Abstract
A 16-year-old man presented to the Accident and Emergence services with a 10-day history of shortness of breath, sore throat, vomiting, diarrhoea, poor oral intake, chest pain, jaundice, diplopia and reduced urine output. He was initially treated for sepsis, however, subsequent imaging and blood cultures confirmed the diagnosis of Lemierre’s syndrome (LS). LS, also known as necrobacillosis or post-pharyngitis anaerobic septicaemia is comprised of a triad of metastatic septic emboli secondary to pharyngitis, bacteraemia, and internal jugular vein thrombophlebitis. Fusobacterium necrophorum, a Gram-negative anaerobe, is the most common culprit of LS, followed by Fusobacterium nucleatum and anaerobic bacteria such as streptococci, staphylococci, and Klebsiella. LS is also called the forgotten syndrome because although use of antibiotics at first decreased the prevalence of LS, resistance to antibiotics has caused a rise in LS and it is no longer a forgotten disease. LS should be on the differential diagnosis of chest empyema if it follows pharyngitis or tonsillitis with neck pain, lymphadenopathy and sepsis, hence taking a thorough history is the key to diagnose it earlier. It is paramount to do chest X-ray, Doppler ultrasound of the neck veins and computed tomography (CT) scan of the neck and chest to look for features of LS. LS can be fatal if not diagnosed and treated properly. Empirical antibiotic therapy should be prescribed for a minimum of 3 weeks and should cover anaerobic bacteria and Gram-negative rods.
LEARNING POINTS
The incidence of Lemierre’s syndrome (LS) is rising possibly to antibiotic resistance and fewer tonsillectomies which should increase awareness of the signs and symptoms of LS. It is no longer the forgotten disease.
Pharyngitis can have serious complications including glomerulonephritis, rheumatic fever, and tonsillar cysts. However, LS is one of the most fatal complications of pharyngitis and must certainly be considered in the differential diagnoses.
Even if the sore throat resolves post oropharyngeal infection or the blood culture comes back negative, if the patient is feeling unwell, this should be treated as a red flag for further investigations. Prompt investigation and management of LS is the key to saving patient’s life as the mortality rate in LS is high.
Keywords: Pharyngitis, emergency medicine, MRI, multi-profession management
INTRODUCTION
Lemierre’s syndrome (LS) was first identified in 1900 by Courmount and Cade when they noticed infection of the oropharynx by anaerobic bacteria could lead to pulmonary infarcts by attaining access to blood. From 1917 to 1925 Goldma, Mosher and Fränkel further explained conversion of simple tonsilitis to metastatic septic emboli in the internal jugular vein. The name Lemierre’s syndrome was identified by the French physician Andre Lemierre in 1936[1]. In the 1990s it was referred to the forgotten disease after introduction of antibiotics in oropharyngeal infections, however there has been a reported rise in the incidence of LS in the last 30 years because of restriction of use of antibiotics by primary care doctors, fewer tonsillectomies, and development of resistance to antibiotics[2]. The most common causative agent of LS is Fusobacterium necrophorum which is part of the normal flora of the oral cavity, gastrointestinal tract and female genitourinary tract. It is also found in dental and peritonsillar abscesses. It can manifest with a sore throat, but clinicians routinely order tests for Streptococcus pyogenes Group A Streptococcus (GAS) because it is the most common cause of pharyngitis. F. necrophorum is hard to culture because it requires a selective medium for anaerobes and currently there is no polymerase chain reaction (PCR) or rapid test available for use. Therefore, worsening symptoms of pharyngitis warrant testing for F. necrophorum.
LS is associated with pharyngitis with either a local invasion of the parapharyngeal space or internal jugular vein leading to septic thrombophlebitis. The diagnostic criteria for LS include:
Clinical findings of pharyngitis or tonsillitis without resolution in less than a week with possible dysphagia, neck swelling, and lymphadenopathy;
Followed by fever, malaise, confusion secondary to septic emboli to other organs for example to lungs causing cavitating pneumonia or empyema;
Thrombosis via internal jugular vein or growth of F. necrophorum in blood culture.
Early diagnosis and management can be lifesaving.
