Abstract
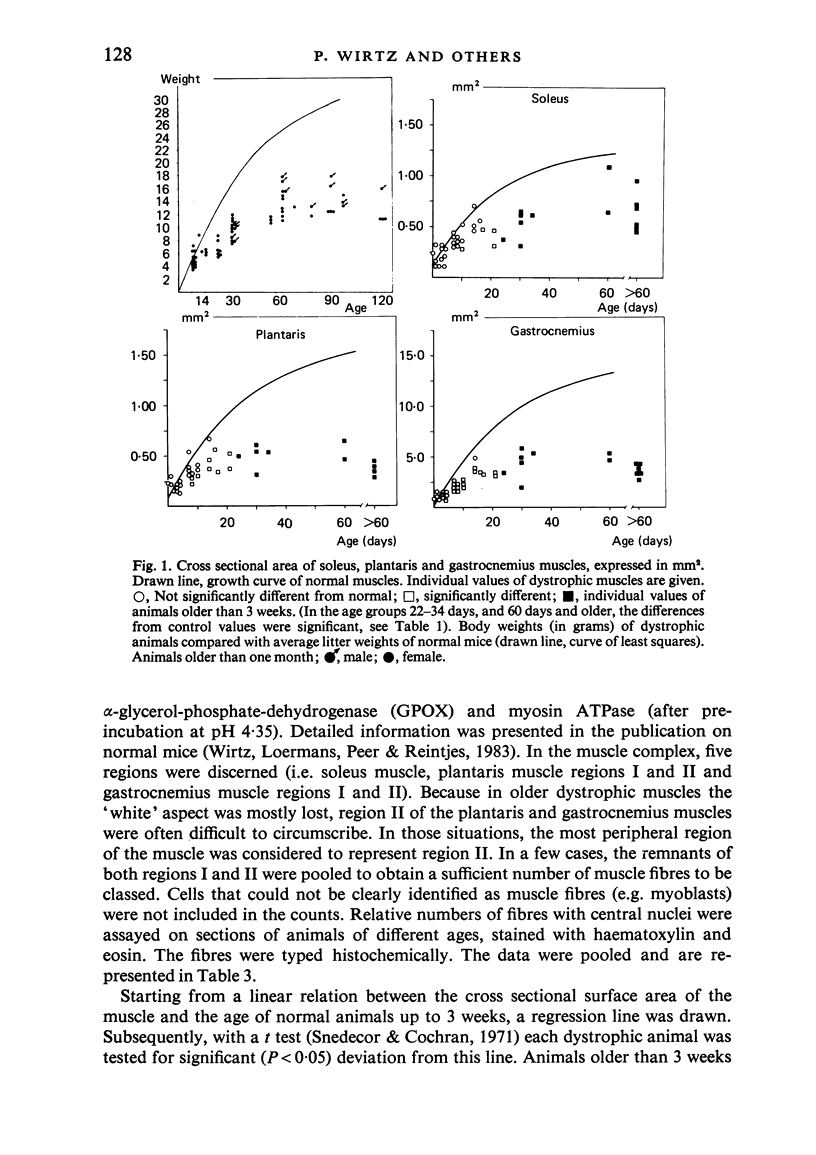
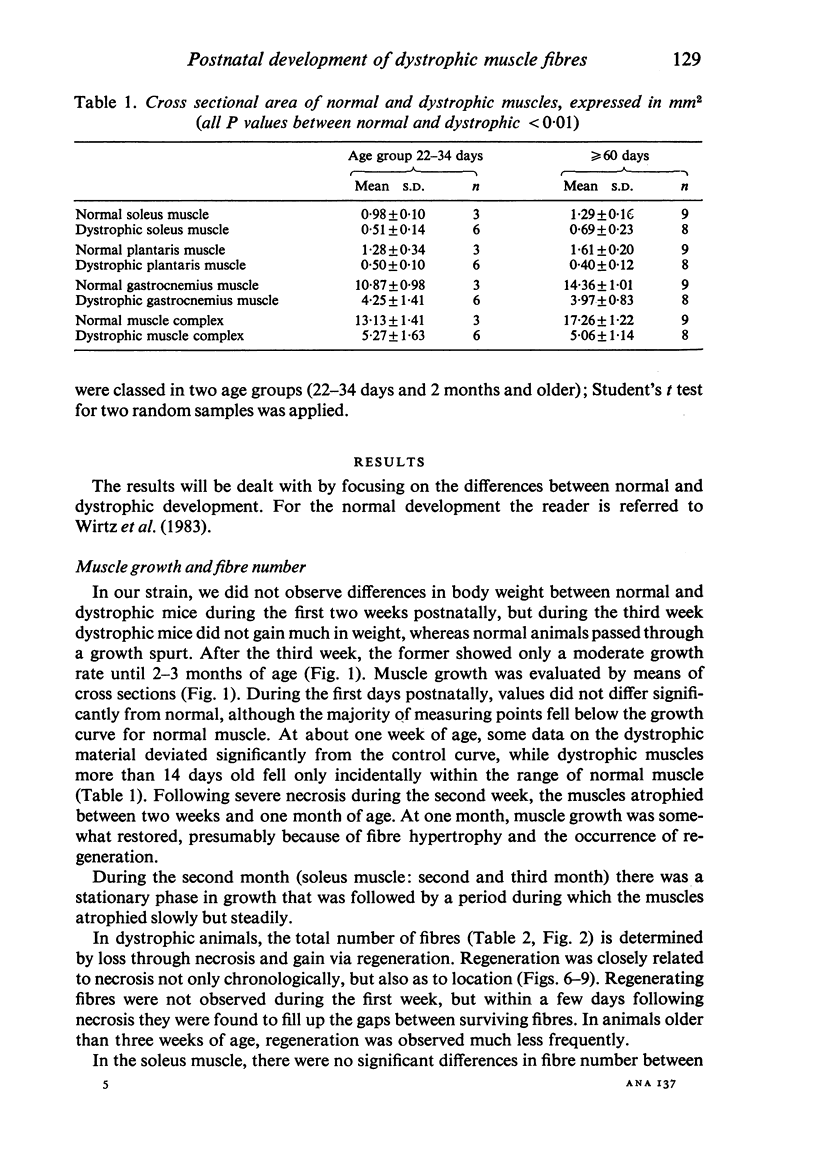
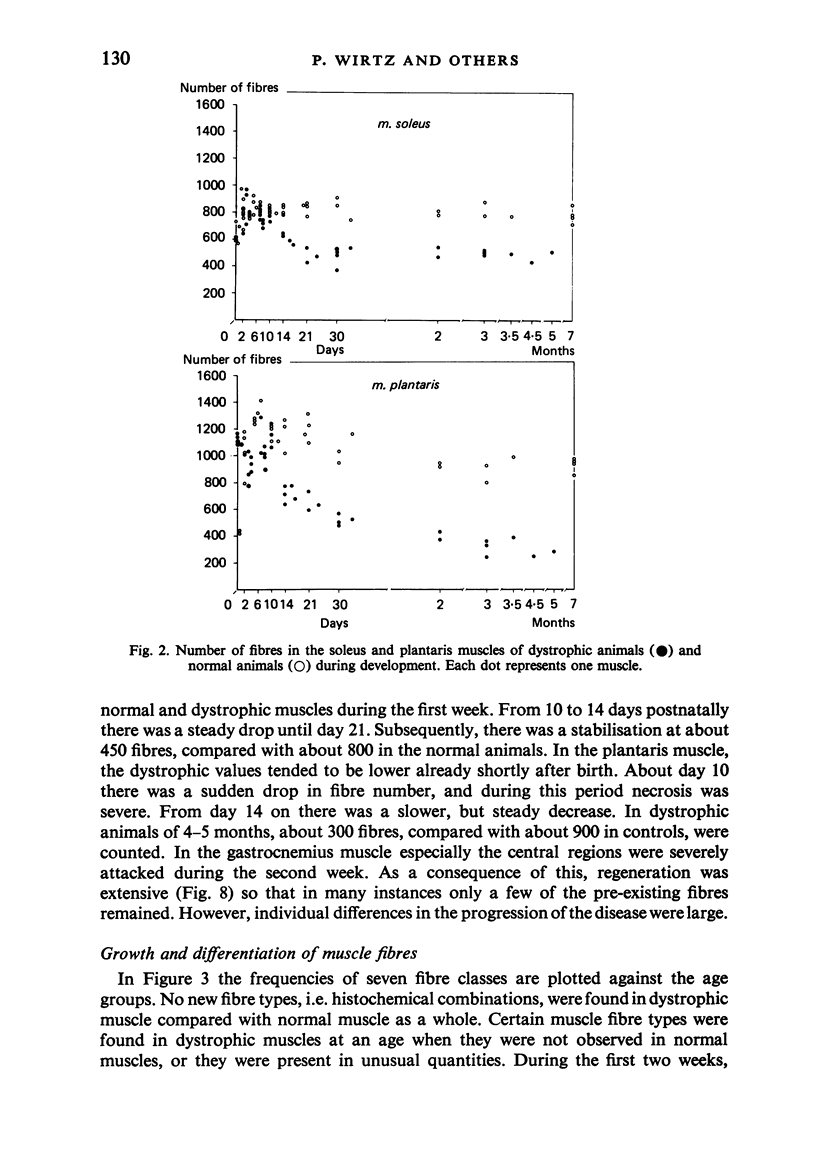
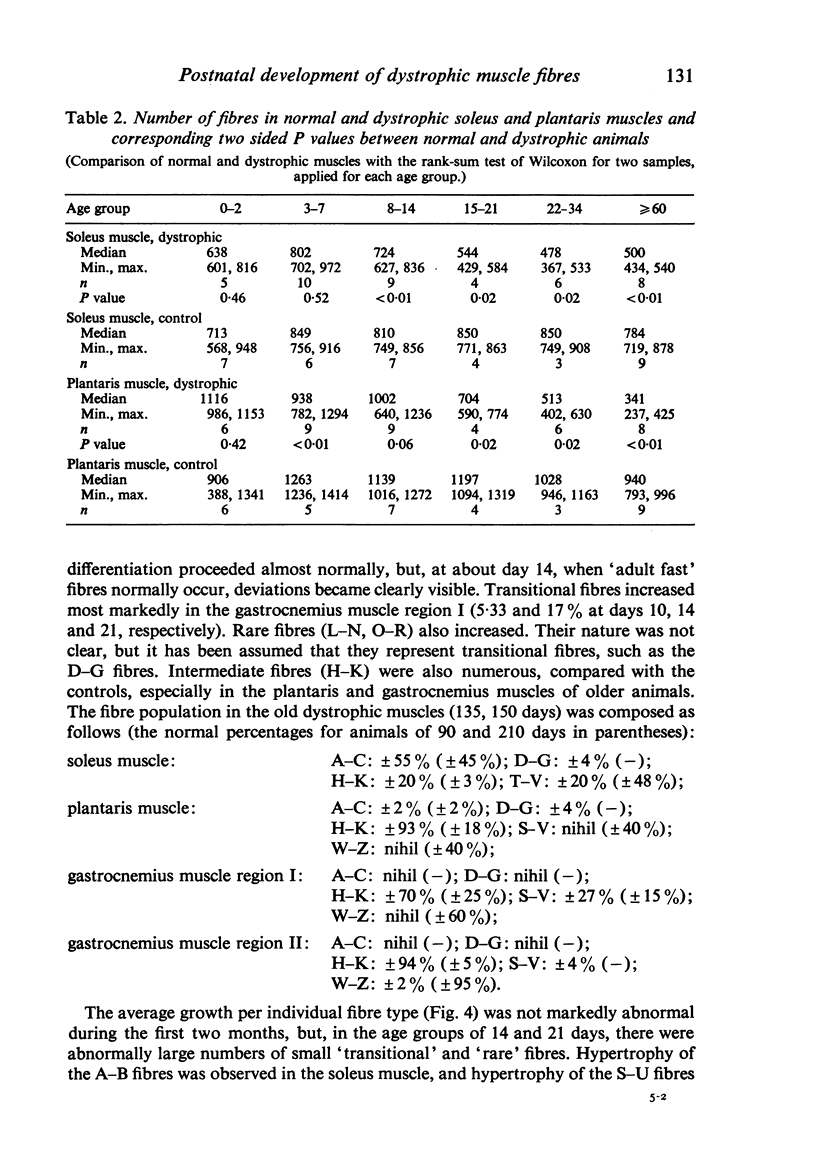
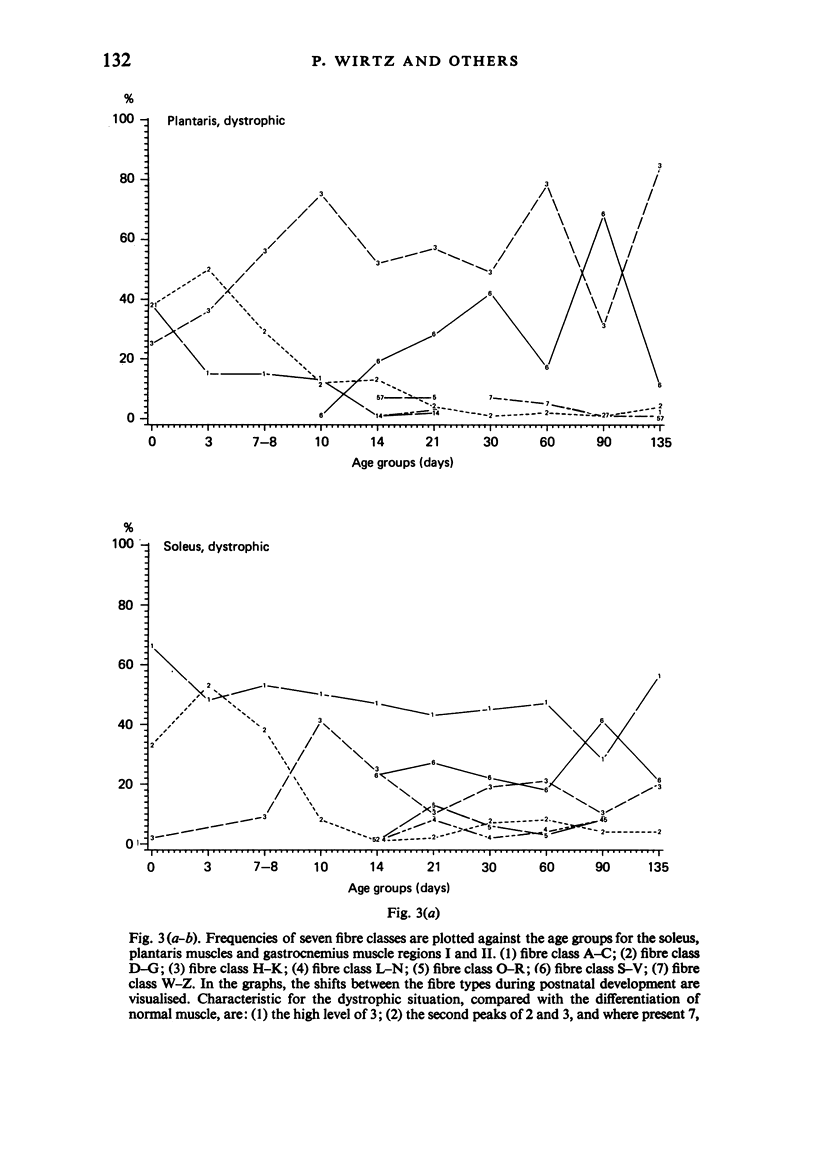
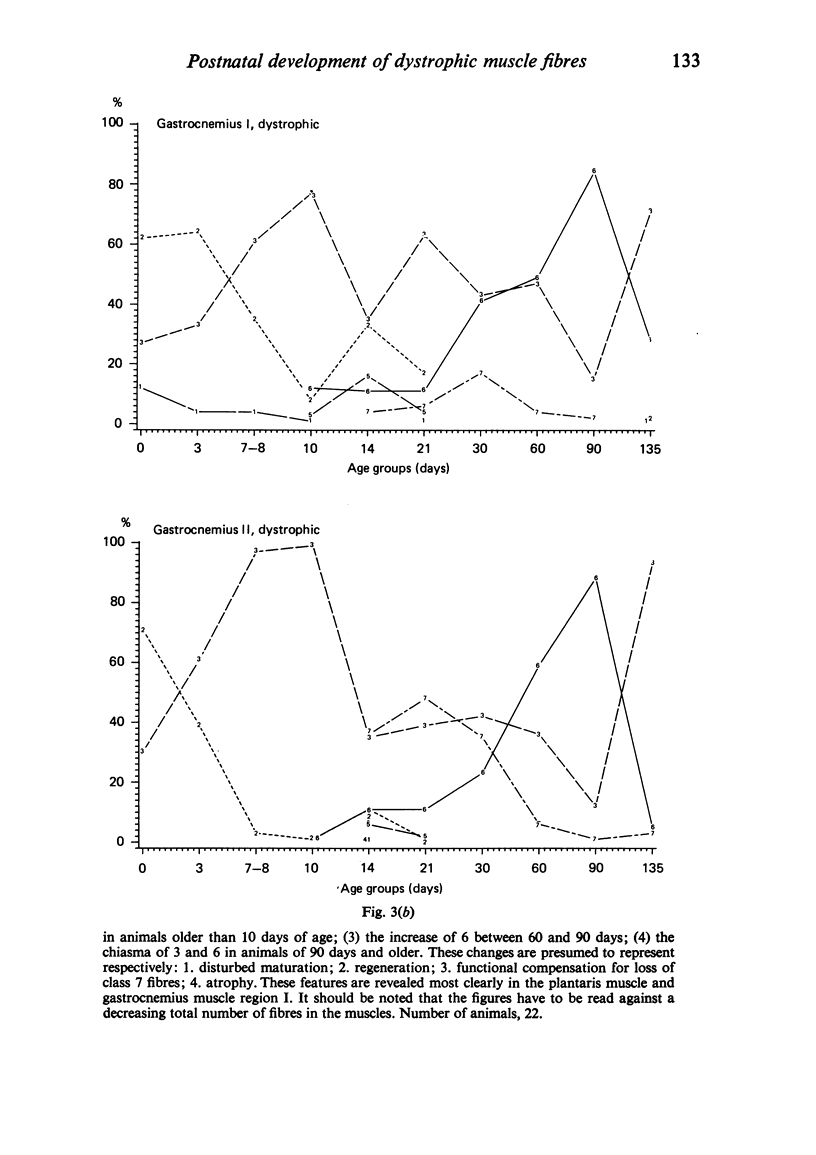
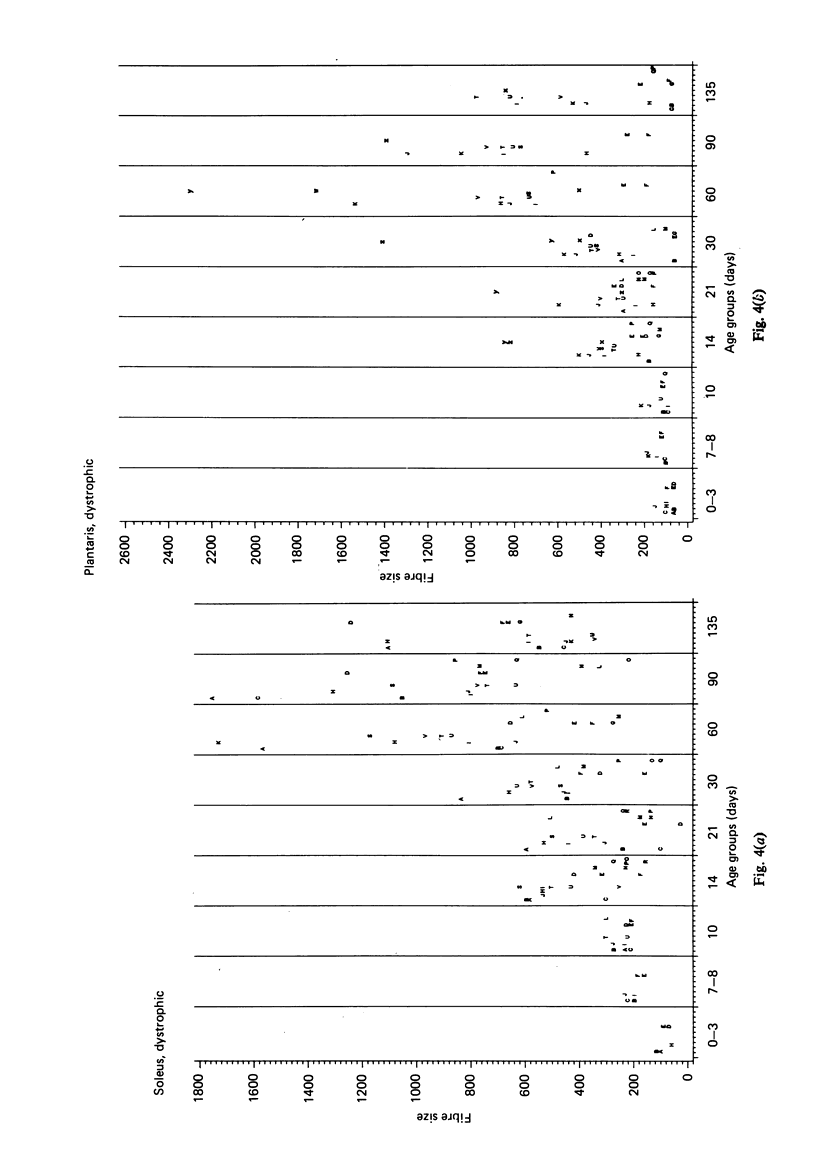
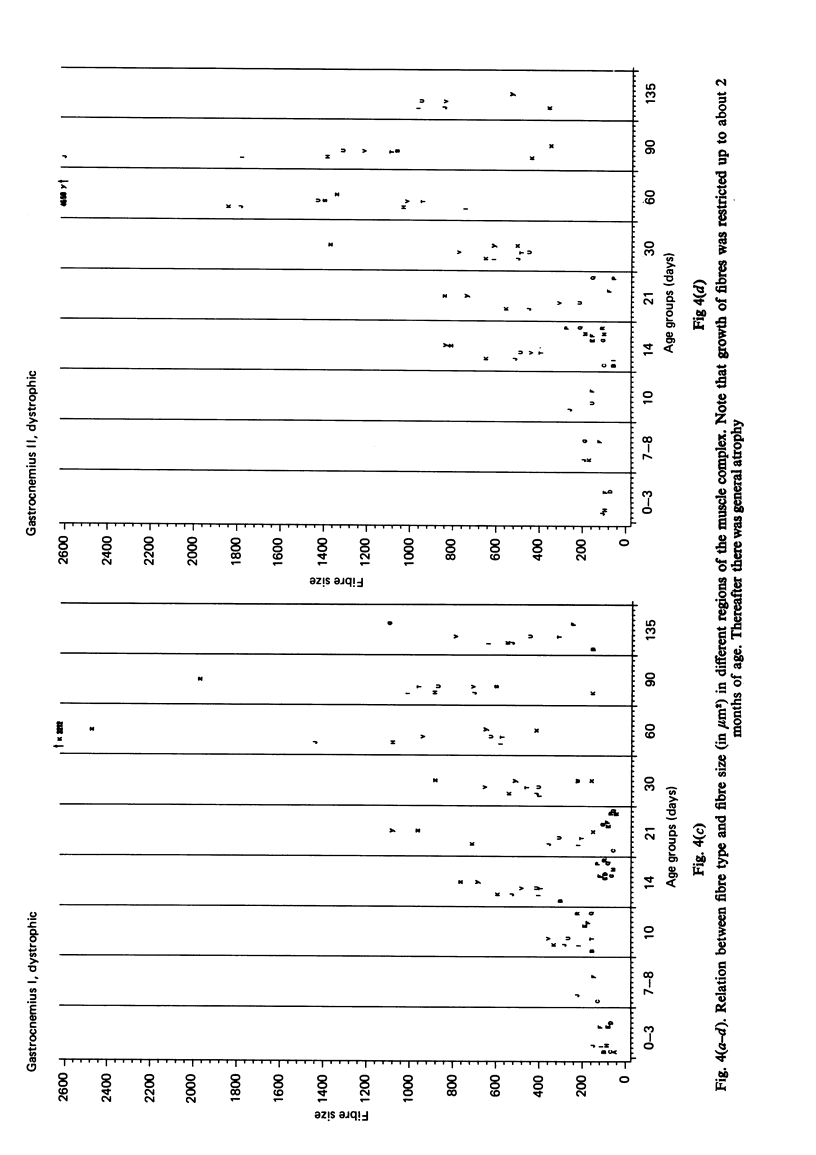
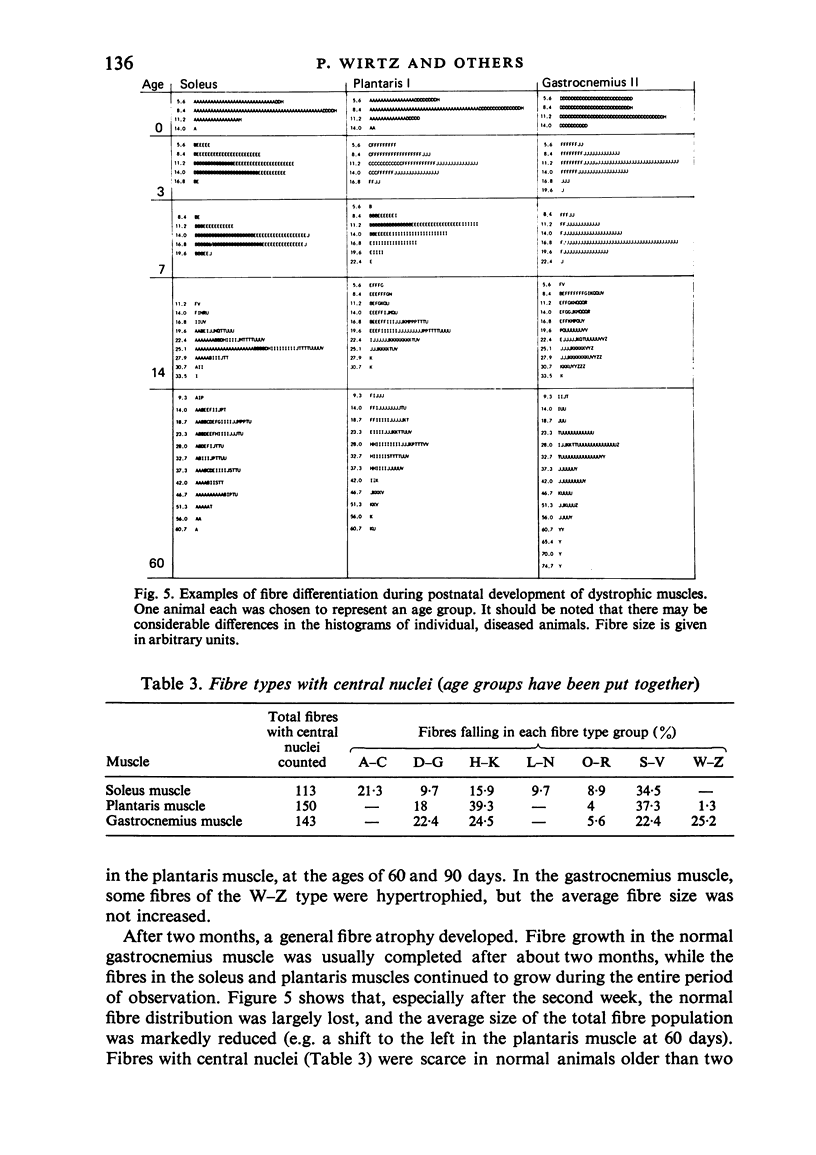
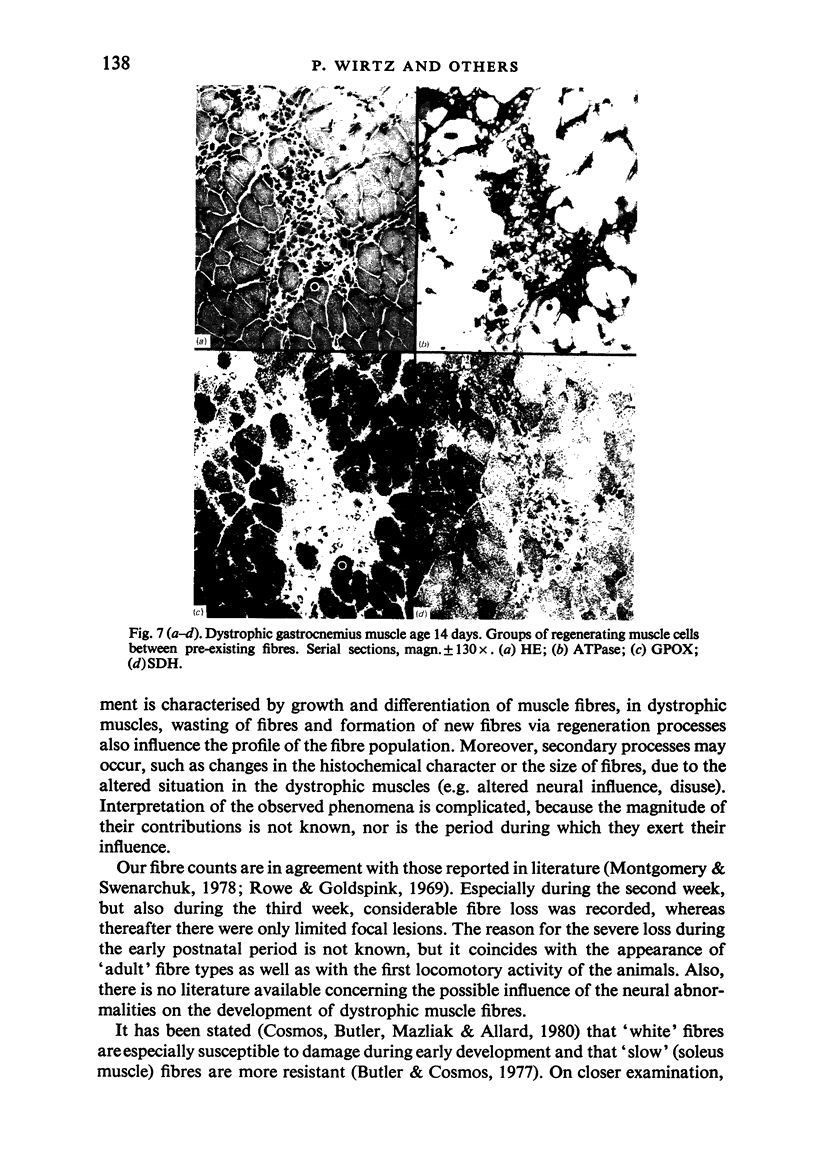
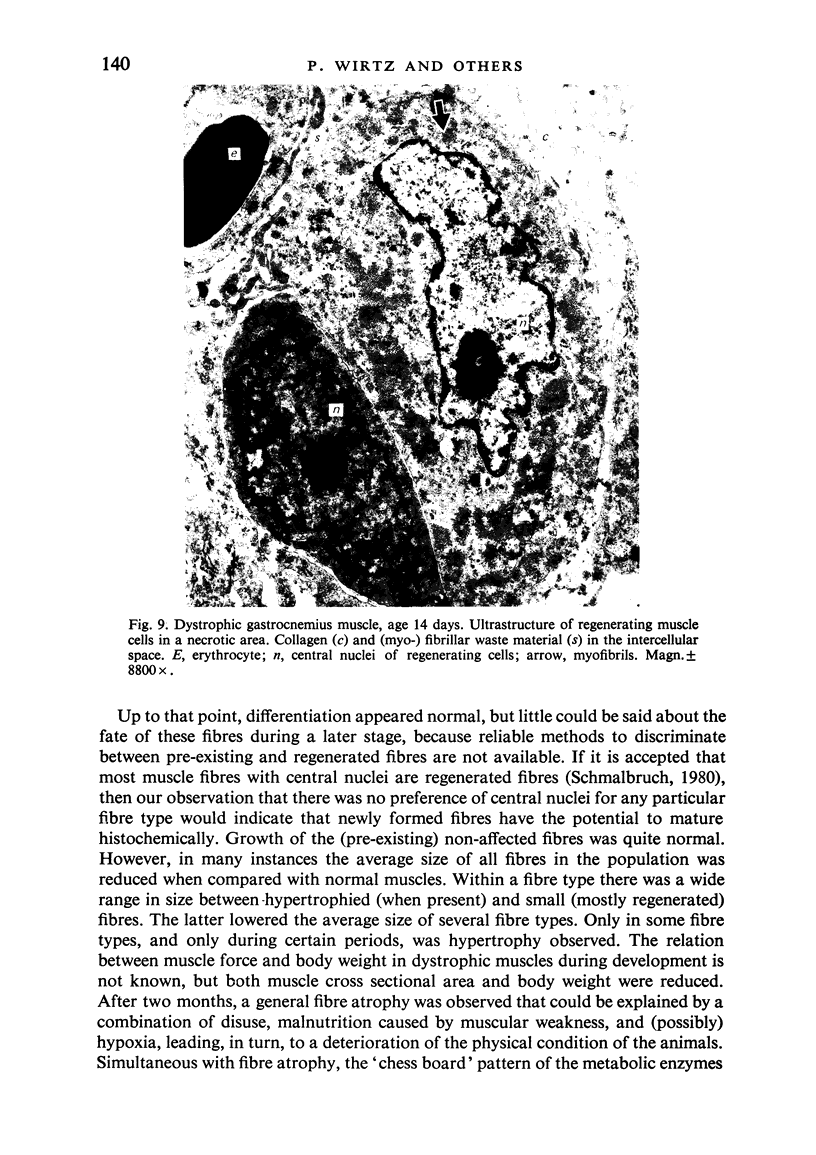
Postnatal development of three hind legs muscles, the soleus, plantaris, and gastrocnemius, of dystrophic mice (ReJ 129) was investigated with histochemical and morphometric methods. The results were compared with normal