Abstract
Boron (B) deficiency results in inhibition of pumpkin (Cucurbia moschata Duchesne) growth that is accompanied by swelling of the cell walls. Monomeric rhamnogalacturonan II (mRG-II) accounted for 80% to 90% of the total RG-II in B-deficient walls, whereas the borate ester cross-linked RG-II dimer (dRG-II-B) accounted for more than 80% of the RG-II in control plants. The results of glycosyl residue and glycosyl linkage composition analyses of the RG-II from control and B-deficient plants were similar. Thus, B deficiency does not alter the primary structure of RG-II. The addition of 10B-enriched boric acid to B-deficient plants resulted within 5 h in the conversion of mRG-II to dRG-II-10B. The wall thickness of the 10B-treated plants and control plants was similar. The formation and possible functions of a borate ester cross-linked RG-II in the cell walls are discussed.
The results of numerous studies have shown that boron (B) deficiency results in changes in cell wall structure, e.g. swelling of the cell walls and the formation of small, irregularly shaped cells (Brown and Hu, 1997; Matoh, 1997). Cell wall swelling has also been reported to be accompanied by changes in the appearance of the middle lamella (Kouchi and Kumazawa, 1976). Inappropriate swelling of the cell wall also occurs in the irregular xylem mutant Arabidopsis, irx4 (Turner and Somerville, 1997). However, the effects of B on the macromolecular organization of the cell wall are poorly understood.
The recent discovery that most of the B in the wall is present as a borate diol diester that cross-links two chains of rhamnogalacturonan II (RG-II) has led to the suggestion that a physiologically important function of B is to cross-link cell wall pectin (Ishii and Matsunaga, 1996; Kobayashi et al., 1996; O'Neill et al., 1996). Such a cross-linked pectic network is likely to have a role in regulating the mechanical and biochemical properties of the wall (Fleischer et al., 1999). Nevertheless, there is only limited evidence on cultured cells showing that borate ester cross-linking of pectin is required for the normal growth and development of plants (Fleischer et al., 1999; Matoh et al., 2000).
We now provide evidence to show that the wall of B-deficient pumpkin (Cucurbia moschata Duchesne, cv Tokyo-Kabocha) plants is swollen compared with the tissue of B-sufficient plants. Adding boric acid to B-deficient plants results in the rapid formation of the borate ester cross-linked RG-II dimer (dRG-II-B) and decrease in wall thickness.
RESULTS
The B Contents of Leaves from Pumpkin Plants Grown in the Presence or Absence of Boric Acid
Pumpkin seeds were germinated in vermiculite. The seedlings were then grown for 1 week on Hoagland liquid medium containing 10 μm boric acid. The plants were then transferred to a B-deficient Hoagland medium with or without 25 μm boric acid and grown for 7 d. The second, third, and fourth leaves were smaller than the control due to the B deficiency (Table I). The second, third, and fourth leaves from B-deficient plants contained between 2.6 and 4.2 μg B g−1 dry weight, whereas the corresponding leaves in control plants contained 32 to 39 μg B g−1 dry weight. At least 90% of the B from the B-deficient leaves (second to fourth) was present in the AIR. In contrast, only 28% to 47% of the B in control leaves (second to fourth) was present in the AIR (Table I). These results confirm that all B is localized in cell walls of squash and tobacco under the B-deficient conditions (Hu and Brown, 1994). The B present in the cotyledons of the plants prior to their transfer to B-deficient medium is not available to newly forming leaves. Such a result is consistent with the notion that B must be supplied continuously during plant growth (Skok, 1957; Loomis and Durst, 1992; Hu and Brown, 1994).
Table I.
