Abstract
Purpose
To describe a rare case of presumed bilateral acute idiopathic maculopathy (AIM) in a pediatric patient.
Observation
An 11-year-old male was evaluated for a “fuzzy Dorito-shaped” spot in the central vision of his right eye (OD) that started 3 days before presenting to our clinic. On examination, best-corrected visual acuity (BCVA) was counting fingers at 5 feet OD, and 20/25 in the left eye (OS). Fundus examination demonstrated a central macular lesion with pigmentary changes OD and mild retinal pigment epithelium (RPE) changes OS. Optical coherence tomography (OCT) imaging showed elevated, irregular RPE with overlying subretinal fluid in the fovea OD and trace ellipsoid zone changes temporal to the fovea OS. Uveitis work-up was unremarkable for any infectious or inflammatory etiologies. Given the severe vision loss in the right eye and the negative infectious work-up, the decision was made to initiate oral corticosteroids in agreement with the patient's family. One week following the initial presentation, the patient developed visual symptoms OS without much improvement OD. The patient was admitted to the hospital, and further work-up revealed elevated coxsackie B3 antibody titers (1:20). Given the characteristic findings on multimodal imaging and elevated coxsackie B3 antibodies, the patient was presumptively diagnosed with bilateral AIM. Over the following 4 months, he would intermittently complain of nonspecific visual complaints such as blurry vision and floaters requiring an increase in corticosteroid dose and addition of immunomodulatory therapy, although the clinical examination and OCT findings remained unchanged. At the patient's follow-up visit 8 months after the initial visit, BCVA was stable at 20/40 OD and 20/30 OS, and OCT demonstrated stable foveal lesions in both eyes.
Conclusion
This case report describes a rare presentation of presumed bilateral AIM in a pediatric patient. This prompts further consideration of this condition in pediatric patients, especially after a prodromal flu-like illness.
Keywords: Acute idiopathic maculopathy, Coxsackie virus, Pediatrics, Uveitis
1. Introduction
Acute idiopathic maculopathy (AIM) is a rare inflammatory disorder first described in 1991 by Yannuzzi et al. in a series of 9 patients.1 The disease typically presents with sudden severe unilateral vision loss (20/200 or worse) in otherwise healthy young adults, and affects both sexes with equal frequency.1,2 A prodromal flulike illness often precedes the visual symptoms, and multiple studies reported association with different infectious etiologies including coxsackievirus,3 group A streptococcus,4 yellow fever,5 and coronavirus disease-19 (COVID-19).6 Different vaccines such as influenza A (H1N1), and COVID-19, have also been reported to be associated with the disease.7, 8, 9 The disease is characterized by an irregular neurosensory retinal detachment overlying a smaller, grayish thickening at the level of the retinal pigment epithelium (RPE).1,2 The visual prognosis with this condition is often favorable with most patients recovering to near-complete restoration of visual acuity with some residual RPE atrophic changes corresponding to the area of previous detachment.2 Although it was initially thought to only present unilaterally, many cases of bilateral disease have been described in the literature.2,10, 11, 12, 13, 14, 15, 16 Herein, we describe a rare presentation of presumed bilateral AIM in a pediatric patient.
2. Case report
An 11-year-old male was evaluated for a “fuzzy Dorito-shaped” (Doritos is an American brand of flavored tortilla chips with a triangular shape) spot in the central vision of the right eye (OD) that started 3 days prior. His mother also stated that the patient had started complaining of intermittently seeing new shapes in his left eye (OS) since the previous evening. The patient denied any systemic symptoms associated with the vision changes, though he did have a mild cough 2–3 weeks prior. Past medical history includes attention-deficit/hyperactivity disorder, which was diagnosed 3 years prior and has been managed with methylphenidate. There is no pertinent surgical history or family history of systemic or ocular disease.
