Abstract
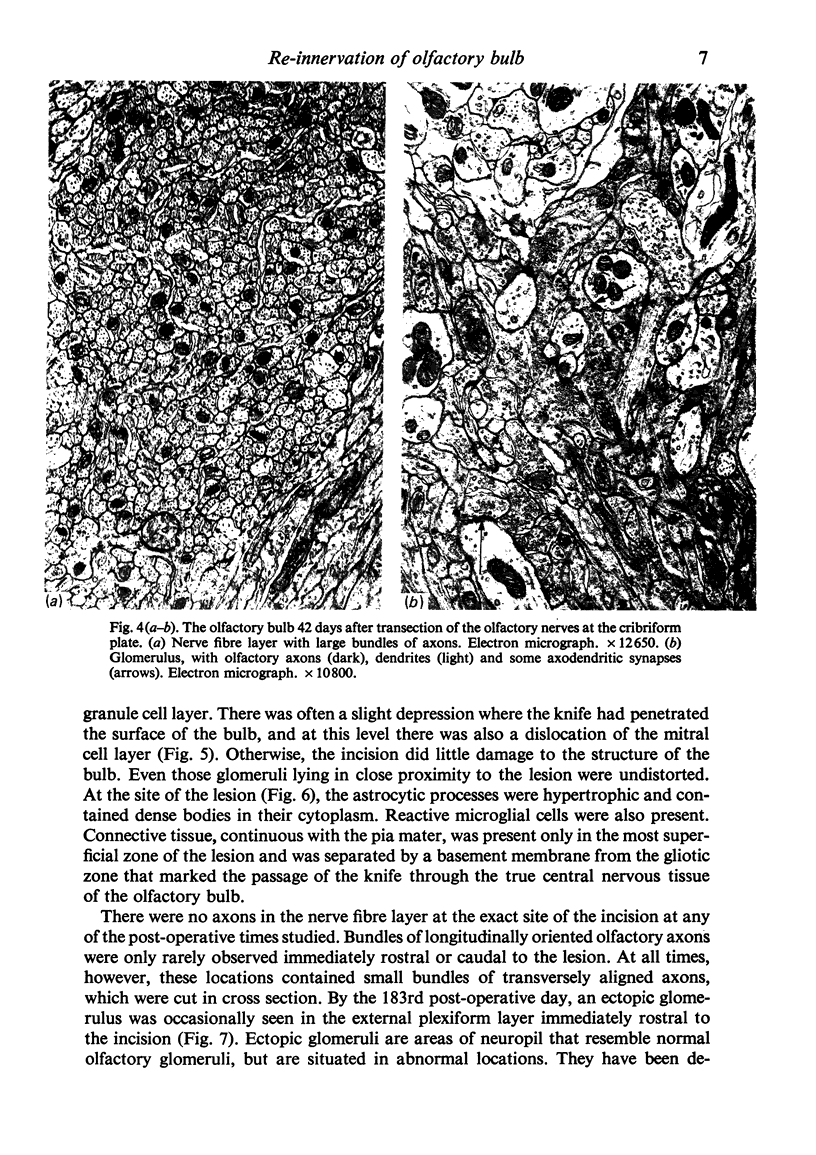
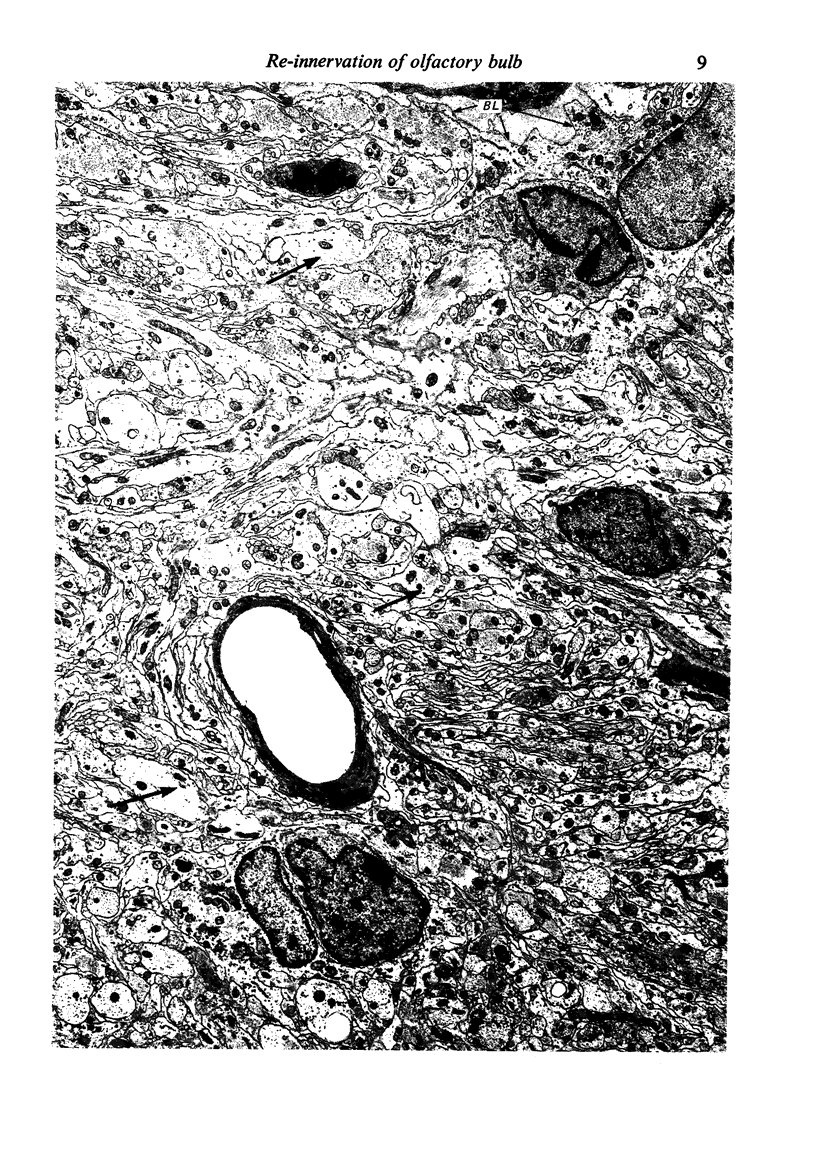
The re-innervation of the olfactory bulb has been studied in rats in which the primary afferent axons were transected either in the peripheral nervous system, on the intracranial side of the cribriform plate, or in the central nervous system, in the nerve fibre layer of the bulb. Both procedures resulted in denervation of glomeruli on the dorsal surface of the olfactory bulb. Re-innervation of these glomeruli was first seen approximately three weeks after operation and was largely completed by the sixth week, irrespective of the site of the lesion. The similarity of the timing of re-innervation following the two procedures indicates that the cut fibres did not regenerate from their sites of transection. It is much more probable that the re-innervation axons were those of neurons newly generated in the olfactory epithelium. This view is supported by the results of other investigations, in which retrograde degeneration and subsequent replacement of the neurons have been found to follow transection of the olfactory nerves. After transection of the olfactory nerves, the new axons entering the bulb grew through the site of the lesion, across the interface between peripheral and central nervous tissue, through the nerve fibre layer and into the glomeruli. Thus, they followed the same course as normally growing primary olfactory axons. After the afferent fibres had been cut within the olfactory bulb, the site of transection was transformed into a scar composed largely of astrocytes. No olfactory axons grew through the scar and none passed beneath it in the deeper layers of the bulb. However, by tracing the anterograde axonal transport of horseradish peroxidase, it has been shown that axons immediately rostral to the lesion terminated in the re-innervated glomeruli. These denervated glomeruli were, therefore, probably re-innervated by axons that grew through the intact central nervous tissue of the nerve fibre layer on either side of the lesion.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. A., Flumerfelt B. A. A light and electron microscopic study of the effects of 3-acetylpyridine intoxication on the inferior olivary complex and cerebellar cortex. J Comp Neurol. 1980 Mar 1;190(1):157–174. doi: 10.1002/cne.901900111. [DOI] [PubMed] [Google Scholar]
