Abstract
Evaporation of water from the guard cell wall concentrates apoplastic solutes. We hypothesize that this phenomenon provides two mechanisms for responding to high transpiration rates. First, apoplastic abscisic acid is concentrated in the guard cell wall. Second, by accumulating in the guard cell wall, apoplastic sucrose (Suc) provides a direct osmotic feedback to guard cells. As a means of testing this second hypothesized mechanism, the guard cell Suc contents at a higher transpiration rate (60% relative humidity [RH]) were compared with those at a lower transpiration rate (90% RH) in broad bean (Vicia faba), an apoplastic phloem loader. In control plants (constant 60% RH), the guard cell apoplast Suc content increased from 97 ± 81 femtomol (fmol) guard cell pair−1 to 701 ± 142 fmol guard cell pair−1 between daybreak and midday. This increase is equivalent to approximately 150 mm external, which is sufficient to decrease stomatal aperture size. In plants that were shifted to 90% RH before daybreak, the guard cell apoplast Suc content did not increase during the day. In accordance, in plants that were shifted to 90% RH at midday, the guard cell apoplast Suc content declined to the daybreak value. Under all conditions, the guard cell symplast Suc content increased during the photoperiod, but the guard cell symplast Suc content was higher (836 ± 33 fmol guard cell pair−1) in plants that were shifted to 90% RH. These results indicate that a high transpiration rate may result in a high guard cell apoplast Suc concentration, which diminishes stomatal aperture size.
Potassium and its counterions are the well-known fluctuating osmotica that cause stomatal movements through regulation of the aqueous volume of guard cells (for review, see Outlaw, 1983; Zeiger, 1983). Thus, an accumulation of potassium causes stomatal opening and a dissipation of potassium may cause stomatal closing. Suc has been found more recently to be an important fluctuating osmolyte in the guard cell symplast (Tallman and Zeiger, 1988; Poffenroth et al., 1992; Talbott and Zeiger, 1993, 1996, 1998; Amodeo et al., 1996) and the guard cell apoplast (Lu et al., 1995). The importance of Suc in stomatal aperture size regulation lies in the difference between Suc concentrations in the guard cell symplast and the guard cell apoplast. Therefore, it is important to know the sources and conditions leading to changes in the Suc concentration in both these compartments, as discussed below.
Three sources have been proposed for the elevation of guard cell symplastic Suc in open stomata: (a) The photosynthetic carbon reduction pathway is the first (Gotow et al., 1988; Poffenroth et al., 1992; Talbott and Zeiger, 1993), but guard cells typically do not have sufficient carbon flux through this pathway (Outlaw, 1989; Tarczynski et al., 1989; Reckmann et al., 1990; Gautier et al., 1991; Lu et al., 1997) to account for Suc accumulation. (b) Starch breakdown in guard cells is a second potential source of Suc (Tallman and Zeiger, 1988; Talbott and Zeiger, 1993). Although light quality (Poffenroth et al., 1992), the availability of external chloride (Raschke and Schnabl, 1978), and osmotic stress (Kopka et al., 1997; Asai et al., 1999) regulate carbon metabolism in guard cells, the amount of starch in guard cells appears to be a limiting factor. (c) Import of Suc by an H+-Suc symporter (Ritte et al., 1999) is a final potential source of guard cell symplastic Suc. Import is consistent with the presence of Suc in the guard cell apoplast (Lu et al., 1995) and of Suc-metabolizing enzymes that are associated with photosynthate sinks (Hite et al., 1993).
Mesophyll-derived leaf apoplastic Suc is the source of guard cell apoplastic Suc (Lu et al., 1997) during midday in broad bean (Vicia faba), an apoplastic phloem loader (Delrot et al., 1983; Kühn et al., 1999). Suc becomes concentrated in the guard cell apoplast because this is the distal point in the evaporative pathway. In accordance, the extent to which Suc accumulates in the guard cell apoplast is hypothesized to be controlled by two interacting physiological parameters: (a) the Suc concentration in the leaf apoplast, which is a function of photosynthesis rate and the transport rate from the leaf (Ntsika and Delrot, 1986; Lohaus et al., 1995); and (b) the rate of transpiration. As discussed below, this second parameter indicates that guard cell apoplast Suc concentration may be a factor in the stomatal closing response to increasing vapor pressure difference (VPD).
In a landmark study, Mott and Parkhurst (1991) found that the stomatal response to humidity is consistent with sensing of the transpiration rate rather than of relative humidity (RH) per se. A reanalysis (Monteith, 1995) of 52 published sets of measurements and subsequent work (Jarvis et al., 1999) support this conclusion. Therefore, Lu et al. (1997) hypothesized that transpiration-linked accumulation of guard cell apoplastic Suc is a mechanism by which plants respond to high transpiration rate. The present study is a test of that hypothesis. Broad bean plants were maintained at 60% RH (control conditions) or shifted to 90% RH, which decreased the transpiration rate. At various times, the guard cell apoplast Suc contents, the guard cell symplast Suc contents, the whole-leaf Suc contents, and the bulk-leaf apoplast Suc concentrations were determined. The results indicate that sufficient Suc accumulates in the guard cell apoplast to diminish stomatal aperture size at a high transpiration rate but not at low transpiration rate.
RESULTS
Lowered VPD Increased Guard Cell Symplast Suc Content, Prevented an Increase in the Guard Cell Apoplast Suc Content, and Decreased Transpiration
The essence of this report is a comparison of the Suc contents of the guard cell symplast and the guard cell apoplast under control conditions (constant 60% RH) and RH shift conditions (90% RH). The results of the control experiments (compare with Lu et al., 1995) and the RH shift experiments will be described (Fig. 1), respectively. Then, results under the two conditions will be compared (Table I), along with changes in the transpiration rate.
