Abstract
Purpose
We aimed to assess outcomes in patients undergoing sequential intragastric balloon (IGB) treatment for obesity.
Materials and Methods
Consecutive patients who underwent treatment between May 2014 and February 2023 were identified. We recorded outcomes including: weight at 3-monthly intervals, progression to definitive bariatric procedure and morbidity.
Results
Forty-five patients were identified. Median weight loss with first IGB was 15.2 kg (8.8%). 11 patients (26.7%) had a second IGB, with median weight loss of 3.3 kg (1.9%). Twenty-one patients (46.7%) were suitable for definitive surgery after first IGB treatment. One further patient (2.2%) was suitable for surgery after a second IGB. During first IGB, median weight loss was observed during the each of the first 3 quartiles (0–3 months: 10.1 kg; 3–6 months: 2.3 kg; 6–9 months: 4.2 kg). There was a median 2 kg weight gain during 9–12 months.
Conclusion
Greatest weight loss was achieved during first IGB treatment. Sequential IGB treatment did not lead to beneficial weight loss or progression to surgery. Weight loss with first IGB was not uniform across the 12-month period of treatment, with net weight gain during the last quartile.
Keywords: General surgery; Weight loss; Obesity, Equipment & supplies
INTRODUCTION
Obesity is quantitatively defined as a body mass index (BMI) of 30 kg/m2 or above [1]. Obesity has become a ‘global epidemic,’ particularly prevalent in Western populations [1]. In 2022, 25.9% of adults aged 18 years or over in England were estimated to be obese, with prevalence rising and equal distribution reported amongst women (26.1%) and men (25.8%) [2]. Crucially, obesity is associated with development of health complications, including cardiac and vascular disease, diabetes mellitus, cancer and mortality [1].
The literature suggests that bariatric surgery provides the most weight loss-sustaining, and hence heath-benefiting, choice for obesity [3,4]. The most common bariatric operations of choice in the UK, at the present time, are Roux-en-Y gastric bypass and sleeve gastrectomy [3,4]. Bariatric surgery, however, carries risk of morbidity and mortality [3,4]. Indeed, patients may be too obese to proceed directly to weight-loss surgery and/or deemed too high-risk candidates for definitive operations [3]. Prospective cohort studies report the intragastric balloon (IGB) as a safe and effective temporary bridging therapy to achieve sufficient weight loss to proceed to definitive bariatric surgery [5,6,7,8,9]. IGBs reduce stomach capacity, promoting the feeling of satiety through stimulation of gastric mechanoreceptors, facilitating weight loss [10]. If patients do not lose sufficient weight to safely progress to definitive bariatric surgery, consideration may be given to a second sequential period of IGB treatment. Several studies [5,6,7] have failed to reach a consensus on the efficacy of sequential IGBs.
Prior to the coronavirus disease 2019 (COVID-19) pandemic, IGBs were endoscopically removed after 6 months [11]. However, during the period of restrictions which led to limited patient interactions and the postponement of elective bariatric surgery [11], duration of IGB treatment increased from 6 to 12 months at our bariatric centre and many other centres around the UK following consultations with balloon suppliers.
Given these changes in IGB treatment, we aimed to consider what the optimal treatment duration is to facilitate maximal weight loss in bariatric patients. We also aimed to consider the associated safety of IGBs where patients undergo sequential IGB insertion. The purpose of our study is important in the context of enhancing the effectiveness of IGB treatment as a safe bridging therapy to definitive bariatric surgery.
MATERIALS AND METHODS
We retrospectively reviewed clinical outcomes for patients with obesity and super obesity who had undergone IGB insertions in a single bariatric centre. The study period accounted for the changes in IGB removal protocol that resulted during the COVID-19 pandemic. The primary outcome was percentage of total body weight loss (%WL) following insertion of sequential IGBs. A sub analysis was performed assessing weight loss trends during different phases of the first IGB treatment. The secondary outcome was to assess safety of sequential IGB treatment.
