Abstract
Like the lines themselves, concerns about facial wrinkles, particularly glabellar lines - the prominent furrows between the eyebrows - intensify with age. These lines can inadvertently convey negative emotions due to their association with negative facial expressions. We investigated the effects of repeated frowning on the development of temporary glabellar lines through the activation of the corrugator muscle. In addition, as communication via facial expressions requires precise control over the muscles of the face in such a way as to avoid contradictory signals, we hypothesized that smiling and activation of the zygomatic major may inhibit the corrugator. Our findings reveal that repeated frowning creates temporary wrinkles between the eyebrows, caused by the slight but cumulative activation of the corrugator muscle. Further we found that the act of smiling activates zygomatic major and suppresses the corrugator reducing the appearance of glabellar lines. The results offer a novel perspective on smiling and suggests that smiling not only facilitates positive emotional exchanges but may also help maintain a youthful facial appearance.
Keywords: Facial muscle, Electromyography, Facial expression, Corrugator Supercilii, Zygomaticus major
Subject terms: Electromyography - EMG, Human behaviour, Ageing
Introduction
The wrinkles between the eyebrows, known as glabellar lines, are often a major age-related concern. These wrinkles, which appear when a person frowns, contribute to the impression of being angry, worried, or aged1. The wrinkles are caused due to loss of skin and muscle fiber elasticity and repeated and habitual contraction of the underlying muscles of facial expression (e.g. corrugator supercilii (CS) muscle)2,3. In other words, it is important not only to care for the physiological changes in the skin that occur with ageing but also to reduce habitual contractions of the CS muscle. The CS muscle is known to be activated when expressing negative emotions such as anger, fear, or sadness through facial expressions4. However, it is also known to be activated in situations other than the expression of these emotions. Reports suggest that individuals sometimes furrow their eyebrows for extended periods while engaging in conversation or exerting mental effort, such as during calculation or pondering5,6. As individuals age, the appearance of glabellar lines can unintentionally convey a negative facial expression, which may be perceived as emotionally inappropriate. This can lead to a decrease in self-esteem and a reduction in quality of life7. Therefore, finding new approaches to reduce activation in the CS muscle may be an effective strategy to reduce glabellar lines and improve overall well-being.
One possible solution to avoid habitual contraction of the CS muscle is to facilitate positive experiences for individuals. CS muscle activation decreases when people imagine a happy situation8. CS muscle deactivation has been also found for pleasant stimuli9,10. Previous researches have shown that viewing photographs of smiling faces leads to decreased CS muscle activity11,12. While positive experiences can reduce CS activation and potentially prevent wrinkles, it is not always feasible for individuals to experience or even imagine positive scenarios. This underscores the need for alternative, cost-effective, and universally accessible methods to reduce CS activity.
One potential intervention for the CS activity reduction could involve leveraging the effects of smiling. Our ability to initiate and switch between various different facial emotion expressions each of which require different combinations of facial muscles may involve inhibition of muscles which are unrelated or the contraction of which would result in a conflicting emotional expression13,14. In addition, William James posited the notion that smiling could enhance happiness, a concept later formalized as the “facial feedback hypothesis”15. Although some controversy surrounds this hypothesis16, feedback information from some positive and negative facial expressions (e.g. happiness, anger, disgust) has the effect, albeit small, of modifying emotional experience more positive and negative, respectively17. A previous study has shown that making smile-like expressions, where a “smile” is induced by holding a pen in the mouth, enhanced the perceived humor of visual stimuli18. In other words, an induced smile (i.e. forced smiling in the absence of positive emotion) may result in inhibition of CS muscles. Zygomaticaus major (ZM) muscle is the critical facial muscle for smiling expression and positive affect, while the CS muscle is critical for negative facial expressions and negative affect9. We thus hypothesized that the CS muscle would be inhibited by facial expressions involving the ZM muscle. In either case, if ZM muscle contraction leads to inhibition of the CS muscle, we may be able to provide a simple intervention to the problem of glabellar lines. We examined this possibility in three ways. The first is determining how much CS activation can persist even during relaxation and accumulate during repetitive frowning. The second is whether any CS residual activation induces glabellar lines. The third is whether activating the ZM muscle via induced smiling can inhibit CS muscle activation and if so, what is the mechanism and to what degree this inhibition reduces medial movement of eyebrows and visible glabellar lines.
It remains unclear to what extent, if any, CS muscle activation persists during relaxation or whether residual activity can accumulate with repetitive frowning, both of which may contribute to the development of glabellar lines. To investigate this, we utilized facial electromyography (EMG), which is sensitive to subtle changes in facial muscle activity8, to assess the ease of CS muscle activation and the difficulty of its relaxation. We hypothesized that the CS muscle is often activated unconsciously and continuously. Therefore, we recorded CS muscle activation during instructed relaxation at two time points: before and after a periorbital massage. Due to the nature of EMG analysis, signals are inherently non-zero. Previous research has indicated that massage can reduce muscle tension and lower EMG signals19,20. We postulated that periorbital massage would significantly reduce CS muscle activity, allowing us to establish a baseline level of activity. A notable decrease in activation post-massage would imply the presence of residual CS activation during relaxation. Furthermore, we examined whether repeated frowning leads to an increase in involuntary baseline activity in the CS muscle.
