Abstract
Valyl-tRNA synthetase (ValRS) has difficulty differentiating valine from structurally similar non-cognate amino acids, most prominently threonine. To minimize errors in aminoacylation and translation the enzyme catalyzes a proofreading (editing) reaction that is dependent on the presence of cognate tRNAVal. Editing occurs at a site functionally distinct from the aminoacylation site of ValRS and previous results have shown that the 3′-terminus of tRNAVal is recognized differently at the two sites. Here, we extend these studies by comparing the contribution of aminoacylation identity determinants to productive recognition of tRNAVal at the aminoacylation and editing sites, and by probing tRNAVal for editing determinants that are distinct from those required for aminoacylation. Mutational analysis of Escherichia coli tRNAVal and identity switch experiments with non-cognate tRNAs reveal a direct relationship between the ability of a tRNA to be aminoacylated and its ability to stimulate the editing activity of ValRS. This suggests that at least a majority of editing by the enzyme entails prior charging of tRNA and that misacylated tRNA is a transient intermediate in the editing reaction.
INTRODUCTION
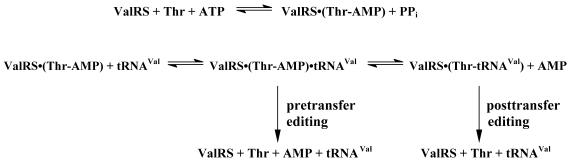
Correct aminoacylation of tRNA with its cognate amino acid is essential to the precision of protein synthesis. Accuracy is achieved by specific recognition of amino acids and tRNAs by their cognate aminoacyl-tRNA synthetases. A variety of identity determinants and anti-determinants ensure the specificity of tRNA binding to its corresponding synthetase (reviewed in 1–4). However, amino acids are small molecules with limited distinctive structural features. Consequently, some synthetases have difficulty discriminating their cognate amino acids from structurally similar ones, resulting in misactivation of non-cognate amino acids and potential misacylation of tRNA (reviewed in 5). To correct such errors, these enzymes catalyze proofreading (editing) reactions at either or both of the two steps in the aminoacylation pathway (Fig. 1): pre-transfer editing, which involves direct hydrolysis of the enzyme-bound non-cognate aminoacyl adenylate, and post-transfer editing, in which misacylated tRNA is deacylated.
Figure 1.
Editing reactions carried out by ValRS. Misactivated threonine (Thr-AMP) may be hydrolyzed before the non-cognate amino acid is transferred to tRNAVal (pre-transfer editing). Any misacylated Thr-tRNAVal formed is rapidly hydrolyzed (post-transfer editing).
Editing has been most extensively studied in class I synthetases, especially valyl-tRNA synthetase (ValRS) and isoleucyl-tRNA synthetase (IleRS). ValRS misactivates threonine and several other non-cognate amino acids (5,6), while IleRS misactivates valine (7). Mutational analysis of ValRS (8,9) and IleRS (8,10) has shown that the editing reaction takes place at a site functionally distinct from the aminoacylation site. A large insert in the active site (connective polypeptide 1) is responsible for editing activity (9,11–14).
The proofreading activity of ValRS is strongly and specifically stimulated by wild-type tRNAVal (15,16), which triggers translocation of misactivated amino acids from the aminoacylation site to the editing site (14,17). Numerous experiments have elucidated the identity elements for aminoacylation of tRNAVal (18–23); however, much less is known about the determinants for editing. Several studies have focused on the universally conserved 3′-CCA end of tRNA. Chemical or enzymatic alteration of the 3′-terminal adenosine can result in misacylation of tRNA (24–28). Systematically mutating the 3′-CCA end of Escherichia coli tRNAVal showed that substituting pyrimidines (C or U) for the normal 3′-terminal adenine produces tRNAVal variants that are active in accepting valine (29,30) but do not stimulate the editing activity of ValRS (6,30), indicating that the 3′-terminus of tRNAVal is recognized differently at the aminoacylation and editing sites of the enzyme. Schulman and Pelka (17) and Schimmel and co-workers (31) reported that replacing 3 nt in the D-loop of tRNAIle inhibits editing of misactivated valine by E.coli IleRS without affecting aminoacylation with isoleucine.
The aim of this work is to identify the tRNA determinants for editing by E.coli ValRS and to establish whether these differ from those for aminoacylation. To compare tRNA recognition at the aminoacylation site of ValRS with that at the editing site, we studied the effects on aminoacylation and editing of mutating known synthetase recognition elements of tRNAVal and of transplanting them into the framework of other tRNAs. Analysis of results with these tRNA transcripts shows that only those that are actively aminoacylated stimulate the editing reaction of ValRS, suggesting that editing by the enzyme requires prior charging of tRNA.
MATERIALS AND METHODS
Materials
Restriction endonucleases were purchased from either New England Biolabs or Promega. T7 RNA polymerase was purified from E.coli BL21/pAR1219 by the procedure of Zawadski and Gross (32). Homogeneous ValRS was prepared from E.coli GRB238/pHOV1 as described by Chu and Horowitz (33). Nucleotide triphosphates, guanosine 5′-monophosphate and [γ-32P]adenosine 5′-triphosphate (3 Ci/mmol) were from Sigma, Amersham Life Sciences, NEN or United States Biochemical Co. [3H]valine (23–32 Ci/mmol) and [3H]threonine (15.2 Ci/mmol) were products of Amersham Life Sciences.
