Abstract
Background:
Leukocyte telomere length (LTL) shortening, a biomarker of telomere attrition, has been linked to multiple diseases. However, the relationship between LTL and digestive diseases remains uncertain. This study aimed to investigate the association between LTL and the risk of digestive diseases.
Methods:
A cohort analysis of over 500,000 participants from the UK Biobank (UKB) between 2006 and 2021 was conducted to estimate the associations of LTL with more than 90 common digestive diseases. LTL was quantified using multiplex quantitative polymerase chain reaction, and cases of each disease were determined according to inpatient and primary care data. Multivariable Cox proportional hazards regression analysis was used to evaluate the associations of LTL with the risk of digestive diseases. Furthermore, such associations were also evaluated after stratification by sex and ethnicity.
Results:
After a mean follow-up time of 11.8 years, over 20 International Classification of Diseases, 10th Revision (ICD-10) codes were showed to be associated with telomere attrition. LTL shortening is associated with an increased risk of several digestive diseases, including gastroesophageal reflux disease (K21: hazard ratio [HR] = 1.30, 95% confidence interval [95% CI]: 1.19–1.42), esophageal ulcer (K221: HR = 1.81, 95% CI: 1.22–2.71), Barrett’s esophagus (K227: HR = 1.58, 95% CI: 1.14–2.17), gastritis (K29: HR = 1.39, 95% CI: 1.26–1.52), duodenal ulcer (K26: HR = 1.55, 95% CI: 1.14–2.12), functional dyspepsia (K30X: HR = 1.36, 95% CI: 1.06–1.69), non-alcoholic fatty liver disease (NAFLD) (K760: HR = 1.39, 95% CI: 1.09–1.78), liver cirrhosis (K74: HR = 4.73, 95% CI: 3.27–6.85), cholangitis (K830: HR = 2.55, 95% CI: 1.30–5.00), and hernia (K43: HR = 1.50, 95% CI: 1.17–1.94; K44: HR = 1.29, 95% CI: 1.17–1.42). The risk of rectal polyps (K621: HR = 0.77, 95% CI: 0.63–0.92) decreased per unit shortening of LTL.
Conclusions:
This study suggests that LTL shortening is associated with an increased risk of most digestive diseases except for rectal polyps. These findings may provide some clues for understanding the pathogenesis of digestive diseases.
Keywords: Telomere shortening, Digestive system diseases, Leukocyte telomere length, Gastrointestinal diseases, Hepatobiliary diseases
Introduction
Telomeres are DNA-protein complexes located at the ends of chromosomes that protect genomic DNA during cell division.[1] Telomere length can shorten during DNA replication in cell proliferation.[2] Extremely short telomeres eventually trigger cellular senescence, making telomere attrition one of the hallmarks of aging.[3,4] Recent studies have found that telomere attrition increases the risk of various diseases and mortality.[5,6] Leukocyte telomere length (LTL) has been widely used as a proxy for telomere length in various tissues.[2] Extensive studies have reported that LTL is related to aging diseases such as diabetes and cardiovascular and pulmonary diseases.[7–9] Several digestive diseases have also been associated with shortened telomere length. For example, Souza et al[10] found that shortened telomeres in the squamous epithelium of the distal esophagus were related to gastroesophageal reflux disease (GERD), regardless of the presence of Barrett’s esophagus. Tahara et al[11] suggested that the severity of Helicobacter pylori (H. pylori)-induced gastritis was closely related to telomere shortening. Donati et al[12] reported an association of short LTL with non-alcoholic fatty liver diseases and hepatocellular carcinoma (NAFLD-HCC). However, the association of LTL with several digestive diseases is still unclear. Kim et al[13] reported that no relationship between NAFLD and LTL was found in the overall population, but it was significant in young adults aged 20–39 years. Although some studies have investigated the association of LTL with certain digestive diseases, few studies have comprehensively evaluated the relationship between telomere attrition and the risk of digestive diseases.[14,15]
This work aimed to assess the relationship between LTL shortening and the risk of digestive diseases using a large-scale population-based cohort from the UK Biobank (UKB), which contains over 500,000 individuals followed by long-term follow-up. Over 90 International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes of typical digestive diseases were included in this study. These mainly consist of gastrointestinal, hepatobiliary, and pancreatic diseases.
Overall, this study was designed to find insights into the relationship between LTL and the risk of digestive diseases and to advance our understanding of the causes of these debilitating conditions.
Methods
Study population
This study utilized data from the UKB cohort between 2006 and 2021. The UKB performed a thorough cross-sectional baseline evaluation of over 500,000 subjects and collected data on them from various databases. The specimens collected at baseline were used for multiple analyses, including analysis of LTL.[16] Extensive biological and medical data were collected from the participants. With their consent, biological samples such as blood, urine, and saliva were provided for testing. The UKB had ethical approval from the North West Multi-Center Research Ethics Committee. The application ID for this study is 84347.