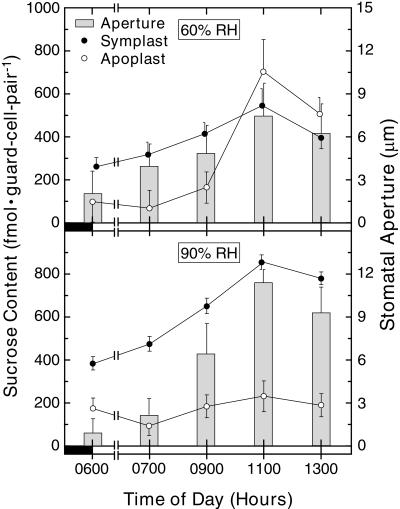
Figure 1.
Time course for Suc content changes in the guard cell symplast (●) and the guard cell apoplast (○). Broad bean plants were cultured at a constant 60% RH in a growth chamber (14-h day, 20°C/25°C, 600 μmol photons m−2 s−1). Control plants (upper; n = 16 [two experiments]) were maintained at 60% RH (Lu et al., 1995), whereas the RH-shifted plants (lower; n = 40 [five experiments]) were transferred to 90% RH 8 h before the onset of the photoperiod. Corresponding stomatal aperture sizes (n = 48–120) are represented by the histogram. Errors are se.
Table I.
Statistical comparisons of the guard cell-apoplast Suc contents, the guard cell-symplast Suc contents, and stomatal aperture sizes at a constant 60% RH versus cognate values obtained when the RH was shifted to 90% 8 h prior to the onset of illumination
| Time |
P Values,
Parameter at 60% RH versus 90% RH
|
||
|---|---|---|---|
| Guard cell Suc
content
|
Stomatal aperture size | ||
| Apoplast | Symplast | ||
| h | |||
| 0600 | 0.51 | 0.41 | 0.95 |
| 0700 | 0.88 | 0.35 | 0.17 |
| 0900 | 0.93 | 0.02 | 0.51 |
| 1100 | 0.01 | 0.02 | 0.02 |
| 1300 | 0.02 | 0.02 | <0.01 |
The compared values are derived from Figure 1, which provides other details.
Control experiments (upper, Fig. 1) were conducted on plants that were maintained at 60% RH. The guard cell apoplast Suc content was low at the onset of the photoperiod (0600 h, 97 ± 81 femtomol [fmol] guard cell pair−1) and was correlated with a small stomatal aperture size (2.0 ± 1.6 μm). No significant changes took place in this Suc pool (P > 0.11, compared with 0600 h) or in stomatal aperture size over the next 3 h. By 1100 h, however, the guard cell apoplast Suc content had increased (P < 0.02 for all earlier values) by 7-fold to 701 ± 142 fmol guard cell pair−1 (upper, Fig. 1). This highest value in guard cell apoplast Suc content during the photoperiod was correlated with the largest stomatal aperture size (7.4 ± 2.3 μm, P < 0.01). The increase in guard cell apoplast Suc content during the photoperiod was approximately 145 mm (for conversion factors, see Ewert et al., 2000). In these experiments, the apparent decreases between 1100 h and 1300 h in guard cell apoplast Suc content (to 504 ± 77 fmol guard cell pair−1, P > 0.11) and in stomatal aperture size (to 6.2 ± 2.1 μm) were not significant.
Under control conditions, the symplast Suc pool size in guard cells increased linearly from the onset of the photoperiod (270 ± 49 fmol guard cell pair−1) to 1100 h (551 ± 88 fmol guard cell pair−1, P = 0.04). The apparent decrease in the guard cell symplast Suc pool size between 1100 h and 1300 h was not significant (P = 0.14). Overall, the results of the control experiments are in general qualitative agreement with those of Lu et al. (1995).
Figure 1 (lower) shows the results for RH-shifted plants (in these experiments, plants were transferred to 90% RH 8 h before the onset of the photoperiod). The guard cell apoplast Suc content was initially low (0600 h, 173 ± 48 fmol guard cell pair−1) and was correlated with a small stomatal aperture size (0.9 ± 1.0 μm). The guard cell apoplast Suc pool size did not change significantly over the course of the experiment. The stomatal aperture size increased significantly (P < 0.01) by 0900 h to 6.5 ± 2.1 μm, and reached a highest value at 1100 h (11.6 ± 2.0 μm). In these experiments, the decrease between 1100 and 1300 h in stomatal aperture size (to 9.3 ± 1.9 μm) was significant.
Under these RH shift conditions, the symplast Suc pool size in guard cells increased linearly from the onset of the photoperiod (383 ± 33 fmol guard cell pair−1, P < 0.01). The apparent decrease in the guard cell symplast Suc pool size between 100 h and 1300 h was not significant (P = 0.37).
Pair-wise comparisons of the guard cell symplast Suc contents, the guard cell apoplast Suc contents, and stomatal aperture sizes under control and RH shift conditions are shown in Table I. At 0600 or 0700 h, altered VPD did not have a significant effect on the guard cell apoplast Suc content, the guard cell symplast Suc content, or the stomatal aperture size, but all three of these parameters were significantly altered by lowered VPD at 1100 and 1300 h.
Stomatal aperture size is proportional to conductance (broad bean; see Zhang and Outlaw, 2001b). Between 0900 and 1100 h, the average increase in stomatal conductance for RH-shifted plants was twice that of control plants (Fig. 1, Table I). At all times, however, the average stomatal conductance of control plants was less than 0.5-fold that of RH-shifted plants.
In summary, the main features that were altered by the shift to 90% RH were exemplified at 1100 h (Fig. 1, Table I): (a) a 1.6-fold greater stomatal aperture size, coupled with a nominal 4-fold decrease in VPD, which led to a less than 2-fold decrease in transpiration rate; (b) a 1.5-fold increase in the guard cell symplast Suc content; and (c) the absence of a 7-fold increase in the guard cell apoplast Suc content.