Preparation of tRNA
Wild-type tRNAVal was transcribed in vitro by T7 RNA polymerase (34) from a DNA template derived from the recombinant phagemid pFVAL119 containing the E.coli wild-type tRNAVal gene (35). Methods similar to those described for tRNAVal (34) were used to construct the phagemid pALA119, which carries the gene for E.coli tRNAAla (22). pFPHE119 is a comparable construct containing the gene for E.coli tRNAPhe (23). It was prepared from pUS618 containing the E.coli tRNAPhe gene, supplied by Dr O. C. Uhlenbeck (University of Colorado, Boulder, CO). Dr Paul Schimmel (Scripps Research Institute, La Jolla, CA) kindly provided a phagemid containing the tRNAIle gene (17). The gene differs from that of natural tRNAIle by having the A1:U72 base pair replaced by G1:C72 to improve transcription efficiency. tRNA transcripts were purified by HPLC (36). Mutations were introduced into the cloned tRNA genes by site-directed mutagenesis (34) using mutagenic oligonucleotides synthesized by the Nucleic Acid Facility at Iowa State University. The sequence of mutant tRNA genes was confirmed by dideoxy sequence analysis performed by the Nucleic Acid Facility.
Editing assay
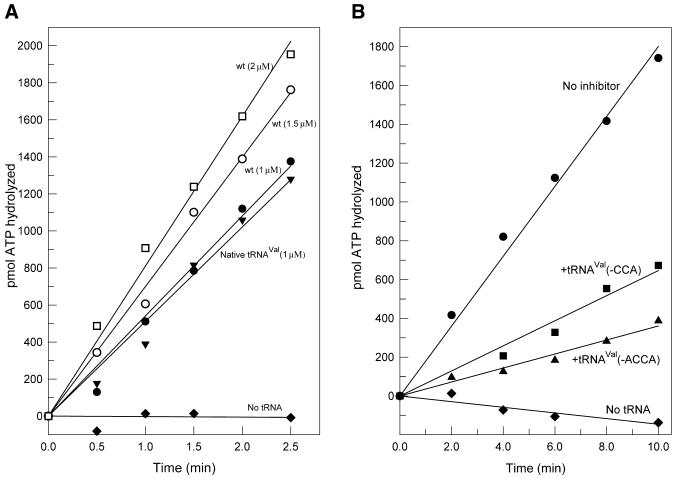
Editing (ATP hydrolysis) assays were carried out essentially as described by Schmidt and Schimmel (10). Reaction mixtures of 60 µl containing 150 mM Tris–HCl pH 7.5, 10 mM MgCl2, 37 mM threonine, 3 mM [γ-32P]ATP (20–30 c.p.m./pmol), 1 µM tRNA and 2 µM ValRS were incubated at 25°C. At intervals over a 10 min time period, 10 µl of the reaction mix was quenched with 25 vol of a solution containing 7% HClO4, 10 mM sodium pyrophosphate and 3% activated charcoal (Sigma). [32P]pyrophosphate released as a result of ATP hydrolysis was separated from charcoal-bound ATP/AMP by centrifugation. Radioactivity in 50 µl of the supernatant was measured by liquid scintillation counting. The rate of ATP hydrolysis is the average of two or more determinations. Control reactions with no added tRNA and with added wild-type tRNAVal were included with each set of assays. Some variation in the rate of ATP hydrolysis was noted with different sources of [γ-32P]ATP, but the relative rates of ATP hydrolysis remained constant. Under the conditions of the experiment, the ATP hydrolysis rate is proportional to tRNA concentration (see Fig. 2A).
Figure 2.
tRNA-dependent editing by ValRS in the presence of threonine. (A) Stimulation of ValRS-catalyzed editing by native tRNAVal (modified) and unmodified in vitro transcribed wild-type (wt) tRNAVal: triangle, 1 µM native (modified) tRNAVal; solid circle, 1 µM unmodified wt tRNAVal transcript; open circle, 1.5 µM wt tRNAVal transcript; square, 2 µM wt tRNAVal transcript; diamond, no tRNA. (B) Inhibition by truncated tRNAs of wt (unmodified) tRNAVal-stimulated translational editing by ValRS: circle, no inhibitor; triangle, tRNAVal(-ACCA); square, tRNAVal(-CCA); diamond, no tRNA. In all experiments, ValRS and tRNAVal concentrations were 2 and 1 µM, respectively; concentration of 3′-truncated tRNAs was 10 µM.
Aminoacylation assays
The time course of aminoacylation was determined at 37°C as previously described (36) in a 60 µl reaction mix containing 100 mM HEPES–KOH pH 7.5, 10 mM KCl, 15 mM MgCl2, 7 mM ATP, 1 mM DTT, 100 µM [3H]valine, 1 nM ValRS and 2 µM tRNA. Aminoacylation kinetics experiments were monitored using a range of tRNA concentrations from 0.5 to 6.0 µM. The kinetic parameters Km and Vmax were resolved by a least squares fit of the double reciprocal plot of the data using the Enzfitter computer program (Elsevier-Biosoft). Results reported are the average of two or more experiments.
RESULTS AND DISCUSSION
Modified nucleosides of E.coli tRNAVal play no role in the ability of the tRNA to stimulate the editing function of ValRS; unmodified wild-type tRNAVal transcripts function as efficiently as native (modified) tRNAVal (Fig. 2A). This has allowed us to use in vitro transcribed tRNAVal mutants to identify nucleotides and structural features of the tRNA that are determinants for translational editing by ValRS. Editing was measured in the presence of the non-cognate amino acid threonine by the tRNA-dependent continuous hydrolysis of ATP (to AMP plus PPi), which is diagnostic of the overall editing reaction (pre-plus post-transfer editing; Fig. 1).