Data extraction
To avoid omissions, we extracted and harmonized information on phenotype and digestive-system outcomes following the protocol provided by Yeung et al[17] using the R package “ukbpheno” (https://github.com/niekverw/ukbpheno). Self-reported data, hospital admission data, and death certificates were included in the harmonized data. Outcomes were defined using the ICD-10 codes, with ICD-9, Read versions 2 and 3 mapped to ICD-10 codes. Digestive diseases included in this study were mainly categorized into gastrointestinal, hepatobiliary, and pancreatic diseases. Diseases with fewer than 200 cases were removed. Definition tables for diseases are provided in Supplementary Table 1, http://links.lww.com/CM9/B868. The start time of this study was defined as the date of attending the assessment center. Therefore, individuals who experienced the disease before the start time were excluded. Participants were followed until the earliest occurrence of disease, death, or end of hospital inpatient data collection (September 30, 2021, for England, July 31, 2021, for Scotland, and February 28, 2018, for Wales). Person-years were calculated from the start to the end.
Assessment of telomere length
The UKB extracted DNA from peripheral blood leukocytes using an automated process for the majority of the samples.[18] The researchers were blinded to the phenotypic information. As reported by Codd et al[18], LTL was measured as the ratio of telomere repeat number (T) relative to that of a single copy gene (S) using a multiplex quantitative polymerase chain reaction (qPCR). The UKB performed statistical adjustments of the T/S ratio to minimize technical variation in three main stages.[18] In the first stage, technical parameters influencing all measurements within a qPCR run were identified using backward selection in linear regression. The second stage involved testing significant main effects and two-way interactions using the same approach. In the third stage, individual-level T/S ratios were further adjusted based on coefficients from the final model. A linear regression model was applied at the individual measurement level, adjusting for the 260/280 ratio of the DNA sample. Missing or extreme 260/280 ratio values were imputed using the mean value. Linear regression was also used to assess the impact of the time between sample collection, DNA extraction, and LTL measurement on the results.[18] Therefore, the adjusted T/S ratio was chosen for this study. In addition to the adjusted T/S ratio, the raw T/S ratio and z-standardized loge-transformed T/S ratio were provided by the UKB, and the latter was chosen to perform sensitivity analyses.
Assessment of covariates
Using a touchscreen questionnaire and physical measurements, the participants provided personal information on age, sex, ethnicity, body mass index (BMI), alcohol drinker status, smoking status, Townsend deprivation index, and physical activity group as covariates. The age when the patients attended the assessment center was chosen for this study. Since most of the individuals were of white ancestry, we divided the ethnic group into white and others. Categories of alcohol drinker status and smoking status were defined as never, previous, and current. Individuals who preferred not to answer were excluded from this study. The physical activity group was measured by the International Physical Activity Questionnaire (IPAQ). Details of the covariates are provided in Supplementary Table 2, http://links.lww.com/CM9/B868.
Statistical analysis
Multivariable Cox regressions were conducted to assess the relationship between LTL and the risk of digestive diseases. The Cox regression models were adjusted for potential confounding factors, including age, sex, ethnicity, BMI, alcohol drinker status, smoking status, Townsend deprivation index, and physical activity group. In addition to these baseline characteristics, specific confounding factors were considered for certain diseases. For instance, models of gastritis and duodenitis were adjusted for H. pylori infection status and the use of non-steroidal anti-inflammatory drugs. Diabetes status was included as a confounder of NAFLD. Detailed information on specific confounders for each disease is provided in Supplementary Tables 2 and 3, http://links.lww.com/CM9/B868. The proportional hazard assumption was tested using Schoenfeld tests. Covariates that violated the assumption were split into time-dependent parts until they met the assumption. The results from different time intervals were reported. Statistical power was calculated. Adjusted hazard ratios (HRs) per unit of LTL shortening and corresponding 95% confidence intervals (CIs) were estimated to quantify the strength of the association between LTL and the risk of digestive diseases.
To ensure the robustness of the results, we conducted numerous sensitivity analyses. First, we performed analyses using sex and ethnicity as stratum variables to adjust the model in a more general way. In the stratified model, each individual was compared to individuals within their own stratum, and the final results were summed over the strata. Second, the z-standardized loge-transformed T/S ratio was chosen to replace the adjusted T/S ratio, allowing comparison with previous studies that used the same measure of LTL.[6] Third, to test trends across quartiles of LTL, we replaced adjusted LTL with quartiles of adjusted LTL while using the longest quartile as a reference group.
