Highlights
-
•
In vivo dosimetry provides independent dose verification during gynaecological brachytherapy.
-
•
Dosimeters can measure dose delivered to the high risk target volume (CTVHR), and organs at risk.
-
•
Differences between measured and calculated dose are related to anatomical and applicator shifts.
-
•
In vivo dosimetry has potential to guide inter-fraction adaptions improving the therapeutic ratio.
Keywords: In vivo dosimetry, Brachytherapy, Gynaecological malignancy
Abstract
Brachytherapy is a key treatment for gynaecological malignancies, delivering high doses to the tumour volume whilst sparing nearby normal tissues due to its steep dose gradient. Accuracy is imperative as small shifts can lead to clinically significant under- or over-dosing of the target volume or organs at risk (OARs), respectively. Independent verification of dose delivered during brachytherapy is not routinely performed but it is important to identify gross errors and define action thresholds to guide inter-fraction treatment decisions. In vivo dosimetry (IVD) is one strategy for improving accuracy and identifying potential errors. Despite promising phantom work, clinical application of IVD is lacking. A literature search was performed using Medline and EMBASE without date limits and based on the PICO framework to evaluate the clinical application of IVD in gynaecological brachytherapy. After screening of titles and abstracts, full text papers were reviewed and 28 studies were identified. Several dosimeters were utilised and measurements were typically taken from the rectum, bladder, vagina and within interstitial catheters. Significant differences between calculated and measured dose were attributed to geometric shifts. The studies reviewed demonstrated the feasibility of IVD in brachytherapy for dose verification but further work is required before IVD can be used to optimise treatment. The purpose of this scoping review is to investigate the clinical application of IVD in gynaecological brachytherapy, understand its challenges and identify the steps required to facilitate integration into everyday clinical practice.
Introduction
Brachytherapy plays an integral role in the treatment of gynaecological malignancies and over the past two decades significant progress including 3D imaging, dose optimization systems and hybrid intracavitary-interstitial applicators has led to an increasingly sophisticated pathway. However, with increasing complexity there is simultaneously less leeway for error and a greater need for accuracy; small deviations (of millimetres) can, in the worst-case scenario, lead to a clinically significant suboptimal dose to the target and or an unexpectedly high dose to nearby normal tissues. Clinical data has shown that 10 % changes to planned dose distribution can have a significant impact on tumour control probability (TCP) between 2–12 % and the American Association of Physicists in Medicine (AAPM) Task Group 100 has defined radiotherapy error margins [1], [2], [3] (Table 1).
Table 1.
AAPM Task Group 100 classification of terminology for severity of error in radiotherapy [3].
| Term | Description of error margin | Outcome Risk |
|---|---|---|
| Wrong distribution | 5–10 % deviation | Reduced tumour control, increased likelihood of grade I-II late toxicities |
| Very wrong distribution | 10–20 % deviation | Tumour recurrence, increased likelihood of grade III-IV late toxicities |
| Wrong location | 3–5 mm | Reduced tumour control, increased likelihood of grade I-II late toxicities |
| Very wrong location | >5mm | Tumour recurrence, increased likelihood of grade III-IV late toxicities |
| Wrong absolute dose | 5–10 % |
Human error is the biggest contributor to errors in brachytherapy [4], [5]. Strategies to reduce this include use of Failure Mode and Effects Analysis (FMEA) [6], [7]. High dose-rate (HDR) after-loaders have eliminated several potential sources of human error and led to greater staff protection from radiation. However, a remotely controlled system introduces a different set of risks and requires its own series of quality control measures and safety checks. Applicator migration can also affect dose; Karlsson et al (2017) calculated longitudinal applicator shifts and found in the majority of cases small displacements of maximum −2.5 mm to 3.5 mm in the craniocaudal direction correlating with a mean change of 0.6 % (SD 2.4 %) to the dose delivered to 90 % of the high risk clinical target volume (HR-CTV), D90. Although the relative differences between applicator shifts were low, the study did also identify a 6.6 % dose reduction in a single fraction, which if maintained across four fractions would result in a 5 Gy EQD2 dose loss from a total combined external beam and brachytherapy dose of between 78.02–90.09 Gy EQD2 for cervical cancer. [8] Applicator reconstruction is outlined using computed tomography (CT) or magnetic resonance (MRI) imaging and can also lead to error [9].
There are also uncertainties in brachytherapy that reduce accuracy. Examples include source calibration; dwell position and timings; inherent assumptions within the treatment planning system (TPS); discrepancies in image reconstruction; variation in target delineation; and applicator, source and internal organ movement [10], [11]. Tanderup et al (2013) reported total uncertainty within gynaecological brachytherapy as 12 % for the target and 21–26 % for organs at risk (OARs) in a single fraction [12].
In an effort to increase knowledge and transparency of brachytherapy errors, there are incident reporting platforms and guidelines have been developed on how to report uncertainties. Despite these measures, it is acknowledged that errors in brachytherapy are potentially still missed due to a lack of detection.
One strategy to improve error detection and reduce uncertainty is through independent verification of actual dose delivered using in vivo dosimetry (IVD).
The Vision 20/20 paper defines IVD:
“…as a radiation measurement that is acquired while the patient is being treated containing information related to the absorbed dose in the patient. This definition implies that an [IVD] system must be able to capture errors due to equipment failure, errors in dose calculation, applicator positioning errors, and patient anatomy changes.” [12].
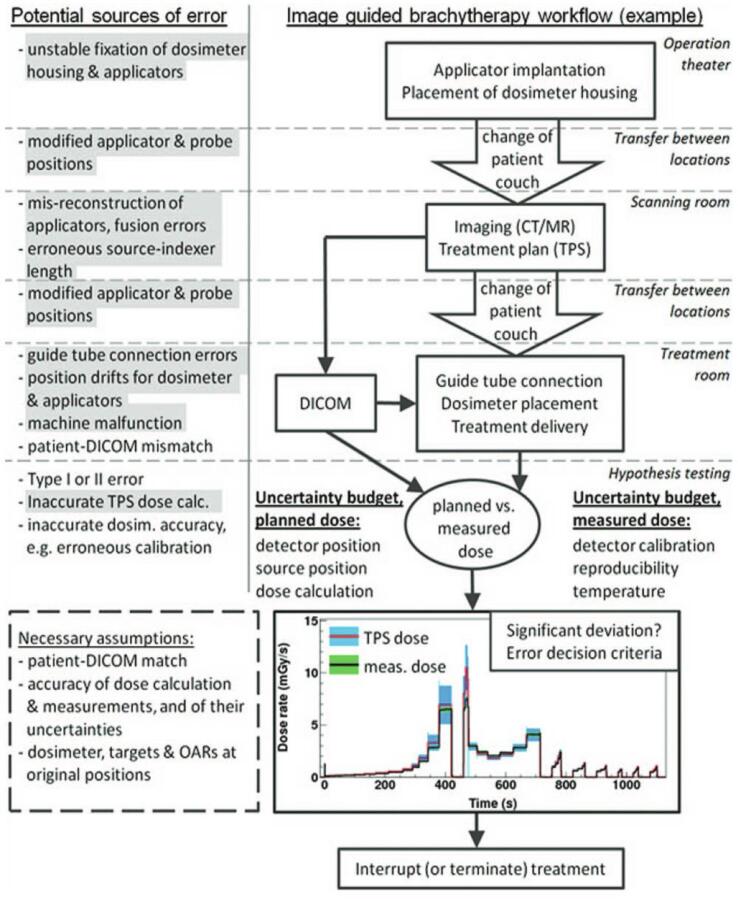
Despite indications for its use, IVD has not been integrated into routine brachytherapy practice due to several obstacles impeding accurate recording of absorbed dose. The potential error points along the brachytherapy pathway are comprehensively summarised by Tanderup et al (2013) in the Vision 20/20 paper (Fig. 1) [12].
