Abstract
In developing seeds, the permeability of the plasma membrane of seed coat parenchyma cells is crucial for the supply of nutrients to the embryo. Here, we report characteristics of the transport of the organic cation choline and the basic amino acid l- histidine (His; cation at pH 5, electroneutral at pH 7) into isolated seed coats of pea (Pisum sativum). Supplied at sub-micromolar concentrations, choline+ accumulated in the seed coat tissue 5.1 ± 0.8-fold, His+ 2.4 ± 0.3-fold, and His0 1.3 ± 0.2-fold. Taking into consideration that at pH 5 His influxes as a cation but effluxes as a neutral molecule, these accumulations are in reasonable agreement with (electro) diffusional uptake at the prevailing membrane potential of −55 ± 3 mV. At a concentration of 100 mm, choline+ and His+, but not His0, depolarized the membrane of the parenchyma cells and neither of the substrates was accumulated. At this concentration, the relative influx (the ratio of influx and external concentration, a measure for membrane permeability) of choline and His was approximately 10 μmol g−1 fresh weight min−1 m−1, similar to that found for neutral amino acids, sucrose, glucose, and mannitol. At lower concentrations, the relative influx of choline+ and His+ increased because of increasingly more negative membrane potentials, giving rise to apparent saturation kinetics. It is suggested that transport of organic cations can proceed by a general, poorly selective pore in the plasma membrane of seed coat parenchyma cells. This pore is thought to be responsible for the unloading of a range of solutes that serve as nutrients for the embryo.
During the development of the pea (Pisum sativum) seed, the embryo receives its nutrients from the surrounding maternal seed coat. These nutrients arrive in the seed coat mainly through the phloem of three vascular bundles and are then distributed by cell-to-cell transport over the seed coat parenchyma (Grusak and Minchin, 1988; Patrick et al., 1995; Tegeder et al., 1999). Because embryo and seed coat are symplasmically isolated from each other, the nutrients have to be unloaded into the apoplast. This implies the transport of the nutrients across the plasma membrane of the seed coat parenchyma cells. The mechanism by which the various nutrients (Suc, amino acids, and inorganic ions) are released from the parenchyma cells is still incompletely understood.
The release of sugars and amino acids from the pea seed coat is biphasic, consisting of a fast and a slow component (De Jong and Wolswinkel, 1995). It is most likely that the fast component represents the efflux across the plasma membrane of seed coat parenchyma cells. The rate constant for the fast component in the release of Suc and amino acids amounts to approximately 1.4 h−1, corresponding to a half-life time of approximately 0.5 h. For a spherical cell, the permeability coefficient (P) is related to the half-life time (t1/2) and the cell radius (r) by P = ln2 × r/3t1/2 (Lanfermeijer et al., 1990). Inasmuch as the diameter of parenchyma cells in the pea seed coat ranges from 20 to 100 μm, the permeability coefficient for Suc and amino acids can be roughly estimated to be in the order of 10−7 cm s−1. For Suc, Glc, mannitol, and several neutral amino acids, membrane permeabilities have been estimated more precisely in uptake experiments with 14C-labeled solutes (De Jong et al., 1996, 1997). Estimated permeability coefficients of all tested solutes turned out to be in the range 3 × 10−7 to 9 × 10−7 cm s−1, which is at least 105-fold higher than expected for diffusion through a lipid bilayer. In addition, it has been found that the efflux of endogenous Suc and amino acids (De Jong and Wolswinkel, 1995) as well as the influx of exogenously supplied substrates could be inhibited by p-chloromercuribenzene sulfonic acid (pCMBS). Therefore, it has been proposed that the plasma membrane of seed coat parenchyma cells contains nonselective, proteinaceous pores that facilitate the unloading of sugars and amino acids (De Jong et al., 1996, 1997).
To probe the permeability of the plasma membrane of seed coat parenchyma cells for molecules bearing a positive electrical charge, we investigated the uptake of the small organic cation choline and the basic amino acid l-His by pea seed coat tissue. Because the pKa value of the imidazole group of His is 6.0, the abundance of the cationic form (His+) decreases from 91% at pH 5 to 9% at pH 7, whereas the abundance of the electroneutral zwitterionic form (His0) increases from 9% to 91% (Segel, 1968). Thus, using His as a substrate the transport of a molecule in its electroneutral or cationic form can be studied in the pH range 5 to 7. We assessed the extent to which choline and His can be accumulated in the seed coat tissue, measured their influxes over a wide concentration range, and studied their effect on the membrane potential. The results indicate that the cations choline+ and His+ are taken up by electrodiffusion through a general, poorly selective pore. The implication of these pores for the regulation of assimilate release by seed coats will be discussed.
RESULTS
Time Course of Uptake and Accumulation of Choline and l-His
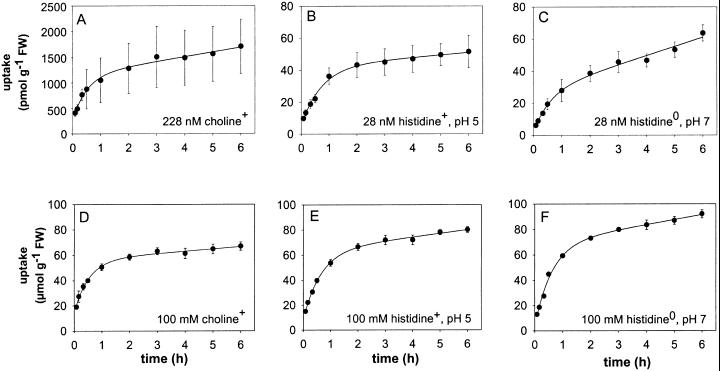
The uptake of choline and l-His by the isolated seed coat halves was determined at a sub-micromolar concentration and at 100 mm. His uptake was determined using incubation medium of either pH 5 or pH 7. Figure 1 shows the time course of uptake of both substrates during 6 h of incubation.
