Abstract
Chemotherapy treatment-related side effects are common and increase the risk of suboptimal outcomes. Exercise interventions during cancer treatment improve self-reported physical functioning, fatigue, anxiety, and depression, but it is unclear whether these interventions improve important clinical outcomes, such as chemotherapy relative dose intensity. The National Cancer Institute funded the Exercise and Nutrition to Improve Cancer Treatment-Related Outcomes (ENICTO) Consortium to address this knowledge gap. This article describes the mechanisms hypothesized to underpin intervention effects on clinically relevant treatment outcomes, briefly outlines each project’s distinct research aims, summarizes the scope and organizational structure of ENICTO, and provides an overview of the integrated common data elements used to pursue research questions collectively. In addition, the article includes a description of consortium-wide activities and broader research community opportunities for collaborative research. Findings from the ENICTO Consortium have the potential to accelerate a paradigm shift in oncology care such that patients with cancer could receive exercise and nutrition programming as the standard of care in tandem with chemotherapy to improve relative dose intensity for a curative outcome.
Despite advances in chemotherapy, treatment-related side effects are common and increase the risk of suboptimal outcomes because patients may be unable to complete guideline-concordant therapy. Cancer treatment-related outcomes include chemotherapy dose reductions and delays, related health-care utilization, and chemotherapy dose-limiting toxicities that occur in close proximity to cancer treatment for curative or life-extending intent (1). Lack of exercise, low physiologic reserves, poor diet, malnutrition, and changes in body composition may increase the risk of adverse treatment-related outcomes (2-8). Exercise and nutrition interventions are known to improve fitness and body composition and can be delivered concurrently with cancer therapies (4,6,9,10). The effects of specific exercise and nutrition therapy on chemotherapy-related outcomes are not well characterized. Most exercise and nutrition intervention research to date has focused on primary cancer prevention or on improving long-term outcomes after the completion of cancer treatment rather than the period shortly before or during chemotherapy, and few studies have targeted a treatment-related outcome as a primary endpoint (11,12).
Evidence is needed to understand the optimal type, frequency, intensity, duration, and overall volume (ie, dose) of exercise necessary to improve cancer treatment-related outcomes. Medical nutrition interventions aimed at treating or preventing malnutrition during treatment, alone or combined with exercise, have been associated with improved maintenance of lean body mass, fewer adverse events, and decreased length of hospital stay in select cancer populations (13). Heterogeneity in the interventions and other methodological challenges, however, have precluded translation into clinical practice guidelines. Given the limited body of specific evidence to guide exercise oncology and oncology nutrition practice, current national health promotion recommendations advise people living with and beyond cancer generally to consume a healthy diet and maintain or pursue recommended levels of physical activity (4,14,15); they also highlight the need for further research to determine intervention effects on cancer treatment-related outcomes.
The Exercise and Nutrition Interventions to Improve Cancer Treatment-Related Outcomes (ENICTO) Consortium was established by the National Cancer Institute (NCI) to determine whether specific exercise prescriptions and medical nutrition interventions before or during chemotherapy affect the receipt of the planned prescribed dose of cancer treatment and related factors. Five projects (4 trials and 1 coordinating center) were selected, by peer review, for funding as part of the ENICTO Consortium (https://www.enicto.bcs.gwu.edu). ENICTO requires that each trial address independent aims, while ENICTO’s consortium structure provides an opportunity for methodological and data harmonization and coordination to pursue research questions collectively that would not be possible individually by harnessing the harmonized dataset that will arise from the 4 trials. This commentary describes the mechanisms hypothesized to underpin intervention effects on clinically relevant treatment outcomes, briefly outlines the distinct research aims of each project, summarizes the scope and organizational structure of ENICTO, and provides an overview of the integrated common data elements that can be used to pursue research questions collectively.
Targeted mechanisms
Exercise and nutrition may drive cancer-related treatment outcomes through several potential mechanisms. Each ENICTO trial prespecified several hypothesized mechanisms by which exercise and nutrition interventions are hypothesized to improve relative dose intensity, the primary outcome across all 4 ENICTO trials (Table 1; Supplementary Figure 1, available online).
Table 1.
Exercise and Nutrition to Improve Treatment-Related Outcomes hypothesized mechanisms by which exercise (and nutrition) may influence relative dose intensity
| Primary hypothesized mechanism | Secondary hypothesized mechanism | Harmonized mechanistic data | |
|---|---|---|---|
| Adaptive Randomization of Aerobic Exercise during Chemotherapy in Colon Cancer | Exercise-induced reductions in fat mass and preservation of lean mass improve the pharmacokinetic properties of chemotherapy | Exercise-induced changes in neutrophil counts, phenotypes, and functions will decrease the probability of developing neutropenia | Anthropometric circumferences, patient-reported symptoms, and nutrition status assessment |
| Tele-exercise during chemotherapy Trial | Reduce systemic reactive oxygen species, improve hematopoietic stem and progenitor cell differentiation and neutrophil apoptosis, and improve hematological profile (eg, neutrophil count, hemoglobin) | N/A | Body composition, patient-reported symptoms |
| Trial of Exercise And Lifestyle | Patient-reported chemotoxicities (neuropathy, arthralgia, cognition, depression, gastrointestinal symptoms), muscle mass, and nutritional status | Inflammation, fatigue, sleep, physical function, improved white cell function | N/A |
| TeleHealth Resistance exercise Intervention to preserve dose intensity and Vitality in Elder Breast Cancer Patients | Preservation of muscle mass, patient reported symptoms nutritional status (protein) | Modifiable elements of the Geriatric Assessment (eg, functional status, depressive symptoms, symptoms of distress) | Patient-reported symptoms |
For example, the primary hypothesized mechanisms through which the aerobic exercise program prescribed in the Adaptive Randomization of Aerobic Exercise during Chemotherapy in Colon Cancer (ACTION) trial is anticipated to improve relative dose intensity are exercise-induced reductions in fat mass and preservation of lean mass, which will, in turn, improve the pharmacokinetic properties of chemotherapy. The Tele-exercise during chemotherapy Trial (TNT) hypothesizes that chemotherapy may also impair hematological function by direct suppression of hematopoiesis and by increasing reactive oxygen species (16,17), which in turn induce apoptosis in circulating neutrophils (18) and hematopoietic stem and progenitor cells (19). Such changes ultimately reduce neutrophil counts and suppress the generation of mature blood cells (eg, neutrophils, erythrocytes). In related clinical work, exercise training reduces systemic reactive oxygen species (20) and promotes rapid hemoglobin and neutrophil recovery after every chemotherapy cycle in people living with and beyond cancer (21,22).
The Trial of Exercise And Lifestyle (TEAL) presumes that chemotherapy causes a decrease in muscle mass and that aerobic and resistance exercise and medical nutrition therapy may improve these outcomes as well as patient-reported chemotoxicities improving relative dose integrity.
TeleHealth Resistance exercise Intervention to preserve dose intensity and Vitality in Elder Breast Cancer Patients (THRIVE-65) hypothesizes that maintenance of muscle mass through resistance training and protein supplementation will lead to improved physical function and fewer patient-reported symptoms, including neuropathy (23), gastrointestinal (GI) distress (24), and fatigue (25). Severe symptom burden among older patients (≥ 65 years of age) could lead to alterations in medical management. Patients may further decrease physical activity because of symptoms while undergoing chemotherapy (26). Findings from randomized controlled trials, however, suggest that exercise and nutrition may improve these common chemotherapy-related signs and symptoms, thus preventing the need for symptom-related chemotherapy dose reductions (14,27).
