Regarding: Viscusi ER, de Leon‐Casasola O, Cebrecos J, Jacobs A, Morte A, Ortiz E, et al. Corrigendum: Celecoxib‐tramadol co‐crystal in patients with moderate‐to‐severe pain following bunionectomy with osteotomy: A phase 3, randomized, double‐blind, factorial, active‐ and placebo‐controlled trial. Pain Pract. 2023;23(1):8–22. doi: 10.1111/papr.13136.
In Figure 1, the number of patients who completed the study in the tramadol group has been corrected to n = 178. In Supplementary Table S2, the numbers of patients in the tramadol and celecoxib groups have been updated to n = 183 and n = 181, respectively. Corrected versions of Figure 1 and Supplementary Table S2 are provided below and have been updated in the online version of the manuscript. The authors regret these errors and apologize for any confusion that may have resulted.
FIGURE 1.
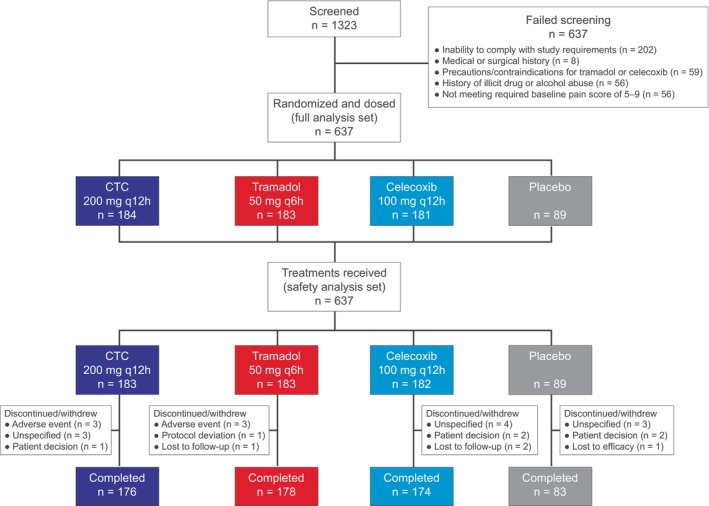
Patient disposition. CTC, celecoxib‐tramadol co‐crystal; q6h, every 6 h; q12h, every 12 h.
TABLE S2.
Responder analyses (full analysis set).
| Treatment group, n (%) | Odds ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| CTC (n = 184) | Tramadol (n = 183) | Celecoxib (n = 181) | Placebo (n = 89) | CTC versus tramadol | CTC versus celecoxib | CTC versus placebo | |
| Responders 50% a , b | 115 (62.5) | 107 (58.5) | 107 (59.1) | 49 (55.1) | 1.204 (0.777, 1.864) | 1.167 (0.752, 1.811) | 1.377 (0.806, 2.353) |
| Responders 30% a , c | 128 (69.6) | 118 (64.5) | 128 (70.7) | 58 (62.5) | 1.303 (0.820, 2.070) | 0.937 (0.582, 1.507) | 1.230 (0.696, 2.174) |
| Responders NRS <4 a , d | 117 (63.6) | 108 (59.0) | 109 (60.2) | 49 (55.1) | 1.245 (0.800, 1.939) | 1.160 (0.743, 1.811) | 1.440 (0.838, 2.473) |
| Responders 50% and NRS <4 a | 113 (61.4) | 105 (57.4) | 105 (58.0) | 46 (51.7) | 1.202 (0.778, 1.855) | 1.164 (0.752, 1.800) | 1.509 (0.887, 2.569) |
| Responders 30% and NRS <4 a | 117 (63.6) | 108 (59.0) | 109 (60.2) | 49 (55.1) | 1.245 (0.800, 1.939) | 1.160 (0.743, 1.811) | 1.440 (0.838, 2.473) |
Abbreviations: CI, confidence interval; CTC, celecoxib‐tramadol co‐crystal; NRS, numerical rating scale.
Logistic regression adjusted for center and baseline pain.
A 50% reduction in pain intensity from baseline sustained until the end of the 48‐h observation period.
A 30% reduction in pain intensity from baseline sustained until the end of the 48‐h observation period.
A pain intensity below 4 on the NRS sustained until the end of the 48‐h observation period.