Figure 1.
Time course of the uptake of choline and l-His by pea seed coats. Uptake was measured when the substrate was supplied at a submicromolar concentration (A, B, and C) or at a concentration of 100 mm (D, E, and F). His uptake was measured at pH 5, where approximately 90% of the amino acid is present as the monovalent cation (B and E) and at pH 7 where approximately 90% of His is present as the electroneutral zwitterion (C and F). Curves were drawn using the parameter values obtained by fitting Equation 2 to the uptake data (Table I). FW, Fresh weight.
The time-dependent uptake [U(t)] of a solute by plant tissue may be described by:
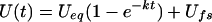 |
Eq. 1 |
where Ueq is the uptake at equilibrium, k is a rate constant, and Ufs represents the uptake in the free space. If, per unit of time, a fraction f of the intracellular substrate is converted into molecular species that cannot leave the cell, Equation 1 has to be transformed into:
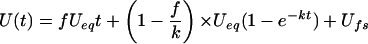 |
Eq. 2 |
Equation 2 was fitted to the data shown in Figure 1 and the resulting parameter values are given in Table I.
Table I.
Parameters describing the time course of uptake of choline and l-His by seed coat halves
| Substrate | External Concentration, [S]out | Ueq | k | f | Ufs | Accumulation Ratio, [S]in/[S]out |
|---|---|---|---|---|---|---|
| nm | pmol g−1 fresh wt | h−1 | pmol g−1 fresh wt | |||
| Choline | 228 | 940 ± 150 | 2.1 ± 0.6 | 0.10 ± 0.06 | 246 ± 35 | 5.1 ± 0.8 |
| l-His+, pH 5 | 21 | 40 ± 5 | 1.1 ± 0.2 | 0.02 ± 0.04 | 6.4 ± 0.7 | 2.4 ± 0.3 |
| l-His0, pH 7 | 27 | 28 ± 5 | 1.4 ± 0.3 | 0.20 ± 0.07 | 3.1 ± 0.4 | 1.3 ± 0.2 |
| mm | μmol g−1 fresh wt | μmol g−1 fresh wt | ||||
| Choline | 100 | 44 ± 2 | 1.9 ± 0.2 | 0.04 ± 0.01 | 13.3 ± 1.2 | 0.55 ± 0.02 |
| l-His+, pH 5 | 100 | 57 ± 3 | 1.5 ± 0.1 | 0.05 ± 0.01 | 8.3 ± 0.4 | 0.71 ± 0.04 |
| l-His0, pH 7 | 100 | 72 ± 7 | 1.1 ± 0.2 | 0.03 ± 0.04 | 6.4 ± 0.6 | 0.90 ± 0.09 |
The equation U(t) = fUeqt + (1 − f/k)Ueq(1 − e−Kt) + Ufs was fitted to the uptake data shown in Fig. 1. The parameter Ueq represents the amount of substrate in the seed coat tissue at equilibrium and f is the fraction of intracellular substrate assumed to be converted per unit of time into immobile molecular species. The concentration of substrate in the tissue, [S]in, was calculated from Ueq assuming that pea seed coats contain 80% of tissue water.
Assuming that pea seed coats contain 80% (w/w) water, substrate concentrations in the tissue at equilibrium ([S]in) were computed from Ueq, so that an estimate could be made of the extent to which the substrate was accumulated. As can be seen in Table I, choline and His were not accumulated by seed coat tissue when present at a concentration of 100 mm in the incubation medium. When the substrate was administered at a sub-micromolar concentration to the incubation medium, choline accumulated about 5-fold and His+ about 2-fold, whereas His0 was not significantly accumulated in the seed coat tissue.
Concentration Dependence of Initial Influx
The uptake of choline and His was investigated over a wide concentration range, from <1 μm to 100 or 200 mm. Initial influxes (v) were determined from the uptake between 5 and 20 min, which was essentially linear with time (Fig. 1). The ratio of initial influx and external substrate concentration is defined here as the relative influx, v/[S]. Concentration dependencies of choline and His influx were analyzed by fitting the equation:
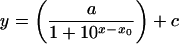 |
Eq. 3 |
which describes the relative influx (y) as a function of the logarithm of the external substrate concentration (x), and where a + c is the maximum relative influx when the substrate concentration approaches zero, x0 equals log[S] at the inflection point of the sigmoid part of the curve, and c is the relative influx at an infinitely high substrate concentration. Parameter values resulting from fits of Equation 3 are given in Table II. Omitting the constant c in Equation 3 resulted in poor fits (not shown).
Table II.
Parameters describing the concentration dependence of the influx of choline and l-His into pea seed coat tissue
| Substrate | a | x0 | c |
|---|---|---|---|
| μmol g−1 fresh wt min−1m−1 | log m | μmol g−1 fresh wt min−1m−1 | |
| Choline | 67.0 ± 5.4 | −2.67 ± 0.14 | 9.6 ± 0.1 |
| l-His+, pH 5 | 11.4 ± 1.0 | −1.77 ± 0.15 | 10.1 ± 0.7 |
| l-His0, pH 7 | 2.3 ± 0.2 | −2.37 ± 0.53 | 10.2 ± 0.03 |
The equation y = a/(1 + 10x−x0) + c was fitted to data of the concentration dependency of the influx shown in Figure 2.