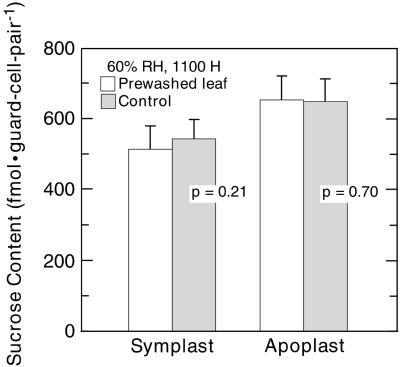
The Changes in Guard Cell Suc Pools Caused by the Shift to 90% RH Were Not Artifacts Resulting from External Liquid Water
At 90% RH and 25°C, the dew point is 23.2°C. This small temperature differential raises the possibility that transient microscopic external water droplets on the leaf surface at 90% RH leach the contents of the guard cell apoplast. As a means of testing this possibility, guard cell Suc was assayed in leaflets that were washed vigorously immediately before sampling. As expected, the symplast Suc content of guard cells (Fig. 2) was not affected by the washing treatment (516 ± 66 fmol guard cell pair−1 versus 541 ± 58 fmol guard cell pair−1 [P = 0.21]). The apoplast Suc content of guard cells also was not affected by the washing treatment (655 ± 68 fmol guard cell pair−1 versus 650 ± 64 fmol guard cell pair−1 [P = 0.70]). Therefore, the diminution of guard cell apoplast Suc content in RH-shifted plants (Fig. 1) cannot be attributed to artifactual dew formation.
Figure 2.
Suc content of the guard cell symplast (left pair of columns) and of the guard cell apoplast (right pair of columns) of control leaflets (░⃞) and of leaflets that were washed before sampling (□). Samples were collected at constant 60% RH. n = 24 (three experiments). P values (t test) are for the paired columns; other details are as in Figure 1.
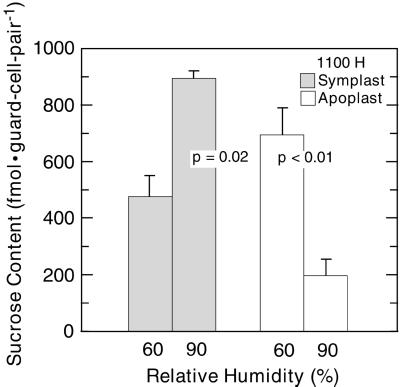
The Changes in Guard Cell Suc Contents Caused by the Shift to 90% RH Were Not Artifacts Resulting from Limited Sample Sizes Used for Quantitative Histochemistry
Suc content varies by as much as 2-fold within an individual broad bean leaflet (Outlaw and Manchester, 1979), raising the possibility that changes in Suc pools attributed to the RH shift (Fig. 1) are sampling artifacts. As a first means of eliminating this possibility, multiple areas of each leaflet were selected randomly for dissection of guard cells (see “Materials and Methods”). As a second means of eliminating this possibility, guard cell Suc contents were studied exhaustively at 1100 h under the control and RH shift conditions of Fig. 1. Altogether (Fig. 3), 624 guard cell pairs, individually dissected from 78 leaflets, were extracted individually and analyzed. In control plants, the guard cell apoplast Suc content was 701 ± 96 fmol guard cell pair−1 (Fig. 3), in contrast to the 205 ± 52 fmol guard cell pair−1 of RH-shifted plants. This significant decrease (P < 0.01) of 496 fmol guard cell pair−1 is equivalent to a concentration change of 120 mm (for conversion factor, see Ewert et al., 2000). In control plants, the guard cell symplast Suc content was 479 ± 69 fmol guard cell pair−1, less than that of RH-shifted plants (894 ± 27 fmol guard cell pair−1). This significant increase (P = 0.02) is 415 fmol guard cell pair−1.
Figure 3.
Suc content of the guard cell symplast (░⃞) and the guard cell apoplast (□) at 60% RH (left column in each pair) and at 90% RH (right column in each pair) at 1100 h. n = 88–224 guard cell pairs (11–28 plants) for each column. P values (t test) are for the paired columns; other details are as in Figure 1.
In summary, at 1100 h, the total guard cell Suc pool at 60% RH was 1,180 fmol guard cell pair−1, of which 59% was apoplastic, whereas the total guard cell Suc pool at 90% RH was 1,099 fmol guard cell pair−1, of which 19% was apoplastic.
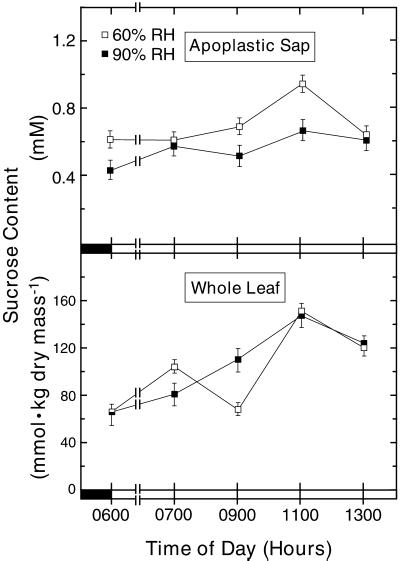
The Changes in Guard Cell Suc Pools Caused by the Shift to 90% RH Were Not Consequences of Changes in the Whole-Leaf Suc Content or the Bulk-Apoplast Suc Concentration
Both the bulk-apoplast Suc concentration and the whole-leaf Suc content may increase during the photoperiod, raising the possibility that either or both are altered by an RH shift and, thus, account for guard cell Suc pool changes (Figs. 1 and 3) independently of the transpiration rate. The bulk-apoplast Suc concentration was, as expected, higher (top, Fig. 4) at 1100 h than at 0600 h in control plants (Δ = 0.3 mm; P < 0.01) but not in RH-shifted plants (Δapp = 0.2 mm; P = 0.08). There was also a significant difference (P < 0.01) in the bulk-apoplast Suc concentration at 1100 h in the control (0.94 mm) and RH-shifted (0.67 mm) plants. However, the absolute difference was much smaller than the corresponding severalfold difference in the guard cell apoplast Suc contents (Fig. 3).