Patients were identified by comprehensive reviews of the hospital electronic theatre management system to identify all patients who had undergone endoscopic IGB insertion and removal between 2014–2023.
Where patients underwent insertion of more than one IGB, this decision had been taken by the bariatric multi-disciplinary team (MDT), comprising bariatric surgeons, bariatric specialist nurses, a dietician and psychologist. The MDT evaluated the weight loss achieved from a single IGB, in addition to patient compliance with dietary instruction, consideration of potential weight loss achievable with further IGB treatment, and whether progression to definitive bariatric surgery was achievable.
The bariatric MDT works on a model of individualised patient centred care and decision making, taking into account both clinical and patient factors. There are no specific inclusion or exclusion criteria for IGB treatment, other than its use as a bridging therapy to potential definitive bariatric surgery, hence only patients deemed fit enough for potential definitive bariatric surgery at presentation were included. Exclusion criteria for IGB treatment largely centre around patient co-morbidities, either specific to the gastrointestinal tract (peptic ulcer disease, previous resection of stomach, oropharyngeal abnormalities, inflammatory bowel disease, gastric cancer, large hiatus hernia, varices) or general health (significant cardiovascular co-morbidities, bleeding disorders, pregnancy, alcohol or substance misuse, significant psychiatric illness) that increase risk associated with IGB treatment.
MDT recommendations were then discussed with patients in a dedicated bariatric outpatient clinic. Where patients then underwent more than one IGB treatment, the new balloon was inserted on removing the existing balloon during the same endoscopic procedure.
Baseline data was collected including patient demographics, co-morbidities and initial weight upon commencing onto the bariatric pathway. Weights and BMIs were recorded at 3 monthly intervals until the date of IGB removal, and for any subsequent periods of IGB treatment. Our Trust uses the Orbera365 Non-Surgical Weight Loss Balloon System® (Boston Scientific Corporation, Marlborough, MA, USA) filled with saline and methylene blue dye, for up to 12 months treatment duration.
We recorded weight loss, %WL, length of treatment, complications and eventual patient outcome, including progression to definitive bariatric surgery (laparoscopic Roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy).
This study was approved by the local clinical governance department of the bariatric centre involved. Data analysis was performed using a Microsoft Excel (Microsoft, Redmond, WA, USA) spreadsheet analysis.
RESULTS
In total 45 patients were identified between May 2014 and February 2023 who had undergone insertion of an IGB. As with the typical bariatric population, the majority were female (73.3%). Approximately one third were diagnosed with type 2 diabetes (31.1%) or hypertension (33.3%). Detailed demographics as well as initial weights are shown in Table 1. The median IGB fill volume was 610 mL, ranging from 540 mL to 660 mL.
Table 1. Patient demographics and initial weights.
| Demographics | Number of patients | Percentage (%) of the total number of patients (n=45) | Median | Range | |
|---|---|---|---|---|---|
| Male | 12 | 26.7 | |||
| Female | 33 | 73.3 | |||
| Age (years) | 50 | 23–71 | |||
| Ethnicity | |||||
| White British | 38 | 84.4 | |||
| White Other | 2 | 4.4 | |||
| Asian | 4 | 8.9 | |||
| Other group | 1 | 2.2 | |||
| Co-morbidities | |||||
| Type 2 diabetes | 14 | 31.1 | |||
| Hypertension | 15 | 33.3 | |||
| Obstructive sleep apnoea | 10 | 22.2 | |||
| Weight | |||||
| Weight on commencement onto the bariatric pathway (kg) | 176.0 | 113.5–242.5 | |||
| Body mass index on commencement onto the bariatric pathway (kg/m2) | 62.4 | 40.3–83.75 | |||
In total 37 patients completed more than 1 month (28 days) of initial IGB treatment. The median duration was 237 days (33.9 weeks or 7 months). Median weight loss with initial IGB treatment was 15.2 kg (8.8%WL), ranging from 54.8 kg (31%) of weight loss to 30.9 kg (19.7%) of weight gain.