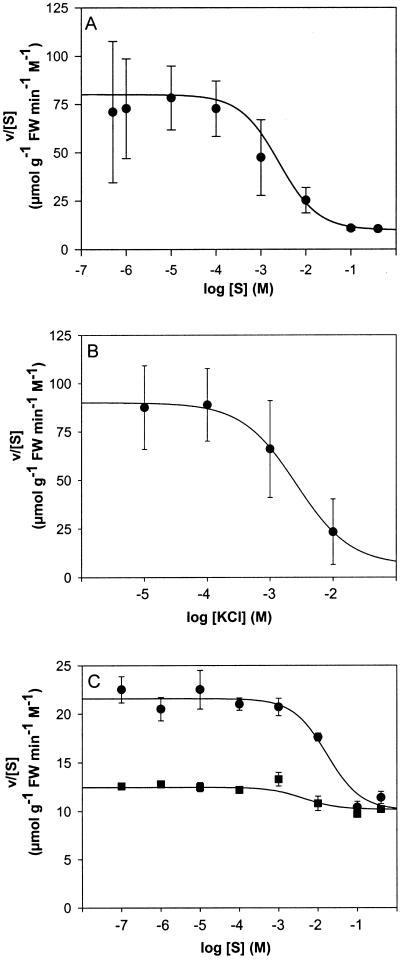
Figure 2A shows the sigmoid relationship between the relative influx of choline and the logarithm of the external concentration. The relative influx was highest at low concentrations, amounting to approximately 75 μmol g−1 fresh weight min−1 m−1, and decreased with increasing substrate concentrations until it attained a value of about 10 μmol g−1 fresh weight min−1 m−1 at concentrations above 100 mm. Increasing concentrations of KCl had a similar effect on choline influx as increasing concentrations of unlabeled choline (Fig. 2B).
Figure 2.
Concentration dependence of the relative influx, v/[S], of choline and l-His. The osmolarity of the incubation medium was adjusted to 400 mm by adding different amounts of mannitol. Data points represent mean values ± se from three to 30 measurements. The curves drawn were calculated from the parameters in Table II. A, Choline in incubation medium, pH 5.5. B, Effect of increasing concentrations of KCl on the relative influx of choline supplied at a concentration of 202 nm. C, L-His in incubation medium with pH5 (●) or pH7 (▪). FW, Fresh weight.
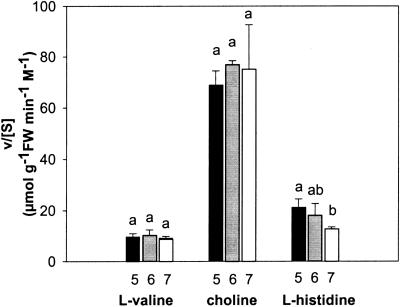
At low concentrations, the relative influx of His+ amounted to approximately 20 μmol g−1 fresh weight min−1 m−1, which is about 4-fold less than that of choline (Fig. 2C). However, at high concentrations the relative influxes of His+ and choline were essentially the same. It is interesting that the relative influx of His0 decreased only slightly with increasing substrate concentrations, from 12.5 to 10 μmol g−1 fresh weight min−1 m−1. Hence, the influx of His at low concentrations decreased when the pH of the incubation medium was raised from 5 to 7. In contrast, the influxes of choline and the neutral amino acid l-Val were essentially constant in this pH range (Fig. 3).
Figure 3.
Influence of the pH of the incubation medium on the relative influxes of l-Val, choline, and l-His. The substrate concentrations in the medium were: l-Val, 90 nm; choline, 200 nm; and l-His, 40 nm. Each bar represents the mean value ± se of at least three measurements. For each solute, bars with different letters were significantly different by the Tamhane's T2 test (P = 0.05) following ANOVA. FW, Fresh weight.
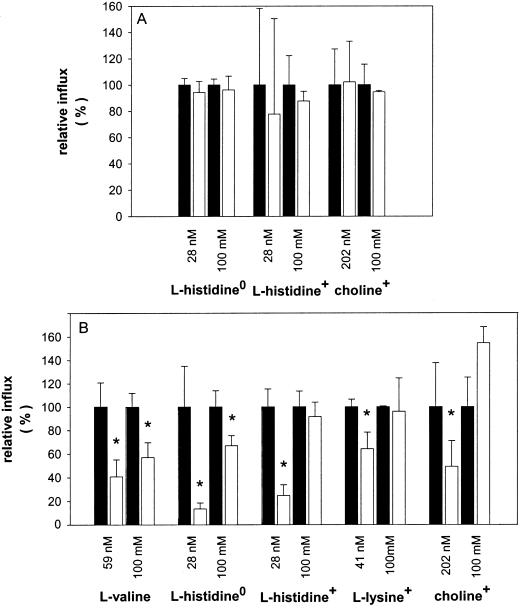
Effect of Carbonylcyanide m-Chlorophenylhydrazone (CCCP) and pCMBS
Previous work has shown that efflux of endogenous sugars and amino acids from pea seed coats, as well as the influx of exogenously supplied substrates, was not influenced by CCCP, whereas it could be partially inhibited by pCMBS (De Jong and Wolswinkel, 1995; De Jong et al., 1996, 1997). This is also true for the influx of choline and His. Addition of 10 μm CCCP had neither an effect on the uptake of choline+ nor on the uptake of neutral and cationic His (Fig. 4A). The sulfhydryl reagent pCMBS supplied at a concentration of 2.5 mm inhibited the influxes of Val, His0, His+, Lys+, and choline+ when the substrates were supplied at concentrations <1 μm. At high substrate concentrations (100 mm), pCMBS still inhibited the uptake of electrically neutral solutes, including His0, but had no effect on the influx of the organic cations choline+, His+, and Lys+ (Fig. 4B). The membrane potential of seed coat parenchyma cells was not affected by the presence of 2.5 mm pCMBS in the incubation solution (not shown).