postnatal development. Especially during the second week postnatally, there was severe fibre necrosis with no apparent preference for any particular fibre type. This period of necrosis was shortly followed by a wave or regeneration during the third week that could not, however, compensate for the loss of fibres. In dystrophic animals of 4-5 months of age, the number of fibres was reduced by 40-70%. Cross sectional areas of dystrophic muscles rarely, if ever, exceeded values for normal animals 14 days of age, while body weights were also drastically reduced. Growth and differentiation of the nonaffected fibres proceeded almost normally during the first month. During the second month, the "slow' fibres in the soleus muscle, and the "fast-oxidative-glycolytic' fibres in the plantaris muscle were hypertrophied, while, incidentally, some "fast-glycolytic' fibres showed hypertrophy; but in this case the average size of the fibre type was not increased. After two months, a general fibre atrophy was observed. The fate of the regenerated fibres was difficult to trace, especially in muscles older than one month. It is assumed that a number of them were capable of developing into "adult' fibre types histochemically. During the course of the disease the percentage of "intermediate' fibres increased markedly, whereas nearly all "fast-glycolytic' fibres disappeared. Because of these shifts in fibre profiles, the plantaris and the gastrocnemius muscles obtained a rather "juvenile' and "oxidative' aspect. Changes in the histochemical character of the soleus muscle were less spectacular. In dystrophic muscles, no new fibre types were found, compared with normal muscles. Rather, fibre types were present at the wrong moment, or occurred in quantities unusual for the age concerned.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Caddy K. W., Pallot D. J., Pehrson U. M. Investigation of cranial and other nerves in the mouse with muscular dystrophy. J Neurol Neurosurg Psychiatry. 1975 Apr;38(4):391–403. doi: 10.1136/jnnp.38.