Abstract
Dengue is the most common arboviral disease. It is typically spread by the bite of an infected female Aedes aegypti or Aedes albopictus mosquitoes. Dengue is endemic in subtropical and tropical regions, but its geographic reach keeps expanding. Ophthalmic manifestations of dengue are common and may present with a wide spectrum of ophthalmic findings. These may range from conjunctival petechiae, retinal hemorrhage, retinal vasculitis to panophthalmitis. Some of these may be vision threatening and may require urgent ophthalmic evaluation. The precise pathophysiologic mechanisms involved in dengue infection involve a complex interplay between host immune responses, virus, and host genes. There is no specific treatment for ocular dengue. Therefore, treatment is supportive. Despite the lack of proven efficacy, corticosteroids have been used in vision-threatening dengue-related ocular complications. Dengue must be considered in endemic areas, and a careful travel history needs to be elicited in nonendemic areas.
Keywords: Dengue maculopathy, dengue ocular, dengue retinopathy, foveolitis, mosquito ocular
Introduction
Arbovirus refers to an arthropod-borne virus or a virus carried by an arthropod. They serve as vectors of several viral diseases such as dengue. The dengue virus (DENV) is a single-stranded positive-sense 11 kb RNA virus comprising four different serotypes (DENV 1–4) with no cross-immunity among them, which belong to the Flavivirus genus of the family Flaviviridae. The viral genome is housed in the nucleocapsid which is enclosed by a lipid envelope. The DENV genome consists of three structural proteins (envelope, capsid, and premembrane) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5).[1]
DENV is commonly spread by the female mosquito of the Aedes genus, most commonly by Aedes aegypti and Aedes albopictus. The bite of an infected female mosquito inoculates the virus into the epidermis, dermis, and the bloodstream of a human. Macrophages, dendritic cells, and Langerhans cells become infected. The virus enters the cells via receptor-mediated endocytosis. Following endocytosis, the endosome containing the viral RNA undergoes pH-dependent fusion with a lysosome. The viral genome is then released into the cytoplasm and transported to the endoplasmic reticulum where it is translated. Posttranslational processing is performed at the Golgi apparatus. Mature viral particles are released from the infected cells via exocytosis. As these infected cells migrate to the regional lymph nodes, macrophages and monocytes are recruited to the lymph nodes which in turn become infected. Infection becomes disseminated via the bloodstream and lymphatic system.[2,3]
Dengue is the most common of the arboviral diseases. Dengue remains out of control as exemplified by the estimated 100–400 million yearly cases.[3,4] It is the only communicable disease that has grown four-fold from 2000 to 2013 and six-fold from 1999 to 2019.[5] Dengue is endemic in subtropical and tropical regions. Almost half of the world’s population, about 4 billion people, live in areas at risk of dengue.[6] Several factors are responsible for the explosive growth of dengue infections. Global warming has led to an increase in the average temperature and amount of rainfall which favor the proliferation of warmer environments in which the mosquitoes reproduce most efficiently. The increased ease of global trade and travel have facilitated the spread of both dengue and its vectors. The trade in used tires in particular has been implicated by collecting water and transporting mosquitoes long distances. Rapid international air travel allows infected travelers to arrive in nonendemic areas during their viremic period. During this period of time, invasive mosquitoes that are currently present in these nonendemic areas can bite the DENV-infected traveler and become infected. Infected mosquitoes can then bite other humans.[3,4,7,8,9,10,11]
Ocular manifestations in dengue fever (DF) are common.[12,13] With the recent explosion in the incidence and geographic distribution of dengue, it is very likely that the ocular manifestations will increase as well. The purpose of this narrative review is to describe the ocular manifestations of dengue.