Figure 4.
Effect of CCCP (A) and pCMBS (B) on the relative influxes (white bars) as a percentage of the relative uptake rates measured without CCCP or pCMBS in the medium (black bars). The substrates were supplied to the incubation medium at a sub-micromolar concentration and at 100 mm. Seed coat halves were pre-incubated for 15 min in incubation medium with 2.5 mm pCMBS, and then transferred to medium containing both substrate and pCMBS. Each bar represents the mean value ± se of at least three measurements. The asterisks indicate a significant decrease of the relative influx as compared to the control (without CCCP or pCMBS) at a 95% confidence interval as tested by the Student's t test.
Effect of Choline, His, and KCl on the Membrane Potential of Seed Coat Parenchyma Cells
From 13 impalements of seed coat parenchyma cells, an average membrane potential of −55 ± 3 mV was determined. In some experiments, dye injection by iontophoresis was used to ascertain the proper impalement of a seed coat parenchyma cell (not shown).
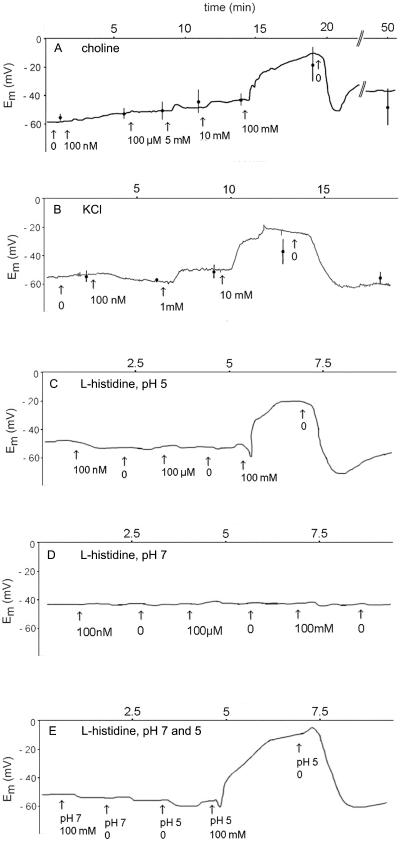
Alterations in the membrane potential at increasing concentrations of choline and His in the bathing solution were recorded using a perfusion system. Addition of choline+ (Fig. 5A), potassium chloride (Fig. 5B), or His+ (Fig. 5C) at concentrations above 1 mm depolarized the membrane. However, when using the pH 7 medium, His at a concentration as high as 100 mm did not result in depolarization (Fig. 5, D and E). Changing the pH of the bathing solution from 7 to 5 had no appreciable effect on the membrane potential (Fig. 5E).
Figure 5.
Effects of various concentrations of choline, potassium chloride, and l-His in the bathing solution on the membrane potential of seed coat parenchyma cells. A slice of seed coat tissue was mounted in a chamber through which various solutions were perfused. A, Perfusion of incubation medium, pH5.5, supplied with various concentrations of choline as indicated. B, Perfusion of incubation medium, pH5.5, supplied with various concentrations of KCl. C, Perfusion of incubation medium, pH 5, with various concentrations of l-His as indicated. D, Perfusion of incubation medium, pH7, supplied with various concentrations of l-His. E, Perfusion of incubation medium, pH7, or incubation medium, pH 5, with or without 100 mm l-His. All traces shown are from a single, representative experiment and the data points present the mean values ± se of at least five determinations.
DISCUSSION
Uptake Mechanism
Analysis of uptake versus time curves allowed the estimation of substrate concentrations in pea seed coat tissue at equilibrium. When supplied at a high concentration (100 mm) to the incubation medium, the equilibrium concentrations of choline, cationic His, and neutral His in the seed coat were determined at 44, 57, and 72 mm, respectively (Table I). Similar uptakes were measured for the neutral solutes Suc, mannitol, Glc, and l-Val (De Jong et al., 1996, 1997). All solutes tested attained, on average, a concentration of approximately 60 mm in the seed coat tissue when their concentration in the outer solution was 100 mm. This may indicate that the seed coat symplast is only partially accessible to exogenously supplied substrates. From the observation that the organic cations choline+ and His+, when supplied at high concentrations, attained approximately the same internal concentrations as neutral solutes it may be concluded that the membrane potential was zero. This is in line with the electrophysiological experiments showing membrane depolarization at high concentrations of choline+ or His+ (Fig. 5).
When choline was supplied at a sub-micromolar concentration, its concentration in the seed coat tissue at equilibrium was calculated to be 5.1- ± 0.8-fold higher than the external concentration (Table I). This accumulation is expected at a membrane potential of −41 ± 0.4 mV, which agrees reasonably with the measured membrane potential of −55 ± 3 mV.
The lower accumulation of His (2.4-fold) can be understood considering the difference in external and internal pH. At an external pH 5, His will be taken up in its cationic form that, on arrival in the symplast (pH is approximately 7), will be converted into the electroneutral zwitterion. As a consequence, the membrane potential will affect the influx, but not the efflux, of His.