Fig. 1.
An example of brachytherapy clinical workflow with potential error points at each step. The grey highlighted errors could be potentially addressed by IVD [12].
Fonseca et al (2020) outlined the considerations for IVD, and described time-integrated and time-resolved methods [50]. Time-integrated IVD provide a dose reading for a single or multiple points whilst time-resolved IVD uses real-time dosimeters that can measure dose, dose rate, and dwell times, and can therefore be used to track source position. The former technique is simpler to employ but is more susceptible to geometric shifts and positional uncertainty, provides information after delivery rather than in real time and is more heavily influenced by dose from nearby dwell positions. Time-resolved methods are more sophisticated using real-time dosimeters to provide information of source position relative to dosimeters and one can construct a 3D dose distribution [50]. However, with increasing complexity there are more variables, each with a degree of uncertainty that could preclude meaningful clinical interpretation.
The purpose of this scoping review is to investigate the literature to date regarding the clinical application of IVD in gynaecological brachytherapy to understand its challenges as well as the steps required to facilitate integration into clinical practice.
There are a variety of dosimeters available to provide measurement in vivo during brachytherapy. The characteristics and types of dosimeter currently available are summarised in Table 2. Unsurprisingly, there is no ideal dosimeter; each dosimeter is selected depending on the specific scenario and or what might be available in the clinical setting at the time.
Table 2.
Qualities of dosimeters suitable for IVD.
| Dosimeter | Thermoluminescent Dosimeters (TLDs) | Semiconductor Diodes | Metal oxide semiconductor field effect transistor (MOSET) | Radioluminescence (RL) | Plastic Scintillator Detector (PSD) | Optically Stimulated Luminescent Detectors (OSL) |
|---|---|---|---|---|---|---|
| Advantages | − Small − High Sensitivity − No angular dependence − Dynamic range − Low cost − Well studied system |
− Variable size − High sensitivity − Dynamic range − Simple calibration − Reasonable cost − Online dosimetry |
− Very small size − High sensitivity − Dynamic range −Low cost − Online dosimetry |
− Very small size − Very sensitive − No angular dependent − No energy dependence − Dynamic range − Online dosimetry |
− Very small size − No angular dependence − No energy dependence − Online dosimetry − Simple calibration |
− Stable − No directional effects − Dose rate independent |
| Disadvantages | − No on-line dosimetry − Energy dependent − Requires calibration and preparation for read-out |
− Energy dependent − Angular dependence |
− Energy dependent − Angular dependence (due to epoxy resin bulb) − Limited lifespan (20,000 mV) |
− Frequent recalibration − Stem effect − Not commercially available |
− Stem/Cherenkov effect* | − Stem/Cherenkov effect* − Need a laser reading |
Stem effect = unwanted light generated in the fibre cable upon irradiation.
Methods
There has been extensive work demonstrating feasibility of in vivo dosimeters in phantoms for brachytherapy. However, translation into the clinical setting is lacking due to labour intensive commissioning and practical challenges of delivering IVD in a clinically meaningful and precise way.
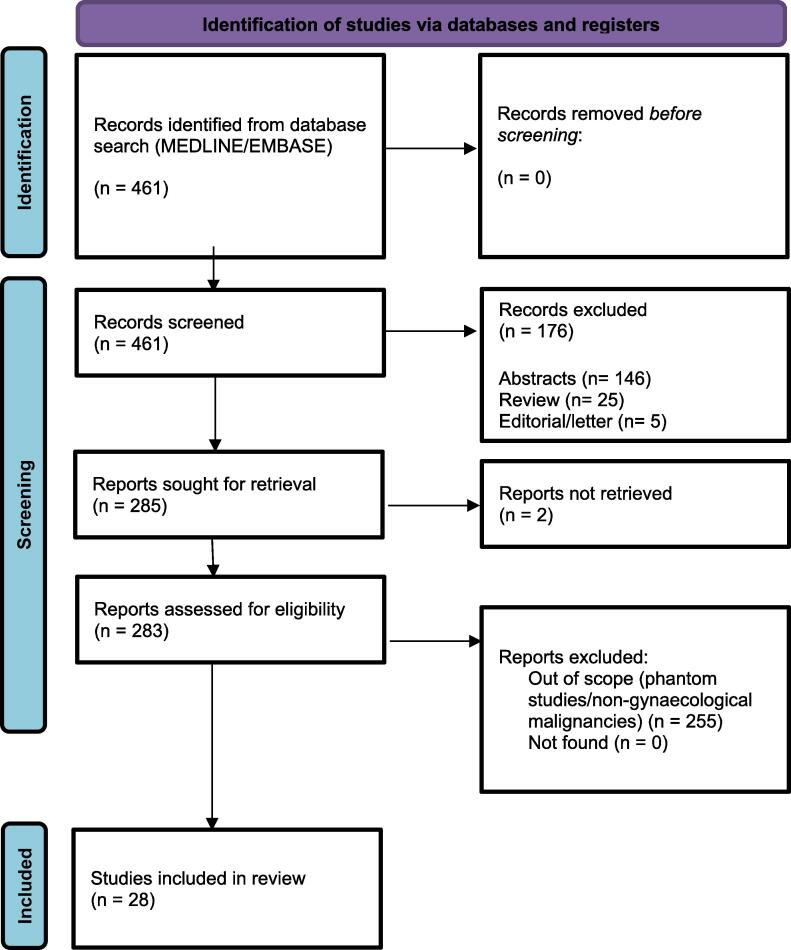
A literature search was performed in July 2023 using the OVID platform to search Medline and EMBASE databases. The search strategy employed was a combination of ‘brachytherapy’ AND ‘in vivo dosimetry’ (see Fig. 2).
Fig. 2.
PRISMA flowchart of study identification and inclusion.
Reference lists of included studies were reviewed for further studies.
Limits to the search were applied to exclude:
-
–
studies carried out in non-gynaecological tumour sites only (studies containing both gynaecological and non-gynaecological work were included)
-
–
studies carried out in phantoms only
-
–
abstracts, reviews, editorials and letters
Results
28 studies were included after screening. Table A.1 summarises the studies identified (see Supplementary materials). The majority of studies (n = 20) investigated IVD in cervical cancer. Other gynaecological malignancies evaluated included vaginal (n = 3) and endometrial cancer (vaginal cuff) (n = 5); two studies did not specify tumour subtype.
Most studies (n = 26) used HDR brachytherapy. One study delivered pulsed dose- rate (PDR) [43] and one used medium dose-rate (MDR) [32]. Eighteen studies used Iridium-192 (192Ir), seven studies used Cobalt-60 (60Co), one used Caesium-137 (137Cs) and two did not specify the source used.
In-vivo dosimeters
Three studies used OSL dosimeters [18], [31], [43]. Of these, two studies used α-Al2-O3:C NanoDot OSL and one study used a dual RL/OSL dosimeter. One study used a Radio-Photoluminescence Glass Dosimeter (RPLGD) [37]; five studies used MOSFETs [21], [22], [28], [29], [35] with one study using a microMOSFET and two using MOSkins; three studies used a plastic scintillator detector (PSD) [17], [19], [27]; one study used an ionisation chamber [36] and 13 studies used a semiconductor diode [16], [20], [22], [23], [24], [26], [30], [32], [33], [38], [39], [41], [42].