Figure 4.
Suc concentration in the apoplastic sap (top) and the Suc content of whole leaf (lower) at 60% RH (□) and at 90% RH (▪) at various times during the photoperiod. Apoplastic sap (n = 9 [three experiments]) was obtained with the pressure chamber. Separate extracts for Suc analysis were made of three random fragments of each freeze-dried whole leaf used in the experiments displayed in Figure 1, which provides additional details.
The whole-leaf Suc content was higher (lower, Fig. 4) at 1100 h than at 0600 h in control (P = 0.04) and in RH-shifted (P < 0.01) plants. The 2-fold increase, nominally 60 mmol kgdry mass−1, occurred independently of RH.
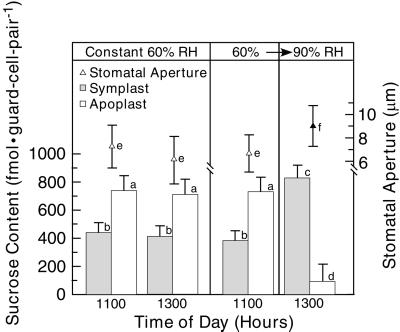
Lowering VPD during Midday Caused an Increase in the Guard Cell Symplast Suc Content, a Decrease in the Guard Cell Apoplast Suc Content, and a Decrease in Transpiration
The previous sections establish that a shift from 60% RH to 90% RH 8 h before the onset of the photoperiod results in an elevation in the guard cell symplast Suc content and prevents an increase in the guard cell apoplast Suc content during the following day (Figs. 1 and 3). These changes were not an artifact of the high ambient humidity (Fig. 2), and they could not be accounted for by RH-induced changes in the whole-leaf Suc content or the bulk-leaf apoplast Suc concentration (Fig. 4). In this section, the effects of an RH shift during the photoperiod, when stomata are open, will be addressed. In these experiments, all plants were maintained at 60% RH until 1100 h; then, some plants were shifted to 90% RH (Fig. 5). At 1100 h in control plants, the guard cell apoplast Suc content was 743 ± 107 fmol guard cell pair−1; the stomatal aperture size, 7.4 ± 1.6 μm; and the guard cell symplast Suc content, 441 ± 71 fmol guard cell pair−1 (Fig. 5). These values were not significantly different from those at 1100 h in plants that were later shifted to 90% RH or from those in control plants at 1300 h. Shifting to 90% RH during the photoperiod corresponded to a decrease in the guard cell apoplast Suc content to 96 ± 120 fmol guard cell pair−1 (P < 0.01), an increase in stomatal aperture size to 9.0 ± 1.8 μm (P < 0.01), and an increase in the guard cell symplast Suc content to 832 ± 92 fmol guard cell pair−1 (P < 0.01). Thus, the RH shift resulted in a decrease of the Suc concentration of 155 mm in the guard cell apoplast.
Figure 5.
The Suc content in the guard cell symplast (░⃞) and in the guard cell apoplast (□) at 1100 h and at 1300 h. The control plants (left) were maintained at constant 60% RH (Fig. 1; Lu et al., 1995). RH-shifted plants (right) were shifted from 60% RH at 1100 h to 90% RH, which required approximately 10 min. The experiments were conducted in duplicate; five leaflets were sampled in each experiment for each datum (n = 80 guard cells per column). Corresponding stomatal aperture sizes (n > 180) are represented by triangles (Δ, plants at 60% RH; ▴, plants at 90% RH). Data labeled by identical alphabetic characters are not significantly different (P > 0.05). Other details are as in Figure 1.
DISCUSSION
The Physiological Relevance of Changes in Guard Cell Suc Contents
The guard cell Suc apoplast concentration varied by 7-fold, depending on conditions and time of day (Figs. 1 and 5; Table I). The difference that coincided with lowering the transpiration rate was 620 fmol guard cell pair−1 (right, Fig. 5). The aqueous cell wall volume of a pair of broad bean guard cells is 4.2 pL (Ewert et al., 2000), indicating that the Suc concentration in the guard cell apoplast changed by approximately 0.16 molal, equivalent to a Δψs = 0.4 MPa (Michel, 1972). Similar calculations for the guard cell symplast, yielding Δψs ≈ 0.2 MPa, are imprecise because the volume of the guard cell symplast increases with stomatal aperture size. (That is, part of the increase in Suc content of the guard cell symplast simply maintains the concentration there as the volume increases.) There are various estimates for Δψs (guard cell) μm−1 (stomatal aperture) for broad bean. Hsiao (1976) cites a range of 0.12 to 0.2 MPa μm−1 from four studies, and Poffenroth et al. (Table III, 1992) use a range of 0.05 to 0.16 MPa μm−1, derived from three different studies, in model calculations. Use of the midpoint of these values, 0.125 MPa μm−1, provides a perspective on the importance of Suc fluctuations on stomatal aperture size. Thus, the change in guard cell apoplast Suc concentration predicts a change in aperture size of about 3 μm. In summary, the most important conclusion is that decreasing the transpiration rate coincides with reciprocal changes in the guard cell Suc pools that are sufficient to increase stomatal aperture size.