Several models have been proposed that describe the relation between ion flux and membrane potential (Smith, 1973). According to the Goldman model (Goldman, 1943; Nobel, 1991; Sha et al., 1996) the rate constants for influx and efflux of a monovalent cation are given by:
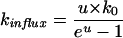 |
Eq. 4 |
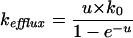 |
Eq. 5 |
in which u = EmF/RT (where Em is the membrane potential and F, R, and T have their usual meaning), and k0 is the rate constant for transport of the cation at zero membrane potential. The Kimizuka-Koketsu equation (Kimizuka and Koketsu, 1964; Sanders et al., 1984; Borst-Pauwels, 1993) alternatively has been used. This model predicts fluxes of monovalent cations to depend on the membrane potential according to:
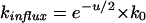 |
Eq. 6 |
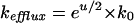 |
Eq. 7 |
For both models, the ratio of the internal and the external concentration of the ion at equilibrium is given by:
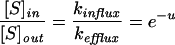 |
Eq. 8 |
which is equivalent to the Nernst equation. However, for His at an external pH 5, the accumulation ratio at equilibrium should be written as:
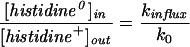 |
Eq. 9 |
which is equal to e−u/2 in the Kimizuka-Koketsu model and to u/(eu − 1) in the Goldman model. At the prevailing membrane potential of −55 mV, the predicted accumulation ratios for His are then 2.9 and 2.4, respectively, which compares favorably with the experimental value of 2.4 ± 0.3 (Table I).
Therefore, the extent to which choline+ and His+ were accumulated in seed coat tissue seems to be determined solely by the membrane potential. No indications were found that their transport is energized by ATP hydrolysis or by ion gradients. These substrates apparently are transported across the cell membrane of seed coat parenchyma cells by a uniport mechanism.
Influx Kinetics
The relative influx, v/[S], is a measure of the membrane permeability. If the specific plasma membrane area for the tissue is known, v/[S] can be recalculated into a permeability coefficient. The relative influx of choline+ and His+ decreased with increasing substrate concentrations, approaching a limiting value at approximately 100 mm (Fig. 2; Table II).
The concentration dependence of the influx conformed to Equation 3. It may be noted that the sigmoid part of Equation 3 is nothing other than the Michaelis-Menten equation in disguise. Two interpretations may apply. The substrates may be taken up by two independent transport routes, one showing saturation kinetics, the other a linear kinetic; alternatively, the cations may be taken up by an electrodiffusional mechanism. Thus the constant c in Equation 3 may represent the permeability of the cell membrane for the cation (at zero membrane potential), which is approached at high external substrate concentrations. The increase in relative influx at lower substrate concentrations may then result from an increasingly more negative membrane potential. This interpretation accounts for the observed accumulation of choline and His (Fig. 1) as well as for the depolarizing effect of high concentrations of choline and His+ (Fig. 5). In addition, it offers a simple explanation for the inhibiting effect of increasing K+ concentrations on the influx of choline+ (Fig. 2B).
The 2.4-fold increase in the relative influx of His+ when the membrane hyperpolarized from 0 to −55 mV is also in agreement with an electrodiffusional uptake mechanism because Equations 4 and 6 predict an increase of 2.4- and 2.9-fold, respectively. In contrast, the relative influx of choline+ increased about 7-fold (Table II), and that for Lys+ 5.7-fold (De Jong et al., 1997). We cannot offer a ready explanation for the different behavior of the three cations. It should be kept in mind that, though seemingly simple, the flow of ions through a membrane pore is a very complex phenomenon (Zambrowicz and Colombini, 1993).
The theory of the apparent saturation kinetics of non-mediated, passive permeation of cations is well documented (Smith, 1973; Borst-Pauwels, 1993), but it has received little attention from experimental workers. Kochian and Lucas (1982), for example, found the concentration dependence of K+ influx into maize roots to conform to a Michaelis-Menten term and a linear term, but did not discuss the effect of the increasing potassium concentration on the membrane potential.
A General, Poorly Selective Pore
An important result of the present work is that the plasma membrane of seed coat parenchyma cells has approximately the same permeabilities for small organic cations as for neutral organic solutes. At zero membrane potential, the “permeabilities” (expressed in μmol g−1 fresh weight min−1 m−1) for choline+, His+, and His0 were 9.6, 10.1, and 10.2, respectively (Table II), as compared with “permeabilities” for Suc (6.3), Glc (4.8), mannitol (5.1), and several neutral amino acids (8.7–15.3) determined in previous studies (De Jong et al., 1996, 1997). These results favor the hypothesis that the organic cations share the same transport route as the neutral solutes. This is supported by the observation that, like for neutral solutes, the influx of choline+ and His+ was not affected by the protonophore CCCP, whereas the organic mercurial pCMBS inhibited the influx partially, at least when the cationic substrates were supplied at concentrations <1 μm. This inhibition cannot be attributed to membrane depolarization because pCMBS did not alter the membrane potential (data not shown). The uptake of the cations added at 100 mm was not affected by pCMBS, possibly because the binding between the sulphydryl reagent and the protein is prevented at high ionic strength.
The finding that the plasma membrane of seed coat parenchyma cells has similar permeabilities for sugars, amino acids, and organic cations supports our hypothesis that seed coat unloading is accomplished by poorly selective pores in the plasma membrane. Extrapolation of the membrane permeability as calculated from uptake experiments to the in vivo unloading process is reasonable because the accumulation of the organic cations accords with the Nernst equation and its derivatives (Table I; Eqs. 8 and 9). The pathway enabling solutes to cross the seed coat parenchyma membrane therefore can be taken as bidirectional symmetric with the electrochemical gradient as the single determining factor for the direction of transport.
