Abstract
Follicular lymphoma (FL) is a disease often characterized by chronic and successive relapses after first-line chemoimmunotherapy. Although chemoimmunotherapy and combination therapy, such as lenalidomide with rituximab, are well established in the treatment sequence of FL, there is a need to streamline treatment options and determine placement of novel agents, such as chimeric antigen receptor T-cell therapy, an enhancer of zeste homolog 2 inhibitor, or a phosphoinositide 3 kinase inhibitor, into the treatment landscape. As such, the purpose of this review is to compare the safety profiles of approved agents in subsequent lines of therapy for relapsed or refractory FL and to assess how the management of adverse events may impact treatment choice.
Follicular lymphoma (FL) is the second-most common lymphoma in the United States (Tan et al., 2013), accounting for 20% of non-Hodgkin lymphoma (NHL) cases (National Institutes of Health [NIH], 2021). It is most frequently diagnosed among people aged 55–64 years (median, 63; NIH, 2021). Relapsed/refractory (R/R) FL remains incurable and is characterized by cycles of treatment–remission–relapse wherein the malignancy may become refractory to treatment (Tan et al., 2013).
Despite recent advances in the FL treatment landscape, overall survival (OS) and progression-free survival (PFS) outcomes decrease with each subsequent line of therapy. In the first-line setting, rituximab (Rituxan) in combination with cytotoxic therapy is the preferred option and is prescribed in approximately 52% to 58% of patients with FL (Batlevi et al., 2020; Friedberg et al., 2009; Link et al., 2019). In the second-line setting, combination lenalidomide (Revlimid)/rituximab (Rituxan; R2) or an anti-CD20 monoclonal antibody in combination with cytotoxic therapy are preferred options (Salles, 2020). Although first- and second-line therapies in FL are well established and effective at achieving remission, no standard of care has been established in the third-line setting and beyond.
The goal of therapy for R/R FL after first-line therapy is to maintain a good quality of life while managing the disease and slowing down its progression. When choosing an optimal therapy, greater emphasis should be placed on the need to balance efficacy with patient preferences in terms of tolerability and management of adverse events (AEs). As such, the purpose of this review is to compare the safety profiles of commonly used agents in subsequent lines of therapy for R/R FL and assess how AE management might impact treatment choice.
TREATMENT OPTIONS
Although many treatment options are available for R/R FL, the optimal therapeutic regimen in this setting has not been established (Figure 1). The main treatment options for these patients include immunomodulatory agents, and targeted therapies, such as chimeric antigen receptor (CAR) T-cell therapy, bispecific antibodies, an enhancer of zeste homolog 2 (EZH2) inhibitor, or a phosphoinositide 3 kinase (PI3K) inhibitor (Dreyling et al., 2021; Huntington et al., 2022; Ngu et al., 2022; Potnis et al., 2023). Because patients with R/R FL experience successive relapses, many are treated with several of these options throughout their disease course (Tan et al., 2013).
Figure 1.
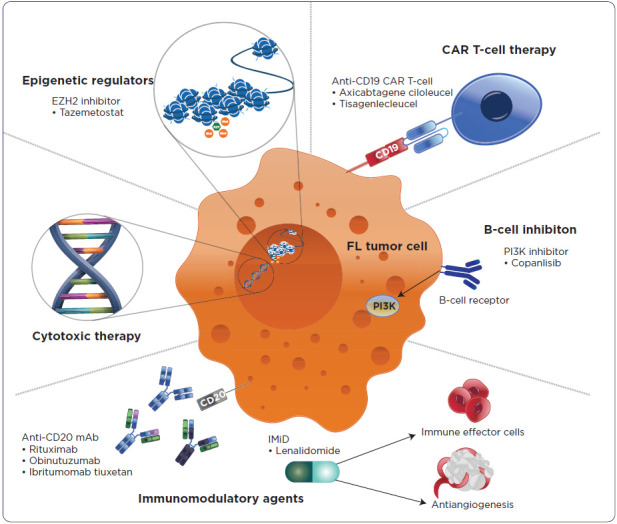
Mechanism of action of approved therapeutic classes in follicular lymphoma.
Note. CAR T-cell = chimeric antigen receptor T-cell therapy; EZH2 = enhancer of zeste homolog 2; FL = follicular lymphoma; IMiD = immunomodulatory imide drug; mAb = monoclonal antibody; PI3K = phosphoinositide 3 kinase.
Monoclonal antibodies directed against CD20 are key components in the treatment of FL. Such anti-CD20 monoclonal antibodies include rituximab and obinutuzumab (Gazyva). Although single-agent rituximab is a viable option for some patients, chemoimmunotherapy remains at the forefront as a treatment option for R/R FL (Dreyling et al., 2023; Skarbnik & Patel, 2023). Chemoimmunotherapy options combine either rituximab or obinutuzumab with chemotherapeutic agents such as bendamustine, combination cyclophosphamide/doxorubicin/vincristine/prednisone, or cyclophosphamide/vincristine/prednisone.
Lenalidomide, an immunomodulatory agent and non-chemotherapeutic option, is approved as combination therapy with rituximab in the R/R setting and may be favorable for select patients (Celgene, 2021). Lenalidomide/rituximab or an anti-CD20 monoclonal antibody in combination with cytotoxic therapy are preferred options in the second-line setting (Salles, 2020).
AUGMENT is a randomized phase III study that compared R2 with rituximab monotherapy in patients who received at least one prior therapy but were not refractory to rituximab (Table 1). In a subset of 295 patients with FL, median PFS (mPFS) was 39.4 months with R2 compared with 13.9 months in the rituximab monotherapy group (Leonard et al., 2019a, 2019b). GADOLIN is another randomized phase III study that compared obinutuzumab/bendamustine vs. bendamustine monotherapy in patients with rituximab-refractory disease. In a subset of 321 patients with FL, mPFS was 24.1 months in the obinutuzumab/bendamustine group vs. 13.7 months in the bendamustine group (Genentech, 2022a; Sehn et al., 2019).
Table 1. Efficacy Results of Studies in FLa.
| Trial | Regimen | Study population | ORR, % | mPFS | mOS |
|---|---|---|---|---|---|
| Immunomodulatory therapy | |||||
| McLaughlin, Piro, and Davis trials | Rituximab | R/R low-grade or follicular NHL (N = 296) | 36–57 | — | — |
| AUGMENT | Lenalidomide + rituximab | R/R FL, ≥ 1 prior systemic therapy, not rituximab-refractory (n = 295) | 80 | 39.4 months | 95% at 2 yearsb |
| Rituximab | 55 | 13.9 months | 86% at 2 yearsb | ||
| CAR T-cell therapy | |||||
| ZUMA-5 | Axicabtagene ciloleucel | 3L R/R FL (n = 123) | 94 | 73% at 18 monthsc | 92% at 18 monthsb |
| ELARA | Tisagenlecleucel | 3L R/R FL (N = 97) | 86 | 67% at 12 monthsc | — |
| Bispecific antibody therapy | |||||
| GO29781 | Mosunetuzumab | 3L R/R FL (N = 90) | 80 | 17.9 months | 90% at 18 monthsb |
| EZH2 inhibitor | |||||
| E7438-G000-101 | Tazemetostat | 3L R/R FLd (N = 99) | 51 | 12 months | NR |
| PI3K inhibitor | |||||
| CHRONOS-1 | Copanlisib | 3L R/R FL (n = 104) | 59 | 11.2 months | — |
Note. 3L = third-line; CAR T-cell therapy = chimeric antigen receptor T-cell therapy; EZH2 = enhancer of zeste homolog 2; FL = follicular lymphoma; mOS = median overall survival; mPFS = median progression-free survival; NHL = non-Hodgkin lymphoma; NR = not reported; ORR = objective response rate; PI3K = phosphoinositide 3 kinase; R/R = relapsed/refractory.
