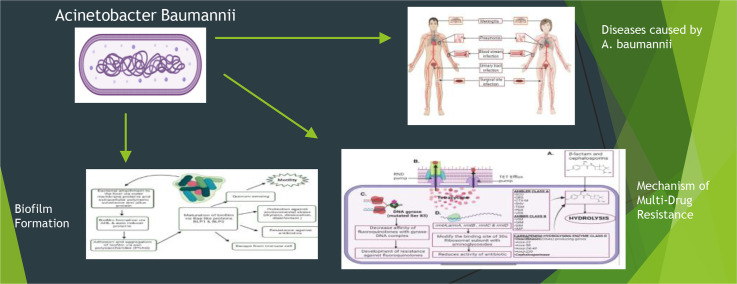
Highlights
-
•
A. baumannii, a challenging nosocomial bacterial pathogen is the main focus here.
-
•
The study elaborates the virulence factors and diseases caused by the bacterium.
-
•
The study also reflects the mechanism of developing resistance against antibiotics.
-
•
This review gives emphasis on the potential therapies against A. baumannii.
Keywords: Acinetobacter baumannii, Hospital acquired pneumonia, Pathogenesis, Urinary tract infection, Multidrug resistant organism
Abstract
The overuse of antibiotics has led to the global dissemination of Acinetobacter baumannii, an increasingly challenging nosocomial pathogen. This review explores the medical significance along with the diverse resistance ability of A. baumannii. Intensive care units (ICUs) serve as a breeding ground for A. baumannii, as these settings harbour vulnerable patients and facilitate the spread of opportunistic microorganisms. A. baumannii belongs to the ESKAPE group of bacterial pathogens that are major contributors to antibiotic-resistant infections. The pathogenic nature of A. baumannii is particularly evident in seriously ill patients, causing pneumonia, wound infections, and other healthcare-associated infections. Historically considered benign, A. baumannii is a global threat due to its propensity for rapid acquisition of multidrug resistance phenotypes. The genus Acinetobacter was formally recognized in 1968 following a comprehensive survey by Baumann et al., highlighting the relationship between previously identified species and consolidating them under the name Acinetobacter. A. baumannii is characterized by its Gram-negative nature, dependence on oxygen, positive catalase activity, lack of oxidase activity, inability to ferment sugars, and non-motility. The DNA G+C content of Acinetobacter species falls within a specific range. For diagnostic purposes, A. baumannii can be cultured on specific agar media, producing distinct colonies. The genus Acinetobacter comprises numerous species those are associated with bloodstream infections with high mortality rates. Therefore, A. baumannii poses a significant challenge to global healthcare due to its multidrug resistance and ability to cause various infections. A comprehensive understanding of the mechanisms underlying its resistance acquisition and pathogenicity is essential for combating this healthcare-associated pathogen effectively.
Graphical abstract
1. Introduction
The excessive and widespread application of antibiotics has caused the global dissemination of Acinetobacter baumannii, a challenging nosocomial pathogen. Its medical significance is primarily attributed to its significant ability to obtain a multitude of resistance determinants. Therefore, it is considered as the formidable multidrug-resistant (MDR) organisms. This poses a significant threat to modern antibiotic therapies, as A. baumannii continues to evolve and jeopardize effective treatment options (Tacconelli, 2017). Hospital intensive care units (ICUs) are known to accommodate critically ill patients who are highly susceptible to infections. These units serve as a conducive environment for the proliferation of opportunistic microorganisms such as A. baumannii, which typically pose no threat to healthy individuals but have developed significant resistance to antibiotics. Consequently, these bacteria can rapidly spread among patients within the ICU, leading to potential epidemics (Dijkshoorn et al., 2007).
A.baumannii, an opportunistic human pathogen, belongs to ESKAPE (Klebsiella pneumoniae, Enterococcus faecium, Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) group of microorganisms (Boucher et al., 2009). A Gram-negative opportunistic nosocomial pathogen leads to the charge for causing pneumonia, along with infections of burns sites and wounds (Towner, 1997). Once noticed to be benign, A. baumannii is now taken into consideration as an international risk within the healthcare setting, specifically because of its propensity to accumulate multidrug and substantial drug resistance phenotypes at previously unexpected rates (Vázquez-López et al., 2020). The organism usually targets patients who have experienced a prolonged hospitalization.
The first records about the genus Acinetobacter were reported in the early twentieth century by Beijerinck (1911). He isolated the bacteria from soil and grew it in the calcium acetate-enriched minimal medium. Later on, it was named Micrococcus calcoaceticus (Beijerinck, 1911). The contemporary definition of the Acinetobacter genus encompasses coccobacilli with a short and variable shape. These bacteria are characterized by being Gram-negative, requiring oxygen for growth, possessing the enzyme catalase while lacking oxidase activity, unable to ferment sugars, and lacking motility.
The G+C content of the Acinetobacter genome falls within the range of 39 –47 % (Peleg et al., 2008). As of 2019, consistent with an evaluation posted through Vijaya kumar et al., fifty-nine species belonging to the genus Acinetobacter, wherein eleven have described names and fifteen are in further discussion to be included as. A. baumannii belongs to the Acinetobacter calcoaceticus-baumannii complex, which includes other related bacteria such as A. pittii, A. nosocomialis, and A. calcoaceticus. Moreover, these are found to be catalase-positive, oxidase-negative, and non-fermenting. They can be both non- pigmented, and pigmented. For diagnostic purposes, Acinetobacter is grown in sheep blood agar and tryptic soy agar, producing greyish-white, smooth, mucoid colonies at 37 °C (Peleg et al., 2008). Bouvet and Grimont classified acinetobacters on the basis of DNA–DNA hybridization studies and divided them into twelve DNA groups/genospecies. These are A. baumannii, A. calcoaceticus, A. johnsonii, A. junii, A. haemolyticus, and A. lwoffii (Beijerinck, 1911; Wisplinghoff et al., 2012). A. baumannii is often responsible for bloodstream infections, particularly those associated with intravascular devices. These infections have high mortality rates ranging from 30 % to 52 %. Other manifestations of A. baumannii include burn infections, osteomyelitis, skin and soft tissue infections (including necrotizing fasciitis), meningitis, and endocarditis (Peleg et al., 2008). The genus designation "Acinetobacter" was coined by Brisou and Pre´vot in 1954. It distinguished non-motile microorganisms and the motile ones of the genus Achromobacter (Brisou, 1954). The acceptance of the genus Acinetobacter was officially established in the year 1968 by Baumann et al. (1968).
2. Study methodology
This review article was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Comprehensive literature search was performed using the following databases: PubMed, Scopus, Google Scholar, and NCBI. The search strategy included the following keywords: "Acinetobacter baumannii," "virulence factors," "antibiotic resistance," "MDR," "treatment," and "COVID-19."
The inclusion criteria for articles were:
-
•
Published in English
-
•
Peer-reviewed
-
•
Original research articles or review articles
-
•
Relevant to the topic of A. baumannii pathogenesis, antibiotic resistance, and treatment
The exclusion criteria were:
-
•
Case reports
-
•
Conference abstracts
-
•
Articles not published in English
The initial search yielded a large number of articles. After screening titles and abstracts, a subset of articles was selected for full-text review. The final selection of articles was based on their relevance to the review topic and their methodological quality.
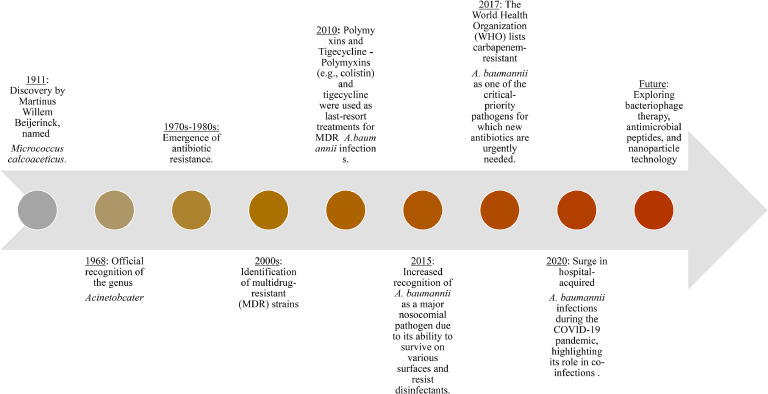
Data extracted from the selected articles included information on A. baumannii virulence factors, mechanisms of antibiotic resistance, current treatment options, and the impact of COVID-19 on A. baumannii infections. This information was synthesized and analysed to provide a comprehensive overview of the current state of knowledge on A. baumannii. This systematic approach ensured that the review was based on a thorough and unbiased assessment of the available literature. A time line on the emergence of A. baumannii is represented in Fig. 1.
Fig 1.
Schematic timeline showing the emergence of Acinetobacter Baumannii.
3. Genomic analysis of A. baumannii
Carbapenem-resistant MDR A. baumannii has been increasing its presence worldwide, and concerns are raised about the credibility of modern antibiotic treatments (Pogue et al., 2013). Methods like comparative genomics analysis evaluates pathogenicity, resistance mechanisms, and the evolution of bacterial pathogens (Fournier et al., 2006). This illustrates diversification of resistance genes, restriction-modification systems, and surface polysaccharides phylogenetically closed strains (Choi et al., 2017). A study by Choi et al. identified 38 isolates those were collected from a patient [16]. The data was summarised to delineate the whole genome sequence of BL1. Annotation and prediction of the open reading frame are done by Prokka (v1.12b) (Seemann, 2014), and the detection of the prophage is commenced by PHASTER (Arndt et al., 2019). The confirmation of resistance genes is done by the RUST server (Aziz et al., 2008) and the Resistance gene identifier (Jia et al., 2016). Another study was conducted on a strain of Acinetobacter baumannii, the strain, referred to as BL1, was analyzed using various techniques and tools to understand its genetic composition and characteristics. The strain's genome was sequenced, and the paired ends of the genome were found to be larger than three gigabytes (Choi & Chan, 2015).
The genomic characteristics of the BL1 strain were determined. It comprised 3.9 million approximate base pairs and contained around 3.8 × 103 coding sequences, 18 tRNA genes, and 73 rRNA genes. The analysis also identified eleven intact insertion sequences (ISs) and three intact prophages among a total of 45 ISs and 4 prophages found. The G+C content of the BL1 strain was approximately 39.1%. Furthermore, the presence of 27 antibiotic-resistance genes was identified, with blaOXA-23 and blaOXA-66 located in the chromosome. Among the 38 SNVs identified, 36 were functional mutations and 2 were synonymous mutations (not changing the amino acid sequence). Overall, this study provides a detailed characterization of the BL1 strain of A. baumannii, including its genetic composition and SNVs (Kim et al., 2018). From the study of Li et al. (2015) it was reported that strains of A. baumannii were subjected to whole-genome sequencing and comparative genomic analysis to investigate their genetic characteristics. Among the strains, 32 were carbapenem-resistant and 3 were carbapenem-susceptible. The analysis revealed the presence of ten types of AbrR resistance, which are associated with resistance-related proteins. Additionally, a previously unreported AbaR island containing a two-component response regulator and RND efflux system proteins was identified in two strains isolated from Zhejiang in 2004. The study also found the presence of multiple transposons or insertion sequences (ISs) in all the strains. These IS elements or transposons showed a tendency to diversify with evolution, and some of them were previously unreported. Most of these elements were predominantly found in strains from two specific provinces. Among the identified IS elements, the study observed the presence of ten types of AbaR resistance islands. Amongst these, type 6 AbaR showed the least amount of homology with the other groups while type 10 AbaR Island comprised the highest no. of resistance genes (Tada et al., 2014). Furthermore, the study identified a highly homogeneous core genome shared among all sequenced A. baumannii strains. This core genome encoded proteins involved in DNA replication, transcription, translation, and various metabolic pathways. Recombination and mutation were found to play a role in the evolution of these strains, as evidenced by the phylogenetic allocation of different alleles of blaADC and blaOXA-51.
4. Disease caused by A. baumannii
The diseases caused by A. baumannii is represented in Table 1.
Table 1.
Diseases caused by Acinetobacter baumannii.
| Disease name | Organ Affected | Symptoms | Treatment | References |
|---|---|---|---|---|
| Hospital-acquired pneumonia (HAP) | Lungs | Fever, cough, chest pain, difficulty breathing | Carbapenems and aminoglycosides, intensive care, mechanical ventilation | (Howard et al., 2012; Peleg et al., 2008a) |
| Bacteremia | Bloodstream | Fever, chills, rapid heartbeat, confusion | Carbapenems and aminoglycosides, mechanical ventilation, intensive care | (Wisplinghoff et al., 2012) |
| Urinary tract infection (UTI) | Urinary tract | Frequent urination, cloudy/strong-smelling urine, burning/pain during urination | Carbapenems and aminoglycosides | (Ayoub Moubareck & Hammoudi Halat, 2020; Peleg et al., 2008b) |
| Wound infection | Skin and soft tissue | Redness, warmth, swelling, discharge from wound | Carbapenems and aminoglycosides, surgery in severe cases | (Ayoub Moubareck & Hammoudi Halat, 2020; Peleg et al., 2008a) |
| Meningitis | Brain and spinal cord Membranes | Fever, neck stiffness, headache, sensitivity to light | Eravacycline, intensive care | (Dexter et al., 2015; Dijkshoorn et al., 2007; Howard et al., 2012) |
4.1. Hospital-acquired pneumonia (HAP)
Acinetobacter baumannii is a gram-negative bacterium that is the causative agent of numerous human infections. This bacterium is especially troublesome in healthcare settings because it can survive for a long time on abiotic surfaces and its potential as a multidrug resistance (Howard et al., 2012). A. baumannii can cause infections in diverse body parts, ranging from mild to severe. Pneumonia is one of the most typical manifestations of infections caused by A. baumannii (Howard et al., 2012). When bacteria get into the lungs, they cause inflammation and damage to the tissue in the lungs. Fever, cough, chest pain, and difficulty in breathing are all signs of pneumonia brought on by A. baumannii. The infection may result in sepsis and death in severe cases. As a gram-negative bacterium that can cause pneumonia and other infections in humans, among them, hospital-acquired pneumonia (HAP) (Fig. 2) can occur 48 hours or more after a patient is admitted to a hospital. It is a serious problem that can make hospital stays longer, increase mortality, and raise costs for medical care. HAP outbreaks in hospitals and long-term care facilities are also known to be brought on by A. baumannii. This could happen because the bacteria can easily spread from one person to another and survive for a long time on surfaces (Jawad et al., 1998). Patients, receiving mechanical ventilation, and those with underlying medical conditions like diabetes and chronic obstructive pulmonary disease (COPD) and who are admitted to the intensive care unit (ICU) are more likely to develop HAP caused by A. baumannii (Bianco et al., 2016). HAP caused by A. baumannii is also more likely to occur in immunocompromised, elderly, or antibiotic-treated patients, because the symptoms of HAP caused by A. baumannii are similar to those of other types of pneumonia, it can be difficult to diagnose this condition. Cultures of sputum, blood, bronchial secretions, or pleural fluid typically provide the diagnosis. The diagnosis of HAP can also be confirmed with a chest X-ray and CT scan (Dexter et al., 2015). HAP caused by A. baumannii can be difficult to treat because the bacteria are resistant to antibiotics. A regimen of carbapenems and aminoglycosides, two types of antibiotics, is the standard treatment for HAP brought on by A. baumannii. However, the resistance of the bacteria may result in treatment failure and prolonged therapy for patients. Patients may also require intensive care and mechanical ventilation in some instances (Dijkshoorn et al., 2007). Good hygiene practices, particularly in healthcare settings, such as frequent hand washing and proper surface cleaning, are essential for preventing HAP caused by A. baumannii (Ayoub Moubareck & Hammoudi Halat, 2020; Dijkshoorn et al., 2007; Peleg et al., 2008).
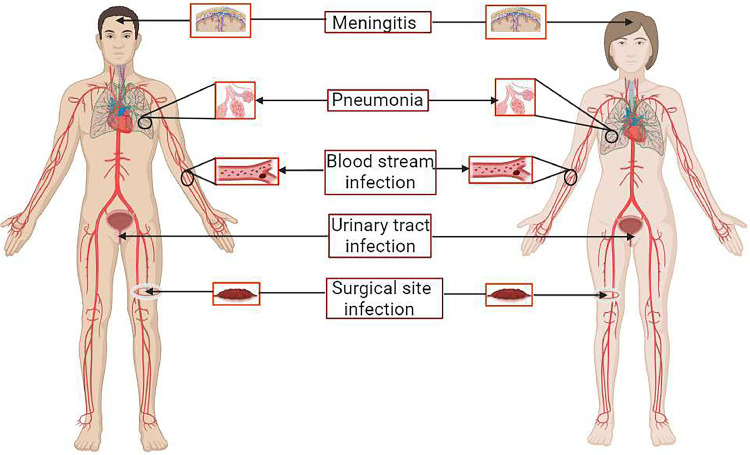
Fig 2.
Schematic diagram of different diseases caused by A. baumannii. Such as the bacterial infection of meninges known as meningitis. The most affected is nosocomial pathogenic pneumonia in patients admitted to hospitals such as HAP (Hospital Acquired Pneumonia) and VAP (Ventilator-associated pneumonia). Septicemia or the bloodstream infection, urinary tract infection, and surgical site infection for its MDR nature.
4.2. Bacteremia
Bacteremia, the condition when bacteria enter the bloodstream and circulate to other body parts, is another common symptom of A. baumannii infections. Sepsis is a potentially fatal condition characterized by a widespread infection that leads to organ failure and tissue damage in the body. Fever, chills, a rapid heartbeat, and confusion are all signs of sepsis (Table 3). Acinetobacter baumannii causes many infections in humans, including BSI (bloodstream infections). BSI is frequently brought on by A. baumannii, particularly in hospitalized patients or those with weakened immune systems (Wisplinghoff et al., 2012). The symptoms of BSI brought on by A. baumannii can differ depending on where the infection is located. In more severe cases, the infection may progress to sepsis, a potentially fatal condition marked by a widespread infection that results in organ failure and tissue damage. Because it is frequently resistant to multiple antibiotics (Wisplinghoff et al., 2012). The ability of the bacteria to produce enzymes that can neutralize antibiotics and biofilms is frequently the cause of this resistance. Biofilms are thin layers of microorganisms that can stick to surfaces and shield the bacteria from antibiotics and the immune system of the host. Patients who are admitted to the ICU, who are receiving life support such as ventilation, catheters and patients who have underlying medical conditions such as diabetes and COPD are more likely to develop BSI caused by A. baumannii. BSI caused by A. baumannii is also more common in the elderly, immunocompromised, and patients with prolonged antibiotic consumption. Because the symptoms of BSI caused by A. baumannii are similar to those of other infections, it can be difficult to diagnose this condition. Typically, a culture of blood, urine, or other body fluids is used to make the diagnosis. The diagnosis of BSI can also be confirmed with blood tests like a complete blood count (CBC) and blood cultures. Antibiotics like carbapenems and aminoglycosides are commonly used to treat BSI caused by A. baumannii. However, the resistance of the bacteria may result in treatment failure and prolonged therapy for patients. Patients may also require mechanical ventilation and intensive care in some instances (Cisneros & Rodríguez-Baño, 2002; Peleg et al., 2008; Wisplinghoff et al., 2012).