Scope and organization
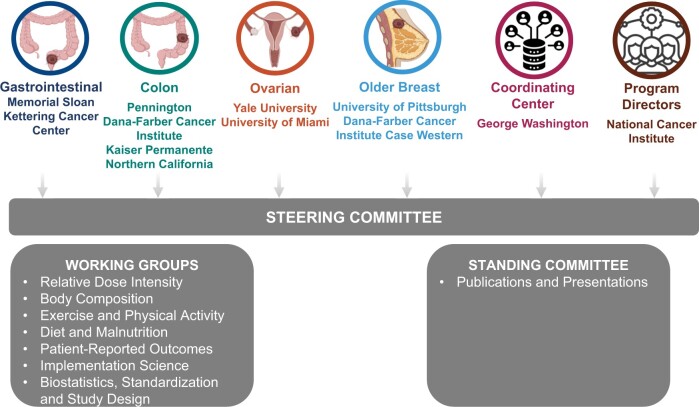
The organizational structure of ENICTO was designed to maintain centralized leadership while facilitating input from and collaboration among the multidisciplinary individual project teams, working groups, and the 2 standing committees (Publications and Presentations, Project Managers) (Figure 1).
Figure 1.
Organizational structure of the Exercise and Nutrition to Improve Treatment-Related Outcomes Consortium.
The Coordinating Center supports the scientific goals of the ENICTO Consortium through curation, harmonization, and analysis of common data elements across consortium sites and coordinating the consortium’s administrative functions as well as internal and external communication. The ENICTO Steering Committee serves as the initiative’s principal governing board. ENICTO working groups were charged with identifying common data elements and standardizing data-collection procedures across consortium projects and identifying potential future cross-consortium research questions.
Overview of projects
All 4 ENICTO projects are randomized controlled trials that include exercise interventions during cancer treatment. Two of the projects also include nutrition interventions. All 4 have a common primary endpoint, but the cancer sites and patient populations vary. In the sections that follow and in Supplementary Table 1 (available online), we briefly describe the projects.
Colon cancer is the third-most common malignancy worldwide (28), and postoperative chemotherapy has been proven to improve overall survival by reducing disease recurrence (29). The ACTION trial uses a bayesian, multistage, response-adaptive, dose-ranging design to characterize the effects of aerobic training on chemotherapy relative dose intensity in patients receiving chemotherapy for stage II and III colon cancer following surgical resection. The study will enroll 219 adults (men and women) from 3 regions of the United States that are socioeconomically, racially, and geographically diverse (Baton Rouge, Louisiana; Boston, Massachusetts; and Oakland, California). The first 80 participants will be randomly assigned to 1 of 5 groups equally: moderate-intensity aerobic training at 75 min/wk, 150 min/wk, 225 min/wk, or 300 min/wk or an attention control condition of static stretching for the duration of chemotherapy (3 or 6 months) using bayesian covariate-adaptive randomization. This phase will be followed by bayesian covariate-adjusted response-adaptive random assignment, in which subsequent participants will be assigned to a specific treatment group in a ratio proportional to the posterior probability that the specific treatment group improves relative dose intensity compared with the control groups (30). The exercise prescription will be chemotherapy periodized, with a smaller volume of aerobic training prescribed on the weeks that chemotherapy is administered to accommodate variations in patient-reported symptoms and a larger volume of exercise prescribed on the weeks that chemotherapy is not administered (31). The ACTION trial uses an innovative design to rigorously and efficiently identify an aerobic training prescription that is patient centered and proven to have a high probability of improving chemotherapy relative dose intensity. The ACTION trial will consider 4 specific aims: 1) Assess the effects of aerobic training on chemotherapy relative dose intensity; 2) assess the effects of aerobic training on GI and peripheral neuropathy symptoms assessed using the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) (32); 3) assess the effects of aerobic training on body composition, measured using dual-energy x-ray absorptiometry; and 4) assess the effects of aerobic training on immune function, measured using neutrophil counts, phenotypes (eg, segmented immature or banded mature), and functions (eg, effector functions, chemotaxis, oxidative burst).
The TNT is a 3-arm randomized trial that will recruit a total of 216 inactive (≤90 minutes of moderate-intensity exercise per week) patients with GI cancers scheduled to initiate neoadjuvant chemotherapy. Poor chemotherapy tolerance is common in patients with GI cancers, and chemotherapy is associated with increased risk of recurrence and death (33-35). Participants will be stratified by cancer type and randomly allocated (1:1:1 ratio) to receive 90 min/wk, 150 min/wk, or 300 min/wk of structured, remotely supervised aerobic training for the length of chemotherapy. The primary goal will be to evaluate the dose response of aerobic training on chemotherapy tolerance and related outcomes. All study assessments and aerobic training will be performed remotely in the patient’s home with remote real-time monitoring. Briefly, following e-consent, each patient will receive a “study kit” containing an iPad device, several Bluetooth-enabled health biodevices (eg, scale, smartwatch), and a treadmill. These biodevices will permit passive measurement of longitudinal physiological responses to aerobic training using high sampling frequency. TNT will address 3 aims: 1) Determine the dose response of aerobic training on treatment tolerance; 2) evaluate aerobic training dose response on hematological function by evaluating systemic reactive oxygen species, hematopoietic stem and progenitor cell enumeration and differentiation, and neutrophil apoptosis; 3) explore aerobic training dose response on tumor clinical outcomes by comparing pathological complete response and disease-free survival rates.
The TEAL study is a multisite (Yale University and University of Miami), 2-arm, randomized trial of an approximately 18-week, remotely delivered lifestyle intervention of medical nutrition therapy and exercise (resistance training and aerobic training) vs usual care. TEAL will recruit a racially and ethnically diverse sample of 200 women diagnosed with ovarian cancer (stages I-IV) and initiating curative-intent chemotherapy, either neoadjuvant or adjuvant to surgery. Women are recruited before the second chemotherapy cycle and randomly assigned 1:1 to either the lifestyle intervention or usual care. The TEAL study is offered in both English and Spanish, and women in the intervention meet weekly via My Wellness Research with a registered dietitian and an exercise physiologist (separately) for the duration of their chemotherapy. My Wellness Research is a research management and communications platform developed at Sylvester Comprehensive Cancer Center and University of Miami Health Systems. It facilitates bidirectional communication (videoconferencing, SMS text messaging, email, and telephone), remote data collection (eg, wearable devices), and patient-reported data collection (eg, daily food and exercise journals), with all data stored within its information technology infrastructure, meeting the highest standards for data security. During the weekly nutrition counseling session, the registered dietitian and/or board-certified specialist in oncology nutrition discusses with the participant whether they are experiencing any nutrition impact symptoms from chemotherapy and approaches to improve diet quality. During the weekly exercise counseling call, the certified exercise trainer leads 1 of the twice-weekly resistance bands sessions with the participant by Zoom (Zoom Video Communications, San Jose, CA) and also counsels on increasing aerobic exercise and reviews Fitbit (FitBit Inc, San Francisco, CA) data. Both weekly calls also include a discussion of the prior week’s nutrition and exercise logging. Exercise intensity is monitored by the Borg Rating of Perceived Exertion.
TEAL is designed to address 4 specific aims: 1) Assess the effect of the physical activity and nutrition intervention on relative dose intensity; 2) assess the effect of the intervention on patient-reported chemotoxicities; 3) assess the effect of the intervention on body composition, determined using computed tomography (CT) scans and urinary D3‐creatine dilution methods; and 4) assess the effects of the physical activity and nutrition intervention on lifestyle behaviors, body composition, chemotoxicities, and health-care utilization, assessed 12 months from diagnosis.