Table 1 does not represent head-to-head trials. Head-to-head trials have not been conducted among the listed agents.
OS; estimated from survival curves when not available.
PFS; estimated from survival curves when not available.
Includes wildtype and mutant-type EZH2.
Patients with FL include elderly and frail individuals who may not be candidates for cytotoxic therapy and its combinations because of its myelosuppressive effects (Javarappa et al., 2018; Skarbnik & Patel, 2023). Although rituximab monotherapy is the preferred regimen for the elderly or those who are infirm and unable to tolerate first-line chemoimmunotherapy regimens (Dreyling et al., 2021; Potnis et al., 2023), safer alternatives and novel targeted therapies are needed. Such agents used to treat R/R FL include CAR T-cell therapy, a bispecific antibody, an EZH2 inhibitor, and a PI3K inhibitor.
Axicabtagene ciloleucel (axi-cel; Yescarta) and tisagenlecleucel (tisa-cel; Kymriah) are CAR T-cell therapies approved for treatment of patients with FL (objective response rate [ORR], 91% and 86%, respectively), whereas tazemetostat (Tazverik) is an EZH2 inhibitor indicated for patients with FL, regardless of EZH2 mutation status (ORR, 51%) (Epizyme, 2020; Kite Pharma, 2022; Novartis Pharmaceuticals Corporation, 2022; Salles et al., 2020). Four PI3K inhibitors were approved by the US Food and Drug Administration (FDA) in the third-line setting and beyond (idelalisib [Zydelig], duvelisib [Copiktra], copanlisib [Aliqopa], and umbralisib [Ukoniq]). Because they target different PI3K subunits (α, β, γ, or δ), the efficacy and toxicity profiles of these inhibitors also differ (Qualls & Salles, 2022). Recently, the indications for use of idelalisib (δ-specific), duvelisib (γ- and δ-specific), and umbralisib (δ- and casein kinase 1-specific) in FL were voluntarily withdrawn from the US market (Gilead, 2022; PR Newswire, 2021; TG Therapeutics, 2022). Concerning trends of poor OS in randomized controlled trials of these drugs led to FDA review of PI3K data. Their investigation concluded that the impact to OS was likely due to increased toxicity, whether from increased risk of infection or immune-mediated AEs, or from long-term effects of PI3K inhibitors that could complicate subsequent anticancer therapy (FDA, 2022). The remaining agent, copanlisib (α- and δ-specific), is relatively effective, with an ORR of 59% (Dreyling et al., 2017). Mosunetuzumab is a first-in-class CD20 × CD3 bispecific antibody recently approved for the treatment of patients with R/R FL following two or more prior systemic therapies (ORR 80%; Budde et al., 2021).
SAFETY PROFILES OF TREATMENT OPTIONS
Once disease relapse has been confirmed, the goal of therapy is to balance effectiveness with maintaining a good quality of life. Careful and thorough assessment of activities of daily living and previous treatment toxicities by health-care professionals is needed to offer each patient an individualized treatment plan.
IMMUNOMODULATORY AGENTS
Rituximab
With more than 20 years of post-marketing surveillance data, rituximab as monotherapy and in combination with chemotherapeutic agents or lenalidomide has a well-defined safety profile. Rituximab carries black box warnings (BBWs) of fatal infusion-related reaction, severe mucocutaneous reaction, hepatitis B virus (HBV) reactivation, and progressive multifocal leukoencephalopathy (Table 2; Genentech, 2021). Premedication with acetaminophen (Tylenol) and an antihistamine is recommended for rituximab because of possible infusion-related reaction. Prior to initiating treatment, patients should be screened for a HBV infection, and complete blood count, with differential and platelet counts, should be obtained to monitor for cytopenia (Genentech, 2021).
Table 2. Safety Profile of Therapeutic Options in Follicular Lymphomaa.
| Agent | Most common adverse events | Black box warning | Contraindication |
|---|---|---|---|
| Immunomodulatory therapy | |||
| Rituximab | Infusion-related reaction, fever, lymphopenia, chills, infection, asthenia | Fatal infusion-related reaction, severe mucocutaneous reaction, HBV reactivation, progressive multifocal leukoencephalopathy | None |
| Obinutuzumab | Infusion-related reaction, fatigue, neutropenia, cough, URTI, musculoskeletal pain | HBV reactivation, progressive multifocal leukoencephalopathy | Hypersensitivity reaction to obinutuzumab or any of the excipients, including serum sickness with prior obinutuzumab use |
| Lenalidomide | Cytopenia (neutropenia, thrombocytopenia, anemia, leukopenia), diarrhea, constipation, nausea, fatigue, pyrexia, cough, URTI, rash | Embryofetal toxicity, hematologic toxicity, venous/arterial thromboembolism | Pregnancy Demonstrated severe hypersensitivity to lenalidomide |
| CAR T-cell therapy | |||
| Axicabtagene ciloleucel | Cytopenia (neutropenia, leukopenia, thrombocytopenia, anemia), CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, febrile neutropenia, nausea, infection with unspecified pathogens, decreased appetite, chills, diarrhea, tremor, musculoskeletal pain, cough, hypoxia, constipation, vomiting, arrhythmias, dizziness | CRS, neurologic toxicity | None |
| Tisagenlecleucel | Cytopenia (neutropenia, leukopenia, thrombocytopenia, anemia), CRS, infections-pathogens unspecified, fatigue, musculoskeletal pain, headache, diarrhea | CRS, neurologic toxicity | None |
| Bispecific antibody therapy | |||
| Mosunetuzumab | Cytopenia (lymphopenia, anemia, leukopenia, neutropenia, thrombocytopenia), CRS, fatigue, rash, pyrexia, headache | CRS | None |
| EZH2 inhibitor | |||
| Tazemetostata | Fatigue, URTI, musculoskeletal pain, nausea, abdominal pain | None | None |
| PI3K inhibitor | |||
| Copanlisib | Hyperglycemia, diarrhea, decreased general strength/energy, hypertension, cytopenia (leukopenia, neutropenia, thrombocytopenia), nausea, lower respiratory tract infection | None | None |
Note. CAR T-cell therapy = chimeric antigen receptor T-cell therapy; CRS = cytokine release syndrome; EZH2 = enhancer of zeste homolog 2; HBV = hepatitis B virus; PI3K = phosphoinositide 3 kinase; URTI = upper respiratory tract infection.
Includes wildtype and mutant-type EZH2.
The most common AEs of at least grade 3 associated with rituximab therapy in clinical trials were lymphopenia (40%) and neutropenia (6%) (Tables 2 and 3; Genentech, 2021). The median duration of these cytopenias was 14 days for lymphopenia and 13 days for neutropenia. In most patients, infusion-related reactions (involving fever, chills/rigors, nausea, pruritus, angioedema, hypotension, bronchospasm, urticaria, rash, vomiting, myalgia, dizziness, or hypertension) occurred during the first rituximab infusion and were resolved by slowing or interrupting the infusion rate and supportive care. Infusion-related reactions typically occurred within 120 minutes during the first rituximab infusion with an incidence of 77%, and the frequency decreased with subsequent infusions. For management of infusion-related reactions, the infusion may be temporarily interrupted or administered at a slower rate and can be continued at one half the previous rate after symptoms improve. In addition to infusion-related reaction, infection (bacterial, viral, fungal, unspecified) was reported in 31% of patients and included cytomegalovirus, herpes simplex virus, varicella zoster virus, HBV, and hepatitis C virus, among others. For serious infections, rituximab should be discontinued and an appropriate anti-infective therapy administered (Genentech, 2021).