Concordance of Transpiration and Suc Fluxes in the Guard Cell Apoplast
From the stomatal aperture sizes at 1100 h (Fig. 5) and ambient conditions, the rate of transpiration is calculated to be 2 mmol water m−2 s−1, or 20 pL guard cell pair−1 min−1. This value is in close agreement with the directly measured transpiration rates for broad bean under similar conditions (1–2.5 mmol m−2 s−1, Ewert et al., 2000) and indicates that the guard cell apoplast water turns over about five times each minute if all evaporation occurs at this site. The rate of delivery of Suc to the guard cell apoplast is the product of evaporation rate and concentration of Suc in the transpiration stream. The concentration of Suc in the transpiration stream near stomata is unknown (see “Materials and Methods”), but labeling experiments indicate that the sap expressed from the petiole provides an underestimate of the Suc concentration near the minor veins (Lu et al., 1997). Thus, use of that sap value (approximately 1 mm, Fig. 4) to calculate the rate of Suc delivery to the guard cell wall, 20 fmol guard cell pair−1 min−1, is similarly underestimated, but is still 5-fold the observed rate of Suc accumulation in the guard cell apoplast (Fig. 1). In summary, delivery of Suc via the transpiration stream can account for Suc accumulation in the guard cell apoplast and is consistent with previous results (Lu et al., 1997) that demonstrated that the source of guard cell apoplastic Suc is the mesophyll. This interpretation is also consistent with a CO2 (Internal)-independent mesophyll signal that prevents further stomatal opening when light is increased (Muschak et al., 1999).
The decline in guard cell apoplast Suc content when the RH was shifted to 90% was 620 fmol guard cell pair−1 (Fig. 5), as discussed above. Thus, the average net loss over this 2-h period was 5 fmol guard cell pair−1 min−1, which is 10-fold less than the unidirectional efflux rate calculated on the basis of 14C compartmentation analysis (Lu et al., 1997). The relationship of these values is consistent and indicates the rapidity with which conductance may be affected by ΔVPD via an apoplastic solute mechanism.
Suc Uptake by Guard Cells
Direct in planta measurements of 14CO2 incorporation by guard cells (Lu et al., 1997) of broad bean plants grown identically to those in this study showed that guard cell photosynthesis is not sufficient as a source of the increased Suc content of guard cells (Fig. 5). The starch content of guard cells of open stomata of broad bean, approximately 500 fmol (anhydroglucosyl units) guard cell pair−1 (Outlaw and Manchester, 1979), is somewhat low to account for the increased Suc content of guard cells upon RH shift (Fig. 5), but guard cell starch measurements under several conditions have not been made. The simplest interpretation is the guard cell symplast Suc increase (Fig. 5) must result from uptake from the apoplast. At 40 mm external Suc, the average rate of 14C-Suc uptake by broad bean guard cells was 225 fmol guard cell pair−1 h−1 (Outlaw, 1995). That rate is similar to the average rate of increase in the guard cell symplast Suc content caused by shifting the RH from 60% to 90% (Fig. 5), indicating that guard cells have sufficient Suc-transport capacity.
The increase in the guard cell symplast Suc content when plants were shifted to 90% RH (Figs. 1 and 5) was unexpected, but may be related to increased symplast volume with increased stomatal aperture size or to observations (Amodeo et al., 1996; Talbott and Zeiger, 1996) that Suc is the major guard cell osmoticum later in the day. Regardless, the increase reveals that environmental control of stomatal aperture size by Suc fluctuations is not simply a physical process that balances evaporative deposition of Suc in the guard cell apoplast against diffusion from this site of accumulation. An explanation requires further understanding of guard cell Suc transport and metabolism, but present evidence indicates regulation of these processes. In this regard, it is relevant to note that Outlaw (1995) and Ritte et al. (1999) observed large variability among experiments in Suc uptake by guard cells, and Kopka et al. (1997) reported a water stress-induced decrease in guard cell expression of the Suc transporter. It is also intriguing that high Suc concentrations suppress expression of a Suc transporter in broad bean (Weber et al., 1997), whereas high guard cell apoplast Suc concentration coincided with reduced guard cell symplast Suc uptake (Figs. 1 and 5). However, the coincidence of these observations must not be overinterpreted, as Suc transporters comprise a family with differently regulated members filling dedicated physiological roles (Williams et al., 2000). In summary, regulation of guard cell Suc uptake is likely to be multifaceted, involving posttranslational (Roblin et al., 1998) or transcriptional or posttranscriptional (Williams et al., 2000) regulation, and requires further study.
The RH-linked change in guard cell Suc contents (Figs. 1 and 5) reconciles reported quantitative differences in broad bean guard cell symplast Suc contents. Thus, Lu et al. (1995) grew plants on a 16-h day, 20°C/25°C, and 60% RH regimen and reported an increase in guard cell symplast Suc contents from 140 to 350 fmol Suc guard cell pair−1 over the day. Talbott and Zeiger (1996) grew plants on a 12-h day, 25°C/15°C, and an 85% RH regimen but in a growth cabinet of similar design and light intensity. They reported a larger increase, from 400 to 1,200 fmol Suc guard cell pair−1, in guard cell symplast Suc contents over the day. The present data (Figs. 1, 3, and 5) imply that transpiration rate is a factor in the above-reported differences. As a further reconciliation, the absence of leaf photosynthesis probably accounts for the small, but significant, changes in guard cell Suc content during light and −CO2-induced stomatal opening (Outlaw and Manchester, 1979) because mesophyll photosynthesis is the source of guard cell Suc (Lu et al., 1997). Altogether, the reported differences in guard cell Suc quantitation appear to reflect different physiological states, suggesting avenues for further investigation.