Pumpkin growth, distribution of B, and dRG-II-B ratio in pumpkin plants
| Treatment | Leaf No. | Fresh Leaf Wt | Leaf B | AIR Ba | dRG-II-B in Total RG-IIb |
|---|---|---|---|---|---|
| mg | μg g−1 dry leaf | % | |||
| 25 μm B | Cotyledon | 660 ± 38 | 99.3 ± 5.2 | 32.9 ± 2.4 | 85 |
| First leaf | 858 ± 62 | 69.5 ± 1.3 | 19.7 ± 1.7 | 82 | |
| Second leaf | 546 ± 43 | 39.3 ± 2.7 | 11.0 ± 0.6 | 87 | |
| Third leaf | 241 ± 44 | 32.5 ± 2.2 | 15.3 ± 1.9 | 86 | |
| Fourth leaf | 26 ± 4 | 32.0 ± 5.0 | 14.7 ± 4.2 | 82 | |
| 0 μm B | Cotyledon | 751 ± 40 | 92.4 ± 6.1 | 41.3 ± 1.8 | 93 |
| First leaf | 530 ± 50 | 21.5 ± 1.2 | 19.1 ± 4.9 | 88 | |
| Second leaf | 216 ± 30 | 4.2 ± 1.6 | 3.8 ± 0.1 | 35 | |
| Third leaf | 131 ± 20 | 3.0 ± 1.0 | 3.0 ± 0.1 | 10 | |
| Fourth leaf | 37 ± 14 | 2.6 ± 0.3 | 2.7 ± 0.1 | 20 | |
Plants were precultured for 7 d in 10 μm B. Seven days prior to harvest, one-half of the plants were transferred to B-free solution and the remaining one-half were transferred to 25 μm B. Fresh leaf wt was expressed as means ± se (n = 8). Alcohol-insoluble residue (AIR) were isolated from each leaf (details in “Materials and Methods”). B was determined in the leaf and AIR samples by inductively coupled plasma-mass spectrometry (ICP-MS). Data are means of three replicates and se are shown.
AIR B was calculated from the B content in AIR determined by ICP-MS and the yield of AIR.
The relative proportion of dRG-II-B to total RG-II was determined by size exclusion chromatography (SEC)/refractive index detector of the endo-polygalacturonase (EPG) digests of the AIR. Data are average from two individual experiments.
Anatomical Observation
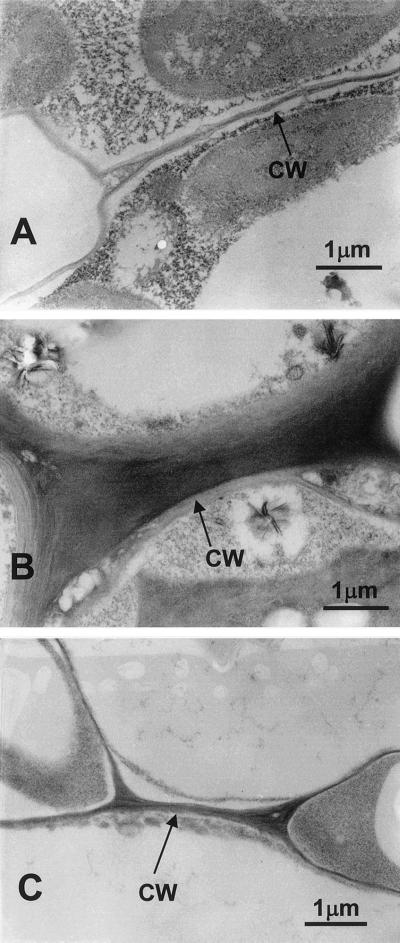
Light microscopic analysis indicated that the cells of the B-deficient second leaf and petiole are smaller and more irregular than their B-sufficient counterparts. The B-deficient cells were shorter by 70% and 20% in the longitudinal and transverse directions, respectively, than those supplied with B. Transmission electron microscope examination showed that the B-deficient cell walls were swollen compared with the thin-walled normal cell (Fig. 1, A and B). When the B-deficient plants were treated for 5 h with boric acid, the thickness of their cell walls and control plants were comparable (Fig. 1, A and C). In general, the cell wall of B-defecient plants had disorganized middle lamellae. A similar phenomenon was observed of B-deficient root tip of Lycopersicon escurentum (Kouchi and Kumazawa, 1976).
Figure 1.
Transmission electron microscope of the second leaf from pumpkin grown in B-normal, B-deficient, and B-treated conditions. A, B-normal leaf; B, B-deficient leaf; C, B-deficient and then B-treated for 5 h leaf. Scale bar is 1 μm. The mid-lamellae region is easily distinguishable as the very dark staining region in the center of the walls.
Characterization of the RG-II from AIR of B-Deficient, B-Grown, and 10B-Treated Pumpkin
We have shown that B-deficient pumpkin petioles and leaves have swollen cell walls when compared with control plants. The addition of boric acid to the B-deficient plants results in the appearance of cells whose wall thickness is comparable to that of control plants. We now show that this morphological change is correlated with increase in the proportion of dRG-II-B in the walls.