The average weight at the time of initial IGB insertion of the 37 patients who completed more than one month of IGB treatment was 144 kg (range 113.5–242.5 kg). Of these, 11 patients went on to have a second IGB treatment (24.4%). At the time of their initial IGB insertion, these 11 patients had an average weight of 200 kg (range 149.8–250 kg). At the time of insertion of their second IGB, their average weight was 184 kg (range 130.2–220 kg). Therefore, patients who required second IGBs were of an overall heavier weight on commencement of initial IGB treatment and had not lost sufficient weight to proceed to definitive bariatric surgery, hence requiring a second IGB.
Of the 11 patients who had a second IGB, one balloon was removed after 14 days due to vomiting and electrolyte imbalance and one balloon burst during treatment. Another patient had only just commenced their second IGB treatment. These patients (n=3) were excluded from subsequent analysis assessing the weight loss associated with IGBs over a 12-month treatment period.
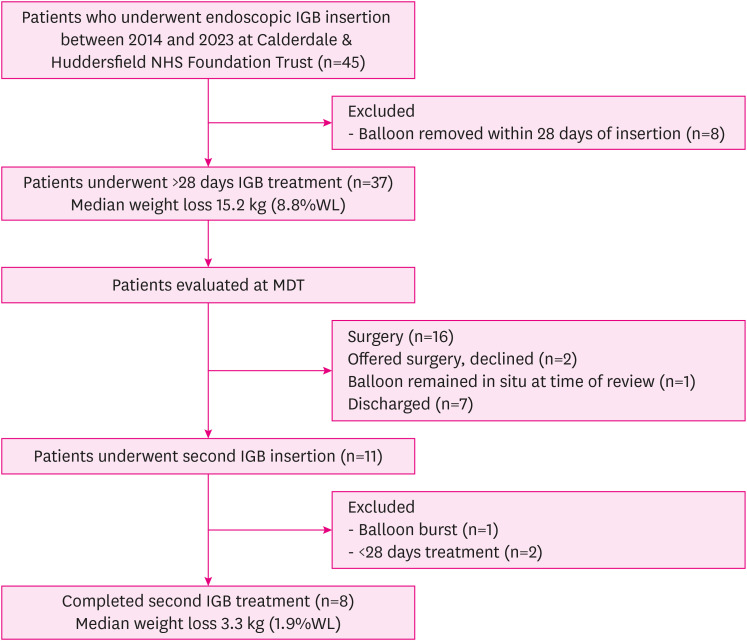
The median length of second IGB treatment was 234 days (33 weeks or 7.5 months). Median weight loss with the second IGB was 3.3 kg (median 1.9%WL), ranging from 21.2 kg weight loss to 5.6 kg weight gain (15.5%WL to 2.8% weight gain). Fig. 1 is a flow diagram of the study.
Fig. 1. Study flow diagram.
IGB = intragastric balloon, %WL = percentage of total body weight loss, MDT = multi-disciplinary team.
Table 2 shows the comparative outcomes with the first versus second IGB.
Table 2. Outcomes of IGB treatment.
| Variables | First IGB (n=37) | Second IGB (n=8) | |
|---|---|---|---|
| Average weight at time of IGB insertion (kg) | 144.2 | 184.0 | |
| Median length of IGB treatment | |||
| Days | 237 | 234 | |
| Weeks | 33.9 | 33.0 | |
| Months | 7.0 | 7.5 | |
| Weight loss (kg) | |||
| Median | 15.2 | 3.3 | |
| Range | −54.8 to +30.9 | −21.2 to +5.6 | |
| Weight loss (%) | |||
| Median | 8.8 | 1.9 | |
| Range | −31 to +19.7 | −15.5 to +2.8 | |
IGB = intragastric balloon.