Table 3.
Prevalence mechanism of different bacteria and overview of treatment options.
| Common bacterial species | Mechanism of resistance | Antibiotic substances | References |
|---|---|---|---|
| Enterobacteriaceae, p. aeruginosa, Acinetobacter spp. | Extended-spectrum or ESBLs | Meropenem+tigecycline, meropenem+colistin, ceftazidime+avibactam, aminoglycosides+meropenem |
(Fritzenwanker et al., 2018a; Karaiskos et al., 2019a) |
| Stenotrophomonas maltophilia, p. aeruginosa, Bacteroides fragilis, A. baumannii | Metallo-β-lactamases, and carbapenemases through the mobile genetic element. VIM, SIM, IMP, NDM-like | Colistin+meropenem, colistin+tigecycline, colistin+meropenem+ ampicillin/ sulbactam, colistin+meropenem+tigecycline |
(Queenan & Bush, 2007; Miriagou et al., 2010) |
|
P. aeruginosa, A. bauamnnii |
Chromosomal mutation: porin mutation (loss of outer membrane permeability) | Meropenem+ colistin | (Ruppé et al., 2015; Karaiskos et al., 2019b) |
| P. aeruginosa, A. bauamnnii | Chrosomal mutation of efflux pumps | Meropenem, aminoglycosides, tigecycline, fluoroquinolones | (Ruppé et al., 2015) |
| Enterobacteriaceae, A. baumannii | Aminoglycosides modifying enzymes: (mobile genetic element aminoglycosides phototransfarase, APH, aminoglycosides nucleotidyltransferases, ANT and aminoglycoside actyletransferases, AAC | Ceftazidime + avibactam, colistin + meropenem, aminoglycoside + meropenem | (Ruppé et al., 2015; Fritzenwanker et al., 2018a; Liu et al., 2016) |
| NDM-1 producing strains | Methlases of the 16s ribosomal subunit | plazomicin | (Karaiskos et al., 2019b)(Fritzenwanker et al., 2018a) |
| P. aeruginosa, K. pneumonia, A. baumannii | Lipid A (LPS) modification | Colistin | (Ruppé et al., 2015) |
4.3. Urinary tract infection (UTIs)
A. baumannii can also induce urinary tract infections (UTIs), which are infections of the urinary tract caused by bacteria. UTIs caused by A. baumannii are characterized by frequent urination, cloudy or strong-smelling urine, and pain or burning during urination (Table 1). The infection may also result in sepsis in some instances. Women are more likely than men to get UTIs caused by A. baumannii. Patients who spend a long duration in the ICU, who are receiving life support such as ventilation, catheters, etc., and patients who have underlying medical conditions such as diabetes and COPD are more likely to develop UTIs caused by A. baumannii. Because the symptoms of A. baumannii-caused UTIs are similar to those of other infections, they can be difficult to diagnose. Urine microscopy, urinalysis, and urine culture are other tests that can be used to confirm a UTI diagnosis. Due to its antibiotic resistance, A. baumannii-caused UTIs can be challenging to treat. A combination of antibiotics, such as carbapenems and aminoglycosides, is the standard treatment for UTIs caused by A. baumannii. However, the resistance of the bacteria may result in treatment failure and prolonged therapy for patients (Ayoub Moubareck & Hammoudi Halat, 2020; Peleg et al., 2008).
4.4. Wound infection
A. baumannii infections frequently present as wound infections as well as when bacteria enter a wound, they cause inflammation and damage to the tissue surrounding the wound. Redness, warmth, swelling, and discharge from the wound are all indicative of infection by A. baumannii (Ayoub Moubareck & Hammoudi Halat, 2020; Peleg et al., 2008). In severe cases, the infection may result in sepsis or even limb amputation. Wound infections caused by A. baumannii are a profound concern, especially on battlefields and other high-risk environments where wounds are more susceptible to bacterial infection.
The risk of developing wound infections induced by A. baumannii patients with compromised immune systems, and those with medical conditions. Patients with underlying medical conditions such as diabetes and COPD, immunocompromised patients, the elderly, or those with a history of antibiotic use are also at increased risk for developing wound infections induced by A. baumannii (Whitman, 2007). Diagnosing a wound infection caused by A. baumannii can be difficult because the symptoms are similar to those of other infections. Diagnosis is usually made by culture of wound swabs or tissue samples. Other tests such as debridement and wound culture may also be used to confirm the diagnosis of wound infection. Treatment of wound infections caused by A. baumannii can be difficult due to antibiotic-resistant bacteria. Standard treatment for wound infections caused by A. baumannii includes a combination of antibiotics, such as carbapenems and aminoglycosides (Table 3). However, due to bacterial resistance, treatment may fail. In some cases, the patient may also require surgery. To prevent wound infection caused by A. baumannii, it is important to follow good hygiene practices, such as frequent hand washing and proper cleaning of surfaces, especially in battlefields and high-temperature environments. In addition, it is important to use antibiotics sensibly, as overuse and unprescribed use of antibiotics can substantially increase the rate of development of antibiotic-resistant strains of bacteria. Infection control measures such as contact precautions and isolating infected patients can also help prevent the spread of bacteria (Ayoub Moubareck & Hammoudi Halat, 2020; Peleg et al., 2008).
4.5. Meningitis
Meningitis, an infection that occurs surrounding the protective membranes of the brain and spinal cord, can also be caused by A. baumannii. This kind of meningitis can cause severe complications like hearing loss, brain damage, and even death. Fever, stiffness in the neck, headache, and sensitivity to light are all signs of meningitis. A. baumannii can also cause a wide range of other infections, including osteomyelitis, endocarditis, and septicemia, in addition to these typical manifestations (Dexter et al., 2015; Howard et al., 2012). Because it is frequently resistant to multiple antibiotics, A. baumannii poses a particular threat to healthcare facilities. The ability of the bacteria to produce enzymes that can neutralize antibiotics and biofilms is frequently the cause of this resistance. Biofilms are thin layers of microorganisms that can stick to surfaces and shield the bacteria from antibiotics and the immune system of the host. In addition, it is essential to use antibiotics appropriately because using too many of them can result in the growth of bacteria that can resist the antibiotics (Ayoub Moubareck & Hammoudi Halat, 2020). Ultimately, A. baumannii is a gram-negative bacterium that causes a wide spectrum of human infections. These infections can be mild to severe and affect the lungs, bloodstream, urinary tract, wound, and meninges, among other places (Tabel 3). These infections can be severe and can progress to sepsis, a potentially fatal condition that results in organ failure and tissue damage. To prevent the spread of A. baumannii, it is essential to practice good hygiene, use antibiotics appropriately, and implement infection control measures (Jawad et al., 1998) (Ayoub Moubareck & Hammoudi Halat, 2020; Dijkshoorn et al., 2007; Peleg et al., 2008).
5. A. baumannii virulence factors
5.1. Outer membrane proteins
The outer membrane proteins of A. baumannii, ompA are involved in invasion, apoptosis of the host cell, and the interaction with the environment. With its attachment to the cell surface of the host, it localizes to the nuclei and mitochondria to induce cell death (Choi et al., 2005a). Induction of apoptosis, biofilm formation, bloodstream infection, and its interaction with the epithelial cell with the help of fibronectin can occur by ompA (Smani et al., 2012). Other outer membrane proteins like omp33 act as transporters of water and their expression is crucial for carbapenem resistance (Smani et al., 2013). This protein induced apoptosis in immune cells and connective tissues by blocking autophagy (Rumbo et al., 2014). Omp33 having decreased activity of adhesion, cytotoxicity, and invasion, these are indicative that omp33 play salient roles in the virulence of A. baumannii depicted by the study of a knockout strain lacking omp33 (Smani et al., 2013). Other proteins such as carO play identical properties in resistance against carbapenem.
5.2. Capsular polysaccharide composition
The capsular o-polysaccharide of the outer membrane plays an important role in the invasion of host cell with the help of pilli, in adherence (Haseley et al., 1997). A. baumannii survival against environmental stress such as dryness, desiccation, and disinfectant are correlated to presence of capsular polysaccharide. The fluidity of the outer membrane possessed by A. baumannii chemically alters the lipo-oligosaccharides and enables them to transport water and nutrients inside the cell (Boll et al., 2015).
5.3. Biofilm formation and quorum sensing
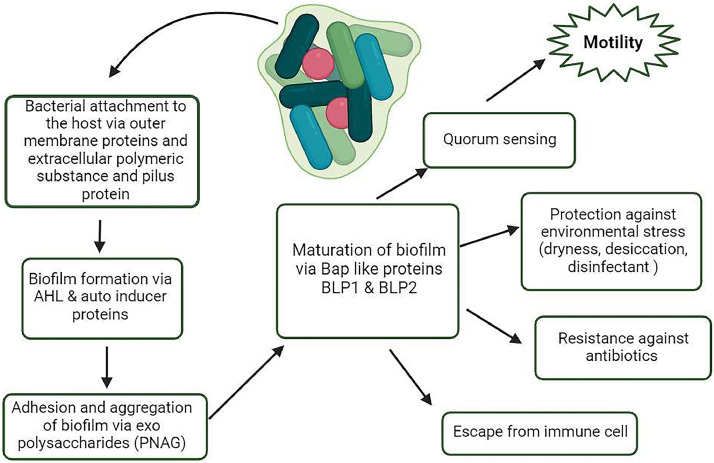
Biofilms are micro-communities of microbes on an extracellular substance rendering microbes less prone to desiccation, antibiotics, and cellular aggregation against the immune system. Biofilm formation is also a primary pathogenesis characteristic of A. baumannii (Doi et al., 2015). Biofilm also mediates pathogen-host interaction in A. baumannii. It uses a network of molecules that influence Csu pili and gene expression that build a defensive capsule in response to antibiotics i.e. the BfmRS two-component (TC) system, (Greene et al., 2016). Alternative TC system GacSA that influences Csu gene expression in A. baumannii carries Bap-like proteins, BLP1 and BLP2 that help in the maturation of biofilm, such as BapAb (Goh et al., 2013). For the adhesion and aggregation of biofilms (Fig. 3). The production of exopolysaccharide poly-β-1,6- N-acetylglucosamine (PNAG) in A. baumannii (Choi et al., 2009).
Fig 3.
Steps leading toward biofilm formation. First, the A. baumannii attaches to the surface and a colony is formed. Individual cells communicate with each other via quorum sensing, releasing molecules such as autoinducers like AHL (Acetylhomoserin lactose). Cells aggregate and adhere to each other via exopolysaccharides. Proteins on the bacterial surface (Bap) help in the maturation of the biofilm. Finally, the biofilm helps in evading immune cells, resistance to antibiotics, and protects against environmental stresses.
To maintain population density A. baumannii use signal molecules to communicate within the community known as autoinducers in a method called quorum sensing (Whiteley et al., 2017). Autoinducers Acyl homoserine lactones (AHLs), responsible for motility and biofilm formation. In A. baumannii AbaI is an inducer and its associated receptor is AbaR. Aba I is a sensor protein that synthesizes AHL molecules, AbaR is the receptor for AHL and this binding regulates biofilm formation (Saipriya et al., 2020).
5.4. Motility
It has long been thought that A. baumannii is non-motile but studies show that this organism uses the type IV pili to propagate by utilizing twitching motility on surfaces (Vijayakumar et al., 2016). In addition to motility, type IV pili help in biofilm formation and gene transfer (Burrows, 2012). A. baumannii can also depend on surface- associated motility which requires quorum sensing, lipo-oligosaccharide, type IV pili, and 1,3- diaminopropane production, rather than solely relying on twitching motility (Ahmad et al., 2020).
5.5. Nutrient acquisition systems
A. baumannii's tenacity to survive as hospital-acquired infections, the nutrient acquisition is a major factor in it. A. baumannii can capture manganese, iron, and zinc even in a metal-limiting environment (Tipton & Rather, 2017). The iron acquisition mechanism includes siderophores in addition to transporters and receptors such as FecA and FecI (Morris et al., 2019). For zinc, A. baumannii relies on ZnuABC transporter and ZigA GTPase, which are high-capacity zinc scavenging systems (Nairn et al., 2016). Transporters belonging to the family natural resistance-associated macrophage protein (NRMP) facilitate accumulation of manganese (Juttukonda et al., 2016).
5.6. Protein secretion system
Interaction with the environment and the host in A. baumannii is through the secretion of proteins from its surface. In A. baumannii Ata construct the trimeric autotransporter responsible for binding with the cell matrix and also in biofilm formation, maintenance, and virulence (Weber et al., 2017). A. baumannii uses the export effector proteins such as alkaline phosphatase, lipase, phospholipases, and elastase in a two- step secretion process by the type II secretion system (T2SS). First, the twin-arginine with N-terminal signals is secreted across the inner membrane. Next, T2SS secretes the protein outside the cell (Weber et al., 2017). T2SS effectors proteins include LipH, LipA, and CapA. Both LipH and LipA help in utilizing exogenous lipids and CapA is a metallo-endopeptidase that degrades fibronectin. Like T2SS, A. baumannii also uses a multi-component secretion protein by type VI secretion system (T6SS) capable of injecting toxic proteins with contact with other bacteria (Hood et al., 2010). It produces toxins like peptidoglycan hydrolases and nucleases.
6. Immune interaction by the host and A. baumannii
6.1. Innate immune response
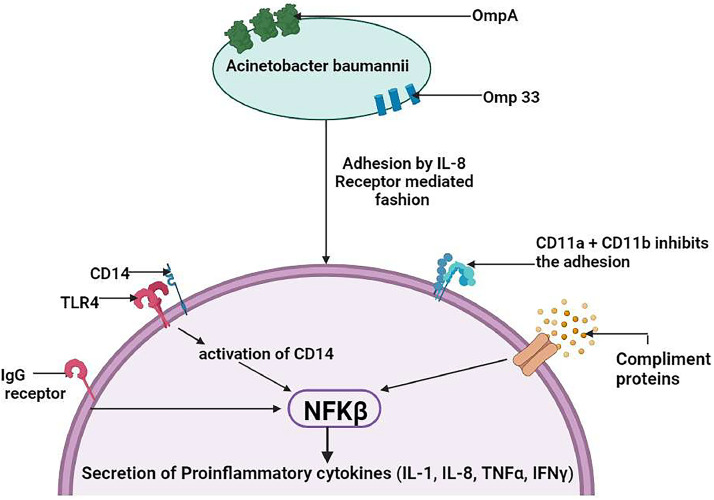
Neutrophils play a crucial role in the initial immune response by exerting antibacterial effects through processes such as phagocytosis, degranulation, and the formation of neutrophil extracellular traps (NETs). Phagocytosis is facilitated either via opsonization IgG or by the binding of the complement- mediated pattern. Once inside the neutrophils, bacteria are eliminated through the generation of reactive oxygen species (ROS), fusion with enzyme-filled granules, lysozymes, and the release of antimicrobial molecules into phagosomes. However, Acinetobacter baumannii has developed strategies to evade the immune system, particularly by evading NETs. It accomplishes this by forming biofilms and adhering to neutrophil surfaces in an IL-8-dependent manner, effectively escaping the entrapment and killing effects of NETs. This mechanism allows A. baumannii to persist and cause infections despite the presence of neutrophils and their immune responses (Kamoshida et al., 2016; Konstantinidis et al., 2016). Forming NETs composed of chromatin constituted with antimicrobial proteins such as myeloperoxidase, neutrophil elastase, and LL-37 kill pathogens. But the mechanism of A.baumannii inhibition of NET is not well understood, neutrophil cell surface receptors CD11a and CD11b are thought to have been implicated in the observed reduction of adhesion (Kamoshida et al., 2018). Macrophages are part of a salient early defense mechanism. They promote neutrophil recruitment and phagocytosis. Similar to neutrophils, the relation between A. baumannii infection and the function of macrophages is controversial. In the lungs, the alveolar macrophages act as the first line of defense against A. baumannii, able to induce microfilament and microtubule-dependent phagocytosis, stimulating high levels of IL-6, tumor necrosis factor TNF-α, and macrophage inflammatory protein. They promote chemotaxis in neutrophils, with additional IL-1β and IL-10 production. Though macrophages kill A.baumannii but at a slower rate than neutrophils (Qiu et al., 2009, 2012). Production of neutrophil chemoattractant, and keratinocyte chemoattractant by Natural killer (NK) cells leads to reduced survival and impaired bacterial clearance (Tsuchiya et al., 2012). The natural cytotoxicity receptors in NK cells have a very low affinity for attachment to A. baumannii. Activation of the dendritic cells (DCs) requires the presence of OmpA resulting in its stimulation, MAP kinase, and NF-κB signaling leading to CD4+ Th2 T cells response or cell apoptosis (Lee et al., 2007). A. baumannii causes the death of DC via targeting the mitochondria and producing ROS, and OmpAs as the mechanism of action, modulation of T cell responses is suggested by the facts.