Detector position and geometric accuracy
The dosimeter positions selected included the rectum (n = 23), bladder (n = 9), vagina (n = 2), perineal skin (n = 1) and within the applicators or interstitial catheters (n = 4).
The majority of studies (n = 23) focussed on OAR dosimetry due its clinical relevance in relating measured dose to bladder and rectal toxicity. Placement of dosimeters within the target volume was less common, likely due to the increased need for precise placement due to greater proximity to the source.
Dosimeters were often inserted at the time of the applicator implant. Post-insertion the dosimeter position was verified using orthogonal x-ray images (n = 8), CT imaging (n = 10), and fluoroscopy (n = 5).
Quality indicators for brachytherapy in cervical cancer
The European Society of Gynaecological Oncology (ESGO) and the European Society for Radiotherapy & Oncology (ESTRO) summarise the dose constraints and targets for cervical cancer (Table 3). These constraints can be extrapolated to other gynaecological malignancies because the dose-fractionation schedules (typically 45–50.4 Gy in 25–28 fractions (1.8 Gy/fraction) with brachytherapy between 28–36 Gy in 4–6 fractions (6–7 Gy/fraction)), the histological subtype (predominantly squamous cell) and the anatomical location i.e. the female pelvis are the same as for cervical cancer. Additionally, these are relatively rare cancers with limited prospective randomised trial data investigating the role of brachytherapy (monotherapy or in combination with EBRT) in their management.
Table 3.
Brachytherapy dose volume histogram target for the high risk clinical target volume (CTVHR), rectum and bladder adapted from ESGO/ESTRO quality indicators for radiation therapy of cervical cancer, Chargari et al (2023) [13].
| Target Dose |
D90CTVHR EQD210 |
D98CTVHR EQD210 |
D98GTVres EQD210 |
D98CTVIR EQD210 |
||
|---|---|---|---|---|---|---|
| Minimum dose constraint (optimal constraints) | >85 Gy (>90 and < 95 Gy) |
>75 Gy (>80 Gy) |
>90 Gy (>95 Gy) |
>60 Gy (−) |
||
| OARs |
Rectum D2cm3 EQD23 |
Bladder D2cm3 EQD23 |
ICRU rectovaginal point D2cm3 EQD23 |
ICRU bladder point D2cm3 EQD23 |
Bowel D2cm3 EQD23 |
Sigmoid D2cm3 EQD23 |
| Maximum dose constraint (optimal constraints) | <75 Gy (<65 Gy) |
<85 Gy (<80 Gy) |
<75 Gy (<65 Gy) |
<85 Gy (<75 Gy) |
<75 Gy (<65 Gy) |
<75 Gy (<70 Gy) |
These constraints are defined to reduce the risk of radiation related toxicities; the 3-year rectal fistula risk reaches 12.5 % for a D2cc > 75 Gy [14] and at three years the cumulative incidence of grade 2 urinary toxicity was 22.8 % +/−2.9 % for doses < 80 Gy versus 61.8 % for doses exceeding 80 Gy. The bladder trigone dose most accurately predicts late urinary toxicity [15].
Strategies to improve geometric accuracy
Due to steep dose gradients brachytherapy creates heterogeneity across a target volume. With a linear source the dose gradient at 4 mm is approximately 50 %/mm decreasing to 6 %/mm at 20 mm and 5 %/mm at 35 mm respectively [12]. The placement of a dosimeter is therefore of great importance as a slight displacement can have a large impact on the measured dose and interpretation of clinical effect. The dosimeter uncertainty threshold is inversely proportional to its distance from the source; dosimeters placed close to the source require even greater accuracy (within millimetres) in order to correctly identify true dose deviations [11]; in order to achieve this, technical skill with accurate and timely imaging is required. The interval between imaging and treatment delivery should be as short as possible to reduce the opportunity for movement. High resolution imaging allows accurate reconstruction of applicators and dosimeter identification. The site of placement needs to be reproducible, not cause additive patient harm and in a place of clinical relevance e.g. OARs or within the HR-CTV.
Location
In the studies reviewed, dosimeter location was selected to provide dose verification in a position of clinical relevance; this could allow for correlation with toxicity and tumour control probability to be investigated.
Rectum
In all studies rectal dosimeters were placed within the rectal cavity. The rectum was the most common site selected for IVD. Seventeen studies used a rectal probe (eight used the PTW 9112 rectal probe) or tube, four papers used a rectal catheter and two studies used a rectal retractor [16], [17], [20], [21], [22], [23], [24], [25], [26], [28], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42].
Bladder
For bladder dosimetry, the measured dose was recorded by insertion of a dosimeter into a urinary Foley catheter, which is routinely inserted during the implant [17], [20], [21], [25], [32], [34], [37], [38], [42].
Vaginal applicators and interstitial catheters
Four studies assessed dose within the applicators or interstitial catheters [19], [27], [29], [43] and two studies assessed vaginal dose with dosimeter placement on vaginal applicators [17], [37]. Although it is more challenging to measure the dose close to the source it is important to confirm that prescribed doses are delivered within the target (and provide a proxy for the dose delivered to the target volume).
Customisation of dosimeters
Simple yet innovative methods of dosimeter customisation are described to minimise uncertainty and allow for clinically relevant positions to be reached. Typically, the studies have adapted required equipment to prevent potential added harm to patients.
Rectum
One study used a rectal retractor and created a groove slot for a NanoDot OSL [31]. This was then covered with a latex sleeve taped to a metal rod to keep the detector clean and reduce movement. The groove helped to ensure the correct surface of the dosimeter surface was facing the source and avoided the need for angle corrections. Another approach was to place a semiconductor rectal probe at 100 mm into the rectum, as identified by a marker on the external surface of the diode [22]. The same study placed a MOSkin anterior to the third rectal diode, 50 mm from the probe tip to provide a separate reading. Encapsulation of dosimeters was also carried out to protect from mucus contamination or other bodily fluids, which could affect readings [37].
Bladder
Dosimeters used for bladder dosimetry were typically arranged in a linear arrangement and threaded through a supplementary lumen of the urinary catheter; most commonly through the balloon port. Whilst customised 3-way catheters created for IVD are available [17], in most cases standard catheters proved sufficient. Spacer inserts allowed dosimeters to be placed at specific lengths [34]. When loading with dosimeters occurred outside of the operating room, catheters required repeat sterilisation prior to insertion. Although this did not impact the treatment delivery time, there was a significant pre-treatment workload impact [25].
Interstitial catheters and applicators
Vaginal cylinders used for vaginal vault brachytherapy (VBT) were customised for IVD by drilling a customised groove on the applicator surface [27] and when interstitial catheters were sited any unused needles were utilised for dosimeter placement [29]. Measures taken, or lack thereof, to prevent incorrect connection of the afterloader to the dosimeter catheter were not discussed. It is important that this is considered due to the potential risk of the source getting stuck in the catheter loaded with a dosimeter. MicroMOSFETs were able to be advanced through an interstitial catheter without additional impact on the patient and placed in a timely fashion (<1 min) [29]. Advancing microMOSFETS to the end of the catheter ensured reproducibility. This was achieved with the polyamide cable attachment, which also connected to a portable reader for real-time dose readout. Whilst some studies had reported uncertainty regarding the angle of the microMOSFET within the catheter, this could be corrected by using a clip fed through the cable which fixes to the interstitial catheter in a specific orientation.
Image optimisation
3D imaging is an essential component of brachytherapy and inter-fraction imaging is required for gold standard adaptive treatment [45]. Imaging also provides the opportunity to verify dosimeter position relative to the applicators and patient anatomy. Dosimeters should therefore be radiologically visible or alternatively placed in close relation to a radiographic marker. The latter option is less desirable as this increases uncertainty regarding position.