THRIVE-65 is a 2-arm randomized controlled trial that will examine the impact of a telehealth delivered as a resistance training, aerobic training, and protein supplementation intervention on relative dose intensity and chemotoxicities in 270 women 65 years of age and older receiving chemotherapy for stage I through III breast cancer. The intervention will last the length of chemotherapy (typically 12-24 weeks, regimen dependent). All participants will be provided with a computer tablet and a fitness watch. Women in the treatment group will receive telehealth delivered 1 on 1 resistance training coaching twice weekly over Zoom and will be asked to accumulate 90 minutes of moderate-intensity aerobic training weekly (intensity monitored by Borg Rating of Perceived Exertion). Women in the supportive care comparison arm will receive nonexercise supportive care on a tablet. Older women experience worse breast cancer–specific outcomes than younger women, likely because of undertreatment of their cancers and increased treatment-related toxicity (36-39). The Cancer and Aging Research Group—Breast Cancer chemotoxicity score (40) will be used at baseline to balance randomization according to likelihood of chemotoxicities. Participants will be recruited before the second chemotherapy cycle for baseline assessments, then they will be randomly assigned to the THRIVE-65 intervention or the supportive care comparison arm. The unique study measures to be conducted in THRIVE-65 at baseline and at the end of chemotherapy are the geriatric assessment (subjective and objective measures of functional status, cognition, review of comorbidities, medication review, nutritional and psychological status). THRIVE-65 will address 3 specific aims: 1) Assess the effects of the intervention on relative dose intensity, 2) assess the effects of the intervention on chemotoxicities, and 3) evaluate intervention implementation requirements as well as collection of facilitators and barriers to implementation from key stakeholders in anticipation of a successful trial and the long-term goals for broad implementation of the intervention.
Integrated common data elements
All ENICTO U01 projects must share all common data elements, data generated in pilot and collaborative projects, and all study resources with the Coordinating Center. At the end of the Consortium funding period, a deidentified dataset will be made available to the broader research community through 1 of the controlled-access data repositories that the National Institutes of Health (NIH) maintains (41,42).
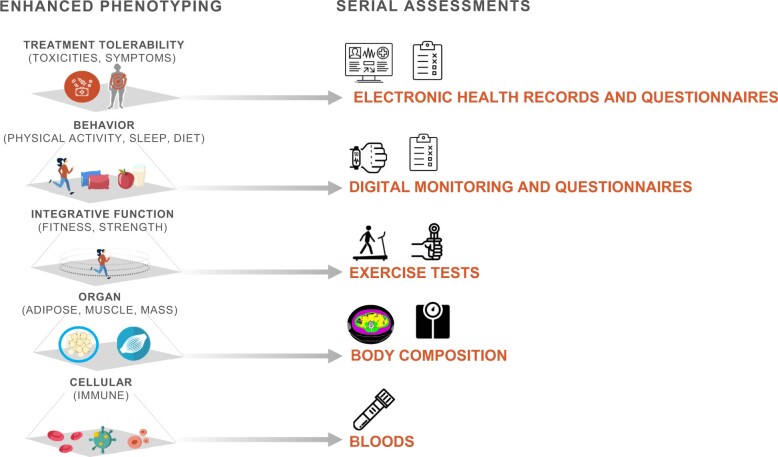
Six working groups promote standardized integration of enhanced phenotyping and serial assessments across the 4 projects (Figure 2). In the sections that follow, we describe these common data elements.
Figure 2.
Standardized phenotyping and serial assessments across trials.
Relative dose intensity
Chemotherapy relative dose intensity is the primary endpoint in all 4 ENICTO intervention trials. It is a single quantitative measure that integrates dose reductions, dose delays, and early discontinuation, all of which are typically the result of treatment-related toxicities. Many studies have demonstrated that chemotherapy relative dose intensities higher than 70%, 80%, or 85% have been associated with improved disease-free and overall survival (43-47). In ENICTO, relative dose intensity will be calculated in 2 ways, each an adaptation of the calculations proposed by Weycker et al. (48). The first method will consider all drugs in any regimen received throughout the entire chemotherapy treatment period. This method will serve as the primary outcome for all 4 trials. The second method will consider only drugs that are part of the first chemotherapy regimen the treating oncologist prescribes (Box 1). Typically, the delivered dose intensity and standard dose intensity are calculated from the first cycle of chemotherapy administered or intended. In the ENICTO projects, however, patients are eligible to be enrolled after the first chemotherapy visit, and intervention effects on relative dose intensity can occur only after the intervention is initiated. Thus, in the ENICTO trials, only cycles of chemotherapy administered after random assignment will be considered in the delivered dose intensity calculation. For example, if the participant is randomly assigned after the first chemotherapy cycle has been delivered, the dose for the second chemotherapy cycle will be used as the threshold for establishing 100% of relative dose intensity. In other words, the standard dose intensity calculation also will use the dose of therapy administered during the first cycle after random assignment as the planned dose. The planned number of cycles will be the number of cycles remaining after random assignment.
Box 1.
Relative dose intensity formula
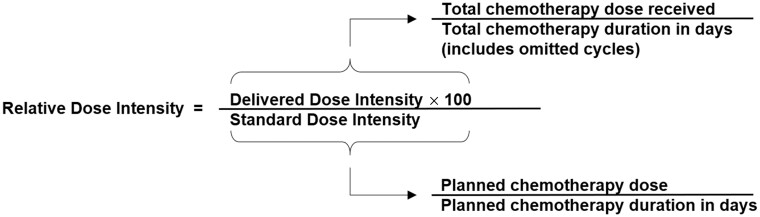
The relative dose intensity formula will be applied to each chemotherapy drug or administration method that is administered as part of the overall chemotherapy regimen. Relative dose intensity values will then be summed and divided by the number of chemotherapy drugs administered to derive the total relative dose intensity score for the overall chemotherapy regimen.
Secondary treatment-related outcomes include dose delays (defined as any delay or a specific toxicity-related delay of any individual drug >5 days), dose reductions (defined as any reduction in the formula used to order chemotherapy (mg/m2, mg/kg, area under the curve) of at least 5% after the first cycle of chemotherapy administered after study randomization), and early stoppage (defined as the termination of at least 1 drug in a regimen before the intended number of cycles).
The use of relative dose intensity as an endpoint in the ENICTO Consortium enhances the rigor because regardless of a patient’s adherence to the intervention or follow-up status and barring withdrawal of consent, relative dose intensity information will be complete, given that it will be obtained from electronic health records for all study participants.
The actual primary outcome varies according to the sample size calculations for each individual trial. ACTION and TEAL are powered to evaluate relative dose intensity as a continuous variable. THRIVE-65 will analyze the proportion of participants who reach a threshold of 85%, based on published evidence that this threshold predicts future recurrence and mortality (46,47,49). TNT will analyze the proportion of participants who reach a threshold of 90%, based on evidence that this relative dose intensity was associated with a reduced risk of recurrence (50). Continuous relative dose intensity will be the common relative dose intensity outcome across the 4 trials.
All ENICTO trials will collect standardized symptom data, including information about the type, grade, and the relationship of symptoms to treatment and to the study intervention. The common instrument used across all trials is the PRO-CTCAE (32). In addition to collecting these data at day 1 of each cycle, most trials are collecting PRO-CTCAE data on day 8 of each cycle, given evidence that symptom profile changes over the course of a chemotherapy cycle (51). TNT will also use a dedicated clinical trial nurse to adjudicate and grade toxicities according to CTCAE, version 5.0 (52). A common core of symptoms is included in the checklist, and each trial will supplement this core list with disease-specific symptom questions. Data on unplanned clinic visits, emergency department visits, and hospitalizations will also be collected. Electronic health records will be reviewed at the start of each chemotherapy infusion cycle to record elements related to relative dose intensity, blood cell counts, kidney function, liver function, and general laboratory tests.