Table 3. Comparison of Common Adverse Events Associated With Therapeutic Options in Follicular Lymphoma .
| Adverse event | Immunotherapy | CAR T-cell | BsAb | EZH2 inhibitor | PI3Ki | |||
|---|---|---|---|---|---|---|---|---|
| Rituximab | Obinutuzumab | Lenalidomide | Axi-cel | Tisa-cel | Mosunetuzumab | Tazemetostat | Copanlisib | |
| Blood and lymphatic system disorders | ||||||||
| Cytopenia | x | x | x | x | x | |||
| Anemia | x | x | x | x | ||||
| Lymphopenia | x | x | ||||||
| Leukopenia | x | x | x | x | x | |||
| Neutropenia | x | x | x | x | x | x | ||
| Thrombocytopenia | x | x | x | x | x | |||
| Febrile neutropenia | x | |||||||
| Cardiac disorders | ||||||||
| Arrhythmias | x | |||||||
| Tachycardia | x | |||||||
| Gastrointestinal disorders | ||||||||
| Abdominal pain | x | |||||||
| Nausea | x | x | x | x | ||||
| Vomiting | x | |||||||
| Diarrhea | x | x | x | x | ||||
| Constipation | x | x | ||||||
| Oral ulceration/stomatitis | ||||||||
| General disorders and administration site conditions | ||||||||
| IRR | x | x | ||||||
| Pyrexia/fever | x | x | x | x | ||||
| Chills | x | x | ||||||
| Asthenia | x | |||||||
| Fatigue | x | x | x | x | x | x | x | |
| Immune system disorders | ||||||||
| Cytokine release syndrome | x | x | x | |||||
| Infections and infestations | ||||||||
| Infection | x | x | x | |||||
| Nasopharyngitis | ||||||||
| URTI | x | x | x | |||||
| Metabolism and nutrition disorders | ||||||||
| Anorexia | ||||||||
| Decreased appetite | x | |||||||
| Weight loss | ||||||||
| Hyperglycemia | x | |||||||
| Musculoskeletal and connective tissue disorders | ||||||||
| Musculoskeletal pain | x | x | x | x | ||||
| Nervous system disorders | ||||||||
| Dizziness | x | |||||||
| Encephalopathy | x | |||||||
| Headache | x | x | x | |||||
| Peripheral neuropathy | ||||||||
| Tremor | x | |||||||
| Respiratory, thoracic, and mediastinal disorders | ||||||||
| Cough | x | x | x | |||||
| Dyspnea | ||||||||
| Hypoxia | x | |||||||
| Skin and subcutaneous tissue disorders | ||||||||
| Alopecia | ||||||||
| Rash | x | x | ||||||
| Vascular disease | ||||||||
| Hypertension | x | |||||||
| Hypotension | x | |||||||
Note. axi-cel = axicabtagene ciloleucel; BsAb = bispecific antibody; CAR T-cell therapy = chimeric antigen receptor T-cell therapy; EZH2 = enhancer of zeste homolog 2; IRR = infusion-related reaction; PI3Ki = phosphoinositide 3 kinase inhibitor; tisa-cel = tisagenlecleucel; URTI = upper respiratory tract infection.
Obinutuzumab
Despite the clinical success of rituximab in the treatment landscape of FL, the majority of patients will relapse and may develop rituximab- refractory disease (Tan et al., 2013). In most clinical trials, rituximab-refractory disease was defined as nonresponse or progression within 6 months of treatment with a rituximab-containing regimen (monotherapy or combination therapy; Acrotech Biopharma, 2019; Genentech, 2022a). Obinutuzumab can be used as an alternative to rituximab in combination with cytotoxic therapy for treatment of R/R FL (Marcus et al., 2017). The FDA has granted accelerated approval to zanubrutinib plus obinutuzumab for the treatment of patients with relapsed/refractory follicular lymphoma following 2 previous lines of treatment (FDA, 2024a). Obinutuzumab carries a BBW of HBV reactivation and progressive multifocal leukoencephalopathy (Genentech, 2022a), which are also associated with rituximab (Genentech, 2021). All patients must be screened for an active HBV infection prior to initiating treatment. Premedication with acetaminophen, an antihistamine, and a glucocorticoid is recommended to reduce the risk of an infusion-related reaction (Genentech, 2022a).
In clinical trials, the most common AEs of at least grade 3 were neutropenia (35%–49%) and infusion-related reaction (11%–12%; Genentech, 2022a). Infusion-related reactions have occurred within 24 hours of obinutuzumab infusion and can occur with subsequent infusions. Reaction on the first day of treatment has been reported in 37% of patients with R/R NHL. The most frequently reported infusion-related symptoms are nausea, fatigue, chest discomfort, dyspnea, dizziness, vomiting, diarrhea, rash, hypertension, hypotension, flushing, headache, pyrexia, and chills. Depending on the severity of these infusion-related reactions, obinutuzumab may be administered at a reduced rate, temporarily interrupted, or discontinued. Fatal cardiac events have been observed with obinutuzumab. Patients with a preexisting cardiac condition should be monitored more frequently during and after infusion (Genentech, 2022a). Severe/life-threatening neutropenia, including febrile neutropenia, has been reported with obinutuzumab, so patients should be monitored for any manifestations of developing infection. Administration of granulocyte colony-stimulating factors should be considered for patients with grade 3 or 4 neutropenia. In some patients, neutropenia can present later after therapy completion whereupon antimicrobial prophylaxis is highly encouraged. Fatal and serious bacterial, fungal, and viral infections have been reported during and after obinutuzumab treatment, with grade 3 to 5 infections occurring in as many as 8% of patients during combination therapy, 13% during monotherapy, and 8% following treatment. Patients with an active infection should not receive obinutuzumab therapy (Genentech, 2022a).
Lenalidomide
The immunomodulatory drug, lenalidomide, in combination with rituximab is frequently used as a non-chemotherapeutic option in the second-line setting and beyond for patients with R/R FL; all safety concerns associated with rituximab should be considered before choosing this option because rituximab is part of this regimen. The recommended starting dose of lenalidomide is 20 mg orally once daily on days 1 through 21 of 28-day cycles for up to 12 treatment cycles, with dose adjustments as needed for hematologic toxicities and renal impairment (Celgene, 2021). Lenalidomide carries a BBW of embryofetal toxicity, hematologic toxicity, and venous/arterial thromboembolism. Lenalidomide is only available through the Risk Evaluation and Mitigation Strategy (REMS) program owing to its teratogenic property. Complete blood counts, including platelet counts, must be monitored because lenalidomide can cause significant neutropenia and thrombocytopenia. Thromboprophylaxis is also recommended because of an increased incidence of venous thromboembolism observed in the AUGMENT trial (4.5% in the R2 arm vs. 1.1% in the control arm; Celgene, 2021). Severe cutaneous reactions have been reported with lenalidomide therapy, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS). Patients with a prior history of grade 4 rash associated with thalidomide treatment should not receive lenalidomide. Treatment interruption or discontinuation should be considered for grade 2 or 3 skin rash, and lenalidomide should be permanently discontinued in cases of grade 4 rash, exfoliative or bullous rash, SJS, TEN, or DRESS (Celgene, 2021).
Grade 3 to 4 neutropenia and leukopenia were reported in 50% and 7% of patients treated with R2 in AUGMENT, respectively, and contributed to the overall increased rates of grade 3 to 4 AEs. Three percent of patients discontinued lenalidomide treatment due to neutropenia and the rest were treated with dose interruptions and/or reductions and growth-factor support. Growth factors were administered in 36% of patients treated with R2 and in 12% of patients treated with rituximab monotherapy. Use of antiplatelets and/or anticoagulants during the study treatment period occurred in 70% of patients receiving R2 and 67% of patients receiving rituximab monotherapy (Leonard et al., 2019a, 2019b).