Particular attention focused on those elements of tRNAVal that are determinants for aminoacylation by E.coli ValRS. These were previously identified (18–23,35) and include the two anticodon nucleotides A35 and C36 (Fig. 3A), which are the major determinants for aminoacylation (18). In addition, G at the discriminator base position (no. 73) acts as a strong anti-determinant for ValRS, the C73 and U73 mutants as well as wild-type tRNAVal (A73) being readily aminoacylated (23). G20, in the D-loop (part of the variable pocket), and G45, in the central core, serve as minor recognition elements (23). Unlike most tRNAs, E.coli tRNAVal has no nucleotide-specific acceptor stem synthetase recognition determinants, but regular A-type RNA helix geometry is essential, especially near the 4:69 base pair (22). Conformation of the anticodon stem of tRNAVal also plays a role in ValRS recognition; mutant tRNAs with rigid, G:C-rich anticodon stems are poor valine acceptors, due largely to an increase in Km (23). Lack of flexibility in the anticodon stem may alter access of ValRS to essential functional groups on the major synthetase recognition nucleotides in the anticodon, A35 and C36 (23). The effects on editing and aminoacylation of mutating these identity determinants or transplanting them into the framework of other tRNAs were examined to compare tRNA recognition at the aminoacylation site of ValRS with that at the editing site.
Figure 3.
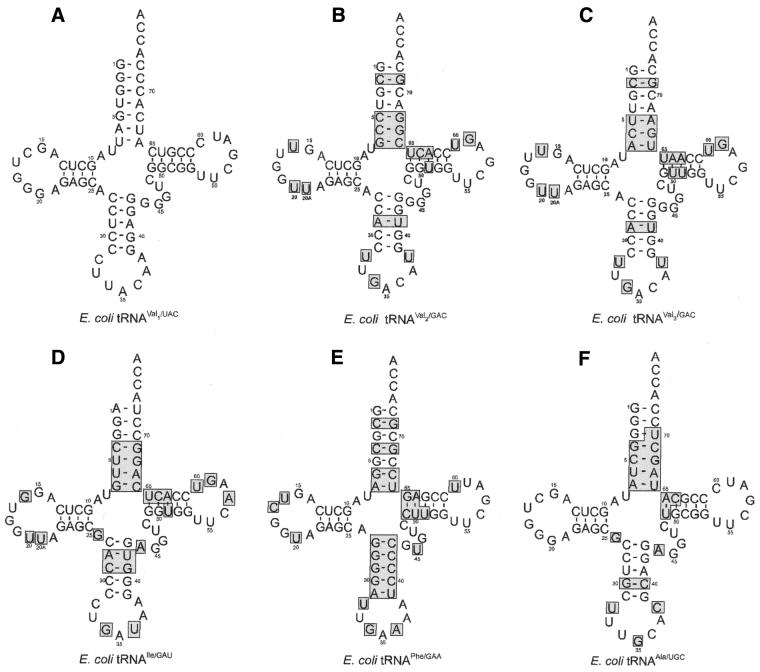
Nucleotide sequences of the tRNAs used in this study. Nucleotide differences between (A) E.coli tRNAVal1(UAC) and (B) E.coli tRNAVal2(GAC), (C) E.coli tRNAVal3(GAC), (D) E.coli tRNAIle, (E) E.coli tRNAPhe and (F) E.coli tRNAAla are indicated by the shaded box areas.
Role of aminoacylation determinants of tRNAVal in translational editing
Discriminator base and 3′-CCA end. We previously showed that the universally conserved 3′-terminal adenosine (A76) is essential for tRNAVal to trigger the editing reaction of ValRS (6; also see Table 1). tRNAVal mutants with any base substituted for A76 fail to stimulate ATP hydrolysis and are misacylated with threonine and several other amino acids (6). Mutations of other bases in the 3′-ACCA sequence of tRNAVal have relatively little effect on the ability of the tRNA to stimulate editing (Table 1). The only exceptions are the G75 and G73 mutants. Valine tRNA variants with the wild-type C75 replaced by either U75 or A75 effectively stimulate the editing reaction of ValRS, although at a somewhat lower rate (Table 1). Substituting G75 for C75, however, reduces the editing activity of the tRNA >95% (Table 1). Similarly, changing the wild-type discriminator base (A73) to either U73 or C73 has relatively little effect on the ability of tRNAVal to stimulate editing (Table 1), but the G73 mutant is almost inactive (Table 1).
Table 1. Editing efficiency of 3′ end mutants of tRNAVal.
| tRNAVal variant | Relative rate of ATP hydrolysisa | Relative aminoacylation efficiencyb (Vmax/Km) |
|---|---|---|
| Wild-type | (1.0) | (1.0) |
| A76→U76 | –0.03c | 0.39 |
| A76→C76 | –0.10c | 0.50 |
| A76→G76 | –0.03c | 0.0 |
| C75→U75 | 0.76 | 0.31 |
| C75→A75 | 0.87 | 0.42 |
| C75→G75 | 0.04 | 0.01 |
| C74→U74 | 0.93 | 0.33 |
| C74→A74 | 0.98 | 0.86 |
| C74→G74 | 0.81 | 1.1 |
| A73→U73 | 0.85 | 0.31 |
| A73→C73 | 0.89 | 0.44 |
| A73→G73 | 0.07 | 0.026 |
| tRNAVal(-CCA) | –0.01 | ndd |
| tRNAVal(-ACCA) | –0.01 | nd |
| No tRNA | –0.09 | nd |
To further define the function of the 3′ end of tRNAVal in the editing reaction, the editing efficiency of tRNAVal lacking the 3′-CCA or 3′-ACCA sequence was examined. These truncated tRNAs fail to stimulate the ValRS-catalyzed hydrolysis of ATP (Table 1). They do, however, inhibit the stimulation of editing by wild-type tRNAVal (Fig. 2B), indicating that they bind efficiently to ValRS.
