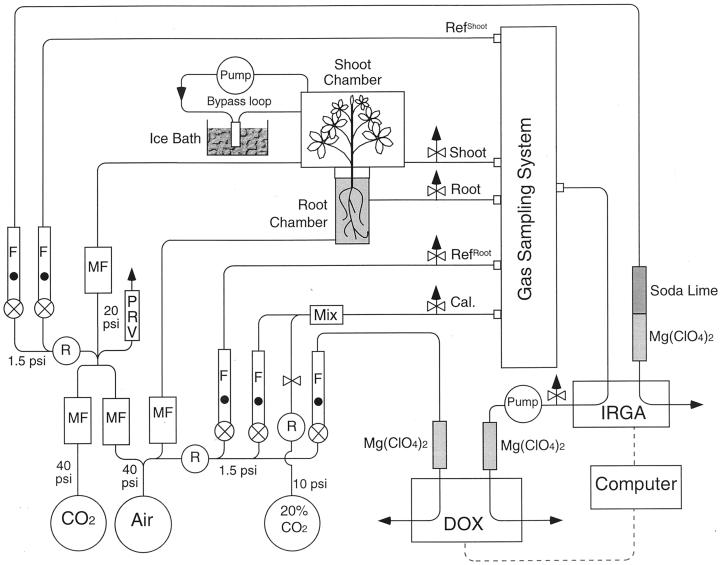
This study was made possible through the use of a differential oxygen analyzer (Willms et al., 1997), which was able to measure less than 2 μL L−1 O2 differentials between a reference and analytical gas stream having the composition of air (20.95% [v/v] or 209,500 μL L−1 O2). Given that the gas exchange system used here (Fig. 5) allowed the plants to generate differentials of 180 to 250 μL L−1, and contained a single calibration system for the O2 and CO2 analyzers (Willms et al., 1999), sufficient precision was available to measure small differences between CO2 and O2 exchange.
Figure 5.
A schematic diagram of a system capable of making continuous, simultaneous measurements of CO2 and O2 exchange in intact roots and shoots. See text for description. Cal, Calibration gas stream; DOX, differential O2 analyzer; IRGA, infrared CO2/H2O analyzer; F, flow meter; MF = mass flow controller; PRV, pressure release valve (set at 20 psi); R, downstream pressure regulator; RefRoot, reference supply gas to root; RefShoot, reference supply gas to shoot.
In roots and shoots, and during light and dark, the production of CO2 or O2 was consistently higher than the corresponding uptake of O2 or CO2 in the same plant organs. This is the gas exchange signature of a plant tissue that is synthesizing biomass, which is more reduced per unit of carbon than carbohydrate. For example, in photosynthetic tissues in which O2 production is greater than CO2 fixation, the difference between the two exchanges represents the flow of reducing power that is diverted to alternative pathways such as nitrate reduction (Bloom et al., 1989; Weger and Turpin, 1989) or oil synthesis (Willms et al., 1999). In a similar manner, in respiring tissues where CO2 production is greater than O2 uptake, the difference between the two exchanges represents the flow of reducing power into processes such as nitrate reduction (Bloom et al., 1989; Weger and Turpin, 1989), oil synthesis (Willms et al., 1999), or other biochemical pathways that results in a final biomass that is more reduced per unit of carbon than the initial biomass.
GER, DRUR, and Reductive Biosynthesis
In the present study with white lupin, the calculated values for gas exchange (GER = [CO2 production]/[O2 uptake] or [O2 production]/[CO2 uptake]) were consistently greater than 1.0, but less than 1.2 (Fig. 3A). This range of values was similar to those obtained in many previous studies (Kaplan and Bjorkman, 1980; Bloom et al., 1989), but differed from other reports for leaves (GER = 0.79; Fock et al., 1971, 1972), shoot tissue (GER = −2 to 1; Tolbert et al., 1995), and roots (GER = 0.5–1.1; Cramer and Lewis, 1993; Van Der Westhuizen and Cramer, 1998).