CASE DESCRIPTION
A 16-year-old male presented to the primary care with sore throat and shortness of breath and was treated for viral pharyngitis. He went back to his general practitioner for worsening shortness of breath for 10 days with intermittent chest pain with electrocardiogram (ECG) changes of scattered saddle shaped ST elevation and a heart rate of 116 bpm. Therefore an ambulance was called. The paramedics noted his oxygen saturation was 90% on 2 l/min of oxygen, and he had a respiratory rate of 36 with difficulty speaking, gasping for air. He looked pale with icteric sclera, but did not have clammy skin. He had 10 days of intermittent chest pain associated with nausea, vomiting, loose stool, fever, shivering and reduced oral intake. The ambulance crew reported a blood pressure of 99/62 mmHg and a temperature of 37.1°C. The patient denied any recent travel, or vaccination. He did not use any illicit drugs. He had no recent history of dental procedures or poor oral hygiene. He reported having a dog. On examination the patient looked pale and exhausted and had a dry mouth. His neck was soft without lymphadenopathy. There was reduced air entry on the left side with oxygen saturation of 100% on 10 l/min of oxygen. His Glascow Coma Scale (GCS) was 15/15 and he did not have any fever. He looked dry and jaundiced. He complained of pain in the left side of the neck and left shoulder, and he had diplopia. The patient was started on piperacillin/tazobactam and clindamycin in the Accident and Emergency department per microbiologist’s advice to cover for chest sepsis. He was given intravenous (IV) fluid, vitamin K, adenosine and verapamil. He was in poor condition and significantly short of breath, hence he was transferred to intensive care unit on the same day where he was intubated, a central venous line, vascath and arterial line were inserted, and he received fresh frozen plasma and platelet transfusion. A chest drain was put in and pus was drained. A nasogastric tube was inserted. Noradrenaline was given to maintain mean arterial pressure (MAP) >65 mmHg. Key results of laboratory tests on the day of admission are shown in Table 1.
Table 1.
Key results of laboratory tests on the day of admission.
| Test | Laboratory values | Reference range |
|---|---|---|
| C-reactive protein (mg/l) | 400 | <5 |
| Ferritin (μg/ml) | 674 | 22–322 |
| Procalcitonin (ng/ml) | >200 | <0.05 |
| Bilirubin (mM/l) | 99 | 0–20 |
| Creatinine (mM/l) | 437 | 54–99 |
| Urea (mM/l) | 40.3 | 2.5–7.8 |
| Sodium (mM/l) | 128 | 133–146 |
| Platelets (×103/μl) | 33 | 170–400 |
| Prothrombin time (s) | 25.6 | 10–14 |
| Pleural fluid analysis | ||
| Microorganisms | Not detected | |
| Pus cells | +++ | |
| WCC | ++ | |
| Bacterial 16S rRNA gene | Detected (gene sequence confirmed F. necrophorum) | |
| Stool sample analysis | ||
| Clostridium difficile GDH screening | Negative | |
| Cryptosporidium | Negative | |
| Campylobacter species | Negative | |
| Salmonella species | Negative | |
| Shigella species | Negative | |
| Urine sample analysis | ||
| Culture | No significant growth | |
| Cells: WCC, RBC, epithelial cells | Negative | |
| Escherichia coli O157 | Negative | |
| Rectal swab | ||
| Candida auris | Negative | |
| CPE | Negative | |
| Repeated blood cultures from different sources such as vein, artery, PICC line tip, CVC line tip | negative | |
Abbreviations: CPE, carbapenemase-producing organism; CVC, central venous catheter; GDH, glutamate dehydrogenase; PICC, peripherally inserted central catheter; RBC, red cell count; RNA, ribonucleic acid; WCC, white cell count.
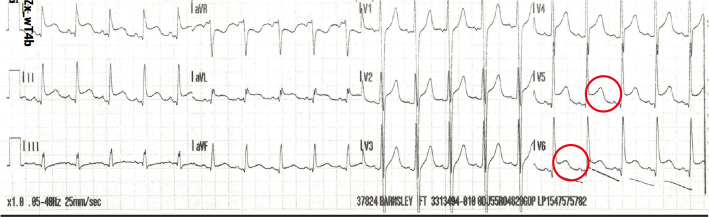
A blood film showed toxic neutrophils suggestive of severe sepsis. A venous blood gas (VBG) showed pH 7.38, partial pressure of carbon dioxide (pCO2) 5.2 kPa, lactate 3.5 mmol/l and base excess −1.8 mmol/l. High sensitivity troponin I was normal, and an ECG showed widespread saddle shaped ST elevation with reciprocal changes in aVR lead. The patient was tachycardic (Fig. 1). An echocardiogram was done which showed moderate to severe left ventricular systolic dysfunction with reduced right ventricular function. He was commenced on beta-blockers and angiotensin-converting enzyme (ACE) inhibitors by the cardiology team. The echocardiogram was repeated 2 weeks later which revealed normal cardiac functioning, therefore his cardiac medications were stopped. Myocardial dysfunction on the initial scan was attributed to severe sepsis which spontaneously resolved after the patient improved.