The contribution of slight activation of the CS muscle to frowning and wrinkles between the eyebrows remains unclear. Emotional experiences do not always manifest visually as facial expressions, particularly when the intensity of the emotion is low and perception is not explicit. Facial muscles, however, often respond to such states even in the absence of facial skin movement6,8. Video recordings of facial expressions, obtained simultaneously with the CS activation measurement, were used to measure the glabellar lines.
Lastly, we investigate whether an induced smile can inhibit the CS muscle activity and its underlying mechanism, aiming to develop a simple, cost-free method to combat glabellar lines. If the induced smile decreases the CS muscle activity and simultaneously contributes to the reduction of frown-induced wrinkles detected by the video recording, it suggests that induced smiles could be a new and easy way to reduce wrinkling and the associated negative impressions. We analyzed the temporal relationship between the activation of the ZM muscle by the induced smile and the deactivation of the CS muscle. By investigating the mechanisms of the antagonistic relationship between the CS and the ZM muscles in detail, we will be able to extend our understanding of the relationship between facial expression and emotion (e.g. facial feedback hypothesis).
Materials and methods
Participants
Thirty-six participants aged between 29 and 59 years old (Female: N = 10, M ± SD = 37.9 ± 6.6; Male: N = 26, M ± SD = 43.0 ± 9.1) received remuneration for participation in this experiment, after providing their written informed consent. They reported normal or corrected-to-normal vision and no history of psychiatric and neurological disorders. This study was performed in accordance with the principles in the Declaration of Helsinki and approved by the Ethical Committee at the Shiseido Global Innovation Center (Approval number: C10069 and C10402).
Stimuli and procedure
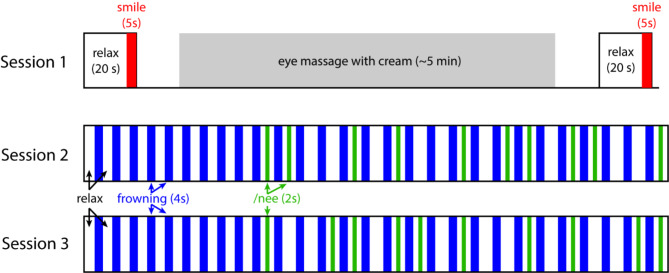
The experiment consisted of 3 sessions and during which the participants sat comfortably on a chair and conducted the task instructed on the PC screen in front of them. The purpose of session 1 was to detect any residual activity of the CS muscle at the beginning of the experiment. The participants were asked to relax for 20 s, after which they were instructed to smile maximally for 5 s. Next the participants performed a massage around their eyes in accordance with the experimenter’s instructions. The massage, using a cream, is a conventional periorbital massage procedure adopted by Shiseido, lasted approximately 5 min and was expected to reduce muscle activity. After the massage, the participants repeated the 20-s relaxation and 5-s maximal smile. The timing of smiling was instructed by the in-house developed Python 3.7.3 (Python software foundation, https://www.python.org/) program made with the PsychoPy® library21.
There were three objectives in Session 2 and 3. The first was to test if involuntary muscle activity can accumulate following repeated frowning. The second was to determine if the induced smile decreases the CS muscle activity and simultaneously contributes to the reduction of frown induced wrinkles. The third was to determine the temporal relationship between the ZM muscle activation by the induced smile and the CS muscle deactivation. For these objectives, we first asked participants to repeatedly frown, instructing them to keep their eyebrows strongly inward and create a wrinkle between them. Between the frowns, they were sometimes instructed to pull the corners of their mouth back in the same direction as when pronouncing of the pseudoword ‘Nee’ (hereafter referred to as induced /nee smiling), so that they could increase the activity of the zygomaticus major muscle without reminding the participants of smiling. Those who were more comfortable saying it out loud were allowed to do so, but most members did not. Actually, both sessions (Session 2 and 3) started with 4-s relaxation as baseline. After this, they consisted of 10 trials of 4-s frowning followed by 4-s relaxation and then, in pseudorandom order, 9 trials of frowning and 6 s relaxations (hereafter referred to as Non trials) and 10 trials of frowning and 2-s relaxation, 2-s induced /nee smiling and a further 2-s relaxation (hereafter referred to as Nee trials; Fig. 1). This approach was used rather than verbal instruction to smile or “holding a pen in the teeth”18 in order to reduce the possibility of incorrect facial expressions while ensuring the minimal contraction of facial muscles around mouth corner.
Fig. 1.
Schematic representation of the experimental design.