Lenalidomide monotherapy is another treatment option (Witzig et al., 2015). While R2 demonstrated greater efficacy than lenalidomide monotherapy in patients with R/R FL, similar toxicity profiles were observed (Leonard et al., 2015). CALGB 50401 compared lenalidomide monotherapy with R2 in patients with recurrent FL who received at least one prior therapy with rituximab either alone or in combination with cytotoxic therapy. Grade 3 to 4 AEs were reported in 58% and 52% of patients treated with lenalidomide monotherapy and R2, respectively; among these AEs was neutropenia (16% vs. 20%, respectively). The two treatment groups had a similar proportion of patients (≈20%) who discontinued therapy due to AEs. At least one dose modification occurred in 67% of patients treated with lenalidomide monotherapy and 80% of patients treated with R2 (Leonard et al., 2015).
CAR T-CELL THERAPY
Despite the initial clinical remission that patients might achieve with immunomodulatory and chemoimmunotherapy regimens, many patients continue to relapse and may require novel approaches (Tan et al., 2013). Two CD19-directed CAR T-cell therapies, axi-cel and tisa-cel, are approved in the United States for patients with R/R FL (Kite Pharma, 2022; Novartis Pharmaceuticals Corporation, 2022). While the benefit of these CAR T-cell therapies has been demonstrated, understanding the potential for early and long-term toxicities is essential for safe use. The most common toxicities are cytokine release syndrome (CRS), immune cell-associated neurotoxicity syndrome (ICANS), and infection (Wudhikarn & Perales, 2022; Yáñez et al., 2019). Strategies to mitigate the toxic side effects of CAR T-cell therapy include prophylaxis, close observation during and after infusion, and prompt treatment of severe AEs. Data regarding the long-term risk of infection in patients treated with CAR T-cell therapy are scarce. However, baseline disease, type, and number of prior anticancer therapies; the dose of CAR T-cell lymphocytes; and the type and intensity of lymphodepleting chemotherapy have been identified as potential risk factors for the development of infection (Los-Arcos et al., 2021). Neutropenia has also been associated with increased risk of infection, especially in patients with prolonged cytopenias. Patients should be screened for latent infections prior to initiating CAR T-cell therapy. Antiviral and anti-pneumocystis prophylaxis is recommended from lymphodepletion until 1 year post-infusion and CD4+ T cells > 200/µL (Gea-Banacloche, 2023). B-cell aplasia and hypogammaglobulinemia can occur in patients receiving CAR T-cell therapy (Kite Pharma, 2022; Novartis Pharmaceuticals Corporation, 2022). Immunoglobulin levels should be monitored following treatment and symptoms managed using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement guidelines (Kite Pharma, 2022; Novartis Pharmaceuticals Corporation, 2022).
Axicabtagene Ciloleucel
New modalities of targeted immunotherapies with CAR T-cell therapy were studied in FL, and axi-cel is the first CAR T-cell therapy to be approved in patients with R/R FL after at least two lines of systemic therapy (Kite Pharma, 2022). Axi-cel carries a BBW of CRS and neurologic toxicity, including ICANS. Prior to the infusion of axi-cel, patients must be pretreated with lymphodepleting cytotoxic therapy and premedicated with acetaminophen and diphenhydramine (Benadryl). Patients must be monitored for at least 7 days at a certified health-care facility post-infusion for possible clinical manifestations of CRS (e.g., fever, hypotension, tachycardia, hypoxia, chills, headache, arrhythmia, fatigue) and ICANS (e.g., encephalopathy, headache, tremor, dizziness, delirium, aphasia, insomnia). Patients are instructed to remain within proximity (i.e., 2 hours of travel) of the certified health-care facility for at least 4 weeks following infusion and to refrain from driving for at least 8 weeks after receiving treatment. The package insert outlines management recommendations for CRS and neurologic toxicity for which patients should be monitored with continuous cardiac telemetry and pulse oximetry for grade 2 and higher events (Kite Pharma, 2022). Tocilizumab (Actemra) and corticosteroids can be used to manage CRS depending on the severity of the event. Corticosteroids are administered for neurologic toxicity depending on severity, and seizure prophylaxis is recommended for neurologic toxicity of any grade, according to the package insert. Axi-cel is only available through the REMS program because of the risks of CRS and neurologic toxicities (Kite Pharma, 2022).
In ZUMA-5, 123 patients with FL previously treated with an anti-CD20 monoclonal antibody and an alkylating agent were treated with axi-cel (Jacobson et al., 2022). The most common grade 3 and higher treatment-emergent AEs (TEAEs) in the safety population were neutropenia (33%), decreased neutrophil count (28%), and anemia (24%). Grade 3 and higher CRS and neurologic events (including ICANS) occurred in 6% and 15% of patients with FL, respectively. In the FL safety population, the median time of CRS onset was 4 days (median duration: 6), whereas the median time to neurologic toxicity onset was 7 days (median duration: 14; Jacobson et al., 2022). Grade 3 or worse infections occurred in 15% of patients with FL, including pneumonia (6%), urinary tract infection (2%), and staphylococcus, sinusitis, and herpes zoster (1% each). Immunoglobulin therapy was administered to 27% of patients with FL, and one event of grade 3 hypogammaglobulinemia was observed (Jacobson et al., 2022). Patients treated with axi-cel should be monitored for signs and symptoms of infection before and after infusion and treated with appropriate prophylactic antimicrobials.
Tisagenlecleucel
Tisa-cel is another CAR T-cell therapy that is also approved for treatment of patients with R/R FL after at least two lines of systemic therapy (Novartis Pharmaceuticals Corporation, 2022). Similar to axi-cel, tisa-cel carries a BBW for CRS and neurologic toxicity. Prior to the infusion of tisa-cel, patients must be pretreated with lymphodepleting cytotoxic therapy and be premedicated with acetaminophen and diphenhydramine or another H1 antihistamine. Patients must be monitored two to three times during the first week following infusion at a certified health-care facility for signs and symptoms of CRS and neurologic toxicities. The clinical management of CRS and neurologic toxicity for axi-cel can also be applied to tisa-cel therapy. Tisa-cel is only available through the REMS program (Novartis Pharmaceuticals Corporation, 2022).
In ELARA, 97 patients with FL who were previously treated with an anti-CD20 monoclonal antibody and an alkylating agent were treated with tisa-cel (Fowler et al., 2022). The most common grade 3 or higher AE in the safety population was neutropenia (42%). No grade 3 or higher CRS events occurred, whereas 3% of patients reported grade 3 or higher neurologic events. The median time to CRS onset and to resolution was 4 days each. The median time to a serious neurologic event onset and to resolution was 9 days and 2 days, respectively (Fowler et al., 2022). Infections of grade 3 or higher occurred in 21% of patients in ELARA (Novartis Pharmaceuticals Corporation, 2022). Patients with an active infection should not start tisagenlecleucel treatment until the infection is resolved. Administration of infection prophylaxis is recommended prior to infusion, and patients should be monitored for signs and symptoms of infection following treatment (Novartis Pharmaceuticals Corporation, 2022). Prolonged grade ≥ 3 cytopenias observed in the ELARA trial included thrombocytopenia (17%) and neutropenia (16%). Hypogammaglobulinemia occurred in 18% of patients.