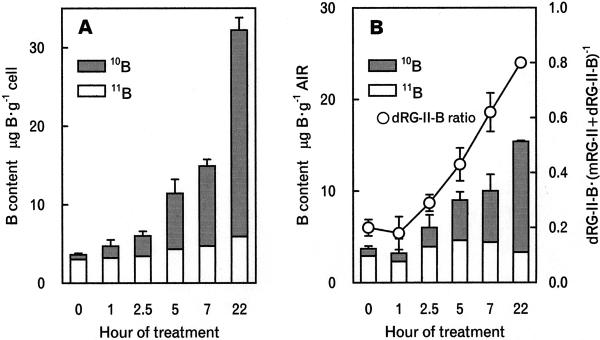
When B-deficient pumpkin plants were transferred to 10B-enriched boric acid medium, the 10B content and abundance in the third leaf and the binding of 10B to the cell wall gradually increased (Fig. 2). The 10B abundance in the leaves increased to 0.6 after 5-h exposure to the 10B boric acid-containing medium.
Figure 2.
The binding of B to B-deficient third leaves and formation of 10B-containing dRG-II-B after the addition of 10B-enriched boric acid. At the indicated times the third leaves were harvested (details in “Materials and Methods”). B content in the leaves and AIR was determined by ICP-MS. The ratio of dRG-II-B and monomeric RG-II (mRG-II) was determined by SEC/refractive index detector. A, B content in the leaf. B, B content in AIR, which was calculated from B content determined by ICP-MS and the yield of AIR. Ratio of dRG-II-B and mRG-II was determined by SEC/refractive index detector. Data are means of three replicates and se are shown.
The AIR prepared from the third leaf of B-deficient, B-normal, and 10B-treated for 22 h pumpkin contained 0.3, 1.4, and 1.5 μ 77 B g−1, respectively (Table II). The AIR from these plants had similar glycosyl residue compositions and contained similar amounts of RG-II (Table II).
Table II.
Glycosyl residue compositions and RG-II and B contents of AIR isolated from pumpkin grown in the presence or absence of boric acid and 10B-treated pumpkin
| Glycosyl Residue | B Normala | B Deficientb | 10B Treatedc |
|---|---|---|---|
| mol % | |||
| Rha | 2 | 2 | 2 |
| Fuc | 1 | 1 | 1 |
| Ara | 9 | 11 | 10 |
| Xyl | 9 | 12 | 8 |
| Man | 4 | 5 | 6 |
| Gal | 13 | 12 | 11 |
| Glc | 40 | 37 | 43 |
| GalA | 22 | 21 | 19 |
| μm g−1 dry wt AIR | |||
| mRG-IId | 0.2 | 2.9 | 0.2 |
| dRG-II-Bd | 1.4 | 0.2 | 1.4 |
| Be | 1.4 | 0.3 | 1.5 |
AIR was isolated from the third leaf. The values given are for those glycosyl residue that accounted for >1 mol % of the leaves of plants. The glycosyl residues that are diagnostic of RG-II (2-O-Me Fuc, 2-O-Me Xyl, and apiose) each accounted for <0.5 mol % of the AIR.
The AIR from pumpkin grown in the presence of B boric acid (25 μm).
The AIR from pumpkin grown in the absence of boric acid.
AIR from B-deficient pumpkin grown for 22 h in the presence of 10B-enriched boric acid (25 μm).
The RG-II content was estimated from the 2-O-Me Fuc and 2-O-Me Xyl contents of the AIR. The relative proportions of dRG-II-B and mRG-II were determined by SEC/refractive index detection (RI) of the EPG digests of the AIR and these values were used to estimate the mRG-II and dRG-II-B contents using molecular masses of 5 and 10 kD, respectively.
The B content of the AIR was determined by ICP-MS.
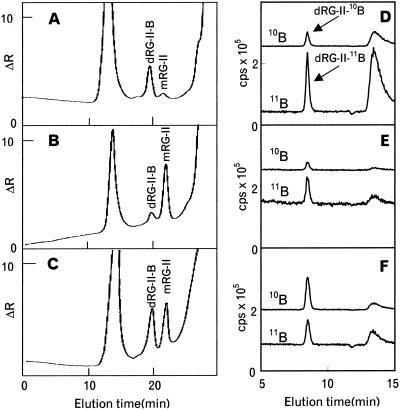
The AIR from the cotyledons and the leaves of B-deficient and B-normal pumpkin was saponified and then treated with EPG. The ratio of dRG-II-B and mRG-II in the EPG-soluble material then was determined by SEC with RI. The RG-II dimer accounted for >80% of the total RG-II in the cotyledon and the first to fourth leaves of the control plant (Fig. 3A; Table I). In contrast, mRG-II accounted for between 80% and 90% of the RG-II in the third and fourth leaves of the B-deficient pumpkin (Fig. 3B; Table I). The proportion of mRG-II decreased to 65%, 12%, and 7% in the second leaf, the first leaf, and the cotyledon, respectively. This result was expected because the cotyledon and first leaf were formed during the period when the plant was grown in the presence of B. Taken together, these results establish that in the B-deficient tissues RG-II is present mainly as a monomer and that dRG-II-B is predominant under the B-sufficient conditions.