The first 9 trials of frowning and relaxation allowed the measurement of the accumulation of muscle CS activity and, following the reduction in activity elicited by the massage in Session 1, this accumulation of activity is necessary to examine the potential activity suppressing effects of the induced /nee smiling. Participants were also asked not to move their eyes to avoid electrical signal artefacts. The in-house developed program was also used to record a video of the participants face (30 frames per second) via a PC built-in camera to detect visible changes of facial skin around the eyebrows. This video recorded facial expressions synchronously with the EMG measurement. For this purpose, at the beginning of each session, the participants adjusted their face position relative to the PC camera via the real-time video feedback which displayed 3 ellipse-shaped locators for positioning their face and eyes (the distance between eye centers was 80 pixels). This allowed us to minimize the error in the face detection and spatial registration within and across participants.
Data acquisition and analysis
Two pairs of active bipolar Ag electrodes (AP-C140-020, Miyuki Giken Co., Ltd., Tokyo, Japan) were attached to the face to acquire the EMG over the CS and ZM muscles on the left side of the face. Recordings were made from the left side because the left face dominates emotional expression6. The detailed positioning was decided in accordance with a published guideline22. The reference electrode (AP-C151-015) was attached on the wrist radial styloid process. A grand electrode (MA-C004-015 and general disposable electrode) was attached on the forehead and covered with a hairband. To lower the electrode conductance, we applied Ten20 conductive paste (Weaver and Company, Aurora, CO, USA) on the electrode tips and attached a pierced double-sided tape between the body of the electrode and facial skin. They were also covered and fixed with elasticated adhesive tape.
EMG signals were recorded by a 32-ch physiological signal recording device (Polymate 1000 (AP1532), Miyuki Giken Co., Ltd., Tokyo, Japan) with a sampling rate of 1000 Hz. For offline data processing, 50 Hz noise in the EMG signal was excluded by a notch filter (48–52 Hz). The filtered signal was rectified and averaged along each 20mec time window with a 1ms shift. The sampling rate of the time series was decreased to 10 Hz just for visual presentation in the figures (Figs. 2 and 3). The preprocessed EMG signals were divided into epochs of each behavioral task based on the trigger signals from the task PC. For the statistics, the median of the EMG signal was calculated within the analysis time window and across trials as the representative value for each participant so that the effects of the sudden motion artefacts and contaminated blink signals were minimized.
Fig. 2.
CS and ZM muscle activation during and after the repeated frowning at each trial with and without induced /nee smiling. The upper panel depicts the time series of these muscles. Blue, red, cyan, magenta lines indicate the CS muscle activation at the Non and Nee trials, and the ZM muscle activation at the Non and Nee trials, respectively. The lower left panel depicts the comparison of the CS EMG amplitude between the trials (Non vs. Nee) during Nee phase. The lower right panel depicts the comparison of the CS EMG amplitude between the trials (Non vs. Nee) after the Nee phase. The phase in which we analyze the data are superimposed in the upper panel as gray boxes.
Fig. 3.
Quantitative assessment of the distance between eyebrows. The upper panel depicts the time series of the normalized distance (B/W; B: Horizontal distance between inner end coordinates of eyebrows, W: The horizontal distance between the left and right outermost landmarks of the face outline). All face landmarks coordinates were superimposed in this panel. Blue and red lines are the time series of the Non and Nee trials, respectively. The left and right gray boxed areas indicate the analysis time windows of during Nee and after Nee phases, respectively. The middle panel depicts the comparison of the distances between the trials (Non vs. Nee) during the Nee phase. The right panel depicts the comparison of the distance between the trials (Non vs. Nee) after the Nee phase.
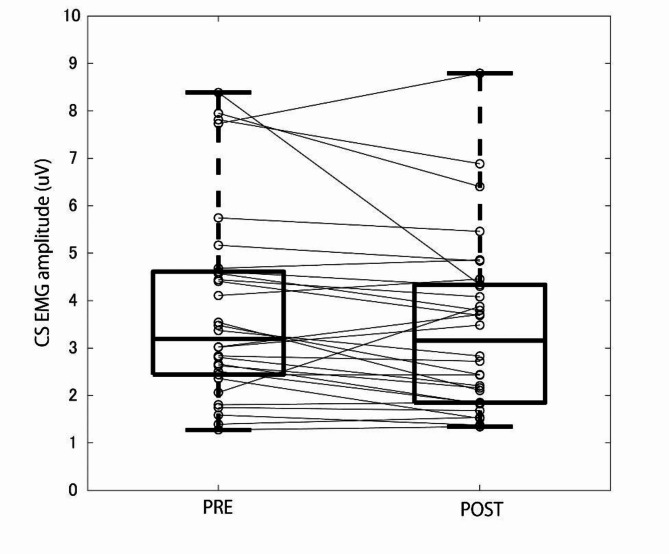
Session 1 EMG data was analyzed was to investigate the effect of periorbital massage on the CS activations (Fig. 4) during relaxation (20 s) but not on those during smiling (5 s), and in order to ascertain if there can be residual activity. The smiling task was inserted to prevent participants from realizing the intention of the experiment. The first 9 trials of Session 2 and 3 EMG data were first analyzed to measure gradual accumulation of the CS muscle activation induced by repeated frowning. The median CS EMG signal during the relaxations of each of the first 9 trials of sessions 2 and 3 (time window of 1 s) were calculated and compared to the 1 s baseline before the first trial (Fig. 5A). We also conducted video analysis during repetitive frowning. Thirty frames of the image around eyebrows were extracted during relaxation period in between each 9 trials of frowning (as corresponding to the EMG analysis). They were averaged and compared with the baseline images (Fig. 5B–E). Note that the baseline image was calculated from 10 frames of images after frowning instruction (~ 330 ms and the frowning did not started yet) because the video recording lacks the data before the first frowning instruction. Next, to investigate the effect of induced smiling on the post-frown CS muscle activity, the CS EMG signals in the remaining trials were compared between conditions. That is, the effect of the induced /nee smiling on the CS muscle activity following frowning was investigated. There were 2 phases to be analyzed: CS activation during induced smile (during Nee phase, i.e. 3–4 s from the end of the frowning) and CS activation after the induced smile (after Nee phase, i.e. 5–6 s from the end of the frowning) (Fig. 2).