The interesting possibility comes up that even inorganic ions may be released from seed coat parenchyma cells through these pores. Potassium, the predominant cation in seed coats, is present in similar quantities as Suc, the predominant organic solute. It is significant that K+ and Suc are released from legume seed coats at comparable rates (Wolswinkel et al., 1992; Walker et al., 1995, 2000). Outward-rectifying K+ channels have been identified in seed coat parenchyma cells of Vicia faba, but it was concluded that their conductances were insufficient to explain the observed K+ effluxes (Zhang et al., 1997). Poorly selective pores therefore may constitute an additional route for efflux of K+ ions. Because the membrane of seed coat parenchyma cells depolarized at high salt concentrations (Fig. 5), these pores should have some degree of preference for cations over anions, and may be responsible, at least partially, for the moderate negative membrane potential (K+ diffusion potential) of seed coat parenchyma cells. Although an H+-ATPase (proton pump) is present in the plasma membrane of seed coat parenchyma cells (Harrington et al., 1997; De Jong and Borstlap, 2000), its contribution to the membrane potential is probably limited (Walker et al., 1995).
Regulation of Assimilate Efflux
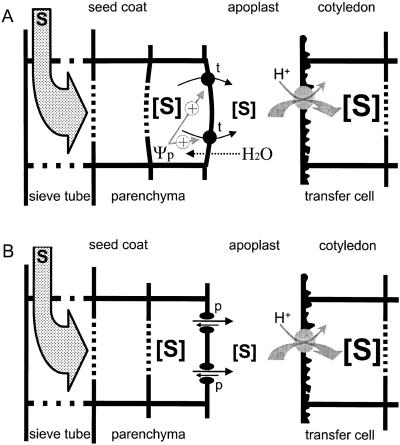
Patrick developed the “Turgor Homeostat” model (Fig. 6A) for the regulation of assimilate efflux from the seed coat (Patrick, 1994; for review, see Patrick and Offler, 1995). Uptake of assimilates by the embryo in situ will result in an immediate decrease in the osmolarity of the apoplastic solution. As a consequence, the seed coat parenchyma cells will take up water from the apoplast and cell turgor will increase. According to the “Turgor Homeostat” model, an increase in turgor that exceeds a certain set point value will emit an “error signal” resulting in an increase of assimilate efflux.
Figure 6.
Models for the regulation of the efflux of assimilates from seed coat parenchyma cells of legume seeds. Assimilates (S) arrive in the seed coat by way of sieve tubes in the vascular strands, and are finally taken up by the embryo. Uptake of the main assimilates (Suc and amino acids) by cotyledonary cells, in particular the transfer cells constituting the epidermis, is envisaged to occur by proton symporters, though it may be effected partly by a transport pathway with a linear kinetic (Lanfermeijer et al., 1990, 1991). The models apply to the seed filling stage, where the fluid endosperm has disappeared, and Suc released from the seed coat cells is not hydrolyzed by cell wall invertase (Weber et al., 1997). A, The Turgor Homeostat model (Patrick, 1994, 1997; Patrick and Offler, 1995). A decrease in the solute concentration in the apoplast will lead to an increase in cell turgor (Ψp), which in turn increases the activity of a transporter (t) responsible for the efflux of assimilates. Thus, the efflux of assimilates is thought to be indirectly controlled by the total concentration of solutes in the apoplast via a hypothetical turgor homeostat operating in the seed coat parenchyma cells. Turgor could affect the activity of the transporter directly or by a signaling cascade. B, The Supply follows Demand model. The efflux of assimilates from seed coat parenchyma cells occurs through poorly selective pores (p) in the plasma membrane. Transport of substrates through these pores has a diffusional kinetic. For any substrate the net efflux will be proportional to the concentration gradient across the plasma membrane of the seed coat parenchyma cells. In the quasi-steady state, the net efflux of assimilates from the seed coat parenchyma cells will be equal to their net influx into the cotyledonary cells. A decrease in substrate concentration in the apoplast will enhance its net efflux from the seed coat parenchyma cells as dictated by the law of mass action. Hence, the supply (net efflux) of any nutrient by the seed coat parenchyma cells will be determined by the demand (net influx) of the cotyledonary cells.
If nutrients are released from seed coat parenchyma cells by poorly selective pores, their uptake from the apoplast by the embryo will spontaneously result in an increased efflux from the seed coat. The net efflux of any nutrient will then simply be “controlled” by the rate at which it is subsequently taken up by the embryo. Hence, a “Supply follows Demand” model would apply for the regulation of assimilate efflux from the seed coat (Fig. 6B). The two models need not be exclusive, as it could be envisaged that the poorly selective pores are responsive to cell turgor. In this respect, it is noteworthy that an increase in cell turgor enhances the Suc efflux as well as the efflux of K+ ions from seed coats (Wolswinkel et al., 1992; Walker et al., 1995), suggesting that these solutes are released from seed coat parenchyma cells by the same turgor-responsive pore.
MATERIALS AND METHODS
Plant Material
Pea (Pisum sativum L. cv Marzia; Nunhems Zaden bv, Haelen, The Netherlands) plants were grown according to De Jong and Wolswinkel (1995), except that no flowers were removed during development. At the end of the seed filling stage, when embryos had a relative water content of about 60% (w/w), seeds were removed from the pod and seed coat halves were isolated according to De Jong et al. (1996).
Uptake Experiments
Uptake experiments were carried out as described by De Jong et al. (1996). Ten isolated seed coat halves were incubated in 5 mL of incubation medium {0.5 mm CaCl2 and 2 mm MES (2-[N-morpholino]-ethanesulfonic acid) adjusted to pH 5.5 (unless stated otherwise) with KOHl and as much mannitol as needed to give all incubation media an overall solute concentration of 400 mm} supplied with unlabeled substrate at the concentrations indicated, together with approximately 370 Bq mL−1 14C-labeled substrate. Specific activities were as follows: l-His, 12.1 GBq mmol−1; choline, 2.0 GBq mmol−1; l-Lys, 11.2 GBq mmol−1; and l-Val, 10.5 GBq mmol−1. CCCP was used at a concentration of 10 μm. In experiments with pCMBS, seed coat halves were preincubated for 15 min in the presence of 2.5 mm pCMBS and subsequently transferred to incubation medium containing the labeled substrate and 2.5 mm pCMBS. Incubation of the seed coats took place in a reciprocating water bath at 25°C.