Systemic Findings
Dengue has been classified into undifferentiated fever, DF, and dengue hemorrhagic fever (DHF). Depending on its severity, DHF was further subclassified into grades I through IV. Grades III and IV were defined as dengue shock syndrome. However, there have been multiple instances where cases could not be classified accordingly. Many have called for a new classification.[14] Individuals infected with the DENV become immune to re-infection with the same serotype. Most severe forms of dengue occur when a previously infected individual is re-infected with a different serotype. In these cases, preexisting antibodies from the primary infection react with dengue particles from a different serotype during the secondary infection.[15]
The clinical manifestations of dengue range from asymptomatic cases to hypovolemic shock, multi-organ failure, and death. Most cases are asymptomatic. When cases become symptomatic, dengue is characterized by an abrupt onset of fever following the incubation period which can range anywhere from 3 to 15 days. Rarely, prodromal signs that include headache, myalgia, chills, malaise, and backache may precede the onset of fever. During the first 2 days, a transient macular rash that blanches under pressure develops. Flushing of the face may appear. Days 2–6 are characterized by nausea, vomiting, cutaneous hyperalgesia, and generalized lymphadenopathy. The fever usually lasts 4–6 days. Viremia typically coincides with the fever. Defervescence is accompanied by profuse sweating. On the last day of fever or soon thereafter, a secondary maculopapular or morbilliform rash may appear for 1–5 days. As the secondary rash appears, fever re-appears giving rise to a saddleback fever profile. As the patient defervesces, the rash starts to fade as well. Petechiae over the lower extremities, hands, and the oral mucosa ensue. Thrombocytopenia and granulocytopenia are typical during this period of time. Most patients recover but in a small percentage, progression to severe disease characterized by plasma leakage with or without hemorrhage may occur. DHF is characterized by petechiae, purpura, gum bleeding, epistaxis, menorrhagia, and gastrointestinal hemorrhages.[16,17]
Diagnosis
The choice of laboratory tests will depend on the timing of patient presentation. Techniques that detect the virus, viral nucleic acid, or viral antigens are recommended during the first 5 days of illness during the febrile phase of the disease. After the 5th day, the virus and its antigen disappear just as specific antibodies begin to appear therefore serological tests such as immunoglobulin (Ig) M enzyme-linked immunoassay/rapid tests or IgG-paired acute and convalescent sera should be employed instead.[18] Cross-reactivity with other Flavivirus gives rise to false-positive results.[19]
Ocular Findings
The true incidence of ocular dengue remains unclear with different studies reporting rates anywhere from 7% to 40%.[20] Most of the patients reported in the literature are from Singapore and India. Affected patients are mostly young adults although the reported range includes patients from 14 to 73 years old.[21]
Dengue can develop a variety of eye symptoms which are common such as blurry vision, floaters, scotomas, micropsia, metamorphopsia, impairment of color vision, red eye, and ocular pain. Of these, blurry vision was the most common followed by scotomas.[21] Most ocular symptoms appear within 1 day of the nadir of thrombocytopenia.[22,23] The severity of the blurry vision and scotomas are directly correlated to the degree of macular edema and the severity of retinal hemorrhage.[22,24,25,26,27,28,29]
Patients present with a wide spectrum of ocular findings that may occur from days to months from the onset of fever.[20,25] Most cases are bilateral but asymmetric.[12] Ocular involvement may depend on DENV serotypes, host responses, and geography.[27] For instance, in the 2005 Singapore epidemic characterized by the DENV1 serotype, 10% of the hospitalized patients with dengue developed maculopathy. In comparison, none of the patients hospitalized during the 2007 Singapore epidemic characterized by the DENV2 serotype developed maculopathy.[27] Serotype-specific differences in dengue severity have been reported as well.[30,31]
Anterior Segment Findings
Conjunctival petechiae are seen in almost half of all patients.[19,20] Subconjunctival hemorrhage is a common finding occurring in up to 60% of cases and appears to be independent of the degree of platelet count. It can present unilaterally or bilaterally.[20,24,25,32,33]
Patients with iridocyclitis typically present with a progressive loss of vision, red eye, perilimbal injection, photophobia, keratic precipitates, and cells in the anterior chamber.[23,25,34,35] In certain patients, the anterior uveitis occurs 3–4 months following the full recovery of the acute febrile illness. In these patients, there were no ocular manifestations during the acute dengue infection.[25,35] In another set of patients, anterior uveitis presents concurrently with dengue maculopathy.[36]
Keratitis has been described since the first ocular dengue reports.[34] More recently punctate lower corneal erosions and complications secondary to exposure keratitis such as corneal perforation and peripheral corneal ulceration have been reported.[24,37] In a case series of 29 eyes of 23 Indian patients with DHF, 10% developed a scleral or corneal melt.[38] Very rarely necrotizing scleritis may occur.[39]
Posterior Segment Findings
The clinical findings in dengue retinopathy are typically inflammatory or occlusive in nature. Both inflammatory and occlusive manifestations may be present in the same eye. The most common posterior segment findings include posterior uveitis and maculopathy.[21]
Dengue maculopathy is typically bilateral but asymmetric. Furthermore, the findings may be different in each eye.[36] Dengue maculopathy typically presents a week after fever onset and coincides with the thrombocytopenia nadir. Maculopathy is typically characterized by parafoveal yellow spots, macular edema, dot blot or flame-shaped retinal hemorrhages, cotton wool spots, Roth spots, perifoveal telangiectasias, microaneurysms, and arteriolar sheathing.[21,27,36,40,41] The retinal capillaries may be involved at all levels giving rise to cotton wool spots, acute macular neuroretinopathy (AMN) lesions [Figure 1], and paracentral acute middle maculopathy (PAMM) lesions.[42,43,44,45,46,47,48]
Figure 1.
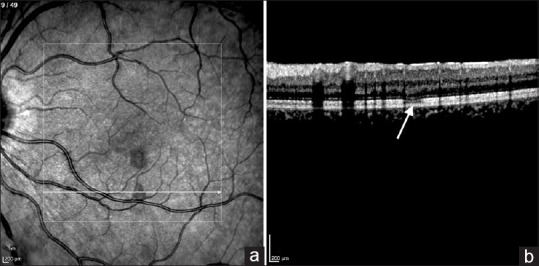
A 27-year-old woman with dengue who developed acute macular neuroretinopathy following dengue infection. (a) Infrared reflectance imaging of the left fundus demonstrating two oval-shaped hyporeflective lesions. (b) Spectral-domain optical coherence tomography horizontal scan across the oval-shaped hyporeflective lesion demonstrating attenuation of the ellipsoid zone
The term foveolitis was coined to describe a well-circumscribed, pale yellow-orange lesion at the center of the fovea which may occur as a solitary lesion or in the presence of other lesions such as vascular occlusions. On optical coherence tomography (OCT), this lesion is seen as a foveal retinal pigment epithelium (RPE)-outer retina thickening which disrupts the outer retina.[36,49] The degree of foveal thickening and the extent of foveolitis correlates with the severity of the central scotoma. Typically, foveolitis is not detected by fluorescein angiography (FA).