6.2. Cell signaling in response to A. baumannii
Toll-like receptor (TLR) signaling is important through which hosts recognize a pathogen, detection of peptidoglycan, porins, lipoproteins, lipoteichoic acid (TLR2), LOS (TLR4), and unmethylated CpG DNA motif (TLR9) for A. baumannii TLR2, 4 and TLR9 are responsible to recognizing them (Knapp et al., 2006). The activation of TLR2, 4 is in the absence or presence of GPI-linked glycoproteins, CD14 led to the NF-κB activation, resulting in secretion of pro-inflammatory cytokines together with IL-1, 8, 10 and 12, TNF-α and IFN-γ (Fig. 4) (Lin et al., 2012b). The IL-4 pathway assumes a central role in directing a type 2 helper T cell (TH2) response, essential for combating this bacterial invasion. The production of IL-4, stimulated by the presence of the bacteria, orchestrates the immune system's shift towards a TH2 response. This is characterized by the activation of dendritic cells and macrophages via receptors such as TLR-4 and TLR-2, which then trigger signaling pathways involving NF-κB and MAPKs, crucial for further IL-4 production. The resulting TH2 cytokine profile fosters humoral immunity, an antibody-mediated defense crucial for neutralizing pathogens (García-Patiño et al., 2017).
Fig 4.
Adhesion and recognition of A. baumannii by host neutrophil. A. baumannii binds with neutrophil via IL-8 receptor interaction. TLRs help in the recognition of A. baumannii cells through their OMPs, lipoproteins, peptidoglycans (TLR4), and lipoteichoic acid (TLR2). IgG and complement protein binding with neutrophil and TLR activation via binding of A. baumannii in IL-8 receptor mediated fashion activates CD14 which in turn activates NF-κβ transcription factor. It results in the secretion of pro-inflammatory cytokines (TNF-α, IFNγ, IL-1,8 and 10). Neutrophil surface receptors CD11a and CD11b reduce the adhesion of A. baumannii cells.
The IL-4 pathway triggers the activation of the M2 macrophage phenotype, which is vital for reducing inflammation and aiding in tissue repair. This activation involves a multi-step process that includes the phosphorylation of STAT6 and JAK1, essential for initiating M2 polarization. Additionally, IL-4 induces an increase in MEK/ERK signaling, crucial for enhancing PPARγ expression. This, in turn, boosts retinoic acid signaling, which is critical for the metabolic reprogramming of M2 macrophages. It leads to heightened oxidative phosphorylation and PPARγ signaling, along with a shift in retinoic acid metabolism (He et al., 2021).
DC recognition is done by TLR2 and is important for A. baumannii OmpA. LpxC inhibitor protects against the lethality of A. baumannii in infected animals, by enhancing TLR stimulating and reducing NF-κB signaling and TNF-α secretion and promoting opsonophagocytic killing in response to increasing surface PNAG (Lin et al., 2012a). Isolates with phosphoethanolamine-modified lipid A render higher levels of TLR4 signaling. On the other hand, bacterial and viral DNA is detected by endolysosomal responsible for TLR9 is an internal receptor. Its stimulation promotes NF-κB activation and pro-inflammatory cytokines response (Noto et al., 2015).
6.3. Macrophage polarisation in A. baumannii infection
The primary interaction of A. baumannii with the host cells is enacted through Toll-Like Receptor (TLR) 4 and 2; CD14 as a coreceptor. TLR4 responds to the lipid A of LPS of gram-negative bacteria such as A. baumannii (Chen, 2020). In in vitro studies done by Kim et al. showed that the TLR signalling is vital for the activation of Bone Marrow Derived Macrophages (BMDMs) against A. baumannii infection. Leading to the activation of MAPKs pathways such as JNK, ERK and P38; NF-κB signalling pathway (C.-H. Kim et al., 2013); mRNA expression of iNOS and NO production; interleukin production of IL-12, IL-6 and tumour necrosis factor (TNF)-α.
TLR2 detects the peptidoglycan and lipoproteins. The role of TLR2 is not well defined, as shown by studies by Knapp et al. and Kim et al., where they demonstrated contradictory results, respectively. Knapp et al. showed TLR2 −/− mice were resistant to A.baumannii infection, whereas Kim et al. showed TLR2 −/− mice were susceptible compared to wild-type mice (Kim et al., 2013; Knapp et al., 2006b).
Depending on the tissue microenvironment and stimuli, macrophages can be polarised into M1 and M2 subtypes. M1 macrophages are pro-inflammatory and produce an antibacterial response. M2 macrophages are anti-inflammatory and responsible for tissue repair (Liu & Xu, 2022). Before infection, all bronchoalveolar lavage fluid (BALF) cells were primarily macrophages (Harris et al., 2019). In the early stages of infection, the LPS of A.baumannii and TNF-α produced by pulmonary epithelial cells induce M1 macrophage activation. TLR4 on the macrophage cells interact with the LPS, and the pro-inflammatory M1 macrophages produce large amounts of cytokines and chemokines. Recruit neutrophils and macrophages from neighbouring tissue sites and the bloodstream (Kim et al., 2013).
6.4. Complement-mediated response
The complement system is a salient non-cellular intrinsic immune response by the host consisting of soluble factor-mediated cell lysis or opsono-phagocytosis (Bruhn et al., 2015) (Fig. 4). But in human serum, an alternative complement system is required as resistance is found often in A. baumannii isolates (Bruhn et al., 2015). Factor H is important to distinguish host cell markers to mediate the alternative complement system. C3 deposition is stimulated with the activation of the alternative complement system, which is deposited to the serum and binds to sensitive strains. If the factor H bacterial cell, it leads to serum resistance in A. baumannii by inhibiting C3 deposition (King et al., 2009). Inhibition of biosynthesis of capsule and pili that prompt serum resistance by the regulation of TCS bfmS (Geisinger et al., 2018). To prevent phagocytosis in the serum and ascites fluid A. baumannii encodes genes such as mitB, epsA, ptK, and pglC that encode putative kinase, lytic transglycosylase, OM polysaccharide exporter, and glycosyltransferase, all these attributes highlight the importance of the capsule in resistance against complement system (Crépin et al., 2018; Russo et al., 2010).
6.5. The effects of lung fibrosis and cytokine storms in A. baumannii infections
Acinetobacter baumannii infections, particularly in immunocompromised individuals, can provoke a robust immune response that may escalate into a cytokine storm. Initially, the immune system's goal is to eradicate the bacteria, but Acinetobacter baumannii resistance to being killed by immune cells like macrophages and neutrophils leads to persistent immune system activation. This ongoing stimulation results in an overproduction of inflammatory factors, culminating in a cytokine storm (Jeffreys et al., 2022; Lee et al., 2017; Wang et al., 2024)
The cytokine storm in Acinetobacter baumannii infections is primarily driven by the TLR2/MyD88/NF-κB signalling pathway. Acinetobacter baumannii possesses Pathogen-Associated Molecular Patterns (PAMPs), such as OmpA, which are recognized by Toll-like receptors (TLRs), specifically TLR2, on immune cells like macrophages. This recognition triggers the TLR2/MyD88/NF-κB signalling pathway, leading to the polarization of macrophages towards the M1 phenotype. M1 macrophages are pro-inflammatory and release large amounts of cytokines, contributing to the cytokine storm (Tansho-Nagakawa et al., 2021).
Excessive or prolonged activation of the M1 macrophage program is harmful to the host. While a moderate M1 response can be protective, an uncontrolled M1 program can lead to a detrimental cytokine storm, causing systemic inflammation, multi-organ failure, and even death (Chen, 2020; Lee et al., 2017).
Neutrophils also play a crucial role in the immune response to Ab infection, but their role in cytokine storms is less clear. Although neutrophils are essential for controlling Acinetobacter baumannii infection, their involvement in cytokine storms requires further investigation. They contribute to the inflammatory response, but their role seems less direct compared to macrophages (Jeffreys et al., 2022). Cytokine storms are a dangerous complication of Acinetobacter baumannii infections and can be fatal. This excessive inflammatory response, characterized by the overproduction of cytokines, can lead to severe complications such as sepsis, organ failure, and death. The severity of Acinetobacter baumannii induced cytokine storms may vary between different strains. Studies suggest that the virulence and immune response triggered by Acinetobacter baumannii can differ depending on the specific strain, which could influence the severity of the cytokine storm and the outcome of the infection (Tansho-Nagakawa et al., 2021).
A recent study by Lee et al. (2020) investigated the impact of alveolar macrophages on the development of A. baumannii pneumonia in a murine model. Alveolar macrophages are a type of immune cell that resides in the alveoli, the tiny air sacs in the lungs responsible for gas exchange. These macrophages are part of the first line of defence against inhaled pathogens. The study found that depletion of alveolar macrophages prior to infection with A. baumannii led to increased bacterial replication, extensive tissue damage, and higher mortality rates in the mice. The researchers also observed a significant increase in neutrophil infiltration into the lungs of macrophage-depleted mice. In this study, the excessive neutrophil infiltration observed in the absence of alveolar macrophages led to increased production of reactive oxygen species (ROS) and the release of lactate dehydrogenase (LDH), a marker of cellular damage. The study also found that macrophage depletion led to a reduction in the levels of pro-inflammatory cytokines in the lungs of infected mice (Lee et al., 2020; Qiu et al., 2012). A reduction in pro-inflammatory cytokines can impair the host's ability to fight off infection. Taken together, these findings suggest that alveolar macrophages play a critical role in controlling A. baumannii infection and preventing lung damage. The absence of these macrophages can lead to increased bacterial replication, excessive neutrophil infiltration, and tissue damage, all of which can contribute to the development of lung fibrosis (Qiu et al., 2012).
Lung fibrosis is a chronic and irreversible condition characterized by excessive deposition of extracellular matrix (ECM) proteins, such as collagen, in the lungs. This excessive deposition can stiffen the lungs and impair their ability to function properly. While the exact mechanisms by which A. baumannii infection contributes to lung fibrosis are still under investigation, the findings of Lee et al. (2020) suggest that the absence of alveolar macrophages can create an environment conducive to the development of fibrosis. Specifically, the increased bacterial replication, excessive neutrophil infiltration, and tissue damage observed in macrophage-depleted mice can all contribute to the activation of fibroblasts, the cells responsible for producing ECM proteins. The activation of fibroblasts can lead to excessive collagen deposition and the development of lung fibrosis (Glasser et al., 2016; Lee et al., 2020).
In addition to the findings of Lee et al., other studies have also suggested a link between A. baumannii infection and lung fibrosis. For instance, a study by Tansho-Nagakawa et al. found that A. baumannii infection can lead to prolonged pulmonary inflammation in a mouse model. This prolonged inflammation can also contribute to fibroblast activation and collagen deposition (Tansho-Nagakawa et al., 2021).
While more research is needed to fully understand the relationship between A.baumannii infection and lung fibrosis, the available evidence suggests that this bacterium can contribute to the development of this chronic condition. The absence of alveolar macrophages, as demonstrated by Lee et al. (2020), can further exacerbate this process.
7. Mechanism of developing resistance against antibiotics
The prevalence mechanism of different bacteria and overview of treatment options is represented in Table 3 and the advantages and limitations of the main classes of antibiotics used against A. baumannii is represented in Table 4.
Table 4.
The advantages and limitations of the main classes of antibiotics used against A. baumannii.
| Class of antibiotics | Advantages | Limitations |
|---|---|---|
| β-lactams | [1] Disrupt peptidoglycan synthesis in bacterial cell wall [2] Broad-spectrum β-lactams are more stable against β-lactamases |
[1] A. baumannii is intrinsically resistant to many β-lactams [2] Carbapenemases are a growing problem |
| Tetracyclines | [1] Block aminoacyl-tRNA from binding to ribosome-mRNA complex | [1] Resistance is typically plasmid-mediated [2] Can be blocked from entering bacterial cell |
| Aminoglycosides | [1] Bind irreversibly to the 30S ribosomal subunit [2] Increase production of abnormal proteins |
[1] Resistance can occur through modification of ribosome target [2] Can be blocked from entering bacterial cell |
| Fluoroquinolones | [1] Block production of enzymes gyrase and topoisomerase IV | [1] Resistance can occur through modification of target enzymes [2] Can be blocked from entering bacterial cell |
| Polymyxins | [1] Disrupt bacterial cell membrane function | [1] Polymyxin resistance is emerging |
7.1. β-lactams
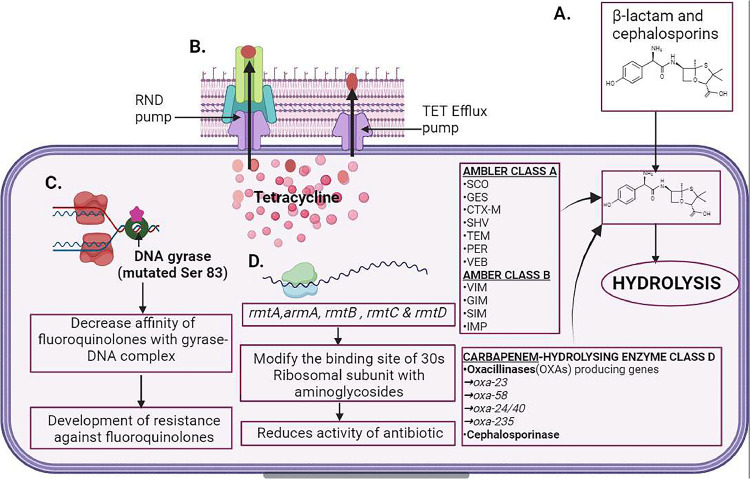
Enzymatic hydrolysis by β-lactamases is the most principal mechanism of resistance exhibited by Acinetobacter baumannii in the presence of β-lactams. Clavulanate and tazobactam can inhibit class A β-lactamases, a serin-dependent enzyme. SCO, GES, CTX-M, SHV, TEM, PER, and VEB are some enzymes belonging to this class. They can hydrolyze all types of penicillins and cephalosporins except cephamycins. The Klebsiella pneumoniae carbapenemase (KPC)-5 ambler class A carbapenemase in MDR A. baumannii was first reported by Robledo et al. in 2010 from Puerto Rico. Ambler class B is metallo-β-lactamases (MBLs) with a cofactor as Zinc which hydrolyzes all β- lactams. NDM-1 is the most concerning MBL of all. VIM, GIM, SIM, and IMP (Fig. 5) including IMP- 55, a new allelic variant of this enzyme, were reported. The prevalence of acquired MBL genes in A. baumannii lies in class 1 integrons, which at the same time harbor other resistant genes, like aminoglycoside resistance.
Fig 5.
Mechanism of antibiotics resistance in A. baumannii. A. β-lactams (penicillin, amoxicillin, etc.) when entering into A. baumannii cells, β-lactamase like Ambler class A and B hydrolyze the antibiotics. Class C cephalosporinase hydrolyzes cephalosporins and class D distorts oxacillin. B. Tetracyclines are actively pumped outside of the cell by efflux pumps upon entry inside the bacterial cells. Nodulation division family type pumps (RND pumps) also participate in the same function. C. Mutation of enzymes such as DNA gyrase at the Ser83 and Gly81 prevents the binding of the fluoroquinolones to the DNA gyrase-DNA complex. D. Different 16S rRNA (rmtA, armA, rmtB) alter the 30S ribosome subunit binding sites for aminoglycosides.
Acinetobacter-derived cephalosporinases (ADCs) are Ambler class C, chromosomally encoded by AmpC cephalosporinases, innate in all A. baumannii strains. The combinational effort of penicillium, cephalosporin, cephamycin, and β-lactam is inhibited by ADCs but β-lactamase inhibitors such as sulbactam and clavulanate don't influence the action of ADCs. Distinguishing from other Gram- negative bacteria, A. baumannii does not yield the manifestation of AmpCs. The overexpression is arbitrated by inserting ISAba1 before AmpCs. Thus, enhancing the A. baumannii resistance towards the extended spectrum of cephalosporins.
The ambler class D β-lactamases, also known as oxacillinases (OXAs), have the cofactor as serin and have a greater capacity to hydrolyze β-lactams than benzylpenicillin. Therefore, named after it. A. baumannii isolates bearing plasmid-encoded OXA-23, OXA-58, OXA-24/40, OXA-143, and OXA- 235 families. The first isolated carbapenem-hydrolyzing class D oxacillin was OXA-23. OXA-23 enzymes have been identified all around the globe in concurrence with other carbapenemases. Non-enzymatic β-lactam Resistance mechanism: the modification of the outer membrane protein associated with imipenem and meropenem resistance; A. baumannii loss 29KDa porinCarO. In β- lactam resistance, multi-drug efflux pumps play a major role. β-lactam, chloramphenicol, aminoglycosides, tetracycline, erythromycin, fluoroquinolones, and trimethoprim are pumped outside of A. baumannii cells with the best-studied multi-drug efflux pump of the resistance-nodulation-division (RND) family AdeABC. Outer membrane protein (adeC), multidrug transporter (adeB) and membrane fusion protein (adeA) construct the AdeABC efflux pump (Karaiskos et al., 2019a) (Table 3).
7.2. Tetracycline and glycylcyclines
The energy-dependent pumps of A. baumannii are attributed not only to the resistance of tetracycline but also to ribosomal proteins to a lesser extent. Two distinct categories of tetracycline efflux pumps such as the resistance-nodulation-division (RND) pumps and the other hand constitutive and Tet efflux pumps (Fig. 5). RND pumps are non-specific. On the other side Tet A provides resistance against tetracycline and Tet B against minocycline and tetracycline but does not affect tigecycline. RND pumps include three parts; Adeb, AdeA, and Adec. Tigecycline is relatively stable against the resistance mechanism of tetracycline including Tet efflux pumps, and ribosomal protection like Tet (O) and Tet (M) comparatively, tigecycline has a broad spectrum of activity and goods tissue penetration than tetracycline (Karaiskos et al., 2019b)(Table 3).
7.3. Fluoroquinolones
The mutation of enzyme genes such as gryA and parC and their encoded protein, enzymes such as DNA gyrase and DNA topoisomerase IV confer resistance in A. baumannii. The fluoroquinolones- resistance determining regions (QRDRs) of target enzymes are mainly mutated, with common amino acid substitution Ser83 and Gly81 within gyrA, and Ser80 and Glu84 within parC (Fig. 5). These mutations decrease the affinity of fluoroquinolones. Chromosomal efflux pumps result in moderate- level fluoroquinolone resistance. The mutation of the regulator (AdeR) and sensor (AdeS) of AdeABC efflux pumps of the RND family results in the higher efflux of fluoroquinolone (Karaiskos et al., 2019b) (Table 3).