Other Consequences of a High-Suc Environment
The Suc concentration in the guard cell apoplast, approximately 150 mM (Figs. 1 and 5), of plants grown at 60% RH is consistent with a role of sugars in guard cell gene expression (Koch, 1996; Pego et al., 2000). In particular, enzymes involved in photosynthetic carbon metabolism are repressed by sugar (Pego et al., 2000). This repression is an explanation for low or undetectable levels for markers of photosynthetic carbon metabolism such as mRNA for Rubisco and plastidic fruP2ase (Kopka et al., 1997), Rubisco protein (Tarczynski et al., 1989), light-induced P-Gly concentration increase (Outlaw and Tarczynski, 1984), and 14CO2 incorporation (Lu et al., 1997). Although most quantitative studies support the conclusion that photosynthetic carbon metabolism is very limited in guard cells (Outlaw, 1989; see also Vaughn and Vaughan, 1988), there are exceptions. As discussed earlier (Outlaw, 1989), the most significant exception is that of Shimazaki and Zeiger (1987), who reported rates of broad bean guard cell CO2 uptake that are typical of mesophyll cells on a chlorophyll basis (but only 2%–4% as much as mesophyll cells on a cell basis because of the low chlorophyll content of guard cells). It is notable that growth conditions in the Shimazaki and Zeiger study were at a higher RH and lower temperature, both of which lower VPD. Therefore, we hypothesize that transpiration rate is an indirect factor in guard cell carbon metabolism. Minimum Rubisco-specific fluorescence in all C3 and C4 guard cells studied and considerable Rubisco-specific fluorescence in many crassulacean acid metabolism guard cells (Madhavan and Smith, 1982) might also be explained by differences in transpiration and, hence, sugar repression. Confidence in an interpretation of the role of Suc in guard cell gene expression requires further investigation, which will be complicated because phosphate (which is high in guard cells; Outlaw et al., 1984) modulates sugar-inducible genes (Sadka et al., 1994) and because the ABA and Suc signal transduction networks overlap (Huijser et al., 2000).
MATERIALS AND METHODS
Plant Growth and Treatments
Broad bean (Vicia faba L. cv Longpod) plants were grown in a growth chamber in Fafard No. 2 soil-less potting medium at a density of two plants per 1-L pot. In brief, culture conditions were a 16-h day with a constant 60% RH and a 25°C/20°C day/night temperature regime. Illumination (photosynthetic photon flux density 600 μmol m−2 s−1) was supplied by a combination of fluorescent and incandescent lamps. Full details of the ramping program for the temperature and light transitions in the growth chamber are given in Lu et al. (1997). The second fully expanded bifoliate of 3-week-old plants was used in all experiments.
As a means of assessing the long-term and short-term effects of lowered transpiration rate on the symplastic and apoplastic pools of guard cell Suc, ambient RH was shifted to 90% RH: (a) at 2000 h the day before sampling, which provided a night of acclimation and stomatal opening under the higher humidity; and (b) at 1100 h on the day of sampling, which provided otherwise steady-state conditions during the RH shift.
Precision of Humidity Treatments and Associated Effects
At a set point of 60% RH, an RH range of 59% to 64% was maintained; at a set point of 90% RH, an RH range of 86% to 92% was maintained. The shift from 60% to 90% RH required approximately 10 min. Ambient temperature was maintained within 0.3°C. Leaf temperature under these conditions (and wind speed of 25 cm s−1) is approximately 1°C higher than ambient temperature (see Gates, 1968). These data indicate that the VPD at 60% RH was 2.9- to 4.6-fold that at 90% RH, or possibly higher if the leaf temperature was higher at the lower transpiration rate at 90% RH. In summary, the RH shift provided the designed decrease in the driving force for transpiration.
Tissue Samples
Whole-Leaf and Guard Cell Samples
Four manipulations in the indicated order were made on each bifoliate: (a) leaf conductance was measured on one side of one leaflet with an LI-600 Steady State Porometer (LI-COR, Inc., Lincoln, NE), and (b) a 1- × 2-cm rectangle, cut from the opposite side of this leaflet, was frozen in liquid N2, freeze dried (−35°C, <0 μm Hg, 4 d), and stored at −20°C under vacuum. Individual guard cell pairs dissected from whole-leaf samples contained both the apoplast and symplast Suc pools. Next, (c) an abaxial epidermal peel was taken from the sister leaflet, rinsed in water for 1 min to remove apoplastic solutes from guard cells (Zhang and Outlaw, 2001a), and then frozen in liquid N2. Individual guard cell pairs subsequently dissected from rinsed freeze-dried epidermal peel contained only the symplast Suc pool. Finally, (d) another peel, from the opposite side of this leaflet, was stained with 0.03% (w/v) aqueous neutral red and stomatal aperture sizes were determined microscopically.
Spurious conductance values were sometimes obtained at 90% RH, so interpretations rely on stomatal aperture sizes, which are linearly related to conductance (r2 = 0.98; Zhang and Outlaw, 2001b; see also Weyers and Meidner, 1990).
Leaf Apoplast Sap Samples
Leaflet apoplast sap was obtained with a pressure chamber (Model 1000, PMS Instrument Co., Corvallis, OR) as detailed by Ewert et al. (2000). Sap extruded by pressurizing the chamber to 0.5 MPa was discarded. The pressure was increased to 1.0 MPa, which resulted in the extrusion of approximately 5 μL sap. This sap was collected as the bulk-leaf apoplast sample and stored at −80°C until analysis. Altogether, the procedures required less than 3 min. It is important to caution against overinterpretation of solute concentrations in sap expressed by the pressure bomb (Jokhan et al., 1996, 1999).
Suc Assay
Guard Cells
Sub-microliter constriction pipettes and the oil well technique were used for Suc analysis of guard cell pairs randomly dissected from freeze-dried tissue (Lu et al., 1997; Outlaw and Zhang, 2001). The guard cell apoplast Suc content was calculated by subtraction of the Suc content of guard cells dissected out of rinsed peels from that of guard cells dissected out of whole leaf. Standard errors for guard cell apoplast Suc contents were calculated by a conservative routine (Rice, 1987) that incorporated the variance of the Suc contents of both types of guard cell samples. P < 0.05 was considered significant.
Whole Leaf and Leaf Apoplast
A freeze-dried leaf fragment (approximately 150 μg) was heated for 30 min at 95°C in 0.5 mL 0.02 N NaOH. Then, the extract was cooled to room temperature and 0.5 mL of double-strength pH-compensated Suc-specific step reagent was added. After 30 min, the increase in NADPH concentration was determined fluorometrically.
