Abstract
Commelina communis stomata closed within 1 h of transferring intact plants from 27°C to 7°C, whereas tobacco (Nicotiana rustica) stomata did not until the leaves wilted. Abscisic acid (ABA) did not mediate cold-induced C. communis stomatal closure: At low temperatures, bulk leaf ABA did not increase; ABA did not preferentially accumulate in the epidermis; its flux into detached leaves was lower; its release from isolated epidermis was not greater; and stomata in epidermal strips were less sensitive to exogenous ABA. Stomata of both species in epidermal strips on large volumes of cold KCl failed to close unless calcium was supplied. Therefore, the following cannot be triggers for cold-induced stomatal closure in C. communis: direct effects of temperature on guard or epidermal cells, long-distance signals, and effects of temperature on photosynthesis. Low temperature increased stomatal sensitivity to external CaCl2 by 50% in C. communis but only by 20% in tobacco. C. communis stomata were 300- to 1,000-fold more sensitive to calcium at low temperature than tobacco stomata, but tobacco epidermis only released 13.6-fold more calcium into bathing solutions than C. communis. Stomata in C. communis epidermis incubated on ever-decreasing volumes of cold calcium-free KCl closed on the lowest volume (0.2 cm3) because the epidermal apoplast contained enough calcium to mediate closure if this was not over diluted. We propose that the basis of cold-induced stomatal closure exhibited by intact C. communis leaves is increased apoplastic calcium uptake by guard cells. Such responses do not occur in chill-sensitive tobacco leaves.
Chill-sensitive plant species often appear wilty and/or exhibit low leaf water potentials, which may limit growth, photosynthetic activity, and even survival (Pardossi et al., 1992). Some chill-tolerant plants maintain water potentials by closing their stomata and preventing transpirational water loss (Kozlowski and Pallardy, 1979; Cornic and Ghashghaie, 1991), and cold-sensitive wilt-prone species often exhibit wide open stomata at low temperatures (Wilson, 1976; Lee et al., 1993). Rapid stomatal closure at low temperature will limit leaf dehydration when the water supply from the roots is restricted at low temperature (Davies et al., 1982), but the mechanism by which this occurs is unknown and is investigated in the present paper.
A plant cannot be said to be cold-tolerant unless stomatal closure (or the appropriate cold tolerance mechanism) occurs before the development of any water deficit, as has been observed to occur in several species (e.g. Teskey et al., 1983; Benzioni and Dunstone, 1988; Day et al., 1991; Wolfe, 1991; Lee et al., 1993). However it is possible that hydraulic signals resulting from decreased root water uptake and reduced flux toward the shoot in the xylem, which remain undetectable in bulk tissue water measurements using current technology, might still be responsible for stomatal closure (Teskey et al., 1983; Day et al., 1991).
It has also been suggested that cold-induced stomatal closure might occur through direct effects of cold on guard cells themselves (e.g. Honor et al., 1995). Low temperature may affect the enzymes and ion channels responsible for the active maintenance of guard cell osmotic potential (Ilan et al., 1995).
Direct effects of cold to reduce photosynthetic processes (DeLucia 1986; Day et al., 1990; Sassenrath et al., 1990) might increase the internal CO2 in the substomatal cavity, which could indirectly reduce stomatal aperture. However increases in internal CO2 are not often observed in response to reduced temperature, or they occur at much colder temperatures than are required to reduce conductance (Day et al., 1991).
The fact that the concentration of the drought-responsive plant hormone abscisic acid (ABA) has many times been shown to increase in cold tissues (e.g. Daie and Campbell, 1981; Chen et al., 1983; Vernieri et al., 1991) and that its presence can be correlated with the amelioration of chilling damage (e.g. Rikin et al., 1979; Eamus and Wilson, 1984; Janowiak and Dörffling, 1996) has led to the hypothesis that ABA might mediate the effect of cold on stomatal aperture (Eamus et al., 1983; Capell and Dörffling, 1993; Lee et al., 1993) among other protective roles. ABA binds to receptors on the outside of the stomatal guard cell plasma membrane and induces a signal transduction cascade involving increases in cytoplasmic calcium, which eventually reduces guard cell osmotic potential via loss of K+ and Cl− to cause stomatal closure (Assmann and Shimazaki, 1999). Although several authors have suggested that low temperature-induced ABA accumulation in plant tissues only results from chilling induced water deficit (Eamus and Wilson, 1983; Lalk and Dörffling, 1985; Vernieri et al., 1991), there are many instances that provide evidence for a more direct effect of cold on ABA biosynthesis (Daie and Campbell, 1981; Zhang et al., 1986; Perez et al., 1997). However, one problem with the hypothesis that ABA mediates cold-induced stomatal closure is the time frame over which the two processes occur. Stomatal closure in cold tolerant species occurs within 1 to 3 h, whereas ABA accumulation occurs much later (usually after approximately 15–30 h; but see Lee et al., 1993). In addition ABA synthesis can actually be markedly delayed upon initiation of chilling (Vernieri et al., 1991; Pardossi et al., 1992), although stomatal aperture only became reduced in chill-sensitive bean once ABA had increased.
An absence of bulk increases in ABA in cold tissues cannot, however, rule out an involvement of ABA in cold-induced stomatal closure as the low “background” level of ABA, which is always present even in warm well-watered plants, may be able to redistribute between the various tissues and compartments of the leaf upon a reduction in temperature. If a reduction in temperature reduced the ability of leaf tissue cells to remove ABA from the apoplast, this might allow even a low concentration of xylem ABA arriving at the leaf to close stomata in the absence of an increase in bulk leaf ABA. This has already been found to occur as a result of soil drying-induced changes in xylem sap pH in several species (Wilkinson, 1999, and references therein). Lee et al. (1993) found that increases in ABA in the xylem sap of a cold-tolerant rice variety correlated with stomatal closure upon transfer of the plants to the cold, whereas increases in bulk leaf ABA only occurred a few hours later. Alternatively, Hartung and co-workers have suggested that water deficit induces the release of sequestered ABA from the epidermal symplast into the apoplast (Hartung et al., 1988). Low temperatures may also give rise to such a phenomenon.
Another problem with the hypothesis that an increase in ABA at its site of action at the guard cell is responsible for cold-induced stomatal closure is that the sensitivity of stomata to ABA is greatly reduced at low temperature in Commelina communis (Honor et al., 1995), maize (Rodriguez and Davies, 1982), and bean (Eamus et al., 1983; Pardossi et al., 1992). Stomata of some chill-sensitive species show a “locking open” effect upon transfer to low temperature, which could be a result of this reduced sensitivity of the guard cells to ABA (Eamus et al., 1983).
The potential involvement of calcium in cold-induced stomatal closure must also be considered in the light of recent findings. It has been shown that in several types of plant cell temperature reduction induces a calcium influx to the cytosol from the external medium, and that the response that this transposes (e.g. gene expression or inositol triphosphate production) only requires a transient calcium increase (Minorsky, 1989; Knight et al., 1991, 1996; Ding and Pickard, 1993; Gilroy et al., 1993; Monroy and Dhindsa, 1995). Calcium has long been known to affect guard cell activity and stomatal aperture by acting as a second messenger in signal transduction cascades in response to a wide variety of signals (Trewavas and Malhó, 1997; Sanders et al., 1999). Since stomatal closure follows increases in guard cell cytosolic Ca2+ (McAinsh et al., 1990) due to the ability of Ca2+ to regulate the activity of ion transporters in the plasma and vacuolar membranes, which control the turgor of guard cells (for reviews, see McAinsh et al., 1997; Assmann and Shimazaki, 1999), we propose that low temperature may reduce stomatal aperture by inducing calcium influx from the leaf apoplast to the guard cell cytosol. At least in C. communis an apoplastic calcium concentration of 0.05 mm exists under control conditions (DeSilva et al., 1996).