7.4. Aminoglycosides
Aminoglycoside-modifying enzymes (AMEs) produced by A. baumannii make them resistant to aminoglycoside. It can be categorized into varied groups based on chemical actions-acetyltransferase, adenyltransferase, and phosphotransferase. By altering the corresponding function groups of Aminoglycosides, AMEs hamper the capacity to bind to the target ribosomal sites of the antibiotics. Aminoglycoside resistance can also occur through an alternative mechanism involving the production of certain 16S rRNA genes, namely rmtA, armA, rmtB, rmtC, and rmtD (Fig. 5). These genes modify the binding site of aminoglycosides within the 30S ribosomal subunit, resulting in reduced effectiveness of the antibiotics (Karaiskos et al., 2019b) (Table 3).
7.5. Polymyxins
Polymyxins are also called colistin. Polymyxins are ionic compounds, its positively charged sides interact with lipid A, a negatively charged constituent of LPS, and reduces the affinity of PetN residues in LPS. It has few resistance mechanisms. A. baumannii also alters its outer membrane structure by polymerizing phosphoethanolamine (Pet N) into lipid A. Mutation of genes required to synthesize lipid A leads to low expression of protein needed to stabilize outer membrane and low concentration of cofactors required to LPS synthesis (Karaiskos et al., 2019b)(Table 3).
8. Existing clinical treatments
The current advent of multi-drug resistance (MDR) among gram-negative Enterobacteriaceae poses significant challenges in the treatment options in healthcare systems around the world (Giamarellou & Poulakou, 2009). Our main focus in this section of the review is on the existing treatments that are available to us against MDR and pan drug resistance (PDR) and the special emphasis on Acinetobacter baumannii. The existing clinical treatments are represented in Table 3.
8.1. Conventional antibiotics
8.1.1. Colistin
Colistin methane sulfonate (CMS) is the systematically administered form of colistin also the last resort antibiotic of recent times due to the absence of new antimicrobial agents (Karaiskos et al., 2017). Colistin exhibits a broad spectrum of antimicrobial activity including MDR and extensive drug-resistance (XDR) Gram-negatives, mainly K. pneumonia, A. baumannii, and P. aeruginosa regardless of their resistance mechanism (Karaiskos et al., 2017). The combination of meropenem with colistin has reduced the mortality of patients with septic shock (Daikos et al., 2014). Colistin has been readily used to treat carbapenem-resistant A. baumannii (CRAB). But using colistin has its downside, for its neurotoxicity and nephrotoxicity (Aksoy et al., 2020) (Table 3).
8.1.2. Fosfomycin
By inhibition of the initial step in peptidoglycan synthesis fosfomycin induces bactericidal activity against susceptible strains (Albur et al., 2015). In vitro studies of fosfomycin show activity against almost 80 % of Staphylococcus aureus and Enterococcus faceium, ESBL-producing E. coli, and carbapenem-resistance K. pneumonia (Vardakas et al., 2016). Fosfomycin shows no activity against A. baumannii strains and there is no clinical threshold value for A. baumannii. In bacteria with colistin resistance and Metallo-β-lactamases, extensive drug-resistance (XDR) and pan drug resistance (PDR), carbapenem resistance Enterobacteriaceae (CRE) infections, fosfomycin will cover the unmet need. In vivo, the synergistic effects with colistin against A. baumannii infections in the future may expand its use (Sirijatuphat & Thamlikitkul, 2014). Intravenous administration of fosfomycin could have adverse effects specifically hypokalemia, hypernatremia, and heart failure (Grabein et al., 2017).
8.1.3. Aminoglycoside
Aminoglycosides are mainly used in urinary tract infections (UTIs) as monotherapy and other instances as a combination regimen. In the presence of XDR pathogens, aminoglycoside may still retain activity (Zavascki et al., 2017). Co-transfer of aminoglycoside modifying enzymes and other resistance genes influence greatly susceptibility in vitro (Almaghrabi et al., 2014). 16s rRNA methyltransferase expression is a concern that exerts aminoglycoside resistance, especially in NDM-producing CRE (Doi et al., 2016). CRE isolates in vitro treated with a combination of aminoglycosides and carbapenem produce hopeful results in treatment (Hirsch et al., 2013). Administering aminoglycosides may show nephrotoxicity, to ensure a non-toxic dose therapeutic drug monitoring should be essayed (Paquette et al., 2015) (Tables 2, 3).
Table 2.
Drug resistance mechanisms and their targets.
| Antibiotics | Resistance mechanism | Targets, permeability defects | Example | Reference |
|---|---|---|---|---|
| β-lactams | β-lactamase, Permeability lesion, Efflux pump activity, Targate mutation. |
Ambler class A, B, C, D Outer membrane porins, RND pump, PBP. |
Carbapenem-hydrolyzing ESBL, VIM, SIM, IMP, AMP-69, 70,71, ACD-169, OXA-23 like, 24/40,58 OmpA, OmpB, OmpC, OmpD, Omp25,Omp33, AdeABC, PBP6d |
(Hammoudi et al., 2015; Heritier et al., 2006; Jeon et al., 2015; Lee et al., 2017; Queenan & Bush, 2007) |
| Tetracycline | Efflux pump activity, Ribosomal protection. |
RND pump, Tet pump, Tetracycline dissociation from ribosome. |
AdeABC, TetA, TetB Tet(O),Tet(M). |
(Huys et al., 2005; Magnet et al., 2001) |
| Fluoroquinolones | Target mutation, Efflux pump activity. |
DNA gyrase, DNA topoisomerase IV, RNA pump. |
Gry A, Par C, AdeABC. |
(Doi et al., 2015; Hamouda & Amyes, 2004) |
| Aminoglycosides | Drag inactivating enzymes, Target mutation, Efflux pump. | Aminoglycoside modifying enzyme, 16s methylase genes, RND pump. | AAC(3)-la, ANT(2′)-la, ArmA,rmt, AdeABC. | (Yamane et al., 2005; Zhu et al., 2009) |
| Polymyxins | Target mutation. | Lipid A modification pet N transferase, Decrease stability of the outer membrane. | PmrC, LpsB, LptD. | (Lima et al., 2018)(Ayoub Moubareck & Hammoudi Halat, 2020) |
8.1.4. Tigecycline
Glycylcyclines and tigecycline is being used to treat infection caused by CRE and CRAB (Garnacho-Montero et al., 2015). A higher dose of tigecycline had been in use for ventilator-associated pneumonia (VAP) and hospital-acquired pneumonia (HAP) infections caused by A. baumannii (Koomanachai et al., 2009). For low serum concentration of drug in the approved regimen. In conclusion, the combination of tigecycline is mandatory, particularly in critically ill patients when administered as monotherapy, resulting in increased mortality (Bassetti et al., 2014). Colistin-tigecycline lowered the mortality of patients with CRE-BSI compared to those who received monotherapy (Durante-Mangoni et al., 2013) (Table 3).
8.1.5. Carbapenem and double carbapenem combinations
It is shown to lower mortality in CRKP BSIs with meropenem treatment. Before the resistance, carbapenem was used as a life-saving therapeutic (Daikos et al., 2014). So, a dual carbapenem is more effective, a high affinity carbapenem can bind pathogen carbapenemases and exhaust it, second carbapenem can have the bactericidal effect against XDR and PDR CRE such as the combination of ertapenem and doripenem (Bulik & Nicolau, 2011). A study done on A. baumannii bloodstream infection showed the superiority of carbapenem and colistin (Cheng et al., 2015) (Table 3).
8.2. Non-conventional antibiotics
8.2.1. Ceftazidime + avibactam
The combination of ceftazidime with a non-β-lactam, β-lactamase inhibitor, avibactam show an effect on ESLBs and carbapenemases like KPCs-Ambler class A, class C AmpC and Class D oxacillinase OXA-48 by restoring the activity of ceftazidime. In short, the CAZ- AVI has proven hopeful against a wide array of MDR bacteria (Keepers et al., 2014). With The FDA and EMA approval CZA-AVI combination is being used in various infections such as ventilator-associated pneumonia (VAP), hospital-acquired pneumonia, complicated urinary tract infections (cUTIs), and intra- abdominal infections (cIAIs) (Karaiskos et al., 2019a). INFORM global surveillance program revealed 99.6 % and 98.5 % susceptibility to CAZ-AVI for all Enterobacteriaceae (Kazmierczak et al., 2018). CZA-AVI inhibits 99.6 % of KPC and 100 % of OXA-48-producing isolated strains. CAZ-AVI only confers modest activity against A. baumannii due to its OXA-type carbapenemase (Castanheira et al., 2014) (Table 3). In conclusion, CAZ-AVI has the potential to act against CRE infection.
8.2.2. Meropenem + vaborbactam
Vaborbactam, β-lactamase inhibitor, inhibiting ESLBs, ambler class A and cephalosporinases class C and show minimal activity against Metallo-β-lactamases and minimal activity against class D of β-lactamases (Castanheira et al., 2017). It has a seminal presence in clinical use as the first available β-lactamase inhibitor. Meropenem + vaborbactam inhibits 99.0 % KPC-producing Enterobacteriaceae, as vaborbactam restores the meropenem activity, which is four times more effective than CAZ-AVI (Hackel et al., 2018). With higher MICs of meropenem + vaborbactam used to treat MBLs, with individual isolates, there was a lower expression of porin, OmpK37, and overexpression of the efflux system AcrAB-TolC (Castanheira et al., 2016).
8.2.3. Plazomicin
Plazomicin is a successive-generation aminoglycoside and acts by inhibiting the synthesis of proteins. It is active against MDR Enterobacteriaceae because plazomicin remains stable against aminoglycoside-modifying enzymes (Zhanel et al., 2012). But it is vulnerable to rRNA methyltransferase enzymes, particularly NDM-1 carbapenemases present in A. bauamnnii and P. aeruginosa (Livermore et al., 2011). OXA- producing A. baumannii is susceptible to plazomicin compared to other aminoglycosides (Landman et al., 2011). Plazomicin showed 79 % susceptibility in isolates producing MBLs type VIM and IMP but in action against NDM-1 for ribosomal methyltransferases (Livermore et al., 2011)(Table 3).
8.2.4. Eravacycline
A synthetic fosfomycin similar to tigecycline in mechanism, and structure. Eravacycline is active against ESLB and KPC-producing Enterobacteriaceae (excluding P. aeruginosa) and MDR A. baumannii and gram-positives (Abdallah et al., 2015) and has superior activity in biofilms. In a recent in vitro study eravacycline is compared against commonly used antibiotics to counter OXA and MBL-producing carbapenem resistance A. baumannii. Eravacycline demonstrated the most efficacious results in vitro compared to other antibacterial agents (Seifert et al., 2018). Eravacycline may well be the potent antibiotic agent in the fight against MDR A. baumannii.
9. The surge of MDR A. baumannii and related infections during the time of COVID-19
In December 2019 a mysterious incidence of viral pneumonia was found, later designated as the coronavirus disease, caused by SARS-CoV-2. The disease quickly spread throughout the world and became a transnational health threat (Sreenath et al., 2021), as of December 2022 about 645 million confirmed cases and 6.6 million deaths globally (http://covid19.who.int/). The symptoms of covid are mild upper respiratory tract disease or asymptomatic infection (Cheng et al., 2020).Infections can lead from severe to critical respiratory failure, septic shock, and multi-organ dysfunction (Verity et al., 2020). Due to COVID-19, many immunocompromised individuals are admitted to the hospital with this impaired immunity because of inadequate CD8 T cells response (Westmeier et al., 2020)and bacteria take advantage of this compromised immune response and cause secondary pneumonia (Ritchie & Singanayagam, 2020). Due to these bacterial infections, morbidity and mortality can increase in COVID-19 patients (Sharifipour et al., 2020). The unambiguous characteristic of the bacterial infection method is not well understood (Rangel et al., 2021). COVID-19 patients often have severe pulmonary symptoms and are taken to the ICU and need ventilator support care. This use of ventilators leads to ventilator-associated pneumonia (VAP), especially MDR A. baumannii responsible for VAP in COVID-19 patients (Lescure et al., 2020). A. baumannii is also found in surgical sites, bloodstream infection associated with the central line, and urinary tract infections related to catheter use (Sievert et al., 2013). These risk factors arise from a patient's prolonged hospitalization, intensive care unit admission, old age, previous infection, and colonization with A. baumannii. Contamination between the healthcare workers and the hospital environment is a major reservoir for infection (Rangel et al., 2021; Sreenath et al., 2021). Sreenath et al. showed A. baumannii strain's resistance to all experimented antibiotics except colistin. Zhang et al. found nearly 55.6 % of patients have a secondary infection with carbapenem resistance A. baumannii. In a new jersey hospital, sustained a cluster of 26 CRAB isolates expressing the OXA-23 carbapenemase and two isolates harbouring the rare New Delhi Metallo-β-lactamase (S. Perez et al., 2020). Both Gottesman et al. and Shinohara et al reported a monoclonal outbreak of CRAB strains (Gottesman et al., 2021; Shinohara et al., 2022). The outbreak of A. baumannii infections is the critical factor responsible for the increase in morbidity and mortality in ICU patients with strains that are only susceptible to colistin. But a pre-optimized two-phage cocktail elucidates signs of effectiveness in CRAB-infected COVID patients (Wu et al., 2021). Co-infection with A. baumannii in SARS-CoV-2 infected patients has emerged multiple times during the COVID-19 pandemic including in Wuhan, Brazil, France, Iran, Italy, Spain, and New York (Rangel et al., 2021). But the most common secondary infection in COVID-19 patients is Staphylococcus aureus, Streptococcus pneumoniae, Klebsiella pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae etc (Wu et al., 2021). Nearly 50 % of all non-surviving patients have a prevalence of secondary infection (Lai et al., 2020).
10. Risk factors of A. baumannii infection to the cancer patients
The acquisition of multidrug-resistant (MDR) Acinetobacter baumannii in cancer patients is predominantly associated with general nosocomial infection risk factors rather than the malignancies themselves. These induced risk factors are akin to those identified in the general ICU population and are largely related to healthcare interventions and the hospital environment. Key induced risk factors include:
Prolonged ICU Stay: Prolonged intensive care unit (ICU) stay is a significant risk factor for MDR A. baumannii infection, with an odds ratio of 19.28. The median ICU stay before MDR A. baumannii detection was 5 days for case patients versus 1 day for control patients. This indicates that the longer a cancer patient remains in the ICU, the greater their risk of exposure and subsequent infection.
Need for Dialysis: Cancer patients requiring dialysis face an increased risk, with an odds ratio of 18.23. This elevated risk is due to factors such as frequent line placements, repeated hospitalizations, and regular contact with dialysis facilities, which increase the potential for exposure to the pathogen. Patients with end-stage renal disease on hemodialysis are particularly vulnerable due to their immunosuppressed state and exposure to invasive devices.
Mechanical Ventilation: Mechanical ventilation is another induced risk factor, as it is an invasive procedure that can create a pathway for A. baumannii to enter the body. A significantly higher percentage of case patients (80.6 %) required intubation compared to controls (17.7 %).
Prior Antimicrobial Use: All case patients had received antimicrobial treatment in the 30 days preceding the detection of MDR A. baumannii, compared to 46.8 % of the control group. Prior exposure to antibiotics can disrupt the normal balance of bacteria in the body, making it easier for drug-resistant strains like MDR A. baumannii to colonize and cause infection.
Tube Feeding: Tube feeding, common in hospitals for critically ill patients, was identified as a significant risk factor. A higher proportion of case patients (67.7 %) received tube feeding compared to control patients (17.7 %). This may be related to potential contamination during tube feeding or an alteration of the gut microbiome, making patients more susceptible to A. baumannii colonization.
Blood Transfusion: A higher percentage of case patients (58.1 %) received blood transfusions compared to the control group (27.4 %). While the specific mechanism is not detailed, transfusions, despite rigorous safety protocols, could represent a potential, albeit rare, route for A. baumannii transmission.
Transfer from Other Health Care Facilities: Patients transferred from other healthcare facilities had a higher risk of acquiring MDR A. baumannii. This aligns with the understanding that A. baumannii is often endemic in healthcare settings, and patients may acquire the bacteria from previous hospitalizations or long-term care facilities.
These risk factors underscore the role of healthcare interventions and the hospital environment in increasing the vulnerability of cancer patients to MDR A. baumannii infection. While cancer and its treatments can weaken the immune system, these findings suggest that general infection control measures are crucial in preventing the spread of this pathogen among this patient population (Fukuta et al., 2013).
11. Potentials of unconventional therapies against A. baumannii
In modern medicine, the effects of vaccination and its advancement enabled us better and longer lives. The emergence of Acinetobacter baumannii as a successful pathogen because of its ability to survive antibiotics in highly arid surfaces present in hospitals (Harding et al., 2018; Ramirez et al., 2020). Since the existing antibiotic treatment shows limited effects and the possibility of getting new antibiotics soon is far few as an effective treatment for multidrug resistance A.baumannii. Unconventional approaches are drastically needed and vaccines against this threat could be an out-of-the-box solution. Guided by the clinical syndromes caused by A. baumannii a prophylactic vaccination would help patients supported by mechanical ventilation, burn, and wounds (Perez & Bonomo, 2014). Developing a vaccine is an important aspect that has to be taken into account in investments by manufacturers. Another thing to developing a vaccine is the large number of trials needed to ensure a vaccine's efficacy.
11.1. Passive immunization
Whole-cell multi-antigen preparation in 2010 was able to increase protective polyclonal antibodies in the serum against A. baumannii (Breslow et al., 2011). The antiserum prepared from it lowered the bacterial concentration in the spleen after infection by a thousand times. An important asset for making vaccines is the A. baumannii outer membrane complex (OMC); its constituent surface proteins will act as antigens. TetR-type transcriptional regulator, ABUW_1645, a virulence regulator of A. baumannii, its expression turns virulent opaque A. baumannii cells into avirulent and translucent. This potential of ABUW_1645 expression can be utilized as a liver vaccine for A. baumannii (Tan & Lahiri, 2022).The surface exopolysaccharide poly-β-1,6-N-acetylglucosamine (PNAG), is a conserved outer membrane protein crucial in maintaining biofilm integrity. A synthetic oligosaccharide is used to mimic the PNAG and its response to the immune system. The PNAG antibody in the serum is highly specific only to the strains that contain PNAG even at minimum amounts (Bentancor, O'Malley, et al., 2012). The K1 capsular polysaccharide of A. baumannii consists of a very high amount of polysaccharides containing trisaccharide repeats units; its high polysaccharide content helps it to adhere to host cells. It can increase the monoclonal antibodies to initiate opsonization and bactericidal activity by increasing neutrophil mediation against the K1 capsule of A. baumannii, along with a significant 4-log decrease in the bacterial load of infectious tissues. The only drawback of this method is of being serotype- specific (Russo et al., 2010). The surface protein of A. baumannii the Acinetobacter trimeric autotransporter (Ata) partakes in biofilm formation and adhesion of the host cells' C-terminal domain encoding 4-β sheets in its C- terminal (Bentancor, Camacho-Peiro, et al., 2012). The anti-recombinant Ata hampers the binding to type IV collagen and promotes complement-dependent bacterial killing and amplification of pathogenesis and oxidative killing activity, and prevents pneumonia in mice, induced by A. baumannii (Bentancor, Routray, et al., 2012).