A P-value below 0.05 was considered statistically significant, and multiple testing was adjusted using the false discovery rate (FDR). All analyses were conducted using R 4.1.3 (https://www.r-project.org/) with the “ukbpheno”, “survival”, and “survivalpwr” packages.
Results
Over 90 ICD-10 codes were included in this study to test their relationship with LTL. After adjusting for the covariates described above, more than 20 codes were found to be associated with LTL. They mainly consisted of gastrointestinal diseases, hernia, and hepatobiliary diseases. No pancreatic disease was found in this study. As anticipated, most of the diseases were related to shorter LTL. Interestingly, rectal polyps were found to be associated with a longer LTL.
Table 1 provides the baseline characteristics of the 472,513 participants stratified by quartiles of LTL. The mean age of the participants was 56.5 years, and 45.8% (216,407/472,513) were male. During the long follow-up period (over 5,000,000 person-years), an average of 11,089 cases were found for each disease. As expected, factors such as older age, male sex, obesity, smoking, and alcohol consumption were related to a shorter LTL. Additionally, white people and those with a high level of affluence had a high prevalence of short LTL. Both excessive and insufficient exercise were associated with a shorter LTL.
Table 1.
Baseline characteristics of the UKB population by quartiles of adjusted telomere length.
| Characteristics | All (n = 472,513) | Quartile 1 (n = 118,130) | Quartile 2 (n = 118,128) | Quartile 3 (n = 118,129) | Quartile 4 (n = 118,126) | P-value |
|---|---|---|---|---|---|---|
| Age (years) | 56.54 ± 8.09 | 58.64 ± 7.63 | 57.14 ± 7.91 | 55.98 ± 8.07 | 54.41 ± 8.16 | <0.001 |
| Sex | <0.001 | |||||
| Female | 25,6106 (54.2) | 57,000 (48.3) | 61,802 (52.3) | 65,928 (55.8) | 71,376 (60.4) | |
| Male | 21,6407 (45.8) | 61,130 (51.7) | 56,326 (47.7) | 52,201 (44.2) | 46,750 (39.6) | |
| BMI (kg/m2) | 27.43 ± 4.79 | 27.69 ± 4.75 | 27.52 ± 4.79 | 27.37 ± 4.79 | 27.13 ± 4.81 | <0.001 |
| Ethnicity | <0.001 | |||||
| White | 427,989 (90.6) | 109,156 (92.4) | 107,989 (91.4) | 106,755 (90.4) | 104,089 (88.1) | |
| Others | 44,524 (9.4) | 8974 (7.6) | 10,139 (8.6) | 11,374 (9.6) | 14,037 (11.9) | |
| Townsend deprivation index | –1.31 ± 3.09 | –1.32 ± 3.08 | –1.34 ± 3.07 | –1.32 ± 3.08 | –1.24 ± 3.12 | <0.001 |
| Smoking status | <0.001 | |||||
| Never | 25,7201 (54.7) | 60,041 (51.1) | 63,334 (53.9) | 65,692 (55.9) | 68,134 (58.0) | |
| Previous | 163,093 (34.7) | 43,872 (37.3) | 41,618 (35.4) | 39,818 (33.9) | 37,785 (32.1) | |
| Current | 49,800 (10.6) | 13,575 (11.6) | 12,544 (10.7) | 12,068 (10.3) | 11,613 (9.9) | |
| Alcohol drinker status | <0.001 | |||||
| Never | 20,887 (4.4) | 4920 (4.2) | 4990 (4.2) | 5279 (4.5) | 5698 (4.8) | |
| Previous | 16,991 (3.6) | 4536 (3.8) | 4196 (3.6) | 4130 (3.5) | 4129 (3.5) | |
| Current | 433,445 (92.0) | 108,384 (92.0) | 108,646 (92.2) | 108,442 (92.0) | 107,973 (91.7) | |
| IPAQ activity group | 0.012 | |||||
| Low | 72,109 (18.9) | 17,976 (19.0) | 18,138 (19.0) | 17,956 (18.8) | 18,039 (18.8) | |
| Moderate | 155,496 (40.7) | 38,213 (40.4) | 38,515 (40.4) | 39,196 (41.0) | 39,572 (41.1) | |
| High | 154,074 (40.4) | 38,287 (40.5) | 38,664 (40.6) | 38,529 (40.3) | 38,594 (40.1) |
Data were presented as mean ± SD or n (percentage). BMI: Body mass index; IPAQ: International Physical Activity Questionnaire; SD: Standard deviation; UKB: UK Biobank.