Posterior uveitis in dengue is commonly manifested as panuveitis, vitritis, intermediate uveitis, retinitis, chorioretinitis, and neuroretinitis[37] [Figure 2]. Other forms include acute posterior multifocal placoid pigment epitheliopathy, acute zonal outer retinopathy, and choroidal effusions.[50,51,52,53,54] Retinal venous occlusions have been reported to occur in 25% of eyes.[36] Retinal hemorrhages have been associated more frequently with vascular sheathing and vasculitis [Figure 3].[12,22,36,55,56] Eyes with severe and widespread retinal vascular leakage may develop neurosensory retinal detachments.[36] Extreme choroidal effusions have led to secondary angle closure glaucoma.[57] Panophthalmitis is a very rare complication that is usually associated with platelet transfusions.[58,59,60,61,62,63,64]
Figure 2.
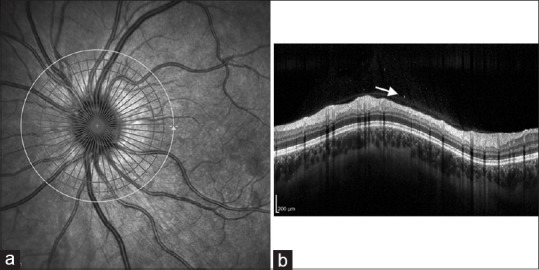
A 27-year-old woman with dengue who developed vitritis in her left eye. (a) Infrared reflectance imaging of the left optic nerve. (b) Spectral-domain optical coherence tomography circular scan of the retinal nerve fiber layer demonstrating hyper-reflective dots (arrow) in the vitreous cavity
Figure 3.
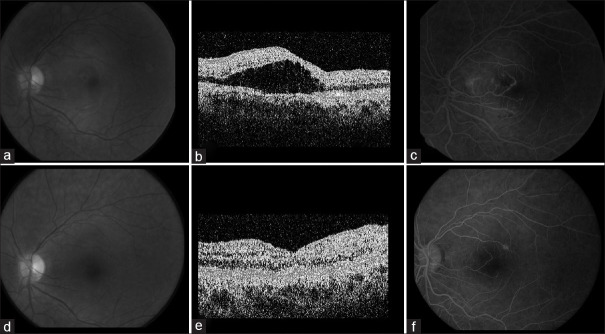
A 20-year-old woman with dengue developed flu-like symptoms and central visual loss of her left eye. The patient was treated with 60 mg of prednisone for 2 weeks. (a) Fundus photograph of the left eye demonstrating loss of the foveal reflex suggesting macular edema. (b) Time-domain optical coherence tomography (TD-OCT) demonstrating macular edema. (c) Fluorescein angiogram demonstrating late leakage of macular retinal vessels. (d) Fundus photograph of the left eye 2 weeks postprednisone demonstrating resolution of macular edema. (e) TD-OCT 2 weeks postprednisone demonstrating resolution of macular edema. (f) Fluorescein angiogram 2 weeks postprednisone demonstrating resolution of fluorescein leakage
Neuro-ophthalmic Findings
Optic neuropathy has been described in multiple case studies in the forms of optic disc swelling, hyperemia, hemorrhages, and neuroretinitis. Some of these patients developed optic atrophy and lost visual acuity to no light perception.[21,36,41,65,66,67] Other neuro-ophthalmic complications include neuromyelitis optica, proptosis due to panophthalmitis or retrobulbar hemorrhage, and cranial nerve III and VI palsies.[21,34,37,68] Severe thrombocytopenia may cause a retrobulbar hemorrhage accompanied by vitreous and suprachoroidal hemorrhage that leads to globe perforation and a phthisical eye.[37,68]
Multimodal Imaging
Multimodal imaging with infrared reflectance imaging, FA, indocyanine green angiography (ICG-A), OCT, and OCT angiography (OCT-A) are useful ancillary tools in the assessment of the posterior segment manifestations of dengue.