One problem in the interpretation of GER values is that they are very sensitive to variations in specific gas exchange rates of the tissues. For example, in the present study, the GER in the shoot in the light (1.04 ± 0.01) was lower than that of roots (1.15 ± 0.02), but it contributed a larger proportion (40%) of whole-plant DRUR than did the root (18%; Fig. 4A). This illustrates the value of the DRUR term over the GER term as a quantitative measure of reductant flow to biosynthetic processes. In essence, the difference between CER and OER provides more valuable information than the ratio of the two gas exchanges (Willms et al., 1999).
When DRUR was calculated for each 2-h period, the values were consistently positive, but higher in shoots than in roots, and greater in the light than in the dark (Fig. 3B). This pattern was observed in the 24- to 25-d-old study plant for which detailed data was provided (Figs. 1 and 2; Table I), and in the five other white lupin plants (ages ranging from 18–33 d) that were treated in a similar fashion (Fig. 3B).
NO3− assimilation in leaves may be higher in the light than in the dark due to an enhanced nutrient uptake from the roots and to the potential for the light reactions of photosynthesis to provide directly, the reductant demand for nitrate reductase (Bloom et al., 1989).
The greater increase in shoot DRUR in the last 2 h of the light period may also be attributed to NO3− assimilation, especially if carbohydrates pools are filled as has been described previously for algal systems (Huppe and Turpin, 1994). In contrast, the higher shoot DRUR during the first 2 h of the dark period may be similar to previous studies (Scaife and Schloemer, 1994; Delhon et al., 1995) in which nitrate reduction did not decline until about 2 h in the dark. However, other reductive biosynthesis in plant such as fatty acids biosynthesis might also be involved in the dynamic changes of DRUR. For example, Willms et al. (1999) reported higher DRUR values in soybean fruit in the light than in the dark, and suggested that this may be due to light stimulated oil synthesis in developing fruits.
Comparison of Measured DRUR and the Deposition of DRUR Products
In the 24- to 25-d-old white lupin plant, the whole-plant DRUR over a 24-h period was measured at 3.2 mmol plant−1 d−1, with 68% of this being attributed to the shoot tissue during the light and dark (Fig. 4A). Although the measured DRUR provided information on the site of reductive biosynthesis, the ultimate deposition of the reduced products within plant tissues may be very different, since the xylem and phloem may redistribute highly oxidized (e.g. organic acids) or highly reduced (e.g. reduced nitrogen, lipids, etc.) compounds around the plant after they have been synthesized.
The deposition of the biosynthetic products that were more (or less) reduced per unit of carbon than carbohydrate was estimated from the growth rate and composition of biomass in the study plant (Fig. 4B). Although this theoretical, whole-plant DRUR was slightly larger (3.5 mmol plant−1 d−1) than that measured by gas exchange (3.2 mmol plant−1 d−1), the former was based on a plant that was 1 to 2 d older than the plant used for gas exchange, and there were many assumptions about the precise tissue composition. As a consequence, the whole-plant values for measured DRUR from gas exchange were considered to be in good agreement with those calculated for deposition of DRUR products from tissue composition.
The deposition of DRUR products in the shoot accounted for 75% of whole-plant DRUR, and 58% of whole-plant DRUR products were associated with nitrogen reduction (nitrate assimilation). In contrast, shoot gas exchange accounted for only 68% of whole plant DRUR, suggesting that some of the nitrogen and other reduced products deposited in shoot tissues were, in fact, reduced in the root and translocated to the shoot, presumably in the xylem (Atkins et al., 1979).
The Site of Nitrate Reduction in White Lupin
A previous study (Pate et al., 1979) estimated that over 90% of the nitrogen assimilation in white lupin roots was associated with root nitrate reduction; the shoot having only a minor role in this process. In making this conclusion, they coupled compositional analyses of xylem and phloem sap in white lupin with an empirical model of whole-plant carbon and nitrogen transport.
The present study offers a very different conclusion from that of Pate et al. (1979), highlighting a much more important role for the shoot in nitrate reduction in white lupin plants. Root DRUR measured by gas exchange accounted for only 32% of whole-plant DRUR (Fig. 4A), and on a whole-plant basis, nitrate reduction to ammonia was estimated to account for 74% of whole-plant DRUR (Fig. 4B). Given these constraints, and assuming that the entire DRUR of roots or shoots could be associated with nitrate reduction, root nitrate reduction could account for 8% to 43% of whole-plant nitrate reduction, whereas shoot nitrate reduction could account for 57% to 92% of whole-plant nitrate reduction (calculations not shown).
