Abstract
Background
Direct measurement of portal venous pressure (PVP) is invasive, so the hepatic venous pressure gradient (HVPG) is commonly measured to evaluate portal hypertension (PH). HVPG is the gold standard for estimating PVP but few reports have covered standardized measurement techniques.
Methods
This study validated standardized techniques for PVP measurement.
Results
In Western countries, electronic transducers are commonly used to measure PVP, whereas the water column method is still frequently applied in Japan. Setting a reference point for accurate PVP measurement is important but complicated. According to Japanese guidelines, the reference point for PVP measurement is 10 cm above the dorsal surface or in the midaxillary line. For simpler determination, the anterior axillary point, defined as the point of convergence between the proximal pectoralis major muscle and arm when both arms are positioned against the trunk in a supine position, can be used as the reference point. New methods, such as endoscopic ultrasound-guided portal pressure gradient, offer less invasive alternatives. Non-invasive methods like elastography measure liver and spleen stiffness, which correlate with HVPG. The Baveno VII criteria incorporate measurements of liver and splenic stiffness for risk stratification. Biomarkers such as type IV collagen, M2BPGi, and FIB-4 score also predict HVPG. The Baveno VII consensus emphasizes the status of HVPG as the gold standard while advocating for non-invasive alternative methods to improve patient care and monitor treatment efficacy.
Conclusions
Continued development of non-invasive tests is crucial for safer, more convenient PH management.
Keywords: PVP, HVPG, WHVP, Elastography, Baveno VII
Introduction
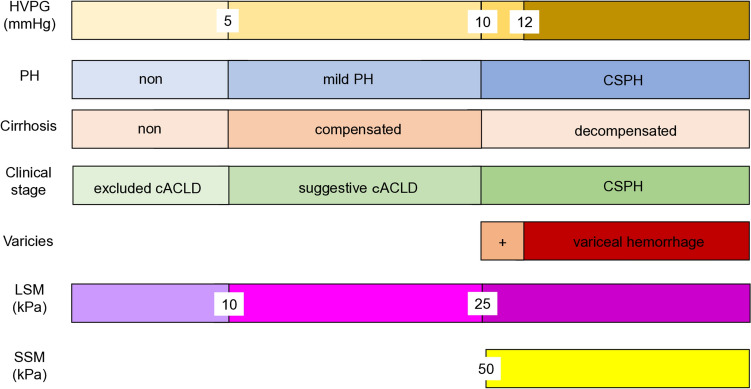
Direct measurement of portal venous pressure (PVP) is invasive and not straightforward. In 1951, Myers and Taylor first reported wedged hepatic venous pressure (WHVP) as an indirect method of measuring PVP [1]. Evaluation of PVP is essential for understanding the pathophysiology and hemodynamics of portal hypertension (PH). As no valves are present in the portal system or splenic vein, increases in blood flow resistance anywhere between the splenic vein and right atrium will lead to increased portal and/or splenic venous pressure, eventually resulting in PH. PH is classified into three types based on the site of increased blood flow resistance: prehepatic, intrahepatic, and posthepatic [2]. Cirrhosis results in intrahepatic PH and is the most common cause of PH [3, 4]. PH is a central underlying pathology for complications seen in patients with chronic liver disease or cirrhosis, and accurate diagnosis of PH holds significant clinical importance for considerations of prognosis. HVPG ≥ 5 mmHg indicates PH, and ≥ 10 mmHg represents clinically significant PH (CSPH) [5–7]. HVPG ≥ 12 mmHg carries a risk of progression to decompensated cirrhosis and variceal bleeding (Fig. 1) [6, 7].
Fig. 1.