In an iron-deficient environment the low molecular weight siderophore, the Iron Regulated Outer Membrane Protein (IROMP) has a vital role in A. baumannii virulence and iron accusation (Roca et al., 2012). In homologous A. baumannii colonies, the Anti-IROMPs show effectiveness in vitro. By blocking the iron acquisition by siderophores, inhibition of growth showed bactericidal activity ranging from 80 % to 90 % and increased the phagocytic activity by 6–8 folds (Goel & Kapil, 2001). The drawback of this method is that IROMPs are only expressed in environments with depleted iron and this limits the spectrum of immunization to iron-dependent. Omp36, the outer membrane protein helps A. baumannii to adhere to the host and activate death receptors in the surface and localizes to mitochondria, causing cell death by activating pro-apoptotic molecules; a cause of pathogenicity of A. baumannii (Choi et al., 2005b). In the early infection stage, the human immune system targets the epitope OmpA in response to antigen. The binding of the H factor with the OmpA renders the A. baumannii strains resistant to the complement system (Kim et al., 2009). OmpA's structure is 99 % conserved in all clinical isolates and showed ≥89 % homology with other strains. The enhancement of opsonophagocytosis by the anti-recombinant antibodies OmpA, but the complement system remains ineffective against MDR A. baumannii (Luo et al., 2012). Outer membrane nuclease (NucAB), Omp22, and OmpW, and their serum antibodies and their effect on the survival of animal models are yet to be decided whether they can be good vaccine candidates (Garg et al., 2016; Huang et al., 2015).
11.2. Active immunisation
To stimulate the body to gain active immunity the preparation of A. baumannii inactive whole cells (IWC) by formalin in addition to aluminum phosphate as an adjuvant can be used. Immunization showed lesser bacterial burden in infected tissues and lower levels of inflammatory cytokines like TNF-α, IL-1β, and IL-6 in serum which typically cause sepsis and avail in securing the high survival rate among vaccinated mice (McConnell & Pachón, 2010). Antibiotic-treated bacterial cells can produce potent vaccines (Shu et al., 2016). The preparation of the IWC vaccine generated hyperimmune antibodies which are mostly confined to the outer membrane and confer protection against a wide array of A. baumannii strains and are easy to prepare, inexpensive, and do not require denaturation of antigens which may change the conformation (McConnell & Pachón, 2010). LPS vaccines have proven to induce a protective level of immunoglobulin and reduce the pro- inflammatory cytokines (García-Quintanilla et al., 2014).
Another way to formulate a vaccine is to use the Sonicated outer membrane complex (OMC) with an adjuvant formulated with adju-phos and used as a vaccine. This type of vaccine is easily produced, and their results show inhibition of cytokines produced after infection leading to a septic shock, other than this lowering of bacterial concentration by 105 folds. An increase in IgG and IgM can be seen in response to the OMC vaccine only against the bacterial outer membrane proteins. With a single jab of the vaccine within 6 days the humoral response was capable of dispensing a protective immune response and able to sustain it for up to 21 days (McConnell, Domínguez-Herrera, et al., 2011). Outer membrane vesicles (OMV) are non-viable, spherical, and acellular nanovesicles that contribute to colonization during infection (Kuehn & Kesty, 2005). A. baumannii secretes OMVs during growth. Mice were vaccinated with OMV with adju-phos, and it was observed that there is an increase of IgG by 60 folds, the bacterial burden in tissue was reduced by 106 fold and the pro-inflammatory cytokines IL-6and IL-1β decreased post-infection (McConnell, Rumbo, et al., 2011).
11.3. Pure proteins-based vaccines
Pure protein can be used as a promised vaccine component against A. baumannii, mainly occurring from OMP and fimbriae proteins (Tan & Lahiri, 2022). One major vaccine could be the use of recombinant OmpA and aluminum hydroxide as adjuvants. Mice immunized showed high antibodies of Omp, and IgG lowering of concentration of bacterial presence in tissue (Brossard & Campagnari, 2012). Another choice is the surface protein Bap, a protein with high molecular weight and acidic in nature. Bap is a salient regulatory component of biofilm formation; it also helps in adherence. It is present in 43% of isolates and makes A. baumannii cells incapable of producing biofilm. With Freund's adjuvant recombinant Bap subunits showed similar effects (Fattahian et al., 2011).
11.4. Breakthroughs in subunits vaccine research
The concoction of CsuA/B and FimA recombinant of pilous protein, resulted in partial protection when injected (Ramezanalizadeh et al., 2020). CipA and PBP-7/8, the serum resistance factors of the outer membrane induce a potent immune response in mouse models. Polysaccharides are an attractive target for vaccine design, but they alone don't obtain enough immune response, there's always a need for a carrier protein (Harding & Feldman, 2019).
A. baumannii capsular polysaccharide recombined with cholera toxin B subunit and adjuvant aluminum hydroxide showed a good immune response (Li et al., 2022). Vaccination can be achieved with antigen expression by immunogenic antigen coding DNA by injecting the host with a plasmid (Grodeland et al., 2016). DNA vaccines are a breakthrough, OmpA coding DNA vaccine induces a CpG oligodeoxynucleotides- based adaptive immune response against A. baumannii using AMD Pal adjuvant (Lei et al., 2019). For controlling the coronavirus pandemic and the success of the mRNA vaccine is our recent achievement, in the future, it would be feasible to produce the A. baumannii mRNA vaccine.
11.5. In-silico, immune informatics and proteomics methods in novel vaccine research
With the advancement in bioinformatics as well as proteomics and genomic databases, the research attempts to bring forth vital proteins as future vaccination candidates (van Gils et al., 2022). Hypothetical proteins (HPs) of 30 different MDR A. baumannii strains are conducted by the in silico immunological analysis method, 4 of them are competent to be vaccine components. Chimeric subunits from TolC and MrcB of A. baumannii are finalized as vaccine candidates through an array of methods relied on such as the combination of T and B cell epitopes, Upstream subtractive proteomics, structural screening of immune receptors and druggability, other 13 druggable protein have been chosen (Solanki & Tiwari, 2018). Two outer membrane proteins, DcaP-like protein and HP-2 are selected for vaccine components by reverse genome analysis of genomes from 33 A. baumannii strains with downstream epitopes of B cell analysis (Fereshteh et al., 2020). Bacterial membrane proteins are predicted, and their topology is studied to finalize vaccine candidates based on antigenicity, solubility, and B-cell epitopes such as OmpH, LptE, CarO, FimF, and PfsR as top A. baumannii vaccine candidates (Beiranvand et al., 2021). A 25 amino acid long vaccine postulate has been characterized in silico by TLR-2 molecular docking, by studying the in-silico prediction of downstream epitopes of T and B cells and comparing A. baumannii strains coding OmpA (Sogasu et al., 2021). Another study has shown the integration of pan-genomic analysis of 4200 genomes, T and B cell epitopes predictions which identified multiple immunological nodes in A. baumannii, combinatorial synergy can be distinguished for different vaccination components of different antigens (McConnell & Martín-Galiano, 2021).
11.6. Unconventional therapies
Four compounds extracted from the plant Zingiber officinale are [6]-dehydrogingedione, [10]- gingerol, [6]-shogaol, [6]-gingerol. [6]-dehydrogingerdioneis the most effective while, [6]-shogaol also had antimicrobial activities on MDR A.baumanii but they were less inhibitory than [10]-gingerol (Wang et al., 2010). The combinational therapy of tetracycline with ginger compounds exponentially decreases their MIC value. The combination of commercial antibiotics with the modulatory activity of plant compounds would be a trend for new drug development, and the addition of monotherapy with commercial antibiotics would be another trend for new drug development. Holarrhena antidysenterica extract is composed of steroidal alkaline conessine. By enhancing the novobiocin activity to modify resistance, the extract increases 1-N-phenyl naphthyl amine uptake and makes the outer membrane of A. baumannii more permeable. The combination of novobiocin or rifampicin with H.antidysenterica extract or conessine lowers the MIC of both antibiotics against all A.bauamnnii strains studied with a synergistic effect (Siriyong et al., 2016).
12. Future aspect
12.1. Bacteriophage therapy
As the usage of antibiotics increases rapidly in the clinical, food industry, and health care system, antibiotics resistance among bacteria increases proportionately (Lemiech-Mirowska et al., 2021). According to the list of ten most serious threats to public health published by WHO in 2019, antibiotic resistance is one of them and in 2018 among the MDR bacteria A. baumannii was classified within the most critical priority group. For this, it's even direr to look for alternatives that can effectively take the place of conventional antibiotics treatment. One such therapeutics could be phage therapy. Discovered over a century ago, bacteriophage therapy is a major potential means for the treatment of bacterial infection when it comes to multiple drug resistance MDRs (Poole, 2003). The best way to procure A.baumannii phages is to go to hospital sewage. Unlike antibiotics, phage therapy doesn't hamper the natural microbiome of animals and the human body. Their growth is only possible in conditions where susceptible bacteria are present. Due to these features, it is possible in future that phage therapy would emerge as a promising alternative for the treatment of MDR A. baumannii. Genetically engineered bacteriophage and phage lytic enzymes avail the treatment of MDR A. baumannii with a natural bacteriophage cocktail (Grygorcewicz et al., 2021). The high virulence of A. baumannii is because A. baumannii can form biofilms, which is why it's hard to treat. Bacteriophages showed antibiotic activity against A. baumannii strains capable of biofilm formation as demonstrated by Vukotic et al. (2020). ISTD and NOVI are two bacteriophages taken from the wastewater sample and tested against 103 A. baumannii isolates 6 h later ISTD bacteriophage showed a decrease in bacterial cell count. The action of bacteriophage and antibiotics on biofilm produced by A. baumannii was tested in vitro (Vukotic et al., 2020). The AB20, A. baumannii strain was selected for this test. The biofilm was produced by immersing the plates for 24 h in human urine. The biofilm has experimented with antibiotics of different MIC values such as levofloxacin, ciprofloxacin, gentamicin, imipenem, etc. A phage cocktail of different Aba strains (1, 2, 3, 4, and 6) is used and applied with antibiotics with a titer of 1 × 107 PFU/mL. The combination of ciprofloxacin with a phage cocktail proceeds a reduction of A. baumannii cells by 93.3 % and 87 % for ½ and ¼ MIC, respectively. Other antibiotics induced a similar result. Trimethoprim/sulfamethoxazole managed to reduce the concentration of cells in biofilm by 98.6 % and 94.3 % with ½ and ¼ MIC (Grygorcewicz et al., 2021).
However, bacteriophage resistance can be acquired by A. baumannii. In vitro study conducted on two A. baumannii strains (AB900 and A9844) showed compatibility to occur resistance against specific bacteriophages (ɸFG02 and ɸC001). As a result, minimum inhibitory concentration (MIC) for ciprofloxacin decreased by 2 folds 16x decrease in MIC for ceftazidime in AB900 strain. Meropenem and ampicillin MIC decreased by 2 folds also. Resistance to bacteriophage of A. baumannii strains results in decreased antibiotic resistance and increased sensitivity to antibiotics (Gordillo Altamirano et al., 2021).
12.2. Antimicrobial proteins
12.2.1. Cathelicidins
In marsupials, they give birth to their newborns, which reside within the maternal pouch for months. In the pouch, the newborn is protected from both gram-positive and gram-negative bacteria, fungi, and protozoa by the action of cathelicidins, a family of antimicrobial peptides. The bipolar interaction of positive and negatively charged subunits interacts with the pathogen membrane (Wang et al., 2011). The best-studied human antimicrobial peptide [AMP] is LL-37, and the other AMP is found in marsupials, the WAM-1. Four MDR A. baumannii strains are treated with WAM-1 and LL-37. Both AMPs show varying activity with WAM-1 having a stronger bacteriostatic effect than LL-37. Both AMPs showed inhibition of biofilm formation by A. baumannii (Feng et al., 2013).WAM-1 is not hemolytic against human erythrocytes indicating its potential for parental use (Challenge, 2008). WAM-1s antimicrobial activity and tolerance of similar salt concentrations found in the human body make it a potential possibility for effective treatment (Jayathilaka et al., 2021).
Another AMP, octominin shows antibacterial and antibiofilm activity at low MIC and MBC of 5 to 10 µg/ml respectively. Octaminin shows a concentration-dependent antibacterial activity, furthermore, it doesn't show hemolytic activity in mammals up to 100 µg/ml concentration. It shows a high eradication of biofilms in A. baumannii. Propidium iodide and ROS generation analysis show that octominin increases membrane permeability (Neshani et al., 2020). 46 anti-Acinetobacter peptides were identified and classified into ten groups: Ceropins, Defensins, Frog AMPs, Cathelicidins, Histatins, Melittins, Mastoparan, Dermicidins, Tachyplesin, and computationally designed AMPs. Histatin-8, Melittin, Omega-76, AM-CATH 36, and two other peptides have the highest anti-A. baumannii effect against sensitive and antibiotic-resistant A. baumannii isolates. Except for Dermacidin which has a net positive charge. AMPs could likely be amongst the salient drugs against A. baumannii in the post-antibiotic era (Wang et al., 2018).
12.2.2. Defensin
The endogenous peptide Human defensin 5 [HD5] has a complex structure and showed antibacterial activity against A. baumannii. By replacing non-cationic and non-hydrophobic residues with positively charged arginine the antibacterial activity can be increased and the new derivative is called HD5d5. In vitro study of HD5d5, conducting irrational wound infection and antibacterial assay in animal models shows that the potential of HD5d5 to eradicate MDR A. baumannii is more than HD5 (Brogden, 2005). The interaction of defensin with its negative subunits with negatively charged membrane proteins A. baumannii enables it to subsequently penetrate the membrane (Hancock & Chapple, 1999). As microbes can't change the ionic properties of the membrane this makes it harder for A. baumannii to develop resistance (Schroeder et al., 2011). HD5 has an exclusive structure conferred by its triple disulfide bonds and HD5 is a 32-residue peptide long. The presence of three disulfide bonds makes it harder for its preparationve, but results show reduction and simplified peptides may be superior at killing bacteria (Kim et al., 2005). Human α- defensin can bind and suppress bacterial toxins other than its antibacterial activity (Mihu et al., 2010; Shankar et al., 2007). The MIC needed for inactivating 50 % of MDR A. baumannii by HD5 is 9.3 µg/ml.
12.3. Bactericidal gene transfer therapy
Bactericidal genes delivered by vectors that are introduced to the recipient pathogen organisms by conjugation using enervated donor cells are known as bactericidal gene therapy. This method of therapeutics could be a potential for the treatment of the multidrug resistance A. baumannii. Positive results have been shown using murine burn infection (Sharkey & Goldenberg, 2011). Using these therapeutics mice injected once with a dose of 1010 CFU of donor cells encoding bactericidal proteins lowers the A. baumannii level in burn wounds compared with untreated mice. The capability of donor cells to lower the A. baumannii cells present in wound infection was detected by quantitative culture.
12.4. Radioimmunotherapy
Radioimmunotherapy (RIT) exploits the antigen-antibody interaction to deliver radionuclides and cytotoxin radiation to target cells (Dadachova & Casadevall, 2005). Using I-131 labeled anti-HLD-DR antibody and I labelled CD-37 IgG, anti-CD20 IgG, and anti-CD22 IgG achieved a remarkable reduction in bulky masses, in patients with non-Hodgkin lymphoma, shows its potential against cancer treatment (Dadachova et al., 2003).The method of treatment has not been fully studied in vitro as an antimicrobial strategy. The use of RIT in the treatment of infectious diseases is easier than its application against cancer therapy because of tissue perforation differences in the sites of microbial infection and normal organs (Dadachova, Howell, et al., 2004). RIT has been shown successfully in treatment experimentally against fungi, bacterial and viral infection (Dadachova, Burns, et al., 2004). Monoclonal antibodies [mABs] can be labelled with α or β emitters which can initiate cell apoptosis. Using this method and specific mABs can be produced to treat A. baumannii, like radiolabelled alpha-emitter bismuth which decreases microbial metabolic activity (Kashef & Hamblin, 2017). Novel treatments can be developed by combining RIT with antimicrobial therapies to treat A. baumannii infection.
12.5. Photodynamic therapy
In photodynamic therapy (PDT) cytotoxins like reactive oxygen species [ROS], oxidize cellular components like DNA, membrane lipids, and proteins with a nontoxic dye known as photosensitizer [PS] and visible light (near infrared) (Fekrirad et al., 2021). Polycationic biopolymer chitosan induced with PDT can affect a larger number of pathogens including A. baumannii (Tsai et al., 2011). Erythrosin B is a food dye and has a photosensitizing effect. The effect of PS can be enhanced by adding biological and chemical molecules. The addition of acetic acid with erythrosine can cause planktonic A. baumannii cells to eradicate and the addition of chitosan and EB in the presence of acetic acid has a lethal effect on biofilm formation (Fekrirad et al., 2021). Methyl blue along with a 660 nm low-level laser is used to treat carbapenem- resistant strains. The number of cells in all isolates is reduced by 62 to 88 % in susceptible isolates and 26 to 97 % in resistant strains by PDT (Marcolan De Mello et al., 2019). In a similar study where the A. baumannii isolates show resistance against carbapenems treated with protoporphyrin IX and methyl blue and halogen lamp. Where methyl blue shows a higher reduction than protoporphyrin IX (Anane et al., 2020). These results show PDT as a potential alternative to treat MDR A. baumannii strains.