There are a number of possible explanations for this discrepancy. First, the white lupin cultivar and growing conditions used in the present study were different from that used previously (Pate et al., 1979), and this might have affected the site of nitrate reduction. Second, the nitrate and amino acid composition of the xylem and phloem saps in the earlier study may have been altered during sampling, and therefore may not have provided a reliable measure of nitrate reduction in plant tissues (Rufty et al., 1982; Andrews, 1986). Third, the DRUR measurements from gas exchange used in the present study may not reflect the site of nitrate reduction in plant tissues. For example, if the shoots were to synthesize highly reduced organic compounds and send them to the root (or if the root were to synthesize highly oxidized compounds and send them to the shoot), the DRUR associated with nitrate reduction would not be able to be distinguished from background rates of reductive (or oxidative) biosynthesis within plant organs.
Allen and Raven (1987) showed there to be a net flux of organic anions from root to shoot to help balance the nitrate anion that is taken up from the medium. Also, organic anions may be synthesized from carbohydrates in the roots and then excreted to and accumulated in the rhizosphere (Loss et al., 1994), thereby lowering the DRUR of the roots. This latter explanation could be tested by measuring DRUR in plant roots and leaves in the presence and absence of added 15NO3− and ammonia.
Using Gas Exchange to Study Reductive Biosynthesis in Plants
The simultaneous measurements of CO2 and O2 exchange, and the use of these data to calculate DRUR offers a potentially valuable tool for the noninvasive study of key metabolic pathways in plants. Integrated over a 24-h period, the measured DRUR of shoots and roots were a good fit to what would be expected from the composition of the plant tissues being synthesized. Further studies are needed to link these DRUR measurements to specific biosynthetic processes, but when this has been done with various tissues and environmental conditions, the method described here should offer a powerful tool for the study of metabolic regulation in intact plants or plant organs.
MATERIALS AND METHODS
Plant Material
Seeds of white lupin (Lupinus albus cv Manitoba) were sterilized (0.3% [w/v]sodium hypochlorite for 3 min) and rinsed with water before being germinated in silica sand in 0.72-L pots that could be sealed for gas exchange measurements (Hunt et al., 1989). The plants were watered twice a day with a nutrient solution (Walsh et al., 1987) containing 1 mm KNO 3 (0–19 d) or 5 mm KNO 3 (20+ d) and were maintained in growth chambers (Conviron model PGV 36, Winnipeg, Manitoba) with a 12-h photoperiod and a temperature of 22°C/17°C (day/night). Photosynthetically active radiation was 500 μmol quanta m−2 s−1 at the plant level and relative humidity was 75%. Gas exchange measurements were conducted over sequential 2- to 4-d periods when the plants were 18 to 33 d old. No nodules were present on the plants used in the study.
The Gas-Exchange System
Plant CO2 and O2 gas exchange measurements were conducted using an open gas exchange system with separate shoot and root chambers as shown in Figure 5. Compressed air drawn from outside of the building was provided directly to the root chamber (approximately 150–250 mL min−1), but was mixed with pure CO2 before being supplied to the shoot chamber (approximately 1–2 L min−1). Sufficient CO2 was provided to give an effluent gas stream of about 360 ± 20 μL L−1. All flow rates for gas mixing and chamber supply were regulated using thermal mass flow controllers (FMA-100 series, OMEGA Engineering, Stamford, CT). The flow rates through the root and shoot chamber, and the size of the plants used were set to provide CO2 and O2 differentials of between 180 and 250 μL L−1. To minimize gas volume and exchange with the environment, all tubing was kept short and made of copper or Bev-A-Line IV (Warehouse Plastic Sales, Toronto).