Here, we aim to elucidate the mechanism whereby rapid cold-induced stomatal closure occurs in cold-tolerant species such as C. communis but not in cold-sensitive species such as tobacco (Nicotiana rustica). Several of the above-mentioned possibilities whereby ABA or apoplastic calcium may mediate cold-induced stomatal closure have been tested.
RESULTS
Effect of Temperature on Stomatal Aperture in Intact Plants
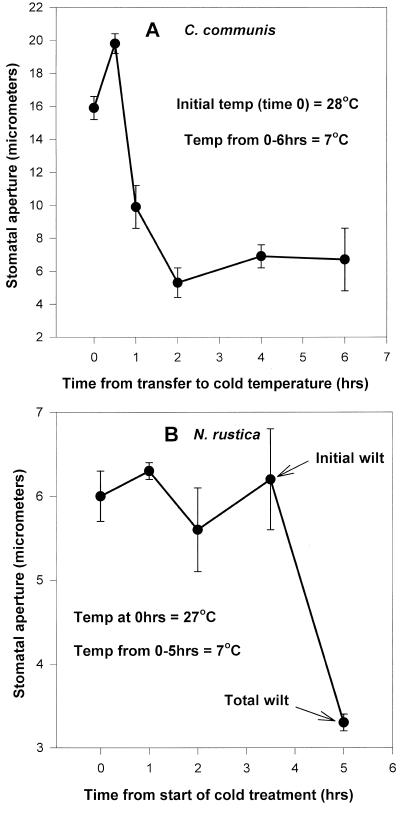
In epidermal strips peeled from leaves of intact cold-tolerant C. communis plants stomatal aperture began to decrease within 1 h after transfer of the plants from 28°C to 7°C (Fig. 1A), whereas bulk leaf ABA concentrations and leaf relative water contents did not change over 6 and 4 h, respectively (Table I). Pore width decreased from approximately 16 μm at room temperature (RT) to 6 μm under chilling conditions (Fig. 1A).
Figure 1.
Rates of stomatal closure in leaves from intact plants of C. communis (A) and tobacco (B) at two different temperatures under a light source with a photosynthetic photon flux density (PPFD) of 350 μmol m−2 s−1. Stomatal aperture (25 stomata from each of three leaves per treatment) was measured under the microscope by peeling epidermal strips from the leaves.
Table I.
Effect of transfer from high to low temperature on bulk leaf ABA and RWC of C. communis leaves from intact plants incubated under a PPFD of 350 μmol m−2 s−1 for up to 6 h
| Time from Start of Treatment | Temperature | Bulk Leaf ABA Concentration | RWC |
|---|---|---|---|
| h | °C | nmol g−1 dry wt | % turgid fresh wt |
| 0 | 28 | 3.6 ± 1.1 | 97.3 ± 0.3 |
| 0.5 | 7 | 4.7 ± 1.6 | 98.2 ± 0.1 |
| 1 | 7 | 1.4 ± 0.6 | 99.2 ± 0.1 |
| 2 | 7 | 4.0 ± 0.6 | |
| 4 | 7 | 3.7 ± 1.3 | 97.9 ± 0.3 |
| 6 | 7 | 4.8 ± 0.7 |
Bulk leaf ABA concentrations are means of three leaves measured by radioimmunoassay (Quarrie et al., 1988) over a time course. RWCs are means of three leaves over a time course, expressed as a percentage of the total turgid fresh wt.
On the other hand in epidermal strips peeled from leaves of cold-sensitive tobacco plants, stomata remained open for up to 3.5 h after transfer of the plants to chilling conditions (Fig. 1B). Stomata were open even when incipient leaf wilt was apparent to the naked eye (after 3.5 h). Only when leaves became severely wilted did the stomata close.
It would appear from these results that stomatal closure may have conferred stress-tolerance upon C. communis plants but that tobacco leaves may not be able to tolerate low temperatures because stomata were not induced to close in response to the change in temperature.
Effect of Temperature on Tissue ABA (Endogenous and Externally Supplied) Distribution in Detached C. communis Leaves
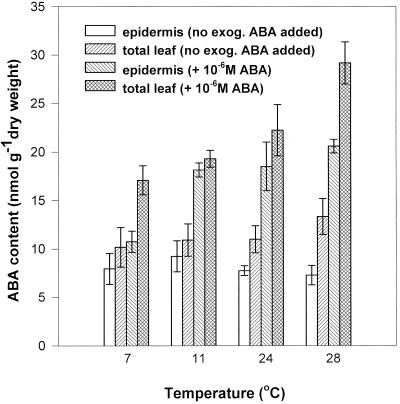
To test whether a transfer of ABA from the mesophyll to the epidermis could occur in the cold to accumulate ABA adjacent to its site of action at the guard cells, the ABA concentration was measured in epidermal tissues peeled from C. communis leaves after incubation at a range of temperatures (Fig. 2). Endogenous epidermal ABA did not increase as temperature decreased from 28°C to 7°C. When extra ABA (10−6 m) was supplied to detached leaves incubated at 7°C for 3 h, the total flux of ABA into the leaf was low compared with that at warmer temperatures. The amount of exogenously supplied ABA that reached the epidermis was also lower at 7°C. These data appear to cast doubt on a role for ABA in the mediation of cold-induced stomatal closure in the short-term.
Figure 2.
The effect of temperature on the ABA concentration in the abaxial epidermis and in the total leaf of C. communis when detached leaves were incubated under a PPFD of 350 μmol m−2 s−1 in the presence or absence of 10−6 m ABA for 3 h. Results are means of five separate experiments at each temperature and in each experiment the epidermis (and the remaining tissue) from 15 leaves was bulked for ABA analysis by radioimmunoassay.
Effect of Temperature on ABA Release from Isolated C. communis Epidermis
It is still possible that the epidermis could release ABA into the apoplast immediately under the guard cells, which may give rise to stomatal closure even in leaves that contain lower bulk and epidermal ABA concentrations than those incubated at higher temperatures (Fig. 2). Table II shows however, that epidermal strips of C. communis incubated at low chilling temperatures did not release more ABA into the bathing medium than those incubated at RT.
Table II.
ABA remaining in external solution (2.0 cm3 70 mm KCl) after incubation of C. communis epidermal strips (1.0 cm2) at different temperatures for 1.5 h
| Temperature | Volume | Mean pmol ABA Detected (±se) | ABA Concentration | n |
|---|---|---|---|---|
| °C | cm3 | nm | ||
| 25 | 2.0 | 0.22 ± 0.03 | 1.5 | 10 |
| 8 | 2.0 | 0.14 ± 0.03 | 0.9 | 12 |
Detection of ABA was by radioimmunoassay.
Effect of Temperature on Stomatal Sensitivity to ABA
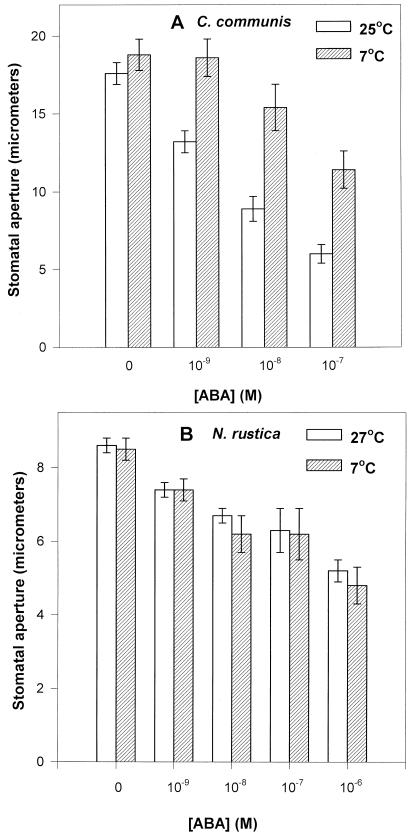
Finally we were also able to show, like Honor et al. (1995), that stomatal sensitivity to a range of ABA concentrations supplied to isolated epidermal strips of C. communis was significantly reduced at low temperature (Fig. 3A). It would appear that something other than ABA mediates rapid cold-induced stomatal closure in C. communis leaves. Counterintuitively, epidermal strips peeled from cold-sensitive tobacco leaves showed no difference in their sensitivity to a range of ABA concentrations between low and high temperature (Fig. 3B). Despite this, stomata remained open in these leaves at low temperatures long enough to induce wilting (Fig. 1B).