Retinal vascular occlusion or leakage is easily assessed with FA[36] [Figure 3]. ICG-A may detect the areas of choroidal involvement that are not clinically evident, particularly the yellow subretinal dots.[36] OCT is a useful tool for both diagnostic and monitoring purposes. Macular edema is best diagnosed by an OCT. Time-domain OCT patterns serve as predictors of visual outcome in dengue maculopathy. Type 1 is characterized by a diffuse edema, type 2 describes cystoid macular edema, and type 3 foveolitis.[26] Spectral-domain OCT in conjunction with OCTA is very helpful in characterizing and defining retinal capillary and choriocapillaris involvement [Figure 1]. Acutely, a paracentral hyper-reflective band is observed. Chronically, the hyper-reflective bands lose their hyper-reflectivity and thin out. OCTA has been used to describe diminished flow signals in the different retinal capillary plexuses and the choriocapillaris.[42,43,44] Electrophysiological tests demonstrate that the inner retina is spared whereas the outer and middle retina are affected in dengue maculopathy.[69]
Mechanisms of Ocular Disease
The precise pathophysiologic mechanisms involved in dengue infection are complex and remain largely unknown. There is a complex interplay between host immune responses, virus, and host genes.[70] Viral factors include variations in the DENV genome that may make the virus more virulent, subgenomic Flavivirus RNA (sfRNA), and DENV NS1.[70] During replication of the DENV RNA genome, the host’s exoribonuclease incompletely breaks it down generating small RNA (0.3–0.5 kb) fragments. These small RNA fragments, also known as sfRNA, accumulate and suppress the host’s antiviral defenses, particularly interferon signaling.[70] A direct cytopathic effect of DENV on ocular cells may contribute to ocular dengue. An in vitro study has shown that both human RPE and retinal endothelial cells are susceptible to infection by the DENV. However, it appears that the RPE is more susceptible than the retinal endothelial cells. Unlike retinal endothelial cells, RPE cells lose their F-actin cellular structure and express less junctional molecules causing increased permeability.[71]
On the other hand, there are several lines of evidence that suggest that pathological immunological responses are at play. Systemically, DENV infections are most severe at the time that the virus is being cleared by the host immune system rather than when the viremia load is at its peak.[72] The late presentation following some cases of anterior uveitis suggests that immune mechanisms are at play.[25,35] In addition, the typical 1-week delay between the onset of fever and visual symptoms that is characteristic of dengue maculopathy also points toward immunological responses rather than direct DENV infection.[13,26,36,44] Serum complement 3 levels are significantly lower in patients with maculopathy.[13]
NS1 has been implicated in the pathogenesis of dengue. During the acute phase of the disease, the NS1 levels are markedly elevated. These levels are correlated with the severity of dengue infection.[73] NS1 activates macrophages and peripheral blood monocytes via toll-like receptor 4 to produce and release inflammatory cytokines which disrupt the endothelial cell monolayer integrity.[74] NS1 also activates the alternative complement system leading to the activation of the membrane attack complex which lyses the target cells and releases a host of pro-inflammatory cytokines.
Host factors include antibody-dependent enhancement, anti-NS1 antibodies, and T-cells.[70] Antibody-dependent enhancement occurs when an individual is secondarily infected with a different DENV serotype. Antibodies to the original DENV serotype cross-react but do not neutralize the secondary DENV forming virion–antibody immune complexes. Host phagocytes expressing the Fcɣ receptor recognize and uptake these immune complexes causing the amplification of viral replication and the release of pro-inflammatory cytokines. Concurrently, there is an enhanced production of interleukin (IL)-10 which skews the immune response toward TH2. TH2 has a limited antiviral effect and is unable to clear the DENV prolonging the infection. Furthermore, elevated IL-10 levels lead to T-cell apoptosis which reduces DENV clearance.[70,75] Ocular autoantibody production may account for the ocular manifestations of DENV.[12] The DENV may induce autoimmunity via molecular mimicry.[76] Antibodies directed against NS1 may cross-react with human platelets and endothelial cells causing thrombocytopenia and an increased vascular permeability.[75]
The release of pro-inflammatory cytokines by all the different mechanisms mentioned above leads to retinal vascular occlusion or a breakdown of the blood-retinal barrier. This explains the common findings of macular edema and retinal hemorrhages. Immune complex deposition may occlude different retinal capillary plexuses giving rise to cotton wool spots and PAMM and AMN lesions. Thrombocytopenia may serve as a potential factor contributing to the onset of AMN.[20,47,77] Ocular hemorrhages are related to the severity of thrombocytopenia.[20]
Current Treatments