A shoot chamber (300 mm wide, 300 mm deep, and 180 mm high, 16-L volume) was made with Plexiglas (clear, 4 mm thick) sides and a glass top. It housed the entire shoot of the plant and was tightly sealed onto a Plexiglas (clear, 6 mm thick) plate designed so that the base of the stem could be sealed with Qubitac sealant (Qubit Systems, Canada). Five 12V electric fans located inside the chamber maintained an air flow of about 1.2 m 3 min−1 and a copper tube (6.5 mm OD ×1.8 m long) was installed in the chamber so that it could be flushed with cold water to maintain the air temperature at 28°C ± 1°C in the day period and 25°C ± 1°C during the night. Three micro-thermocouples (model N-965, OMEGA Engineering) were used to monitor the air temperature at three different locations within the chamber. To control humidity, a pump was used to draw air (about 2–6 L min−1) from the chamber and pass it through a copper coil to a glass container (69 mL) immersed in an ice bath before returning to the chamber. The flow rate of this bypass pump was adjusted to maintain a relative humidity in the shoot chamber of about 85% during the light period, and 75% during the dark period. The chamber was illuminated from above by an array of 6 × 100 W halogen lamp (General Electric, Canada) to give a photon flux density of 700 μmol quanta m−2 s−1 (photosynthetically active radiation) at the plant level. The light was passed through a water filter to absorb infrared radiation.
A multi-channel gas sampling system (Layzell et al., 1989) was used to select one of five gas streams for analysis, including the effluent from the shoot and root chambers, reference gas streams similar to those provided to the shoot (Refshoot) and root (Refroot) chambers, and a calibration gas stream. The calibration gas stream was prepared by mixing (1 L min−1) the outside air (21% [v/v] O2) with a small volume (0.25–1 mL min−1) of 20.2% (v/v) CO2 in N2. This resulted in the calibration gas being enriched in CO2 and diluted in O2 by about 50 to 200 μL L−1, but at a precise ratio of 0.96 (Willms et al., 1997, 1999). This gas stream allowed simultaneous calibration of the infrared CO2 and the differential O2 analyzers.
The selected gas from the sampling system was passed through an IRGA (LI-6262, LI-COR, Lincoln, NE) before it was subsampled, dried (15-mL column of magnesium perchlorate), and provided to a DOX (S-3A/DOX, AEI Technologies, Pittsburgh). Information on the water vapor content of the effluent gas from the shoot chamber was used to monitor and maintain the humidity in the chamber by manual adjustment of the pump moving gas past the dehumidifier on the bypass loop.
The signals from the IRGA (absolute CO2 and absolute H2O) and DOX (differential and absolute O2, differential and absolute pressure, and temperature of the differential O2 block) were collected using Workbench software (Version 4.01, Strawberry Tree Software, Sunnyvale, CA) running on a Macintosh computer (Apple Computer, Cupertino, CA). The same computer and software was used to control the mass flow controllers. The shoot chamber was tested with various combinations of flow rate (400–5,000 mL min−1), CO2 concentration (0–1,500 μL L−1), and relative humidity (0%, 50%, and 95%). Under steady-state conditions, no significant differences were found in the inlet and effluent gas streams over this range of the measurement conditions (data not shown).
Plant Growth, Nitrogen, and Sulfur Analysis
Plant dry weight and growth rate were measured by harvesting randomly selected plants from the same population at intervals through the study period. In addition, plants randomly selected for gas exchange measurements were harvested at the end of the study. Leaves, stems, petioles, and roots were separated and dried in an oven (70°C) for 5 d before dry weights were recorded. RGR rates were calculated according to Radford (1967) using the equation of RGR = (ln dry weight2 − ln dry weight1)(d2 − d1), where the d is plant age in days and subscripts “1” and “2” refer to values obtained at the start and at the end of the study period, respectively. Total nitrogen, sulfur, and nitrate nitrogen were measured in dried plant samples. Nitrogen content was determined by automated combustion method (McGeehan and Naylor, 1988; Sweeney, 1989). Level of nitrate nitrogen was measured by reducing to nitrite at pH 7.5 in a copper-cadmium column (Atkins et al., 1979) and was determined colorimetrically (Wood et al., 1967). Sulfur determination was according to Tabatabi and Bremner (1970) with oxidation temperature at 1,350°C.