Figure 3.
The effect of ABA concentration on stomatal aperture in detached abaxial epidermal strips of C. communis (A) and tobacco (B) at two different temperatures. Strips (approximately 35–40) were pre-incubated on 25cm3 70 mm KCl at RT for 3 h, and then transferred to treatment solutions, prechilled where necessary, of 70 mm KCl (4.0 cm3) ± ABA for 1.5 h. All leaves received a PPFD of 350 μmol m−2 s−1 at all times. Stomatal aperture was measured under the microscope: 25 apertures in each of three strips per treatment.
Effect of Temperature on Stomatal Aperture in Isolated Epidermal Strips
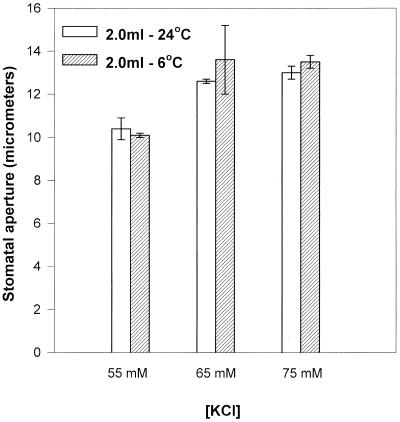
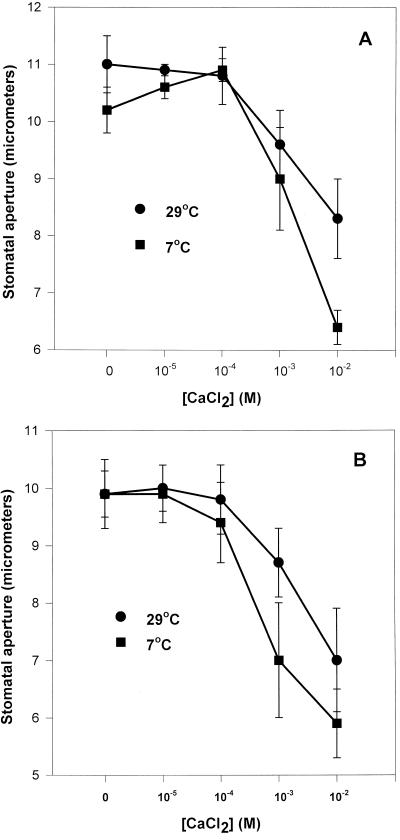
Despite the fact that stomata in epidermal strips peeled from intact cold-treated C. communis plants had greatly reduced apertures, when identical strips were peeled from warm leaves and placed on petri dishes of KCl solution before transfer to and incubation at low temperatures, the stomata remained wide open (Fig. 4, see also Fig. 3A). This also occurred in the absence of ABA in cold-treated epidermal strips of tobacco (Fig. 3B). These wide apertures persisted for up to 4 h in C. communis (longest time tested; results not shown). This result rules out the possibility that direct effects of cold on guard cell ion leakage or reductions in active solute uptake/efflux activity might be responsible for closure. Cold-induced changes in the endogenous leaf calcium concentration (or stomatal sensitivity to it) could explain the fact that stomata in intact leaves closed in response to low temperature, whereas those in isolated epidermis did not. Stomata in isolated epidermal strips floating on KCl solutions (Fig. 4) would remain open in the cold unless an adequate concentration of calcium, at least equivalent to that present in the apoplast in vivo, was also supplied in the external medium, if a role for calcium exists in controlling stomatal aperture at low temperatures.
Figure 4.
The effect of temperature and KCl concentration on stomatal aperture in isolated abaxial epidermal strips of C. communis. Strips (15) were pre-incubated on the appropriate KCl concentration (25 cm3) at RT for 3 h and then transferred to the appropriate watch glass of KCl solution (2.0 cm3), which had been prechilled where necessary and incubated under a PPFD of 350 μmol m−2 s−1 for 1.5 h. Stomatal aperture was measured as described for Figure 3.
Effect of Temperature on Stomatal Sensitivity to [CaCl2]
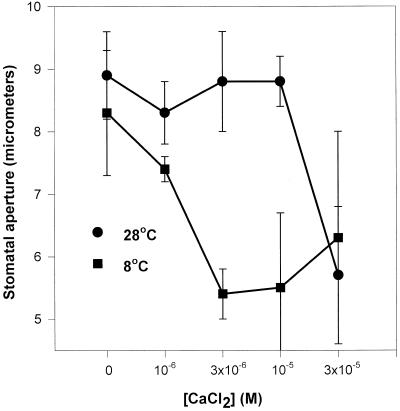
A role for calcium in cold-induced stomatal closure was investigated by floating isolated epidermal strips peeled from the leaves of both species on KCl solutions of differing temperature. The solutions contained a range of CaCl2 concentrations thought to approximate the in planta apoplastic concentration. This has never been directly measured but is estimated to be approximately 10−5 m (DeSilva et al., 1996). Figure 5 shows that stomata of C. communis were much more sensitive to calcium in the cold than in the warm, in total contrast to their sensitivity to ABA. In the presence of approximately 3 × 10−6 m CaCl2 we could restore the ability of stomata in isolated epidermal strips to respond to the cold by closing and mimic what happens in vivo in the intact leaf. This concentration of calcium had no effect on aperture at the warmer temperature.
Figure 5.
The effect of CaCl2 concentration on stomatal aperture in abaxial epidermal strips of C. communis at two different temperatures. Strips (approximately 35–40) were pre-incubated on 25 cm3 70 mm KCl at RT for 3 h, then transferred to a second KCl (4.0 cm3) solution with the appropriate concentration of CaCl2 added, which had been prechilled where necessary. Incubations were carried out under a PPFD of 350 μmol m−2 s−1 for 1 h; stomatal aperture was measured as in Figure 3.
However Figure 6, A and B indicate that the situation was rather complex, as there was a biphasic response to increasing calcium concentration in the cold that was absent at higher temperatures. When C. communis stomata experienced very high calcium concentrations they closed to a lesser degree than those in epidermal strips supplied with mid-range concentrations, such that stomata in warm epidermes were more sensitive to high calcium concentrations than those incubated at lower temperatures.
Figure 6.
The effect of CaCl2 concentration on stomatal aperture in abaxial epidermal strips of C. communis at 7°C (A) and 28°C (B). Strips were treated as in Figure 5, and stomatal apertures were measured as in Figure 3.