Figure 3.
SEC/RI and SEC/ICP-MS of the EPG-soluble material from the AIR of pumpkin grown in the presence or absence of boric acid and treated with 10B-enriched boric acid. AIR was prepared from the third leaf. A, SEC/RI profile of the AIR from plants grown in the presence of boric acid (25 μm). B, SEC/RI profile of the AIR from plants grown in the absence of boric acid. C, SEC/RI profile of the AIR from growing B-deficient plants that had been treated for 7 h with 10B-enriched boric acid (25 μm). The chromatograms A, B, and C were obtained with the Superdex-75 column. The Superdex-75 column was calibrated with sugar beet dRG-II-B (approximately 9.4 kD) and sugar beet mRG-II (approximately 4.7 kD), which have retention times at 21.0 and 23.2 min, respectively. Pullulans of 23.7 and 5.8 kD eluted at 15.8 and 22.4 min, respectively. The inner volume of the column using Glc was 31.8 min. D through F, the 10B and 11B profiles obtained by SEC/ICP-MS analysis of the 11B-normal, B-deficient, and 10B-treated pumpkin AIR, respectively. The chromatograms D through F, were obtained with the Diol–120 column (details in “Materials and Methods”). dRG-II-B and boric acid were eluted at 8.1 and 13.2 min, respectively. A large peak of boric acid in chromatogram D was due to the boric acid contaminant in the enzyme buffer.
dRG-II-B and mRG-II were isolated from the B-grown pumpkin AIR, and mRG-II was isolated from the B-deficient plant. The glycosyl composition and glycosyl linkage analyses of these RG-IIs were similar (Tables III and IV). The apiosyl residues in mRG-II are 3′-linked, whereas in dRG-II-B they are 3′- and 2, 3, 3′-linked (Table IV), confirming that 3′-linked apiosyl residues are the site of borate esterification (O'Neill et al., 1996; Ishii et al., 1999). The mRG-II from the B-deficient plants was converted to dRG-II-B in vitro by treatment with boric acid and lead acetate (O'Neill et al., 1996), showing that all of the mRG-II synthesized by these plants is capable of forming a dimer.
Table III.
Glycosyl residue compositions of the mRG-II and dRG-II-B isolated from pumpkin leaf in the presence or absence of boric acid and 10B-treated pumpkin
| Glycosyl Residue | B Normala
|
B-Deficientb mRG-IId |
10B Treatedc
|
||
|---|---|---|---|---|---|
| dRG-II-Bd | mRG-IId | dRG-II-Bd | mRG-IId | ||
| 2-Me Fuc | 3 | 3 | 1 | 2 | 2 |
| Rha | 7 | 6 | 12 | 7 | 5 |
| Fuc | 2 | 2 | 2 | 2 | 1 |
| 2-Me Xyl | 4 | 3 | 2 | 2 | 2 |
| Ara | 9 | 12 | 12 | 12 | 14 |
| Api | 5 | 6 | 7 | 8 | 8 |
| AcerA | 3 | 3 | 4 | 4 | 4 |
| Gal | 10 | 9 | 11 | 12 | 13 |
| GalA | 43 | 38 | 39 | 35 | 40 |
| GlcA | 5 | 5 | 3 | 4 | 2 |
| Kdo | 4 | 8 | 4 | 5 | 4 |
| Dha | 5 | 7 | 3 | 7 | 5 |
| 10B/(10B +11B)e | 0.19 | –f | –f | 0.73 | –f |
AIR was isolated from the second to the fourth leaves. RG-II was isolated by SEC from EPG digests of the AIR of pumpkin leaf grown in the presence (25 μm) or absence of boric acid and 10B-treated leaf. The neutral and acidic glycosyl residues were determined by gas-liquid chromatography analysis of alditol acetates and trimethyl silyl derivatives, respectively.
Pumpkin grown in the presence of B boric acid (25 μm).