Hepatic venous pressure gradient and portal hypertension. Relationships between HVPG, other noninvasive tests, and clinical manifestations. cACLD compensated advanced chronic liver disease, CSPH clinically significant portal hypertension, HVPG hepatic venous pressure gradient, LSM liver stiffness measurement, PH portal hypertension, SSM splenic stiffness measurement. Modified from the figures of De Franchis et al. [5], Suk [17], Garcia-Tsao et al. [6], and Albilllos et al. [7]
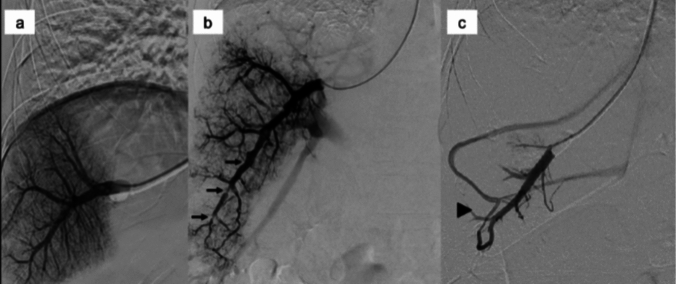
The current standard for estimating PVP is measurement of HVPG [8–12]. In the Baveno VII consensus, while the necessity for the promotion of non-invasive tests is mentioned, HVPG is considered as the gold standard for diagnosing PH [5]. Furthermore, measuring HVPG can provide more clinical information by performing hepatic venography during catheter insertion, such as irregularities in the hepatic veins suggestive of cirrhosis and the presence of hepatic venous shunts (Fig. 2) [13]. In addition, utility of HVPG is supported by a long history of accumulated evidence, such as its ability to predict the risk of esophageal variceal rupture [6, 7].
Fig. 2.
Hepatic venography image during HVPG measurement. a Homogeneous findings on sinusoidgram. b Inhomogeneous findings on sinusoidgram and irregular findings of the hepatic vein (arrow). c Hepatic venous anastomosis (arrowhead)
Although HVPG measurement is widely practiced, few reports have summarized standard techniques for the devices and methods used to achieve this measurement. This study aimed to provide an overview and validation of standardized examination techniques for measuring portal pressure in Japan.
Baveno VII consensus and HVPG
In 2021, the Baveno VII consensus meeting was held, announcing new recommendations regarding PH as the Baveno VII consensus. New criteria for compensated advanced chronic liver disease (cACLD), CSPH, individualized management of esophagogastric varices, and other aspects were introduced [14]. Recommendations regarding HVPG were also provided. In the Baveno VII consensus, HVPG remains the gold standard for diagnosing PH. In addition, measurement of HVPG is recommended when assessing the efficacy of treatment for CSPH. The Baveno VII consensus also mentioned potential variations in HVPG values based on underlying liver pathologies such as viral hepatitis and non-alcoholic steatohepatitis, although careful interpretation of measured HVPG is needed [5].
While highlighting the importance of HVPG in diagnosing CSPH, the Baveno VII consensus also discussed the utility of non-invasive tests. The previous Baveno VI consensus proposed risk stratification for CSPH using LSM measured by elastography [15]. The Baveno VII consensus stated that LSM ≤ 15 kPa and platelet count > 150 × 109/dL can be used to rule out CSPH. Further, in cases of patients with virus- and/or alcohol-related and non-obese non-alcoholic steatohepatitis-related cACLD, LSM ≥ 25 kPa is sufficient to identify CSPH. Moreover, the Baveno VII consensus suggested that risk stratification for CSPH could also be achieved using SSM. SSM can be used to rule out and rule in CSPH (SSM < 21 kPa and SSM > 50 kPa, respectively) [5].
Methods of measuring PVP
PVP
PVP is considered normal at 100–150 mmH2O (7.4–11.0 mmHg), with 1 mmH2O equivalent to 0.0736 mmHg and 1 mmHg equivalent to 13.6 mmH2O. PH is defined as a constant PVP ≥ 200 mmH2O (14.7 mmHg). Direct and indirect methods can be used for measuring portal pressure (Table 1). Devices used for pressure measurement include the water column method and electronic transducers [16]. While the use of electronic transducers is more common in Western countries, many facilities in Japan still utilize the water column due to the lack of a requirement for specialized equipment. The antecubital, right jugular or femoral veins are commonly selected as puncture routes [17]. In Japan, Yamamoto et al. reported that puncture of antecubital veins is less invasive and more convenient in terms of risk of bleeding and postoperative management [18].
Table 1.
Methods of measuring portal vein pressure
| Direct portal pressure measurement |
| Intraoperative portal pressure measurement |
| Transhepatic percutaneous portal pressure measurement |
| Indirect portal pressure measurement |
| Wedged hepatic venous pressure (WHVP) measurement |
| Hepatic venous pressure gradient (HVPG) measurement |
Methods for measuring WHVP and HVPG: are WHVP and FHVP being evaluated correctly?
Measurement of WHVP: The procedure involves inserting a balloon catheter into the hepatic vein via the right antecubital, right jugular, or femoral veins and inflating the balloon within the hepatic vein for pressure measurement, or wedging the catheter tip into the hepatic vein to occlude the vessel while measuring pressure [19, 20]. When wedging the catheter tip into the hepatic vein for measurement, it is important to note that the degree of fibrosis varies between different regions of each vein, which may lead to fluctuations in measured values [21]. In addition, if an anastomosis is observed at the distal end of the hepatic vein, portal pressure may be underestimated, so the presence of hepatic vein anastomosis needs to be ruled out by pre-measurement imaging [22].