- Berthold C. H., Carlstedt T. Observations on the morphology at the transition between the peripheral and the central nervous system in the cat. II. General organization of the transitional region in S1 dorsal rootlets. Acta Physiol Scand Suppl. 1977;446:23–42. [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M., Harris E. W. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev. 1981 Jul;61(3):684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- Doucette J. R., Kiernan J. A., Flumerfelt B. A. Two different patterns of retrograde degeneration in the olfactory epithelium following transection of primary olfactory axons. J Anat. 1983 Jun;136(Pt 4):673–689. [PMC free article] [PubMed] [Google Scholar]
- Foerster A. P. Spontaneous regeneration of cut axons in adult rat brain. J Comp Neurol. 1982 Oct 1;210(4):335–356. doi: 10.1002/cne.902100403. [DOI] [PubMed] [Google Scholar]
- Graziadei G. A., Graziadei P. P. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979 Apr;8(2):197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Graziadei P. P., DeHan R. S. Neuronal regeneration in frog olfactory system. J Cell Biol. 1973 Nov;59(2 Pt 1):525–530. doi: 10.1083/jcb.59.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei P. P., Graziadei G. A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979 Feb;8(1):1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Graziadei P. P., Monti Graziadei G. A. Neurogenesis and neuron regeneration in the olfactory system of mammals. III. Deafferentation and reinnervation of the olfactory bulb following section of the fila olfactoria in rat. J Neurocytol. 1980 Apr;9(2):145–162. doi: 10.1007/BF01205155. [DOI] [PubMed] [Google Scholar]
- Graziadei P. P., Samanen D. W. Ectopic glomerular structures in the olfactory bulb of neonatal and adult mice. Brain Res. 1980 Apr 14;187(2):467–472. doi: 10.1016/0006-8993(80)90217-6. [DOI] [PubMed] [Google Scholar]
- Harding J., Graziadei P. P., Monti Graziadei G. A., Margolis F. L. Denervation in the primary olfactory pathway of mice. IV. Biochemical and morphological evidence for neuronal replacement following nerve section. Brain Res. 1977 Aug 19;132(1):11–28. doi: 10.1016/0006-8993(77)90703-x. [DOI] [PubMed] [Google Scholar]
- Kerr F. W. The potential of cervical primary afferents to sprout in the spinal nucleus of V following long term trigeminal denervation. Brain Res. 1972 Aug 25;43(2):547–560. doi: 10.1016/0006-8993(72)90407-6. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. Hypotheses concerned with axonal regeneration in the mammalian nervous system. Biol Rev Camb Philos Soc. 1979 May;54(2):155–197. doi: 10.1111/j.1469-185x.1979.tb00871.x. [DOI] [PubMed] [Google Scholar]
- LIU C. N., CHAMBERS W. W. Intraspinal sprouting of dorsal root axons; development of new collaterals and preterminals following partial denervation of the spinal cord in the cat. AMA Arch Neurol Psychiatry. 1958 Jan;79(1):46–61. [PubMed] [Google Scholar]