Body composition
The ENICTO trials assess body composition in 3 ways, with varying overlap across trials. Three trials are using CT scans (ACTION, TEAL, TNT), 1 is using dual-energy x-ray absorptiometry (ACTION), and 2 are using the urinary biomarker D3‐creatine dilution method (TEAL, THRIVE-65) (53). Standard operating procedures were developed for each modality. In addition to these methods, all trials will capture anthropometric data (including waist to hip ratio and abdominal circumference) before and after cancer treatment using a research-quality, repeated-use tape measure and body mass index based on measured weight and height.
Three ENICTO trials (ACTION, TEAL, TNT) will segment pretreatment diagnostic CT scans to determine the cross-sectional area (ie, quantity) and radiodensity (ie, quality) of skeletal muscle, visceral adipose tissue, and subcutaneous adipose tissue. TEAL and TNT will also interpret CT scans at other time points during follow-up, including after cancer treatment. A single-slice CT scan at the L3 vertebra (54-56) will be segmented using a semiautomated, commercially available software set with prespecified Hounsfield units for each tissue type.
Exercise and activity monitoring
A key eligibility criterion across all ENICTO trials is physical activity levels below the intervention volume. Accordingly, 3 of the 4 trials employ validated, structured questionnaires to assess baseline physical activity levels (57-59), whereas 1 trial uses self-report of less than 60 min/wk. At baseline and follow-up, 3 of the 4 trials (ACTION, TEAL, THRIVE-65) use ActiGraph accelerometry (ActiGraph, Pensacola, FL) for 1-week periods to measure minutes of light-, moderate-, and vigorous-intensity physical activity, with cut points based on population-specific values (60,61). Finally, all sites are using wrist-based devices (Fitbit, Withings Scan Watch [Withings, Issy-les-Moulineaux, France) to monitor all activity (structured and volitional) throughout the intervention. Data from these devices will be harmonized across sites using Monitor Independent Movement Summary units (62).
An essential methodological consideration when designing exercise trials is the fundamental components of the exercise prescription (ie, frequency, intensity, time, type, and progression) for aerobic training or resistance training. Accordingly, all trials have defined exercise prescriptions using these principles, with 2 of the trials using aerobic training alone and the other 2 using a combination of aerobic and resistance training (Supplementary Table 2, available online). Monitoring of exercise adherence has typically focused on the proportion of those individuals lost to follow-up (ie, the ratio of participants completing follow-up assessments to the total number enrolled) and attendance (ie, the ratio of attended to planned chemotherapy treatments), and few trials have rigorously monitored exercise safety (9). Therefore, data on the actual tolerability and safety of exercise training during chemotherapy to date are limited. Accordingly, all trials will evaluate exercise adherence, as previously described (63,64), and will report the frequency and type of serious (eg, life-threatening, requiring hospitalization, causing notable incapacity) and nonserious (eg, knee or back pain) adverse events during all exercise training sessions. Each site also will use the Borg Rating of Perceived Exertion scale (65) and the Exercise Feeling Scale (66) at each exercise session to monitor patient-reported exercise experience.
Harmonization of exercise-related biomarkers is important to determine the effects of exercise training on physiological endpoints. All trials will collect preintervention and postintervention data on cardiorespiratory fitness, using either a submaximal exercise test or a 6-minute walk test (67), and strength, using a hand grip-strength test.
Diet and malnutrition
All projects will collect 24-hour dietary recalls from participants on 2 randomly assigned nonconsecutive days at baseline and the end of the intervention period. This is accomplished with interviewer-assisted multipass dietary recalls conducted by the University of Arizona Behavioral Measurement and Interventions Shared Resource (68) (TEAL and THRIVE-65) or interviewer-assisted completion of the NCI’s Automated Self-Administered Dietary Assessment Tool (69) dietary recall (ACTION and TNT). Dietary supplement use will also be assessed. Overall diet quality using dietary pattern scores such as the Healthy Eating Index-2020 (70) and the World Cancer Research Fund and American Institute for Cancer Research Adherence Score (71,72) will be calculated. TEAL participants will be asked to complete the Arizona Food Frequency Questionnaire (73) at baseline to collect data on usual dietary intake before treatment, which also uses the US Department of Agriculture Food and Nutrient Database for Dietary Studies (74).
In addition, TEAL and THRIVE-65 participants will monitor adherence to study-specified dietary goals during the trial, in the intervention arms. TEAL participants will complete an app-based food log to report aspects of their dietary intake during the intervention (dietary fiber, fruit and vegetable intake, protein, added sugars, and processed and red meat). THRIVE-65 participants will complete daily high-protein food checklists.
Malnutrition status will be determined either directly using the Patient-Generated Subjective Global Assessment (75) (TEAL) or indirectly using changes in daily weight measurements (TNT) and responses from the PRO-CTCAE (76) measurement system (ACTION, TEAL, THRIVE-65, and TNT). PRO-CTCAE data will be used to determine the prevalence of nutrition impact symptoms (77) during each chemotherapy cycle.
Patient-reported outcomes
A major goal of the PRO instrument selection process was to ensure harmonization across several ENICTO domains of interest (eg, pain, disease-specific symptom burden, health-related quality of life [HRQOL]) and determine the optimal timing of administration for key measures such as the PRO-CTCAE (32), which captures symptomatic treatment toxicity by self-report. The process for selecting common PRO data elements also considered 1) alignment with the NCI requests for application (eg, symptom toxicities, health behaviors), 2) evidence of well-validated instruments for use across multiple cancer populations and ethnic groups, 3) relevance to the participants in each project, 4) study hypotheses (eg, impact of physical activity on symptom burden), and 5) expert input and discussion through workgroup meetings in consultation with the project principal investigators. Instrument selection was prioritized based on the validity of the measure and expected participant burden.
The PRO data elements include multiple constructs across 2 broad domains: HRQOL and physical function. Within the HRQOL domain, measures were selected to assess general HRQOL, depression, anxiety, emotional well-being, social activity and function, and social support. General HRQOL, including emotional well-being, is a common data element across all projects captured with the 36-Item Short Form Health Survey (78), a widely used and well-validated measure that assesses multiple HRQOL domains. Another common data element assessed across all ENICTO projects is anxiety, which is captured by the PROMIS Anxiety Short-Form questionnaire (79). Regarding the physical function domain, all projects assess overall physical function and cancer-specific symptom burden using the 36-Item Short Form Health Survey or the Functional Assessment of Cancer Therapy cancer-specific subscales (78,80).
Implementation science
Members from all projects, the Coordinating Center, and the NCI have adapted the process described by Jolles Perez et al. (81) and reviewed grant proposals, protocols, and manuals of procedure to develop a function and form matrix outlining each intervention’s core functions (basic purpose of the intervention), subfunctions (required processes), and forms (activities related to intervention implementation). Adaptations will be documented systematically across all projects. Elements of the interventions required for implementation will be updated as adaptations are made during the projects. The working group has collectively reviewed validated program satisfaction questionnaires, adapted relevant questions for the ENICTO initiative, and developed a program satisfaction and intervention acceptability instrument for patient assessment. Future work for this working group includes operationalization of the dimensions of the Reach, Effectiveness, Adoption, Implementation, and Maintenance framework (82,83) and identification of which data are available to measure these outcomes in each ENICTO research project.
Current status and future directions
All 4 of the ENICTO Consortium projects were opened to recruitment in 2023. The consortium funding mechanisms (U01/U24) include set-aside funds for new cross-consortium data collection to address novel and emerging areas in the field and to pursue questions with collective data that are not feasible for individual projects, given a harmonized dataset that will include relative dose intensity, symptom data, body composition, exercise relative dose intensity, diet, and patient-reported outcomes.