Comparison of the ability of these tRNAVal mutants to accept valine and to stimulate the editing reaction shows a good correlation between the two activities (Table 1). Mutant valine tRNAs that are active in stimulating editing are also readily aminoacylated (Table 1; see also later results). However, the converse is not true; not all tRNAs able to accept valine trigger the editing function of ValRS. tRNAVal with pyrimidine 3′-termini are quite actively aminoacylated (29) but fail to stimulate editing (6; Table 1).
Acceptor stem and anticodon stem. ValRS directly recognizes the acceptor helix of tRNAVal (23). Although there are no identity nucleotides in the acceptor stem, the efficiency of aminoacylation of tRNAVal is sensitive to distortions in the A-form acceptor helix resulting from the introduction of G:U base pairs (22). Wobble base pairs in the acceptor helix also inhibit the ability of the tRNA to stimulate editing. Replacing the U4:A69 base pair of E.coli tRNAVal with G4:U69 results in a 6-fold reduction in editing activity relative to wild-type tRNAVal (Table 2A). The U4:G69 variant of tRNAVal, which is more efficiently aminoacylated than the G4:U69 mutant (21; Table 2A), is also more active in stimulating editing, having 45% of the activity of wild-type tRNAVal (Table 2A).
Table 2. Efficiency of tRNAVal variants in stimulating translational editing.
| tRNAVal variant | Relative rate of ATP hydrolysisa | Relative aminoacylation efficiencyb (Vmax/Km) |
|---|---|---|
| Wild-type (UAC) | (1.0) | (1.0) |
| (A) Acceptor stem mutants | ||
| U4:A69→G4:U69 | 0.18 | 0.026 |
| U4:A69→U4:G69 | 0.45 | 0.17 |
| (B) Anticodon stem mutants | ||
| U29:A41→C29:G41 | –0.04 | 0.022 |
| (C) Anticodon mutants | ||
| A35→G35 | 0.05 | 7.5 × 10–6 |
| C36→U36 | 0.07 | 8.6 × 10–4 |
| (D) Central core mutants | ||
| G45→U45 | 0.62 | 0.57 |
| G45→A45 | 0.49 | 0.48 |
| No tRNA | –0.08 | – |
Correspondence between aminoacylation and editing activities is also observed with anticodon stem mutants of tRNAVal. Increasing the G:C content of the anticodon stem by mutating U29:A41 to C29:G41 decreases both the aminoacylation and editing efficiencies of the tRNA (Table 2B).
Anticodon and central core. The major identity nucleotides in E.coli tRNAVal, A35 and C36 (18,20,23), were mutated to determine their role as editing determinants. Replacing A35 with G or changing C36 to U36 results in a major (93–95%) reduction in the ability of these tRNAVal mutants to stimulate the rate of ATP hydrolysis relative to that of wild-type tRNAVal (Table 2C).
Mutation of G45, in the central core, reveals a small contribution of this minor aminoacylation identity element to the editing efficiency of tRNAVal. Changing G45 to U45 or A45 produces tRNAVal variants with a 40–50% reduction in editing efficiency (Table 2D). These tRNAVal mutants are aminoacylated by ValRS at a rate 50–60% that of wild-type tRNAVal (Table 2D).
Variable pocket (D- and T-loops). The D- and T-loops of tRNAVal, which interact in the corner of the L-shaped molecule to form the variable pocket (nucleotides 16, 17, 20, 59 and 60), were also modified to determine their role in the editing reaction catalyzed by ValRS. Hale et al. (31) had reported that nucleotides G16, D20 and G21 in the D-loop of E.coli tRNAIle are specific determinants for editing by IleRS; mutation of these bases affects editing without affecting aminoacylation.
Nucleotide G20 in the D-loop is a minor synthetase recognition determinant for the aminoacylation of tRNAVal (23). Converting G20 to any other base consistently reduces aminoacylation efficiency 3–4-fold (23; Table 3) Similar reductions are observed in the editing efficiency of G20 mutants of tRNAVal compared with wild-type tRNAVal (Table 3). In contrast, substitution of U59 and C60 in the T-loop of tRNAVal has only a relatively small effect on both editing and aminoacylation efficiency (Table 3). Nucleotides in the D-loop that have not been directly tested for their effect on editing are conserved or semi-conserved in E.coli tRNAs. Clearly, there are no unique translational editing determinants in the D-loop of tRNAVal, i.e. bases essential for editing but not required for aminoacylation.
Table 3. Stimulation of translational editing by D- and T-loop variants of E.coli tRNAVal.
| tRNAVal variant | Relative rate of ATP hydrolysisa | Relative rate of aminoacylation |
|---|---|---|
| Wild-type | (1.0) | (1.0) |
| G20→U20 | 0.11 | 0.31b |
| G20→A20 | 0.18 | 0.39b |
| G20→C20 | 0.21 | 0.28b |
| C16 + G20→U16 + U20U20a | 0.54 | 0.60 |
| C16 + G20→G16 + U20U20a | 0.57 | 0.44 |
| U59 + C60→G59 + U60 | 0.66 | 0.95 |
| C16 + G20 + U59 + C60→U16 + U20U20a + G59 + U60 | 0.43 | 0.60 |
| No tRNA | –0.06 | – |
aNegative values are the product of small changes in the background rate of ATP hydrolysis.
bResults are from Horowitz et al. (23); included for comparison.