Measurement of HVPG: HVPG is currently the most commonly used indicator of portal pressure [5]. HVPG is calculated as the difference between WHVP and free hepatic venous pressure (FHVP) [23, 24]. Both WHVP and FHVP are influenced by intra-abdominal pressure and the position of the reference point, whereas HVPG is unaffected by these factors.
Setting the reference point for PVP measurement
When measuring portal pressure, the reference point corresponds to the height of the transducer and is conventionally positioned at the level of the right atrium (midaxillary line) [16, 25]. While it is common in central venous pressure (CVP) measurement to use the superior vena cava as the reference point, methods for estimating the position of the superior vena cava from the body surface can be inconsistent. Such inconsistency is likely due to the ambiguity of the axillary line as an imaging reference. Specifically, variations arise depending on whether the axillary line is drawn curved along the trunk axis, leading to differences in height for setting the reference point in the axilla or on the chest or abdomen. According to Japanese guidelines (The General Rules for Study of Portal Hypertension, 4th edition), the reference point for PVP measurement is typically established 10 cm above the back of the patient in the supine position or the midaxillary line. In this guideline, the utility of using CT to measure the height of the portal vein from the back and employing this as the reference point has been suggested. In addition, for simplicity in determining the reference point for a supine individual, the “anterior axillary point” is considered beneficial as the reference point for portal pressure measurement and defined as the convergence point between the proximal pectoralis major and the arm with both arms positioned against the trunk (Fig. 3).
Fig. 3.
Anterior axillary point as a reference point for PVP measurement. Point A: Anterior axillary point is the convergence point between the proximal pectoralis major and the arm with both arms positioned against the trunk. The height of the anterior axillary point is approximately equal to the height of the midaxillary line (Line B) of the upper abdomen
New methods for measuring portal pressure
HVPG serves as the gold standard for evaluating PH, but poses issues due to its invasive nature. HVPG cannot assess presinusoidal or prehepatic PH and is not useful in the presence of hepatic venous anastomosis (Fig. 2c) [16, 22, 26]. In addition, the diagnostic accuracy of HVPG in metabolic-associated fatty liver disease (MAFLD), a major cause of chronic liver disease, is under scrutiny [27]. Given the widespread use of endoscopic ultrasound (EUS) in routine gastrointestinal practice, this modality may be applicable to methods of PVP measurement. As an alternative to HVPG measurement, a new technique has been reported involving direct puncture of the portal vein under EUS guidance, followed by measurement of portal pressure using a digital pressure gage. Huang et al. established the clinical feasibility of measuring EUS-guided portal pressure gradient (EUS-PPG) and examined its correlation with HVPG [28]. Several cohort studies have validated the safety and feasibility of EUS-PPG in clinical settings [29–31]. In terms of procedural skills, EUS-PPG primarily relies on the technique of fine needle aspiration needles, leveraging an already acquired skill set, and holds potential to overcome most of the aforementioned limitations of HVPG. However, reports correlating EUS-PPG with HVPG simultaneously are scarce [32]. The ongoing ENCOUNTER trial (NCT 04987034) aims to prospectively evaluate the correlation between EUS-PPG and HVPG, and the results are eagerly anticipated.
Hemodynamics and HVPG
Cardiac output and HVPG
In PH, the increase in intrahepatic vascular resistance and hyperdynamic circulation of splanchnic blood flow, and the development of the collateral circulation termed “splanchnic caput medusa” by Chikamori et al. lead to alterations in systemic hemodynamics [33, 34]. In a state of PH, the production of nitric oxide from vascular endothelial cells is increased, causing vasodilation and consequent decreases in systemic vascular resistance index [35]. In addition, the increases in stroke volume and heart rate in PH lead to an increase in cardiac index (CI), resulting in a hyperdynamic circulatory state.
CI shows positive correlations with WHVP, HVPG, and Child–Pugh score, and negative correlations with prothrombin time and hepaplastin test [33]. Child–Pugh Grade A shows lower HVPG and cardiac output compared to Child–Pugh Grades B and C [36]. Furthermore, Chikamori et al. reported a significant increase in CI with increasing severity esophageal varices [37].