Uptake was terminated by decanting the incubation solution followed by rinsing the seed coats three times with demineralized water and carefully blotting the seed coats with a soft tissue to remove adhering water. This took about 1 min. Radioactivity taken up by the seed coat halves was determined by scintillation counting after destruction of the tissue in 1 mL of a 1:1 mixture of 50% (v/v) H2O2 and 30% (v/v) HClO4. Initial influxes were calculated from the uptake between 5 and 20 min of incubation and expressed on a fresh weight basis.
Substrate concentrations in the seed coat parenchyma cells were estimated by assuming that the water content of a seed coat is 80% (w/w; Lanfermeijer et al., 1992). Curve fitting was carried out using SigmaPlot-4.0 (Jandel Scientific, San Rafael, CA). Reciprocals of the variance were used as weighting factors.
Electrophysiology and Iontophoresis
Membrane potentials of seed coat parenchyma cells were recorded in various bathing solutions using a gravity driven perfusion system (Blatt, 1991). Perfusate was drained from the perfusion chamber into a waste beaker via a cotton thread. The perfusion chamber was placed under a binocular (SV8, Zeiss, Oberkochen, Germany). A tangential slice of 2- to 3-mm thickness was cut from the lateral side of a seed of which the embryo had a relative water content of about 60% (w/w). This slice was fixed to the bottom of the perfusion chamber with a little droplet of Secure Adhesive (B-400, Factor II Inc., Lakeside, AZ). After perfusing the incubation medium for 0.5 h, the remaining slice of the cotyledon was removed from the seed coat.
Glass microelectrodes were made from borosilicate capillaries with an inner filament (GC100TF-10, Clark Electromedical Instruments, Reading, UK) using a vertical electrode puller (P-30, Sutter Instruments Inc., Novato, CA). Electrodes were backfilled with 1 m KCl and clamped in a microelectrode holder-Ag/AgCl-halfcell (MEH1SF, WPI Inc., Sarasota, FL). Only electrodes with a resistance of about 20 MΩ were used. The electrode was connected to a high-input impedance amplifier (610C, Keithley, Cleveland; or an Intra 767 electrometer, WPI Inc.). The output was continuously recorded with a chart recorder (B10 8 multi-range; Kipp and Zonen, Delft, The Netherlands). A reference electrode (DRIREF-2, WPI Inc.) was placed in the medium at the inlet side of the perfusion chamber. The measurement electrode was impaled into a seed coat parenchyma cell with a micromanipulator (MMO-204, Narishige, Tokyo). Perfusion solutions were changed when stable membrane potentials were recorded.
Dye injection was used to check whether the measured electrical potentials were real transmembrane potential differences. Iontophoretic injections were performed under an epifluorescence microscope (Olympus BH2-RFL equipped with a dichroic mirror [U {DM-400 + l-240}], an excitation filter combination [IF49 + EY 455], and a barrier filter [Y 495], Olympus Optical Co. Europa, Hamburg, Germany) with hand-cut tangential slices of pea seed coat tissue in incubation medium. The tip of a glass electrode was backfilled with about 5 nL of 1% (w/v) Lucifer Yellow CH and potassium salt (Molecular Probes Inc., Eugene, OR). The rest of the electrode was filled with 3 m KCl. The electrical resistance of the electrodes used was between 30 and 50 MΩ. The electrode was impaled in a seed coat parenchyma cell and after recording a stable potential, Lucifer Yellow CH was ejected using a Dagan 2400 extra cellular preamplifier current pump system (Dagan Corp., Minneapolis). The intermittent current pulse of −15 nA lasted no more than 10 s.
ACKNOWLEDGMENTS
The authors wish to thank Judith Koerselman-Kooij for technical assistance. We are also grateful to Dr. Jurriaan Ton and Dr. Frank Bretschneider for valuable advice about statistics and electrophysiological measurements, respectively. Finally, we would like to acknowledge Dr. John Patrick and Dr. Alan Walker for stimulating discussions.