The D-loops of two minor valine isoacceptors of E.coli, tRNAVal2(GAC) and tRNAVal3(GAC), differ from that of the major valine isoacceptor, tRNAVal1(UAC) in having 9 nt rather than 8, with U20U20a replacing G20 and U16 in place of C16 (see Fig. 3B and C). There are also differences in the T-loop, at positions 59 and 60 (part of the variable pocket); the minor tRNA species have G59 and U60 in place of U59 and C60. Transplanting the D-loop of the minor E.coli tRNAVal isoacceptors into tRNAVal1 results in a tRNA with ∼50% of the editing activity of tRNAVal1 (Table 3).
Furthermore, replacing the T-loop of tRNAVal1 with that of the minor isoacceptors (changing U59 and C60 to G59 and U60) has only a relatively modest effect on editing efficiency (Table 3). Similar results are found when both the T- and D-loops of the major valine isoacceptor are exchanged for those of the minor tRNA species (Table 3). As observed with other variants of tRNAVal, these substitutions affect the aminoacylation and editing activities to about the same degree (Table 3).
Stimulation of translational editing by non-cognate tRNAs
To further test the importance of synthetase recognition elements of tRNAVal as determinants for editing and to identify possible anti-determinants, the known identity elements of tRNAVal were transplanted into the framework of non-cognate tRNAs. This approach was used successfully to identify editing determinants in E.coli tRNAIle (31).
Escherichia coli tRNAIle. We had previously shown that E.coli tRNAAla(UGC), E.coli tRNAPhe(GAA) and yeast tRNAPhe(GAA) could be readily transformed into efficient valine acceptors by introducing the ValRS recognition nucleotides and removing negative determinants (22,23). It is also possible to convert E.coli tRNAIle(GAU) into a substrate for aminoacylation by ValRS (Table 4).
Table 4. Efficiency of E.coli tRNAIle variants in accepting valine and stimulating translational editing.
| tRNA | Relative rate of ATP hydrolysisa | Relative rate of aminoacylation with valine |
|---|---|---|
| Escherichia coli tRNAVal | ||
| tRNAVal(UAC) (wild-type) | (1.0) | (1.0) |
| tRNAVal(GAC) | – | 0.63b |
| Escherichia coli tRNAIle | ||
| tRNAIle(GAU) (wild-type) | –0.01 | –0.002 |
| tRNAIle(GAC) | 0.05 | 0.05 |
| tRNAIle(GAC) A68 | 0.07 | 0.08 |
| tRNAIle(GAC) Val anticodon stem | 0.13 | 0.19 |
| tRNAIle(GAC) A68 + Val anticodon stem | 0.16 | 0.31 |
| tRNAIle(GAC) G20 + Val anticodon stem | 0.38 | 0.45 |
| tRNAIle(GAC) + G20 + A68 + Val anticodon stem | 0.48 | 0.70 |
| No tRNA | –0.01 | – |
aNegative values are the result of small changes in the background rate of ATP hydrolysis.
bResult is from Horowitz et al. (23); included for comparison.
Wild-type tRNAIle(GAU) is not aminoacylated with valine under standard conditions (Table 4). The nucleotide sequence of E.coli tRNAIle(GAU) differs from that of E.coli tRNAVal1 at 28 positions located in all regions of the molecule (see shaded areas in Fig. 3D). It is missing the ValRS recognition nucleotides C36 and G20, has a G:U wobble base pair in the acceptor stem that acts as an anti-determinant for ValRS and possesses a G:C-rich anticodon stem that is known to decrease valine acceptance (23). tRNAIle(GAU) also has G34 in the anticodon, rather than the U34 present in tRNAVal1, but the G34 variant of tRNAVal1 is a good acceptor of valine (Table 4). It is interesting to note that the minor E.coli valine isoacceptors also have G34 and that the sequence of their D-loops closely resemble that of E.coli tRNAIle, differing only at position 16, where tRNAIle has G16 in place of U16 (Fig. 3).
Inserting C36 in place of U36 improves aminoacylation efficiency only slightly (5%; Table 4). Further, converting the U5:G68 wobble base pair to a U5:A68 Watson–Crick base pair, by mutating G68 to A68, results in little enhancement of valine accepting activity (Table 4). Decreasing the G:C content of the lower part of the anticodon stem of tRNAIle(GAC), by replacing the tRNAIle anticodon stem with that of tRNAVal, moderately improves valine aminoacylation (19%; Table 4). The combined effect of inserting the valine anticodon stem and A68 into E.coli tRNAIle is a further modest improvement of aminoacylation efficiency to 31% that of wild-type tRNAVal (Table 4). Inserting additional ValRS recognition elements gradually increases valine acceptance in the tRNAIle framework until the mutant tRNAIle(GAC) + G20 + A68 + valine anticodon stem is as active as tRNAVal(GAC) (Table 4).
Comparison of the valine accepting efficiencies of tRNAIle variants with their ability to stimulate editing by ValRS shows a close correlation between the two activities (Table 4). As additional ValRS identity determinants for aminoacylation are introduced into the tRNAIle framework, the editing activity of the tRNA increases in parallel with its ability to be charged with valine. The mutant tRNAIle(GAC) + G20 + A68 + valine anticodon stem is quite active in stimulating the editing reaction of ValRS.
Escherichia coli tRNAPhe. Wild-type E.coli tRNAPhe(GAA) is not aminoacylated with valine by ValRS (Table 5; 23). Its sequence differs from that of E.coli tRNAVal at 31 positions, including most of the acceptor stem, the entire anticodon stem, part of the anticodon loop, part of the T-stem and loop and three positions in the D-loop (see shaded areas in Fig. 3E). tRNAPhe(GAA) has two of the synthetase recognition determinants essential for aminoacylation by ValRS, A35 and A73 (Fig. 3). Introducing the ValRS recognition determinants C36, G20 and G45 does not improve valine acceptance (Table 5), but substituting the anticodon stem of tRNAVal for that of tRNAPhe and converting U45 to G45 transforms tRNAPhe into a good substrate for ValRS (Table 5; 23). Once again, there is generally a direct correlation between the valine acceptance of the tRNAPhe variants and their ability to stimulate the editing reaction (Table 5).