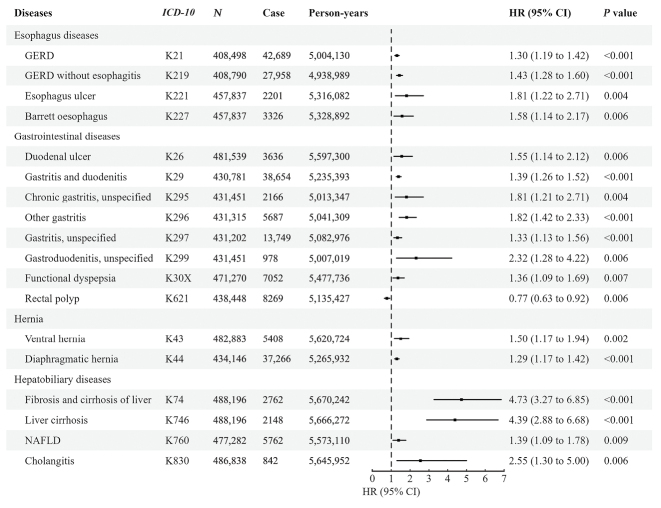
After multiple testing corrections, diseases significantly correlated with the shortening of the LTL are illustrated in Figure 1. GERDs (K21: HR = 1.30, 95% CI: 1.19–1.42) and GERD without (K219: HR = 1.43, 95% CI: 1.28–1.60) esophagitis showed similar trends per unit shortening of LTL. However, GERD with esophagitis (K210) was not identified in this study. Similar trends were observed in sensitivity analyses. As one of the complications of GERD, the incidence of Barrett’s esophagus (K227: HR = 1.58, 95% CI: 1.14–2.17) significantly increased per unit LTL shortening. Furthermore, a decrease in LTL was associated with an increased risk of esophageal ulcers (K221: HR = 1.81, 95% CI: 1.22–2.71). Further analysis showed that a shorter LTL increased the risk of gastritis (K29: HR = 1.39, 95% CI: 1.26–1.52; K295: HR = 1.81, 95% CI: 1.21–2.71; K296: HR = 1.82, 95% CI: 1.42–2.33, K297: HR = 1.33, 95% CI: 1.13–1.56). However, no increased risk of acute gastritis (K290, K291) or chronic atrophic gastritis (K295) was associated with a short LTL. Compared to gastritis, no significant correlation was found between duodenitis and LTL except for gastroduodenitis (K299: HR = 2.32, 95% CI: 1.28–4.22). Interestingly, the risk of duodenal ulcers (K26: HR = 1.55, 95% CI: 1.14–2.12) was observed to be related to shorter LTL. Moreover, functional dyspepsia (K30X: HR = 1.36, 95% CI: 1.06–1.69) was correlated with a short LTL. Five major types of hernia (K40, K41, K42, K43, K44) were included in this study, and it was found that individuals with ventral hernia (K43: HR = 1.50, 95% CI: 1.17–1.94) and diaphragmatic hernia (K44: HR = 1.29, 95% CI: 1.17–1.42) tended to have shorter LTLs. Surprisingly, per unit shortening of LTL was associated with a decreasing risk of rectal polyps (K621: HR = 0.77, 95% CI: 0.63–0.92), contradicting common sense. Compared to rectal polyps, colon polyps (K635) and anal polyps (K620) showed no associations with LTL.
Figure 1.
The significant association of adjusted LTL with the risk of digestive diseases adjusted for covariates. A P-value below 0.05 was considered statistically significant. 95% CI: 95% confidence interval; GERD: Gastroesophageal reflux disease; HR: Hazard ratio generated by multivariable Cox regression; ICD-10: International Statistical Classification of Diseases, Tenth Revision; LTL: Leukocyte telomere length; N: Sample size.
Further analysis showed a correlation between hepatobiliary diseases and LTL. Individuals with shorter LTLs had a higher risk of liver cirrhosis and fibrosis (K74: HR = 4.73, 95% CI: 3.27–6.85, K746: HR = 4.39, 95% CI: 2.88–6.68). Similar trends were found for NAFLD (K760: HR = 1.39, 95% CI: 1.09–1.78) and cholangitis (K830: HR = 2.55, 95% CI: 1.30–5.00) per unit increase in LTL. Due to the violation of the proportional hazard assumption, several datasets were split into time-dependent parts. Among them, diverticular disease of the intestine (K57: HR = 1.20, 95% CI: 1.08–1.33), celiac disease (K900: HR = 3.76, 95% CI: 2.34–6.03), alcoholic liver disease (K70: HR = 21.96, 95% CI: 7.32–65.85), and portal hypertension (K766: HR = 19.79, 95% CI: 7.89–49.61) showed significant associations with short LTL in the first episode of follow-up. Similar trends were also observed in the second and fifth time intervals of alcoholic liver disease. Details of all diseases are provided in Supplementary Table 4, http://links.lww.com/CM9/B868.