Beta-blockers and HVPG
Beta-blockers are used to prevent the rupture of esophageal varices by lowering portal pressure [38, 39]. Non-selective beta-blockers (NSBBs) reduce portal pressure through two main mechanisms: a decrease in cardiac output via beta-1 blockade and reductions in portal blood flow due to the constriction of abdominal visceral blood vessels via beta-2 blockade [40].
Cases in which HVPG decreased by > 10% with NSBB treatment have been observed to show significant reductions in variceal bleeding [41]. In addition, the incidence of esophagogastric varices is also significantly reduced in cases where NSBBs decrease HVPG by > 10% [42]. Measuring HVPG is therefore important to confirm the portal pressure-lowering effects of beta-blockers. However, as frequent measurement of HVPG is difficult, heart rate monitoring is often used as a substitute in practice [12, 43].
Compared to traditional beta-blockers like propranolol, the third-generation beta-blocker carvedilol has been reported to offer superior efficacy in lowering HVPG. Further, carvedilol significantly reduces mortality in cirrhotic patients with esophagogastric varices and shows a lower incidence of adverse events compared to classical beta-blockers [44, 45].
Non-invasive tests for assessing liver fibrosis and HVPG
Ultrasound elastography and HVPG
Measurement of HVPG is considered the gold standard for evaluating PH, but has the problem of being invasive. This makes it difficult to perform repeated tests within short periods, such as for monitoring treatment efficacy. Attempts have therefore been made to substitute HVPG with safe and convenient non-invasive tests (Table 2).
Table 2.
Diagnosis of CSPH by HVPG measurement and other testing methods
| Method | Reference value | Study (references) |
|---|---|---|
| HVPG | 10 mmHg | Baveno VII [5] |
| LSM (TE) | 25 kPa | Baveno VII [5] |
| SSM (TE) | 50 kPa | Baveno VII [5] |
| SSM (RTE) | 8.24 | Hirooka et al. [49] |
| Diameter of cisterna chyli | 5.4 mm | Yano et al. [60] |
| Diameter of tDT | 3.7 mm | Yano et al. [60] |
| M2BPGi | 2.33 C.O.I | Wu et al. [71] |
HVPG hepatic venous pressure gradient, LSM liver stiffness measurement, M2BPGi Mac-2 binding protein glycosylation isomer, SSM splenic stiffness measurement, tDT terminal thoracic duct, TE transient elastography, RTE real-time tissue elastography
Liver biopsy has been considered the gold standard for diagnosing liver fibrosis, but less invasive tests have become more widely used in recent years. Elastography using ultrasound can measure liver and spleen stiffness noninvasively. Two main methods are applied in elastography: one measures the strain distribution when tissue is compressed to visualize the relative stiffness distribution (real-time tissue elastography, etc.), and the other measures the shear wave propagation velocity distribution when tissue is vibrated to visualize the quantitative stiffness distribution (transient elastography, etc.) [46]. Correlations have been shown between liver stiffness as measured by elastography and HVPG [47]. Pons et al. reported that liver stiffness as measured by transient elastography predicts CSPH, defined as HVPG ≥ 10 mmHg, when liver stiffness measurement (LSM) is ≥ 25 kPa [48]. This diagnostic criterion has also been adopted in the Baveno VII criteria [5].
In addition, spleen stiffness reportedly correlates with HVPG [49–52]. Hirooka et al. set a cutoff value of 8.24 for spleen stiffness as measured by real-time tissue elastography to predict HVPG ≥ 10 mmHg, achieving 90% diagnostic accuracy for esophagogastric varices. Splenic stiffness measurement (SSM) as measured by transient elastography has also been reported to correlate with HVPG [53]. The increase in spleen stiffness is attributed to PH, leading to hyperplasia of reticular tissue, elongation of terminal arterioles, enlargement of the white pulp, and fibrosis between trabeculae [49]. SSM correlates with HVPG better than LSM does [49, 54]. Elastography offers the advantages of bedside applicability and real-time information. However, caution is needed as measurement results may be affected by liver inflammation, liver congestion, and dietary intake [55, 56].