12.6. Nanoparticle technology
Nitric oxide (NO) can prevent skin infection, modulate immune response and wound healing (Mowbray et al., 2008). According to Mihu et al., a saline hydrogel nanoparticle (np) that is capable of the stable release of NO reduced the A. baumannii cells in murine burn wounds compared to control animals. NO-np- treated mice have suppurative inflammation, minimum bacterial burden, and less collagen degradation by bacterial collagenase (Mihu et al., 2010). NO-np is capable of completely inhibiting A. baumannii growth. The addition of chitosan, a cationically charged polymer, disrupts the cellular membrane and damages the cell wall increasing antimicrobial potential. A. baumannii growth with the concentration of 10 to 20 mg/ml of nanoparticle (np) has a significant reduction in bacterial growth after 12 h due to the addition of chitosan in the control np. Nanotechnology renders many ways of medical care. Like nano-silver for antimicrobial surfaces, chitosan for enhanced antibiotic delivery and NO-np showed significant effects against Gram-positive and Gram-negative organisms. Its ability to easily penetrate the lipid by layer and reach important metabolic enzymes and DNA, it renders biological processes from functioning (Friedman et al., 2011).
13. Conclusion
In the last few decades, our understanding of multi-drug resistant Acinetobacter baumannii has significantly increased with the aid of advanced tools like whole genome sequencing and other bioinformatics analysis. Here in this review, we emphasize the significance of virulence factors in A. baumannii pathogenesis as well as its antibiotic resistance mechanisms which include resistance against carbapenem, a popular drug currently used in most healthcare facilities. Additionally, it highlights the emergence of polymyxin B as a potential therapeutic alternative. The concept of antimicrobial peptides as a promising class of antimicrobial agents has also been discussed here. We tried to emphasize the genetic distinctions between contemporary clinical isolates and commonly used laboratory strains which may underline the importance of studying modern clinical isolates for a more accurate understanding of A. baumannii's virulence mechanisms. Over 30 species of this bacterium and their differential efficacy as multi-drug resistant superbug is of great concern to the World Health Organization (WHO).
Acinetobacter baumannii, a pathogen of significant clinical concern, has indeed presented challenges in the development of new antibiotics. The bacterium's adeptness at acquiring resistance determinants, including those against carbapenems and polymyxins, has been a formidable barrier in treatment. The situation is further complicated by the variability in study designs and the definitions of multidrug resistance, which impedes the ability to draw broad conclusions from research findings. The economic impact of A.baumannii is evident in the prolongation of hospital stays and the escalation of healthcare costs. However, the direct correlation between A. baumannii infections and mortality rates is obscured by various confounding factors and the heterogeneity of patient demographics in different studies. Some studies have small patient numbers, leading to a lack of statistical power and potentially unreliable conclusions.
The transition from in vitro to in vivo is a critical step in drug development. It involves the consideration of pharmacokinetics, pharmacodynamics, and the drug's interaction with complex physiological systems. The discrepancies between in vitro and in vivo effects can be attributed to factors such as metabolic differences, immune responses, and the presence of other comorbidities in the host organism. These differences underscore the importance of animal models and clinical trials in the drug development process, as they provide a more accurate representation of how a therapeutic agent will perform in humans.
In the case of A. baumannii, the need for novel therapeutics is urgent. The bacterium's resistance to last-resort antibiotics such as aminoglycosides, polymyxins etc, poses a significant threat to public health. The development of new drugs with novel mechanisms of action is imperative to combat this resilient pathogen. Research efforts must focus on bridging the gap between in vitro and in vivo studies to expedite the translation of experimental therapies into clinically effective treatments. Furthermore, standardization of study designs and definitions of multidrug resistance could enhance the comparability and generalizability of research findings, providing a clearer picture of the pathogen's impact on mortality and informing better strategies for management and treatment.
In conclusion, this study tends to serve a summarized view of the genomic organization, bacterial resistance mechanism, and plausible therapeutic strategies which could be potentially enough in the translational medicinal field in the future.
CRediT authorship contribution statement
H.M. and A.B and A.D.R. contributed in preparing the manuscript. A.M. and A.K.P. prepared the tables and figures. A.N. supervised the whole task and contributed in referencing and editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was not supported by any funding agency. H.M., A.B, A.M., A.K.P. A.D.R. and A.N. would like to acknowledge NCBI, BIORENDER, ENDNOTE and MENDELEY CITE for preparing the manuscript.
Data availability
The authors do not have permission to share data.
References
- Abdallah M., Olafisoye O., Cortes C., Urban C., Landman D., Quale J. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob. Agents Chemother. 2015;59(3):1802–1805. doi: 10.1128/AAC.04809-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad I., Nygren E., Khalid F., Myint S.L., Uhlin B.E. A Cyclic-di-GMP signalling network regulates biofilm formation and surface associated motility of Acinetobacter baumannii 17978. Sci. Rep. 2020;10(1):1991. doi: 10.1038/s41598-020-58522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy G., Ozyazici-Ozkan S., Tezel G., Dayar G., Köşker M., DOĞAN C. Assesment of colistin related side effects in premature neonates. Turk. J. Pediatr. 2020;62(5) doi: 10.24953/turkjped.2020.05.011. [DOI] [PubMed] [Google Scholar]
- Albur M.S., Noel A., Bowker K., MacGowan A. The combination of colistin and fosfomycin is synergistic against NDM-1-producing Enterobacteriaceae in in vitro pharmacokinetic/pharmacodynamic model experiments. Int. J. Antimicrob. Agents. 2015;46(5):560–567. doi: 10.1016/j.ijantimicag.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Almaghrabi R., Clancy C.J., Doi Y., Hao B., Chen L., Shields R.K., Press E.G., Iovine N.M., Townsend B.M., Wagener M.M. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob. Agents Chemother. 2014;58(8):4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anane Y.A., Apalata T., Vasaikar S., Okuthe G.E., Songca S.P. In vitro antimicrobial photodynamic inactivation of multidrug-resistant Acinetobacter baumannii biofilm using Protoporphyrin IX and Methylene blue. Photodiagnosis. Photodyn. Ther. 2020;30 doi: 10.1016/j.pdpdt.2020.101752. [DOI] [PubMed] [Google Scholar]
- Arndt D., Marcu A., Liang Y., Wishart D.S. PHAST, PHASTER and PHASTEST: Tools for finding prophage in bacterial genomes. Brief. Bioinform. 2019;20(4):1560–1567. doi: 10.1093/bib/bbx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub Moubareck C., Hammoudi Halat D. Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics. 2020;9(3):119. doi: 10.3390/antibiotics9030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz R.K., Bartels D., Best A.A., DeJongh M., Disz T., Edwards R.A., Formsma K., Gerdes S., Glass E.M., Kubal M. The RAST Server: rapid annotations using subsystems technology. BMC. Genomics. 2008;9(1):1–15. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M., Poulakou G., Giamarellou H. Vol. 40. Springer; 2014. Is there a future for tigecycline? pp. 1039–1045. (Intensive Care Medicine). [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. A study of the Moraxella group II. Oxidative-negative species (genus Acinetobacter) J. Bacteriol. 1968;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijerinck M. Pigmenten als oxydatieproducten gevormd door bacterien. Versl Koninklijke Akad Wetensch Amsterdam. 1911;19:1092–1103. [Google Scholar]
- Beiranvand S., Doosti A., Mirzaei S.A. Putative novel B-cell vaccine candidates identified by reverse vaccinology and genomics approaches to control Acinetobacter baumannii serotypes. Infect. Genet. Evol. 2021;96 doi: 10.1016/j.meegid.2021.105138. [DOI] [PubMed] [Google Scholar]
- Bentancor L.V, Camacho-Peiro A., Bozkurt-Guzel C., Pier G.B., Maira-Litrán T. Identification of Ata, a multifunctional trimeric autotransporter of Acinetobacter baumannii. J. Bacteriol. 2012;194(15):3950–3960. doi: 10.1128/JB.06769-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentancor L.V, O'Malley J.M., Bozkurt-Guzel C., Pier G.B., Maira-Litrán T. Poly-N-acetyl-β-(1-6)-glucosamine is a target for protective immunity against Acinetobacter baumannii infections. Infect. Immun. 2012;80(2):651–656. doi: 10.1128/IAI.05653-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentancor L.V, Routray A., Bozkurt-Guzel C., Camacho-Peiro A., Pier G.B., Maira-Litrán T. Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect. Immun. 2012;80(10):3381–3388. doi: 10.1128/IAI.06096-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A., Quirino A., Giordano M., Marano V., Rizzo C., Liberto M.C., Focà A., Pavia M. Control of carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit of a teaching hospital in Southern Italy. BMC. Infect. Dis. 2016;16(1):1–7. doi: 10.1186/s12879-016-2036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll J.M., Tucker A.T., Klein D.R., Beltran A.M., Brodbelt J.S., Davies B.W., Trent M.S. Reinforcing lipid A acylation on the cell surface of Acinetobacter baumannii promotes cationic antimicrobial peptide resistance and desiccation survival. mBio. 2015;6(3) doi: 10.1128/mbio.00478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B., Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Breslow J.M., Meissler J.J., Jr, Hartzell R.R., Spence P.B., Truant A., Gaughan J., Eisenstein T.K. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance. Infect. Immun. 2011;79(8):3317–3327. doi: 10.1128/IAI.00069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisou J. Studies on bacterial taxonomy. X. The revision of species under Acromobacter group. Annales de l'Institut Pasteur. 1954;86(6):722–728. [PubMed] [Google Scholar]
- Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Brossard K.A., Campagnari A.A. The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect. Immun. 2012;80(1):228–233. doi: 10.1128/IAI.05913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn K.W., Pantapalangkoor P., Nielsen T., Tan B., Junus J., Hujer K.M., Wright M.S., Bonomo R.A., Adams M.D., Chen W. Host fate is rapidly determined by innate effector-microbial interactions during Acinetobacter baumannii bacteremia. J. Infect. Dis. 2015;211(8):1296–1305. doi: 10.1093/infdis/jiu593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik C.C., Nicolau D.P. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2011;55(6):3002–3004. doi: 10.1128/AAC.01420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows L.L. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 2012;66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- Castanheira M., Costello S.E., Woosley L.N., Deshpande L.M., Davies T.A., Jones R.N. Evaluation of clonality and carbapenem resistance mechanisms among Acinetobacter baumannii-Acinetobacter calcoaceticus complex and Enterobacteriaceae isolates collected in European and Mediterranean countries and detection of two novel β-lactamases, GES-22 and VIM-35. Antimicrob. Agents Chemother. 2014;58(12):7358–7366. doi: 10.1128/AAC.03930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanheira M., Rhomberg P.R., Flamm R.K., Jones R.N. Effect of the β-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2016;60(9):5454–5458. doi: 10.1128/AAC.00711-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanheira M., Huband M.D., Mendes R.E., Flamm R.K. Meropenem-vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2017;61(9):10–1128. doi: 10.1128/AAC.00567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challenge S. Cathelicidin administration protects mice. J. Immunol. 2008;181:4989–5000. doi: 10.4049/jimmunol.181.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. Host innate immune responses to Acinetobacter baumannii infection. Front. Cell Infect. Microbiol. 2020;10:486. doi: 10.3389/fcimb.2020.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A., Chuang Y.-C., Sun H.-Y., Sheng W.-H., Yang C.-J., Liao C.-H., Hsueh P.-R., Yang J.-L., Shen N.-J., Wang J.-T. Excess mortality associated with colistin-tigecycline compared with colistin-carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit. Care Med. 2015;43(6):1194–1204. doi: 10.1097/CCM.0000000000000933. [DOI] [PubMed] [Google Scholar]
- Cheng M.P., Papenburg J., Desjardins M., Kanjilal S., Quach C., Libman M., Dittrich S., Yansouni C.P. Diagnostic testing for severe acute respiratory syndrome–related coronavirus 2: a narrative review. Ann. Intern. Med. 2020;172(11):726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Chan A.P. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31(16):2745–2747. doi: 10.1093/bioinformatics/btv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C.H., Lee E.Y., Lee Y.C., Park T.I., Kim H.J., Hyun S.H., Kim S.A., Lee S., Lee J.C. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7(8):1127–1138. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- Choi C.H., Lee E.Y., Lee Y.C., Park T.I., Kim H.J., Hyun S.H., Kim S.A., Lee S., Lee J.C. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7(8):1127–1138. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- Choi A.H.K., Slamti L., Avci F.Y., Pier G.B., Maira-Litrán T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-β-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 2009;191(19):5953–5963. doi: 10.1128/JB.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.J., Kil M.C., Choi J.-Y., Kim S.J., Park K.-S., Kim Y.-J., Ko K.S. Characterisation of successive Acinetobacter baumannii isolates from a deceased haemophagocytic lymphohistiocytosis patient. Int. J. Antimicrob. Agents. 2017;49(1):102–106. doi: 10.1016/j.ijantimicag.2016.09.024. [DOI] [PubMed] [Google Scholar]
- Cisneros J.M., Rodríguez-Baño J. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin. Microbiol. Infect. 2002;8(11):687–693. doi: 10.1046/j.1469-0691.2002.00487.x. [DOI] [PubMed] [Google Scholar]
- Crépin S., Ottosen E.N., Peters K., Smith S.N., Himpsl S.D., Vollmer W., Mobley H.L.T. The lytic transglycosylase MltB connects membrane homeostasis and in vivo fitness of Acinetobacter baumannii. Mol. Microbiol. 2018;109(6):745–762. doi: 10.1111/mmi.14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadachova E., Casadevall A. Antibodies as delivery vehicles for radioimmunotherapy of infectious diseases. Expert. Opin. Drug Deliv. 2005;2(6):1075–1084. doi: 10.1517/17425247.2.6.1075. [DOI] [PubMed] [Google Scholar]
- Dadachova E., Nakouzi A., Bryan R.A., Casadevall A. Ionizing radiation delivered by specific antibody is therapeutic against a fungal infection. Proc. Natl Acad. Sci. 2003;100(19):10942–10947. doi: 10.1073/pnas.1731272100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadachova E., Burns T., Bryan R.A., Apostolidis C., Brechbiel M.W., Nosanchuk J.D., Casadevall A., Pirofski L. Feasibility of radioimmunotherapy of experimental pneumococcal infection. Antimicrob. Agents Chemother. 2004;48(5):1624–1629. doi: 10.1128/AAC.48.5.1624-1629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadachova E., Howell R.W., Bryan R.A., Frenkel A., Nosanchuk J.D., Casadevall A. Susceptibility of the human pathogenic fungi Cryptococcus neoformans and Histoplasma capsulatum to γ-radiation versus radioimmunotherapy with α-and β-emitting radioisotopes. J. Nucl. Med. 2004;45(2):313–320. [PubMed] [Google Scholar]
- Daikos G.L., Tsaousi S., Tzouvelekis L.S., Anyfantis I., Psichogiou M., Argyropoulou A., Stefanou I., Sypsa V., Miriagou V., Nepka M. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob. Agents Chemother. 2014;58(4):2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter C., Murray G.L., Paulsen I.T., Peleg A.Y. Community-acquired Acinetobacter baumannii: clinical characteristics, epidemiology and pathogenesis. Expert. Rev. Anti. Infect. Ther. 2015;13(5):567–573. doi: 10.1586/14787210.2015.1025055. [DOI] [PubMed] [Google Scholar]
- Dijkshoorn L., Nemec A., Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007;5(12):939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- Doi Y., Murray G.L., Peleg A.Y. Acinetobacter baumannii: evolution of antimicrobial resistance—treatment options. Semin. Respir. Crit. Care Med. 2015;36(01):85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y., Wachino J., Arakawa Y. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect. Dis. Clin. 2016;30(2):523–537. doi: 10.1016/j.idc.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante-Mangoni E., Signoriello G., Andini R., Mattei A., De Cristoforo M., Murino P., Bassetti M., Malacarne P., Petrosillo N., Galdieri N. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin. Infect. Dis. 2013;57(3):349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- Fattahian Y., Rasooli I., Gargari S.L.M., Rahbar M.R., Astaneh S.D.A., Amani J. Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm associated protein (Bap) Microb. Pathog. 2011;51(6):402–406. doi: 10.1016/j.micpath.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Fekrirad Z., Darabpour E., Kashef N. Eradication of Acinetobacter baumannii planktonic and biofilm cells through erythrosine-mediated photodynamic inactivation augmented by acetic acid and chitosan. Curr. Microbiol. 2021;78:879–886. doi: 10.1007/s00284-021-02350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Sambanthamoorthy K., Palys T., Paranavitana C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides. 2013;49:131–137. doi: 10.1016/j.peptides.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Fereshteh S., Abdoli S., Shahcheraghi F., Ajdary S., Nazari M., Badmasti F. New putative vaccine candidates against Acinetobacter baumannii using the reverse vaccinology method. Microb. Pathog. 2020;143 doi: 10.1016/j.micpath.2020.104114. [DOI] [PubMed] [Google Scholar]
- Fournier P.-E., Vallenet D., Barbe V., Audic S., Ogata H., Poirel L., Richet H., Robert C., Mangenot S., Abergel C. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS. Genet. 2006;2(1):e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A., Blecher K., Sanchez D., Tuckman-Vernon C., Gialanella P., Friedman J.M., Martinez L.R., Nosanchuk J.D. Susceptibility of Gram-positive and-negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence. 2011;2(3):217–221. doi: 10.4161/viru.2.3.16161. [DOI] [PubMed] [Google Scholar]