Fig. 4.
CS muscle median activities before and after periorbital massage.
Fig. 5.
Accumulation of unintentional CS muscle activation and wrinkle production through repeated frowning across the first 9 trials in session 2 and 3. (A) The CS muscle activation extracted 1-s period after each frowning and “pre” means those before the 1st frowning B Wrinkle images in each trial subtracted by a “pre” wrinkle image in session 2. They were extracted 1-s period before each frowning and averaged across participants for session 2. “pre” means those before the 1st frowning. Note that the only the wrinkle image at “pre” is computed from 10 frames (see Materials and methods). C Wrinkle images in session 3 as in (B) . (D) t-value map of the wrinkle image for 9th trial in session 2. The image was resized (1 grid = 5 × 5 pixels) for statistics and only the results above the threshold of corrected p values were represented. E t-value map of wrinkle image for 9th trial in session 3. For panel (D) and (E) the averaged position of the face landmarks are superimposed.
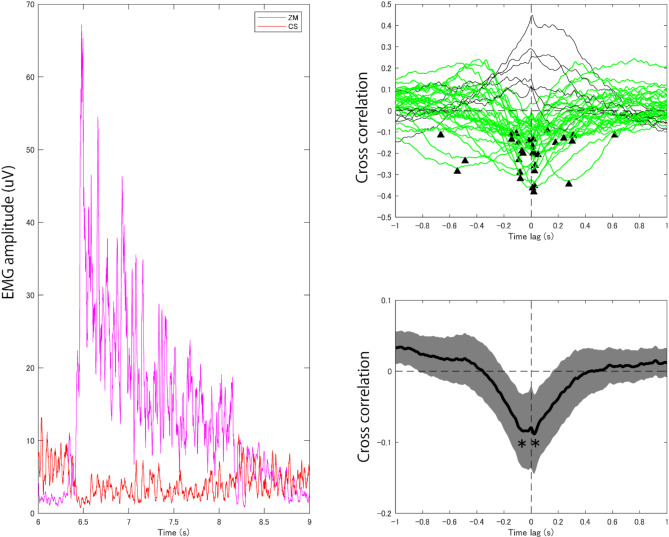
To investigate the relationship between the ZM and CS muscle, we adopted 2 analyses. One was the simple regression analysis with a linear mixed model, using R software and the lme4 and lmerTest packages. We calculated the regression between the median of ZM muscle activation (log transformed value) during the Nee phase and the median CS muscle activation (log transformed value) during the same phase for each trial and participant by the random slope and intercept effect model. The other analysis was a time series analysis between the ZM muscle activation around the Nee phase (6–9 s from the onset of the frowning instruction, Fig. 6 left panel) and the CS muscle deactivation during the same period, we computed 2 types of time lags in the cross-correlation of their signals (Fig. 6, right panels). Firstly, we calculated the cross correlation between the CS and ZM EMG signals for each trial and averaged as representative data for each participant. Then we selected typical cross correlation functions so that their negative peaks ranged from − 1 to 1 s in the time lag (individual time lags) and averaged them. We also averaged the cross-correlation functions of all participants first, then we picked up the negative peak (grand averaged time lag).
Fig. 6.
Temporal relationship between the ZM muscle activation and the CS muscle deactivation by the induced smile. The left panel depicts signal of the ZM muscle activation (magenta) and CS muscle deactivation (red) in a typical single trial. Right upper panel depicts cross correlation functions of each panel separately. Green lines indicate the cross correlation functions whose lowest values were localized as a negative peak (indicated as arrows) from − 1 s to 1 s for the time lag. Right lower panel depicts grand average cross correlation function across all participants. Gray area indicates the standard error of mean of the cross correlation.