Imaging of the lymphatic vascular system and HVPG
In PH, sinusoidal pressure elevation leads to an approximately 30-fold increase in lymph fluid production by the liver [57]. Lymph fluid produced by the liver flows into the veins through the cisterna chyli and thoracic duct, and changes the shape of these vessels due to changes in portal pressure [58]. Yano et al. evaluated the cisterna chyli and thoracic duct using ultrasound and computed tomography, reporting a positive correlation between HVPG and diameters of the cisterna chyli and thoracic duct. They reported that the diameters of the cisterna chyli (≤ 4.5 mm to exclude CSPH; ≥ 5.4 mm to diagnose CSPH) and terminal thoracic duct (≤ 2.1 mm to exclude CSPH; ≥ 3.7 mm to diagnose CSPH) are useful for diagnosing CSPH. They also noted that during the compensated phase, these vessels tend to narrow, posing a risk for the development of refractory ascites. In addition, by incorporating lymphatic vessel diameter into the Baveno VII criteria, non-invasively and accurately identifying CSPH patients is suggested to become feasible [59].
Other imaging tests and HVPG
Magnetic resonance elastography (MRE) is a method that uses an external actuator to propagate elastic waves through the liver and measures these with MRI [60]. Reports suggest that MRE has a higher diagnostic accuracy for liver fibrosis than ultrasound elastography [61]. Liver and spleen stiffnesses measured by MRE correlate well with HVPG, with spleen stiffness measured by 3D-MRE showing the best correlation [62, 63].
Subharmonic-aided pressure estimation (SHAPE) is a diagnostic method that utilizes contrast-enhanced ultrasound [64]. The size of microbubbles within blood vessels changes with variations in hydrostatic pressure. SHAPE measures these pressure changes using signals in the subharmonic band, which are most sensitive to these changes in microbubble size [65]. SHAPE has been reported to correlate with HVPG and is also useful for monitoring treatment effects [66, 67].
Biomarkers of liver fibrosis and HVPG
Attempts have been made to predict HVPG using serum biomarkers of liver fibrosis. Type IV collagen is a major component of the extracellular matrix found in the digestive tract, skin, kidneys, and liver. In chronic liver diseases, levels rise with the progression of liver fibrosis. Levels of type IV collagen are reportedly significantly elevated in cases where HVPG is ≥ 10 mmHg [68].
M2BPGi is a glycosylated isoform of Mac-2 binding protein produced by hepatic cells and has been developed as a novel glycan marker reflecting the progression of liver fibrosis [69, 70]. Wu et al. reported that M2BPGi levels ≥ 2.33 C.O.I. can predict CSPH (HVPG ≥ 10 mmHg) [71].
FIB-4 score and APRI are non-invasive diagnostic scoring systems used to assess liver fibrosis. FIB-4 score is calculated using parameters such as age, aspartate aminotransferase (AST) concentration, alanine aminotransferase (ALT) concentration, and platelet count. APRI is calculated using parameters such as AST, ALT, and platelet count. FIB-4 score and APRI reportedly correlate with HVPG and predict CSPH [72–74].
Conclusion
Measurement of HVPG remains the gold standard for diagnosing PH and continues to hold a significant position in clinical practice. However, the active development of safer and more convenient non-invasive testing methods that could potentially be substituted for HVPG is expected to continue.
Abbreviations
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- cACLD
Compensated advanced chronic liver disease
- CI
Cardiac index
- CSPH
Clinically significant portal hypertension
- CVP
Central venous pressure
- EUS
Endoscopic ultrasound
- EUS-PPG
Endoscopic ultrasound-guided portal pressure gradient
- FHVP
Free hepatic venous pressure
- HVPG
Hepatic venous pressure gradient
- LSM
Liver stiffness measurement
- MAFLD
Metabolic-associated fatty liver disease
- MRE
Magnetic resonance elastography
- NSBBs
Non-selective beta-blockers
- PH
Portal hypertension
- PVP
Portal venous pressure
- SHAPE
Subharmonic-aided pressure estimation
- SSM
Splenic stiffness measurement
- tDT
Terminal thoracic duct
- TE
Transient elastography
- RTE
Real-time tissue elastography
- WHVP
Wedged hepatic venous pressure
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Myers JD, Taylor WJ. An estimation of portal venous pressure by occlusive catheterization of a hepatic venule. J Clin Invest. 1951;30:662–3. [Google Scholar]