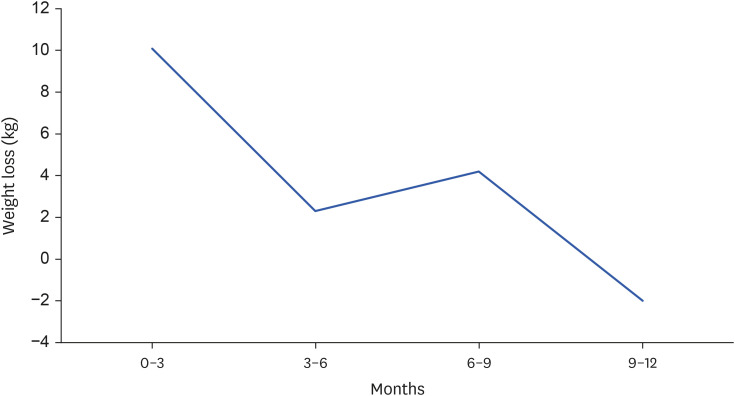
We analysed weight loss with the first IGB across the 12-month treatment period, broken down into quartiles. Greatest weight loss was observed in the initial 3 months of IGB treatment (median 10.1 kg, 7.1%). During 9–12 months of IGB treatment, the median weight change was a gain of 2 kg (1%). This is shown in Fig. 2. Analysis of weight loss by quartiles was not performed for the second IGB due to the small number of patients undergoing this treatment.
Fig. 2. Weight loss profile (first intragastric balloon only).
Across the study period, 14 (31.1%) initial IGBs were removed before 12 months of treatment due to complications (8 vomiting, 5 acute kidney injury with serum electrolyte disturbance and 1 perforated gastric ulcer); 9 (20%) of these complications were within the first month (28 days) of IGB treatment. The overall complication rate requiring re-attendance to hospital, but not necessarily removal of IGB, was 40%. Six patients (13.3%) had multiple hospital attendances with complications. Average length of stay related to IGB complications was 1 day (range 0–19 days). The average time from insertion of IGB to presentation with a complication was 14 days (range 1–272 days). One patient was admitted to intensive care due to severe electrolyte disturbance. There was no mortality related to any of these complications, including the perforated gastric ulcer which was managed non-operatively with intravenous antibiotics, proton pump inhibitor infusions and endoscopic closure using clips. This patient however had a prolonged total length of stay of 19 days. This patient’s subsequent contrast study revealed no evidence of ongoing leak related to the ulcer. IGB complications are shown in Table 3.
Table 3. IGB complications.
| Complication | First IGB (n=37) | Second IGB (n=8) |
|---|---|---|
| Vomiting with electrolyte disturbance/acute kidney injury | 3 (6.6) | 1 (2.2) |
| Perforated gastric ulcer | 1 (2.2) | |
| Intensive care unit admission | 1 (2.2) | |
| Burst balloon | 1 (2.2) |
Values are presented as number of patients (%).
IGB = intragastric balloon.
In terms of final outcomes of therapy, 22 patients (48.8%) were deemed suitable for surgery following their IGB treatment. Only one of these patients (4.5%) had a second IGB. Seventeen patients went on to have surgery (12 Roux-en-Y gastric bypass, 5 sleeve gastrectomy), 3 patients were offered surgery but declined and 1 patient had an abandoned Roux-en-Y gastric bypass due to limited intra-abdominal space. One patient is current awaiting surgery (Roux-en-Y gastric bypass). Sixteen patients were discharged from the MDT and underwent no further bariatric intervention. Two patients were discharged to the private sector and the outcome of these patients is unknown. Two patients remained under the MDT, awaiting further clinical review but had undergone no further bariatric intervention at the time of writing. Table 4 shows the outcomes on completion of the bariatric pathway.