- Fritzenwanker M., Imirzalioglu C., Herold S., Wagenlehner F.M., Zimmer K.-P., Chakraborty T. Treatment options for carbapenem-resistant gram-negative infections. Deutsches Ärzteblatt International. 2018;115(20–21):345. doi: 10.3238/arztebl.2018.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta Y., Muder R.R., Agha M.E., Clarke L.G., Wagener M.M., Hensler A.M., Doi Y. Risk factors for acquisition of multidrug-resistant Acinetobacter baumannii among cancer patients. Am. J. Infect. Control. 2013;41(12):1249–1252. doi: 10.1016/j.ajic.2013.04.003. [DOI] [PubMed] [Google Scholar]
- García-Patiño M.G., García-Contreras R., Licona-Limón P. The immune response against Acinetobacter baumannii, an emerging pathogen in nosocomial infections. Front. Immunol. 2017;8:441. doi: 10.3389/fimmu.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Quintanilla M., Pulido M.R., Pachon J., McConnell M.J. Immunization with lipopolysaccharide-deficient whole cells provides protective immunity in an experimental mouse model of Acinetobacter baumannii infection. PLoS. One. 2014;9(12) doi: 10.1371/journal.pone.0114410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg N., Singh R., Shukla G., Capalash N., Sharma P. Immunoprotective potential of in silico predicted Acinetobacter baumannii outer membrane nuclease, NucAb. Int. J. Med. Microbiol. 2016;306(1):1–9. doi: 10.1016/j.ijmm.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Garnacho-Montero J., Dimopoulos G., Poulakou G., Akova M., Cisneros J.M., De Waele J., Petrosillo N., Seifert H., Timsit J.F., Vila J. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intens. Care Med. 2015;41:2057–2075. doi: 10.1007/s00134-015-4079-4. [DOI] [PubMed] [Google Scholar]
- Geisinger E., Mortman N.J., Vargas-Cuebas G., Tai A.K., Isberg R.R. A global regulatory system links virulence and antibiotic resistance to envelope homeostasis in Acinetobacter baumannii. PLoS. Pathog. 2018;14(5) doi: 10.1371/journal.ppat.1007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellou H., Poulakou G. Multidrug-resistant Gram-negative infections: what are the treatment options? Drugs. 2009;69:1879–1901. doi: 10.2165/11315690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Glasser S.W., Hagood J.S., Wong S., Taype C.A., Madala S.K., Hardie W.D. Mechanisms of lung fibrosis resolution. Am. J. Pathol. 2016;186(5):1066–1077. doi: 10.1016/j.ajpath.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V.K., Kapil A. Monoclonal antibodies against the iron regulated outer membrane proteins of Acinetobacter baumannii are bactericidal. BMC. Microbiol. 2001;1(1):1–8. doi: 10.1186/1471-2180-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh H.M.S., Beatson S.A., Totsika M., Moriel D.G., Phan M.-D., Szubert J., Runnegar N., Sidjabat H.E., Paterson D.L., Nimmo G.R. Molecular analysis of the Acinetobacter baumannii biofilm-associated protein. Appl. Environ. Microbiol. 2013;79(21):6535–6543. doi: 10.1128/AEM.01402-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo Altamirano F., Forsyth J.H., Patwa R., Kostoulias X., Trim M., Subedi D., Archer S.K., Morris F.C., Oliveira C., Kielty L. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat. Microbiol. 2021;6(2):157–161. doi: 10.1038/s41564-020-00830-7. [DOI] [PubMed] [Google Scholar]
- Gottesman T., Fedorowsky R., Yerushalmi R., Lellouche J., Nutman A. An outbreak of carbapenem-resistant Acinetobacter baumannii in a COVID-19 dedicated hospital. Infect. Prev. Pract. 2021;3(1) doi: 10.1016/j.infpip.2021.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabein B., Graninger W., Baño J.R., Dinh A., Liesenfeld D.B. Intravenous fosfomycin—back to the future. Systematic review and meta-analysis of the clinical literature. Clin. Microbiol. Infect. 2017;23(6):363–372. doi: 10.1016/j.cmi.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Greene C., Wu J., Rickard A.H., Xi C. Evaluation of the ability of Acinetobacter baumannii to form biofilms on six different biomedical relevant surfaces. Lett. Appl. Microbiol. 2016;63(4):233–239. doi: 10.1111/lam.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodeland G., Fredriksen A.B., Løset G.Å., Vikse E., Fugger L., Bogen B. Antigen targeting to human HLA class II molecules increases efficacy of DNA vaccination. J. Immunol. 2016;197(9):3575–3585. doi: 10.4049/jimmunol.1600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorcewicz B., Wojciuk B., Roszak M., Łubowska N., Błażejczak P., Jursa-Kulesza J., Rakoczy R., Masiuk H., Dołęgowska B. Environmental phage-based cocktail and antibiotic combination effects on Acinetobacter baumannii biofilm in a human urine model. Microb. Drug Resist. 2021;27(1):25–35. doi: 10.1089/mdr.2020.0083. [DOI] [PubMed] [Google Scholar]
- Hackel M.A., Lomovskaya O., Dudley M.N., Karlowsky J.A., Sahm D.F. In vitro activity of meropenem-vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob. Agents Chemother. 2018;62(1):10–1128. doi: 10.1128/AAC.01904-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoudi D., Moubareck C.A., Hakime N., Houmani M., Barakat A., Najjar Z., Suleiman M., Fayad N., Sarraf R., Sarkis D.K. Spread of imipenem-resistant Acinetobacter baumannii co-expressing OXA-23 and GES-11 carbapenemases in Lebanon. International Journal of Infectious Diseases. 2015;36:56–61. doi: 10.1016/j.ijid.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Hamouda A., Amyes S.G.B. Novel gyrA and parC point mutations in two strains of Acinetobacter baumannii resistant to ciprofloxacin. Journal of Antimicrobial Chemotherapy. 2004;54(3):695–696. doi: 10.1093/jac/dkh368. [DOI] [PubMed] [Google Scholar]
- Hancock R.E., Chapple D.S. Peptide antibiotics. Antimicrobial agents and chemotherapy. 1999;43(6):1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C.M., Feldman M.F. Glycoengineering bioconjugate vaccines, therapeutics, and diagnostics in E. coli. Glycobiology. 2019;29(7):519–529. doi: 10.1093/glycob/cwz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C.M., Hennon S.W., Feldman M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018;16(2):91–102. doi: 10.1038/nrmicro.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G., KuoLee R., Xu H.H., Chen W. Acute intraperitoneal infection with a hypervirulent Acinetobacter baumannii isolate in mice. Sci. Rep. 2019;9(1):6538. doi: 10.1038/s41598-019-43000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseley S.R., Pantophlet R., Brade L., Holst O., Brade H. Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter junii strain 65. Eur. J. Biochem. 1997;245(2):477–481. doi: 10.1111/j.1432-1033.1997.t01-1-00477.x. [DOI] [PubMed] [Google Scholar]
- He L., Jhong J.-H., Chen Q., Huang K.-Y., Strittmatter K., Kreuzer J., DeRan M., Wu X., Lee T.-Y., Slavov N. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021;37(5) doi: 10.1016/j.celrep.2021.109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritier C., Poirel L., Nordmann P. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clinical Microbiology and Infection. 2006;12(2):123–130. doi: 10.1111/j.1469-0691.2005.01320.x. [DOI] [PubMed] [Google Scholar]
- Hirsch E.B., Guo B., Chang K.-T., Cao H., Ledesma K.R., Singh M., Tam V.H. Assessment of antimicrobial combinations for Klebsiella pneumoniae carbapenemase–producing K. pneumoniae. J. Infect. Dis. 2013;207(5):786–793. doi: 10.1093/infdis/jis766. [DOI] [PubMed] [Google Scholar]
- Hood R.D., Singh P., Hsu F., Güvener T., Carl M.A., Trinidad R.R.S., Silverman J.M., Ohlson B.B., Hicks K.G., Plemel R.L. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host. Microbe. 2010;7(1):25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A., O'Donoghue M., Feeney A., Sleator R.D. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3(3):243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Wang S., Yao Y., Xia Y., Yang X., Long Q., Sun W., Liu C., Li Y., Ma Y. OmpW is a potential target for eliciting protective immunity against Acinetobacter baumannii infections. Vaccine. 2015;33(36):4479–4485. doi: 10.1016/j.vaccine.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Huys G., Cnockaert M., Vaneechoutte M., Woodford N., Nemec A., Dijkshoorn L., Swings J. Distribution of tetracycline resistance genes in genotypically related and unrelated multiresistant Acinetobacter baumannii strains from different European hospitals. Research in Microbiology. 2005;156(3):348–355. doi: 10.1016/j.resmic.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Jawad A., Seifert H., Snelling A.M., Heritage J., Hawkey P.M. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J. Clin. Microbiol. 1998;36(7):1938–1941. doi: 10.1128/jcm.36.7.1938-1941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayathilaka E.H.T.T., Rajapaksha D.C., Nikapitiya C., De Zoysa M., Whang I. Antimicrobial and anti-biofilm peptide octominin for controlling multidrug-resistant Acinetobacter baumannii. Int. J. Mol. Sci. 2021;22(10):5353. doi: 10.3390/ijms22105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys S., Chambers J.P., Yu J.-J., Hung C.-Y., Forsthuber T., Arulanandam B.P. Insights into Acinetobacter baumannii protective immunity. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1070424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J.H., Lee J.H., Lee J.J., Park K.S., Karim A.M., Lee C.-R., Jeong B.C., Lee S.H. Structural basis for carbapenem-hydrolyzing mechanisms of carbapenemases conferring antibiotic resistance. International Journal of Molecular Sciences. 2015;16(5):9654–9692. doi: 10.3390/ijms16059654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B., Raphenya A.R., Alcock B., Waglechner N., Guo P., Tsang K.K., Lago B.A., Dave B.M., Pereira S., Sharma A.N. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic. Acids. Res. 2016:gkw1004. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juttukonda L.J., Chazin W.J., Skaar E.P. Acinetobacter baumannii coordinates urea metabolism with metal import to resist host-mediated metal limitation. mBio. 2016;7(5) doi: 10.1128/mbio.01475-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshida G., Tansho-Nagakawa S., Kikuchi-Ueda T., Nakano R., Hikosaka K., Nishida S., Ubagai T., Higashi S., Ono Y. A novel bacterial transport mechanism of Acinetobacter baumannii via activated human neutrophils through interleukin-8. J. Leucocyte Biol. 2016;100(6):1405–1412. doi: 10.1189/jlb.4AB0116-023RR. [DOI] [PubMed] [Google Scholar]
- Kamoshida G., Kikuchi-Ueda T., Nishida S., Tansho-Nagakawa S., Ubagai T., Ono Y. Pathogenic bacterium Acinetobacter baumannii inhibits the formation of neutrophil extracellular traps by suppressing neutrophil adhesion. Front. Immunol. 2018;9:178. doi: 10.3389/fimmu.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaiskos I., Souli M., Galani I., Giamarellou H. Colistin: still a lifesaver for the 21st century? Expert. Opin. Drug Metab. Toxicol. 2017;13(1):59–71. doi: 10.1080/17425255.2017.1230200. [DOI] [PubMed] [Google Scholar]
- Karaiskos I., Lagou S., Pontikis K., Rapti V., Poulakou G. The “old” and the “new” antibiotics for MDR gram-negative pathogens: for whom, when, and how. Front. Public Health. 2019;7:151. doi: 10.3389/fpubh.2019.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaiskos I., Lagou S., Pontikis K., Rapti V., Poulakou G. The “old” and the “new” antibiotics for MDR gram-negative pathogens: for whom, when, and how. Front. Public Health. 2019;7:151. doi: 10.3389/fpubh.2019.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashef N., Hamblin M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updates. 2017;31:31–42. doi: 10.1016/j.drup.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K.M., de Jonge B.L.M., Stone G.G., Sahm D.F. In vitro activity of ceftazidime/avibactam against isolates of Enterobacteriaceae collected in European countries: INFORM global surveillance 2012–15. J. Antimicrob. Chemother. 2018;73(10):2782–2788. doi: 10.1093/jac/dky266. [DOI] [PubMed] [Google Scholar]
- Keepers T.R., Gomez M., Celeri C., Nichols W.W., Krause K.M. Bactericidal activity, absence of serum effect, and time-kill kinetics of ceftazidime-avibactam against β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014;58(9):5297–5305. doi: 10.1128/AAC.02894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Gajendran N., Mittrücker H.-W., Weiwad M., Song Y.-H., Hurwitz R., Wilmanns M., Fischer G., Kaufmann S.H.E. Human α-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl Acad. Sci. 2005;102(13):4830–4835. doi: 10.1073/pnas.0500508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Choi C.H., Moon D.C., Jin J.S., Lee J.H., Shin J.-H., Kim J.M., Lee Y.C., Seol S.Y., Cho D.T. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol. Lett. 2009;301(2):224–231. doi: 10.1111/j.1574-6968.2009.01820.x. [DOI] [PubMed] [Google Scholar]
- Kim C.-H., Jeong Y.-J., Lee J., Jeon S.-J., Park S.-R., Kang M.-J., Park J.-H., Park J.-H. Essential role of toll-like receptor 4 in Acinetobacter baumannii-induced immune responses in immune cells. Microb. Pathog. 2013;54:20–25. doi: 10.1016/j.micpath.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Kim Y.-J., Ko K.S. Genomic analysis of consecutive Acinetobacter baumannii strains from a single patient. Front. Microbiol. 2018;9:2840. doi: 10.3389/fmicb.2018.02840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.B., Swiatlo E., Swiatlo A., McDaniel L.S. Serum resistance and biofilm formation in clinical isolates of Acinetobacter baumannii. FEMS Immunol. Med. Microbiol. 2009;55(3):414–421. doi: 10.1111/j.1574-695X.2009.00538.x. [DOI] [PubMed] [Google Scholar]
- Knapp S., Wieland C.W., Florquin S., Pantophlet R., Dijkshoorn L., Tshimbalanga N., Akira S., van der Poll T. Differential roles of CD14 and toll-like receptors 4and 2 in murine Acinetobacter pneumonia. Am. J. Respir. Crit. Care Med. 2006;173(1):122–129. doi: 10.1164/rccm.200505-730OC. [DOI] [PubMed] [Google Scholar]
- Knapp S., Wieland C.W., Florquin S., Pantophlet R., Dijkshoorn L., Tshimbalanga N., Akira S., van der Poll T. Differential roles of CD14 and toll-like receptors 4and 2 in murine Acinetobacter pneumonia. Am. J. Respir. Crit. Care Med. 2006;173(1):122–129. doi: 10.1164/rccm.200505-730OC. [DOI] [PubMed] [Google Scholar]
- Konstantinidis T., Kambas K., Mitsios A., Panopoulou M., Tsironidou V., Dellaporta E., Kouklakis G., Arampatzioglou A., Angelidou I., Mitroulis I. Immunomodulatory role of clarithromycin in Acinetobacter baumannii infection via formation of neutrophil extracellular traps. Antimicrob. Agents Chemother. 2016;60(2):1040–1048. doi: 10.1128/AAC.02063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomanachai P., Kim A., Nicolau D.P. Pharmacodynamic evaluation of tigecycline against Acinetobacter baumannii in a murine pneumonia model. J. Antimicrob. Chemother. 2009;63(5):982–987. doi: 10.1093/jac/dkp056. [DOI] [PubMed] [Google Scholar]
- Kuehn M.J., Kesty N.C. Bacterial outer membrane vesicles and the host–pathogen interaction. Genes Dev. 2005;19(22):2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- Lai C.-C., Wang C.-Y., Hsueh P.-R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020;53(4):505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman D., Kelly P., Bäcker M., Babu E., Shah N., Bratu S., Quale J. Antimicrobial activity of a novel aminoglycoside, ACHN-490, against Acinetobacter baumannii and Pseudomonas aeruginosa from New York City. J. Antimicrob. Chemother. 2011;66(2):332–334. doi: 10.1093/jac/dkq459. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Lee J.C., Lee C.-M., Jung I.D., Jeong Y.-I., Seong E.-Y., Chung H.-Y., Park Y.-M. Outer membrane protein A of Acinetobacter baumannii induces differentiation of CD4+ T cells toward a Th1 polarizing phenotype through the activation of dendritic cells. Biochem. Pharmacol. 2007;74(1):86–97. doi: 10.1016/j.bcp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Lee C.-R., Lee J.H., Park M., Park K.S., Bae I.K., Kim Y.B., Cha C.-J., Jeong B.C., Lee S.H. Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell Infect. Microbiol. 2017;7:55. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.H., Aslanyan L., Vidyasagar A., Brennan M.B., Tauber M.S., Carrillo-Sepulveda M.A., Dores M.R., Rigel N.W., Martinez L.R. Depletion of alveolar macrophages increases pulmonary neutrophil infiltration, tissue damage, and sepsis in a murine model of Acinetobacter baumannii pneumonia. Infect. Immun. 2020;88(7):10–1128. doi: 10.1128/IAI.00128-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L., Yang F., Zou J., Jing H., Zhang J., Xu W., Zou Q., Zhang J., Wang X. DNA vaccine encoding OmpA and Pal from Acinetobacter baumannii efficiently protects mice against pulmonary infection. Mol. Biol. Rep. 2019;46:5397–5408. doi: 10.1007/s11033-019-04994-2. [DOI] [PubMed] [Google Scholar]
- Lemiech-Mirowska E., Kiersnowska Z.M., Michałkiewicz M., Depta A., Marczak M. Nosocomial infections as one of the most important problems of healthcare system. Ann. Agric. Environ. Med. 2021;28(3) doi: 10.26444/aaem/122629. [DOI] [PubMed] [Google Scholar]
- Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu F., Zhang Y., Wang X., Zhao C., Chen H., Zhang F., Zhu B., Hu Y., Wang H. Evolution of carbapenem-resistant Acinetobacter baumannii revealed through whole-genome sequencing and comparative genomic analysis. Antimicrob. Agents Chemother. 2015;59(2):1168–1176. doi: 10.1128/AAC.04609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Pan C., Liu Z., Sun P., Hua X., Feng E., Yu Y., Wu J., Zhu L., Wang H. Safety and immunogenicity of a new glycoengineered vaccine against Acinetobacter baumannii in mice. Microb. Biotechnol. 2022;15(2):703–716. doi: 10.1111/1751-7915.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima W.G., Alves M.C., Cruz W.S., Paiva M.C. Chromosomally encoded and plasmid-mediated polymyxins resistance in Acinetobacter baumannii: a huge public health threat. European Journal of Clinical Microbiology & Infectious Diseases. 2018;37:1009–1019. doi: 10.1007/s10096-018-3223-9. [DOI] [PubMed] [Google Scholar]