BISPECIFIC ANTIBODY THERAPY
Mosunetuzumab
Mosunetuzumab is a first-in-class T-cell–engaging CD20 × CD3 bispecific monoclonal antibody designed as an off-the-shelf immunotherapy for patients with R/R FL following two or more prior systemic therapies (Genentech, Inc.; Sun et al., 2015). It is administered as an intravenous infusion in 21-day cycles with a step-up dosing regimen: 1 mg on cycle 1 day 1 (C1D1), 2 mg on C1D8, 60 mg on C1D15 and C2D1, and 30 mg on C3D1 and day 1 of each cycle thereafter (Genentech, 2022b). Mosunetuzumab carries a BBW of CRS. Premedication consisting of dexamethasone, diphenhydramine, and acetaminophen is administered to all patients prior to infusion during the first two cycles and should be continued for patients who experience any-grade CRS with the previous dose. At the first sign of CRS or neurologic toxicity (including ICANS), patients should be evaluated and supportive therapy provided based on severity. Serious/fatal infections have been observed with mosunetuzumab. Patients must be monitored for infection prior to and during treatment, and caution should be exercised when considering treatment for patients with a history of recurrent or chronic infections or patients with significant prior immunosuppressive therapy (Genentech, 2022b).
In GO29781, 90 patients with R/R FL and at least two prior systemic therapies received mosunetuzumab monotherapy (Budde et al., 2022). The most common grade 3 to 4 AEs were neutropenia or decreased neutrophil count (27%), hypophosphatemia (17%), hyperglycemia (8%), and anemia (8%) (Budde et al., 2022). Grade 3 and higher CRS and neurologic events occurred in 2.5% and 3% of patients, respectively (Genentech, 2022b). Grade 3 or 4 infections occurred in 14% of patients, most commonly pneumonia, sepsis, and upper respiratory tract infection.
EZH2 INHIBITOR
Tazemetostat
EZH2 is an epigenetic regulator of cellular differentiation programs and is required for germinal center B cells to proliferate and mutate (Béguelin et al., 2013). Somatic gain-of-function mutations in EZH2 that confer oncogenic dependency have been described in approximately 25% of patients with FL (Baker et al., 2015; Bödör et al., 2013). Tazemetostat is a first-in-class, oral, selective methyltransferase inhibitor of EZH2 activity, including both mutant type (MT) and wildtype (WT), approved for treatment of R/R FL in patients with EZH2 mutation who have received at least two prior therapies or those who have no satisfactory alternative treatment options (Epizyme, 2020). Tazemetostat does not carry a BBW. The recommended dose of tazemetostat is 800 mg orally twice daily, with dose adjustments required for coadministration with strong and moderate cytochrome P450 3A inhibitors and certain hematologic AEs (neutropenia, thrombocytopenia, and anemia; Epizyme, 2020). The risk of developing secondary malignancies increases following tazemetostat treatment, and long-term monitoring for secondary malignancies is recommended (Epizyme, 2020).
In E7438-G000-101, 99 patients (45 with MT EZH2, 54 with WT EZH2) with R/R FL who previously received at least two systemic therapies were treated with tazemetostat (Morschhauser et al., 2020). The most common grade 3 and higher TEAEs were thrombocytopenia and anemia (both 5%). TEAEs that led to treatment reduction, interruption, and discontinuation occurred in nine, 27, and eight patients, respectively (Morschhauser et al., 2020).
A matching-adjusted indirect comparison study demonstrated that tazemetostat is associated with a lower relative risk of safety outcomes vs. copanlisib (Proudman et al., 2021). For any grade 3 and higher TEAE, tazemetostat had a relative risk of 0.37 vs. copanlisib (p < .001), supporting the favorable safety profile of tazemetostat (Proudman et al., 2021).
When treatment decisions are being made, the patient characteristics of age and frailty should be considered, in addition to safety concerns. Because elderly and frail patients have a decreased ability to tolerate standard cytotoxic therapy, there is a need for safer alternatives. While rituximab monotherapy remains the preferred second-line therapy option for the elderly or those who are infirm, tazemetostat may be an appropriate treatment option for this patient population (author opinion).
PI3K INHIBITOR
Copanlisib
Copanlisib is an intravenously administered α and δ isoform PI3K inhibitor approved in 2017 for treatment of R/R FL in patients who received at least two prior systemic therapies (Bayer, 2021). Copanlisib does not carry a BBW. Its recommended dose is 60 mg via intravenous administration, with dose adjustments required for hepatic impairment, coadministration with a strong cytochrome P450 3A inhibitor, or for certain AEs (Bayer, 2021). Because of its α isoform-specific inhibition, copanlisib is associated with unique toxicities among PI3K inhibitors, including hyperglycemia and hypertension, which may limit its use in certain populations, such as patients with insulin resistance or cardiovascular disease (Qualls & Salles, 2022).
In CHRONOS-1, 104 patients with FL were treated with copanlisib, including 80 patients whose disease was rituximab refractory and 61 patients whose disease was double refractory to rituximab and alkylating agents (Dreyling et al., 2017). The most common grade 3 or higher TEAEs included hyperglycemia (41%), hypertension (24%), and decreased neutrophil count (24%). For management of hyperglycemia, if pre-dose fasting serum glucose level was at least 160 mg/dL or random/nonfasting glucose level was at least 200 mg/dL, copanlisib would be temporarily interrupted until fasting glucose or random/non-fasting glucose values recovered to normal levels; however, if the pre-dose or post-dose blood glucose value was at least 500 mg/dL, then dose reductions were required (Bayer 2021). Noninfectious pneumonitis, lung infection, and hyperglycemia were AEs that led to dose discontinuation in 25% of patients (Dreyling et al., 2017).
An expert panel comprising specialists in lymphoma, hypertension, and diabetes developed recommendations for optimal management of hyperglycemia and hypertension associated with copanlisib treatment (Cheson et al., 2019). In patients with diabetes or prediabetes, blood sugar levels should be closely monitored and endocrinologist consultation is suggested prior to treatment. Patients with preexisting hypertension should be treated prior to initiating copanlisib; however, in those who have copanlisib-induced hypertension, short-acting antihypertensives may be preferred given the anticipated short duration of increased blood pressure (Cheson et al., 2019).
SPECIAL POPULATIONS
Treatment decisions for patients with R/R FL must include a number of considerations, including baseline risk factors, prior systemic therapies, AE frequency and severity, dosing regimen, route of administration, and treatment access. Furthermore, some populations may have specific circumstances that drive treatment decisions. For example, patients treated in a community oncology setting may not have reasonable access to a certified CAR T-cell therapy hospital or research center. Patients with multiple comorbidities or those who are frail may benefit from less aggressive therapies that carry a lower risk of serious, long-term AEs. Conversely, patients with multiple prior therapies for refractory disease or those with early-relapsing progression of disease within 24 months of initial chemoimmunotherapy (POD24) may require a more aggressive treatment plan despite the potential risks.
POD24 is a robust marker of poor outcomes in patients with FL, and optimal second-line treatment strategies are not well defined (Casulo et al., 2015; Rodgers et al., 2021). For fit patients without histologic transformation, aggressive cellular strategies such as autologous stem cell transplant or CAR T-cell therapy could be an option (Rodgers et al., 2021). Novel therapies also have shown promise in the POD24 population. Immunomodulatory R2 treatment demonstrated significantly improved PFS vs. rituximab regardless of POD24 status (Leonard et al., 2019a, 2019b). Similar efficacy findings have been reported for POD24 vs. non-POD24 populations in clinical trials of tazemetostat, mosunetuzumab, and copanlisib (Budde et al., 2021; Chauhan & Cheson, 2021; Morschhauser et al., 2020).
FUTURE PERSPECTIVES
With the success of many of these targeted agents, new emerging agents are also being studied. TIDAL (NCT03768505) is a phase II study of zandelisib (ME-401) in patients with R/R FL who have failed at least two prior lines of systemic therapy (MEI Pharma, Inc., 2021). Zandelisib, an investigational drug, is an oral PI3K inhibitor that also has promising initial results, although data are not yet mature (MEI Pharma, Inc., 2021).