Table 5. Efficiency of E.coli tRNAPhe variants in stimulating translational editing.
| tRNA variant | Relative rate of ATP hydrolysisa | Relative aminoacylation efficiencyb (Vmax/Km) |
|---|---|---|
| Escherichia coli tRNAVal | ||
| tRNAVal(UAC) (wild-type) | (1.0) | (1.0) |
| Escherichia coli tRNAPhe | ||
| tRNAPhe(GAA) (wild-type) | –0.10 | 0 |
| tRNAPhe(GAC) G20 + G45 | –0.03 | 0.03 |
| tRNAPhe(GAC) G20 + Val anticodon arm | 0.01 | 0.37 |
| tRNAPhe(GAC) G20 + G45 + Val anticodon stem + Phe anticodon loop | 0.76 | 0.83 |
| tRNAPhe(GAC) G20 + G45 + Val anticodon arm | 1.03 | 1.04 |
| No tRNA | –0.14 | – |
aNegative values are the result of small changes in the background rate of ATP hydrolysis.
bResults are from Horowitz et al. (23); included for comparison.
Note, however, that whereas G45 is a minor recognition element for aminoacylation by ValRS, it is essential for the editing-stimulatory activity of tRNA. tRNAPhe variants, even those that are reasonable substrates for aminoacylation with valine, do not stimulate editing by ValRS unless G45 is substituted for U45 (Table 5). Position 45 in the variable loop of tRNAs is involved in a tertiary interaction (base triple) with G10:C25 of the D-arm. This region of tRNA, where the D- and T-loops interact in the corner of the L-shaped structure (the elbow), has been implicated in the editing reaction. Farrow et al. (18) and Hale et al. (31) reported that modifying nucleotides G16, D20 and G21 in the D-loop of E.coli tRNAIle affects editing without influencing aminoacylation. While we have failed to identify discrete tRNA determinants in the D-loop of tRNAVal that are essential for editing but are not required for aminoacylation, our observation that G45 contributes to editing efficiency and that mutation of G20 decreases the editing effectiveness of tRNAVal (Table 3) support the conclusion that the elbow region of tRNA is important for editing. Although there is no evidence for direct contacts between the D-loop and the synthetase (13,14), mutation of nucleotides in this region of the tRNA may induce structural changes that influence its ability to trigger the editing reaction.
Escherichia coli tRNAAla. Experiments to explore the effects on the editing reaction of mutating ValRS recognition determinants in the framework of still another non-cognate tRNA were carried out with E.coli tRNAAla. The sequence of this tRNA differs from E.coli tRNAVal at 19 positions (Fig. 3F) and wild-type tRNAAla is not a substrate for aminoacylation by ValRS (Table 6; 22). Two substitutions, G35→A35, which inserts a major ValRS recognition determinant in the anticodon, and G3→A3, which converts the G3:U70 wobble base pair in the acceptor stem to an A3:U70 Watson–Crick base pair, increases valine accepting activity to the level of wild-type tRNAVal (22; Table 6). Here, too, the editing efficiency of tRNAAla mutants parallels the aminoacylation efficiency; as the ability to accept valine increases, the editing activity also increases (Table 6).
Table 6. Efficiency of E.coli tRNAAla variants in stimulating translational editing.
| tRNA variant | Relative rate of ATP hydrolysisa | Relative aminoacylation efficiencyb (Vmax/Km) |
|---|---|---|
| Escherichia coli tRNAVal | ||
| tRNAVal(UAC) (wild-type) | (1.0) | (1.0) |
| Escherichia coli tRNAAla | ||
| tRNAAla(UGC) (wild-type) | –0.07 | 0 |
| tRNAAla(UAC) | 0.14 | 0.078 |
| tRNAAla(UAC) A3:U70 | 0.44 | 1.06 |
| No tRNA | –0.08 | – |
aNegative values are the result of small changes in the background rate of ATP hydrolysis.
bResults are from Liu et al. (22); included for comparison.
Conclusions
Mutational analysis of E.coli tRNAVal and several non-cognate tRNAs converted to accept valine in identity swap experiments shows that only those tRNAs that can be aminoacylated by E.coli ValRS stimulate the editing activity of the enzyme. The results indicate that all known aminoacylation identity determinants are involved in the recognition of tRNAVal by the editing site of ValRS.
Although all tRNAs that actively promote editing can be aminoacylated, not all tRNAs that can be aminoacylated are able to trigger the editing response of E.coli ValRS. The most significant example of a determinant essential for editing but not for aminoacylation is the 3′-terminal adenosine of tRNA. Mutants of tRNAVal that terminate in 3′-uridine or 3′-cytidine (i.e. lacking the normal 3′-adenosine) are readily aminoacylated by ValRS (29,30). These tRNAs, however, do not stimulate the editing reaction of the enzyme (Table 1). They cannot be deacylated by ValRS and are stably misacylated with threonine and several other amino acids (6). Clearly the 3′-terminal nucleotide of tRNA is recognized differently at the editing site of ValRS than at the aminoacylation site. Structural analysis of the complex of Thermus thermophilus ValRS, tRNAVal and an analog of the valyl adenylate intermediate shows that the editing domain of the enzyme specifically recognizes the N1 atom and the 6-NH2 group of the 3′-terminal adenosine of the tRNA (14).