- 2.Lu Q, Leong S, Lee KA, et al. Hepatic venous- portal gradient (HVPG) measurement: pearls and pitfalls. Br J Radiol. 2021;94:20210061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tetangco EP, Silva RG, Lerma EV. Portal hypertension: etiology, evaluation, and management. Review Dis Mon. 2016;62:411–26. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni AV, Rabiee A, Mohanty A. Management of portal hypertension. J Clin Exp Hepatol. 2022;12:1184–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII—renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2017;65:310–35. [DOI] [PubMed] [Google Scholar]
- 7.Albilllos A, Garcia-Tsao G. Classification of cirrhosis: the clinical use of HVPG measurements. Dis Markers. 2011;31:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feu F, Garcia-Pagan JC, Bosch J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet. 1995;346:1056–9. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva C, Balanzo J, Novella MT, et al. Nadolol plus isosorbide mononitrate compared with sclerotherapy for the prevention of variceal rebleeding. N Engl J Med. 1996;334:1624–9. [DOI] [PubMed] [Google Scholar]
- 10.Villanueva C, Minana J, Ortiz J, et al. Endoscopic ligation compared with combined treatment with nadolol and isosorbide mononitrate to prevent recurrent variceal bleeding. N Engl J Med. 2001;345:647–55. [DOI] [PubMed] [Google Scholar]
- 11.McCormick PA, Patch D, Greenslade L, et al. Clinical vs hemodynamic response to drugs in portal hypertension. J Hepatol. 1998;28:1015–9. [DOI] [PubMed] [Google Scholar]
- 12.Patch D, Sabin CA, Goulis J, et al. A randomized, controlled trial of medical therapy versus endoscopic ligation for the prevention of variceal rebleeding in patients with cirrhosis. Gastroenterology. 2002;123:1013–9. [DOI] [PubMed] [Google Scholar]
- 13.Fukunaga M. Diagnostic significance of hepatic venography and retrograde portography in portal hypertension. Kanzo. 1987;28:939–48. [Google Scholar]
- 14.Yoshiji H. Portal hypertension: advances in clinical practice from the Baveno VII consensus statement. Kanzo. 2024;65:49–57. [Google Scholar]
- 15.De Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- 16.Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280–2. [DOI] [PubMed] [Google Scholar]
- 17.Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014;20:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto Y, Kawada N, Jogo A, et al. Utility of minimally invasive measurement of hepatic venous pressure gradient via the peripheral antecubital vein. Gut. 2021;70:1199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perello A, Escorsell A, Bru C, Gilabert R, et al. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology. 1999;30:1393–7. [DOI] [PubMed] [Google Scholar]
- 20.Bochnakova T. Hepatic venous pressure gradient. Clin Liver Dis. 2021;17:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keiding S, Vilstrup H. Intrahepatic heterogeneity of hepatic venous pressure gradient in human cirrhosis. Scand J Gastroenterol. 2002;37:960–4. [DOI] [PubMed] [Google Scholar]
- 22.Ma J, Gong X, Luo J, et al. Impact of intrahepatic venovenous shunt on hepatic venous pressure gradient measurement. J Vasc Interv Radiol. 2020;31:2081–8. [DOI] [PubMed] [Google Scholar]
- 23.Procopet B, Berzigotti A. Diagnosis of cirrhosis and portal hypertension: imaging, non-invasive markers of fibrosis and liver biopsy. Gastroenterol Rep. 2017;5:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–8. [DOI] [PubMed] [Google Scholar]
- 25.Berzigotti A, Seijo S, Reverter E, et al. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141–55. [DOI] [PubMed] [Google Scholar]
- 26.Laleman W, Vanderschueren E, Van der Merwe S, et al. The use of endoscopic ultrasound in the diagnosis and management of portal hypertension. Best Pract Res Clin Gastroenterol. 2022;101811:60–1. [DOI] [PubMed] [Google Scholar]
- 27.Bassegoda O, Olivas P, Turco L, et al. Decompensation in advanced nonalcoholic fatty liver disease may occur at lower hepatic venous pressure gradient levels than in patients with viral disease. Clin Gastroenterol Hepatol. 2022;20:2276–86. [DOI] [PubMed] [Google Scholar]
- 28.Huang JY, Samarasena JB, Tsujino T, et al. EUS-guided portale pressure gradient measurement with a novel 25-gauge needle device versus transjugular approach: a comparison animal study. Gastrointest Endosc. 2016;84:358–62. [DOI] [PubMed] [Google Scholar]