Table 4. Outcomes from the bariatric pathway for all patients included in the study.
| Outcome post-IGB treatment | Total number of patients (n=45) | Percentage of total number of patients (%) |
|---|---|---|
| Eligible for surgery | 22 | 48.9 |
| Roux-en-Y gastric bypass | 12 | 26.7 |
| Sleeve gastrectomy | 5 | 11.1 |
| Surgery abandoned | 1 | 2.2 |
| Listed for surgery | 1 | 2.2 |
| Declined by patient | 3 | 6.7 |
| Discharged by bariatric MDT | 16 | 35.6 |
| Discharged to private sector | 2 | 4.4 |
| Remain under MDT | 2 | 4.4 |
| Lost to follow-up | 1 | 2.2 |
| Second IGB still in-situ | 2 | 4.4 |
IGB = intragastric balloon, MDT = multi-disciplinary team.
DISCUSSION
There is limited literature on the effect of sequential IGBs on weight loss [5,6,7]. Alfredo et al. [6] recruited 83 patients with a mean BMI 43 kg/m2, in whom conservative medical obesity management had failed and definitive bariatric surgery declined by patients. The criterion for insertion of sequential IGBs was regain of > or equal to 50% of weight loss achieved with the previous IGB [6]. Up to 4 sequential IGBs, each for a 6-month treatment period, were inserted and all patients underwent insertion of a second IGB [6]. The study found that after first IGB treatment, statistically significant weight loss was achieved (P<0.001), with mean reduction in BMI of 7.8 kg/m2 [6]. No statistically significant weight loss was found following the second IGB [6]. Moreover, the mean BMI (37.6 kg/m2) upon completion of the 72-month study period was similar to the mean BMI of patients prior to insertion of the second IGB (37.9 kg/m2) [6]. The present study differs from Alfredo et al. [6] in that sequential IGBs were considered in patients as a bridging therapy to definitive bariatric surgery; significant weight gain was considered as failure of IGB treatment and in fact indication for removal, and there was no interval balloon-free period between IGBs. Nevertheless, the findings of the present study concur and build on the findings of Alfredo et al. [6], highlighting the limited effect of sequential IGB treatment on weight loss when an IGB is left in-situ for 12 months. In addition, the present study assesses how weight loss is distributed over 12 months, important for evaluating the optimal treatment period for IGBs, questioning whether treatment beyond 9 months facilitates weight loss.
The safety of IGB treatment is a prominent consideration across the literature. One systematic review by Yorke et al. [12] aimed to evaluate the safety of IGBs left in-situ for 6 months. It concluded that ‘IGBs are associated with marked short-term weight loss with limited serious complications’ [12]. However, the review did not specify whether sequential IGBs were included or excluded in the selection criteria, unlike the focus of the present study. In a separate prospective cohort study, Wiggins et al. [13] assessed the safety of IGBs left in-situ for 12 months in 1,100 patients. The study found that 60 patients (5.2%) had an adverse outcome, including 50 patients (4.3%) who required early IGB removal due to intolerance, irrespective of anti-emetic treatment [13]. Thirty-eight of these patients (3.4%) underwent IGB removal between 8 and 38 days post-insertion [13]. There were 8 cases of IGB rupture (0.7%) and these affected patients passed the balloon spontaneously [13]. There were 2 severe complications (0.1%) of gastric outlet obstruction which resolved with conservative management, and gastric perforation requiring laparotomy [13]. This study utilised an Obera365 IGB, as in the present study [13], with similarity in the nature and profile of complications experienced across both studies. Compared to the present study, Wiggins et al. [13] reported a lower incidence of adverse events (5.2% vs. 40%) and early removal of IGB (4.3% vs. 31.1%). Importantly, both studies highlight the tendency of most complications, such as intolerance, to occur 1–2 months post-insertion, and hence unrelated to the 12-month treatment duration. However, significant differences in patient characteristics between the studies should be highlighted and may account for the difference in outcomes. In Wiggins et al. [13], the median BMI was 36.3 kg/m2 and the majority of patients did not have any obesity-related complications. By comparison, the mean BMI was 62.4 kg/m2 in the present study, and a third of patients had type 2 diabetes and hypertension respectively. Hence, the present study adds greater weight to the argument for the overall tolerability and acceptability of IGBs as a bridging treatment to definitive bariatric surgery.