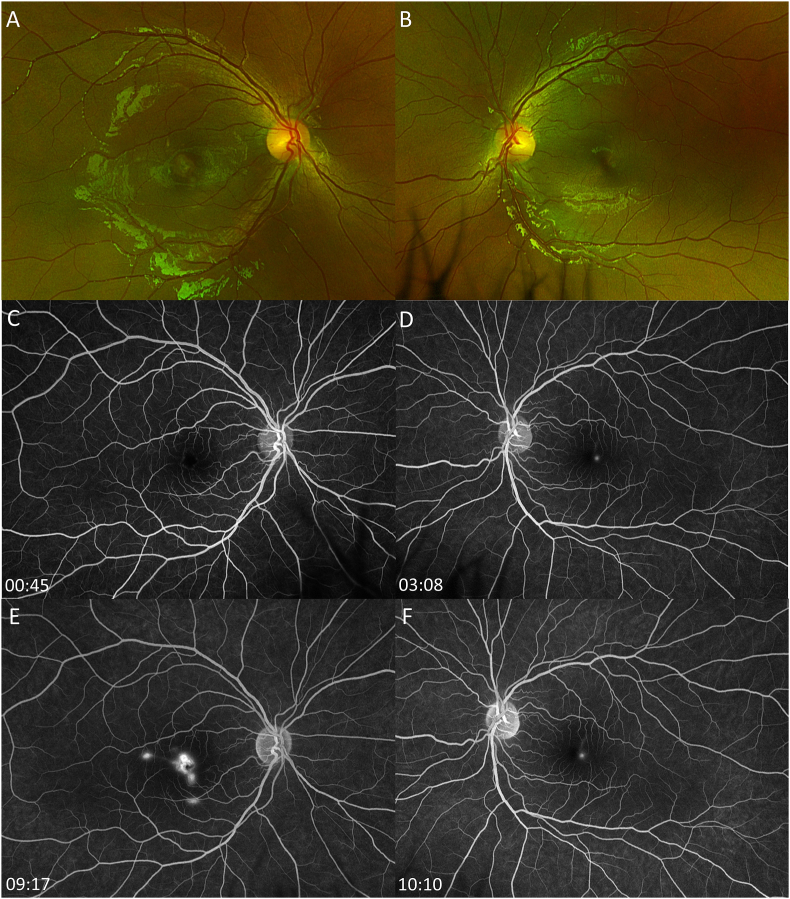
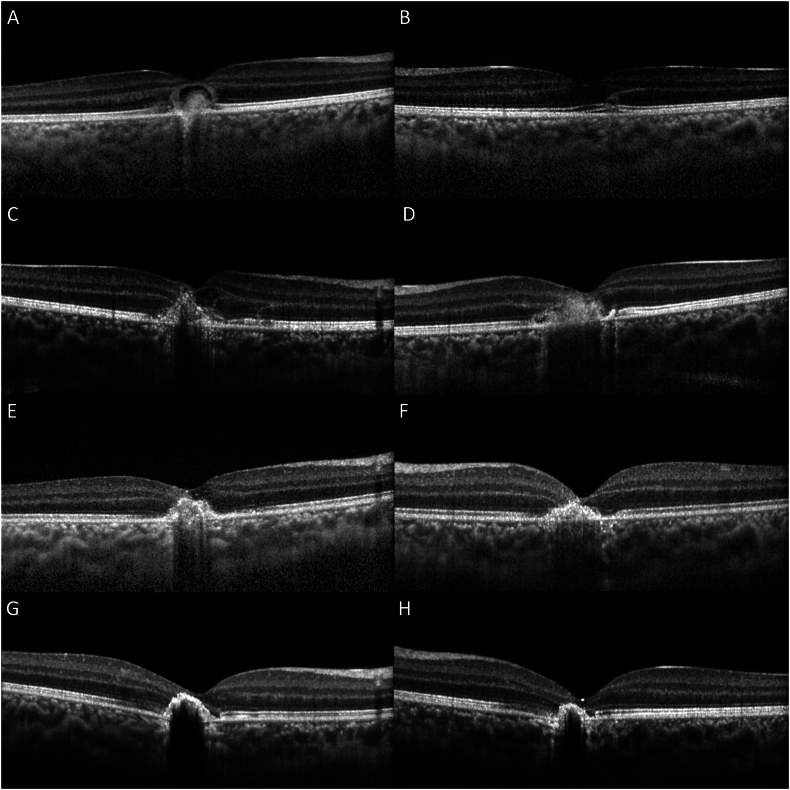
On examination, best-corrected visual acuity (BCVA) was counting fingers at 5 feet OD, and 20/25 OS. Intraocular pressure was 11 mmHg OD, and 13 mmHg OS, and pupils, confrontational visual fields, and anterior segment exam were unremarkable bilaterally. Slit-lamp examination revealed rare vitreous cell OD, and fundus examination demonstrated a central macular lesion with pigmentary changes OD (Fig. 1A), and mild RPE changes OS (Fig. 1B). Fluorescein angiography (FA) demonstrated early hyperfluorescence surrounding the fovea in a ring-like pattern with a hypofluorescent center (Fig. 1C) and late staining OD (Fig. 1E), and temporal foveal pinpoint hyperfluorescence in mid and late phase OS (Fig. 1D, F). Optical coherence tomography (OCT) imaging showed elevated, irregular RPE with overlying subretinal fluid (SRF) in the fovea OD (Fig. 2A), and trace ellipsoid zone changes temporal to the fovea OS (Fig. 2B). Indocyanine green (ICG) angiography showed central hypofluorescence OD, and late temporal hyperfluorescence OS. OCT-angiography showed no evidence of choroidal neovascularization in either eye.