Gas-Exchange Calculations
Measurements of CO2 and O2 differentials between the inlet (Refshoot or Refroot) and effluent gas streams of shoots or roots where used to calculate CO2 (CER) and O2 (OER) exchange rates (+, production; −, uptake). Since the CO2 concentration in the gas stream was measured before dehumidification, corrections for the effects of water vapor on CER were carried out according to Long and Hallgren (1985). As described previously (Willms et al., 1999), the OER calculations were corrected for variations in differential pressure between the sample and reference streams, and for any imbalance in CO2 and O2 exchange that would have diluted or concentrated the effluent gas stream. For example, if CO2 production was greater than O2 uptake, the net gas production (CER and OER) would dilute the O2 within the effluent stream. This was corrected for using Equation 2 in Willms et al. (1999).
Simultaneous CER and OER measurements of shoots and of roots were used to calculate the DRUR (units of mmol e− plant−1 h−1) for each plant part:
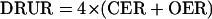 |
2 |
where 4 is the number of electrons associated CO2 or O2 exchange. DRUR is a measure of the energy flow in plant tissues associated with biosynthesis or metabolism to yield a biomass that is more reduced (positive values) or less reduced (negative values) per unit of carbon, than carbohydrate (photosynthesis) or the initial substrate of carbon oxidation (respiration; Willms et al., 1999).
Tissue Deposition of DRUR Products
To determine how the measured DRUR values from gas exchange compared with the deposition of the products of reductive metabolism the following measurements, assumptions, and calculations were carried out:
1. The elemental composition for nitrogen and sulfur were made in leaves, stems + petioles, and roots of the 24- to 25-d-old study plants (items 1 and 2, Table II). Other mineral element (Item 3, Table II) and organic matter constituents (items 4–10, Table II) were assumed as per Penning De Vries et al. (1974). The relative tissue composition was assumed to be stable with time (i.e. mg g−1 dry weight = mg Δg−1 dry weight).
2. The value of βDRUR (units of μmol e− mg−1 element) was calculated as the number of electrons required to reduce each NO3− to NH3 or each SO42− to SH− (items 1 and 2, Table II). This was based on 8e− per nitrogen or sulfur reduced and atomic weights of 14 and 32 g m−1 for nitrogen and sulfur, respectively. Other minerals were assumed to not require reduction before incorporation into tissues (i.e. βDRUR = 0).
3. The CO2 and O2 exchanges (μmol mg−1 compound) associated with producing each mg of the various organic constituents of tissues were calculated from the known biochemical pathways for converting carbohydrate into the respective compounds, assuming that the only other products or substrates were NH3, SH−, O2, H2O, or CO2. If other compounds were required, pathways were included for how they could be made from carbohydrate. In a similar manner, if other products were made, pathways were included to ensure the full oxidation of these to CO2 and H2O. βDRUR values (units of μmol e− mg−1 compound) were calculated as four times the sum of net CO2 and O2 exchange, assuming 4e− are associated with each net CO2 or O2 exchange. The tissue production of carbohydrate for direct incorporation into biomass (item 10, Table II), or as a substrate for other organic compounds (not shown), had a βDRUR value of 0.
4. The theoretical RD (mmol e− Δg−1 dry weight of each plant part) for the synthesis of leaf, stem + petiole, and root tissues was calculated as the product of the biomass composition and βDRUR (Table II).
5. The theoretical DRUR (mmol e− plant−1 d−1) in the 24- to 25-d-old study plant used for gas exchange measurements was calculated as the product of the theoretical reductant demand (Table II), the RGR, and the tissue biomass at harvest (26 d).
ACKNOWLEDGMENTS
The authors thank Nicholas Dowling and Jennifer Willms for technical assistance.
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada (research grants to D.B.L. and D.H.T.).