Spyropoulos et al. [9] assessed efficacy and safety of IGB treatment in 26 high-risk super obese patients undergoing a single 6-month period of IGB treatment. The study reported nausea and occasional vomiting as the only adverse outcomes, recorded in 65% of patients, with only one patient requiring readmission to hospital [9]. Their patient cohort was comparable to the present study, in that the mean BMI was 65.3 kg/m2 with an average weight of 193.9 kg. It differed in the proportion of male patients (88.5%) compared to female patients in the present study (73.3%). Interestingly, despite their higher overall complication rate (65% vs. 40%), the authors observed a lower re-admission rate (3.8%) and early balloon removal rate (11.6% vs. 31.1%) than the present study.
Alfredo et al. [6] considered safety of sequential IGBs as a secondary outcome in their aforementioned prospective cohort study. In this study, all 83 patients (100%) underwent treatment with a second IGB, 18 patients (22.2%) undertook a third IGB, and 1 patient (1.2%) had a fourth IGB [6]. The study reported a longer duration of complications—nausea, vomiting and epigastric pain—with the second IGB (4.0 days) compared to the first IGB (2.5 days) [6]. These symptoms were effectively medically managed [6], but it is unclear whether this required hospital admission. No major complication, such as gastric ulcer, perforation or death, was reported [6]. One patient (1.45%) underwent early IGB removal for intolerance, but in the absence of IGB rupture, oesophagitis or uncontrolled vomiting [6]. Each sequential IGB was left in-situ for 6 rather than 12 months in Alfredo et al. [6], but this does not explain the lower incidence of complications in comparison with the present study, given most complications presented one month from the time of IGB insertion. Super obesity (BMI >60 kg/m2) and associated co-morbidity of patients in the present study might have contributed to complication risk and help account for the difference in findings.
There are limitations of our present study: the overall number of patients who underwent sequential IGB treatment was small. This is in keeping with current practice in our centre where, in those patients fit enough, definitive surgery is the treatment of choice. The longitudinal nature of the study (9 years) demonstrates the reasonably small number of patients who undergo IGB insertion as a bridging treatment to definitive bariatric surgery, and the even smaller number of patients who may be offered sequential IGBs. Although the results of our study to do not support the routine use of sequential IGBs, there may be specific situations where they are indicated, for example patients with previous major abdominal surgery or other technical factors precluding definitive bariatric surgery, as well as patient choice to avoid surgery. The present study also provides a retrospective overview of the practice and clinical outcomes at one bariatric centre.
Proposals for future work could include a prospective longitudinal study evaluating the use of IGBs in different regional bariatric centres in the post-Covid-19 pandemic era. Differences in brands of IGB used, IGB treatment duration, use of sequential IGBs, their effect on weight loss, and complication rates could be evaluated. Conclusions could help inform IGB use in clinical practice to optimise weight loss outcomes. We foresee increased use of injectable pharmacological therapies for weight loss within the coming years, which may in fact surpass the IGB in terms of efficacy and acceptability as a bridging therapy to definitive surgery. Once injectable therapies are more widely available, a study comparing clinical outcomes of IGB use versus injectable pharmacological treatment would aid decision making in the context of bridging therapies.
CONCLUSION
The present study concludes that, although not associated with increased complication risk, sequential IGB use as a bridging treatment to definitive bariatric surgery does not significantly contribute to weight loss in super obese patients. Optimal treatment is achieved with a single IGB, although weight loss is not uniform across a 12-month treatment period and weight gain can result after 9 months.
Footnotes
Presentation: Presented at British Obesity & Metabolic Surgery Society 15th Annual Scientific Meeting 4–5th June 2024, Harrogate, UK.
Funding: No funding was obtained for this study.