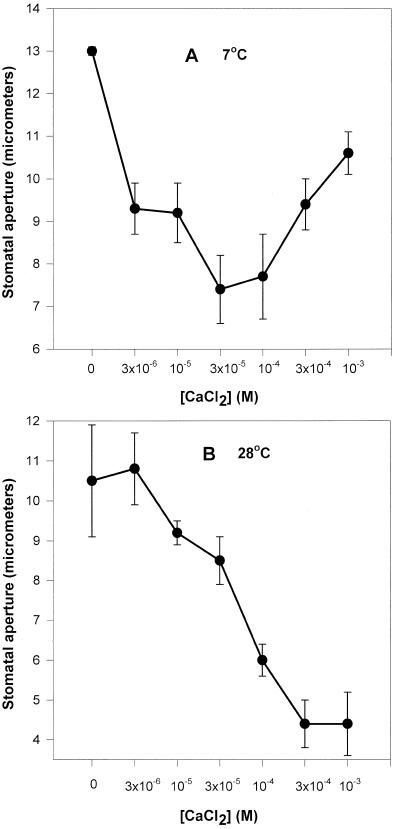
When we carried out the same experiments with isolated strips of tobacco epidermis, we found that tobacco guard cells were much less sensitive to calcium at both high and low temperature (Fig. 7) and did not close until calcium was supplied at the millimolar range. Closure occurred in the micromolar range in C. communis in the cold. The maximum difference in sensitivity to calcium between the two temperatures was only ever as high as 20% (Fig. 7A) in tobacco (and was usually insignificant; Fig. 7B), whereas this difference was invariably approximately 50% in C. communis. In the cold tobacco guard cells were 300- to 1,000-fold less sensitive to calcium than C. communis guard cells in epidermal strips floating on media containing the same KCl concentration. Tobacco stomata did not exhibit a biphasic response to increasing calcium at either temperature.
Figure 7.
The effect of CaCl2 concentration on stomatal aperture in abaxial epidermal strips of tobacco. Strips were treated as in Figure 5, and stomatal apertures were measured as in Figure 3. Results of two separate experiments (A and B) are shown.
Comparison of Apoplastic Calcium between C. communis and Tobacco
We then attempted to obtain a comparison between the endogenous resting concentration of calcium in the epidermal apoplasts of the two species. We measured the calcium released within 5 min by epidermal strips of each species peeled directly from intact warm plants into media of identical volume at two temperatures (Table III). Calcium release per cm2 of epidermal tissue per cm3 of bathing solution was only 13-fold greater for tobacco than for C. communis at both high and low temperatures, rather than the 300-fold figure that we have estimated is required for tobacco to contain enough calcium to mediate cold-induced stomatal closure. It is not known what actual concentration these figures represent in the intact apoplast.
Table III.
Ca2+ in the external solution (4.0 cm3 of 70 mm KCl) after a 5-min incubation in the light (PPFD 350 μmol m−2 s−1) at the appropriate temperature of freshly-peeled epidermal strips (approximately 1.0 by 1.5 cm2, exact size noted) from intact C. communis and tobacco plants kept at RT under lights
| Species | Temperature | Apoplast CaCl2 Concentration cm2/cm3 | Increase from C. communis to Tobacco |
|---|---|---|---|
| °C | m | ||
| C. communis | 27 | 2.2 ± 0.8 | – |
| ×10−5 | |||
| C. communis | 7 | 2.0 ± 0.5 | – |
| ×10−5 | |||
| Tobacco | 27 | 3.0 ± 1.0 | 13.6-Fold |
| ×10−4 | |||
| Tobacco | 7 | 2.4 ± 0.7 | 12.0-Fold |
| ×10−4 |
Ca2+ concentration remaining in the solution as measured with a calcium electrode was expressed on a per cm2 epidermis per cm3 medium basis (n = 6).
Effect of Incubation Volume on the Response of Stomata of C. communis Epidermis to Low Temperatures
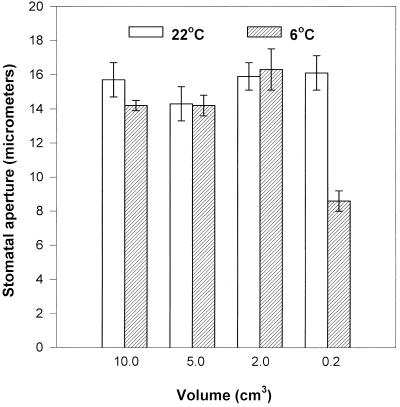
Because the epidermal strips appeared to release substantial amounts of calcium into the incubation medium (Table III) we decided to investigate whether enough calcium already exists in the C. communis epidermal strip itself to induce stomatal closure in the cold. We placed epidermal strips of equal sizes peeled from intact warm plants, which had been pre-incubated for 3 h at RT, on to ever decreasing volumes of warm or cold 70 mm KCl solution and incubated them for 1 h. Stomatal apertures on larger volumes at both temperatures remained high and were comparable with those exhibited at RT (Fig. 8). However, on smaller volumes of solution (0.2 cm3) the stomata in the epidermal strips were able to close in response to the cold to the same extent as those in the intact leaves in the absence of externally supplied calcium. On large volumes of solution the calcium released by the epidermes would become greatly diluted and only on low volumes would it become concentrated enough to cause stomatal closure. The latter situation, with epidermal strips floating on very small volumes of solution, is most akin to that found in vivo due to the extremely low volumes of solution present in the apoplast.
Figure 8.
The effect of volume on stomatal aperture in detached epidermal strips from C. communis at two different temperatures. Equal-sized strips (1.0 cm2, approximately 35–40) were pre-incubated on 25cm3 60 mm KCl for 3 h, then transferred to solutions, prechilled where necessary, of 60 mm KCl of different volumes for a further hour (all under a PPFD of 350 μmol m−2 s−1). Apertures of 25 stomata in each of three strips were measured per treatment.
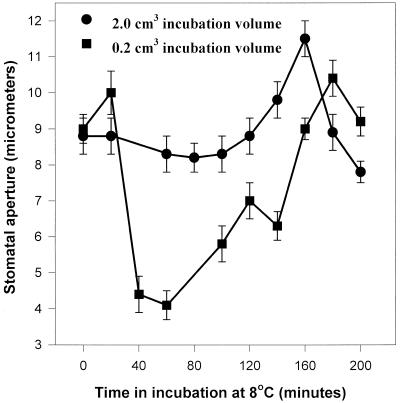
Figure 9 shows a time course of stomatal aperture upon transfer of C. communis epidermal strips from 25°C to 7°C on 2.0- and 0.2 cm3 of incubation solution. Stomatal closure occurred between 20 and 40 min after transfer to the cold when strips were floating on 0.2 but not on 2.0 cm3 of incubation solution, however these started to re-open after approximately 2 h, and re-aquired full pore width 3 to 4 h after the initial transfer to the cold. Re-opening did not occur over this time scale in vivo (Fig. 1A) or in the presence of externally supplied calcium on larger volumes (data not shown).
Figure 9.
A time course for the effect of transfer from RT to 8°C on stomatal aperture in epidermal strips of C. communis floating on 2.0 or 0.2 cm3 75 mm KCl for up to 3.5 h. 1.0-cm2 strips (approximately 35–40) were pre-incubated on 25cm3 75 mm KCl for 3 h in the light (PPFD 350 μmol m−2 s−1) before transfer to the cold room (under same PPFD). Each time point is represented by the mean of 25 stomata in a single strip.
The following set of experiments were designed to confirm the involvement of calcium in the cold-induced stomatal closure response on low volumes of solution.