- Lin L., Tan B., Pantapalangkoor P., Ho T., Baquir B., Tomaras A., Montgomery J.I., Reilly U., Barbacci E.G., Hujer K. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio. 2012;3(5) doi: 10.1128/mbio.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Tan B., Pantapalangkoor P., Ho T., Baquir B., Tomaras A., Montgomery J.I., Reilly U., Barbacci E.G., Hujer K. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio. 2012;3(5):10–1128. doi: 10.1128/mBio.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xu W. Neutrophil and macrophage response in Acinetobacter Baumannii Infection and their relationship to lung injury. Front. Cell Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.890511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-Y., Wang Y., Walsh T.R., Yi L.-X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious Diseases. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- Livermore D.M., Mushtaq S., Warner M., Zhang J.-C., Maharjan S., Doumith M., Woodford N. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J. Antimicrob. Chemother. 2011;66(1):48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- Luo G., Lin L., Ibrahim A.S., Baquir B., Pantapalangkoor P., Bonomo R.A., Doi Y., Adams M.D., Russo T.A., Spellberg B. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS. One. 2012;7(1):e29446. doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet S., Courvalin P., Lambert T. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrobial Agents and Chemotherapy. 2001;45(12):3375–3380. doi: 10.1128/AAC.45.12.3375-3380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcolan De Mello M., De Barros P.P., de Cassia Bernardes R., Alves S.R., Ramanzini N.P., Figueiredo-Godoi L.M.A., Prado A.C.C., Jorge A.O.C., Junqueira J.C. Antimicrobial photodynamic therapy against clinical isolates of carbapenem-susceptible and carbapenem-resistant Acinetobacter baumannii. Lasers. Med. Sci. 2019;34:1755–1761. doi: 10.1007/s10103-019-02773-w. [DOI] [PubMed] [Google Scholar]
- McConnell M.J., Martín-Galiano A.J. Designing multi-antigen vaccines against Acinetobacter baumannii using systemic approaches. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.666742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M.J., Pachón J. Active and passive immunization against Acinetobacter baumannii using an inactivated whole cell vaccine. Vaccine. 2010;29(1):1–5. doi: 10.1016/j.vaccine.2010.10.052. [DOI] [PubMed] [Google Scholar]
- McConnell M.J., Domínguez-Herrera J., Smani Y., López-Rojas R., Docobo-Pérez F., Pachón J. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii. Infect. Immun. 2011;79(1):518–526. doi: 10.1128/IAI.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M.J., Rumbo C., Bou G., Pachón J. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine. 2011;29(34):5705–5710. doi: 10.1016/j.vaccine.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Mihu M.R., Sandkovsky U., Han G., Friedman J.M., Nosanchuk J.D., Martinez L.R. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence. 2010;1(2):62–67. doi: 10.4161/viru.1.2.10038. [DOI] [PubMed] [Google Scholar]
- Miriagou V., Cornaglia G., Edelstein M., Galani I., Giske C.G., Gniadkowski M., Malamou-Lada E., Martinez-Martinez L., Navarro F., Nordmann P. Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clinical Microbiology and Infection. 2010;16(2):112–122. doi: 10.1111/j.1469-0691.2009.03116.x. [DOI] [PubMed] [Google Scholar]
- Morris F.C., Dexter C., Kostoulias X., Uddin M.I., Peleg A.Y. The mechanisms of disease caused by Acinetobacter baumannii. Front. Microbiol. 2019;10:1601. doi: 10.3389/fmicb.2019.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbray M., Tan X., Wheatley P.S., Morris R.E., Weller R.B. Topically applied nitric oxide induces T-lymphocyte infiltration in human skin, but minimal inflammation. J. Invest. Dermatol. 2008;128(2):352–360. doi: 10.1038/sj.jid.5701096. [DOI] [PubMed] [Google Scholar]
- Nairn B.L., Lonergan Z.R., Wang J., Braymer J.J., Zhang Y., Calcutt M.W., Lisher J.P., Gilston B.A., Chazin W.J., de Crécy-Lagard V. The response of Acinetobacter baumannii to zinc starvation. Cell Host. Microbe. 2016;19(6):826–836. doi: 10.1016/j.chom.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neshani A., Sedighian H., Mirhosseini S.A., Ghazvini K., Zare H., Jahangiri A. Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections. Microb. Pathog. 2020;146 doi: 10.1016/j.micpath.2020.104238. [DOI] [PubMed] [Google Scholar]
- Noto M.J., Boyd K.L., Burns W.J., Varga M.G., Peek R.M., Jr, Skaar E.P. Toll-like receptor 9 contributes to defense against Acinetobacter baumannii infection. Infect. Immun. 2015;83(10):4134–4141. doi: 10.1128/IAI.00410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette F., Bernier-Jean A., Brunette V., Ammann H., Lavergne V., Pichette V., Troyanov S., Bouchard J. Acute kidney injury and renal recovery with the use of aminoglycosides: a large retrospective study. Nephron. 2015;131(3):153–160. doi: 10.1159/000440867. [DOI] [PubMed] [Google Scholar]
- Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 2008;21(3):538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F., Bonomo R.A. Vaccines for Acinetobacter baumannii: thinking “out of the box. Vaccine. 2014;32(22):2537. doi: 10.1016/j.vaccine.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S., Innes G.K., Walters M.S., Mehr J., Arias J., Greeley R., Chew D. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions—New Jersey, February–July 2020. Morb. Mort. Weekly Rep. 2020;69(48):1827. doi: 10.15585/mmwr.mm6948e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue J.M., Mann T., Barber K.E., Kaye K.S. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert. Rev. Anti. Infect. Ther. 2013;11(4):383–393. doi: 10.1586/eri.13.14. [DOI] [PubMed] [Google Scholar]
- Poole K. Overcoming multidrug resistance in gram-negative bacteria. Curr. Opin. Invest. Drugs (London, England: 2000) 2003;4(2):128–139. [PubMed] [Google Scholar]
- Qiu H., KuoLee R., Harris G., Chen W. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect. Immun. 2009;77(3):1015–1021. doi: 10.1128/IAI.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H., KuoLee R., Harris G., Van Rooijen N., Patel G.B., Chen W. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS. One. 2012;7(6):e40019. doi: 10.1371/journal.pone.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan A.M., Bush K. Carbapenemases: the versatile β-lactamases. Clinical Microbiology Reviews. 2007;20(3):440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezanalizadeh F., Owlia P., Rasooli I. Type I pili, CsuA/B and FimA induce a protective immune response against Acinetobacter baumannii. Vaccine. 2020;38(34):5436–5446. doi: 10.1016/j.vaccine.2020.06.052. [DOI] [PubMed] [Google Scholar]
- Ramirez M.S., Bonomo R.A., Tolmasky M.E. Carbapenemases: Transforming Acinetobacter baumannii into a yet more dangerous menace. Biomolecules. 2020;10(5):720. doi: 10.3390/biom10050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel K., Chagas T.P.G., De-Simone S.G. Acinetobacter baumannii infections in times of COVID-19 pandemic. Pathogens. 2021;10(8):1006. doi: 10.3390/pathogens10081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword. Lancet. 2020;395(10230):1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca I., Espinal P., Vila-Farrés X., Vila J. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front. Microbiol. 2012;3:148. doi: 10.3389/fmicb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbo C., Tomás M., Fernandez Moreira E., Soares N.C., Carvajal M., Santillana E., Beceiro A., Romero A., Bou G. The Acinetobacter baumannii Omp33-36 porin is a virulence factor that induces apoptosis and modulates autophagy in human cells. Infect. Immun. 2014;82(11):4666–4680. doi: 10.1128/IAI.02034-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppé É., Woerther P.-L., Barbier F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Annals of Intensive Care. 2015;5:1–15. doi: 10.1186/s13613-015-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo T.A., Luke N.R., Beanan J.M., Olson R., Sauberan S.L., MacDonald U., Schultz L.W., Umland T.C., Campagnari A.A. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect. Immun. 2010;78(9):3993–4000. doi: 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saipriya K., Swathi C.H., Ratnakar K.S., Sritharan V. Quorum-sensing system in Acinetobacter baumannii: a potential target for new drug development. J. Appl. Microbiol. 2020;128(1):15–27. doi: 10.1111/jam.14330. [DOI] [PubMed] [Google Scholar]
- Schroeder B.O., Wu Z., Nuding S., Groscurth S., Marcinowski M., Beisner J., Buchner J., Schaller M., Stange E.F., Wehkamp J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature. 2011;469(7330):419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- Seifert H., Stefanik D., Sutcliffe J.A., Higgins P.G. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int. J. Antimicrob. Agents. 2018;51(1):62–64. doi: 10.1016/j.ijantimicag.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Shankar R., He L.-K., Szilagyi A., Muthu K., Gamelli R.L., Filutowicz M., Wendt J.L., Suzuki H., Dominguez M. A novel antibacterial gene transfer treatment for multidrug-resistant Acinetobacter baumannii-induced burn sepsis. J. Burn Care Res. 2007;28(1):6–12. doi: 10.1097/BCR.0b013e31802c8861. [DOI] [PubMed] [Google Scholar]
- Sharifipour E., Shams S., Esmkhani M., Khodadadi J., Fotouhi-Ardakani R., Koohpaei A., Doosti Z., Ej Golzari S. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC. Infect. Dis. 2020;20(1):1–7. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey R.M., Goldenberg D.M. Cancer radioimmunotherapy. Immunotherapy. 2011;3(3):349–370. doi: 10.2217/imt.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara D.R., dos Santos Saalfeld S.M., Martinez H.V., Altafini D.D., Costa B.B., Fedrigo N.H., Tognim M.C.B. Outbreak of endemic carbapenem-resistant Acinetobacter baumannii in a coronavirus disease 2019 (COVID-19)–specific intensive care unit. Infect. Control Hosp. Epidemiol. 2022;43(6):815–817. doi: 10.1017/ice.2021.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu M.-H., MatRahim N., NorAmdan N., Pang S.-P., Hashim S.H., Phoon W.-H., AbuBakar S. An inactivated antibiotic-exposed whole-cell vaccine enhances bactericidal activities against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2016;6(1):22332. doi: 10.1038/srep22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert D.M., Ricks P., Edwards J.R., Schneider A., Patel J., Srinivasan A., Kallen A., Limbago B., Fridkin S. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013;34(1):1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- Sirijatuphat R., Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2014;58(9):5598–5601. doi: 10.1128/AAC.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriyong T., Chusri S., Srimanote P., Tipmanee V., Voravuthikunchai S.P. Holarrhena antidysenterica extract and its steroidal alkaloid, conessine, as resistance-modifying agents against extensively drug-resistant Acinetobacter baumannii. Microb. Drug Resist. 2016;22(4):273–282. doi: 10.1089/mdr.2015.0194. [DOI] [PubMed] [Google Scholar]
- Smani Y., McConnell M.J., Pachón J. Role of fibronectin in the adhesion of Acinetobacter baumannii to host cells. PLoS. One. 2012;7(4):e33073. doi: 10.1371/journal.pone.0033073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smani Y., Dominguez-Herrera J., Pachón J. Association of the outer membrane protein Omp33 with fitness and virulence of Acinetobacter baumannii. J. Infect. Dis. 2013;208(10):1561–1570. doi: 10.1093/infdis/jit386. [DOI] [PubMed] [Google Scholar]
- Sogasu D., Girija A.S.S., Gunasekaran S., Priyadharsini J.V. Molecular characterization and epitope-based vaccine predictions for ompA gene associated with biofilm formation in multidrug-resistant strains of A. baumannii. Silico Pharmacol. 2021;9:1–11. doi: 10.1007/s40203-020-00074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki V., Tiwari V. Subtractive proteomics to identify novel drug targets and reverse vaccinology for the development of chimeric vaccine against Acinetobacter baumannii. Sci. Rep. 2018;8(1):9044. doi: 10.1038/s41598-018-26689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenath K., Batra P., Vinayaraj E.V, Bhatia R., SaiKiran K.V.P., Singh V., Singh S., Verma N., Singh U.B., Mohan A. Coinfections with other respiratory pathogens among patients with COVID-19. Microbiol. Spectr. 2021;9(1):e00163. doi: 10.1128/spectrum.00163-21. -21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E. Global priority list of antibiotic-resistant bacteria to guide research. Discov. Develop. 2017 [Google Scholar]
- Tada T., Miyoshi-Akiyama T., Shimada K., Shimojima M., Kirikae T. Dissemination of 16S rRNA methylase ArmA-producing Acinetobacter baumannii and emergence of OXA-72 carbapenemase coproducers in Japan. Antimicrob. Agents Chemother. 2014;58(5):2916–2920. doi: 10.1128/AAC.01212-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.C., Lahiri C. Promising acinetobacter baumannii vaccine candidates and drug targets in recent years. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.900509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansho-Nagakawa S., Sato Y., Ubagai T., Kikuchi-Ueda T., Kamoshida G.O., Nishida S., Ono Y. Histopathological analysis of lung infection in a Mouse model. Pol. J. Microbiol. 2021;70(4):469–477. doi: 10.33073/pjm-2021-044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton K.A., Rather P.N. An ompR-envZ two-component system ortholog regulates phase variation, osmotic tolerance, motility, and virulence in Acinetobacter baumannii strain AB5075. J. Bacteriol. 2017;199(3) doi: 10.1128/jb.00705-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner K.J. Clinical importance and antibiotic resistance of Acinetobacter spp: Proceedings of a symposium held on 4-5 November 1996 at Eilat, Israel. J. Med. Microbiol. 1997;46(9):721–746. doi: 10.1099/00222615-46-9-721. [DOI] [PubMed] [Google Scholar]
- Tsai T., Chien H.-F., Wang T.-H., Huang C.-T., Ker Y.-B., Chen C.-T. Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 2011;55(5):1883–1890. doi: 10.1128/AAC.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Nakao N., Yamamoto S., Hirai Y., Miyamoto K., Tsujibo H. NK1. 1+ cells regulate neutrophil migration in mice with Acinetobacter baumannii pneumonia. Microbiol. Immunol. 2012;56(2):107–116. doi: 10.1111/j.1348-0421.2011.00402.x. [DOI] [PubMed] [Google Scholar]
- Vardakas K.Z., Legakis N.J., Triarides N., Falagas M.E. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int. J. Antimicrob. Agents. 2016;47(4):269–285. doi: 10.1016/j.ijantimicag.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar S., Rajenderan S., Laishram S., Anandan S., Balaji V., Biswas I. Biofilm formation and motility depend on the nature of the Acinetobacter baumannii clinical isolates. Front. Public Health. 2016;4:105. doi: 10.3389/fpubh.2016.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukotic G., Obradovic M., Novovic K., Di Luca M., Jovcic B., Fira D., Neve H., Kojic M., McAuliffe O. Characterization, antibiofilm, and depolymerizing activity of two phages active on carbapenem-resistant Acinetobacter baumannii. Front. Med. (Lausanne) 2020;7:426. doi: 10.3389/fmed.2020.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-López R., Solano-Gálvez S.G., Juárez Vignon-Whaley J.J., Abello Vaamonde J.A., Padró Alonzo L.A., Rivera Reséndiz A., Muleiro Álvarez M., Vega López E.N., Franyuti-Kelly G., Álvarez-Hernández D.A. Acinetobacter baumannii resistance: a real challenge for clinicians. Antibiotics. 2020;9(4):205. doi: 10.3390/antibiotics9040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen C., Chen H., Huang W., Lin W., Chen T., Lin C., Chien H., Lu P., Lin C. Zingiber officinale (ginger) compounds have tetracycline-resistance modifying effects against clinical extensively drug-resistant Acinetobacter baumannii. Phytother. Res. 2010;24(12):1825–1830. doi: 10.1002/ptr.3201. [DOI] [PubMed] [Google Scholar]
- Wang J., Wong E.S.W., Whitley J.C., Li J., Stringer J.M., Short K.R., Renfree M.B., Belov K., Cocks B.G. Ancient antimicrobial peptides kill antibiotic-resistant pathogens: Australian mammals provide new options. PLoS. One. 2011;6(8):e24030. doi: 10.1371/journal.pone.0024030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhao G., Wang S., Chen Y., Gong Y., Chen S., Xu Y., Hu M., Wang X., Zeng H. A simplified derivative of human defensin 5 with potent and efficient activity against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2018;62(2) doi: 10.1128/aac.01504-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Xu Q., Heng H., Zhao W., Ni H., Chen K., Chan B.K.W., Tang Y., Xie M., Peng M. High mortality of Acinetobacter baumannii infection is attributed to macrophage-mediated induction of cytokine storm but preventable by naproxen. EBioMedicine. 2024;108 doi: 10.1016/j.ebiom.2024.105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B.S., Kinsella R.L., Harding C.M., Feldman M.F. The secrets of Acinetobacter secretion. Trends. Microbiol. 2017;25(7):532–545. doi: 10.1016/j.tim.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmeier J., Paniskaki K., Karaköse Z., Werner T., Sutter K., Dolff S., Overbeck M., Limmer A., Liu J., Zheng X. Impaired cytotoxic CD8+ T cell response in elderly COVID-19 patients. mBio. 2020;11(5):10–1128. doi: 10.1128/mBio.02243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M., Diggle S.P., Greenberg E.P. Progress in and promise of bacterial quorum sensing research. Nature. 2017;551(7680):313–320. doi: 10.1038/nature24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman T.J. Infection control challenges related to war wound infections in the ICU setting. J. Trauma Acute Care Surg. 2007;62(6):S53. doi: 10.1097/TA.0b013e318065aa71. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H., Paulus T., Lugenheim M., Stefanik D., Higgins P.G., Edmond M.B., Wenzel R.P., Seifert H. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J. Infect. 2012;64(3):282–290. doi: 10.1016/j.jinf.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Wu N., Dai J., Guo M., Li J., Zhou X., Li F., Gao Y., Qu H., Lu H., Jin J. Pre-optimized phage therapy on secondary Acinetobacter baumannii infection in four critical COVID-19 patients. Emerg. Microbes. Infect. 2021;10(1):612–618. doi: 10.1080/22221751.2021.1902754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Wachino J., Doi Y., Kurokawa H., Arakawa Y. Global spread of multiple aminoglycoside resistance genes. Emerging Infectious Diseases. 2005;11(6):951. doi: 10.3201/eid1106.040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavascki A.P., Klee B.O., Bulitta J.B. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert. Rev. Anti. Infect. Ther. 2017;15(6):519–526. doi: 10.1080/14787210.2017.1316193. [DOI] [PubMed] [Google Scholar]
- Zhanel G.G., Lawson C.D., Zelenitsky S., Findlay B., Schweizer F., Adam H., Walkty A., Rubinstein E., Gin A.S., Hoban D.J. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert. Rev. Anti. Infect. Ther. 2012;10(4):459–473. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- Zhu J., Wang C., Wu J., Jiang R., Mi Z., Huang Z. A novel aminoglycoside-modifying enzyme gene aac (6′)-Ib in a pandrug-resistant Acinetobacter baumannii strain. Journal of Hospital Infection. 2009;73(2):184–185. doi: 10.1016/j.jhin.2009.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.