Although the ENICTO Consortium was funded and began before the January 2023 changes to the NIH Data Management and Sharing Policy (84), ENICTO requires extensive data and protocol sharing, and the Coordinating Center staff have developed a joint consortium-wide agreement (incorporating data user agreements, material sharing agreements, and confidentiality agreements) that has been approved by the individual U01 performance sites.
The Coordinating Center has developed and maintains an external (ENICTO.org) and an internal study website. In addition, the ENICTO Consortium has launched the Exercise Oncology/Oncology Nutrition Network (https://enicto.bsc.gwu.edu/web/enicto/eon-network) to engage the broader community of researchers and clinicians in the work of the consortium and facilitate networking and collaboration between the 2 fields.
Summary
The 4 ENICTO projects will inform clinical practice guidelines recognizing exercise and medical nutrition therapy as critical for delivering high-quality, evidence-based cancer care. Findings from the ENICTO Consortium have the potential to accelerate a paradigm shift in oncology care such that patients with cancer could receive exercise and nutrition programming as the standard of care in tandem with chemotherapy to improve relative dose intensity for a curative outcome.
Supplementary Material
Acknowledgements
ENICTO is an NIH/NCI cooperative agreement and the funder (NCI) has no role in in ENICTO’s individual project aims; study design; data collection, analysis, or interpretation; or the writing and submission of manuscripts for publication, but NIH/NCI project scientists may participate in consortium-wide activities. Given that this article includes information about the ENICTO cooperative agreement structure and the initiative generally, the NCI authors (Frank Perna, Tanya Agurs-Collins, Joanne Elena) participated in the writing of the manuscript and the decision to submit the manuscript for publication.
Contributor Information
Kathryn H Schmitz, Hematology and Oncology, University of Pittsburgh, UPMC Hillman Cancer Center, Pittsburgh, PA, USA.
Justin C Brown, Department of Cancer Energetics, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Melinda L Irwin, Department of Chronic Disease Epidemiology, Yale School of Public Health, Yale University, New Haven, CT, USA.
Kim Robien, Department of Exercise and Nutrition Sciences and Department of Epidemiology, Milken Institute School of Public Health, George Washington University, Washington, DC, USA.
Jessica M Scott, Department of Medicine, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine, New York, NY, USA.
Nathan A Berger, Department of Medicine, Division of Hematology and Oncology, Case Western Reserve University and Case Comprehensive Cancer Center, Cleveland, OH, USA.
Bette Caan, Division of Research, Kaiser Permanente of Northern California, Oakland, CA, USA.
Andrea Cercek, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Tracy E Crane, Department of Medicine, Division of Medical Oncology, University of Miami, Sylvester Comprehensive Cancer Center, Miami, FL, USA.
Scott R Evans, Biostatistics Center and Department of Biostatistics and Bioinformatics, Milken Institute School of Public Health, George Washington University, Washington, DC, USA.
Jennifer A Ligibel, Division of Breast Oncology, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Jeffrey A Meyerhardt, Gastrointestinal Cancer Center, Dana-Farber Cancer Institute, Boston, MA, USA.
Tanya Agurs-Collins, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA.
Karen Basen-Engquist, Department of Health Disparities Research, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Jennifer W Bea, Health Promotion Sciences, University of Arizona and University of Arizona Cancer Center, Tucson, AZ, USA.
Sheng F Cai, Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Brenda Cartmel, Department of Chronic Disease Epidemiology, Yale School of Public Health, Yale University, New Haven, CT, USA.
Vernon M Chinchilli, Department of Public Health Sciences, The Pennsylvania State University College of Medicine, Hershey, PA, USA.
Wendy Demark-Wahnefried, Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, USA.
Christina M Dieli-Conwright, Division of Population Sciences, Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA, USA.
Loretta DiPietro, Department of Exercise and Nutrition Sciences, Milken Institute School of Public Health, George Washington University, Washington, DC, USA.
Shawna E Doerksen, Hematology and Oncology, University of Pittsburgh, UPMC Hillman Cancer Center, Pittsburgh, PA, USA.
Sharon L Edelstein, Biostatistics Center, Milken Institute School of Public Health, George Washington University, Washington, DC, USA.
Joanne Elena, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA.
William Evans, Department of Nutritional Sciences and Toxicology, University of California, Berkeley, Berkeley, CA, USA.
Leah M Ferrucci, Department of Chronic Disease Epidemiology, Yale School of Public Health and Yale Cancer Center, New Haven, CT, USA.
Julia Foldi, Hematology and Oncology, University of Pittsburgh, UPMC Hillman Cancer Center, Pittsburgh, PA, USA.
Sarah Freylersythe, Department of Medicine, Division of Medical Oncology, University of Miami, Sylvester Comprehensive Cancer Center, Miami, FL, USA.
Helena Furberg, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Lee W Jones, Department of Medicine, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine, New York, NY, USA.
Ross Levine, Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Chaya S Moskowitz, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Cynthia Owusu, Department of Medicine, Division of Hematology and Oncology, Case Western Reserve University, Cleveland, OH, USA.
Frank Penedo, Departments of Psychology and Medicine and Sylvester Comprehensive Cancer Center, University of Miami, Miami, FL, USA.
Borsika A Rabin, Herbert Wertheim School of Public Health and Human Longevity Science, University of California San Diego, La Jolla, CA, USA.
Elena Ratner, Department of Obstetrics, Gynecology, and Reproductive Sciences, Yale University, New Haven, CT, USA.
Margaret Rosenzweig, School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA.
Talya Salz, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Tara Sanft, Section of Medical Oncology, Yale University, New Haven, CT, USA.
Matthew Schlumbrecht, Division of Gynecologic Oncology, Sylvester Comprehensive Cancer Center, Miami, FL, USA.
Guillaume Spielmann, School of Kinesiology, Louisiana State University, Baton Rouge, LA, USA.
Cynthia A Thomson, Department of Health Promotion Sciences, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ, USA.
Ashley H Tjaden, Biostatistics Center, Milken Institute School of Public Health, George Washington University, Washington, DC, USA.
Martin R Weiser, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Shengping Yang, Department of Biostatistics, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Anthony F Yu, Department of Medicine, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine, New York, NY, USA.
Frank M Perna, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA.