Due to the success of many novel targeted therapies and their indications as single-agent options for treatment of R/R FL, some agents are being studied in combination with other FL therapies. The combination tazemetostat/rituximab study, SYMPHONY-1 (NCT04224493), is a phase Ib/III study of tazemetostat/R2 compared with R2 in patients with R/R FL (Batlevi et al., 2021; Patel et al., 2021). A PI3K inhibitor and rituximab combination is being studied in COASTAL (NCT04745832), a phase III study of zandelisib/rituximab vs. standard chemoimmunotherapy in patients with R/R indolent NHL (Jurczak et al., 2021; Matasar et al., 2021).
Trials of new investigational bispecific antibodies as monotherapy agents are ongoing and have promising preliminary data. Epcoritamab and odronextamab, CD20 × CD3 bispecific T-cell engagers in development for patients with R/R FL, have demonstrated durable clinical responses and manageable safety in heavily pretreated patients (Bannerji et al., 2022; Hutchings et al., 2020; Thieblemont et al., 2022). Epcoritamab was granted accelerated approval for adult patients with R/R FL after two or more lines of systemic therapy in June 2024 (FDA, 2024b). Adverse events associated with bispecific antibodies are similar to those observed with CAR T-cell therapy, including CRS and neurotoxicity (Zhou et al., 2021). However, they generally occur at a lower rate and with less severity with bispecific antibody treatment. Administered as subcutaneous injections, these therapies may emerge as convenient off-the-shelf treatment options.
CONCLUSIONS
Treatment of R/R FL depends on disease burden, goal of therapy, and patient preference. It requires shared decision-making between health-care professionals and patients. In the R/R setting, tolerability and patient needs should be a major consideration while preserving quality of life, without compromising effectiveness, when choosing treatment options.
Although chemoimmunotherapy remains as the standard of care for initial therapy and can be used in later lines, approvals of novel non-chemotherapeutic agents may shift the FL landscape. R2 is a well-established regimen for patients with FL at first relapse, thereby placing any of the novel targeted agents as fair options for patients at second relapse and beyond. In the third-line setting, tazemetostat demonstrates promising clinical efficacy and tolerability and, because of its favorable safety profile, is being studied with R2 in the second-line setting. However, with the development of bispecific antibodies and allogeneic CAR T-cell therapies, their maturing response data and durability of benefit will provide insight into how these agents will fit in the current treatment paradigm. Finally, treatment selection in the relapsed setting is also dependent on multiple factors that include the patient's lifestyle and treatment goals, comorbidities, prior AEs, and potential long-term side effects associated with each therapeutic agent.
Acknowledgment
Medical writing and editorial support were provided by Sarah Huh, PharmD, of Peloton Advantage, an OPEN Health company, and funded by Epizyme, Inc, an Ipsen company.
Footnotes
Ms. Bradley has served on an advisory board and received honoraria from Epizyme, Inc. Mr. Davis has served as a member of a speakers bureau for Bristol Myers Squibb, GlaxoSmithKline, Incyte, Janssen, and Takeda, and has served as a paid consultant for Incyte. Ms. Martin has served on an advisory board for Epizyme, Inc. and serves on a speakers bureau for the Clinical Education Alliance. Ms. Woodward serves on a speakers bureau for BeiGene, and has served on advisory boards for Epizyme, Inc.
This study was sponsored by Epizyme, Inc, an Ipsen company. Medical writing and editorial support were provided by Sarah Huh, PharmD, of Peloton Advantage, an OPEN Health company, and funded by Epizyme, Inc., an Ipsen company.
References
- Acrotech Biopharma. (2019). Zevalin (ibritumomab tiuxetan) package insert. https://zevalin.com/wp-content/uploads/2019/04/PI-ZEVALIN-092019.pdf
- Baker, T., Nerle, S., Pritchard, J., Zhao, B., Rivera, V. M., Garner, A., & Gonzalvez, F. (2015). Acquisition of a single EZH2 D1 domain mutation confers acquired resistance to EZH2-targeted inhibitors. Oncotarget, 6(32), 32646–32655. 10.18632/oncotarget.5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerji, R., Arnason, J. E., Advani, R. H., Brown, J. R., Allan, J. N., Ansell, S. M.,…Topp, M. S. (2022). Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): Results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematology, 9(5), e327–e339. 10.1016/s2352-3026(22)00072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlevi, C. L., Park, S. I., Phillips, T., Nastoupil, L., Amengual, J., Andorsky, D.,…Morschhauser, F. (2021). Interim analysis of the randomized phase 1b/3 study evaluating the safety and efficacy of tazemetostat plus lenalidomide and rituximab in patients with relapsed/refractory follicular lymphoma. Blood, 138(Supplement 1), 2207. 10.1182/blood-2021-148199 [DOI] [Google Scholar]
- Batlevi, C. L., Sha, F., Alperovich, A., Ni, A., Smith, K., Ying, Z.,…Younes, A. (2020). Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer Journal, 10(7), 74. 10.1038/s41408-020-00340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer. (2021). Aliqopa (copanlisib) package insert. http://labeling.bayerhealthcare.com/html/products/pi/Aliqopa_PI.pdf
- Béguelin, W., Popovic, R., Teater, M., Jiang, Y., Bunting, K. L., Rosen, M.,…Melnick, A. M. (2013). EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell, 23(5), 677–692. 10.1016/j.ccr.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bödör, C., Grossmann, V., Popov, N., Okosun, J., O'Riain, C., Tan, K.,…Fitzgibbon, J. (2013). EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood, 122(18), 3165–3168. 10.1182/blood-2013-04-496893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde, L. E., Sehn, L. H., Matasar, M., Schuster, S. J., Assouline, S., Giri, P.,…Bartlett, N. L. (2022). Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: A single-arm, multicentre, phase 2 study. Lancet Oncology, 23(8), 1055–1065. 10.1016/S1470-2045(22)00335-7 [DOI] [PubMed] [Google Scholar]
- Budde, L. E., Sehn, L. H., Matasar, M. J., Schuster, S. J., Assouline, S., Giri, P.,…Bartlett, N. L. (2021). Mosunetuzumab monotherapy is an effective and well-tolerated treatment option for patients with relapsed/refractory (R/R) follicular lymphoma (FL) who have received ≥2 prior lines of therapy: Pivotal results from a phase I/II study. Blood, 138(Supplement 1), 127. 10.1182/blood-2021-145872 [DOI] [Google Scholar]
- Casulo, C., Byrtek, M., Dawson, K. L., Zhou, X., Farber, C. M., Flowers, C. R.,…Friedberg, J. W. (2015). Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. Journal of Clinical Oncology, 33(23), 2516–2522. 10.1200/JCO.2014.59.7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celgene. (2021). Revlimid (lenalidomide) package insert. https://packageinserts.bms.com/pi/pi_revlimid.pdf