Development of an anti-dengue vaccine is challenging since it requires to be equally preventative against all four serotypes to reduce the risk of immune-mediated enhancement.[78] Since 2015, the CYD-TDV (Dengvaxia) vaccine has been approved in several countries. It is a tetravalent live attenuated vaccine where the DENV proteins prM and E of each one of the four serotypes were included. The reported efficacy was 66%, but those with the DENV2 serotype only achieved a 43% efficacy.[79] Furthermore, seronegative individuals had a lower efficacy than those who were seropositive at the time of vaccination. Furthermore, there was an unexplained increase in hospitalizations in children ≤9 years of age when compared to unvaccinated children. Therefore, Dengvaxia is only recommended in dengue-endemic areas where the seropositivity is at least 70% and only in individuals 9–45 years of age.[80]
Treatment is supportive. There is no known treatment for dengue maculopathy. Despite the lack of proven efficacy, corticosteroids have been used.[36,41] The anterior uveitis responds well to topical corticosteroids.[26,35] Patients with extensive retinal vasculitis and exudative retinal detachments and poor vision upon presentation did not show an improvement despite aggressive treatment with systemic corticosteroids.[23]
Visual Outcomes
Ocular manifestations typically resolve spontaneously, and the prognosis is overall favorable.[20,21,22,41] Typically, the visual prognosis for anterior segment manifestations is better than the posterior segment.[81] Most patients with maculopathy regain a best-corrected visual acuity ≥20/40.[26] Recovery time varies from days to months. However, 5%–8% of eyes may develop permanent visual loss.[13] In eyes with maculopathy, scotomata may persist for ≥2 years even after resolution of the disease. Foveolitis is a marker for poor vision. Only 50% of eyes recuperated a visual acuity ≥20/40. These eyes are also at an increased risk of persistent scotomata.[26] The visual outcome of eyes that develop retinal vasculitis will depend on the degree of ischemia and maculopathy.[82] Patients with extensive retinal vasculitis and exudative retinal detachments and poor vision upon presentation did not show an improvement despite aggressive treatment with systemic corticosteroids.[23] Panophthalmitis typically portends blindness and even phthisis.[58,59,60,61,62,63,64]
Future Directions
As illustrated by the ever-increasing number of global cases, current mosquito vector control measures have been inadequate. These have typically relied on using insecticides and breeding site reduction. Mosquitoes are some of the most ubiquitous and resilient organisms in the planet evolving resistance to these insecticides.[83]
Suppressing wild-type mosquito populations with biological and genetic manipulations are promising methods to better control the vectors of disease.[84,85] Wolbachia pipientis is a natural bacterial parasite of insects that has an inhibitory effect on the replication of positive sense RNA viruses. Wolbachia endosymbionts of A. aegypti that block DENV transmission have been deployed into wild-type A. aegypti populations. The Wolbachia then spreads via normal mating, and eventually, most wild-type mosquitoes become infected with the Wolbachia.[84] Alternatively, sterile male mosquitoes induced by different means such as precision-guided sterile insect technique or the radiation-based sterile insect technique have also been released into wild-type mosquito populations.[85]
There are no specific anti-dengue treatments available at the time, and this represents a huge unmet medical need.
Method of Literature Search
The PubMed database was searched in December 2022 and again on June 15, 2023. The search strategy was as follows: (Dengue) and (ocular) OR (retina) OR (macula). There were no language filters or restriction for publication date. All articles were screened by their titles and abstracts by the senior author (LW) for relevance. The full-text articles were retrieved and distributed among the rest of the authors. The reference list of each retrieved full-text article was scanned for any omissions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patients consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Conflicts of interest
Dr. Lihteh Wu received lecture fees from Bayer, Roche, and Lumibird Medical. None of the other authors have anything to declare.
Funding Statement
Nil
References
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: A continuing global threat. Nat Rev Microbiol. 2010;8:S7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henchal EA, Putnak JR. The dengue viruses. Clin Microbiol Rev. 1990;3:376–96. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nat Rev Dis Primers. 2016;2:16055. doi: 10.1038/nrdp.2016.55. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.New Delhi, India: WHO; 2021. WHO. Virtual Meeting of Regional Technical Advisory Group for Dengue and Other Arbovirus Diseases. [Google Scholar]
- 6.Gubler DJ. Dengue, urbanization and globalization: The unholy trinity of the 21(st) century. Trop Med Health. 2011;39:3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36:143–52. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 8.Simmons CP, Farrar JJ, Nguyen VV, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 9.Thomson MC, Stanberry LR. Climate change and vectorborne diseases. N Engl J Med. 2022;387:1969–78. doi: 10.1056/NEJMra2200092. [DOI] [PubMed] [Google Scholar]