- 29.Choi AY, Chang KJ, Samarasena JB, Lee JG, et al. Endoscopic ultrasound-guided porto systemic pressure gradient measurement correlates with histological hepatic fibrosis. Dig Dis Sci. 2022;67:5685–92. [DOI] [PubMed] [Google Scholar]
- 30.Hajifathalian K, Westerveld D, Kaplan A, et al. Simultaneous EUS-guided portosystemic pressure measurement and liver biopsy sampling correlate with clinically meaningful outcomes. Gastrointest Endosc. 2022;95:703–10. [DOI] [PubMed] [Google Scholar]
- 31.Choi AY, Kolb J, Shah S, Chahine A, et al. Endoscopic ultrasound-guided portal pressure gradient with liver biopsy: 6 years of endo-hepatology in practice. J Gastroenterol Hepatol. 2022;37:1373–9. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Peng C, Zhang S, et al. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest Endosc. 2021;93:565–72. [DOI] [PubMed] [Google Scholar]
- 33.Chikamori F, Kuniyoshi N, Okamoto H, et al. Systemic hemodynamic derangement in portal hypertension. Kanzo. 2012;53:316–23. [Google Scholar]
- 34.Chikamori F, Sharma N, Ito S, et al. Stepwise partial splenic embolization for portal hypertension based on a new concept: splanchnic caput medusae. Radiol Case Rep. 2020;16:564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henriksen JH, Møller S. Cardiac and systemic haemodynamic complications of liver cirrhosis. Scand Cardiovasc J. 2009;43:218–25. [DOI] [PubMed] [Google Scholar]
- 36.Meng HC, Lin HC, Tsai YT, et al. Relationships between the severity of cirrhosis and haemodynamic values in patients with cirrhosis. J Gastroenterol Hepatol. 1994;9:148–53. [DOI] [PubMed] [Google Scholar]
- 37.Chikamori F, Inoue A, Okamoto H, et al. Relationships between types of esophagogastric varices and systemic hemodynamics in patients with liver cirrhosis. Hepatogastroenterology. 2011;58:909–15. [PubMed] [Google Scholar]
- 38.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823–32. [DOI] [PubMed] [Google Scholar]
- 39.Bosch J, Abraldes JG. Variceal bleeding: pharmacological therapy. Dig Dis. 2005;23:18–29. [DOI] [PubMed] [Google Scholar]
- 40.Sugimachi K, Futagawa S, Katoh H, et al. Assessment of the prophylactic effect and safety of propranolol in upper gastrointestinal bleeding associated with portal blood circulation disorder (portal hypertension). Kanzo. 2004;43:248–60. [Google Scholar]
- 41.Kerbert AJ, Chiang FW, van der Werf M, et al. Hemodynamic response to primary prophylactic therapy with nonselective β-blockers is related to a reduction of first variceal bleeding risk in liver cirrhosis: a meta-analysis. Eur J Gastroenterol Hepatol. 2017;29:380–7. [DOI] [PubMed] [Google Scholar]
- 42.Groszmann R, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–61. [DOI] [PubMed] [Google Scholar]
- 43.Tripathi D, Stanley AJ, Hayes PC, et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung KS, Mok CH, Lam LK, et al. Carvedilol versus other nonselective beta blockers for variceal bleeding prophylaxis and death: a network meta analysis. J Clin Transl Hepatol. 2023;11:1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tripathi D, Hayes PC. Beta-blockers in portal hypertension: new developments and controversies. Liver Int. 2014;34:655–67. [DOI] [PubMed] [Google Scholar]
- 46.Iijima H, Nishimura T. Ultrasound diagnosis of hepaticfibrosis and steatosis. Kanzo. 2018;59:384–92. [Google Scholar]
- 47.Kumar A, Khan NM, Anikhindi SA, et al. Correlation of transient elastography with hepatic venous pressure gradient in patients with cirrhotic portal hypertension: a study of 326 patients from India. World J Gastroenterol. 2017;23:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pons M, Augustin S, Scheiner B, et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2020;116:723–32. [DOI] [PubMed] [Google Scholar]
- 49.Hirooka M, Ochi H, Koizumi Y, et al. Splenic elasticity measured with real-time tissue elastography is a marker of portal hypertension. Radiology. 2011;261:960–8. [DOI] [PubMed] [Google Scholar]
- 50.Hirooka M, Koizumi Y, Nakamura Y, et al. Spleen stiffness in patients with chronic liver disease evaluated by 2-D shear wave elastography with ultrasound multiparametric imaging. Hepatol Res. 2023;53:93–103. [DOI] [PubMed] [Google Scholar]
- 51.Vizzutti F, Arena U, Romanelli RG, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–7. [DOI] [PubMed] [Google Scholar]
- 52.Karagiannakis DS, Stefanaki K. Spleen stiffness: a predictive factor of dismal prognosis in liver cirrhosis. Clin J Gastroenterol. 2023;16:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buechter M, Manka P, Theysohn JM, et al. Spleen stiffness is positively correlated with HVPG and decreases significantly after TIPS implantation. Dig Liver Dis. 2018;50:54–60. [DOI] [PubMed] [Google Scholar]