Conflict of Interest: None of the authors have any conflict of interest.
References
- 1.World Health Organization. Obesity [Internet] Geneva: World Health Organization; 2024. [cited 2024 Feb 3]. Available from: https://www.who.int/health-topics/obesity. [Google Scholar]
- 2.Office for Health Improvement & Disparities (UK) Obesity profile: short statistical commentary [Internet] London: Office for Health Improvement & Disparities; 2023. [cited 2024 Feb 3]. Available from: https://www.gov.uk/government/statistics/obesity-profile-update-may-2023/obesity-profile-short-statistical-commentary-may-2023. [Google Scholar]
- 3.Kim SH, Chun HJ, Choi HS, Kim ES, Keum B, Jeen YT. Current status of intragastric balloon for obesity treatment. World J Gastroenterol. 2016;22:5495–5504. doi: 10.3748/wjg.v22.i24.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Diabetes and Digestive and Kidney Diseases (US) Treatment for overweight & obesity [Internet] Bethesda (MD): National Institute for Diabetes and Digestive and Kidney Diseases; 2023. [cited 2023 Oct 22]. Available from: https://www.niddk.nih.gov/health-information/weight-management/adult-overweight-obesity/treatment. [Google Scholar]
- 5.Peker Y, Coskun H, Bozkurt S, Cin N, Atak T, Genc H. Comparison of results of laparoscopic gastric banding and consecutive intragastric balloon application at 18 months: a clinical prospective study. J Laparoendosc Adv Surg Tech A. 2011;21:471–475. doi: 10.1089/lap.2010.0439. [DOI] [PubMed] [Google Scholar]
- 6.Alfredo G, Roberta M, Massimiliano C, Michele L, Nicola B, Adriano R. Long-term multiple intragastric balloon treatment--a new strategy to treat morbid obese patients refusing surgery: prospective 6-year follow-up study. Surg Obes Relat Dis. 2014;10:307–311. doi: 10.1016/j.soard.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Dumonceau JM, François E, Hittelet A, Mehdi AI, Barea M, Deviere J. Single vs repeated treatment with the intragastric balloon: a 5-year weight loss study. Obes Surg. 2010;20:692–697. doi: 10.1007/s11695-010-0127-x. [DOI] [PubMed] [Google Scholar]
- 8.Ball W, Raza SS, Loy J, Riera M, Pattar J, Adjepong S, et al. Effectiveness of intra-gastric balloon as a bridge to definitive surgery in the super obese. Obes Surg. 2019;29:1932–1936. doi: 10.1007/s11695-019-03794-8. [DOI] [PubMed] [Google Scholar]
- 9.Spyropoulos C, Katsakoulis E, Mead N, Vagenas K, Kalfarentzos F. Intragastric balloon for high-risk super-obese patients: a prospective analysis of efficacy. Surg Obes Relat Dis. 2007;3:78–83. doi: 10.1016/j.soard.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Tate CM, Geliebter A. Intragastric balloon treatment for obesity: review of recent studies. Adv Ther. 2017;34:1859–1875. doi: 10.1007/s12325-017-0562-3. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Wang C, Shikora S, Kow L. Recommendations for metabolic and bariatric surgery during the COVID-19 pandemic from IFSO. Obes Surg. 2020;30:2071–2073. doi: 10.1007/s11695-020-04578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yorke E, Switzer NJ, Reso A, Shi X, de Gara C, Birch D, et al. Intragastric balloon management of severe obesity: a systematic review. Obes Surg. 2016;26:2248–2254. doi: 10.1007/s11695-016-2307-9. [DOI] [PubMed] [Google Scholar]
- 13.Wiggins T, Sharma O, Sarfaraz Y, Fry H, Baker J, Singhal R. Safety and efficacy of 12-month intra-gastric balloon – series of over 1100 patients. Obes Surg. 2024;34:176–182. doi: 10.1007/s11695-023-06953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]