Fig. 1.
Ultra-widefield fundus photos demonstrate a central macular lesion with pigmentary changes in the right eye (OD) (1A), and mild retinal pigment epithelium (RPE) changes in the left eye (OS) (1B). Fluorescein angiography shows early hyperfluorescence surrounding the fovea in a ring-like pattern with a hypofluorescent center (1C) and late staining OD (1E), and temporal foveal pinpoint hyperfluorescence in mid and late phase OS (1D, F).
Fig. 2.
Optical coherence tomography (OCT) shows elevated, irregular RPE with overlying subretinal fluid (SRF) in the fovea OD (2A), and trace ellipsoid zone changes temporal to the fovea OS (2B) at the initial presentation. OCT 1 week after the initial presentation demonstrates resolution of SRF OD (2C) with additional subfoveal hyperreflective material OS (2D). Stable subfoveal hyperreflective materials are present at 17 days (2E, F) and 6 months (2G,H) after the initial visit.
Uveitis laboratory work-up including complete blood count, complete metabolic panel, Treponema pallidum immunoglobulin (Ig) G, Quantiferon-Tuberculosis Gold, toxoplasmosis IgG and IgM, angiotensin-converting enzyme, lysozyme, erythrocyte sedimentation rate (ESR), and anti-nuclear antibody was unremarkable except an elevated ESR of 24 mm/hr (normal range: 0–15mm/hr). Given the severity of vision loss in the right eye and the negative infectious work-up, the decision was made to initiate oral corticosteroids 40mg/daily with tapering down by 10mg every 2 weeks in agreement with the patient's family. At this time, the differential diagnosis consisted of handheld laser-induced maculopathy, acute retinal pigment epitheliitis (ARPE), acute macular neuroretinopathy (AMN), and AIM.
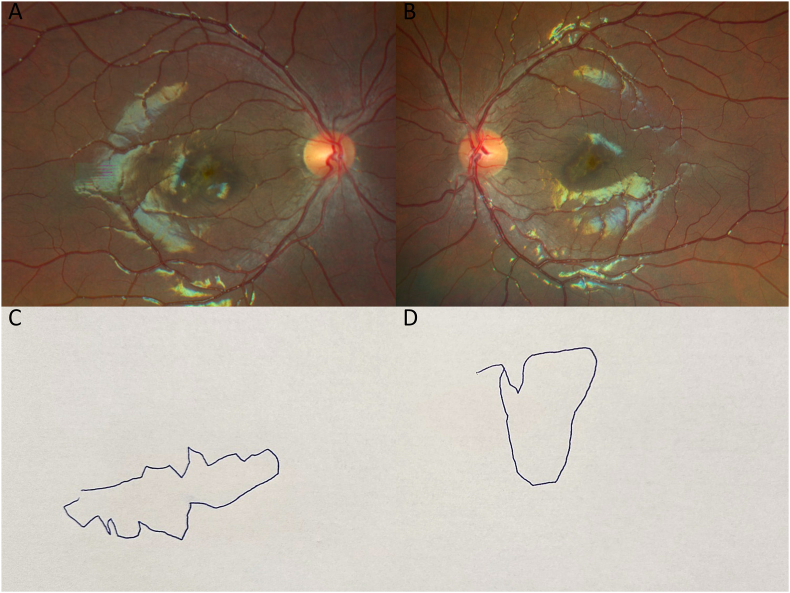
One week following the initial presentation, BCVA worsened to count fingers at 5 feet OS, and the patient endorsed seeing another “fuzzy Dorito” in this eye. Fundus examination remained unchanged OD (Fig. 3A) but revealed an irregular pigmentary lesion OS (Fig. 3B). The patient was able to draw out the specific shapes of his scotomas (Fig. 3C, D). Repeat OCT demonstrated additional subfoveal hyperreflective material in both eyes (OU) (Fig. 2C, D). The patient was admitted to the hospital for magnetic resonance imaging (MRI) of the brain and orbits with and without contrast, and intravenous steroids. Additional laboratory testing was completed including coxsackie antibodies. Coxsackie antibody titers greater than 1:10 may indicate current or past infection, and our patient's coxsackie B3 antibody titers were measured at 1:20. Given the respiratory symptoms 2–3 weeks prior to ocular symptoms, the presence of these antibodies likely indicated recent coxsackie B3 infection. Titers for coxsackie B1 (<1:10), B2 (<1:10), B4 (<1:10), B5 (<1:10), B6 (<1:10) and A9 (<1:8) antibodies were all negative. No pathology was identified on MRI. The patient's vision subjectively improved with corticosteroids, and he endorsed shrinking size of his “Doritos”. Subsequently, he was discharged from the hospital on high-dose oral prednisone. At close outpatient follow-up 3 days after hospital discharge, his BCVA was 20/80 OD and 20/60 OS, and there was a reduction of size and elevation of the patient's abnormal foveal lesions on OCT (Fig. 2E, F). Given the characteristic findings on multimodal imaging and clinical assessment, the patient was presumptively diagnosed with AIM. The patient's recent history of upper respiratory symptoms and elevated coxsackie B3 titers are supportive of this presumed diagnosis given the known association between prodromal viral illness and AIM. The patient was closely monitored for any further clinical symptoms.