- 10.Kamal M, Kenawy MA, Rady MH, Khaled AS, Samy AM. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS One. 2018;13:e0210122. doi: 10.1371/journal.pone.0210122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romi R. History and updating on the spread of Aedes albopictus in Italy. Parassitologia. 1995;37:99–103. [PubMed] [Google Scholar]
- 12.Lim WK, Mathur R, Koh A, Yeoh R, Chee SP. Ocular manifestations of dengue fever. Ophthalmology. 2004;111:2057–64. doi: 10.1016/j.ophtha.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Su DH, Bacsal K, Chee SP, Flores JV, Lim WK, Cheng BC, et al. Prevalence of dengue maculopathy in patients hospitalized for dengue fever. Ophthalmology. 2007;114:1743–7. doi: 10.1016/j.ophtha.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Bandyopadhyay S, Lum LC, Kroeger A. Classifying dengue: A review of the difficulties in using the WHO case classification for dengue haemorrhagic fever. Trop Med Int Health. 2006;11:1238–55. doi: 10.1111/j.1365-3156.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 15.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–8. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer TJ, Panda PK, Wolford RW. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Dengue fever. [Google Scholar]
- 17.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucky P, Peeling R. New edition. Geneva: World Health Organization; 2009. Laboratory Diagnosis and Diagnostic Tests: Dengue Guidelines for Diagnosis, Treatment, Prevention and Control; pp. 93–4. [Google Scholar]
- 19.Merle H, Donnio A, Jean-Charles A, Guyomarch J, Hage R, Najioullah F, et al. Ocular manifestations of emerging arboviruses: Dengue fever, Chikungunya, Zika virus, West Nile virus, and yellow fever. J Fr Ophtalmol. 2018;41:e235–43. doi: 10.1016/j.jfo.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Kapoor HK, Bhai S, John M, Xavier J. Ocular manifestations of dengue fever in an East Indian epidemic. Can J Ophthalmol. 2006;41:741–6. doi: 10.3129/i06-069. [DOI] [PubMed] [Google Scholar]
- 21.Yip VC, Sanjay S, Koh YT. Ophthalmic complications of dengue fever: A systematic review. Ophthalmol Ther. 2012;1:2. doi: 10.1007/s40123-012-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan DP, Teoh SC, Tan CS, Nah GK, Rajagopalan R, Prabhakaragupta MK, et al. Ophthalmic complications of dengue. Emerg Infect Dis. 2006;12:285–9. doi: 10.3201/eid1202.050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teoh SC, Chan DP, Nah GK, Rajagopalan R. A re-look at ocular complications in dengue fever and dengue hemorrhagic fever. Dengue Bull. 2006;30:184–90. [Google Scholar]
- 24.Chhavi N, Venkatesh C, Soundararajan P, Gunasekaran D. Unusual ocular manifestations of dengue fever in a young girl. Indian J Pediatr. 2013;80:522–3. doi: 10.1007/s12098-012-0871-0. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Srinivasan R, Setia S, Soundravally R, Pandian DG. Uveitis following dengue fever. Eye (Lond) 2009;23:873–6. doi: 10.1038/eye.2008.124. [DOI] [PubMed] [Google Scholar]
- 26.Teoh SC, Chee CK, Laude A, Goh KY, Barkham T, Ang BS, et al. Optical coherence tomography patterns as predictors of visual outcome in dengue-related maculopathy. Retina. 2010;30:390–8. doi: 10.1097/IAE.0b013e3181bd2fc6. [DOI] [PubMed] [Google Scholar]
- 27.Chee E, Sims JL, Jap A, Tan BH, Oh H, Chee SP. Comparison of prevalence of dengue maculopathy during two epidemics with differing predominant serotypes. Am J Ophthalmol. 2009;148:910–3. doi: 10.1016/j.ajo.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Humayoun MA, Waseem T, Jawa AA, Hashmi MS, Akram J. Multiple dengue serotypes and high frequency of dengue hemorrhagic fever at two tertiary care hospitals in Lahore during the 2008 dengue virus outbreak in Punjab, Pakistan. Int J Infect Dis. 2010;14(Suppl 3):e54–9. doi: 10.1016/j.ijid.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Seet RC, Quek AM, Lim EC. Symptoms and risk factors of ocular complications following dengue infection. J Clin Virol. 2007;38:101–5. doi: 10.1016/j.jcv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Balmaseda A, Hammond SN, Pérez L, Tellez Y, Saborío SI, Mercado JC, et al. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg. 2006;74:449–56. [PubMed] [Google Scholar]
- 31.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 32.Mehta S, Mehta S, Jiandani P. Ocular features of dengue septic shock (DSS) J Assoc Physicians India. 2006;54:866. [PubMed] [Google Scholar]
- 33.Mehta S. Ocular lesions in severe dengue hemorrhagic fever (DHF) J Assoc Physicians India. 2005;53:656–7. [PubMed] [Google Scholar]
- 34.Richardson S. Ocular symptoms and complications observed in dengue. Trans Am Ophthalmol Soc. 1933;31:450–77. [PMC free article] [PubMed] [Google Scholar]
- 35.Antlanger M, Shaw SJ, Kurup SK. Presumed dengue-associated immune-mediated uveitis. Can J Ophthalmol. 2011;46:92–3. doi: 10.3129/i10-071. [DOI] [PubMed] [Google Scholar]
- 36.Bacsal KE, Chee SP, Cheng CL, Flores JV. Dengue-associated maculopathy. Arch Ophthalmol. 2007;125:501–10. doi: 10.1001/archopht.125.4.501. [DOI] [PubMed] [Google Scholar]