CT imaging has been shown to allow accurate identification of dosimeters between 0.4 to 3.2 mm, with an average of 1 mm inter-observer variation. Sharma et al found that with a distance of 3 cm between source and NanoDot dosimeters on a rectal retractor the mean dose gradient was 0.12 Gy/mm for a tandem and ovoid treatment; based on this an absolute dose difference of 0.05 Gy-0.39 Gy between measured and calculated dose was found to occur simply due to the discrepancy in identifying the dosimeter position accurately [31]. Consensus on the impact of this dose difference is lacking and needs to consider individual clinical, biological and treatment factors. Novel digital tools such as EviGUIDE [46], which models TCP and normal tissue control probability (NTCP) for patients having brachytherapy for locally advanced cervical cancer can help guide this process provided there is real time data exchange between EviGUIDE and TPS.
In some cases use of multiple imaging modalities at different points in the treatment pathway can improve accuracy of position verification. Examples include fluoroscopic and in-room cone beam CT (CBCT) imaging [18]. However, extra radiation exposure needs to be justified and one has to be mindful of its potential proportional effect on passive dosimeters. Dedicated in-room imaging is an attractive option, which minimises the need for patient transfer and reduces opportunity for geometric shift. Furthermore, use of hybrid imaging and treatment apparatus can reduce plan-to-treatment time thereby minimising time for anatomical shifts to occur. Reduced movement can improve agreement between the TPS and IVD.
One study found that the diode dosimeters can cause a scattering artefact making it harder to discriminate between tissues when contouring [26]. It was acknowledged that this may lead to a larger target volume, and in turn, unnecessarily higher dose to OARs. It was also noted on imaging that the rectal IVD probes can push the rectal wall towards the applicators potentially increasing rectal dose [26]. Jaberi et al (2022) also reported careful use of rectal probes with micro silica TLDs on the surface to avoid pushing the anterior rectal wall closer to the target volume [25].
Placing dosimeters into the balloon port lumen can aid visualisation of dosimeters as radiopaque agents such as meglumine compound [25] or urographine [32] can be injected into the lumen. In centres that do not have or use 3D imaging such measures still allow visualisation using x-rays.
Dosimeter fixation
In addition to customisation, dosimeters require fixation to minimise movement following insertion in the desired position; the studies reviewed describe some of the approaches that could be taken to address this issue.
Rectum
Dosimeters can be sutured into position for example within the anterior rectal wall. One study used a rigid rectoscope to place the dosimeter and did not report any adverse side effects [37]; however, how this was assessed was unclear. Using routine equipment to also fix dosimeters minimises additional instrumentation and potential harm for the sake of dosimetry. Similar to fixation of applicators, packing and adhesive tape has also been used to stabilise dosimeters [22], [30], [35]. Despite these measures, significant movement can still occur and gross visual checks are also valuable prior to treatment.
Bladder
Although a urinary catheter offers an opportune conduit for dosimeters, fixation can be difficult as the catheter can freely float within the bladder and is influenced by bladder filling. Traction to hold the catheter balloon at the bladder neck is a reproducible location [25]. This can then be secured using an external attachment, which can be placed on the imaging and treatment couch, or adhesive tape securing the catheter to the patient's external surface [34], [35]. A complicating factor is that the interstitial catheters can pierce the catheter balloon leading to catheter and dosimeter migration. One study tackled this problem by suturing the catheter to the urethral orifice [37]; toxicity and impact on patient comfort was not discussed. Whilst there is some data to demonstrate safety and efficacy, this technique requires technical expertise to prevent long term complications [47].
Applicators and interstitial catheters
It can be difficult to identify the sensitive component on imaging of some dosimeters or correlate a measurement with the precise point across the dosimeter. Using pre-set windows and setting a range lock to contour the dosimeter within specific Hounsfield units has been used with PSD. The calculated dose was then obtained for the dosimeter itself as the minimum dose covering 90 % of each PSD contour to reduce uncertainty from imaging artefact [19].
Dosimeters placed within interstitial catheters cannot easily be distinguished from catheters and so alternative measures such as advancing the dosimeter to the catheter tip or placing dosimeters along the whole length of the catheter can mitigate for this.
Calculated versus measured dose
The studies identified compared calculated to measured dose deviation. Some studies quantified this in terms of the range, mean and standard deviation, whilst others have built upon uncertainty analysis and the AAPM definitions of dose deviation based on 5–10 % increments.
Rectum
The percentage dose difference between calculated and measured range was large across all studies (range −85 % to +50 %) with a modest mean (−8.3 % to +11.0 %) and standard deviation (Table 4, Table 5). Semiconductor diodes were the most commonly used dosimeters; in all studies a flexible PTW probe (Type 9,112 from M/S PTW, Germany), which comprises of five semiconductor diodes with 1.5 cm spacing; readings were taken from each diode along the length of the rectum; this could explain the variability between calculated and measure dose [16], [20], [22], [23], [24], [30], [32], [33], [38], [39], [41], [42]. Similar to other studies higher discrepancies between the calculated dose and measured dose occurred by a factor of 1.7 with a longer treatment planning time. Using IVD it was demonstrated that categorising rectal anatomy using 3D sagittal slices into anterior (rectum in parallel to the cervico-uterus) or posterior (rectum angled towards the sacrococcygeal bone) lead to lower doses in the latter scenario [16]. For these patients it was concluded that the brachytherapy team may feel more confident to increase dose within the target without compromising rectal constraints. Difficulties in placing dosimeters at the precise ICRU rectal point also accounted for variation between the calculated ICRU point and the proxy measured dose. Stronger correlation was seen when comparing measured dose to its corresponding calculated dose based on the dosimeter position on imaging [23]. Whilst one study reported ‘no untoward’ events when comparing IVD to TPS for rectal toxicity, data relating toxicity to measured dose was lacking [23]. Applicator artefact reported in some studies was overcome by using CT compatible applicators. Interestingly, one study did not find a correlation with rectal volume and measured or calculated dose but did find an inversely proportional relationship with bladder volume and rectal measured dose [24]; the mechanism for this is not clear. There was also a positive correlation with the positioning of the vaginal applicator. Movement within the rectum, internal gas and variation in positioning resulted in a lack of consensus and wide variation. Creating customised or 3D printed conduits, which can sit comfortably within the rectum and hold dosimeters against the anterior rectal wall represent one strategy to tackle this.
Table 4.
