The compound 2 was incorrectly drawn in Figures 5 and 6; the correct structure has the biphenyl moiety in para and not in meta position. Corrected Figures 5 and 6 are provided.
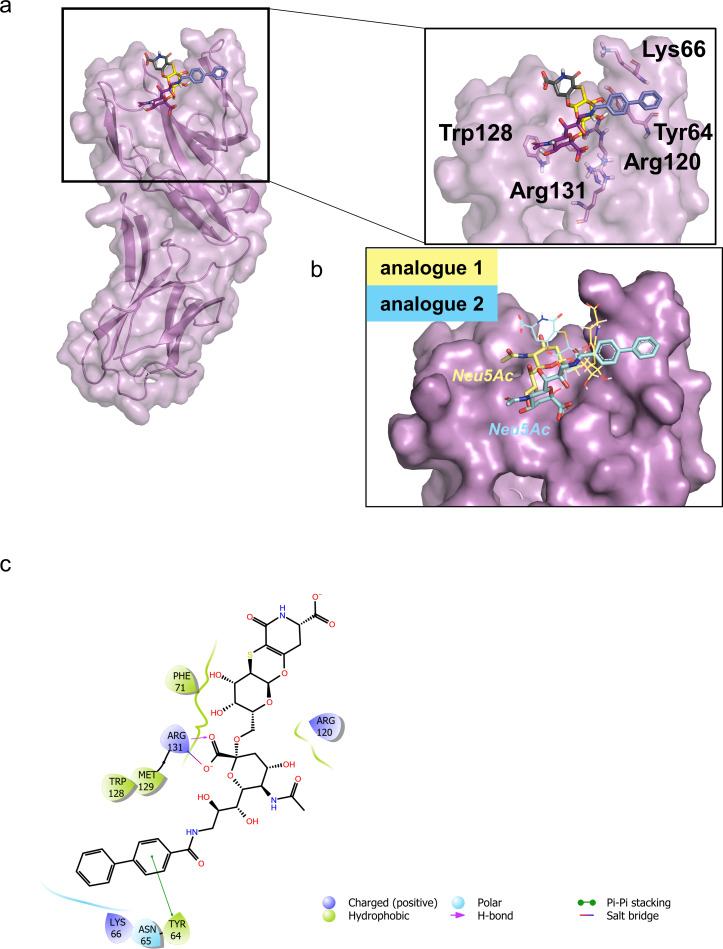
Figure 5.
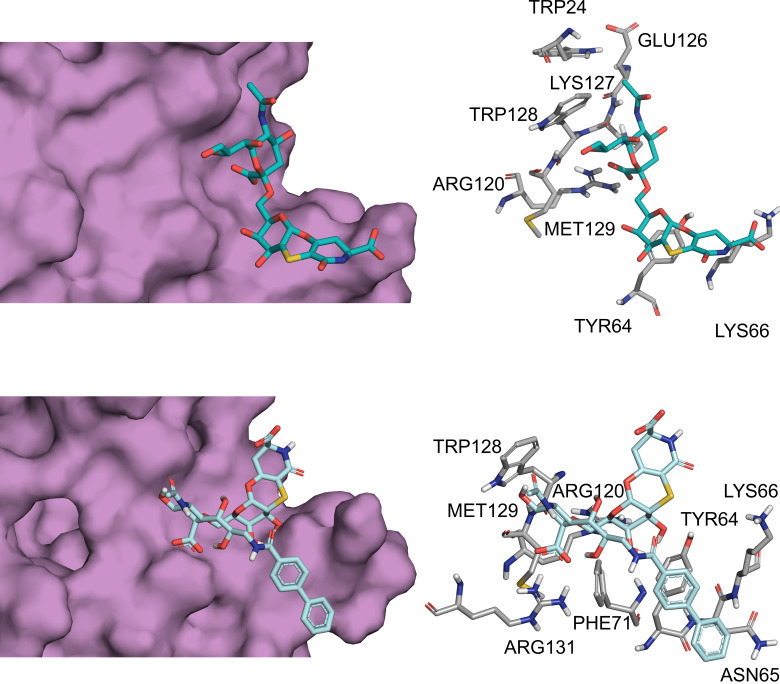
Interaction between CD22 and analogue 2 by molecular modelling. a) 3D model derived by docking calculations of 2 bound to h‐CD22. The lowest energy cluster binding mode was considered to depict the complex. b) Superimposition of the previously obtained h‐CD22/1 complex with the analogue 2 bound model. For clarity, the galactose residue and the aglycon moiety were depicted as lines. c) Two‐dimensional plots representing the interactions between the analogue 2 and the binding site residues of h‐CD22.