- 37.Ng AW, Teoh SC. Dengue eye disease. Surv Ophthalmol. 2015;60:106–14. doi: 10.1016/j.survophthal.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Vijitha VS, Dave TV, Murthy SI, Ali MJ, Dave VP, Pappuru RR, et al. Severe ocular and adnexal complications in dengue hemorrhagic fever: A report of 29 eyes. Indian J Ophthalmol. 2021;69:617–22. doi: 10.4103/ijo.IJO_1588_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamoi K, Mochizuki M, Ohno-Matsui K. Dengue fever-associated necrotizing scleritis: A case report with long-term follow-up. Medicine (Baltimore) 2018;97:e11875. doi: 10.1097/MD.0000000000011875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nah G, Tan M, Teoh S, Chong CH. Maculopathy associated with dengue fever in a military pilot. Aviat Space Environ Med. 2007;78:1064–7. doi: 10.3357/asem.2138.2007. [DOI] [PubMed] [Google Scholar]
- 41.Teoh SC, Chan DP, Nah GK, Rajagopalan R, Laude A, Ang B. Eye institute dengue-related ophthalmic complications workgroup. A re-look at ocular complications in dengue fever and dengue haemorrhagic fever. Dengue Bull. 2006;30:184–93. [Google Scholar]
- 42.Aggarwal K, Agarwal A, Katoch D, Sharma M, Gupta V. Optical coherence tomography angiography features of acute macular neuroretinopathy in dengue fever. Indian J Ophthalmol. 2017;65:1235–8. doi: 10.4103/ijo.IJO_485_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guardiola GA, Villegas VM, Cruz-Villegas V, Schwartz SG. Acute macular neuroretinopathy in dengue virus serotype 1. Am J Ophthalmol Case Rep. 2022;25:101250. doi: 10.1016/j.ajoc.2021.101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Zhang X, Ji Y, Ye B, Wen F. Acute macular neuroretinopathy in dengue fever: Short-term prospectively followed up case series. JAMA Ophthalmol. 2015;133:1329–33. doi: 10.1001/jamaophthalmol.2015.2687. [DOI] [PubMed] [Google Scholar]
- 45.Rajurkar K, Thakar M. Optical coherence tomography angiography features of dengue retinopathy manifesting as acute macular neuroretinopathy, branch vein vasculitis and neurosensory detachment. Oman J Ophthalmol. 2023;16:177–9. doi: 10.4103/ojo.ojo_210_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Translateur A, Perez-Rueda M. Acute macular neuroretinopathy associated to dengue disease. Am J Ophthalmol Case Rep. 2022;26:101474. doi: 10.1016/j.ajoc.2022.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munk MR, Jampol LM, Cunha Souza E, de Andrade GC, Esmaili DD, Sarraf D, et al. New associations of classic acute macular neuroretinopathy. Br J Ophthalmol. 2016;100:389–94. doi: 10.1136/bjophthalmol-2015-306845. [DOI] [PubMed] [Google Scholar]
- 48.Ooi KG, Inglis H, Paramanathan N, Downie JA, Hennessy MP. Dengue fever-associated maculopathy and panuveitis in Australia. Case Rep Ophthalmol Med. 2016;2016:5704695. doi: 10.1155/2016/5704695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loh BK, Bacsal K, Chee SP, Cheng BC, Wong D. Foveolitis associated with dengue fever: A case series. Ophthalmologica. 2008;222:317–20. doi: 10.1159/000144074. [DOI] [PubMed] [Google Scholar]
- 50.Cruz-Villegas V, Berrocal AM, Davis JL. Bilateral choroidal effusions associated with dengue fever. Retina. 2003;23:576–8. doi: 10.1097/00006982-200308000-00031. [DOI] [PubMed] [Google Scholar]
- 51.Teh D, Azira SA, Hassan M, Loo AV, Iqbal TB, Subrayan V. Ocular manifestations of dengue fever in University Malaya Medical Centre. Trop Biomed. 2016;33:799–806. [PubMed] [Google Scholar]
- 52.Goldhardt R, Patel H, Davis JL. Acute posterior multifocal placoid pigment epitheliopathy following dengue fever: A new association for an old disease. Ocul Immunol Inflamm. 2016;24:610–4. doi: 10.3109/09273948.2015.1125513. [DOI] [PubMed] [Google Scholar]
- 53.Tabbara K. Dengue retinochoroiditis. Ann Saudi Med. 2012;32:530–3. doi: 10.5144/0256-4947.2012.30.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai TY, Mohamed S, Chan WM, Lai RY, Lam DS. Multifocal electroretinography in dengue fever-associated maculopathy. Br J Ophthalmol. 2007;91:1084–5. doi: 10.1136/bjo.2006.104240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quek DT, Barkham T, Teoh SC. Recurrent bilateral dengue maculopathy following sequential infections with two serotypes of dengue virus. Eye (Lond) 2009;23:1471–2. doi: 10.1038/eye.2008.149. [DOI] [PubMed] [Google Scholar]
- 56.Tan CS, Teoh SC, Chan DP, Wong IB, Lim TH. Dengue retinopathy manifesting with bilateral vasculitis and macular oedema. Eye (Lond) 2007;21:875–7. doi: 10.1038/sj.eye.6702748. [DOI] [PubMed] [Google Scholar]
- 57.Pierre Filho Pde T, Carvalho Filho JP, Pierre ET. Bilateral acute angle closure glaucoma in a patient with dengue fever: Case report. Arq Bras Oftalmol. 2008;71:265–8. doi: 10.1590/s0004-27492008000200025. [DOI] [PubMed] [Google Scholar]
- 58.Arya D, Das S, Shah G, Gandhi A. Panophthalmitis associated with scleral necrosis in dengue hemorrhagic fever. Indian J Ophthalmol. 2019;67:1775–7. doi: 10.4103/ijo.IJO_2050_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanjay S, Agrawal S, Jain P, Mahendradas P, Kawali A, Shetty N. Permanent visual impairment in dengue fever following platelet transfusion: A series of 5 cases. Ann Acad Med Singap. 2021;50:588–92. [PubMed] [Google Scholar]
- 60.Saranappa SB, Sowbhagya HN. Panophthalmitis in dengue fever. Indian Pediatr. 2012;49:760. doi: 10.1007/s13312-012-0142-1. [DOI] [PubMed] [Google Scholar]