for the ENICTO Consortium:
Bette Caan, Sydney Anderson, Harmenjit Bahia, Adrienne Castillo, Elizabeth Feliciano, Kristina Johnson, Michelle Ross, Erin Weltzein, Justin C Brown, Baylea Albarado, Stephanie Compton, Tamara Green, Ryan Nash, Phillip Nauta, Meredith Welch, Shengping Yang, Jeffrey A Meyerhardt, Christina M Dieli-Conwright, Danny Nguyen, Amalia Perez Pena, Guillaume Spielmann, Youyoung Kim, William J Evans, Jennifer W Bea, Robert M Blew, Tracy E Crane, Atif Bhatti, Reanna Clavon, Stefan Spee Erlandsen, Sarah Freylersythe, Kenna Hollander, Melissa Lopez-Pentecost, Frank J Penedo, LaShae Rolle, Paola Rossi, Matthew Schlumbrecht, Madalyn Wheeler, Melinda L Irwin, Anlan Cao, Brenda Cartmel, Leah M Ferrucci, Linda Gottlieb, Maura Harrigan, Fang-Yong Li, Courtney McGowan, Leah Puklin, Elena Ratner, Tara Sanft, Michelle Zupa, Nathan A Berger, Stephen Cerne, Carissa Mills, Sandy Conochan, Jasmin Hundal, Cynthia Owusu, Jennifer A Ligibel, Nancy Campbell, Kaedryn DiGuglielmo, Wendy Kemp, Christopher Maples-Campbell, Truong Nguyen, Jay Oppenheim, Anna Tanasijevic, Cynthia Thomson, Angela Yung, Karen Basen-Engquist, Preena Loomba, Vernon M Chinchilli, Kathryn H Schmitz, Jenna Binder, Shawna E Doerksen, Julia Foldi, Sara Garrett, Raymond Scalise, Michele Sobolewski, Lacey White, Jessica M Scott, Andrea Cercek, Sheng F Cai, Stephanie Cao, Helena Furberg, Jenna Harrison, Lee W Jones, Catherine Lee, Ross Levine, Meghan Michalski, Chaya S Moskowitz, Robert Novo, Julia Rabazzi, Kurtis Stoeckel, Talya Salz, Martin R Weiser, Anthony F Yu, Wendy Demark-Wahnefried, Kim Robien, Scott R Evans, Loretta DiPietro, Bao Duong, Sharon L Edelstein, Lorens Helmchen, Daisy Le, Caitlin McCleary, Ashley H Tjaden, Heather Wopat, Borsika A Rabin, Frank M Perna, Tanya Agurs-Collins, Susan M Czajkowski, Joanne Elena, Linda C Nebeling, and Wynne E Norton
Data availability
In accordance with the NIH Public Access Policy, we will provide all manuscripts, including this article, to PubMed Central. At the end of the funding period, a deidentified dataset will be made available to the broader research community through 1 of the controlled-access data repositories the NIH maintains, such as the database of Genotypes and Phenotypes. No new data were generated or analyzed for this manuscript.
Author contributions
Kathryn H. Schmitz, PhD (Conceptualization; Funding acquisition; Methodology; Project administration; Writing—original draft; Writing—review & editing); Leah M. Ferrucci, PhD, MPH (Writing—review & editing); Julia Foldi, MD, PhD (Writing—review & editing); Sarah Freylersythe, NBC-HWC (Writing—review & editing); Helena Furberg, PhD, MSPH (Writing—original draft; Writing—review & editing); Lee W. Jones, PhD (Writing—review & editing); Ross Levine, MD (Writing—review & editing); Chaya S. Moskowitz, PhD (Writing—review & editing); Cynthia Owusu, MD, MS (Writing—review & editing); Frank Penedo, PhD (Writing—original draft; Writing—review & editing); William Evans, PhD (Writing—review & editing); Borsika A. Rabin, PhD, MPH, PharmD (Writing—original draft; Writing—review & editing); Maragaret Rosenzweig, PhD, CRNP-C, AOCNP, FAAN (Writing—review & editing); Talya Salz, PhD (Writing—review & editing); Tara Sanft, MD (Writing—review & editing); Matthew Schlumbrecht, MD (Writing—review & editing); Guillaume Spielmann, PhD (Writing—review & editing); Cynthia A. Thomson, PhD, RD (Writing—original draft; Writing—review & editing); Ashley H. Tjaden, MPH (Writing—review & editing); Martin R. Weiser, MD (Writing—review & editing); Shengping Yang, PhD (Writing—review & editing); Elena Ratner, MD, MBA (Writing—review & editing); Anthony F. Yu, MD (Writing—review & editing); Joanne Elena, PhD, MPH (Writing—review & editing); Shawna E. Doerksen, PhD (Writing—review & editing); Justin C. Brown, PhD (Funding acquisition; Writing—original draft; Writing—review & editing); Melinda L. Irwin, PhD, MPH (Funding acquisition; Writing—original draft; Writing—review & editing); Kim Robien, PhD (Conceptualization; Funding acquisition; Writing—original draft; Writing—review & editing); Jessica M. Scott, PhD (Conceptualization; Funding acquisition; Writing—original draft; Writing—review & editing); Nathan A. Berger, MD (Funding acquisition; Writing—review & editing); Bette Caan, DrPH (Funding acquisition; Writing—original draft; Writing—review & editing); Andrea Cercek, MD (Funding acquisition; Writing—review & editing); Tracy E. Crane, PhD, RDN (Funding acquisition; Writing—original draft; Writing—review & editing); Scott R. Evans, PhD, MS (Funding acquisition; Writing—review & editing); Sharon L. Edelstein, ScM (Writing—review & editing); Jennifer A. Ligibel, MD (Funding acquisition; Writing—review & editing); Tanya Agurs-Collins, PhD, RD (Writing—review & editing); Karen Basen-Engquist, PhD, MPH (Writing—original draft; Writing—review & editing); Jennifer W. Bea, PhD (Writing—review & editing); Sheng F. Cai, MD, PhD (Writing—review & editing); Brenda Cartmel, PhD (Writing—review & editing); Vernon M. Chinchilli, PhD (Writing—review & editing); Wendy Demark-Wahnefried, PhD, RD (Writing—review & editing); Christina M. Dieli-Conwright, PhD, MPH, FACSM, CSCS (Writing—review & editing); Loretta DiPietro, PhD, MPH (Writing—review & editing); Jeffrey A. Meyerhardt, MD, MPH, FASCO (Funding acquisition; Writing—review & editing); Frank Perna, EdD, PhD (Writing—review & editing).
Funding
This work was supported by the following awards from the NCI: ACTION: U01CA271279-02; TEAL: U01CA271278-02; THRIVE-65: U01CA271277-03; TNT: U01CA271287-02, Coordinating Center: U24CA271246-02. Memorial Sloan Kettering Cancer Center investigators are also supported by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Conflicts of interest
Sheng F. Cai was a consultant for and held equity interest in Imago Biosciences, none of which is directly related to the content of this article; is a consultant for Daiichi-Sankyo and Ursamin; was previously a consultant for Dava Oncology; and held equity interest in Imago Biosciences, none of which are directly related to the content of this article. Andrea Cercek received research funding from Seagan, GlaxoSmithKline and is a member of several advisory boards, including Merck, Seagen/Pfizer, Roche, GlaxoSmithKline, and AbbVie. Scott Evans received funding from Degruyter (editor in chief, Statistical Communications in Infectious Disease) and Taylor & Francis book royalties as well as consulting fees from Genentech, AstraZeneca, Takeda, Microbiotix, Johnson & Johnson, Endologiz, ChemoCentryx, Becton Dickenson, Atricure, Roivant, eovasc, Nobel Pharma, Horizon, International Drug Development Institute, SVB Leerink, Medtronic, Regeneron, Wake Forest University, Recor Janssen, and IDDI. He further received honoraria or travel funds from Analgesic, Anesthetic, and Addition Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION); Liver Forum; and the Paris NASH meeting. Scott Evans received support for attending meetings from the US Food and Drug Administration and participated in the following data safety and monitoring boards or advisory boards: NIH, Biomedical Advanced Research and Development Authority, Breast International Group, University of Pennsylvania, Washington University, Duke University, Roche, Pfizer, Takeda, Akouos, Apellis, Teva, Vir, DayOneBio, Alexion, Tracon, Rakuten, AbbVie, GlaxoSmithKline, Eli Lilly, Nuvelution, Clover, FHI Clinical, Lung Biotech, SAB Biopharm, Advantagene, Candel, and Novartis. He reports leadership or fiduciary roles in other boards, societies, or committees (unpaid): American Statistical Association, Society for Clinical Trials, and Frontier Science Foundation. Lee W. Jones has stock ownership in Pacylex Inc and Illumisonics Inc. Ross Levine is on the supervisory board of Qiagen and is a scientific advisor to Loxo (until 2019), Imago, C4 Therapeutics, Mana, Auron, Ajax, Kurome, Mission Bio, Scorpion, and Isoplexis, each of which includes an equity interest. He receives research support from and consulted for Celgene and Roche; has received research support from Constellation, Roche, and Prelude Therapeutics; and has consulted for Bridge Therapeutics, BMS, Lilly, Incyte, Novartis, and Janssen. He has received honoraria from Astra Zeneca, Constellation, Lilly, and Amgen for invited lectures and from Gilead for grant reviews. Ross Levine reports honoraria, consultant fees, travel expenses, research support, stock shareholdership, or cash compensation from Novartis, Janssen, Incyte, Gilead, Lilly, Constellation, Bridge Therapeutics, BMS, Jubilant, Celgene, Qiagen, Imago, C4 Therapeutics, Isoplexis, Prelude Therapeutics, Zentalis, Mana, Auron, Ajax, Kurome, Mission Bio, Scorpion, Cure Breast Cancer Foundation, ECOG-ACRIN Cancer Research Group, Mark Foundation, Genome Canada, Syndax, Stelexis, Vida Ventures, Anovia, Bakx Tx, Bridge Bio, Bridge Medicines, Daiichi, Epiphanes, and MorphoSys AG. All other authors report no conflicts of interest.