Fig. 3.
One week following the initial presentation, fundus appearance remains unchanged OD (3A) but an irregular pigmentary lesion OS (3B) appears. Patient's drawings of the specific shapes of his scotomas OD (3C) and OS (3D) are shown.
Over the following 4 months, he would intermittently complain of nonspecific visual complaints such as blurry vision and floaters although the clinical examination and OCT remained unchanged. His corticosteroids were increased twice due to visual complaints. Subsequently, corticosteroids were tapered gradually while methotrexate was added in conjunction with a pediatric rheumatologist. However, the patient developed hives and itching in reaction to methotrexate, and he was switched to adalimumab. In the most recent follow-up visit 8 months after the initial visit, BCVA was stable at 20/40 OD and 20/30 OS, and OCT demonstrated stable foveal lesions OU (Fig. 2G, H). The patient continues to be followed routinely for further visual changes while on adalimumab. We plan to discontinue adalimumab between 18 and 24 months if the pain remains stable with the therapy.
3. Discussion
In this case report, we have described a unique case of presumed bilateral AIM in a pediatric patient who required long-term treatment with immunosuppressive therapy for the management of the disease. AIM traditionally presents with unilateral painless central vision loss (20/200 or worse) in otherwise healthy young adults. In addition to vision loss, patients may complain of central scotomas and metamorphopsias. In the initial report by Yannuzzi et al., patients’ age at presentation ranged from 15 to 45 years old with an average age of 32.1 At the time of writing, only 4 pediatric cases of AIM have been reported in the literature, all of which were unilateral.17,18 Three of these patients were found incidentally on routine eye examination without any visual complaints,18 and one patient presented with a visual complaint in their left eye.17 These patients did not require any therapy and recovered spontaneously without any long-term visual implications.17,18 In contrast, our patient presented with bilateral severe vision loss that required long-term treatment with immunosuppression. Therefore, a diagnosis of AIM should be considered in the differential diagnosis in pediatric patients with acute vision loss, especially after a prodromal flu-like illness.
Our patient's differential diagnosis consisted of laser-induced maculopathy, ARPE, AMN, and AIM. Given the patient's unremarkable ocular history, iatrogenic causes such as laser-induced maculopathy could be eliminated. While ARPE has also been associated with prior viral infections, ARPE often demonstrated a hypo-reflective center and hyper-reflective border at the level of ellipsoid zone as seen on OCT, and the majority of ARPE cases resolve spontaneously without residual subretinal deposits.19 In contrast to our case, the subretinal deposits persisted after the acute stage of the disease. Similarly, AMN could be the likely diagnosis given the presence of angular sign of Henle fiber layer hyperreflectivity (ASHH) lesions.20 However, the presence of subretinal fluid and hyperreflective deposits was more consistent with the presumed diagnosis of AIM. Additionally, other causes of endogenous uveitis were also excluded by laboratory results and the lack of supporting clinical features. Thus, our patient was presumptively diagnosed with bilateral AIM.