- Chauhan, A. F., & Cheson, B. D. (2021). Copanlisib in the treatment of relapsed follicular lymphoma: Utility and experience from the clinic. Cancer Management Research, 13, 677–692. 10.2147/cmar.s201024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson, B. D., O'Brien, S., Ewer, M. S., Goncalves, M. D., Farooki, A., Lenz, G.,…Dreyling, M. (2019). Optimal management of adverse events from copanlisib in the treatment of patients with non-Hodgkin lymphomas. Clinical, Lymphoma, Myeloma, and Leukemia, 19(3), 135–141. 10.1016/j.clml.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyling, M., Ghielmini, M., Rule, S., Salles, G., Ladetto, M., Tonino, S. H.,…Jerkeman, M. (2021). Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 32(3), 298–308. 10.1016/j.annonc.2020.11.008 [DOI] [PubMed] [Google Scholar]
- Dreyling, M., Santoro, A., Mollica, L., Leppä, S., Follows, G. A., Lenz, G.,…Zinzani, P. L. (2017). Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. Journal of Clinical Oncology, 35(35), 3898–3905. 10.1200/jco.2017.75.4648 [DOI] [PubMed] [Google Scholar]
- Epizyme. (2020). Tazverik (tazemetostat) package insert. https://d2rkmuse97gwnh.cloudfront.net/a88aa6d6-3ca0-4362-a711-d53c45ae33ff/522a2033-f01b-422d-81b6-78692eb7a6da/522a2033-f01b-422d-81b6-78692eb7a6da_source__v.pdf
- Fowler, N. H., Dickinson, M., Dreyling, M., Martinez-Lopez, J., Kolstad, A., Butler, J.,…Thieblemont, C. (2022). Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: The phase 2 ELARA trial. Nature Medicine, 28(2), 325–332. 10.1038/s41591-021-01622-0 [DOI] [PubMed] [Google Scholar]
- Friedberg, J. W., Taylor, M. D., Cerhan, J. R., Flowers, C. R., Dillon, H., Farber, C. M.,…Link, B. K. (2009). Follicular lymphoma in the United States: First report of the national LymphoCare study. Journal of Clinical Oncology, 27(8), 1202–1208. 10.1200/jco.2008.18.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gea-Banacloche, J. C. (2023). Infectious complications of chimeric antigen receptor (CAR) T-cell therapies. Seminars in Hematology, 60(1), 52–58. 10.1053/j.seminhematol.2023.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genentech. (2021). Rituxan (rituximab) package insert. https://www.gene.com/download/pdf/rituxan_prescribing.pdf
- Genentech. (2022a). Gazyva (obinutuzumab) package insert. https://www.gene.com/download/pdf/gazyva_prescribing.pdf
- Genentech. (2022b). Lunsumio (mosunetuzumab) package insert. https://www.gene.com/download/pdf/lunsumio_prescribing.pdf
- Gilead. (2022). Gilead statement on Zydelig U.S. indication for follicular lymphoma and small lymphocytic leukemia. https://www.gilead.com/news-and-press/company-statements/gilead-statement-on-zydelig-us-indication-for-follicular-lymphoma-and-small-lymphocytic-leukemia
- Huntington, S. F., Appukkuttan, S., Wang, W., Du, Y., Hopson, S., & Babajanyan, S. (2022). Treatment patterns of follicular lymphoma in the United States: A claims analysis. Journal of Health Economics and Outcomes Research, 9(2), 115–122. 10.36469/001c.38070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings, M., Mous, R., Clausen, M. R., Johnson, P., Linton, K. M., Chamuleau, M. E. D.,…Lugtenburg, P. J. (2020). Subcutaneous epcoritamab induces complete responses with an encouraging safety profile across relapsed/refractory B-cell non-Hodgkin lymphoma subtypes, including patients with prior CAR-T therapy: Updated dose escalation data. Blood, 136(Supplement 1), 45–46. 10.1182/blood-2020-133820 [DOI] [Google Scholar]
- Jacobson, C. A., Chavez, J. C., Sehgal, A. R., William, B. M., Munoz, J., Salles, G.,…Neelapu, S. S. (2022). Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): A single-arm, multicentre, phase 2 trial. Lancet Oncology, 23(1), 91–103. 10.1016/s1470-2045(21)00591-x [DOI] [PubMed] [Google Scholar]
- Javaruppa, K. K., Tsallos, D., & Heckman, C. A. (2018). A multiplexed screening assay to evaluate chemotherapy-induced myelosuppression using healthy peripheral blood and bone marrow. SLAS Discovery, 23(7), 687–696. 10.1177/2472555218777968 [DOI] [PubMed] [Google Scholar]
- Jurczak, W., Zinzani, P. L., Cunningham, D., Yavrom, S., Huang, W., Gorbatchevsky, I., & Ribrag, V. (2021). Coastal: A phase 3 study of the PI3Kδ inhibitor zandelisib with rituximab (R) versus immunochemotherapy in patients with relapsed indolent non-Hodgkin's lymphoma (iNHL). Journal of Clinical Oncology, 39(15 suppl), TPS7573. 10.1200/JCO.2021.39.15_suppl.TPS7573 [DOI] [Google Scholar]
- Kite Pharma. (2022). Yescarta (axicabtagene ciloleucel) package insert. https://www.gilead.com/-/media/files/pdfs/medicines/oncology/yescarta/yescarta-pi.pdf
- Leonard, J. P., Jung, S. H., Johnson, J., Pitcher, B. N., Bartlett, N. L., Blum, K. A.,…Cheson, B. D. (2015). Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). Journal of Clinical Oncology, 33(31), 3635–3640. 10.1200/jco.2014.59.9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, J. P., Trneny, M., Izutsu, K., Fowler, N., Hong, X., Zhang, H.,…Gribben, J. (2019a). AUGMENT phase III study: Lenalidomide/rituximab (R2) improved efficacy over rituximab/placebo in relapsed/refractory follicular patients irrespective of POD24 status. Hematological Oncology, 37(S2), 114–115. 10.1002/hon.75_2629 30793818 [DOI] [Google Scholar]
- Leonard, J. P., Trneny, M., Izutsu, K., Fowler, N. H., Hong, X., Zhu, J.,…Gribben, J. G. (2019b). AUGMENT: A phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. Journal of Clinical Oncology, 37(14), 1188–1199. 10.1200/jco.19.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link, B. K., Day, B. M., Zhou, X., Zelenetz, A. D., Dawson, K. L., Cerhan, J. R.,…Friedberg, J. W. (2019). Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: Data from the observational National LymphoCare Study. British Journal of Haematology, 184(4), 660–663. 10.1111/bjh.15149 [DOI] [PubMed] [Google Scholar]
- Los-Arcos, I., Iacoboni, G., Aguilar-Guisado, M., Alsina-Manrique, L., Díaz de Heredia, C., Fortuny-Guasch, C.,…Ruiz-Camps, I. (2021). Recommendations for screening, monitoring, prevention, and prophylaxis of infections in adult and pediatric patients receiving CAR T-cell therapy: A position paper. Infection, 49(2), 215–231. 10.1007/s15010-020-01521-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, R., Davies, A., Ando, K., Klapper, W., Opat, S., Owen, C.,…Hiddemann, W. (2017). Obinutuzumab for the first-line treatment of follicular lymphoma. New England Journal of Medicine, 377(14), 1331–1344. 10.1056/NEJMoa1614598 [DOI] [PubMed] [Google Scholar]
- Matasar, M. J., Capra, M., Özcan, M., Lv, F., Li, W., Yanez, E.,…Zinzani, P. L. (2021). Copanlisib + rituximab versus rituximab + placebo in patients with relapsed follicular (FL) or marginal zone lymphoma (MZL): Subset analysis from the phase III CHRONOS-3 trial. Journal of Clinical Oncology, 39(15_suppl), 7510. 10.1200/JCO.2021.39.15_suppl.7510 [DOI] [Google Scholar]
- MEI Pharma, Inc. (2021). MEI Pharma and Kyowa Kirin announce data from the ongoing global phase 2 TIDAL study evaluating zandelisib as a single agent in patients with relapsed or refractory follicular lymphoma. https://www.kyowakirin.com/media_center/news_releases/2021/e20211130_01.html
- Morschhauser, F., Tilly, H., Chaidos, A., McKay, P., Phillips, T., Assouline, S.,…Salles, G. (2020). Tazemetostat for patients with relapsed or refractory follicular lymphoma: An open-label, single-arm, multicentre, phase 2 trial. Lancet Oncology, 21(11), 1433–1442. 10.1016/S1470-2045(20)30441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. (2021). Non-Hodgkin Lymphoma Treatment (PDQ®)–Health Professional Version. https://www.cancer.gov/types/lymphoma/hp/adult-nhl-treatment-pdq