The relative contribution of the pre- and post-transfer editing pathways to the proofreading activity of ValRS has been difficult to assess, largely because cognate tRNA is required to activate the editing reaction. Aminoacyl-tRNA synthetases are known to deacylate aminoacyl-tRNAs (reviewed in 5) and Fersht and Kaethner (15) have shown, by rapid quenching techniques, transient formation of threonyl-tRNAVal by the ValRS from Bacillus stearothermophilus (which is then rapidly deacylated). It is, however, not clear that this post-transfer mechanism represents the major editing pathway. tRNA binding may enhance editing by inducing an allosteric change in the synthetase without being transiently mischarged. This possibility is illustrated by evidence showing that a DNA aptamer, selected for its ability to bind to the tRNAIle-binding site of IleRS, stimulates editing by IleRS even though it cannot be aminoacylated (37).
The close correlation between aminoacylation efficiency of a tRNA and its effectiveness in stimulating the editing reaction of ValRS, together with our earlier finding that tRNAVal variants that cannot be deacylated by ValRS do not promote the editing reaction (6), support the notion that the majority of editing by ValRS occurs after transfer of misactivated threonine from threonyl-AMP to tRNA and indicates that misacylated tRNAVal is a transient intermediate in the editing reaction. This may occur by the post-transfer pathway in which the 3′ end of the misacylated tRNA is transferred to the editing site of ValRS and hydrolyzed. A second possibility is the model recently proposed by Bishop et al. (38) for editing by IleRS, a synthetase closely related to ValRS, in which translocation of misacylated tRNA to the editing site primes the enzyme for succeeding cycles of pre-transfer editing.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Daniel Spielbauer, Diane Shogren and Lene Larsen for their technical assistance. We are grateful to Dr Olke Uhlenbeck (University of Colorado, Boulder, CO) for the tRNAPhe-containing plasmid and to Dr Paul Schimmel (Scripps Research Institute, La Jolla, CA) for the phagemid containing the tRNAIle gene. This work was supported in part by grant MCB 95-13932 from the National Science Foundation. This is Journal Paper J-19639 of the Iowa Agriculture and Home Economic Experiment Station (Ames, IA), project no. 3552.
REFERENCES
- 1.Schulman L.H. (1991) Recognition of tRNAs by aminoacyl-tRNA synthetases. Prog. Nucleic Acid Res. Mol. Biol., 41, 23–57. [PubMed] [Google Scholar]
- 2.Giegé R., Puglisi,J.D. and Florentz,C. (1993) tRNA structure and aminoacylation efficiency. Prog. Nucleic Acid Res. Mol. Biol., 45, 129–205. [DOI] [PubMed] [Google Scholar]
- 3.Giegé R., Sissler,M. and Florentz,C. (1998) Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res., 26, 5017–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClain W. (1993) Rules that govern tRNA identity in protein synthesis. J. Mol. Biol. 234, 257–280. [DOI] [PubMed] [Google Scholar]
- 5.Jakubowski H. and Goldman,E. (1992) Editing of errors in selection of amino acids for protein synthesis. Microbiol. Rev., 56, 412–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tardif K.D., Liu,M., Vitseva,O., Hou,Y.-H. and Horowitz,J. (2001) Misacylation and editing by E. coli valyl-tRNA synthetase: evidence for two tRNA binding sites. Biochemistry, 40, 8118–8125. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin A.N. and Berg,P. (1966) Transfer ribonucleic acid-induced hydrolysis of valyladenylate bound to isoleucyl ribonucleic acid synthetase. J. Biol. Chem., 241, 839–845. [PubMed] [Google Scholar]
- 8.Lin L. and Schimmel,P. (1996) Mutational analysis suggests the same design for editing activities of two tRNA synthetases. Biochemistry, 35, 5596–5601. [DOI] [PubMed] [Google Scholar]
- 9.Lin L., Hale,S.P. and Schimmel,P. (1996) Aminoacylation error correction. Nature, 384, 33–34. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt E. and Schimmel,P. (1994) Mutational isolation of a sieve for editing in a transfer RNA synthetase. Science, 264, 265–268. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt E. and Schimmel,P. (1995) Residues in a class I tRNA synthetase which determine selectivity of amino acid recognition in the context of tRNA. Biochemistry, 34, 11204–11210. [DOI] [PubMed] [Google Scholar]
- 12.Nureki O., Vassylyev,D.G., Tateno,M., Shimada,A., Nakama,T., Fukai,S., Konno,M., Hendrickson,T.L., Schimmel,P. and Yokoyama,S. (1998) Enzyme structure with two catalytic sites for double-sieve selection of substrate. Science, 280, 578–582. [DOI] [PubMed] [Google Scholar]
- 13.Silvian L.F., Wang,J. and Steitz,T.A. (1999) Insights into editing from an Ile-tRNA synthetase structure with tRNAIle and mupirocin. Science, 285, 1074–1077. [PubMed] [Google Scholar]
- 14.Fukai S., Nureki,O., Sekine,S., Shimada,A., Tao,J., Vassylyev,D.G. and Yokoyama,S. (2000) Structural basis for double-sieve discrimination of L-valine from L-isoleucine and L-threonine by the complex of tRNA(Val) and valyl-tRNA synthetase. Cell, 103, 793–803. [DOI] [PubMed] [Google Scholar]