- 61.Dave TV, Sharma S, Lakshmi V, Rangaiahgari A, Murthy SI, Ali MJ, et al. Evidence of dengue virus in eviscerated specimens of panophthalmitis secondary to dengue fever: A possible cause-effect phenomenon. Indian J Ophthalmol. 2022;70:965–9. doi: 10.4103/ijo.IJO_1732_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamal R, Shah D, Sharma S, Janani MK, Kar A, Saurabh K, et al. Culture-positive unilateral panophthalmitis in a serology-positive case of dengue hemorrhagic fever. Indian J Ophthalmol. 2018;66:1017–9. doi: 10.4103/ijo.IJO_113_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar V, Deorari V, Swaroop S, Biswas A. Panophthalmitis in a patient with dengue fever. BMJ Case Rep. 2019;12:e229588. doi: 10.1136/bcr-2019-229588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramananda K, Sundar M D, Mandal S, Ravani R, Kumar V. Platelet transfusion related panophthalmitis and endophthalmitis in patients with dengue hemorrhagic fever. Am J Trop Med Hyg. 2018;99:1053–4. doi: 10.4269/ajtmh.18-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beral L, Merle H, David T. Ocular complications of dengue fever. Ophthalmology. 2008;115:1100–1. doi: 10.1016/j.ophtha.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 66.Sanjay S, Wagle AM, Au Eong KG. Optic neuropathy associated with dengue fever. Eye (Lond) 2008;22:722–4. doi: 10.1038/eye.2008.64. [DOI] [PubMed] [Google Scholar]
- 67.Carrillo-Soto MA. Case series of ocular involvement due to dengue. First reported cases in Guatemala. Arch Soc Esp Oftalmol (Engl Ed) 2018;93:329–35. doi: 10.1016/j.oftal.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Nagaraj KB, Jayadev C, Yajmaan S, Prakash S. An unusual ocular emergency in severe dengue. Middle East Afr J Ophthalmol. 2014;21:347–9. doi: 10.4103/0974-9233.142276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chia A, Luu CD, Mathur R, Cheng B, Chee SP. Electrophysiological findings in patients with dengue-related maculopathy. Arch Ophthalmol. 2006;124:1421–6. doi: 10.1001/archopht.124.10.1421. [DOI] [PubMed] [Google Scholar]
- 70.Bhatt P, Sabeena SP, Varma M, Arunkumar G. Current understanding of the pathogenesis of dengue virus infection. Curr Microbiol. 2021;78:17–32. doi: 10.1007/s00284-020-02284-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carr JM, Ashander LM, Calvert JK, Ma Y, Aloia A, Bracho GG, et al. Molecular responses of human retinal cells to infection with dengue virus. Mediators Inflamm 2017. 2017 doi: 10.1155/2017/3164375. 3164375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis. 2006;19:429–36. doi: 10.1097/01.qco.0000244047.31135.fa. [DOI] [PubMed] [Google Scholar]
- 73.Thomas L, Najioullah F, Verlaeten O, Martial J, Brichler S, Kaidomar S, et al. Relationship between nonstructural protein 1 detection and plasma virus load in Dengue patients. Am J Trop Med Hyg. 2010;83:696–9. doi: 10.4269/ajtmh.2010.10-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, et al. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med. 2015;7:304ra142. doi: 10.1126/scitranslmed.aaa3863. [DOI] [PubMed] [Google Scholar]
- 75.Lucena-Neto FD, Falcão LF, Moraes EC, David JP, Vieira-Junior AS, Silva CC, et al. Dengue fever ophthalmic manifestations: A review and update. Rev Med Virol. 2023;33:e2422. doi: 10.1002/rmv.2422. [DOI] [PubMed] [Google Scholar]
- 76.Lin YS, Yeh TM, Lin CF, Wan SW, Chuang YC, Hsu TK, et al. Molecular mimicry between virus and host and its implications for dengue disease pathogenesis. Exp Biol Med (Maywood) 2011;236:515–23. doi: 10.1258/ebm.2011.010339. [DOI] [PubMed] [Google Scholar]
- 77.Hwang CK, Sen HN. Concurrent vascular flow defects at the deep capillary plexus and choriocapillaris layers in acute macular neuroretinopathy on multimodal imaging: A case series. Am J Ophthalmol Case Rep. 2020;20:100866. doi: 10.1016/j.ajoc.2020.100866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scherwitzl I, Mongkolsapaja J, Screaton G. Recent advances in human flavivirus vaccines. Curr Opin Virol. 2017;23:95–101. doi: 10.1016/j.coviro.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372:113–23. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 80.Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373:1195–206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 81.Ranjan R, Ranjan S. Ocular pathology: Role of emerging viruses in the Asia-Pacific region-a review. Asia Pac J Ophthalmol (Phila) 2014;3:299–307. doi: 10.1097/APO.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 82.Siqueira RC, Vitral NP, Campos WR, Oréfice F, de Moraes Figueiredo LT. Ocular manifestations in Dengue fever. Ocul Immunol Inflamm. 2004;12:323–7. doi: 10.1080/092739490500345. [DOI] [PubMed] [Google Scholar]
- 83.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017;11:e0005625. doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ant TH, Mancini MV, McNamara CJ, Rainey SM, Sinkins SP. Wolbachia-virus interactions and arbovirus control through population replacement in mosquitoes. Pathog Glob Health. 2023;117:245–58. doi: 10.1080/20477724.2022.2117939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li M, Yang T, Bui M, Gamez S, Wise T, Kandul NP, et al. Suppressing mosquito populations with precision guided sterile males. Nat Commun. 2021;12:5374. doi: 10.1038/s41467-021-25421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.