Figure 1.
Electrocardiogram on admission.
Microbiological tests were ordered (Table 2). Respiratory PCR included: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), adenovirus, bocavirus, coronavirus 229E, NL63, and OC43, human metapneumovirus A and B, influenza A, AH1, AH1N1 pdm-0, H3, influenza B, parainfluenza 1–4, respiratory syncytial virus A and B, rhinovirus, Bordetella pertussis, Legionella pneumoniae, and Mycoplasma pneumoniae. Pleural fluid analysis showed transudative picture per Light’s criteria (Table 3).
Table 2.
Microbiology tests.
| Test | Result |
|---|---|
| SARS-CoV-2 screen | Negative |
| Influenza A and B RNA | Negative |
| Mycoplasma pneumoniae | Negative |
| Chlamydia pneumoniae | Negative |
| Toxoplasma antibody | Negative |
| Syphilis antibody | Negative |
| HIV 1 and 2 antigen/antibody | Negative |
| Cytomegalovirus IgM | Negative |
| Cytomegalovirus IgG | Detected |
| Hepatitis A IgM | Negative |
| Hepatitis B surface Ag | Negative |
| Hepatitis C antibody | Negative |
| Anti-streptolysin O titre (IU/ml) | 400 (<200 is negative) |
| Anti-DNase B titre for group A streptococci (IU/ml) | 100 U/ml (<200 is negative) |
| QuantiFERON test | Negative |
| Epstein Barr VCA IgM | Negative |
| Epstein Barr VCA and EBNA IgG | Detected |
| Respiratory PCR | All negative |
| Beta-D glucan | Negative |
| Meningococcal PCR | Negative |
| Pneumococcal PCR | Negative |
| Haemophilus influenzae DNA PCR | Negative |
| Toxocariasis serology (diplopia - patient has a dog) | Negative |
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RNA, ribonucleic acid; HIV, human immunodeficiency virus; Ag, antigen; DNA, deoxyribonucleic acid; Ig, immunoglobulin; PCR, polymerase chain reaction; VCA, viral capsid antigen; EBNA, Epstein-Barr virus nuclear antigen.
Table 3.
Pleural fluid profile.
| Test | Result |
|---|---|
| Fluid LDH (U/L) | 38900 |
| Fluid total protein (g/l) | 25 |
| Fluid amylase (U/L) | <20 (30–110 normal range) |
| Protein LDH/serum ratio | 0.005 |
| Protein fluid/serum ratio | 0.5 |
Abbreviations: LDH, lactate dehydrogenase.
On day 1 post-admission, chest X-ray revealed left pleural effusion, and multiple pulmonary lesions including cavitating lesions in the left upper zone (Fig. 2). Pus from pleural fluid and blood culture showed growth of F. necrophorum. Antibiotics were escalated to piperacillin/tazobactam 2.4 mg three times a day and metronidazole 500 mg IV four times a day which resulted in a gradual decrease in the levels the C-reactive protein (CRP), ferritin and procalcitonin. Transthoracic echocardiogram did not demonstrate any obvious source of septic emboli, vegetation or valvular abnormalities. On insertion of an intercostal drain, the left sided pleural effusion improved leading to re-expansion of left lower lobe. A peripherally inserted central catheter (PICC) line was inserted 4 days after admission. On day 5, there was re-accumulation of a small left sided pleural effusion which increased to a moderate left loculate effusion the next day. The patient was transferred to respiratory critical care on day 7 of admission. The chest drain was removed on day 9.
Figure 2.