Apoplast sap was diluted 1,000-fold in 0.02 N NaOH and subsequently analyzed by the oil well technique.
ACKNOWLEDGEMENTS
Ping Lu is thanked for technical assistance. T. Jiang, Yun Kang, Fanxia Meng, Nedra Outlaw, Danielle Sherdan, and Dr. William M. Outlaw are thanked for manuscript suggestions.
Footnotes
This work was supported by the U.S. Department of Energy (grant to W.H.O.).
LITERATURE CITED
- Amodeo G, Talbott LD, Zeiger E. Use of potassium and sucrose by onion guard cells during a daily cycle of osmoregulation. Plant Cell Physiol. 1996;37:575–579. [Google Scholar]
- Asai N, Nakajima N, Kondo N, Kamada H. The effect of osmotic stress on the solutes in guard cells of Vicia fabaL. Plant Cell Physiol. 1999;40:843–849. [Google Scholar]
- Delrot S, Faucher M, Bonnemain JL, Bonmort J. Nycthemeral changes in intracellular and apoplastic sugars in Vicia fabaleaves. Physiol Veg. 1983;21:459–467. [Google Scholar]
- Ewert MS, Outlaw WH, Jr, Zhang S, Aghoram K, Riddle KA. Accumulation of an apoplastic solute in the guard-cell wall is sufficient to exert a significant effect on transpiration in Vicia fabaleaflets. Plant Cell Environ. 2000;23:195–203. [Google Scholar]
- Gates DM. Transpiration and leaf temperature. Annu Rev Plant Physiol. 1968;19:211–238. [Google Scholar]
- Gautier H, Vavasseur A, Gans P, Lascève G. Relationship between respiration and photosynthesis in guard cell and mesophyll cell protoplasts of Commelina communisL. Plant Physiol. 1991;95:636–641. doi: 10.1104/pp.95.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotow K, Taylor S, Zeiger E. Photosynthetic carbon fixation in guard cell protoplasts from Vicia fabaL.: evidence from radiolabel experiments. Plant Physiol. 1988;86:700–705. doi: 10.1104/pp.86.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hite DRC, Outlaw WH, Jr, Tarczynski MC. Elevated levels of both sucrose-phosphate synthase and sucrose synthase in Viciaguard cells indicate cell-specific carbohydrate interconversions. Plant Physiol. 1993;101:1217–1221. doi: 10.1104/pp.101.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao TC. Stomatal ion transport. In: Lüttge U, Pitman MG, editors. Encyclopedia of Plant Physiology. 2B, Transport in Plants. Berlin: Springer-Verlag; 1976. pp. 195–221. [Google Scholar]
- Huijser C, Kortstee A, Pego J, Weisbeek P, Wisman E, Smeekens S. The ArabidopsisSUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: involvement of abscisic acid in sugar responses. Plant J. 2000;23:577–585. doi: 10.1046/j.1365-313x.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- Jarvis AJ, Young PC, Taylor CJ, Davies WJ. An analysis of the dynamic response of stomatal conductance to a reduction in humidity over leaves of Cedrella odorata. Plant Cell Environ. 1999;22:913–924. [Google Scholar]
- Jokhan AD, Else MA, Jackson MB. Delivery rates of abscisic acid in xylem sap of Ricinus communisL. plants subjected to part-drying of the soil. J Exp Bot. 1996;47:1595–1599. [Google Scholar]
- Jokhan AD, Harink RJ, Jackson MB. Concentration and delivery of abscisic acid in xylem sap are greater at the shoot base than at a target leaf nearer to the shoot apex. Plant Biol. 1999;1:253–260. [Google Scholar]
- Koch K. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Kopka J, Provart NJ, Müller-Röber B. Potato guard cells respond to drying soil by a complex change in the expression of genes related to carbon metabolism and turgor regulation. Plant J. 1997;11:871–882. doi: 10.1046/j.1365-313x.1997.11040871.x. [DOI] [PubMed] [Google Scholar]
- Kühn C, Barker L, Bürkle L, Frommer WB. Update on sucrose transport in higher plants. J Exp Bot. 1999;50:935–953. [Google Scholar]
- Lohaus G, Winter H, Riens B, Heldt HW. Further studies of the phloem loading process in leaves of barley and spinach: the comparison of metabolite concentrations in the apoplastic compartment with those in the cytosolic compartment and in the sieve tubes. Bot Acta. 1995;108:270–275. [Google Scholar]
- Lu P, Outlaw WH, Jr, Smith BG, Freed GA. A new mechanism for the regulation of stomatal aperture size in intact leaves: accumulation of mesophyll-derived sucrose in the guard-cell wall of Vicia faba. Plant Physiol. 1997;114:109–118. doi: 10.1104/pp.114.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Zhang SQ, Outlaw WH., Jr Sucrose: a solute that accumulates in the guard-cell apoplast and guard-cell symplast of open stomata. FEBS Lett. 1995;362:180–184. doi: 10.1016/0014-5793(95)00239-6. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Smith BN. Localization of ribulose bisphosphate carboxylase in the guard cells by an indirect, immunofluorescence technique. Plant Physiol. 1982;69:273–277. doi: 10.1104/pp.69.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel BE. Solute potentials of sucrose solutions. Plant Physiol. 1972;50:196–198. doi: 10.1104/pp.50.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith JL. A reinterpretation of stomatal responses to humidity. Plant Cell Environ. 1995;18:357–364. [Google Scholar]
- Mott KA, Parkhurst DF. Stomatal responses to humidity in air and helox. Plant Cell Environ. 1991;14:509–515. [Google Scholar]
- Muschak M, Willmitzer L, Fisahn J. Gas-exchange analysis of chloroplastic fructose-1,6-bisphosphatase antisense potatoes at different air humidities and at elevated CO2. Planta. 1999;209:104–111. doi: 10.1007/s004250050611. [DOI] [PubMed] [Google Scholar]
- Ntsika G, Delrot S. Changes in apoplastic and intracellular leaf sugars induced by the blocking of export in Vicia faba. Physiol Plant. 1986;68:145–153. [Google Scholar]