References
- 1. National Cancer Institute. Exercise and Nutrition Interventions to Improve Cancer Treatment-Related Outcomes (ENICTO) in Cancer Survivors Consortium. https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-21-031.html. Accessed August 15, 2024.
- 2. Fung C, Dinh P Jr, Ardeshir-Rouhani-Fard S, et al. Toxicities associated with cisplatin-based chemotherapy and radiotherapy in long-term testicular cancer survivors. Adv Urol. 2018;2018:8671832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mowls DS, Brame LS, Martinez SA, et al. Lifestyle behaviors among US cancer survivors. J Cancer Surviv. 2016;10(4):692-698. [DOI] [PubMed] [Google Scholar]
- 4. Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwedhelm C, Boeing H, Hoffmann G, et al. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev. 2016;74(12):737-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li T, Wei S, Shi Y, et al. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2016;50(6):339-345. [DOI] [PubMed] [Google Scholar]
- 7. Steffens D, Beckenkamp PR, Young J, et al. Is preoperative physical activity level of patients undergoing cancer surgery associated with postoperative outcomes? A systematic review and meta-analysis. Eur J Surg Oncol. 2019;45(4):510-518. [DOI] [PubMed] [Google Scholar]
- 8. Loughney L, West MA, Kemp GJ, et al. Exercise intervention in people with cancer undergoing neoadjuvant cancer treatment and surgery: a systematic review. Eur J Surg Oncol (EJSO). 2016;42(1):28-38. [DOI] [PubMed] [Google Scholar]
- 9. Scott JM, Zabor EC, Schwitzer E, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36(22):2297-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel AV, Friedenreich CM, Moore SC, et al. American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanft T, Harrigan M, McGowan C, et al. Randomized trial of exercise and nutrition on chemotherapy completion and pathologic complete response in women with breast cancer: the lifestyle, exercise, and nutrition early after diagnosis study. J Clin Oncol. 2023;41(34):5285-5295. doi: 10.1200/JClinOncol.23.00871:JCO2300871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caan BJ, Meyerhardt JA, Brown JC, et al. Recruitment strategies and design considerations in a trial of resistance training to prevent dose-limiting toxicities in colon cancer patients undergoing chemotherapy. Contemp Clin Trials. 2021;101:106242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parsons HM, Forte ML, Abdi HI, et al. Nutrition as prevention for improved cancer health outcomes: a systematic literature review. JNCI Cancer Spectr. 2023;7(3):pkad035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rock CL, Thomson CA, Sullivan KR, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72(3):230-262. [DOI] [PubMed] [Google Scholar]
- 15. Ligibel JA, Bohlke K, May AM, et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol. 2022;40(22):2491-2507., 10.1200/JClinOncol.22.00687:JCO2200687. [DOI] [PubMed] [Google Scholar]
- 16. Lees JG, White D, Keating BA, et al. Oxaliplatin-induced haematological toxicity and splenomegaly in mice. PLoS One. 2020;15(9):e0238164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barreto JN, McCullough KB, Ice LL, et al. Antineoplastic agents and the associated myelosuppressive effects: a review. J Pharm Pract. 2014;27(5):440-446. [DOI] [PubMed] [Google Scholar]
- 19. Shao L, Wang Y, Chang J, et al. Hematopoietic stem cell senescence and cancer therapy-induced long-term bone marrow injury. Transl Cancer Res. 2013;2(5):397-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ikizler TA, Robinson-Cohen C, Ellis C, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol. 2018;29(1):250-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Courneya KS, Jones LW, Peddle CJ, et al. Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: a randomized controlled trial. Oncologist. 2008;13(9):1012-1020. [DOI] [PubMed] [Google Scholar]
- 22. Dimeo F, Fetscher S, Lange W, et al. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90(9):3390-3394. [PubMed] [Google Scholar]
- 23. Bhatnagar B, Gilmore S, Goloubeva O, et al. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. Springerplus. 2014;3:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boussios S, Pentheroudakis G, Katsanos K, et al. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol. 2012;25(2):106-118. [PMC free article] [PubMed] [Google Scholar]
- 25. Groopman JE, Itri LM.. Chemotherapy-induced anemia in adults: Incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634. [DOI] [PubMed] [Google Scholar]
- 26. Vilhauer RP. A qualitative study of the experiences of women with metastatic breast cancer. Palliative & Supportive Care. 2008;6(3):249-258. [DOI] [PubMed] [Google Scholar]
- 27. Ligibel JA, Pierce LJ, Bender CM, et al. Attention to diet, exercise, and weight in oncology care: results of an American Society of Clinical Oncology national patient survey. Cancer. 2022;128(14):2817-2825. doi: 10.1002/cncr.34231. [DOI] [PubMed] [Google Scholar]
- 28. Morgan E, Arnold M, Gini A, et al. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338-344. [DOI] [PubMed] [Google Scholar]
- 29. Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27(6):872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adaptive Platform Trials Coalition. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18(10):797-807. [DOI] [PubMed] [Google Scholar]
- 31. Kirkham AA, Bland KA, Zucker DS, et al. "Chemotherapy-periodized" exercise to accommodate for cyclical variation in fatigue. Med Sci Sports Exerc. 2020;52(2):278-286. [DOI] [PubMed] [Google Scholar]
- 32. Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(91):dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morton D, Seymour M, Magill L, et al. ; FOxTROT Collaborative Group. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J Clin Oncol. 2023;41(8):1541-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6):e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin J, Tang Y, Hu C, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol. 2022;40(15):1681-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shayne M, Culakova E, Poniewierski MS, et al. Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer. 2007;110(7):1611-1620. [DOI] [PubMed] [Google Scholar]
- 37. Ladwa R, Kalas T, Pathmanathan S, et al. Maintaining Dose Intensity of Adjuvant Chemotherapy in Older Patients With Breast Cancer. Clin Breast Cancer. 2018;18(5):e1181-e1187. [DOI] [PubMed] [Google Scholar]
- 38. Weiss A, Noorbakhsh A, Tokin C, et al. Hormone receptor-negative breast cancer: Undertreatment of patients over 80. Ann Surg Oncol. 2013;20(10):3274-3278. [DOI] [PubMed] [Google Scholar]
- 39. Strader LA, Helmer SD, Yates CL, et al. Octogenarians: noncompliance with breast cancer treatment recommendations. Am Surg. 2014;80(11):1119-1123. [PubMed] [Google Scholar]
- 40. Magnuson A, Sedrak MS, Gross CP, et al. Development and validation of a risk tool for predicting severe toxicity in older adults receiving chemotherapy for early-stage breast cancer. J Clin Oncol. 2021;39(6):608-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tryka KA, Hao L, Sturcke A, et al. NCBI's Database of Genotypes and Phenotypes: DbGaP. Nucleic Acids Res. 2014;42(Database issue):D975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. National Library of Medicine, National Center for Biotechnology Information. dbGaP/Database of Genotypes and Phenotypes.https://ncbi.nlm.nih.gov/gap/. Accessed August 2024.