Effects of Pretreatment Conditions on Cold-Induced Stomatal Closure in C. communis Epidermal Strips
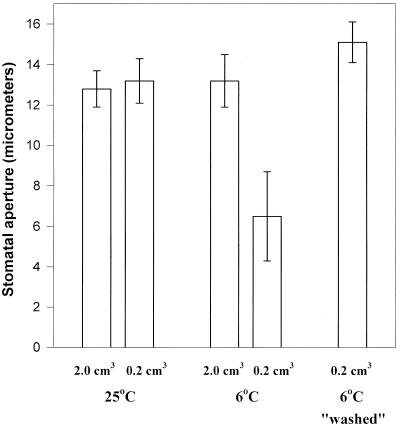
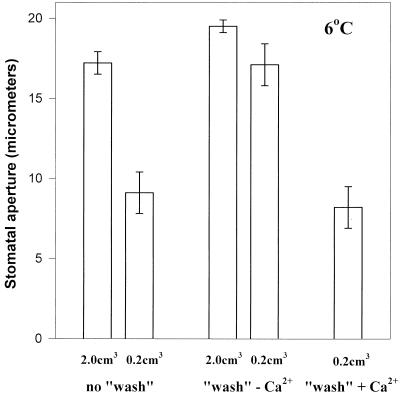
Stomata in isolated epidermal strips were allowed to open in the light at 25°C, then some of the strips were “rinsed” and “washed” by floating them on a second fresh solution to remove the agent CaCl2, which we have already found to be required for stomatal closure. The strips were then transferred singly to watch glasses of 0.2 and 2.0 cm3 of fresh KCl as normal for analysis of the effects of subsequent cold treatments. The store of calcium required for cold-induced stomatal closure should have been removed from the strips by this pretreatment regime. The stomata in the epidermal strips, which had been “washed,” were no longer able to close when introduced to the cold, even on volumes of 0.2 cm3 (Fig. 10). However the normal response to the cold on 0.2 cm3 was restored (i.e. stomatal closure occurred) if calcium (0.05 mm) was resupplied in a second pretreatment period after the initial “wash” (Fig. 11), although it was not present in the final treatment medium (the strips were rinsed quickly before transfer). The calcium store in the epidermal strips must have been replenished, making apoplastic calcium available for symplastic uptake in response to low temperature.
Figure 10.
The effect of prewashing C. communis epidermal strips on the subsequent response of the stomata that they contain to temperature in 0.2 cm3 65 mm KCl. Stomata were allowed to open in the light (350 μmol m−2 s−1) in one group of 20 and one group of five epidermal strips floating on two petri dishes of 25 cm3 65 mm KCl at RT for 1 h. After 1 h the smaller group to be “washed” were rinsed (floated for 30 s on 25cm3 fresh KCl) and transferred to a second dish containing 25 cm3 fresh 65 mm KCl at RT, and the larger group was left where it was. All were incubated for a further hour in the light. Finally, the strips were transferred to the appropriate treatment solutions described above for 1 h before measuring stomatal apertures (means of 25 stomata per strip in five strips).
Figure 11.
The effect of prewashing C. communis epidermal strips and then resupplying the lost calcium, on the subsequent response of the stomata in these strips to low temperature, on 0.2 cm3 65 mm KCl. Epidermal strips (1.0 cm2) were pooled and then divided into two groups (1 of 10 and 1 of 15) on two petri dishes of 25 cm3 65 mm KCl, and stomata were allowed to open over the next hour in the light (PPFD 350 μmol m−2 s−1). After 1 h 10 of the larger group to be “washed” were rinsed as described for Figure 10 and transferred to 25 cm3 fresh 65 mm KCl minus calcium, whereas the other five were rinsed and transferred to 25 cm3 fresh 65 mm KCl containing 0.05 mm CaCl2. Stomata in all three groups (unwashed, washed − Ca, and washed + Ca) were incubated in the light at RT for a further hour. Finally strips were rinsed and transferred to the appropriate treatment solutions (all without calcium) and incubated in the cold room for a final hour, after which time stomatal apertures in 25 stomata from each of five strips were measured.
DISCUSSION
The results described above provide convincing evidence that ABA does not have a role in rapid chill-induced stomatal closure in C. communis leaves, a process that confers chill-tolerance to this species. ABA did not accumulate in the leaf (either via de novo synthesis, increased transport to the leaf, or decreased transport of ABA out of the leaf in the phloem) in response to a drop in temperature (Table I) over the same time frame in which stomatal closure occurred (Fig. 1A). It did not become redistributed between the tissues of the intact leaf, ruling out the possibility that existing ABA could preferentially accumulate in the epidermis at low temperatures (Fig. 2). Supplying extra ABA to detached leaves at 7°C did not even increase the epidermal concentration above endogenous levels, due to a reduction in the total flux of ABA into the leaves at low temperatures. Even though rates of symplastic sequestration of 3H-ABA into both epidermis and mesophyll tissue of C. communis leaves have been shown to decrease with temperature (Clephan, 1996), the reduced flux of ABA into the leaf and the reduced sensitivity of stomata to ABA in the cold (Fig. 3A; Honor et al., 1995) make it very unlikely that any low-temperature-induced apoplastic ABA accumulation is going to be sufficient to cause stomatal closure. In addition the concentration of ABA remaining in the KCl solution after incubating epidermal strips at different temperatures did not increase as temperature decreased (Table II), ruling out the possibility that cold-induced symplastic ABA efflux may have a role in cold-induced stomatal closure.
It is still possible that both apoplastic ABA accumulation and de novo ABA synthesis could prolong stomatal closure in vivo during extended periods of low temperature, perhaps after attenuation of stomata to the chill-induced calcium-sensitization response (see below). A recent consensus of opinion, however, is that induction of new ABA synthesis at low temperature is geared toward other forms of plant protection (Pan, 1990).
We have been able to mimic stomatal closure in an intact leaf by incubating isolated epidermal strips in the presence of calcium or simply on small volumes of solution, and we have shown that stomata remain open in the cold in the absence of calcium or on larger volumes. These findings provide us with much information about the mechanism of cold-induced stomatal closure. Intact leaves are not required for the initial induction of stomatal closure by chilling temperatures. We can therefore rule out the possibility that hydraulic signals from the root, or water relations of the epidermis or the guard cell itself take part in the closure response. These processes may well influence aperture in an intact plant, but they are not part of the underlying mechanism whereby closure is initiated upon chilling. We can also provide evidence that chill-induced changes in photosynthesis are not factors in the stomatal closure response, as presumably photosynthesis will be affected to the same magnitude by the cold in both high and low volumes of the KCl bathing solution. Transport of ABA from roots and leaf tissues other than the epidermis must also be ruled out as primary causes of cold-induced reductions in stomatal aperture, but again these processes will undoubtedly have a role in longer-term plant protection.
That calcium uptake from the apoplast is the trigger for rapid stomatal closure in response to temperature reduction is a novel finding. Recent work has shown that low temperature can increase Arabidopsis guard cell calcium levels and cause a series of oscillations in cytosolic Ca2+ that are required to elicit stomatal closure (Allen et al., 2000). However these cytosolic calcium transients occurred in guard cells in epidermal strips perfused with a calcium-free buffer. In addition Arabidopsis stomata in epidermal strips floating on cold calcium-free buffer were able to close, in contrast to our findings (Figs. 3–11). It must be concluded that different species possess different mechanisms whereby low temperature induces stomatal closure. Perhaps Arabidopsis stomata are particularly sensitive to the cold with closure occurring in response to the calcium released from guard cell stores only, without a requirement for an external source.
From our data we can envisage stomatal closure occurring in the cold in intact leaves simply by an increase in the ability that guard cells have to take up calcium from the apoplastic pool already present in the leaves. DeSilva et al. (1996) have estimated that this is approximately 10−5 m in C. communis. In the warmth, stomata in epidermal strips are unaffected by this concentration of external calcium, but in the cold stomata are stimulated to close in its presence (Figs. 5 and 6). In other plant cells cold shock-induced increases in cytosolic calcium were due to the opening of inward rectifying plasma membrane Ca2+ channels, as the influx was inhibited by Ca2+ channel blockers (De Nisi and Zocchi, 1996). The apoplast has been shown to be the source for cold-shock-induced calcium uptake in tobacco (Knight et al., 1991) and alfalfa (Monroy and Dhindsa, 1995). It seems likely in our experiments with C. communis that low temperatures increase guard cell plasma membrane calcium channel activity as has been found in other cell types (Puhakainen et al., 1999, winter rye; Jian et al., 1999, winter wheat), and that the resultant increase in cytoplasmic Ca2+ induces the well-known signal cascade leading to the loss of guard cell turgor and stomatal closure (Assmann and Shimazaki, 1999). It is thought that cold-induced lipid membrane rigidification causes re-organization of the associated microfilament array in the cytoplasm, which normally creates the tension required to keep membrane-associated channels closed. This process may be responsible for the opening of plasma membrane-integral calcium channels (Örvar et al., 2000). This sequence of events, from membrane rigidification to cytoplasmic calcium oscillations, may well be the basis for chill tolerance in species that close their stomata to endure the cold.