The video analysis of experimental sessions 2 and 3 was undertaken to measure the movement of the skin and detect changes in the depth of the glabellar lines. Skin movement was measured by automatically detecting face landmarks in each frame of the video using Dlib face landmark detector (Enox Software, https://assetstore.unity.com/packages/tools/integration/dlib-facelandmark-detector-64314). These landmarks were used to determine the horizontal distance between the inner end coordinates of the eyebrows, normalized by the face width (i.e. the distance between the left and right outermost coordinates of the face outline (Fig. 3, left panel). This was done for the second half of the 2 s period of the induced /nee smiling during the /Nee/ trials and the corresponding time in the /Non/ trials (Fig. 3, center panel, labeled as During Nee), and also for a 1 s period after the induced /nee smiling in the /Nee/ trials and the corresponding time in the /Non/ trials (Fig. 3, right panel, labelled as After Nee). Changes in the depth of the glabellar lines was measured by calculating the average grey-scale intensity (i.e. brightness) of the face around the center of left and right eyebrows’ inner ends (vertical 50 x horizontal 50 pixels, and resized to 10 × 10 grids (i.e. 5 pixels by grid) for further statistics) during the above-described period of the induced /nee smiling during the /Nee/ trials and corresponding time during /Non/ trials.
A Wilcoxon signed-rank test with a Bonferroni correction was applied for the comparisons in this study except for the mixed-model regression analysis and videographic analysis of wrinkles. Signed rank (V), sample size (n), p-value, and effect size (r = Z/√n) was shown as statistics. For the video analysis of wrinkles, the brightness data around eyebrows were compared at each pixel using a t test with a Bonferroni correction (N = 240 for all grids). The map of t values was presented when the corrected p value < 0.05.
Results
The data in 6 out of 36 participants were excluded from statistical analysis due to a human error in sending timestamp data via in the program, especially early in the experiment (e.g. before the eye massage). The first analysis investigated CS muscle activity before and after periorbital massage. The median EMG amplitude of CS muscle decreased 0.04 µV after the eye massage relative to before (Fig. 4) and this decrease was statistically significant (z = 2.36, n = 30, p = 0.017, r = 0.430), indicating that there is residual CS muscle activity even during relaxing.
Next, we investigated how repeated frowning affects CS muscle activation and whether or not involuntary muscle activity accumulates (Fig. 5). The data of all participants (N = 36) were used for analysis as all timestamp data was available for these sessions. As can be seen in Fig. 5, repeated frowning significantly increases muscle activity during the relaxation period following frowning (corrected p value at session 2, all trials: < 0.001; session 3, trials 3–9: < 0.001, 19 comparisons). Video analysis illustrated the temporary wrinkle were getting deeper from the baseline images (i.e. at “pre” in session 2) at the first trial of session 2 as corresponding to the CS muscle activation (Fig. 5B). For the session 3, the wrinkles were not clear before the session 3 (i.e. at “pre” in session 3) but from the first trial, the wrinkles were shown (Fig. 5C). Statistical test for the difference of the brightness between 9th images (for both session 2 and 3) against the baseline image confirmed that the wrinkles in the medial area of the eyebrows were temporarily deepen by the frowning repetition (corrected p value was all < 0.05 in the area illustrated from green to blue, 100 comparisons, Fig. 5D, E). Taken together, these results indicated even a few microvolt CS activation caused the temporary wrinkle at the medial area of the eyebrows.
The investigation of the effect of the induced /nee smiling on the CS muscle activity following frowning revealed that the induced smile reduced median CS muscle activation both during and after the induced smile (D median = 0.83 µV and 0.94 µV respectively) relative to those without induced smile (Fig. 2). These decreases were statistically significant (z = 3.38, n = 36, p < 0.001, r = 0.563; z = 3.31, n = 36, p < 0.001, r = 0.552; respectively).
To analyze the temporal relationship between the ZM activation and the CS deactivation, we computed the cross-correlation function in 2 ways. One is detecting the time delay for each participant correlation function and summarize them (i.e. median), the other is detecting the time delay from the grand averaged correlation function. Figure 6 (right upper panel) shows the cross correlations of all participants and that the time lags around 0 and ranged from − 666 ms to 616 ms, while the median was 12.5 ms. The grand averaged cross correlation between the CS and ZM EMG signals showed bimodal negative peaks (Fig. 6, right lower panel). The time lag of the lowest cross correlation value was 22 ms and the negative correlation was significant against 0 (t(35) = 3.10, corrected p = 0.008, d = 0.517, 2 comparisons). The time lag of the second lowest cross correlation value was − 58 ms and the negative correlation was also significant against 0 (t(35) = 3.18, corrected p = 0.006, d = 0.530, 2 comparisons).
The analysis of the video showed that the distance between eyebrows normalized by the face width changed depending on the frowning and induced smile of the participant (Fig. 3). When the participant frowns, the distance decreased first. The distance increased during Nee trial induced smile but not in the Non trials, which had no induced smile. This difference is statistically significant (z = 5.15, n = 36, p < 0.001, r = 0.859). This effect continued after the end of the induced smile and this was also statistically significant relative to the Non trials (z = 4.13, n = 36, p < 0.001, r = 0.688).