Pumpkin grown in the absence of boric acid.
B-deficient pumpkins that were grown for 16 h in the presence of 10B-enriched boric acid (25 μm).
dRG-II-B and mRG-II were isolated from the EPG digest of the AIR by SEC.
The relative abundance of 11B and 10B in dRG-II-B were determined by SEC/ICP-MS analysis of the EPG digest of the AIR.
mRG-II contains no detectable amounts of B.
Table IV.
Glycosyl linkage composition of the mRG-II and dRG-II-B isolated from pumpkin grown in the presence or absence of boric acid and 10B-treated pumpkin
| Glycosyl Linkage | B Normala
|
B-Deficientb mRG-IId |
10B Treatedc
|
||
|---|---|---|---|---|---|
| dRG-II-Bd | mRG-IId | dRG-II-Bd | mRG-IId | ||
| T-Rha | 3 | 4 | 2 | 5 | 4 |
| 2-Rha | 1 | 2 | 7 | 1 | 2 |
| 3-Rha | 5 | 5 | 3 | 5 | 5 |
| 2, 4-Rha | – | – | 1 | – | – |
| 2, 3, 4-Rha | 7 | 6 | 8 | 4 | 4 |
| T-Fuc | 3 | 4 | 3 | 4 | 3 |
| 3, 4-Fuc | 5 | 5 | 4 | 5 | 4 |
| T-Araf | 7 | 7 | 6 | 7 | 10 |
| T-Arap | 3 | 4 | 2 | 5 | 5 |
| 2-Arap | 1 | 1 | 1 | 1 | 1 |
| T-Xyl | 5 | 6 | 5 | 6 | 5 |
| 3′-Api | 7 | 10 | 10 | 8 | 12 |
| 2, 3, 3′-Api | 5 | – | – | 6 | – |
| T-Gal | 5 | 5 | 5 | 6 | 6 |
| 2, 4-Gal | 4 | 4 | 3 | 4 | 5 |
| 2-GlcA | 5 | 7 | 4 | 6 | 5 |
| T-GalA | 13 | 13 | 12 | 7 | 8 |
| 4-GalA | 5 | 6 | 9 | 8 | 8 |
| 3, 4-GalA | 4 | 3 | 4 | 4 | 4 |
| 2, 4-GalA | 5 | 3 | 4 | 4 | 4 |
| 2, 3, 4-GalA | 8 | 5 | 7 | 4 | 5 |
AIR was isolated from the second to the fourth leaves. RG-II was isolated from EPG digest of the AIR of pumpkin leaf grown in the presence (25 μm) or absence of boric acid and 10B-treated leaf.
Pumpkin grown in the presence of B boric acid (25 μm).
Pumpkin grown in the absence of boric acid.
B-deficient pumpkins that were grown for 16 h in the presence of 10B-enriched boric acid (25 μm).
dRG-II-B and mRG-II were isolated from the EPG digest of the AIR by SEC.
To establish that adding 10B-enriched boric acid to B-deficient plants results in the formation of dRG-II-10B in muro, the AIR treated with 10B-enriched boric acid was digested with EPG and the solubilized material analyzed by SEC/RI and by SEC in combination with ICP-MS. SEC/ICP-MS analysis showed that the dRG-II-B isolated from 10B-enriched boric acid-treated plants was enriched with 10B (10B abundance = 0.6, Fig. 3F), whereas the dRG-II-B from pumpkins grown in the presence of 11B boric acid contained B in the expected natural abundance ratio (10B abundance = 0.2, Fig. 3D). dRG-II-B accounted for approximately 80% of the total RG-II in 10B-enriched boric acid-treated for 22 h plants (Fig. 2B). dRG-II-10B and mRG-II isolated from the 10B-treated for 16 h pumpkin and the RG-II from plants grown continuously in the presence of B have similar glycosyl residue and glycosl linkage compositions (Tables III and IV). These results confirm that normal RG-II is synthesized under B-deficient conditions and that the mRG-II is able to form dRG-II-B in the B-sufficient conditions in muro.
DISCUSSION
mRG-II in B-Deficient Plants Has Similar Glycosyl Residue Composition with dRG-II-B in B-Normal Plants
We have shown that pumpkin cell walls contain similar amounts of RG-II, irrespective of whether they are grown in the presence or absence of B. The RG-IIs isolated from the B-deficient, B-normal, and 10B-treated pumpkin were structurally similar. However, the relative proportions of dRG-II-B and mRG-II are correlated with the amount of water-insoluble wall B. We conclude that in muro B deficiency results in a significant reduction in the borate ester cross-linking of RG-II.