Our results have also made it possible to explain the basis of chill-sensitivity in tobacco. We approximated that the tobacco epidermal apoplast contained a 13-fold greater calcium concentration than that of C. communis (Table III), yet tobacco stomata were 300- to 1,000-fold less sensitive to externally supplied calcium in the cold (compare Figs. 5–7). Logically we can presume that the tobacco epidermis does not contain enough calcium to confer cold-sensitivity to its stomata, although calcium channels may well become stimulated (to a lesser extent than in C. communis) to take up more calcium than at higher temperatures (see Fig. 7A). Tobacco stomata in general seem to be less sensitive to external stimuli than C. communis, as stomata in epidermal strips only opened in the light when floating on KCl concentrations of at least 70 mm (results not shown). It is also interesting that tobacco stomata were not desensitized to ABA at low temperatures (Fig. 3B), indicating that at least calcium release from internal stores was not impaired at low temperature in this species.
The stomata of C. communis began to re-open after a few hours in the cold in vitro (Fig. 9) when relying on the calcium supplied by the epidermal strip itself, whereas this did not occur in vivo (for up to 6 h) or in the presence of externally supplied calcium on larger volumes. This could be explained by our finding that when apoplastic calcium was supplied in the millimolar range the stomata in epidermal strips of C. communis incubated at low temperature were more open than they were in the presence of a lower calcium concentration of approximately 10−5 m (Fig. 6A). Thus over a time-course (Fig. 9) the calcium may have accumulated in the low volume of solution to a high enough concentration to induce this re-opening response. It is not known whether this phenomenon occurs in vivo. At low temperatures, water (and therefore calcium) flux through the xylem is decreased, making it seem unlikely that apoplastic calcium would accumulate enough to reach the millimolar concentrations required to potentially re-open the stomata. This could occur, however, in response to an increase in soil temperature and restoration of calcium flux to the leaves, whereby calcium would be classed as a long-distance signaling molecule (see e.g. Ruiz et al., 1993). Alternatively the re-opening response could be a factor in cold acclimation.
It has long been known that periods of cold stress provide plants with protection against subsequent stresses of a different nature, such as water deficit, in that stomatal closure can occur much more rapidly and/or sensitively in response to the second stress (Wilson, 1976). This phenomenon has become known as “chill-hardening.” Our work demonstrates that this could be a result of the cold-induced uptake of calcium into the various cells of the leaf, including the guard cells, such that internal stores of calcium become replenished. In effect the guard cells are “primed” by the cold pretreatment, for closure in response to a subsequent exposure to ABA (the agent mediating the effects of water deficit on stomatal aperture): As mentioned above ABA-induced stomatal closure is partially mediated by calcium release from internal guard cell stores, as well as by calcium uptake from the apoplast. Support for this hypothesis comes from the work of Allan et al. (1994). These authors found that guard cells in epidermes isolated from cold-acclimated C. communis leaves (10°C–17°C) did not require additional cytoplasmic calcium for ABA to cause stomatal closure. However, it is difficult to reconcile these findings with those of Knight et al. (1998), which describe how prior cold pretreatments prevented calcium oscillations in response to subsequent mannitol (artificial drought) treatments in Arabidopsis tissues other than guard cells. It is likely that different cells and tissues respond in entirely different ways to cold stress and subsequent additional stresses, just as we have found disparities in the responses of the guard cells of different species to low temperature and external calcium.
MATERIALS AND METHODS
Plant Material
Seeds of Commelina communis were sown in John Innes No. 2 compost. After emergence, seedlings were transplanted into 90 × 90-mm pots and grown in a controlled-environment cabinet with a day/night temperature of 24°C and 16°C and a 14-h photoperiod with a PPFD of 350 μmol m−2 s−1. The pots were watered daily to the drip point, and once a week they were watered with a full-strength, modified Hoagland nutrient solution (Epstein, 1972). When the plants were 4 or 5 weeks old, the fourth leaf, which was the youngest fully expanded leaf, was used as a source of experimental material.
Tobacco (Nicotiana rustica) seeds were surface sterilized in 10% (v/v) bleach, rinsed six times with sterile distilled water, and placed (30 per dish) in covered petri dishes of one-half strength Murashige and Skoog nutrient tissue culture medium (Murashige and Skoog, 1962) in agar (0.6% [w/v]) containing 1% (v/v) Suc, under sterile conditions. Dishes were placed under the same controlled environment cabinet conditions as described above. Seeds were allowed to germinate for approximately 8 to 10 d, after which time seedlings were transferred individually to 90 × 90-mm pots and sown into John Innes No. 2 compost. These were watered and fed as above. After approximately 3 to 4 weeks seedlings were transplanted to 2-L pots. When the plants were 7 to 10 weeks old, the mid-region of the almost mature (still slightly crinkly) leaves were used as a source of experimental material. Leaf bases contained closed stomata, and leaf tips contained wide-open stomata that were less responsive to ABA. Fully expanded leaves contained almost-closed stomata. Standardization of leaf material in this species is critical.
Chemicals and Radiochemicals
[G-3H](±)-ABA, specific activity 2.0 TBq mmol−1, was obtained from Amersham International (Amersham Place, Buckinghamshire, UK). This was diluted with phosphate buffer saline (100 mm NaH2PO4, 100 mm Na2HPO4, 200 mm NaCl, pH 6.0; plus 5.0 mg cm−3 globulin) giving a stock concentration of 8.9 nm, which was stored at −20°C in the dark.
Synthetic racemic (±)-ABA was obtained from Lancaster Synthesis (Morecambe, Lancashire, UK). Working solutions were made up as required by dilution from stock solutions in degassed distilled water, which were refrigerated in darkness. The monoclonal antibody AFRC MAC 252 used in the radioimmunoassay was specific for (+)-ABA and was generously provided by Steve A. Quarrie (Cereals Research Department, John Innes Centre, Norwich, UK). General reagents used in experiments were all BDH Analar grade from Sigma (Poole, Dorset, UK).
Effect of Low Temperature on Stomatal Aperture in Intact Plants
Intact C. communis or tobacco plants were placed in a cold room under light boxes so that leaves to be tested experienced a PPFD of approximately 350 μmol m−2 s−1, an air temperature of between 6°C and 8°C, and a vapor-pressure difference of approximately 0.18 KPa. Before transfer to the cold room, plants were maintained in the laboratory under light boxes such that the air temperature was approximately 28°C (vapor pressure difference approximately 2.4 KPa). Before and after transfer to the cold room sections of intact leaf were cut from either side of the midrib (C. communis) or from the inter-veinal regions (tobacco), and the abaxial epidermis was carefully peeled away from the rest of the leaf following the technique described by Weyers and Meidner (1990). The epidermal strip (approximately 1.0 × 1.5cm) was immediately placed in a few drops of 50 mm KCl on a glass slide and covered with a coverslip, and pore widths of approximately 25 stomata from each strip (usually three to four replicates) were measured under a projection microscope. This was repeated every hour for up to 6 h after transfer of plants to the cold room such that a time course for stomatal aperture was obtained.