Studies comparing difference between calculated and measured dose reporting the range, mean and standard deviation for rectal in vivo dosimetry.
| Study | Detector | Range (% or Gy) | Mean (% or Gy) | Standard Deviation (%) | Median | Comments |
|---|---|---|---|---|---|---|
| Johan et al (2023) [16] | Semiconductor diode | −19.5 % to + 24.0 % | 0.7 % | +/− 9.1 % | − | − |
| Jamalludin et al (2020) [22] | Semiconductor diode | −16.4 % to + 14.9 % | 3.2 % | +/−10.1 % | − | − |
| Villafuerte et al (2020) [23] | Semiconductor diode | +0.05 % to + 50.3 % (0.02–1.9 Gy) |
0.19 Gy | − | − | p = 0.1578 (−0.78 to 0.46) no sig difference between rectal dose in vivo max and ICRU rectal |
| Sharma et al (2013) [31] | OSL | −14.9 to + 13.7 % | − | − | − | − |
| Jaberi et al (2022) [25] | TLD | − | −8.3 % | +/− 19.5 % | − | − |
| Bergau et al (2020) [24] | Semiconductor diode | 0.00 to 6.74 Gy | 0.59 Gy | − | − | Absolute differences |
| Carrar et al (2017) [28] | MOSFET | − | −2.2 % | +/− 6.9 % | − | |
| Van Gellekom et al (2018) [29] | MOSFET | − | 3 % | +/− 14.0 % | − | Disregarding the eight measurements with the large difference, the mean and standard deviation decrease to respectively 1 % and 5 %. |
| Allahverdi et al (2012) [33] | Semiconductor diode | –22.0 to + 39.0 % | 6.5 % | − | − | |
| Toossi et al (2012) [34] | TLD | 1.72–18.55 Gy | 7.62 Gy | − | − | |
| Nose et al (2008) [37] | RPLGD | −11.1 Gy to + 0.5 Gy | − | − | −0.7 Gy | Compatibility ratio between measured and calculated dose 0.99 +/− 0.20 |
| Waldhäusl et al (2005) [38] | Semiconductor diode | −31 to + 90 % −1.4 Gy to + 2.1 Gy |
11.0 % 0.4 Gy |
− | − | −61 % to + 156 % with an average of 29 % (range −3.6 to 4.2 Gy, average 1.0 Gy). 12/55 applications showed a lower dose in the ICRU rectum reference point and 43/55 applications showed higher dose. Only 4/55 had a lower dose at the ICRU rectum point. |
| Eich et al (2000) [39] | Semiconductor diode | − | − | − | − | Measured maximum doses 1.5 less than the mean calculated doses |
| Inoue et al (1980) [42] | Semiconductor diode | − | 101.5 % | +/−9.4 | − | − |
| Hayashi et al (2021) [18] | OSLD | −30 to + 40 % | 3.9 % | 12.7 % | − | − |
| Herreros et al (2022) [17] | PSD | − | − | − | − | Planned and measured dwell times were compared in this study: − The range was −0.5 to + 0.5 s (s), with a mean of −0.003 s absolute difference between dwell times and a standard deviation of +/− 0.2 s − Median dwell times were 0.1 s and 0.060 s for first and last dwell position respectively |
| Muenkel et al (2021) [19] | PSD (OARtrac) | −26 % to + 19.5 % | −0.22 % | +/−5.98 % | ||
| Soror et al (2021) [20] | Semiconductor diodes | − | − | − | − | No difference in gastrointestinal toxicity regardless of dose. Increased acute and later bladder toxicity corresponding with higher measured dose. |
| Alecu et al (1999) [41] | Semiconductor diodes | +/−15 % | 1.0 % | +/−5.5 % | − | |
| Belley et al (2018) [27] | Nanoscintillator fibre optic dosimeter | − | − | − | TLD/TPS: 1.01 (IQR 0.98–1.04)NanoFOD/TPS: 1.0 (IQR: 0.94–1.02) |
|
| Andersen et al (2009) [43] | RL/OSL | − | − | <3% | − | 3/5 patients showing no statistically significant difference between calculated and measured doses for dose per dwell position, dose per applicator, or total dose per pulse. However 2/5 showed significant deviations for 3 individual pulses and for one dosimeter probe hypothesised to be related to applicator movement during the treatment and an incorrectly positioned dosimeter probe, respectively. |
| Shin et al (1999) [40] | TLD | − | − | − | − | |
| Zaman et al (2014) [30] | Semiconductor diode | 8.5 % to 41.2 % (absolute difference 0.3–1.5 Gy) | − | − | 1.4–5.4 % (0–1.4 Gy) | |
| Allahverdi et al (2013) [32] | Semiconductor | −85 % to + 36 % | −3% | − | − | − |
Table 5.
Studies comparing in vivo rectal dosimetry by categorising calculated: measured dose difference < 5 %, 5–10 % and > 10 % groups.
| Study | Dosimeter | Within 5 % | 5–10 % | >10 % |
|---|---|---|---|---|
| Johan et al (2023) [16] | Semiconductor | − | − | 26.4 % |
| Singh et al (2020) [21] | MOSFET | 31/50 | 8/50 | 7/50 |
| Carrar et al (2017) [28] | MOSFET | 44.6 % | 44.6 % | 10.8 % |
| Allahverdi et al (2012) [33] | Semiconductor (9112 PTW) | 20 pts | 11 pts | 9 pts |
| Zaman et al (2014) [30] | Semiconductor (9112 PTW) | − | − | 3/11 (R1-R4 diodes) |
| Jamalludin et al (2020) [22] | (MOSFET) MOSkin | 27.8 % | 27.8 % | 44.4 % |
| Jamalludin et al (2020) [22] | Semiconductor (9112 PTW) RP3 | 16.7 % | 22.2 % | 61.1 % |
| Muenkel et al (2021) [19] | PSD | 75 % | 18.3 % | 6.7 % |
| Inoue et al (1980) [42] | Semiconductor | 44.8 % (read form histogram) | 32.8 % * read from histogram | 22.4 % *read from histogram) |
| Hayashi et al (2021) [18] | OSLD | 36.0 % | 31.0 % | 33 % |
| Villafuerte (2020) [23] | Semiconductor diode | − | − | |
| Belley et al (2018) [27] | nanoFOD & TLD | nanoFOD: 63 % TLD: 70 % |
nanoFOD: 26 % TLD: 22 % |
nanoFOD:11 % (10–20 %) TLD: 7 % (10–20 %) |
| Waldhäusl (2005) [38] | Semiconductor diodes | − | 19/55 rectumBladder 17/29 | 36/55 rectum12/29 bladder |
| Sha et al (2011) [36] | Ionisation chamber | 52/86 | 26/86 | 8/86 |
| Van Gellekom (2018) [29] | MOSFET | − | 50/50 readings within < 9 % | − |
Bladder
Four studies reported on bladder dosimetry using IVD (Table 6) [25], [32], [38], [42]. Three studies found a lower measured dose compared to the TPS [32], [38], [42], whilst one study found the opposite [25]. In the former scenarios this was related to geometric shifts and in the latter scenario this was thought to be due to high mass density of the micro silica bead TLD not accounted for by TG43. Correlating the ICRU bladder point was challenging as the dosimeters were placed within the catheters rather than on the catheter balloon surface hence measuring a lower dose due to increased distance from the source.
Table 6.
Studies comparing difference between calculated and measured dose using the range, mean and standard deviation for bladder in vivo dosimetry.
| Study | Dosimeter | Range (% or Gy) | Mean (% or Gy) | Standard Deviation (%) | Median | Comments |
|---|---|---|---|---|---|---|
| Allahverdi et al (2013) [32] | Semiconductor diode | –22 to +48 % | 11 % | − | − | − |
| Inoue et al (1980) [42] | Semiconductor diode | − | 99.5 % | +/−11.2 % | − | − |
| Waldhäusl et al (2005) [38] | Semiconductor diode | −27 % to + 26 % | 4 % | − | − | ICRU bladder reference point was underestimated by the calculated in-vivo dose by a range of 12 to 162 % (mean 58 %) (range 0.5 to 5.3 Gy, average 2 Gy). All applications showed a higher dose at the ICRU bladder reference point. |
| Jaberi et al (2022) [25] | TLD | − | −7.2 % | +/−14.6 % | − | − |
Vaginal vault
Herreros et al (2022) compared deviations in calculated and measured dwell time in VBT for postoperative endometrial cancer [17]. The absolute deviations showed a mean of 0.0 +/−0.2 s. Of 121 dwell positions 10 % (n = 13) were undetectable due to stem signal affecting the signal–noise ratio and blurring the differentiation of adjacent dwell positions. The detector efficiency was > 89 % and the total deviation in treatment times was < 0.3 % in the 20 treatments measured. There was good linear agreement between planned and measured dwell times with all deviations < 1.5 % in keeping with other similar studies [48], [49]. Transit time of the source in this study was not taken into account by the TPS because in VBT this was significantly less relative to the high dwell time. However, it was noted that in intracavitary or interstitial brachytherapy for cervical cancer this would likely be more relevant [44].