- Mesulam M. M., Hegarty E., Barbas H., Carson K. A., Gower E. C., Knapp A. G., Moss M. B., Mufson E. J. Additional factors influencing sensitivity in the tetramethyl benzidine method for horseradish peroxidase neurohistochemistry. J Histochem Cytochem. 1980 Nov;28(11):1255–1259. doi: 10.1177/28.11.6159394. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978 Feb;26(2):106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Monti Graziadei G. A., Karlan M. S., Bernstein J. J., Graziadei P. P. Reinnervation of the olfactory bulb after section of the olfactory nerve in monkey (Saimiri sciureus). Brain Res. 1980 May 12;189(2):343–354. doi: 10.1016/0006-8993(80)90095-5. [DOI] [PubMed] [Google Scholar]
- Oley N., DeHan R. S., Tucker D., Smith J. C., Graziadei P. P. Recovery of structure and function following transection of the primary olfactory nerves in pigeons. J Comp Physiol Psychol. 1975 Feb;88(2):477–495. doi: 10.1037/h0076401. [DOI] [PubMed] [Google Scholar]
- Pinching A. J., Powell T. P. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci. 1971 Sep;9(2):347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- Price J. L., Powell T. P. An electron-microscopic study of the termination of the afferent fibres to the olfactory bulb from the cerebral hemisphere. J Cell Sci. 1970 Jul;7(1):157–187. doi: 10.1242/jcs.7.1.157. [DOI] [PubMed] [Google Scholar]
- Price J. L., Powell T. P. An experimental study of the origin and the course of the centrifugal fibres to the olfactory bulb in the rat. J Anat. 1970 Sep;107(Pt 2):215–237. [PMC free article] [PubMed] [Google Scholar]
- Raisman G. Neuronal plasticity in the septal nuclei of the adult rat. Brain Res. 1969 Jun;14(1):25–48. doi: 10.1016/0006-8993(69)90029-8. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. Synaptic organization of the mammalian olfactory bulb. Physiol Rev. 1972 Oct;52(4):864–917. doi: 10.1152/physrev.1972.52.4.864. [DOI] [PubMed] [Google Scholar]
- VALENSTEIN E. S. A note on anesthetizing rats and guinea pigs. J Exp Anal Behav. 1961 Jan;4:6–6. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey T. J. The ultrastructure of the cat olfactory bulb. J Comp Neurol. 1973 Dec 1;152(3):211–232. doi: 10.1002/cne.901520302. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Ghetti B., Horoupian D. S. The fate of synaptic membranes of degenerating optic nerve terminals, and their role in the mechanism of trans-synaptic changes. J Neurocytol. 1972 Oct;1(3):297–310. doi: 10.1007/BF01099940. [DOI] [PubMed] [Google Scholar]