Figure 6.
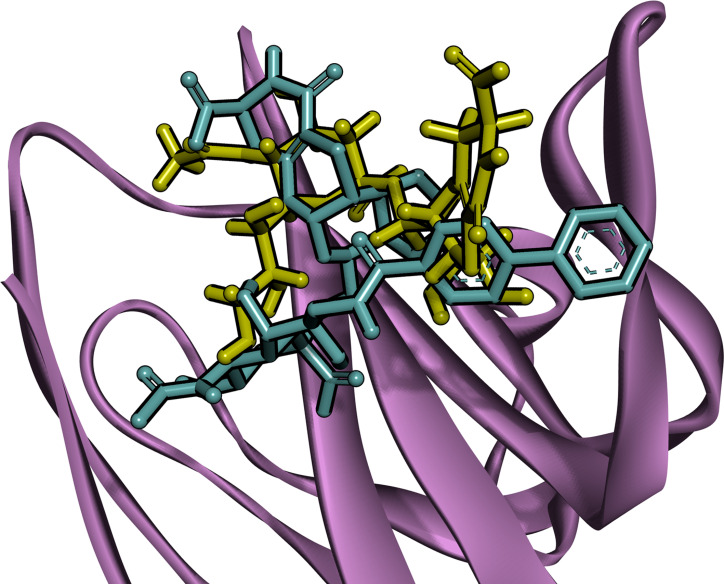
Comparison of the binding modes of analogues 1 and 2 upon interaction with h‐CD22.
Slight corrections to the corresponding text have been made as described below:
– Pag 5, first column:
“The Neu5Ac displayed polar contacts with Arg131 and Arg120, whereas the N‐acetyl moiety was not oriented toward the canonical hydrophobic cleft”
instead of
“The Neu5Ac displayed few polar contacts with Arg120, Trp24 and Trp128 whereas the N‐acetyl moiety was not oriented toward the canonical hydrophobic cleft”
– Pag 5, second column:
“Consistently with the STD‐NMR, the aglycone moiety was not in contact with the receptor surface.”
instead of
“Consistently with the STD‐NMR, Gal and the aglycone moieties were not in contact with the receptor surface.”
– Pag 6, first column:
“This may be due to the steric hindrance given by the combination of the biphenyl and the aglycon moiety, that makes the sialic acid unit not canonically oriented within the receptor binding pocket, establishing polar interactions at the CD22 interface, mainly mediated by the residue of Arg131.”
instead of
“This may be due to the steric hindrance given by the combination of the biphenyl and the aglycon moiety, that makes the sialic acid unit not optimally oriented within the receptor binding pocket, although it still established weaker polar interactions at the CD22 interface.”