The averaged grey-scale brightness value of the face after Nee period quantitatively different between when participants made an induced smile (Nee) and they did not (Non) (Fig. 7), with area-based statistics showing a significant increase in the brightness value with the induced smile at 3 clusters of pixels In the medial area of the brow heads. (cluster #1–3, right panel of the bottom row in Fig. 7; cluster #1: t(35) = 4.45, corrected p = 0.008, d = 0.742; cluster #2: t(35) = 4.91, corrected p = 0.002, d = 0.818; cluster #3: t(35) = 4.83, corrected p = 0.003, d = 0.804, 100 comparisons).
Fig. 7.
Photographic assessment of the face around the center of the 2 eyebrows. Panel (A) illustrates 3 most effective examples of the participants (P1-3). The top and middle rows of panel A depict the photograph around eye when the participants were relaxing without and with the induced smile (Non and Nee), respectively. The bottom row in A depicts the difference in brightness value between the Non and Nee trials (Nee-Non so that the yellow-colored portion indicates that the darker places at the face (e.g. wrinkle) got brighter). Panel (B) illustrates the averaged difference in brightness across participants. Panel (C) indicates the t-value map of the cluster-based statistics (10 × 10 = 100 grids for the correction of multiple comparisons). Only the results above the threshold of corrected p values were represented. For panel (B) and (C) the averaged position of the face landmarks are superimposed.
Discussion
The current study investigated a simple, no cost method to combat the problem of glabellar lines. This investigation had three aspects: The extent to which CS activation persists during relaxation, whether any CS residual activation results in glabellar lines and if an induced smile can inhibit CS muscle activity. We demonstrated that CS muscle activity is lower following a periorbital massage, indicating residual activity in the CS muscle during relaxation. Moreover, repeated frowning can increase this residual activity. Importantly, we demonstrated that this residual activity could result in the appearance of glabellar lines. Finally, we found that induced smiling, which activates the ZM muscle, inhibits CS muscle activity, and reduce glabellar lines. This can be used as a simple and cost-free method to combat glabellar lines. Furthermore, it is reasonable to infer that genuine smiling, which also activates the ZM muscle, would be effective in reducing CS activation and thereby lessening the appearance of glabellar lines.
Our findings revealed that baseline CS muscle activation during relaxation is higher than activation following periorbital massage (Fig. 4), and we demonstrated that CS activation can be persistent and difficult to lower. Additionally, through repeated instances of frowning, we observed an accumulation of involuntary CS muscle activation by up to 15 microvolts (Fig. 5). Our study demonstrated that even at this relatively low level of activation, there is measurable skin movement leading to the temporary formation of the wrinkle (Fig. 5). In other words, everyday activities like frowning during negative emotions or periods of concentration can contribute to the accumulation of CS muscle activation, which is difficult to reduce even during relaxation, ultimately exacerbating the development or progression of glabellar lines.
Although CS muscle activation can persist and accumulate by repetitive frowning, we found that an induced smile significantly decreases this activation (Fig. 2). Nevertheless, the importance of this finding is contingent upon whether the lines themselves decrease or disappear. In our study, we observed a decrease in the appearance of lines, as evidenced by an increase in the distance between the eyebrows (Fig. 3) and a decrease in the depth of the glabellar lines (Fig. 7). Both the reduction of CS activity and the visible effect of this remained for at least 2 s after the end of the induced smile, suggesting that the effect does not immediately revert back to its original state once the induced smiling is stopped. Previous studies have categorized wrinkles into two types: dynamic and static2. The dynamic wrinkles are caused by a habitual muscle contraction of the facial muscle and it is the target of the Botulinum approach. The wrinkles we are targeting are similar to dynamic wrinkles, suggesting that, in principle, our approach could have a similar effect to botulinum toxin treatment.
In this study, we also explored the mechanisms underlying CS muscle deactivation. One of our initial predictions was that an induced smile would result in a positive state (i.e. facial feedback hypothesis15,17) and this positive state reduces the CS muscle activation8–10, resulting in the reduction of the wrinkles between eyebrows. Time series analysis of the CS and ZM EMG signals, however, showed that the CS muscle deactivation occurred not only after the ZM muscle activation but also before the activation (Fig. 6). It is thus plausible that the antagonistic relationship between the ZM muscle activation and the CS muscle deactivation could be regulated by short-term inhibitory mechanisms rather than via emotional experience. While the CS deactivation that continues for a few seconds after the induced smile can be explained by the facial feedback hypothesis, the short-term antagonistic relationship requires a different mechanism. In this study, direct emotional evaluations during the test by subjective assessments were not conducted in order to avoid revealing the experiment’s intent. Although past research focused on the enhancing effect of facial feedback information rather than directly changing emotion, such effects remain controversial16. Hence, doubt remains as to whether subjective emotional changes occur with the unobtrusive intervention of induced /nee smiling in this study. From this perspective, it is difficult to consider the current finding as the CS muscle suppression due to actually feeling happy or pleasant, as previously known8–10. One of the candidates of the short-term inhibitory mechanisms is Ia-inhibition among agonist and antagonist muscles of skeletal muscles. The inter-muscular Ia inhibition in the general skeletal muscle is achieved by the sensory organ in the muscle named muscle spindle (e.g. Lloyd, 1946). However, it is believed that the facial muscles (mimetic muscles) do not contain the spindle6,23 although this remains controversial24. One possible alternative is facial muscle proprioception by the sensors in the skin24. The latency of the feedback, approximately 22 ms, is too long for transfer, but the delay might be explained by a sensory system specific to the mimetic muscle.