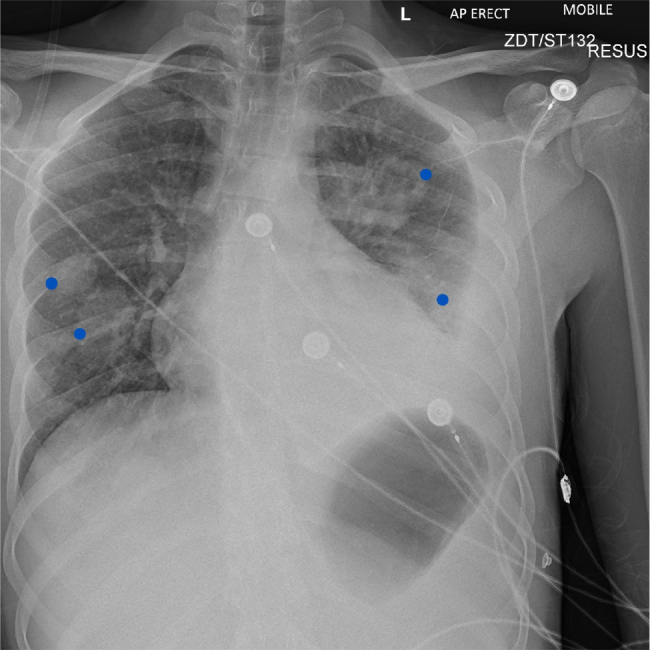
Chest X-ray on admission showing multiple bilateral soft tissue opacities (blue dots) and left-sided pleural effusion.
A contrast-enhanced computed tomography (CT) scan of the chest, abdomen, and pelvis revealed a possible pulmonary embolus, left sided pleural effusion with collapse consolidation of the left lower lobe and cavitation along with multiple wedge-shaped opacifications within the right lung associated with emboli of the right lung and focal splenic infarction (Fig. 3). CT scan of the chest showed left sided pleural effusion with cavitating lesion and air pockets. CT scan of the head was unremarkable. Magnetic resonance imaging (MRI) of the head was done on day 12 due to diplopia with right superior oblique and inferior rectus muscle weakness, which ruled out an intracranial collection. Subsequently, MRI orbits was performed and showed oedema and inflammation of the right superior oblique and inferior rectus muscles in keeping with myositis. CT neck and chest with contrast confirmed a left peritonsillar and parapharyngeal collection measuring 27 × 22 × 37 mm. It caused displacement of the left submandibular gland inferiorly (Fig. 4). Another collection in the strap muscles in the right side of the neck inferior to the thyroid gland extending retrosternally was noted which measured 58 × 22 × 5 mm. The left internal jugular vein was occluded with a thrombus with enhancement of its wall which indicated infective component (Fig. 5). The images confirmed LS secondary to a left tonsillar/parapharyngeal infection with consequent ipsilateral internal jugular vein septic thrombus and pulmonary sepsis.
Figure 3.
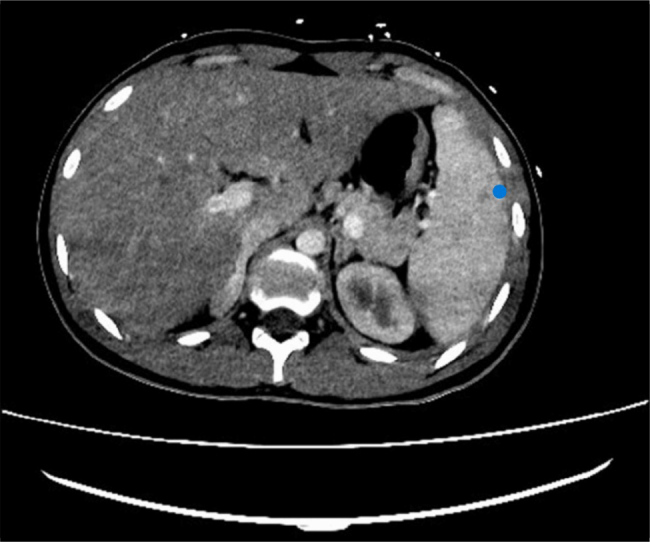
Computed tomography scan of the abdomen with contrast showing focal splenic infarction (blue dot).
Figure 4.