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley W. G., Jenkison M. Abnormalities of peripheral nerves in murine muscular dystrophy. J Neurol Sci. 1973 Feb;18(2):227–247. doi: 10.1016/0022-510x(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Brust M. Relative resistance to dystrophy of slow skeletal muscle of the mouse. Am J Physiol. 1966 Mar;210(3):445–451. doi: 10.1152/ajplegacy.1966.210.3.445. [DOI] [PubMed] [Google Scholar]
- Butler J., Cosmos E. Histochemical and structural analyses of the phenotypic expression of the dystrophic gene in the 129/ReJ dy/dy and the C57BL/6J dy2J/dy2J mice. Exp Neurol. 1977 Dec;57(3):666–681. doi: 10.1016/0014-4886(77)90100-5. [DOI] [PubMed] [Google Scholar]
- Cosmos E., Butler J., Mazliah J., Allard E. P. Animal models of muscle diseases. Part II: murine dystrophy. Muscle Nerve. 1980 Jul-Aug;3(4):350–359. doi: 10.1002/mus.880030413. [DOI] [PubMed] [Google Scholar]
- Jaros E., Bradley W. G. Development of the amyelinated lesion in the ventral root of the dystrophic mouse. Ultrastructural, quantitative and autoradiographic study. J Neurol Sci. 1978 May;36(3):317–339. doi: 10.1016/0022-510x(78)90041-2. [DOI] [PubMed] [Google Scholar]
- MEIER H., WEST W. T., HOAG W. G. PRECLINICAL HISTOPATHOLOGY OF MOUSE MUSCULAR DYSTROPHY. Arch Pathol. 1965 Aug;80:165–170. [PubMed] [Google Scholar]