- 43. Aspinall SL, Good CB, Zhao X, et al. Adjuvant chemotherapy for stage III colon cancer: Relative dose intensity and survival among veterans. BMC Cancer. 2015;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bandera EV, Lee VS, Rodriguez-Rodriguez L, et al. Impact of chemotherapy dosing on ovarian cancer survival according to body mass index. JAMA Oncol. 2015;1(6):737-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nielson CM, Bylsma LC, Fryzek JP, et al. Relative dose intensity of chemotherapy and survival in patients with advanced stage solid tumor cancer: a systematic review and meta-analysis. Oncologist. 2021;26(9):e1609-e1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qi W, Wang X, Gan L, et al. The effect of reduced RDI of chemotherapy on the outcome of breast cancer patients. Sci Rep. 2020;10(1):13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang L, Yu Q, Wu XC, et al. Impact of chemotherapy relative dose intensity on cause-specific and overall survival for stage I-III breast cancer: ER+/PR+, HER2- vs. triple-negative. Breast Cancer Res Treat. 2018;169(1):175-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weycker D, Barron R, Edelsberg J, et al. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat. 2012;133(1):301-310. [DOI] [PubMed] [Google Scholar]
- 49. Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330(18):1253-1259. [DOI] [PubMed] [Google Scholar]
- 50. Sclafani F, Brown G, Cunningham D, et al. PAN-EX: A pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. 2016;27(8):1557-1565. [DOI] [PubMed] [Google Scholar]
- 51. Rohrl K, Guren MG, Smastuen MC, et al. Symptoms during chemotherapy in colorectal cancer patients. Support Care Cancer. 2019;27(8):3007-3017. [DOI] [PubMed] [Google Scholar]
- 52. National Cancer Institute. Division of Cancer Treatment and Diagnosis. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v5.0 (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed August 15, 2024.
- 53. Clark RV, Walker AC, O'Connor-Semmes RL, et al. Total body skeletal muscle mass: Estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol (1985). 2014;116(12):1605-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997-1006. [DOI] [PubMed] [Google Scholar]
- 55. Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97(6):2333-2338. [DOI] [PubMed] [Google Scholar]
- 56. Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: Relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80(2):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rubenstein JH, Morgenstern H, Kellenberg J, et al. Validation of a new physical activity questionnaire for a sedentary population. Dig Dis Sci. 2011;56(9):2678-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kriska AM, Sandler RB, Cauley JA, et al. The assessment of historical physical activity and its relation to adult bone parameters. Am J Epidemiol. 1988;127(5):1053-1063. [DOI] [PubMed] [Google Scholar]
- 59. Godin G. The Godin-Shephard Leisure-time physical activity questionnaire. The Health and Fitness Journal of Canada. 2011;4(1):18-22. [Google Scholar]
- 60. Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188. [DOI] [PubMed] [Google Scholar]
- 61. Barnett A, van den Hoek D, Barnett D, et al. Measuring moderate-intensity walking in older adults using the ActiGraph accelerometer. BMC Geriatr. 2016;16(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Welk GJ. Harmonizing monitor-and report-based estimates of physical activity through calibration. Kinesiol Rev. 2019;8(1):16-24. [Google Scholar]
- 63. Nilsen TS, Scott JM, Michalski M, et al. Novel methods for reporting of exercise dose and adherence: an exploratory analysis. Med Sci Sports Exerc. 2018;50(6):1134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fairman CM, Nilsen TS, Newton RU, et al. Reporting of resistance training dose, adherence, and tolerance in exercise oncology. Med Sci Sports Exerc. 2020;52(2):315-322. [DOI] [PubMed] [Google Scholar]
- 65. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92-98. [PubMed] [Google Scholar]
- 66. Hardy CJ, Rejeski WJ.. Not what, but how one feels: the measurement of affect during exercise. Journal of Sport and Exercise Psychology. 1989;11(3):304-317. [Google Scholar]
- 67. Scott JM, Stene G, Edvardsen E, et al. Performance Status in Cancer: Not Broken, But Time for an Upgrade? J Clin Oncol. 2020;38(25):2824-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. University of Arizona Cancer Center. Behavioral Measurement and Interventions. https://cancercenter.arizona.edu/researchers/shared-resources/behavioral-measurement-and-interventions. Accessed August 15, 2024.
- 69. US Department of Health and Human Services (HHS), National Cancer Institute (NCI), Division of Cancer Control and Population Sciences. Automated Self-Administered 24-hour (ASA24) Dietary Assessment Tool. http://epi.grants.cancer.gov/asa24/. Accessed August 15, 2024.
- 70. Shams-White MM, Pannucci TE, Lerman JL, et al. Healthy eating index-2020: review and update process to reflect the dietary guidelines for Americans, 2020-2025. J Acad Nutr Diet. 2023;123(9):1280-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shams-White MM, Romaguera D, Mitrou P, et al. Further Guidance in Implementing the Standardized 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) score. Cancer Epidemiol Biomarkers Prev. 2020;29(5):889-894. [DOI] [PubMed] [Google Scholar]
- 72. Shams-White MM, Brockton NT, Mitrou P, et al. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) cancer prevention recommendations: a standardized scoring system. Nutrients. 2019;11(7):1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Martinez ME, Marshall JR, Graver E, et al. Reliability and validity of a self-administered food frequency questionnaire in a chemoprevention trial of adenoma recurrence. Cancer Epidemiol Biomarkers Prev. 1999;8(10):941-946. [PubMed] [Google Scholar]
- 74.U.S. Department of Agriculture, Food Surveys Research Group. Food and Nutrient Database for Dietary Studies. Beltsville, MD. https://ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds/. Accessed August 15, 2024.
- 75. Bauer J, Capra S, Ferguson M.. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56(8):779-785. [DOI] [PubMed] [Google Scholar]
- 76.Institute NC. PRO-CTCAE Measurement System Website. https://healthcaredelivery.cancer.gov/pro-ctcae/.Accessed January 28, 2022.
- 77. Baracos VE. Cancer-associated cachexia and underlying biological mechanisms. Annu Rev Nutr. 2006;26:435-461. [DOI] [PubMed] [Google Scholar]
- 78. Ware JE, Snow KK, Kosinski MK.. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: Nimrod Press; 1993. [Google Scholar]
- 79. Cella D, Choi SW, Condon DM, et al. PROMIS((R)) adult health profiles: efficient short-form measures of seven health domains. Value Health. 2019;22(5):537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570-579. [DOI] [PubMed] [Google Scholar]
- 81. Perez Jolles M, Lengnick-Hall R, Mittman BS.. Core functions and forms of complex health interventions: a patient-centered medical home illustration. J Gen Intern Med. 2019;34(6):1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Glasgow RE, Vogt TM, Boles SM.. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Glasgow RE, Harden SM, Gaglio B, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. National Institutes of Health. NOT-OD-21-013. Final NIH Policy for Data Management and Sharing. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-21-013.html. Accessed August 15, 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In accordance with the NIH Public Access Policy, we will provide all manuscripts, including this article, to PubMed Central. At the end of the funding period, a deidentified dataset will be made available to the broader research community through 1 of the controlled-access data repositories the NIH maintains, such as the database of Genotypes and Phenotypes. No new data were generated or analyzed for this manuscript.