LITERATURE CITED
- Allen S, Raven JA. Intracellular pH regulation in Ricinus communis grown with ammonium or nitrate as N source: the role of long distance transport. J Exp Bot. 1987;36:580–596. [Google Scholar]
- Andrews M. The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ. 1986;9:511–519. [Google Scholar]
- Atkins CA, Pate JS, Layzell DB. Assimilation and transport of nitrogen in non-nodulated (NO3− grown) Lupinus albus L. Plant Physiol. 1979;64:1078–1082. doi: 10.1104/pp.64.6.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Caldwell RM, Finazzo J, Warner RL, Weissbart J. Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol. 1989;91:352–356. doi: 10.1104/pp.91.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu TM, Aspinall D, Paleg LG. Stress metabolism: VI. Temperature stress and the accumulation of proline in barley and radish. Aust J Plant Physiol. 1974;1:87–97. [Google Scholar]
- Cramer MD, Lewis OAM. The influence of NO3− and NH4+ nutrition on the gas exchange characteristics of the roots of wheat (Triticum aestivum) and maize (Zea mays) plants. Ann Bot. 1993;72:37–46. [Google Scholar]
- Delhon P, Gojon A, Tillard P, Passama L. Diurnal regulation of NO3− uptake in soybean plants: I. Changes in NO3− influx, efflux, and N utilization in the plant during the day/night cycle. J Exp Bot. 1995;46:1585–1594. [Google Scholar]
- Eamus D, Myers B, Duff G, Williams R. A cost-benefit analysis of leaves of eight Australian savanna tree species of differing leaf life-span. Photosynthetica. 1999;36:575–586. [Google Scholar]
- Fock H, Canvin DT, Grant BR. Effects of oxygen and carbon dioxide on photosynthetic O2 evolution and CO2 uptake in sunflower and chlorella. Photosynthetica. 1971;5:389–394. [Google Scholar]
- Fock H, Hilgenberg W, Egle K. O2 and CO2 exchange of illuminated leaves and the CO2/O2 ratios at normal and low CO2 partial pressure. Planta. 1972;106:355–361. doi: 10.1007/BF00384772. [DOI] [PubMed] [Google Scholar]
- Gary C, Frossard JS, Chenevard D. Heat of combustion, degree of reduction and carbon content: three interrelated methods of estimating the construction cost of plant tissues. Agronomie. 1995;15:59–69. [Google Scholar]
- Griffin KL. Calorimetric estimates of construction cost and their use in ecological studies. Funct Ecol. 1994;8:551–562. [Google Scholar]
- Hunt SB, King J, Layzell DB. Effects of gradual increases in O2 concentration on nodule activity in soybean. Plant Physiol. 1989;91:315–321. doi: 10.1104/pp.91.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppe HC, Turpin DH. Integration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:577–607. [Google Scholar]
- Kaplan A, Bjorkman O. Ratio of CO2 uptake to O2 evolution during photosynthesis in higher plants. Z Pflanzenphysiol. 1980;96:185–188. [Google Scholar]
- Layzell DB, Hunt S, King BJ, Walsh KB, Weagle GE. A multichannel system for steady-state and continuous measurements of gas exchanges from legume roots and nodules. In: Torrey JG, Winship LJ, editors. Applications of Continuous and Steady-State Methods to Root Biology. Boston: Kluwer Academic Publishers; 1989. pp. 1–28. [Google Scholar]
- Long SP, Hallgren J-E. Measurement of CO2 assimilation by plants in the field and the laboratory. In: Coombs J, Hall DO, Long SP, Scurlock JMO, editors. Techniques in Bioproductivity and Photosynthesis. New York: Pergamon Press; 1985. pp. 62–93. [Google Scholar]
- Loss SP, Robson AD, Ritchie GSP. Nutrient uptake and organic acid anion metabolism in lupins and peas supplied with nitrate. Ann Bot. 1994;74:69–74. doi: 10.1093/aob/74.1.69. [DOI] [PubMed] [Google Scholar]
- McDermitt DK, Loomis RS. Elemental composition of biomass and its relation to energy content, growth efficiency and growth yield. Ann Bot. 1981;48:275–290. [Google Scholar]
- McGeehan SL, Naylor DV. Automated instrumental analysis of carbon and nitrogen in plant and soil samples. Soil Sci Plant Anal. 1988;19:493–505. [Google Scholar]