Feedback mechanisms alone are not enough to explain the inhibitory mechanism preceding the ZM activation. The other candidate is thus feedforward inhibitory (i.e. anticipatory) control of facial muscles. This suggests that feedforward inhibitory control CS muscle is linked with the preparatory control of ZM muscle. One possible mechanism is surround inhibition in motor system which is a functional inhibition contributing to shape desired motor program via suppressing other possible, but undesired movements25. The whole phenomenon of surround inhibition is restricted to the movement initiation phase (just before and during the first phase of EMG-onset)26.
The inhibition of the CS muscle activity may also be caused by stretching of the CS muscle via skin movements that occurred in conjunction with induced smile. However, the present intervention was intentionally limited to the area around the corners of the mouth, and the distance to the CS muscle is such that the occurrence of this seems improbable. It is also questionable whether, if the skin around the eyebrows were externally stretched, it would cause a stretching effect (Ib inhibition) that would reduce the activity of the CS muscle. This is because the threshold of inhibition by passive stretching is higher than that of active contraction27, and to inhibit involuntary muscle activity (e.g. spasticity), active contraction is required.
Our findings may also help explain previous findings. For example, when exposed to a highly pleasant stimuli (e.g. pictures and sounds), ZM and CS muscle are activated and deactivated, respectively28. In addition, the facial mimicry response to another’s smiling induces CS muscle deactivation11,12,29. These phenomena can be explained by our finding that the ZM muscle activation induced by positive stimuli also inhibits CS muscle activation. Moreover, our finding that CS muscle is easy to contract but hard to fully relax can explain why there can be CS activation (necessary for deactivation to be possible) prior to the stimulation (e.g. pleasant stimuli).
The current study has some limitations. Firstly, our study only demonstrated that the CS activity was inhibited when activating ZM and could not identify the specific inhibitory mechanisms associated with ZM muscle contraction, only suggesting three potential mechanisms: positive emotion via facial feedback, feedforward control, and reflexive inhibition via ZM activation and its sensory afferent. Future research should aim to narrow down the specific mechanisms. Unlike other interventions such as Botox injections or cosmetic surgery, the method tested in our study is no cost and available to anyone. However, our study only examined the immediate effects of CS deactivation on temporary wrinkles and longer-term studies are needed to assess whether these effects are sustained over time and whether regular smiling can prevent or lead to a noticeable reduction in glabellar lines. For this purpose, future studies should investigate the phenomenon we identified with regard to the types of wrinkles as well as other age-related changes perhaps using more precise measurement of the depth of wrinkles.
Conclusion
Our study presents a novel and cost-free approach to reducing glabellar lines, by utilizing facial expressions. We found CS can have residual activation and this can easily accumulate with repeated frowning. The results indicate that a simple periorbital massage can lower the baseline activation of the CS muscle, and that an induced smile can further inhibit CS muscle activity, leading to a reduction in the depth and appearance of glabellar lines.
Our findings suggest that everyday facial expressions, such as frowning, can contribute to the development of glabellar lines due to the accumulation of residual CS muscle activity. However, smiling which can be easily induced by saying “Nee”, or mimicking the facial expression that doing so induces, may serve as a practical intervention to counteract this effect. This study also explored the underlying mechanisms of CS muscle deactivation, proposing potential inhibitory controls that may be at play.
Acknowledgements
This study was supported by Shiseido Co. Ltd.
Author contributions
S.O. and M.S. conceived and conducted the experiments. S.O. analysed the results. S.O. and K.J.K.D. wrote the paper. All authors reviewed the manuscript.
Data availability
Due to the confidentiality agreements with the participants, the data in this study are available only at Shiseido co. Ltd. The data that supports the findings of this study are available upon request. If you wish to access the data, please reach out to the corresponding author (shuntaro.okazaki@shiseido.com).
Declarations
Competing interests
All authors are employed as researchers in Shiseido Co., Ltd.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qaqish, C. Botulinum Toxin Use in the Upper Face. Atlas Oral Maxillofac. Surg. Clin. N. Am.24, 95–103 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Dastoor, S. F., Misch, C. E. & Wang, H. L. Botulinum Toxin (Botox) to Enhance Facial macroesthetics: a Literature Review. J. Oral Implantology. 33, 164–171 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Kim, H. J., Seo, K. K., Lee, H. K. & Kim, J. Clinical Anatomy of the Face for Filler and Botulinum Toxin Injection (Springer Singapore, 2016). 10.1007/978-981-10-0240-3
- 4.Rosenberg, E. L. & Ekman, P. What the Face Reveals: Basic and Applied Studies of Spontaneous Expression Using the Facial Action Coding System (FACS) (Oxford University Press, 2020).