- National Institutes of Health. (2021). SEER cancer stat facts: NHL — follicular lymphoma. https://seer.cancer.gov/statfacts/html/follicular.html
- Ngu, H., Takiar, R., Phillips, T., Okosun, J., & Sehn, L. H. (2022). Revising the treatment pathways in lymphoma: New standards of care-How do we choose? American Society of Clinical Oncology Educational Book, 42, 1–14. 10.1200/EDBK_349307 [DOI] [PubMed] [Google Scholar]
- Novartis Pharmaceuticals Corporation. (2022). Kymriah (tisagenlecleucel) package insert. https://www.novartis.us/sites/www.novartis.us/files/kymriah.pdf
- Patel, K., Bailey, N., Miller, K., O'Connor, H., Yang, J., Lingaraj, T.,…Pagel, J. M. (2021). Trial in progress: SYMPHONY-2, a phase 2, single-arm, open-label, multicenter study of tazemetostat in combination with rituximab for the treatment of relapsed or refractory follicular lymphoma [poster 3541]. Annual Meeting and Exposition of the American Society of Hematology, Atlanta, GA. [Google Scholar]
- Potnis, K. C., Di, M., Isufi, I., Gowda, L., Seropian, S. E., Foss, F. M.,…Huntington, S. F. (2023). Cost-effectiveness of chimeric antigen receptor T-cell therapy in adults with relapsed or refractory follicular lymphoma. Blood Advances, 7(5), 801–810. 10.1182/bloodadvances.2022008097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PR Newswire. (2021). Secura Bio announces Copiktra (duvelisib) strategic focus on T-cell lymphoma and voluntary U.S. withdrawal of the relapsed or refractory follicular lymphoma indication. https://www.prnewswire.com/news-releases/secura-bio-announces-copiktra-duvelisib-strategic-focus-on-t-cell-lymphoma-and-voluntary-us-withdrawal-of-the-relapsed-or-refractory-follicular-lymphoma-indication-301436834.html
- Proudman, D., Nellesen, D., Gupta, D., Adib, D., Yang, J., & Mamlouk, K. (2021). A matching-adjusted indirect comparison of single-arm trials in patients with relapsed/refractory follicular lymphoma who received at least 2 prior systemic treatments: Tazemetostat was associated with a lower risk for safety outcomes versus the PI3-kinase inhibitors idelalisib, duvelisib, copanlisib, and umbralisib [poster]. Advances in Therapy, 39, 1678–1696. https://link.springer.com/article/10.1007/s12325-022-02054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualls, D., & Salles, G. (2022). Prospects in the management of patients with follicular lymphoma beyond first-line therapy. Haematologica, 107(1), 19–34. 10.3324/haematol.2021.278717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers, T. D., Casulo, C., Boissard, F., Launonen, A., Parreira, J., & Cartron, G. (2021). Early relapse in first-line follicular lymphoma: A review of the clinical implications and available mitigation and management strategies. Oncology and Therapy, 9(2), 329–346. 10.1007/s40487-021-00161-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles, G. (2020). How do I sequence therapy for follicular lymphoma? Hematology, 2020(1), 287–294. 10.1182/hematology.2020000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles, G., Tilly, H., Chaidos, A., McKay, P., Phillips, T. J., Assouline, S. E.,…Morschhauser, F. (2020). Analyzing efficacy outcomes from the phase 2 study of single-agent tazemetostat as third-line therapy in patients with relapsed or refractory follicular lymphoma to identify predictors of response. Blood, 136(suppl 1), 47–49. https://ashpublications.org/blood/article/136/Supplement%201/47/471807/Analyzing-Efficacy-Outcomes-from-the-Phase-2-Study [Google Scholar]
- Sehn, L. H., Trnný, M., Bouabdallah, K., Dueck, G., Gribben, J. G., Lugtenburg, P. J.,…Cheson, B. D. (2019). Sustained overall survival benefit of obinutuzumab plus bendamustine followed by obinutuzumab maintenance compared with bendamustine alone in patients with rituximab-refractory indolent non-Hodgkin lymphoma: Final results of the gadolin study. Blood, 134(Supplement_1), 2822. 10.1182/blood-2019-123422 [DOI] [Google Scholar]
- Skarbnik, A. Z., & Patel, K. (2023). Treatment selection for patients with relapsed or refractory follicular lymphoma. Frontiers in Oncology, 13, 1120358. 10.3389/fonc.2023.1120358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L. L., Ellerman, D., Mathieu, M., Hristopoulos, M., Chen, X., Li, Y.,…Ebens, A. J. (2015). Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Science Translational Medicine, 7(287), 287ra270. 10.1126/scitranslmed.aaa4802 [DOI] [PubMed] [Google Scholar]
- Tan, D., Horning, S. J., Hoppe, R. T., Levy, R., Rosenberg, S. A., Sigal, B. M.,…Advani, R. H. (2013). Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood, 122(6), 981–987. 10.1182/blood-2013-03-491514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TG Therapeutics. (2022). TG Therapeutics announces voluntary withdrawal of the BLA/sNDA for U2 to treat patients with CLL and SLL [press release]. https://ir.tgtherapeutics.com/news-releases/news-release-details/tg-therapeutics-announces-voluntary-withdrawal-blasnda-u2-treat#:~:text=%C2%AB%20Back-,TG%20Therapeutics%20Announces%20Voluntary%20Withdrawal%20of%20the%20BLA%2FsNDA%20for,Patients%20with%20CLL%20and%20SLL&text=NEW%20YORK%2C%20April%2015%2C%202022,)%20%2D%2D%20TG%20Therapeutics%2C%20Inc
- Thieblemont, C., Phillips, T., Ghesquieres, H., Cheah, C. Y., Clausen, M. R., Cunningham, D.,…Lugtenburg, P. J. (2022). Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: Dose expansion in a phase I/II trial. Journal of Clinical Oncology, 41(12), 2238–2247. 10.1200/jco.22.01725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration. (2024a). FDA grants accelerated approval to zanubrutinib for relapsed or refractory follicular lymphoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zanubrutinib-relapsed-or-refractory-follicular-lymphoma
- US Food and Drug Administration. (2024b). FDA grants accelerated approval to epcoritamab-bysp for relapsed or refractory follicular lymphoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-epcoritamab-bysp-relapsed-or-refractory-follicular-lymphoma
- US Food and Drug Administration. (2022). Phosphatidylinositol 3-kinase (PI3K) inhibitors in hematologic malignancies. FDA Oncologic Drugs Advisory Committee briefing document. https://www.fda.gov/media/157762/download
- Witzig, T. E., Nowakowski, G. S., Habermann, T. M., Goy, A., Hernandez-Ilizaliturri, F. J., Chiappella, A.,…Czuczman, M. S. (2015). A comprehensive review of lenalidomide therapy for B-cell non-Hodgkin lymphoma. Annals of Oncology, 26(8), 1667–1677. 10.1093/annonc/mdv102 [DOI] [PubMed] [Google Scholar]
- Wudhikarn, K., & Perales, M. A. (2022). Infectious complications, immune reconstitution, and infection prophylaxis after CD19 chimeric antigen receptor T-cell therapy. Bone Marrow Transplant, 57(10), 1477–1488. 10.1038/s41409-022-01756-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez, L., Sánchez-Escamilla, M., & Perales, M. A. (2019). CAR T cell toxicity: Current management and future directions. Hemasphere, 3(2), e186. 10.1097/hs9.0000000000000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S., Liu, M., Ren, F., Meng, X., & Yu, J. (2021). The landscape of bispecific T cell engager in cancer treatment. Biomarker Research, 9(1), 38. 10.1186/s40364-021-00294-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