Each time a piece of C. communis epidermis was removed and tested for stomatal aperture, an equivalent leaf was cut and immediately frozen in liquid nitrogen for future bulk leaf ABA determination in the radioimmunossay (for details, see below). In addition a second equivalent leaf was cut and its fresh weight (FW) was obtained. Its petiole was then placed in a beaker of water overnight in the dark, and it was reweighed in the morning before freeze drying, obtaining a dry weight (DW) to determine relative leaf water content (relative water content = [experimental FW − DW]/[turgid FW − DW] × 100%).
Effect of Low Temperature on Stomatal Aperture in Isolated Abaxial Epidermal Strips
The abaxial epidermis of either C. communis or tobacco was carefully removed from intact leaf tissue as described above. The strips were pooled, cut into equal sizes (1.0 cm × 1.5 cm), and floated mesophyll-side down for up to 3 h in large plastic petri dishes containing 25 cm3 of 55 to 75 mm KCl at approximately 25°C under a light source (PPFD = 350 μmol m−2 s−1) to open the stomatal pores. From this homogenous pool of tissue, single strips were randomly transferred to 4.0 cm2 glass watch glasses with 2.0-cm diameter indentations containing 2.0 to 4.0 cm3 of 55 to 75 mm KCl (unless otherwise stated) with or without specific additions (ABA or CaCl2; see figure legends), which had been acclimated to the required temperature (inside a cold room where necessary) under light sources of equal magnitude (see “Results” and figure legends). It was necessary to stagger the transfer of the strips so that each had been incubated for the same length of time by the end of the experiment. After 1 to 1.5 h in the final treatment solution (or over a time course) the epidermal strips were removed and immediately placed on a few drops of the appropriate incubation medium on glass slides and covered with coverslips. Pore widths of 25 stomata from each strip (usually three to five replicates) were immediately measured under a projection microscope.
Some experiments required additional “washing” pretreatments (up to two) of some of the C. communis strips in large petri dishes containing 25cm3 of the appropriate medium (with or without 0.05 mm CaCl2), in which case all strips were subsequently rinsed (floated for 30 s) on fresh KCl solutions before the final transfer.
At the end of some of the experiments the solution remaining in the watch glass was accurately weighed for volume determination, sealed into an eppendorf, and refrigerated until the following day. The epidermal strip, which had been floating on the solution, was also weighed after blotting off any excess liquid. The ABA concentration in the incubation solution was determined using a radioimmunoassay with the AFRC MAC 252 antibody (for protocol, see Quarrie et al., 1988).
Effect of Temperature on ABA Distribution within C. communis Leaves
Leaves were removed from intact C. communis plants at RT, and petioles were recut under distilled water to avoid embolism. Leaves were immediately placed into 6.0-cm3 plastic vials containing distilled water ± 10−6 m ABA. The vial tops were covered with aluminum foil to prevent evaporation of solution from the surface, and the leaves were inserted through slits cut across the top. The vials were then transferred to a controlled environment cabinet at the required air temperature: 7°C, 11°C, 24°C, or 28°C (vapor pressure differences were 0.18, 0.44, 1.64 and 2.53 KPa respectively); and leaves were incubated for 3 h under a PPFD of 350 μmol m−2 s−1, after which time the abaxial epidermis of 15 leaves was quickly peeled away from the remainder of the leaf tissues and pooled in liquid nitrogen. Epidermal and remaining leaf tissues were freeze-dried, then ground in the appropriate volume of water for ABA extraction (1:25 leaf DW:solvent), and tissue ABA analysis was carried out using the radioimmunoassay described by Quarrie et al. (1988). The experiment was repeated five times at each temperature so that bulked tissue ABA could be expressed as a mean with an se.
Comparison of Apoplastic Calcium Release from Epidermal Tissue
Epidermal strips were peeled from intact room-temperature-incubated plants of each species and immediately transferred mesophyll-side down into media of identical volume for 5 min. Strip area was measured. Both intact plants and epidermal strips were incubated under a PPFD of 350 μmol m−2 s−1. It was assumed that in the first 5 min the only calcium entering the bathing medium would be that flushed from the apoplast, before symplastic efflux became a factor (auxin efflux from 2-mm segments of zucchini stem tissue was apoplastic for the first 5–10 min; Allan and Rubery, 1991). The calcium concentration found in the bathing medium was determined using a calibrated calcium ion selective electrode (model EE-Ca, Philip Harris Scientific, Manchester, UK). Calcium efflux from each species was calculated as calcium release per cm2 of epidermal tissue per cm3 of bathing solution. The actual concentration that this represents in the intact apoplast cannot be calculated, but these figures serve as a comparison of the calcium found in the apoplast of each species.
ACKNOWLEDGMENTS
The authors would like to thank Geoff Holroyd and Catherine Clarke for generously providing the tobacco seeds and the petri dishes of tissue culture medium, respectively.
Footnotes
This work was supported by the Biotechnology and Biological Science Research Council, UK.
LITERATURE CITED
- Allan AC, Fricker MD, Ward JL, Beale MH, Trewavas AJ. Two transduction pathways mediate rapid effects of abscisic-acid in Commelina guard-cells. Plant Cell. 1994;6:1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AC, Rubery PH. Calcium deficiency and auxin transport in Cucurbita pepo L. seedlings. Planta. 1991;183:604–612. doi: 10.1007/BF00194283. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. doi: 10.1126/science.289.5488.2338. [DOI] [PubMed] [Google Scholar]
- Assmann SM, Shimazaki K-L. The multisensory guard cell, stomatal responses to blue light and abscisic acid. Plant Physiol. 1999;119:809–816. doi: 10.1104/pp.119.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzioni A, Dunstone RL. Effect of air and soil temperature on water balance of jojoba growing under controlled conditions. Physiol Plant. 1988;74:107–112. [Google Scholar]
- Capell B, Dörffling K. Genotype-specific differences in chilling tolerance of maize in relation to chilling-induced changes in water status and abscisic acid accumulation. Physiol Plant. 1993;88:638–646. doi: 10.1111/j.1399-3054.1993.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Chen THL, Li PH, Brenner ML. Involvement of abscisic acid in potato acclimation. Plant Physiol. 1983;71:362–365. doi: 10.1104/pp.71.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clephan AL. The role of abscisic acid in the regulation of stomatal aperture in Commelina communis L.: how do stomata “read” ABA signals under different environmental conditions? PhD thesis. UK: Lancaster University; 1996. [Google Scholar]
- Cornic G, Ghashghaie J. Effect of temperature on net CO2 assimilation and photosystem II quantum yield of electron transfer of French bean (Phaseolus vulgaris L.) leaves during drought stress. Planta. 1991;185:255–260. doi: 10.1007/BF00194068. [DOI] [PubMed] [Google Scholar]
- Daie J, Campbell WF. Response of tomato plants to stressful temperature: increase in ABA concentration. Plant Physiol. 1981;67:26–29. doi: 10.1104/pp.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Rodriguez JL, Fiscus EL. Stomatal behavior and water-movement through roots of wheat plants treated with abscisic acid. Plant Cell Environ. 1982;5:485–493. [Google Scholar]
- Day TA, DeLucia EH, Smith WK. Effect of soil temperature on stem sap flow, shoot gas exchange and water potential of Picea engelmannii (Parry) during snowmelt. Oecologia. 1990;84:474–481. doi: 10.1007/BF00328163. [DOI] [PubMed] [Google Scholar]