Bergau et al (2020) also collected data on 80 VBT (four sessions for 20 patients) [24]. The mean of calculated maximum dose versus measured dose for 80 sessions was 3.95 Gy +/− 1.02 (range 1.95–9.80) and 3.79 Gy +/−0.80 (range 0.34–5.40) respectively. The mean absolute difference between maximum calculated and measured doses for each of the treatments was 0.61 Gy +/−0.91 (range 0.01–6.61 Gy); this was not statistically significant.
Concurrent use of different dosimeters
Two studies used two different dosimeters as a comparator against the TPS [22], [27].
Jamalluddin et al (2020) used a MOSkin and PTW semiconductor diode [22]. The MOSkins showed good agreement with the TPS in phantom studies with a difference between measured and calculated dose < 3 %. Both dosimeters showed equal variation with respect to the TPS (coefficient of variation 33 % and 27 % respectively) in vivo. The mean dose measured for the dosimeters was lower than the TPS in keeping with other studies. The percentage deviation was 32 % and absolute dose deviation was 1.0 Gy for both dosimeters. Again discrepancies in dose were related to applicator shift, which was felt to have occurred during patient transfer, vaginal packing, and internal organ motion. These shifts were felt more likely to occur with increased time from imaging to treatment. Previous studies have shown the location of the MOSkin on a CT planning image to be +/−0.5 mm. The total uncertainty budget for a MOSkin was 5.2 %, while dose point calculation uncertainty with TPS was 6.6 %. The MOSkin did record higher doses relative to the semiconductor diode; a consequence of lying closer to the source.
Belley et al (2018) treated nine women who altogether received a total of 30 fractions [27]. 27 fractions were suitable for evaluation. TLDs and a nanoscintillator-based fibre-optic dosimeter (nanoFOD) were compared to the calculated dose. The median ratio of nanoFOD/TPS was 1.00 (0.94–1.02 IQR) and TLD/TPS 1.02 (1.00–1.07). On two occasions the dosimeters were not able to be read due to a trapped air pocket at the sensor tip, which was rectified subsequently by altering the method of sealing the nanocrystal and checking the integrity of the fibre with a laser point to confirm transmission. Two nanoFODs were used and received cumulative doses in excess of 76 Gy and 108 Gy respectively with no deterioration in radiation performance. The effect of the treatment planning algorithm was considered: TG-43 vs TG-186 was found to have a small impact on the final calculated dose. As expected TG-186 gave smaller values than TG-43, being able to account for tissue inhomogeneity and the presence of applicator; this demonstrated better agreement of TG-186 with the dosimeters. 89 % of nanoFOD doses and 93 % of TLD doses agreed within 10 % of the TPS and none exceeded 20 % agreement. However, it was also acknowledged that in order to determine a dose difference threshold for intervention the uncertainty of both the dosimeter (nanoFOD 13.9 %) and for TPS (15.4 %) needed to be considered. Finally it was recognised that VBT is a simple setup as compared to intracavitary and interstitial implants where therapeutic window will be much narrower and the complexity much greater.
IVD to guide inter-fraction treatment
Only one study used initial measurement at fraction one to guide subsequent treatment decisions when delivering VBT [20]. In this retrospective study the prescription point was either the applicator surface or a depth of 5 mm from the applicator using two different dose-fractionation schedules. Moving the prescription location to the applicator surface pulled dose off the rectum and bladder. Prior to treatment the rectal semiconductor probe was verified with X-ray imaging. In 27.3 % of cases the dose prescription point was altered if the first fraction was felt to be too high. Despite reducing the depth of the dosimetry, excellent local control rates were maintained. In this study the dose threshold at first fraction for reducing the prescription depth was not defined.
Discussion
The accuracy seen in phantom studies is yet to be replicated in the clinical setting, primarily due to ongoing complexities including geometric shift of applicators, dosimeters and internal anatomy. Common difficulties encountered and the corrective strategies employed for the clinical implementation of IVD in gynaecological brachytherapy are summarised in this review.
The findings from this review demonstrate the clinical utility of vivo dosimetry and advocate its use in brachytherapy as an independent verification check. However, whilst the true number of errors related to brachytherapy may be unknown, brachytherapy does remain a highly efficacious treatment and safe delivery is the norm as evidenced by clinical studies to date. Further work investigating the role of IVD may therefore also focus on guiding inter-fraction decisions to further improve the therapeutic ratio between target volume and OARs.
Limitations
A limitation of this review is that the studies were retrospective and prospective cohort studies with small patient populations demonstrating feasibility; the largest study comprised 121 patients. Several studies used imaging techniques such as orthogonal x-rays alone to verify dosimeter positioning and for treatment planning. Whilst these imaging modalities are valid, 3D planning, using CT or MRI, is considered the optimal modality because it allows for more accurate target delineation and better tumour coverage whilst still meeting dose constraints.
Imaging
Verification of IVD is achieved by radiological imaging, which represents another strategy for dose verification in itself. Similar to online CBCT imaging used in EBRT, one study recommended use of C-arm fluoroscopy to verify position just prior to treatment to mitigate against organ and applicator motion effects [16]. Another study found that there was greater agreement between calculated and measured dose in patients receiving treatment within 90 min of imaging versus more than 90 min, with a mean dose difference of 4.7 ± 3.6 % versus 7.1 ± 5.0 % respectively [28]. More recently, increasing advocacy for development of in vivo source tracking has been discussed in the context of brachytherapy; Fonseca et al (2020) also described the use of electromagnetic tracking whereby a small sensor in the form of a coil is tracked using an electromagnetic (EM) field generator [50]. The coil can be tracked relative to the generator with a precision of under 0.2 mm. If incorporated into the source cable this would allow source tracking and be integrated into the IVD system. The RADPOS system, produced by Best Medical Canada, comprises a MOSFET detector paired with a position sensor. In their study, Reniers et al (2012) demonstrated with accuracy of 0.2–0.3 mm between 5–30 cm distance from the EM generator and dose measurement uncertainty of 5 % for a detector 10 mm from the source in phantom studies [51].
OARs vs target volume
Majority of the studies reviewed have focussed on IVD use for bladder and rectal absorbed dose with little focus on the tumour volume. Focus on OARs allows for quality assurance and correlates measured dose with acute and late toxicity. Although six studies looked at the dose delivered in the applicator/vaginal surface, none of the studies explored the dose delivered to the recto-vaginal point, which is a surrogate marker for vaginal stenosis. This may be due in part to the fact that the dose constraints to this region were developed after many of the studies were performed. The lack of measurements for the target volume or applicators is explained by the steep dose gradients about the source meaning that very slight shifts can lead to significant discrepancies, which will be difficult to interpret. Progress in this area has developed with the advent of increasingly small dosimeters, which can be placed within interstitial catheters. Whilst placing a dosimeter within a catheter is one means of ensuring the dosimeter location, movement of the applicators between imaging and treatment still introduces uncertainty.