Our results are consistent with the notion that a primary function of B is as a structural component of the cell wall (Loomis and Durst, 1992; Brown and Hu, 1997; Matoh, 1997; Fleischer et al., 1999). Our data confirm that B-deficient cells and cells grown in the presence of B have walls with similar glycosyl residue compositions (Goldbach and Amberger, 1986; Fleischer et al., 1999).
The Relationship between Cell Wall Physical Properties and the dRG-II-B Formation
The primary wall of dicots and non-graminaeous monocots consists of a rigid, rod-like cellulose/xyloglucan load-bearing network that is embedded in and interacts with a compression-resistant pectin network (Carpita and Gibeaut, 1993). Cross-linking cell wall polymers and alternation in cell wall compositions are most likely to determine wall mechanical properties. We have shown that the cell wall thickness of the leaves is correlated inversely with the dRG-II-B formation of their walls. The addition of boric acid to the B-deficient plant results in the binding of B to the cell walls, the formation of dRG-II-B from mRG-II, and repacking the matrix into a firm, thin wall. The swelling is due to the lack of cross-linking of borate ester of RG-II, not to the increase in density of the wall. A similar type of swelling of cell wall is observed during certain stages of development or in mutants. The classic swelling phenomenon is that of softening fruits, such as tomato and kiwifruit, which is attributed to the breakage of cross-linking of tightly packed pectins (Redgwell et al., 1997). Inappropriate swelling occurs in the irregular xylem mutant, irx4 (Turner and Somerville, 1997). In this instance, a disruption in normal lignin distribution in the tracheary elements results in the swelling of the cellulosic matrix into the lumen. It should be emphasized that in contrast to above phenomena, the swelling of wall caused by B deficiency is reversible. When B-deficient pumpkin plants were transferred to B-sufficient medium, B binding to the cell occurred over a period of hours. 10B binding to the cell wall and the formation of dRG-II-10B was observed within 2.5 h and after 7 h dRG-II-10B accounted for approximately 60% of RG-II. B-deficient suspension-cultured Chenopodium album cells increased in wall pore size (Fleischer et al., 1999). Adding boric acid to growing B-deficient cells results within 10 min in the formation of dRG-II-B from mRG-II and a reduction in wall pore size. The differences in the rate of dimer formation in plant tissue and in cultured cells are likely to be due to the rate at which B gets to the wall. B has immediate access to the walls of cultured cells, whereas in plants B must be taken up by the root and then transported to the developing leaves. However, the possibility cannot be discounted that the delay of B binding and formation of dRG-II-B in B-deficient pumpkin is also due to the impairment of growth and development of meristematic tissue kept under B deficiency for a week.
To compensate for loss of negative charged borate ester, B deficiency would increase in free-charged density of pectin by de-esterification. One interesting question concerns degree of methyl esterification and methyl esterase activity due to B deficiency.
In summary, we have shown that the form of RG-II in the walls of B-deficient pumpkin is mainly mRG-II. The formation in muro of dRG-II-B from mRG-II following the addition of boric acid is accompanied by wall condensation. Our data provide additional support for the hypothesis that B is a structural component of the cell wall and suggest that B is required for the formation of a covalently cross-linked pectic network (Ishii and Matsunaga, 2001) and this network has a role in determining cell wall mechanical properties.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Pumpkin (Cucurbia moschata Duchesne cv Tokyo-Kabocha) seeds were purchased from Sakata Seed company (Yokohama, Japan). The seeds contain on average 18 μg B g−1 and average seed weight was 200 ± 5 mg (se, n = 25). Seeds were sown in washed vermiculite and grown for 7 d at 22.5°C, 12-h day length. The plants were then transferred to 4-L plastic containers containing one-quarter-strength Hoagland solution (Hoagland and Arnon, 1950) supplemented with 10 μm B. After 1 week, the plant roots were rinsed thoroughly with B-free distilled water and the plants were then transferred to fresh medium containing 25 μm B or no B (<0.14 μm B). To avoid boric acid contamination, 2 mL of the borate-binding ion-exchange resin IRA-743 was added to the B-deficient medium. Fifteen plants were grown in the container. The plants were grown for 1 additional week and then harvested. At this stage, plants had developed four leaves. The leaves from the same relative positions on each plant were separately collected, immediately frozen in liquid nitrogen, and then freeze dried. The major veins of the leaves were removed and the remaining leaf blade was cut into small pieces and then divided into two equal portions, 0.2 to 0.5 g dry weight. One portion was used to determine total B content, and the second portion was used to prepare the AIR.