- Day TA, Heckathorn SA, DeLucia EH. Limitations of photosynthesis in Pinus taeda L. (Loblolly pine) at low soil temperatures. Plant Physiol. 1991;96:1246–1254. doi: 10.1104/pp.96.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia EH. Effect of soil temperature on net photosynthesis, stomatal conductance and carbohydrate concentration in Engelmann spruce (Picea engenmannii Parry ex Engelm.) seedlings. Tree Physiol. 1986;2:143–154. doi: 10.1093/treephys/2.1-2-3.143. [DOI] [PubMed] [Google Scholar]
- De Nisi P, Zocchi G. The role of calcium in the cold shock responses. Plant Sci. 1996;121:161–166. [Google Scholar]
- DeSilva DLR, Honor SJ, Mansfield TA. Estimations of apoplastic concentrations of K+ and Ca2+ in the vicinity of stomatal guard cells. New Phytol. 1996;134:463–469. [Google Scholar]
- Ding JP, Pickard BG. Modulation of mechanosensitive calcium-selective cation channels by temperature. Plant J. 1993;3:713–720. [PubMed] [Google Scholar]
- Eamus D, Fenton R, Wilson JM. Stomatal behavior and water relations of chilled P. vulgaris and P. sativum. J Exp Bot. 1983;34:434–441. [Google Scholar]
- Eamus D, Wilson JM. ABA levels and effects in chilled and hardened P. vulgaris leaves. J Exp Bot. 1983;34:1001–1006. [Google Scholar]
- Eamus D, Wilson JM. A model for interaction of low temperature, ABA, IAA and CO2 in the control of stomatal behavior. J Exp Bot. 1984;35:91–98. [Google Scholar]
- Epstein E. The media of plant nutrition. In: Epstein E, editor. Mineral Nutrition of Plants: Principles and Perspectives. New York: John Wiley & Sons; 1972. pp. 29–49. [Google Scholar]
- Gilroy S, Bethke PC, Jones RL. Calcium homeostasis in plants. J Cell Sci. 1993;106:453–462. doi: 10.1242/jcs.106.2.453. [DOI] [PubMed] [Google Scholar]
- Hartung W, Radin JW, Hendrix DL. Abscisic acid movement into the apoplastic solution of water stressed cotton leaves: role of apoplastic pH. Plant Physiol. 1988;86:908–913. doi: 10.1104/pp.86.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honor SJ, Webb AAR, Mansfield TA. The response of stomata to abscisic acid and temperature are interrelated. Proc R Soc Lond B Biol Sci. 1995;259:301–306. [Google Scholar]
- Ilan N, Moran N, Schwartz A. The role of potassium channels in the temperature control of stomatal aperture. Plant Physiol. 1995;108:1161–1170. doi: 10.1104/pp.108.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowiak F, Dörffling Chilling of maize seedlings: changes in water status and abscisic acid concentration in ten genotypes differing in chilling tolerance. J Plant Physiol. 1996;147:582–588. [Google Scholar]
- Jian LC, Li JH, Chen WP, Li PH, Ahlstrand GG. Cytochemical localization of calcium and Ca2+-ATPase activity in plant cells under chilling stress: a comparative study between the chilling-sensitive maize and the chilling-insensitive winter wheat. Plant Cell Physiol. 1999;40:1061–1071. [Google Scholar]
- Knight H, Brandt S, Knight MR. A history of stress alters drought calcium signalling pathways in Arabidopsis. Plant J. 1998;16:681–687. doi: 10.1046/j.1365-313x.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Kozlowski TT, Pallardy GG. Effects of low temperature on leaf diffusion resistance of Ulmus americana and Fraxinus pennsylvanica seedlings. Can J Bot. 1979;57:2466–2470. [Google Scholar]
- Lalk I, Dörffling K. Hardening, abscisic acid, proline and freezing resistance in two winter wheat varieties. Physiol Plant. 1985;63:287–292. [Google Scholar]
- Lee TM, Lur HS, Chu C. Role of abscisic acid in chilling tolerance of rice (Oriza sativa L.) seedlings: I. Endogenous abscisic acid levels. Plant Cell Environ. 1993;16:481–490. [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Abscisic acid-induced elevation of guard cell cytoplasmic Ca2+ precedes stomatal closure. Nature. 1990;343:186–188. [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Calcium ions as second messengers in guard cell signal transduction. Physiol Plant. 1997;100:16–29. [Google Scholar]
- Minorsky PV. Temperature sensing by plants: a review and hypothesis. Plant Cell Environ. 1989;12:119–135. [Google Scholar]
- Monroy AF, Dhindsa RS. Low temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25°C. Plant Cell. 1995;7:321–331. doi: 10.1105/tpc.7.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Örvar BL, Sangwen V, Omann F, Dhindsa RS. Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J. 2000;23:785–794. doi: 10.1046/j.1365-313x.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- Pan RC. The role of abscisic acid in chilling resistance. In: Pharis RP, Rood SB, editors. Plant Growth Substances 1988. Berlin: Springer-Verlag; 1990. [Google Scholar]
- Pardossi A, Vernieri P, Tognoni F. The involvement of ABA in ameliorating plant water status of Phaseolus vulgaris during chilling. Plant Physiol. 1992;100:1243–1250. doi: 10.1104/pp.100.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JJ, Irigoyen JJ, Sanchez-Diaz M. Chilling of drought-hardened and non-hardened plants of different chilling-sensitive maize lines: changes in water relations and ABA contents. Plant Sci. 1997;122:71–79. [Google Scholar]
- Puhakainen T, Pihakaski-Maunsbach K, Widell S, Sommarin M. Cold acclimation enhances the activity of plasma membrane Ca2+ ATPase in winter rye leaves. Plant Physiol Biochem. 1999;37:231–239. [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SJ, Henson IE, Loveys BR. A monoclonal antibody to (S)-abscisic acid: its characterization and use in radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta. 1988;173:330–339. doi: 10.1007/BF00401020. [DOI] [PubMed] [Google Scholar]
- Rikin A, Atsmon D, Gitler C. Chilling injury in cotton: prevention by abscisic acid. Plant Cell Physiol. 1979;20:1537–1546. [Google Scholar]
- Rodriguez JL, Davies WJ. The effects of temperature and ABA on stomata of Zea mays L. J Exp Bot. 1982;33:977–987. [Google Scholar]
- Ruiz LP, Atkinson CJ, Mansfield TA. Calcium in the xylem and its influence on the behavior of stomata. Philos Trans R Soc London Ser B Biol Sci. 1993;341:67–74. [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenrath GF, Ort DR, Portis AR., Jr Impaired reductive activation of stromal bisphosphatases in tomato leaves following low temperature exposure at high light. Arch Biochem Biophys. 1990;282:302–308. doi: 10.1016/0003-9861(90)90121-e. [DOI] [PubMed] [Google Scholar]
- Teskey RO, Hinckley TM, Grier CC. Effect of interruption of flow path on stomatal conductance of Abies amabilis. J Exp Bot. 1983;34:1251–1259. [Google Scholar]
- Trewavas AJ, Malhó R. Signal perception and transduction: the origin of the phenotype. Plant Cell. 1997;9:1181–1195. doi: 10.1105/tpc.9.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernieri P, Pardossi A, Tognoni F. Influence of chilling and drought on water relations and abscisic acid accumulation in bean. Aust J Plant Physiol. 1991;18:25–35. [Google Scholar]
- Weyers JDB, Meidner H. Epidermal strips. In: Weyers JDB, Meidnes H, editors. Methods in Stomatal Research. Ed 1. Harlow, UK: Longman Scientific and Technical; 1990. pp. 129–155. [Google Scholar]
- Wilkinson S. PH as a stress signal. Plant Growth Regul. 1999;29:87–99. [Google Scholar]
- Wilson JM. The mechanism of chill- and drought-hardening of Phaseolus vulgaris leaves. New Phytol. 1976;76:257–270. [Google Scholar]
- Wolfe DW. Low temperature effects on early vegetative growth, leaf gas exchange and water potential of chilling-sensitive and chilling-tolerant crop species. Ann Bot. 1991;67:205–212. [Google Scholar]
- Zhang C-L, Li PH, Brenner ML. Relationship between mefluidide treatment and abscisic acid metabolism in chilled corn leaves. Plant Physiol. 1986;81:699–701. doi: 10.1104/pp.81.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]