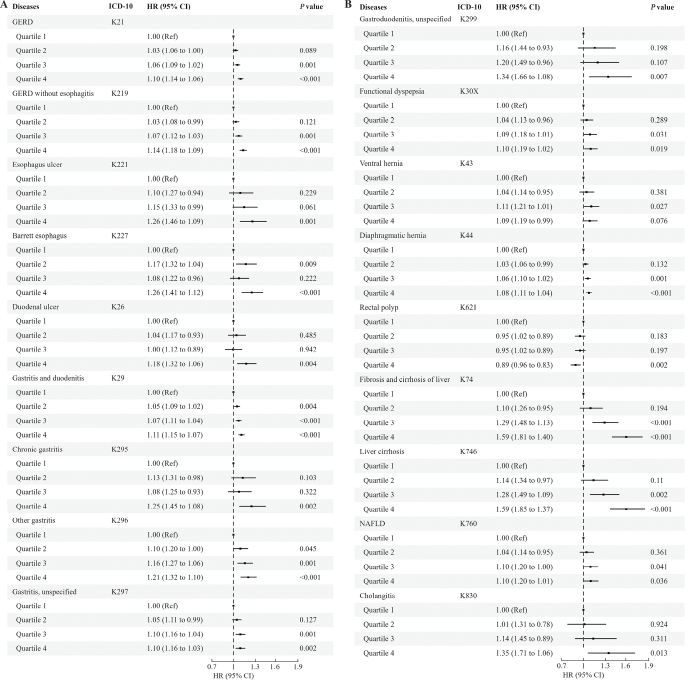
To ensure the robustness of our findings, we conducted several sensitivity analyses. First, we included sex and ethnicity as strata variables rather than covariates in the Cox regression model. The results were the same as the primary results. Second, we chose standardized LTL for the regression. Partially in line with the primary result, we found an increased risk of functional dyspepsia (K30X: HR = 1.04, 95% CI: 1.01–1.07), constipation (K590: HR = 1.02, 95% CI: 1.01–1.03), and functional diarrhea (K591: HR = 1.06, 95% CI: 1.02–1.11) per standard deviation shortening of LTL. The results showed that functional gastrointestinal disorders were closely related to a short LTL. Furthermore, hepatic failure (K72: HR = 1.10, 95% CI: 1.02–1.18) and acute pancreatitis (K85: HR = 1.07, 95% CI: 1.02–1.12) were associated with short standardized LTL. Finally, to test the trends across quartiles of the LTL, we included quartiles of LTL in the regression. As illustrated in Figure 2, it is apparent that the risk of most diseases increased per quartile increase in LTL. Compared to the reference (quartile 1), individuals with the shortest LTL (quartile 4) had a statistically significant risk increase in most diseases. In line with the primary results, rectal polyps (K621 quartile 4: HR = 0.89, 95% CI: 0.83–0.96) were associated with a decrease in LTL. Detailed results of sensitivity analyses are provided in Supplementary Tables 5–7, http://links.lww.com/CM9/B868.
Figure 2.
The association of quartiles of LTL with diseases found in the previous results (A, B). The longest quartile (quartile 1) was chosen for reference. The risk of diseases was statistically increased in the shortest quartile (quartile 4), except for rectal polyps. This is in line with the primary result. 95% CI: 95% confidence interval; GERD: Gastroesophageal reflux disease; HR: Hazard ratio generated by multivariable Cox regression; ICD-10: International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; LTL: Leukocyte telomere length.
Discussion
In this study, we examined the relationship between LTL and the risk of digestive diseases, including gastrointestinal, hepatobiliary, and pancreatic diseases. After adjusting for potential covariates, we observed that more than 20 ICD-10 codes were significantly associated with LTL. No pancreatic diseases were identified in this study. Partially in line with previous studies, we confirmed that shortening of the LTL was related to most of the diseases found in this study. The results were robust in sensitivity analyses. Interestingly, rectal polyps (K621) was the only disease associated with a long LTL. This significant relationship was replicated in a series of sensitivity analyses.