Current and future status of IVD in gynaecological brachytherapy
Despite recognition of its value, uptake of IVD in brachytherapy is lacking globally. The 2008 ‘Towards Safer Radiotherapy’ reported IVD use to be between 30–40 % at the beginning of treatment in the UK referring only to EBRT [52]. In Denmark, Sweden and France it is a legal requirement to incorporate IVD alongside EBRT for independent verification but its role in brachytherapy is less clear [12], [52]. Patterns of care studies carried out in the early 2000 s, showed that in Latin America 19 % of centres used IVD and in Japan a reported 30–40 % used IVD in the context of rectal dose monitoring between 1999–2001 and 2003–2005 [53]. However it is not clear what systems are used and what impact this has on error identification and treatment delivery. In 2020, ESTRO established a task group to facilitate the use of in vivo dosimetry in brachytherapy and to work toward defining thresholds for error detection and treatment adaptation. Larger studies will allow for the clinical utility, or lack thereof, to be realised as well as relevant action levels and clinical thresholds to be defined. In addition to dose verification, IVD could be used to adapt treatment inter-fractionally to reduce OAR and or escalate dose to the target volume. This is yet to be fully explored in the modern era of gynaecological brachytherapy and may be of particular importance in the context of re-irradiation.
Conclusion
The studies reviewed have demonstrated proof of principle for IVD in the clinical setting for quality assurance. Whilst inter-fraction dose adjustment using IVD may be potentially more useful, clinical application for dose verification represents the first stage to advancing this process. Alongside this there is an increasing focus to develop IVD for in vivo source tracking; on the one hand a more sophisticated verification system can circumvent the limitations inherent to time-integrated IVD, however, whether this translates, on a global scale, to a clinically meaningful and cost-effective improvement is yet to be defined. The incidence of cervical cancer is inversely proportional to the income status of a country; time-integrated IVD may be more cost effective and easily integrated for verification and or adaption of brachytherapy.
In combination with sustainable strategies such as a streamlined clinical workflow; minimising imaging-to-treatment time and sharing of rigorous protocols for commissioning dosimeters, IVD represents a powerful tool to improve brachytherapy delivery for gynaecological malignancies.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tipsro.2024.100290.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Uzan J., Nahum A.E., Syndikus I. Prostate dose-painting radiotherapy and radiobiological guided optimisation enhances the therapeutic ratio. Clin Oncol. 2016;28(3):165–170. doi: 10.1016/j.clon.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Pasalic D., Kuban D.A., Allen P.K., Tang C., Mesko S., Grant S.G., et al. Dose escalation for prostate adenocarcinoma: a long-term update on the outcomes of a phase 3, single institution randomized clinical trial. Int J Radiat Oncol Biol Phys. 2019;104(4):790–797. doi: 10.1016/j.ijrobp.2019.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huq M.S., Fraass B.A., Dunscombe P.B., Gibbons J.P., Ibbott G.S., Mundt A.J., et al. The report of Task Group 100 of the AAPM: Application of risk analysis methods to radiation therapy quality management. Med Phys. 2016;43(7):4209–4262. doi: 10.1118/1.4947547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SAFRON Updates on Patient Safety in Radiotherapy; 2017. Available at: https://www.iaea.org/sites/default/files/18/01/17-12-safron-update.pdf [Accessed 30 July 2023].
- 5.Poder J., Rivard M.J., Howie A., Tedgren Å.C., Haworth A. Risk and quality in brachytherapy from a technical perspective. Clin Oncol. 2023;35(8):541–547. doi: 10.1016/j.clon.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Mayadev J., Dieterich S., Harse R., Lentz S., Mathai M., Boddu S., et al. A failure modes and effects analysis study for gynecologic high-dose-rate brachytherapy. Brachytherapy, [online] 2015;14(6):866–875. doi: 10.1016/j.brachy.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Damato A.L., Lee L.J., Bhagwat M.S., Buzurovic I., Cormack R.A., Finucane S., et al. Redesign of process map to increase efficiency: Reducing procedure time in cervical cancer brachytherapy. Brachytherapy. 2015;14(4):471–480. doi: 10.1016/j.brachy.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson L., Thunberg P., With A., Mordhorst L.B., Persliden J. 3D image-based adapted high-dose-rate brachytherapy in cervical cancer with and without interstitial needles: measurement of applicator shift between imaging and dose delivery. J Contemporary Brachytherapy. 2017;1:52–58. doi: 10.5114/jcb.2017.66110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanderup K., Hellebust T.P., Lang S., Granfeldt J., Pötter R., Lindegaard J.C., et al. Consequences of random and systematic reconstruction uncertainties in 3D image based brachytherapy in cervical cancer. Radiother Oncol [online] 2008;89(2):156–163. doi: 10.1016/j.radonc.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Kirisits C., Rivard M.J., Baltas D., Ballester F., De Brabandere M., Rob Y.N., et al. Review of clinical brachytherapy uncertainties: analysis guidelines of GEC-ESTRO and the AAPM. Radiother Oncol. 2014;110(1):199–212. doi: 10.1016/j.radonc.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kertzscher G., Rosenfeld A., Beddar S., Tanderup K., Cygler J.E. In vivo dosimetry: trends and prospects for brachytherapy. Br J Radiol. 2014;87(1041):20140206. doi: 10.1259/bjr.20140206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanderup K., Beddar S., Andersen C.E., Kertzscher G., Cygler J.E. In vivo dosimetry in brachytherapy. Med Phys. 2013;40(7) doi: 10.1118/1.4810943. [DOI] [PubMed] [Google Scholar]
- 13.Chargari C., Tanderup K., Planchamp F., Chiva L., Humphrey P., Sturdza A., et al. ESGO/ESTRO quality indicators for radiation therapy of cervical cancer. Int J Gynecologic Cancer, [online] 2023;33(6):862–875. doi: 10.1136/ijgc-2022-004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazeron R., Fokdal L.U., Kirchheiner K., Georg P., Jastaniyah N., Šegedin B., et al. Dose–volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: results from the prospective multicenter EMBRACE study. Radiother Oncol. 2016;120(3):412–419. doi: 10.1016/j.radonc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Manea E., Escande A., Bockel S., Khettab M., Dumas I., Lazarescu I., et al. Risk of late urinary complications following image guided adaptive brachytherapy for locally advanced cervical cancer: refining bladder dose-volume parameters. Int J Radiation Oncology*Biology*Physics. 2018;101(2):411–420. doi: 10.1016/j.ijrobp.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Johan S.K., Lobo D., Athiyamaan M.S., Srinivas C., Banerjee S., Abhishek K., et al. In-vivo Comparison of Planned and Measured Rectal Doses during Cobalt-60 HDR CT-based Intracavitary Brachytherapy Applications of Cervical Cancer Using the PTW 9112 Semiconductor Probe. Asian Pacific Journal Cancer Prevention: APJCP [online] 2023;24(3):897–907. doi: 10.31557/APJCP.2023.24.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herreros A., Pérez-Calatayud J., Ballester F., Barrera-Gómez J., Abellana R., Melo J., et al. In vivo verification of treatment source dwell times in brachytherapy of postoperative endometrial carcinoma: a feasibility study. J Personalized Med, [online] 2022;12(6):911. doi: 10.3390/jpm12060911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi H, Kimoto N, Maeda T, Tomita E, Asahara T, Goto S, et al. A disposable OSL dosimeter for in vivo measurement of rectum dose during brachytherapy. Medical Physics, [online] 2021; 48(8): 621–4635. Doi: 10.1002/mp.14857. [DOI] [PubMed]