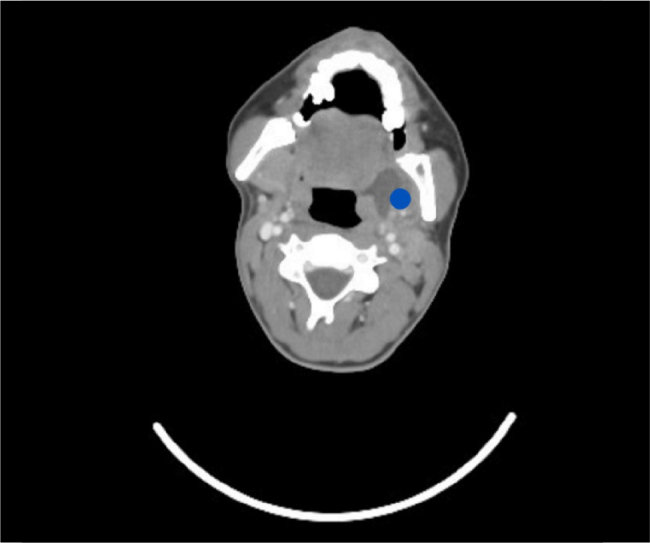
Computed tomography scan of the neck with contrast: axial view showing left peritonsillar/parapharyngeal abscess (blue dot).
Figure 5.
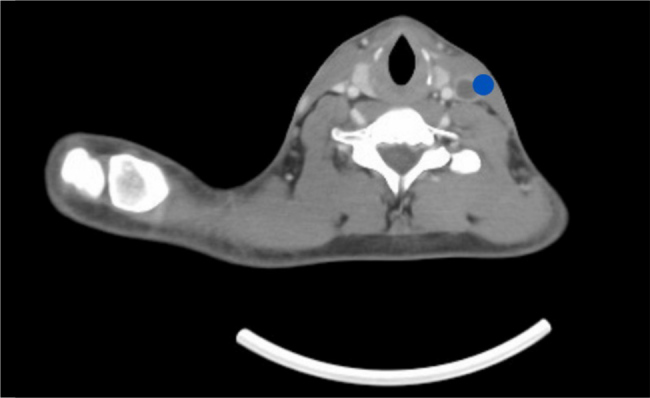
Computed tomography scan of the neck with contrast: axial views showing the occluded left internal jugular vein (blue dot).
The patient ended up having bilateral tonsillectomy along with drainage of the parapharyngeal and retrosternal collection on day 12. He was under close observation by the ophthalmology team who ruled out toxocariasis as the cause of the diplopia as the patient had reported exposure to dogs. On day 16th, the diplopia was less severe and there was no restriction of extraocular motility. However, there was a white spot near the upper temporal arcade in the right eye which could be a sign of infarction of the nerve fibre.
After 16 days of IV antibiotic therapy, the patient’s symptoms completely resolved. The white blood cells and other inflammatory markers were within normal range. His ECG became normal, and echocardiogram showed normal heart functioning. He was started on heparin infusion for 4 days which was changed to apixaban.
The patient was discharged after 20 days of hospitalisation in a stable condition with 625 mg of oral amoxicillin/clavulanic acid every 12 hours for 14 days, apixaban 5 mg twice a day, bisoprolol 1.25 mg once a day and omeprazole 20 mg once a day. He stayed on apixaban and bisoprolol for 3 months and had follow ups with respiratory, ophthalmology and ear nose and throat (ENT) teams with repeated chest X-rays to monitor the resolution of his pulmonary lesions. Chest X-ray 3 months later showed clear lungs and pleural space.