- Myers J. The pattern of photosynthesis in Chlorella. In: Franck J, Loomis WE, editors. Photosynthesis in Plants. Cedar Falls, IA: Iowa State College Press; 1949. pp. 349–369. [Google Scholar]
- Pate JS, Layzell DB, Atkins CA. Economy of carbon and nitrogen in a nodulated and non-nodulated (NO3− grown) legume. Plant Physiol. 1979;64:1083–1088. doi: 10.1104/pp.64.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning De Vries FWT, Brunsting AHM, Van Laar HH. Products, requirements and efficiency of biosynthesis: a quantitative approach. J Theor Biol. 1974;45:339–377. doi: 10.1016/0022-5193(74)90119-2. [DOI] [PubMed] [Google Scholar]
- Radford PJ. Growth analysis formulae: their use and abuse. Crop Sci. 1967;7:171–175. [Google Scholar]
- Ramshaw JAM. Structure of plant proteins. In: Boulter D, Parthier B, editors. Nucleic Acids and Proteins in Plants: I. Structure, Biochemistry and Physiology of Proteins. New York: Springer-Verlag; 1982. pp. 231–233. [Google Scholar]
- Rufty TW, Volk RJ, McClure PR, Israel DW, Raper CD. Relative content of NO3− and reduced N in xylem exudate as an indicator of root reduction of concurrently absorbed 15NO3−. Plant Physiol. 1982;69:166–170. doi: 10.1104/pp.69.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife A, Schloemer S. The diurnal pattern of nitrate uptake and reduction by spinach (Spinacia oleracea L.) Ann Bot. 1994;73:337–343. [Google Scholar]
- Scheurwater I, Cornelissen C, Dictus F, Welschen R, Lambers H. Why do fast- and slow-growing grass species differ so little in their rate of root respiration, considering the large differences in rate of growth and ion uptake? Plant Cell Environ. 1998;21:995–1005. [Google Scholar]
- Spencer DF, Ryan FJ, Ksander GG. Construction costs for some aquatic plants. Aquatic Bot. 1997;56:203–214. [Google Scholar]
- Sweeney RA. Generic combustion method for determination of crude protein in feeds: collaborative study. J Assoc Off Anal Chem. 1989;72:770–774. [PubMed] [Google Scholar]
- Tabatabi MA, Bremner JM. Use of the Leco automatic 70-second carbon analyzer for total carbon analysis of soils. Soil Sci Soc Am Proc. 1970;34:608–610. [Google Scholar]
- Tolbert NE, Benker C, Beck E. The oxygen and carbon dioxide compensation points of C3 plants: possible role in regulating atmospheric oxygen. Proc Natl Acad Sci USA. 1995;92:11230–11233. doi: 10.1073/pnas.92.24.11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Westhuizen MM, Cramer MD. The influence of elevated rhizosphere dissolved inorganic carbon concentrations on respiratory O2 and CO2 flux in tomato roots. J Exp Bot. 1998;49:1977–1985. [Google Scholar]
- Walton EF, Fowke PJ. Estimation of the annual cost of kiwifruit vine growth and maintenance. Ann Bot. 1995;76:613–623. [Google Scholar]
- Walton EF, Wunsche JN, Palmer JW. Estimation of bioenergetic costs of fruit and other organ synthesis in apple. Physiol Plant. 1999;106:129–134. [Google Scholar]
- Walsh KB, Vessey JK, Layzell DB. Carbohydrate supply and N2 fixation in soybean: the effect of varied day length and stem girdling. Plant Physiol. 1987;85:137–144. doi: 10.1104/pp.85.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger HG, Turpin DH. Mitochondrial respiration can support NO3− and NO2− reduction during photosynthesis. Plant Physiol. 1989;89:409–415. doi: 10.1104/pp.89.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Percival F, Merino J, Mooney HA. Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant Cell Environ. 1987;10:725–734. [Google Scholar]
- Willms JR, Dowling AN, Dong Z-M, Hunt S, Shelp B, Layzell DB. The simultaneous measurement of low rate of CO2 and O2 exchange in biological systems. Anal Biochem. 1997;254:272–282. doi: 10.1006/abio.1997.2416. [DOI] [PubMed] [Google Scholar]
- Willms JR, Salon C, Layzell DB. Evidence for light-stimulated fatty acid synthesis in soybean fruit. Plant Physiol. 1999;120:1117–1127. doi: 10.1104/pp.120.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ED, Armstrong FAJ, Richards FA. Determination of nitrate in sea water by cadmium copper reduction to nitrite. J Mar Biol Assoc. 1967;47:23–31. [Google Scholar]