Although the pathogenesis of AIM is a subject of ongoing investigation, the prodromal flu-like symptoms indicate that an infectious etiology could be the trigger of immune activation in the pathogenesis of the disease. Many cases of AIM have been reported in association with a preceding episode of hand, foot, and mouth disease (HFMD), and a strong connection has been made with coxsackievirus infection as demonstrated by positive virus titers during the acute onset of visual symptoms.3,21, 22, 23, 24 More recently, several cases of AIM have been reported with other infectious etiologies including group A streptococcus,4 yellow fever,5 and COVID-19.6 These findings suggest that infectious antigens or molecular mimicry could play a key role in driving the immune response in these patients. Furthermore, coxsackievirus has been shown to directly infect RPE in vitro suggesting that direct hematogenous spread to RPE could potentially contribute to the development of the disease.25 However, further studies are needed to better understand the pathogenesis of the disease.
AIM is clinically diagnosed, but certain characteristics on multimodal imaging can aid clinicians with the diagnosis. Fundus examination typically reveals a solitary central macular lesion with irregular areas of white-grey discoloration, which can be circular, triangular, or wedge-shaped.1,22,26 These lesions correspond to areas of underlying exudative neurosensory retinal detachment. Intraretinal hemorrhages, mild papillitis, and inflammatory cells in the posterior vitreous have also been described along with the macular findings.1,2 Following recovery, the macula develops a residual “bulls-eye” appearance due to RPE hyperpigmentation surrounded by hypopigmented atrophic changes.1,2,22,26 On OCT, the most consistent findings are subfoveal neurosensory detachment, thickening of the RPE with hyper-reflective outer retinal material, and photoreceptor layer disruption.22,26 FA demonstrates early hyper- and hypofluorescence at the level of the RPE, as well as late-stage hyperfluorescence corresponding to combined subretinal staining and pooling in the overlying detachment.22,26 On ICG angiography, lesions appear as hypofluorescent areas that are more obvious on late phase imaging, and the choroidal vasculature may show a “moth-eaten” appearance.22,26 Fundus autofluorescence shows a mixed pattern of hypo- and hyperautofluorescence.22
Typically, the long-term visual prognosis for these patients is favorable, with spontaneous complete or near-complete recovery of vision to baseline over a period of weeks to months. The severe nature of our patient's visual symptoms, bilateral involvement, and relatively prolonged disease course underscores the uniqueness of this case. Symptom improvement following administration of intravenous administration corticosteroids and high-dose oral prednisone suggests a potential role for anti-inflammatory therapy in the treatment of AIM than previously thought. Interestingly, the recurrence of visual symptoms and the chronicity of the disease favored long-term treatment with immunomodulatory therapy. With AIM, visual recovery corresponds to the anatomic improvement as seen on OCT scans; however, structural changes may lag behind vision recovery.22,26 There are some reports of steroid usage accelerating the rate of visual symptom resolution by limiting tissue damage,1,27 although this is controversial as some believe that corticosteroids may worsen the viral-mediated process in these patients. Given the bilateral significant visual loss in our patient, the decision was made to initiate oral corticosteroids and subsequent, corticosteroid-sparing immunosuppression after a careful discussion with the patient's family.
4. Conclusion
Although AIM often presents with unilateral disease in otherwise healthy young adults, we have described a rare case of presumed bilateral AIM in a pediatric patient. A diagnosis of AIM can be presumptively considered in pediatric patients presenting with acute vision loss and characteristic imaging findings, especially after a prodromal flu-like illness.
CRediT authorship contribution statement
Marc Ohlhausen: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Nam V. Nguyen: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Conceptualization. Timothy Kaftan: Writing – review & editing, Formal analysis. Helen Song: Writing – review & editing, Investigation. Sean Kim: Writing – review & editing, Investigation. Daniel Reiff: Writing – review & editing, Investigation. Steven Yeh: Writing – review & editing, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Statement of informed consent
The written informed consent for patient information was not sought for the case report because all patient identifier information was not reported.