Pumpkin grown in B-deficient medium for 1 week was transferred to 10B-enriched boric acid (95 atom %, 25 μm) containing medium and grown further. At certain periods, the third leaf was harvested and treated with the same procedures as the normal boric acid samples for the determination of 10B and formation of dRG-II-10B. The second to fourth leaves of the pumpkin grown in 10B-enriched boric acid for 16 h was used for structural analysis of RG-II.
Preparation of the AIR from Pumpkin Leaves
The dried leaf was suspended in aqueous 80% (v/v) ethanol and homogenized for 0.5 min at 1,500 rpm using a Polytron blender (Physco-Troller NS-610, Nichion Rikakikai Co, Tokyo). The suspensions were centrifuged at 2,500g for 5 min. The insoluble residue was then washed with 80% (v/v) ethanol, 95% (v/v) ethanol, 100% (v/v) ethanol, chloroform: methanol (1:1, v/v), and acetone and then air-dried.
Solubilization of RG-II from AIR of B-Deficient, 10B-Treated, and B-Normal Pumpkin
The AIR was treated for 4 h at 4°C with 0.1 n sodium hydroxide to saponify the methyl and acetyl esters. The suspensions were adjusted to pH 5.0 with 10% (v/v) glacial acetic acid and then treated for 16 h at 30°C with homogeneous preparation of EPG from Aspergillus niger (2.5 units, Megazyme, Wicklow, Ireland; 1 unit releases 1 μmol of reducing sugar min−1 from a 1% [w/v] solution of poly-GalUA at pH 5.0 and 25°C). The suspensions were centrifuged and the insoluble residues washed with water. The EPG-soluble fractions were dialyzed (1.0-kD cutoff tubing) against deionized water and freeze dried. EPG treatment solubilized 10% and 15% of the weight of the B-normal and B-deficient AIR, respectively.
Pumpkin RG-II was purified from the EPG-solubilized material by SEC on a Sephadex G-75 (2.5 × 90 cm) and Superdex Prep 75 (Amersham Pharmacia Biotech Inc., Uppsala, 1.6 × 38 cm) columns. For structural analysis of RG-II, AIR isolated from the second to the fourth leaves was used.
Determination of Ratio of the dRG-II-B and mRG-II in AIR
The AIR (10 mg) was saponified and digested with EPG (2 units). The suspension was centrifuged. The supernatant was subjected to SEC/RI with a Superdex 75 column as described below.
Anatomical Determination
A strip of leaf tissue (approximately 1 cm2) was excised from the second leaf of B-deficient, B-normal, and the B-deficient plant grown for 5 h in the presence of B boric acid (25 μm ). Four leaves of each plant were used and eight strips were cut from each plant. The leaf strips were fixed for 60 min in 2.5% (w/v) glutaraldehyde in 0.1 m potassium phosphate, pH 6.8, and then washed with distilled water. The leaf strips were dehydrated using a graded series of ethanol (30%, 50%, 70%, 80%, 90%, 95%, and 100% [v/v] ethanol) and propylene-oxide and then embedded in epoxy resin. Ultra-thin sections (0.1-μm thickness) were cut with a diamond knife and stained with 0.1% (w/v) uranyl acetate and lead citrate in 0.1 m sodium hydroxide solution. Stained sections were examined with a transmission electron microscope (JEOL 2000 EX, JEOL, Tokyo) at 200 kV. Sections (1 or 2 μm thick) for light microscopy were cut using a glass knife.
Analytical Methods
SEC/RI was performed with an LC-6A system (Shimadzu, Kyoto) with an RI (Shimadzu model RID-6A) connected to a Superdex-75 HR 10/30 column (Amersham Pharmacia Biotech Inc.) eluted at 0.6 mL min−1 with 50 mm ammonium formate, pH 5.3, as described (Ishii and Matsunaga, 1996). The mRG-II and dRG-II-B in the EPG digests was confirmed by comparing their retention time with those of the authentic mRG-II and dRG-II-B from sugar beet and red wine. SEC/ICP-MS was performed with a Diol–120 column (8 × 300 mm, YMC, Kyoto) connected to the ICP source of a mass spectrometer SII-SPQ9000A (Seiko Instruments Inc, Chiba, Japan). The column was eluted at 1 mL min−1 with 200 mm ammonium formate, pH 6.5. The mass spectrometer was operated to selectively detect 10B and 11B. The B content and 10B abundance of the AIR was determined by the ICP-MS.