- 15.Fersht A. and Kaethner,M. (1976) Enzyme hyperspecificity. Rejection of threonine by the valyl-tRNA synthetase by misacylation and hydrolytic editing. Biochemistry, 15, 3342–3346. [DOI] [PubMed] [Google Scholar]
- 16.Jakubowski H. and Fersht,A. (1981) Alternative pathways for editing non-cognate amino acids by aminoacyl-tRNA synthetases. Nucleic Acids Res., 9, 3105–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulman L.H. and Pelka,H. (1988) Anticodon switching changes the identity of methionine and valine transfer RNAs. Science, 242, 765–768. [DOI] [PubMed] [Google Scholar]
- 18.Farrow M.A., Nordin,B.E. and Schimmel,P. (1999) Nucleotide determinants for tRNA-dependent amino acid discrimination by a class I tRNA synthetase. Biochemistry, 38, 16898–16903. [DOI] [PubMed] [Google Scholar]
- 19.Pallanck L. and Schulman,L.H. (1991) Anticodon-dependent aminoacylation of a noncognate tRNA with isoleucine, valine and phenylalanine in vivo. Proc. Natl Acad. Sci. USA, 88, 3872–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K., Himeno,H., Ahahara,H., Hawegawa,T. and Shimizu,M. (1991) Identity determinants of E. coli tRNAVal. Biochem. Biophys. Res. Commun., 177, 619–623. [DOI] [PubMed] [Google Scholar]
- 21.Derrick W., Feiz,V., Chu,W.-C. and Horowitz,J. (1991) Specific interaction between E. coli valine tRNA and its cognate synthetase. FASEB J., 5, A808. [Google Scholar]
- 22.Liu M., Chu,W.-C., Liu,J.C.-H. and Horowitz,J. (1997) Role of acceptor stem conformation in tRNAVal recognition by its cognate synthetase. Nucleic Acids Res., 25, 4883–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horowitz J., Chu,W.-C., Derrick,W.B., Liu J.C.-H., Liu,M. and Yue,D. (1999) Synthetase recognition determinants of E. coli valine transfer RNA. Biochemsitry, 38, 7737–7746. [DOI] [PubMed] [Google Scholar]
- 24.Best A.N. and Novelli,G.D. (1971) Studies with tRNA adenylyl(cytidylyl)transferase from Escherichia coli B. II. Regulation of AMP and CMP incorporation into tRNApCpC and tRNApC. Arch. Biochem. Biophys., 142, 539–547. [DOI] [PubMed] [Google Scholar]
- 25.Tal J., Deutscher,M.P. and Littauer,U.Z. (1972) Biological activity of Escherichia coli tRNAPhe modified in its C-C-A terminus. Eur. J. Biochem., 28, 478–491. [DOI] [PubMed] [Google Scholar]
- 26.Rether B., Gangloff,J. and Ebel,J.-P. (1974) Studies on tRNA nucleotidyltransferase from baker’s yeast. Replacement of the terminal CCA sequence in yeast tRNA Phe by several unusual sequences. Eur. J. Biochem., 50, 289–295. [DOI] [PubMed] [Google Scholar]
- 27.Von der Haar F. and Gaertner,E. (1975) Phenylalanyl-tRNA synthetase from baker’s yeast: role of 3′-terminal adenosine of tRNAPhe in enzyme–substrate interaction studied with 3′-modified tRNAPhe species. Proc. Natl Acad. Sci. USA, 72, 1378–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprinzl M. and Cramer,F. (1979) The –C-C-A end of tRNA and its role in protein biosynthesis. Prog. Nucleic Acid Res. Mol. Biol., 22, 1–69. [DOI] [PubMed] [Google Scholar]
- 29.Liu M. and Horowitz,J. (1994) Functional transfer RNAs with modifications in the 3′-CCA end: differential effects on aminoacylation and polypeptide synthesis. Proc. Natl Acad. Sci. USA, 91, 10389–10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K., Nobukazu,N., Tsunemi,H., Shimizu,M. and Himeno,H. (1994) Role of the CCA terminal sequence of tRNAVal in aminoacylation with valyl-tRNA synthetase. J. Biol. Chem., 269, 22173–22177. [PubMed] [Google Scholar]
- 31.Hale S.P., Auld,D.S., Schimdt,E. and Schimmel,P. (1997) Discrete determinants in transfer RNA for editing and aminoacylation. Science, 276, 1250–1252. [DOI] [PubMed] [Google Scholar]
- 32.Zawadzki V. and Gross,H.J. (1991) Rapid and simple purification of T7 RNA polymerase. Nucleic Acids Res., 19, 1948–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu W.-C. and Horowitz,J. (1991) Recognition of Escherichia coli valine transfer RNA by its cognate synthetase: a fluorine-19 NMR study. Biochemistry, 30, 1655–1663. [DOI] [PubMed] [Google Scholar]
- 34.Chu W.-C. and Horowitz,J. (1989) 19F NMR of 5-fluorouracil-substituted transfer RNA transcribed in vitro: resonance assignment of fluorouracil-guanine base pairs. Nucleic Acids Res., 17, 7241–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M. and Horowitz,J. (1993) In vitro transcription of transfer RNAs with 3′-end modifications. Biotechniques, 15, 264–266. [PubMed] [Google Scholar]
- 36.Kintanar A., Yue,D. and Horowitz,J. (1994) Effect of nucleoside modifications on the structure and thermal stability of Escherichia coli valine tRNA. Biochimie, 76, 1192–1204. [DOI] [PubMed] [Google Scholar]
- 37.Hale S.P. and Schimmel,P. (1996) Protein synthesis editing by a DNA aptamer. Proc. Natl Acad. Sci. USA, 93, 2755–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bishop A.C., Nomanbhoy,T.K. and Schimmel,P. (2002) Blocking site-to-site translocation of a misactivated amino acid by mutation of a class I tRNA synthetase. Proc. Natl Acad. Sci. USA, 99, 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]