In this study, we examined the relationship between hepatobiliary diseases and LTL. We demonstrated that NAFLD (K760) was significantly associated with a shorter LTL after adjusting for potential confounding factors, including age, sex, ethnicity, BMI, alcohol consumption status, smoking status, Townsend deprivation index, physical activity group, and diabetes status. Scholars in several studies have reported that LTL is associated with NAFLD in the presence of other diseases. However, to our knowledge, no relationship was found in the overall population. Nakajima et al[19] reported that the histological degree of steatosis was significantly higher in NAFLD patients with shorter telomere lengths than in those with normal telomere lengths. Moreover, short telomeres in peripheral blood and telomerase reverse transcriptase (TERT) mutations were observed in NAFLD patients developing hepatocellular carcinoma.[12] After a six-year follow-up of 70 type 2 diabetes (T2D) patients, Ping et al[20] reported that T2D patients who developed NAFLD tended to have shorter telomeres. Kim et al[13] conducted a cross-sectional study in all age groups for the general population and suggested that there was no significant difference in LTL between NAFLD patients and controls based on the whole population. However, partially in line with our results, differences were found in 20-year-old to 39-year-old and over 60-year-old persons.[13] The inconsistency with our result may be due to different statistical power. Compared to the large population (cases = 5762, controls = 471,520) in this study, the sample size (n = 6738) of the Kim et al[13] study is relatively small. Furthermore, the association of LTL with NAFLD was statistically significant in our sensitivity analyses. It can be suggested that the shortening of the LTL is closely related to NAFLD in the whole population, especially in 20-year-old to 39-year-old adults. Kim et al[13] reported that telomere length was significantly associated with advanced fibrosis in NAFLD. It can be concluded that the progression of NAFLD is closely related to short telomere length. In line with previous studies, we found that fibrosis and cirrhosis of the liver (K74, K746) were significantly associated with shorter LTL.[13,21] A possible explanation for this might be the existence of gene mutations that result in telomerase deficiency. Studies of telomerase-deficient mice found that shortened telomeres were related to the acceleration of cirrhosis.[22] The recovery of telomerase would reduce cirrhosis and improve liver function.[22] Calado et al[23] reported that mutations in TERT and TERC were more frequent in cirrhotic patients. Furthermore, the presence of shorter telomeres in peripheral blood leukocytes was associated with liver cirrhosis.[24] It can be assumed that telomerase deficiency caused a reduction in LTL and played an important role in the development of liver cirrhosis.
The relationship between gastrointestinal diseases and LTL is another important finding of this study. Partially consistent with previously published results, gastritis and its associated subtypes (K29, K295, K296, K297, K299) were found to be associated with shorter telomere length.[25] After testing gastric biopsies between NSAID users and non-users, Tahara et al[11] found that NSAID users had shorter telomere lengths than non-users. Furthermore, telomere shortening was closely related to the severity of H. pylori-induced gastritis and CDH1 methylation status. This result suggests that the erosion of telomeres plays an important role in H. pylori and NSAID-induced gastritis. Moreover, Muhsen et al[26] reported that individuals with past H. pylori infection and serological evidence of atrophic gastritis tended to have shorter LTL. However, this study failed to demonstrate a significant association between chronic atrophic gastritis (K294, CAG) and a shorter LTL. Several factors could explain this observation. First, the number of cases (319) was too small to test the association. Second, to avoid bias, we excluded individuals with other types of gastritis from the cases of chronic atrophic gastritis. Individuals with H. pylori-induced CAG are generally diagnosed with other types of gastritis as well. Therefore, the CAG cases (K294) included in this study mainly consisted of autoimmune CAG patients. The results indicated no association between autoimmune CAG and shorter telomere length. Another interesting finding was that duodenal ulcer rather than duodenitis was associated with shorter LTL. This finding supported the work of Tuo et al[27], who demonstrated that the lack of telomerase resulted in duodenal mucosal atrophy and reduced duodenal HCO3– secretion in telomerase-deficient mice. Further research is required to investigate whether telomere length shows different trends between duodenal ulcers and duodenitis.
We confirmed that a shorter LTL was related to several esophageal diseases, including GERD (K21, K219), Barrett’s esophagus (K227), and esophageal ulcers (K221). This is consistent with previous studies. For instance, Souza et al[10] reported that telomeres in the squamous epithelium of the distal esophagus were significantly shorter in GERD patients with and without Barrett’s esophagus. Esophageal ulcer (K221) is a condition of GERD. Interestingly, although GERD (K21) and GERD without esophagitis (K219) were identified in this study, the relationship between GERD with esophagitis (K210) and LTL was not significant. Similar trends were observed in sensitivity analyses. As reported in the methods section, we excluded individuals with K219 from the cases of K210. Therefore, it can be inferred that short telomere length is associated with the development of GERD rather than the progression from GERD to esophagitis. Furthermore, as one of the complications of GERD, the link between Barrett’s esophagus and telomere length has been established. The results of numerous studies have suggested that shortening of telomere length increases the risk of Barrett carcinoma.[28–30] Finley et al[31] suggested that chromosome instability was related to short telomeres in Barrett’s esophagus.
Surprisingly, this study found a decreased risk of rectal polyps with shortening of the LTL. Similar trends were replicated in sensitivity analyses. Partially contrary to our finding, Hardikar et al[32] suggested that short telomere length was associated with an increased risk of adenoma and serrated colorectal polyps. However, to our knowledge, few studies have investigated the relationship between telomere length and rectal polyps. Another unexpected finding was the association with ventral and diaphragmatic hernia. Little evidence of short telomeres in hernia patients has been reported before. No significant association with cancer was observed. A similar trend was found by Schneider et al[6], who reported that cancer-related mortality was not related to telomere attrition.[6] These are interesting issues for future research.