Uronic acid was determined by the m-hydroxybiphenyl method (Blumenkrantz and Asboe-Hansen, 1973). The neutral and acidic glycosyl residue compositions of the AIR and RG-II were determined by GC-MS analysis of the alditol acetate derivatives and trimethylsilyl methyl glycoside derivatives, respectively (York et al., 1985). The glycocyl linkage compositions were determined by a modified Hakomori procedure method (Ishii et al., 1999).
ACKNOWLEDGMENTS
We thank Malcolm A. O'Neill (Complex Carbohydrate Research Center, University of Georgia, Athens) for critical reading of the manuscript. Masako Ishikawa (Forestry and Forest Products Research Institute) is acknowledged for growing the pumpkin plants and preparing the manuscript.
Footnotes
This work was supported by the Ministry of Agriculture, Forestry, and Fisheries (Research Grant no. BDP–01–II–2).
LITERATURE CITED
- Blumenkrantz NJ, Asboe-Hansen B. A new method for the determination of uronic acid. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brown PH, Hu H. Does boron play only a structural role in the growing tissues of higher plants? Plant Soil. 1997;196:211–215. [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structures with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R. The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 1999;121:829–838. doi: 10.1104/pp.121.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach H, Amberger A. Influence of boron nutrition on cell wall polysaccharides in cell cultures of Daucus carota L. J Plant Physiol. 1986;123:263–269. [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular 347. Berkeley: The College of Agriculture, University of California; 1950. pp. 1–39. [Google Scholar]
- Hu H, Brown PH. Localization of boron in the cell walls of squash and tobacco and its association with pectin. Plant Physiol. 1994;105:681–689. doi: 10.1104/pp.105.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Matsunaga T. Isolation and characterization of a boron-rhamnogalacturonan II complex from cell walls of sugar beet pulp. Carbohydr Res. 1996;284:1–9. [Google Scholar]
- Ishii T, Matsunaga T (2001) Rhamnogalacturonan II is covalently cross-linked to homogalacturonan. Phytochemistry (in press) [DOI] [PubMed]
- Ishii T, Matsunaga T, Pellerin P, O'Neill MA, Darvill A, Albersheim P. The plant cell wall polysaccharide rhamnogalacturonan II self-assembles into a covalently cross-linked dimer. J Biol Chem. 1999;274:13098–13104. doi: 10.1074/jbc.274.19.13098. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Matoh T, Azuma J. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 1996;110:1017–1020. doi: 10.1104/pp.110.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi H, Kumazawa K. Anatomical responses of root tips to boron deficiency: III. Effect of boron deficiency on sub-cellular structure of root tips, particularly on morphology of cell wall and its related organelles. Soil Sci Plant Nutr. 1976;22:53–71. [Google Scholar]
- Loomis WD, Durst RW. Chemistry and biology of boron. Biofactors. 1992;3:229–239. [PubMed] [Google Scholar]
- Matoh T. Boron in plant cell walls. Plant Soil. 1997;193:59–70. [Google Scholar]
- Matoh T, Takasaki M, Kobayashi M, Takabe K. Boron nutrition of cultured tobacco BY-2 cells: III. Characterization of the boron-rhamnogalacturonan II complex in cells acclimated to low levels of boron. Plant Cell Physiol. 2000;41:363–366. doi: 10.1093/pcp/41.3.363. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P. Rhamnogalacturonan II, a pectic polysaccharide in the walls of growing plant cells, forms a dimer that is covalently cross-linked by a borate ester. J Biol Chem. 1996;271:22923–22930. doi: 10.1074/jbc.271.37.22923. [DOI] [PubMed] [Google Scholar]
- Redgwell RJ, MacRae E, Hallett I, Fisher M, Perry J, Harker R. In vivo and in vitro swelling of cell walls during fruit ripening. Planta. 1997;203:162–173. [Google Scholar]
- Skok J. The substitution of complexing substances for boron in plant growth. Plant Physiol. 1957;32:308–312. doi: 10.1104/pp.32.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SR, Somerville CR. Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell. 1997;9:689–701. doi: 10.1105/tpc.9.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P. Isolation and characterization of plant cell walls and cell wall constituents. Methods Enzymol. 1985;118:3–40. [Google Scholar]