- 54.Ma X, Wang L, Wu H, et al. Spleen stiffness is superior to liver stiffness for predicting esoph ageal varices in chronic liver disease: a meta analysis. PLoS ONE. 2016;11: e0165786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yada N, Sakurai T, Minami T, et al. Influence of liver inflammation on liver stiffness measurement in patients with autoimmune hepatitis evaluation by combinational elastography. Oncology. 2017;92:10–5. [DOI] [PubMed] [Google Scholar]
- 56.Kondo R, Kage M, Iijima H, et al. Pathological findings that contribute to tissue stiffness in the spleen of liver cirrhosis patients. Hepatol Res. 2018;48:1000–7. [DOI] [PubMed] [Google Scholar]
- 57.Hirooka M, Koizumi Y, Yano R, et al. Cisterna chyli as an optimal marker of tolvaptan response in severe cirrhotic ascites. Sci Rep. 2022;12:8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka M, Iwakiri Y. The hepatic lymphatic vascular system: structure, function, markers, and lymphangiogenesis. Cell Mol Gastroenterol Hepatol. 2016;2:733–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yano R, Hirooka M, Koizumi Y, et al. Lymphatic drainage dysfunction via narrowing of the lumen of cisterna chyli and thoracic duct after luminal dilation. Hepatol Int. 2023;17:1557–69. [DOI] [PubMed] [Google Scholar]
- 60.Rouvière O, Yin M, Alex Dresner M, et al. MR elastography of the liver: preliminary results. Radiology. 2006;240:440–8. [DOI] [PubMed] [Google Scholar]
- 61.Chen J, Yin M, Talwalkar JA, et al. Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology. 2017;283:418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kennedy P, Stocker D, Guillermo Carbonell G, et al. MR elastography outperforms shear wave elastography for the diagnosis of clinically significant portal hypertension. Eur Radiol. 2022;32:8339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi Y, Qi YF, Lan GY, et al. Three-dimensional MR elastography depicts liver inflammation, fibrosis, and portal hypertension in chronic hepatitis B or C. Radiology. 2021;301:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eisenbrey JR, Dave JK, Halldorsdottir VG, et al. Chronic liver disease: noninvasive subharmonic aided pressure estimation of hepatic venous pressure gradient. Radiology. 2013;268:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta I, Eisenbrey JR, Machado P, et al. Diagnosing portal hypertension with noninvasive subharmonic pressure estimates from a US contrast agent. Radiology. 2021;298:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta I, Fenkel JM, Eisenbrey JR, et al. A noninvasive ultrasound based technique to identify treatment responders in patients with portal hypertension. Acad Radiol. 2021;28:S128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forsberg F, Gupta I, Machado P, et al. Contrast-enhanced subharmonic aided pressure estimation (SHAPE) using ultrasound imaging with a focus on identifying portal hypertension. J Vis Exp. 2020. 10.3791/62050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leeming DJ, Karsdal MA, Byrjalsen I, et al. Novel serological neo-epitope markers of extracellular matrix proteins for the detection of portal hypertension. Aliment Pharmacol Ther. 2013;38:1086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuno A, Ikehara Y, Tanaka Y, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamasaki K, Tateyama M, Abiru S, et al. Elevated serum levels of WFA+ -M2BP predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu PS, Hsieh YC, Lee KC, Huang YH, et al. Mac-2 binding protein glycosylation isomer is a potential biomarker to predict portal hypertension and bacterial infection in cirrhotic patients. PLoS ONE. 2021;6: e0258589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Procopet B, Cristea VM, Robic MA, et al. Serum tests, liver stiffness and artificial neural networks for diagnosing cirrhosis and portal hypertension. Dig Liver Dis. 2015;47:411–6. [DOI] [PubMed] [Google Scholar]
- 73.Le W, Yuemin F, Xiaowen M, et al. Diagnostic efficacy of noninvasive liver fibrosis indexes in predicting portal hypertension in patients with cirrhosis. PLoS ONE. 2017;12: e0182969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brol MJ, Gödiker J, Uschner FE, et al. Non-invasive assessment of clinically significant portal hypertension. Curr Hepatol Rep. 2023;22:206–15. [Google Scholar]