- Meier H. Histochemical observations in preclinical mouse muscular dystrophy. Am J Pathol. 1967 Apr;50(4):691–706. [PMC free article] [PubMed] [Google Scholar]
- Montgomery A., Swenarchuk L. Further observations on myelinated axon numbers in normal and dystrophic mice. J Neurol Sci. 1978 Aug;38(1):77–82. doi: 10.1016/0022-510x(78)90247-2. [DOI] [PubMed] [Google Scholar]
- PLATZER A. C., CHASE W. H. HISTOLOGIC ALTERATIONS IN PRECLINICAL MOUSE MUSCULAR DYSTROPHY. Am J Pathol. 1964 Jun;44:931–946. [PMC free article] [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. II. Dystrophic mice. J Anat. 1969 May;104(Pt 3):531–538. [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H. Entwicklung, Untergang und Regeneration von Skelettmuskelfasern. Dtsch Med Wochenschr. 1980 Apr 25;105(17):614–617. doi: 10.1055/s-2008-1070717. [DOI] [PubMed] [Google Scholar]
- Summers P. J., Parsons R. Ultrastructural evidence of "abortive" regeneration in murine muscular dystrophy. J Neurol Sci. 1978 Dec;39(2-3):295–301. doi: 10.1016/0022-510x(78)90129-6. [DOI] [PubMed] [Google Scholar]
- Wirtz P., Loermans H. M., Peer P. G., Reintjes A. G. Postnatal growth and differentiation of muscle fibres in the mouse. I. A histochemical and morphometrical investigation of normal muscle. J Anat. 1983 Aug;137(Pt 1):109–126. [PMC free article] [PubMed] [Google Scholar]