Ethical approval
This case report was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)-compliant manner.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Funding
This project was supported by the National Eye Institute of the National Institutes of Health under award number R01 EY029594 (SY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Funding support is also provided by the Macula Society Retina Research Foundation Cox Family Grant, Association for Research in Vision and Ophthalmology Mallinckrodt Foundation Young Investigator Award, and the Stanley M. Truhlsen Family Foundation, Inc.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Steven Yeh reports financial support was provided by National Eye Institute. Steven Yeh reports financial support was provided by Macula Society Retina Research Foundation Cox Family Grant. Steven Yeh reports financial support was provided by Association for Research in Vision and Ophthalmology Mallinckrodt Foundation Young Investigator Award. Steven Yeh reports financial support was provided by Stanley M. Truhlsen Family Foundation, Inc. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Yannuzzi L.A., Jampol L.M., Rabb M.F., Sorenson J.A., Beyrer C., Wilcox L.M., Jr. Unilateral acute idiopathic maculopathy. Arch Ophthalmol. Oct 1991;109(10):1411–1416. doi: 10.1001/archopht.1991.01080100091049. [DOI] [PubMed] [Google Scholar]
- 2.Freund K.B., Yannuzzi L.A., Barile G.R., Spaide R.F., Milewski S.A., Guyer D.R. The expanding clinical spectrum of unilateral acute idiopathic maculopathy. Arch Ophthalmol. May 1996;114(5):555–559. doi: 10.1001/archopht.1996.01100130547007. [DOI] [PubMed] [Google Scholar]
- 3.Hughes E.H., Hunyor A.P., Gorbatov M., Ho I.V. Acute idiopathic maculopathy with coxsackievirus infection. Retin Cases Brief Rep. Winter. 2012;6(1):19–21. doi: 10.1097/ICB.0b013e3181f7f7ee. [DOI] [PubMed] [Google Scholar]
- 4.Shute C.L., Chakravarthy U., McAvoy C.E. Unilateral acute idiopathic maculopathy associated with streptococcal pharyngitis, A case report. Ocul Immunol Inflamm. Aug 2022;30(6):1511–1514. doi: 10.1080/09273948.2021.1880603. [DOI] [PubMed] [Google Scholar]
- 5.Dompieri R.C., Manzano R.P.A., Frazao M.A.M., Kurimori H.Y., Chao J.C.T., Lui A.C.F. Unilateral acute idiopathic maculopathy secondary to yellow fever disease: a case report. Am J Ophthalmol Case Rep. Sep 2019;15 doi: 10.1016/j.ajoc.2019.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesh R., Reddy N.G., Mishra P., Gupta A., Mahendradas P., Yadav N.K. Unilateral acute idiopathic maculopathy following severe acute respiratory syndrome corona virus (SARS-CoV-2) infection. Ocul Immunol Inflamm. Feb 2023;31(2):445–448. doi: 10.1080/09273948.2022.2028287. [DOI] [PubMed] [Google Scholar]
- 7.Jorge L.F., Queiroz R.P., Gasparin F., Vasconcelos-Santos D.V. Presumed unilateral acute idiopathic maculopathy following H1N1 vaccination. Ocul Immunol Inflamm. Aug 18 2021;29(6):1151–1153. doi: 10.1080/09273948.2020.1734213. [DOI] [PubMed] [Google Scholar]
- 8.Bleicher I.D., Brill D., Wu F., Sobrin L., Patel N. Acute idiopathic maculopathy following SARS-CoV-2 vaccination. Ocul Immunol Inflamm. Aug 2023;31(6):1232–1235. doi: 10.1080/09273948.2022.2114915. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa T., Sannomiya Y., Toyoda M., Maruko I., Iida T. Acute idiopathic maculopathy after COVID-19 vaccination. Am J Ophthalmol Case Rep. Jun 2022;26 doi: 10.1016/j.ajoc.2022.101479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Energy Drinks . 2012. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. [PubMed] [Google Scholar]
- 11.Fernandes J.S., Gomes P.P., Neves P., Marques J.P. Idiopathic acute exudative polymorphous vitelliform maculopathy: the importance of multimodal imaging, systemic workup and genetic testing. BMJ Case Rep. Jun 28 2023;16(6) doi: 10.1136/bcr-2022-253969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghazi N.G., Daccache A., Conway B.P. Acute idiopathic maculopathy: report of a bilateral case manifesting a macular hole. Ophthalmology. May 2007;114(5) doi: 10.1016/j.ophtha.2006.08.055. e1-6. [DOI] [PubMed] [Google Scholar]