DISCUSSION
This case shows an uncommon complication of pharyngitis in a 16-year-old male with no relevant medical history who was initially treated for a simple viral cause. LS is no longer a forgotten disease, and awareness needs to be raised. LS is characterised by septic thrombophlebitis of internal jugular vein from head and neck area. LS requires prompt diagnosis and commencement of empiric antibiotics initially to target F. necrophorum and other oropharynx streptococci. F. necrophorum has been found to be resistant to beta lactamase and fluoroquinolones, but sensitive to amoxicillin/clavulanic acid, metronidazole, imipenem, clindamycin and chloramphenicol[3]. Macrolides and cephalosporins have shown a variable response. The most common empiric treatments are piperacillin/tazobactam 3.375 mg IV four times a day and metronidazole 500 mg IV three times a day together, however piperacillin/tazobactam can be replaced by imipenem 500 mg IV four times a day or ceftriaxone 2 g IV once a day. It is paramount to have a multidisciplinary approach including microbiology, ENT, radiology, intensive care, haematology, internal medicine and respiratory specialists. The average recommended duration of antibiotics is 3 to 5 weeks with at least 2 weeks of IV antibiotics. If the bacteria are concealed in pus pockets or in thrombi, longer course of antibiotics is advised which could be up to 8 weeks[1]. The role of anticoagulants is controversial. Some experts argue that the clot is a sequalae of the infection and it will consequently resolve once the infection is treated, while others believe it is beneficial in resolving infections where bacteria are concealed in the thrombus[4]. If the clinical presentation of LS is less severe and there is no evidence of a massive thrombotic event, the use of anticoagulants might not be required, on the contrary, if LS features multi-organ failure and extensive clotting, it could be beneficial to use anticoagulant therapy[5]. Various anticoagulants have been used including low molecular weight heparin, fondaparinux, direct oral anticoagulants and unfractionated heparin for 70–84 days. Despite thrombocytopenia and renal impairment, the use of anticoagulants does not increase the risk of bleeding.
Surgical intervention may be required. This includes procedures such as internal jugular vein ligation, chest drain, abscess drain, mastoidectomy, tonsillectomy, debridement of necrotic tissues, and invasive cranial surgery. Complications of LS could be pylephlebitis, or suppurative thrombophlebitis of the portomesenteric venous system leading to colitis, vomiting and diarrhoea. Pylephlebitis comprising extension of the thrombus in the superior mesenteric vein, intrahepatic branches of portal vein and inferior mesenteric vein in descending order of frequency. LS could cause intestinal ischaemia. Central nervous system thrombosis could cause several complications including meningitis, encephalitis, cavernous sinus thrombosis, brain abscesses, and intracranial ischaemia. LS patients could face long-term sequelae such as cranial nerve palsy, blindness, septic arthritis of the knee, purulent otitis, recurrent tonsillitis, language disorder and dysphagia. Other complications of metastasis of septic emboli could be pericarditis, splenic and hepatic abscesses, muscle abscess, soft tissue abscess and ophthalmological complications[2].
CONCLUSION
This case is important because the incidence of LS is rising, and it is no longer a forgotten disease. It highlights remarkable ECG changes with normal level of troponin I in a very young person with no past medical history which is accountable by severe sepsis. The ECG changes reverted to normal once patient was stable. Long-lasting pharyngitis or feeling unwell after a sore throat has resolved, should be treated as red flags for LS. The most common bacterium associated with LS is F. necrophorum which should be part of the investigation. The thrombi caused by the bacterial toxin could cause multi-organ failure if not diagnosed and treated promptly.
In this article we discussed symptoms of LS and highlighted the importance of performing imaging assessment in a timely manner and working as a multi-disciplinary team.
There is no recent epidemiological study on LS, hence our case can be a reminder that the incidence of LS is rising, and the clinicians must be aware of this and act swiftly and appropriately by doing the right investigations to reach the right diagnosis.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
Patient Consent: We confirm the acquisition of the signed patient consent.
REFERENCES
- 1.Amarnani S, Ranjan A. Lemierre’s Syndrome: A Lethal Complication of Acute Tonsillitis. Cureus. 2022;14:e30072. doi: 10.7759/cureus.30072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiwari A. Lemierre’s Syndrome in the 21st Century: A Literature Review. Cureus. 2023;15:e43685. doi: 10.7759/cureus.43685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohiuddin Z, Manes T, Emerson A. Fusobacterium necrophorum Bacteremia With Evidence of Cavitary Pulmonary Lesion. Cureus. 2021;13:e19537. doi: 10.7759/cureus.19537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie M, Liu J, Zheng J, Wang J, Han D. Lemierre Syndrome: Report of a Case with an Innovative Diagnostic Method and Literature Review. Infect Drug Resist. 2024;17:1–10. doi: 10.2147/IDR.S439069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recchia A, Cascella M, Altamura S, Borrelli F, De Nittis N, Dibenedetto E, et al. Early Diagnosis and Antibiotic Treatment Combined with Multicomponent Hemodynamic Support for Addressing a Severe Case of Lemierre’s Syndrome. Antibiotics (Basel) 2021;10:1526. doi: 10.3390/antibiotics10121526. [DOI] [PMC free article] [PubMed] [Google Scholar]