– A correction to the Supporting Information is also noted:
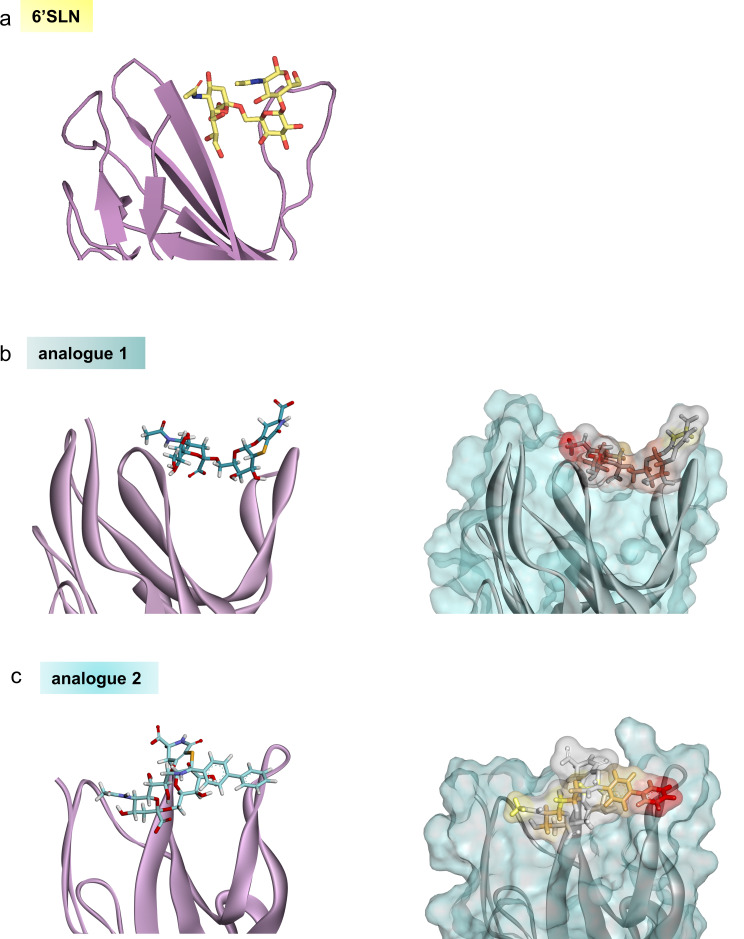
The Figure S16 has been updated and we added the following sentence in “Docking calculations” section (pag 22): “The starting structure of the molecular system h‐CD22 ‐ 1 was built by using the previously published crystal structure (PDB ID: 5VKM) as a reference. The h‐CD22 ‐ 2 starting complex was obtained by the superimposition of 2 on the previously obtained h‐CD22/1 model.”
Figure 16.
3D view of the h‐CD22/6’SLN, h‐CD22/1 and h‐CD22/2 complexes.
– The TOC was updated.
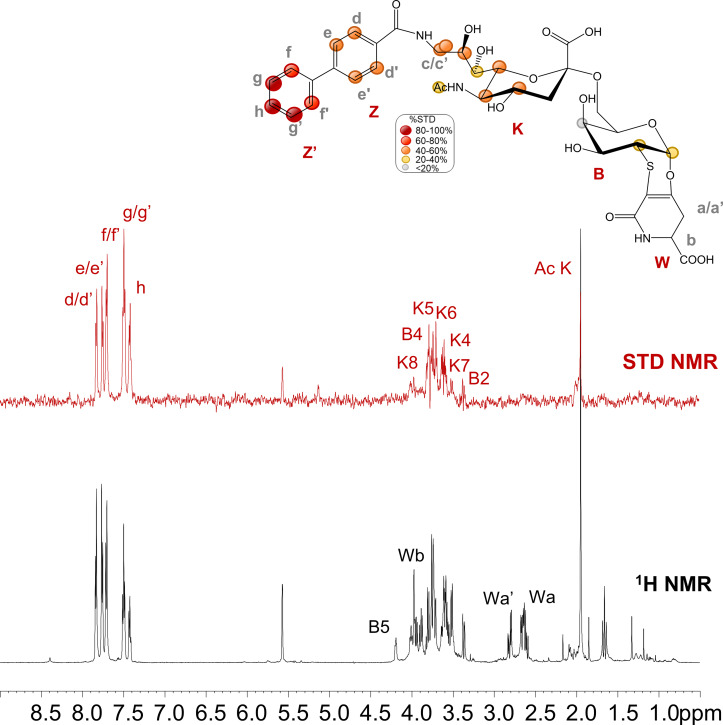
– A minor layout change of figure 4 (in which two labels were placed in the wrong position) was made.
Figure 4.
STD NMR analysis of analogue 2 in the interaction with CD22.