- 13.Khundkar T., Hasan S.R., Breazzano M.P., Mei C., Johnson B.B. Choroidal vascular changes in acute idiopathic maculopathy as demonstrated by multimodal imaging including optical coherence tomography angiography. Case Rep Ophthalmol Med. 2021;2021 doi: 10.1155/2021/6680020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakazawa T., Yamaguchi K., Shimura M., Yoshida M., Yoshioka Y., Tamai M. Clinical features of bilateral acute idiopathic maculopathy. Jpn J Ophthalmol. Jul-Aug 2003;47(4):385–391. doi: 10.1016/s0021-5155(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Costa S., Penas S., Carneiro A., et al. Idiopathic acute exudative polymorphous vitelliform maculopathy: insight into imaging features and outcomes. Case Rep Ophthalmol Med. 2020;2020 doi: 10.1155/2020/7254038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaz-Pereira S., Macedo M., De Salvo G., Pal B. Multimodal imaging of exudative maculopathy associated with hand-foot-mouth disease. Ophthalmic Surg Lasers Imaging Retina. Apr 4 2014;45 doi: 10.3928/23258160-20140331-01. Online:e14-7. [DOI] [PubMed] [Google Scholar]
- 17.Ava S., Hazar L., Karahan M., Erdem S., Dursun M.E. Multimodal imaging of unilateral acute idiopathic maculopathy in a child patient. Retin Cases Brief Rep. May 1 2023;17(3):285–287. doi: 10.1097/ICB.0000000000001189. [DOI] [PubMed] [Google Scholar]
- 18.Georgiou M., McAnena L., Michaelides M., Reddy M.A. Incidental unilateral idiopathic maculopathy in children. J AAPOS. Dec 2020;24(6) doi: 10.1016/j.jaapos.2020.08.009. 357 e1-357 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iu L.P.L., Lee R., Fan M.C.Y., Lam W.C., Chang R.T., Wong I.Y.H. Serial spectral-domain optical coherence tomography findings in acute retinal pigment epitheliitis and the correlation to visual acuity. Ophthalmology. Jun 2017;124(6):903–909. doi: 10.1016/j.ophtha.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Bhavsar K.V., Lin S., Rahimy E., et al. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. Sep-Oct 2016;61(5):538–565. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Beck A.P., Jampol L.M., Glaser D.A., Pollack J.S. Is coxsackievirus the cause of unilateral acute idiopathic maculopathy? Arch Ophthalmol. Jan 2004;122(1):121–123. doi: 10.1001/archopht.122.1.121. [DOI] [PubMed] [Google Scholar]
- 22.Jung C.S., Payne J.F., Bergstrom C.S., et al. Multimodality diagnostic imaging in unilateral acute idiopathic maculopathy. Arch Ophthalmol. Jan 2012;130(1):50–56. doi: 10.1001/archophthalmol.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyerle C.B., Yannuzzi L.A. Acute positive titers of antibody to coxsackievirus in acute idiopathic maculopathy. Retin Cases Brief Rep. Winter. 2008;2(1):34–35. doi: 10.1097/01.iae.0000243065.91330.e0. [DOI] [PubMed] [Google Scholar]
- 24.Yen C.Y., Fang I.M. Unilateral acute idiopathic maculopathy related to hand-foot-mouth disease: case report and literature review. Taiwan J Ophthalmol. Jan-Mar 2024;14(1):133–136. doi: 10.4103/tjo.TJO-D-22-00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huemer H.P., Larcher C., Kirchebner W., Klingenschmid J., Gottinger W., Irschick E.U. Susceptibility of human retinal pigment epithelial cells to different viruses. Graefes Arch Clin Exp Ophthalmol. Mar 1996;234(3):177–185. doi: 10.1007/BF00462030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoang Q.V., Strauss D.S., Pappas A., Freund K.B. Imaging in the diagnosis and management of acute idiopathic maculopathy. Int Ophthalmol Clin. Fall. 2012;52(4):263–268. doi: 10.1097/IIO.0b013e31826861db. [DOI] [PubMed] [Google Scholar]
- 27.de la Fuente M.A., Cuadrado R. Unilateral acute idiopathic maculopathy: angiography, optical coherence tomography and microperimetry findings. J Ophthalmic Inflamm Infect. Sep 2011;1(3):125–127. doi: 10.1007/s12348-010-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]