In this study, we investigated the relationship between the risk of digestive diseases (over 90 ICD-10 codes) and LTL using the large UKB dataset. We confirmed the association with several well-documented diseases, such as GERD, gastritis, and liver cirrhosis. This finding broadens previous views that telomeres are only associated with fibrosis and carcinoma in NAFLD patients. Furthermore, the association with cholangitis, specific types of hernia, and functional dyspepsia was also demonstrated in this study. Due to the limited evidence, future research is required to test the potential mechanisms.
Although fruitful results were found, our analysis has some limitations. First, due to the violation of the proportional hazard assumption, the regression for some diseases was split into episodes. Therefore, the relationships with several diseases were tested in specific episodes of follow-up. Despite this limitation, most findings were not split. Second, some cases were excluded from this study. Due to the retrospective design of this study, we removed cases that occurred before the start time of the follow-up. Individuals diagnosed with related diseases were excluded from the study. This may reduce the statistical power of the analysis. Nevertheless, due to the large sample size of the UKB, many cases were available in this study. Third, this study is based on a single cohort. The generalizability to the whole population is relatively limited. Nevertheless, a series of sensitivity analyses were performed to ensure the robustness of the results. Finally, most UKB participants are White. The findings of this study should be interpreted cautiously in other ethnicities.
In conclusion, we demonstrated the relationship between LTL and the risk of digestive diseases in a large cohort. Shortening of the LTL was observed to be associated with the risk of gastrointestinal and hepatobiliary diseases such as GERD, gastritis, NAFLD, and liver fibrosis. The risk of rectal polyps decreased with the shortening of the LTL. These findings may provide some clues for understanding the pathogenesis of digestive diseases. Further research could be focused on underlying mechanisms as well as potential interventions for preventing telomere attrition and its associated risks.
Funding
This research was supported by the grant from the National Key Research and Development Program (No. 2021YFC2400603).
Conflicts of interest
None.
Supplementary Material
Footnotes
Hongqun Yang and Lanlan Chen contributed equally to this work.
How to cite this article: Yang HQ, Chen LL, Liu YH. Association of leukocyte telomere length with the risk of digestive diseases: A large-scale cohort study. Chin Med J 2025;138:60–67. doi: 10.1097/CM9.0000000000002994
References
- 1.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 2.Demanelis K Jasmine F Chen LS Chernoff M Tong L Delgado D, et al. Determinants of telomere length across human tissues. Science 2020;369:eaaz6876. doi: 10.1126/science.aaz6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov 2016;6:584–593. doi: 10.1158/2159-8290.CD-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rode L, Nordestgaard BG, Bojesen SE. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Natl Cancer Inst 2015;107:djv074. doi: 10.1093/jnci/djv074. [DOI] [PubMed] [Google Scholar]
- 6.Schneider CV Schneider KM Teumer A Rudolph KL Hartmann D Rader DJ, et al. Association of telomere length with risk of disease and mortality. JAMA Intern Med 2022;182:291–300. doi: 10.1001/jamainternmed.2021.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol 2013;10:274–283. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- 8.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adaikalakoteswari A, Balasubramanyam M, Ravikumar R, Deepa R, Mohan V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis 2007;195:83–89. doi: 10.1016/j.atherosclerosis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Souza RF Lunsford T Ramirez RD Zhang X Lee EL Shen Y, et al. GERD is associated with shortened telomeres in the squamous epithelium of the distal esophagus. Am J Physiol Gastrointest Liver Physiol 2007;293:G19–G24. doi: 10.1152/ajpgi.00055.2007. [DOI] [PubMed] [Google Scholar]
- 11.Tahara T Shibata T Kawamura T Ishizuka T Okubo M Nagasaka M, et al. Telomere length in non-neoplastic gastric mucosa and its relationship to H. pylori infection, degree of gastritis, and NSAID use. Clin Exp Med 2016;16:65–71. doi: 10.1007/s10238-014-0335-0. [DOI] [PubMed] [Google Scholar]
- 12.Donati B Pietrelli A Pingitore P Dongiovanni P Caddeo A Walker L, et al. Telomerase reverse transcriptase germline mutations and hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Cancer Med 2017;6:1930–1940. doi: 10.1002/cam4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D, Li AA, Ahmed A. Leucocyte telomere shortening is associated with nonalcoholic fatty liver disease-related advanced fibrosis. Liver Int 2018;38:1839–1848. doi: 10.1111/liv.13886. [DOI] [PubMed] [Google Scholar]