- 5.Hamas, R. S. Reducing the subconscious frown by endoscopic resection of the corrugator muscles. Aesthetic Plast. Surg.19, 21–25 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Rinn, W. E. The neuropsychology of facial expression: a review of the neurological and psychological mechanisms for producing facial expressions. Psychol. Bull.95, 52–77 (1984). [PubMed] [Google Scholar]
- 7.Michaud, T., Gassia, V. & Belhaouari, L. Facial dynamics and emotional expressions in facial aging treatments. J. Cosmet. Dermatol.14, 9–21 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Schwartz, G. E., Fair, P. L., Salt, P., Mandel, M. R. & Klerman, G. L. Facial muscle patterning to affective imagery in depressed and nondepressed subjects. Science192, 489–491 (1976). [DOI] [PubMed] [Google Scholar]
- 9.Cacioppo, J. T., Petty, R. E. & Kim, H. S. Electromyographic Activity over facial muscle regions can differentiate the Valence and Intensity of affective reactions. J. Personal. Soc. Psychol.50, 260–268 (1986). [DOI] [PubMed] [Google Scholar]
- 10.Lang, P. J., Greenwald, M. K., Bradley, M. M. & Hamm, A. O. looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology30, 261–273 (1993). [DOI] [PubMed] [Google Scholar]
- 11.Künecke, J., Hildebrandt, A., Recio, G., Sommer, W. & Wilhelm, O. Facial EMG responses to emotional expressions are related to emotion perception ability. PLoS ONE. 9, e84053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimberg, U. & Lundquist, L. O. Gender differences in facial reactions to facial expressions. Biol. Psychol.30(2), 151–159 (1990). [DOI] [PubMed]
- 13.Otte, E., Habel, U., Schulte-Rüther, M., Konrad, K. & Koch, I. Interference in simultaneously perceiving and producing facial expressions—evidence from electromyography. Neuropsychologia49, 124–130 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Shangguan, C. et al. Inhibition and production of anger cost more: evidence from an ERP Study on the production and switch of Voluntary Facial Emotional expression. Front. Psychol.10, 1276 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buck, R. Nonverbal behavior and the theory of emotion: the facial feedback hypothesis. J. Personal. Soc. Psychol.38, 811–824 (1980). [DOI] [PubMed] [Google Scholar]
- 16.Wagenmakers, E. J. et al. Registered Replication Report: Strack, Martin, & Stepper Perspectives on Psychological Science 11, 917–928 (2016). (1988). [DOI] [PubMed]
- 17.Coles, N. A., Larsen, J. T. & Lench, H. C. A meta-analysis of the facial feedback literature: effects of facial feedback on emotional experience are small and variable. Psychol. Bull.145, 610–651 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Strack, F., Leonard, M. L. & Stepper, S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test. J. Personal. Soc. Psychol.54, 768–777 (1988). [DOI] [PubMed] [Google Scholar]
- 19.Fraser, J. & Kerr, J. R. Psychophysiological effects of back massage on elderly institutionalized patients. J. Adv. Nurs.18, 238–245 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Roberts, L. Effects of patterns of pressure application on resting Electromyography during Massage. IJTMB4, 4–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peirce, J. et al. PsychoPy2: experiments in behavior made easy. Behav. Res. Methods. 51, 195–203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridlund, A. J. & Cacioppo, J. T. Guidelines for human electromyographic research. Psychophysiology23, 567–589 (1986). [DOI] [PubMed] [Google Scholar]
- 23.Frayne, E., Coulson, S., Adams, R., Croxson, G. & Waddington, G. Proprioceptive ability at the lips and jaw measured using the same psychophysical discrimination task. Exp. Brain Res.234, 1679–1687 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Cattaneo, L. & Pavesi, G. The facial motor system. Neurosci. Biobehavioral Reviews. 38, 135–159 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Hallett, M. Pathophysiology of writer’s cramp. Hum. Mov. Sci.25, 454–463 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Beck, S. et al. Short Intracortical and Surround Inhibition are selectively reduced during Movement Initiation in Focal Hand Dystonia. J. Neurosci.28, 10363–10369 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houk, J. & Henneman, E. Responses of golgi tendon organs to active contractions of the soleus muscle of the cat. J. Neurophysiol.30, 466–481 (1967). [DOI] [PubMed] [Google Scholar]
- 28.Larsen, J. T., Norris, C. J. & Cacioppo, J. T. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology40, 776–785 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Dewied, M., Vanboxtel, A., Zaalberg, R., Goudena, P. & Matthys, W. Facial EMG responses to dynamic emotional facial expressions in boys with disruptive behavior disorders. J. Psychiatr. Res.40, 112–121 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the confidentiality agreements with the participants, the data in this study are available only at Shiseido co. Ltd. The data that supports the findings of this study are available upon request. If you wish to access the data, please reach out to the corresponding author (shuntaro.okazaki@shiseido.com).