LITERATURE CITED
- Blatt MR. A primer in plant electrophysiological methods. In: Hostettmann K, editor. Methods in Plant Biochemistry. 6, Assays for Bioactivity. San Diego: Academic Press; 1991. pp. 281–321. [Google Scholar]
- Borst-Pauwels GWFH. Mutual interaction of ion uptake and membrane potential. Biochim Biophys Acta. 1993;1145:15–24. doi: 10.1016/0005-2736(93)90376-b. [DOI] [PubMed] [Google Scholar]
- De Jong A, Borstlap AC. A plasma membrane-enriched fraction isolated from the coats of developing pea seeds contains H+-symporters for amino acids and sucrose. J Exp Bot. 2000;51:1671–1677. doi: 10.1093/jexbot/51.351.1671. [DOI] [PubMed] [Google Scholar]
- De Jong A, Koerselman-Kooij JW, Schuurmans JAMJ, Borstlap AC. Characterization of the uptake of sucrose and glucose by isolated seed coat halves of developing pea seeds: evidence that a sugar facilitator with diffusional kinetics is involved in seed coat unloading. Planta. 1996;199:486–492. [Google Scholar]
- De Jong A, Koerselman-Kooij JW, Schuurmans JAMJ, Borstlap AC. The mechanism of amino acid efflux from seed coats of developing pea seeds as revealed by uptake experiments. Plant Physiol. 1997;114:731–736. doi: 10.1104/pp.114.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong A, Wolswinkel P. Differences in release of endogenous sugars and amino acids from attached and detached seed coats of developing pea seeds. Physiol Plant. 1995;94:78–86. [Google Scholar]
- Goldman DE. Potential, impedance, and rectification in membranes. J Gen Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusak MA, Minchin PEH. Seed coat unloading in Pisum sativum: osmotic effects in attached versus excised empty ovules. J Exp Bot. 1988;39:543–559. [Google Scholar]
- Harrington GN, Franceschi VR, Offler CE, Patrick JW, Tegeder M, Frommer WB, Harper JF, Hitz WD. Cell specific expression of three genes involved in plasma membrane sucrose transport in developing Vicia faba seed. Protoplasma. 1997;197:160–173. [Google Scholar]
- Kimizuka H, Koketsu K. Ion transport through cell membrane. J Theor Biol. 1964;6:290–305. doi: 10.1016/0022-5193(64)90035-9. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. Potassium transport in corn roots: I. Resolution of kinetics into a saturable and linear component. Plant Physiol. 1982;70:1723–1731. doi: 10.1104/pp.70.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfermeijer FC, Koerselman-Kooij JW, Borstlap AC. Changing kinetics of L -valine uptake by immature pea cotyledons during development: an unsaturable pathway is supplemented by a saturable system. Planta. 1990;181:576–582. doi: 10.1007/BF00193013. [DOI] [PubMed] [Google Scholar]
- Lanfermeijer FC, Koerselman-Kooij JW, Borstlap AC. Osmosensitivity of sucrose uptake by immature pea cotyledons disappears during development. Plant Physiol. 1991;95:832–838. doi: 10.1104/pp.95.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfermeijer FC, van Oene MA, Borstlap AC. Compartmental analysis of amino-acid release from attached and detached pea seed coats. Planta. 1992;187:75–82. doi: 10.1007/BF00201626. [DOI] [PubMed] [Google Scholar]
- Nobel PS. Physicochemical and Environmental Plant Physiology. San Diego: Academic Press; 1991. pp. 120–134. [Google Scholar]
- Patrick JW. Turgor-dependent unloading of photosynthates from coats of developing seed of Phaseolus vulgaris L. and Vicia faba L.: turgor homeostasis and set points. Physiol Plant. 1994;90:367–377. [Google Scholar]
- Patrick JW. Phloem unloading: sieve element unloading and post-sieve element transport. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:191–222. doi: 10.1146/annurev.arplant.48.1.191. [DOI] [PubMed] [Google Scholar]
- Patrick JW, Offler CE. Post-sieve element transport of sucrose in developing seeds. Aust J Plant Physiol. 1995;22:681–702. [Google Scholar]
- Patrick JW, Offler CE, Wang XD. Cellular pathway of photosynthate transport in coats of developing seed of Vicia faba L. and Phaseolus vulgaris L.: I. Extent of transport through the coat symplast. J Exp Bot. 1995;46:35–47. [Google Scholar]
- Sanders D, Hansen UP, Gradmann D, Slayman CL. Generalized kinetic analysis of ion-driven cotransport systems: a unified interpretation of selective ionic effects on Michaelis parameters. J Membr Biol. 1984;77:123–152. doi: 10.1007/BF01925862. [DOI] [PubMed] [Google Scholar]
- Segel IH. Biochemical Calculations. New York: John Wiley & Sons, Inc.; 1968. p. 348. [Google Scholar]
- Sha Q, Romano C, Lopatin AN, Nichols CG. Spermidine release from Xenopus oocytes: electrodiffusion through a membrane channel. J Biol Chem. 1996;271:3392–3397. doi: 10.1074/jbc.271.7.3392. [DOI] [PubMed] [Google Scholar]
- Smith PG. Dependence of ion flux across a membrane on ionic concentration. J Theor Biol. 1973;41:269–286. doi: 10.1016/0022-5193(73)90119-7. [DOI] [PubMed] [Google Scholar]
- Tegeder M, Wang XD, Frommer WB, Offler CE, Patrick JW. Sucrose transport into developing seeds of Pisum sativum L. Plant J. 1999;18:151–161. doi: 10.1046/j.1365-313x.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- Walker NA, Patrick JW, Zhang WH, Fieuw S. Efflux of photosynthate and acid from developing seed coats of Phaseolus vulgaris L.: a chemiosmotic analysis of pump-driven efflux. J Exp Bot. 1995;46:539–549. [Google Scholar]
- Walker NA, Zhang WH, Harrington G, Holdaway N, Patrick JW. Effluxes of solutes from developing seed coats of Phaseolus vulgaris L. and Vicia faba L.: locating the effect of turgor in a coupled chemiosmotic system. J Exp Bot. 2000;51:1047–1055. doi: 10.1093/jexbot/51.347.1047. [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. Sugar import and metabolism during seed development. Trends Plant Sci. 1997;2:169–174. [Google Scholar]
- Wolswinkel P, Ammerlaan A, Koerselman-Kooij J. Effect of the osmotic environment on K+ and Mg2+ release from the seed coat and cotyledons of developing seeds of Vicia faba and Pisum sativum: evidence for a stimulation of efflux from the vacuole at high cell turgor. J Exp Bot. 1992;43:681–693. [Google Scholar]
- Zambrowicz EB, Colombini M. Zero-current potentials in a large membrane channel: a simple theory accounts for complex behavior. Biophys J. 1993;65:1093–1100. doi: 10.1016/S0006-3495(93)81148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Walker NA, Tyerman SD, Patrick JW. Mechanism of solute efflux from seed coats: whole-cell K+ currents in transfer cell protoplasts derived from coats of developing seeds of Vicia faba L. J Exp Bot. 1997;48:1565–1572. [Google Scholar]