- Outlaw WH., Jr Current concepts on the role of potassium in stomatal movements. Physiol Plant. 1983;59:302–311. [Google Scholar]
- Outlaw WH., Jr Critical examination of the quantitative evidence for and against photosynthetic CO2fixation by guard cells. Physiol Plant. 1989;77:275–281. [Google Scholar]
- Outlaw WH., Jr . Sucrose and stomata: a full circle. In: Madore MA, Lucus WJ, editors. Carbon Partitioning and Sucrose-Sink Interactions in Plants. Rockville, MD: American Society of Plant Physiologists; 1995. pp. 56–67. [Google Scholar]
- Outlaw WH, Jr, Manchester J. Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol. 1979;64:79–82. doi: 10.1104/pp.64.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Jr, Tarczynski MC. Guard cell starch biosynthesis regulated by effectors of ADP-glucose pyrophosphorylase. Plant Physiol. 1984;74:424–429. doi: 10.1104/pp.74.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Jr, Tarczynski MC, Miller WI. Histological compartmentation of phosphate in Vicia fabaL. leaflet: possible significance to stomatal functioning. Plant Physiol. 1984;74:430–433. doi: 10.1104/pp.74.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Jr, Zhang SQ. Single-cell dissection and microdroplet chemistry. J Exp Bot. 2001;52:605–614. [PubMed] [Google Scholar]
- Pego JV, Kortstee AJ, Huijser C, Smeekens SCM. Photosynthesis, sugars and the regulation of gene expression. J Exp Bot. 2000;51:407–416. doi: 10.1093/jexbot/51.suppl_1.407. [DOI] [PubMed] [Google Scholar]
- Poffenroth M, Green DB, Tallman G. Sugar concentrations in guard cells of Vicia fabailluminated with red or blue light: analysis by high performance liquid chromatography. Plant Physiol. 1992;98:1460–1471. doi: 10.1104/pp.98.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke K, Schnabl H. Availability of chloride affects the balance between potassium chloride and potassium malate in guard cells of Vicia fabaL. Plant Physiol. 1978;62:84–87. doi: 10.1104/pp.62.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckmann U, Scheibe R, Raschke K. Rubisco activity in guard cells compared with the solute requirement for stomatal opening. Plant Physiol. 1990;92:246–253. doi: 10.1104/pp.92.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JA. Mathematical Statistics and Data Analysis. Pacific Groove, CA: Wadsworth & Brooks/Cole Advanced Books & Software; 1987. [Google Scholar]
- Ritte G, Rosenfeld J, Rohrig K, Raschke K. Rates of sugar uptake by guard cell protoplasts of Pisum sativumL. related to the solute requirement for stomatal opening. Plant Physiol. 1999;121:647–655. doi: 10.1104/pp.121.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin G, Sakr S, Bonmort J, Delrot S. Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Lett. 1998;424:165–168. doi: 10.1016/s0014-5793(98)00165-3. [DOI] [PubMed] [Google Scholar]
- Sadka A, DeWald DB, May GD, Park WD, Mullet JE. Phosphate modulates transcription of soybean VspBand other sugar-inducible genes. Plant Cell. 1994;6:737–749. doi: 10.1105/tpc.6.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki KI, Zeiger E. Red light-dependent CO2 uptake and oxygen evolution in guard cell protoplasts of Vicia faba L.: evidence for photosynthetic CO2fixation. Plant Physiol. 1987;84:7–9. doi: 10.1104/pp.84.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. Sugar and organic acid accumulation in guard cells of Vicia fabain response to red and blue light. Plant Physiol. 1993;102:1163–1169. doi: 10.1104/pp.102.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. Central roles for potassium and sucrose in guard cell osmoregulation. Plant Physiol. 1996;111:1051–1057. doi: 10.1104/pp.111.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. The role of sucrose in guard cell osmoregulation. J Exp Bot. 1998;49:329–337. [Google Scholar]
- Tallman G, Zeiger E. Light quality and osmoregulation in Viciaguard cells: evidence for involvement of three metabolic pathways. Plant Physiol. 1988;88:887–895. doi: 10.1104/pp.88.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarczynski MC, Outlaw WH, Jr, Arold N, Neuhoff V, Hampp R. Electrophoretic assay for ribulose 1,5-bisphosphate carboxylase/oxygenase in guard cells and other leaf cells of Vicia fabaL. Plant Physiol. 1989;89:1088–1093. doi: 10.1104/pp.89.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn KC, Vaughan MA. Is ribulose bisphosphate carboxylase present in guard cell chloroplasts. Physiol Plant. 1988;74:409–413. [Google Scholar]
- Weber H, Borisjuk L, Heim U, Sauer N, Wobus U. A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell. 1997;9:895–908. doi: 10.1105/tpc.9.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers JDB, Meidner H. Methods in Stomatal Research. Essex, UK: Longman Scientific and Technical; 1990. [Google Scholar]
- Williams LE, Lemoine R, Sauer N. Sugar transporters in higher plants—a diversity of roles and complex regulation. Trends Plant Sci. 2000;5:283–290. doi: 10.1016/s1360-1385(00)01681-2. [DOI] [PubMed] [Google Scholar]
- Zeiger E. The biology of stomatal guard cells. Annu Rev Plant Physiol. 1983;34:441–475. [Google Scholar]
- Zhang SQ, Outlaw WH., Jr The guard-cell apoplast as a site of abscisic acid accumulation in Vicia fabaL. Plant Cell Environ. 2001a;24:347–355. [Google Scholar]
- Zhang SQ, Outlaw WH Jr (2001b) Abscisic acid introduced into the transpiration stream accumulates in the guard-cell apoplast and causes stomatal closure. Plant Cell Environ (in press)