- 14.Wennerström EC Risques RA Prunkard D Giffen C Corley DA Murray LJ, et al. Leukocyte telomere length in relation to the risk of Barrett’s esophagus and esophageal adenocarcinoma. Cancer Med 2016;5:2657–2665. doi: 10.1002/cam4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee WP Hou MC Lan KH Li CP Chao Y Lin HC, et al. Helicobacter pylori-induced chronic inflammation causes telomere shortening of gastric mucosa by promoting PARP-1-mediated non-homologous end joining of DNA. Arch Biochem Biophys 2016;606:90–98. doi: 10.1016/j.abb.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Sudlow C Gallacher J Allen N Beral V Burton P Danesh J, et al. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeung MW, van der Harst P, Verweij N. Ukbpheno v1.0: An R package for phenotyping health-related outcomes in the UK Biobank. STAR Protoc 2022;3:101471. doi: 10.1016/j.xpro.2022.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Codd V Denniff M Swinfield C Warner SC Papakonstantinou M Sheth S, et al. Measurement and initial characterization of leukocyte telomere length in 474,074 participants in UK Biobank. Nat Aging 2022;2:170–179. doi: 10.1038/s43587-021-00166-9. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima T Moriguchi M Katagishi T Sekoguchi S Nishikawa T Takashima H, et al. Premature telomere shortening and impaired regenerative response in hepatocytes of individuals with NAFLD. Liver Int 2006;26:23–31. doi: 10.1111/j.1478-3231.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- 20.Ping F Li ZY Lv K Zhou MC Dong YX Sun Q, et al. Deoxyribonucleic acid telomere length shortening can predict the incidence of non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Diabetes Investig 2017;8:174–180. doi: 10.1111/jdi.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitada T, Seki S, Kawakita N, Kuroki T, Monna T. Telomere shortening in chronic liver diseases. Biochem Biophys Res Commun 1995;211:33–39. doi: 10.1006/bbrc.1995.1774. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 2000;287:1253–1258. doi: 10.1126/science.287.5456.1253. [DOI] [PubMed] [Google Scholar]
- 23.Calado RT Brudno J Mehta P Kovacs JJ Wu C Zago MA, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology 2011;53:1600–1607. doi: 10.1002/hep.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carulli L. Telomere shortening as genetic risk factor of liver cirrhosis. World J Gastroenterol 2015;21:379–383. doi: 10.3748/wjg.v21.i2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aslan R Bektas A Bedir A Alacam H Aslan MS Nar R, et al. Helicobacter pylori eradication increases telomere length in gastric mucosa. Hepatogastroenterology 2013;60:601–604. doi: 10.5754/hge12691. [DOI] [PubMed] [Google Scholar]
- 26.Muhsen K, Sinnreich R, Merom D, Nassar H, Cohen D, Kark JD. Helicobacter pylori infection, serum pepsinogens as markers of atrophic gastritis, and leukocyte telomere length: A population-based study. Hum Genomics 2019;13:32. doi: 10.1186/s40246-019-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuo B Ju Z Riederer B Engelhardt R Manns MP Rudolph KL, et al. Telomere shortening is associated with reduced duodenal HCOFormula secretory but normal gastric acid secretory capacity in aging mice. Am J Physiol Gastrointest Liver Physiol 2012;303:G1312–G1321. doi: 10.1152/ajpgi.00035.2012. [DOI] [PubMed] [Google Scholar]
- 28.Risques RA Vaughan TL Li X Odze RD Blount PL Ayub K, et al. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev 2007;16:2649–2655. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 29.Gertler R, Doll D, Maak M, Feith M, Rosenberg R. Telomere length and telomerase subunits as diagnostic and prognostic biomarkers in Barrett carcinoma. Cancer 2008;112:2173–2180. doi: 10.1002/cncr.23419. [DOI] [PubMed] [Google Scholar]
- 30.Aida J Takubo K Vieth M Neuhaus H Fujiwara M Arai T, et al. Telomere lengths in Barrett’s esophagus as a precancerous lesion. Esophagus 2022;19:287–293. doi: 10.1007/s10388-021-00884-4. [DOI] [PubMed] [Google Scholar]
- 31.Finley JC Reid BJ Odze RD Sanchez CA Galipeau P Li X, et al. Chromosomal instability in Barrett’s esophagus is related to telomere shortening. Cancer Epidemiol Biomarkers Prev 2006;15:1451–1457. doi: 10.1158/1055-9965.EPI-05-0837. [DOI] [PubMed] [Google Scholar]
- 32.Hardikar S, Burnett-Hartman AN, Phipps AI, Upton MP, Zhu LC, Newcomb PA. Telomere length differences between colorectal polyp subtypes: A colonoscopy-based case-control study. BMC Cancer 2018;18:513. doi: 10.1186/s12885-018-4426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]