- 19.Muenkel J., Xu Z., Traughber B.J., Baig T., Xu K., Langmack C., et al. Feasibility of improving patient’s safety with in vivo dose tracking in intracavitary and interstitial HDR brachytherapy. Brachytherapy. 2021;20(2):353–360. doi: 10.1016/j.brachy.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Soror T., Chafii R., Lancellotta V., Tagliaferri L., Kovács G. Biological Planning of Radiation Dose Based on In Vivo Dosimetry for Postoperative Vaginal-Cuff HDR Interventional Radiotherapy (Brachytherapy) Biomedicines. 2021;9(11):1629. doi: 10.3390/biomedicines9111629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh N., Ahamed S., Sinha A., Srivastava S., Painuly N.K., Mandal A., et al. Rectal and Bladder Dose Measurements in the Intracavitary Applications of Cervical Cancer Treatment with HDR Afterloading System: Comparison of TPS Data with MOSFET Detector. J Biomed Phys Engineering, [online] 2020;10(2):141–146. doi: 10.31661/jbpe.v0i0.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamalludin Z., Malik R.A., Ung N.M. Correlation analysis of CT-based rectal planning dosimetric parameters with in vivo dosimetry of MOSkin and PTW 9112 detectors in Co-60 source HDR intracavitary cervix brachytherapy. Phys Eng Sci Med, [online] 2020;44(3):773–783. doi: 10.1007/s13246-021-01026-x. [DOI] [PubMed] [Google Scholar]
- 23.Villafuerte C.V.L., Fragante E.J.V., Candado M.B. Comparison of ICRU 38 rectal reference point dose estimates with measured dose in vivo in cobalt-60 HDR brachytherapy for cervical cancer. J Radiat Oncol. 2020;9(1–2):37–43. doi: 10.1007/s13566-020-00420-4. [DOI] [Google Scholar]
- 24.Bergau P.F.L., Schirmer M.A., Leha A., Leu M., Emons G., Hess C.F., et al. The impact of rectal/bladder filling and applicator positioning on in vivo rectal dosimetry in vaginal cuff brachytherapy using an enhanced therapy setting. Brachytherapy. 2020;19(2):168–175. doi: 10.1016/j.brachy.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Jaberi R., Babaloui S., Siavashpour Z., Moshtaghi M., Shirazi A., Joya M., et al. 3D in vivo dosimetry of HDR gynecological brachytherapy using micro silica bead TLDs. J Appl Clin Med Phys, [online] 2022;23(9):e13729. doi: 10.1002/acm2.13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fröhlich G., Kovács K.D., Major T., Polgár C. In vivo dosimetry of the rectum in image-guided adaptive interstitial-intracavitary brachytherapy of cervix cancer - a feasibility study. Reports of Practical Oncology and Radiotherapy: Journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology, [online] 2019;24(2):158–164. doi: 10.1016/j.rpor.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belley M.D., Craciunescu O., Chang Z., Langloss B.W., Stanton I.N., Yoshizumi T.T., et al. Real-time dose-rate monitoring with gynecologic brachytherapy: Results of an initial clinical trial. Brachytherapy. 2018;17(6):1023–1029. doi: 10.1016/j.brachy.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Carrara M, Romanyukha A, Tenconi C, Mazzeo D, Cerrotta A, Borroni M, et al. Clinical application of MOSkin dosimeters to rectal wall in vivo dosimetry in gynecological HDR brachytherapy. Physica medica: PM: an international journal devoted to the applications of physics to medicine and biology: official journal of the Italian Association of Biomedical Physics (AIFB), [online] 2017; 41: 5–12. Doi: 10.1016/j.ejmp.2017.05.003. [DOI] [PubMed]
- 29.Van Gellekom M.P.R., Canters R.A.M., Dankers F.J.W.M., Loopstra A., van der Steen-Banasik E.M., Haverkort M.A.D. In vivo dosimetry in gynecological applications—A feasibility study. Brachytherapy. 2018;17(1):146–153. doi: 10.1016/j.brachy.2017.04.240. [DOI] [PubMed] [Google Scholar]
- 30.Zaman ZK, Ngie Min Ung, Rozita Abdul Malik, Gwo Fuang Ho, Chee, V., Zulaikha Jamalludin, Mohamad, Kwan Hoong Ng. Comparison of planned and measured rectal dose in-vivo during high dose rate Cobalt-60 brachytherapy of cervical cancer. 2014; 30(8): 980–984. Doi: 10.1016/j.ejmp.2014.07.002. [DOI] [PubMed]
- 31.Sharma R., Jursinic P.A. In vivo measurements for high dose rate brachytherapy with optically stimulated luminescent dosimeters. Med Phys. 2013;40(7) doi: 10.1118/1.4811143. [DOI] [PubMed] [Google Scholar]
- 32.Allahverdi M., Jaberi R., Aghili M., Ghahremani F., Geraily G. In vivo dosimetry with semiconductors in medium dose rate (MDR) brachytherapy for cervical cancer. Jpn J Radiol. 2013;31(3):160–165. doi: 10.1007/s11604-012-0160-x. [DOI] [PubMed] [Google Scholar]
- 33.Allahverdi M., Sarkhosh M., Aghili M., Jaberi R., Adelnia A., Geraily G. Evaluation of treatment planning system of brachytherapy according to dose to the rectum delivered. Radiat Prot Dosim. 2012;150(3):312–315. doi: 10.1093/rpd/ncr415. [DOI] [PubMed] [Google Scholar]
- 34.Toossi M.T., Ghorbani M., Makhdoumi Y., Taheri M., Homaee Shandiz F., Zahed Anaraki S., et al. A retrospective analysis of rectal and bladder dose for gynecological brachytherapy treatments with GZP6 HDR afterloading system. Rep Pract Oncol Radiotherapy [online] 2012;17(6):352–357. doi: 10.1016/j.rpor.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haughey A, Coalter G, Koki Mugabe. Evaluation of linear array MOSFET detectors for in vivo dosimetry to measure rectal dose in HDR brachytherapy 2011; 34(3): 361–366. Doi: 10.1007/s13246-011-0084-2. [DOI] [PubMed]
- 36.Sha R.L., Reddy P.Y., Rao R., Muralidhar K.R., Kudchadker R.J. Evaluation of rectal dose during high-dose-rate intracavitary brachytherapy for cervical carcinoma. Med Dosim. 2011;36(4):377–382. doi: 10.1016/j.meddos.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Nose T., Koizumi M., Yoshida K., Nishiyama K., Sasaki J., Ohnishi T., et al. In vivo dosimetry of high-dose-rate interstitial brachytherapy in the pelvic region: use of a radiophotoluminescence glass dosimeter for measurement of 1004 points in 66 patients with pelvic malignancy. Int J Radiation Oncology*Biology*Physics. 2008;70(2):626–633. doi: 10.1016/j.ijrobp.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 38.Waldhäusl C., Wambersie A., Pötter R., Georg D. In-vivo dosimetry for gynaecological brachytherapy: physical and clinical considerations. Radiother Oncol. 2005;77(3):310–317. doi: 10.1016/j.radonc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Eich H., Haverkamp U., Micke O., Prott F.-J., Müller R.-P. Dosimetric analysis at ICRU reference points in HDR-brachytherapy of cervical carcinoma. PubMed. 2000;53(2):62–66. [PubMed] [Google Scholar]
- 40.Shin KH, Huh SJ, Chie EK, Choi DR, Lim DH, Kim MK et al. Analysis of correlation between rectal complications and rectal dose following high dose rate intracavitary radiotherapy in patients with uterine cervix cancer: in vivo dosimetric analysis. Radiation Medicine, [online] 1999; 17(4), pp.289–293. Available at: https://pubmed.ncbi.nlm.nih.gov/10510902/ [Accessed 31 Aug. 2023]. [PubMed]
- 41.Alecu R., Alecu M. In-vivo rectal dose measurements with diodes to avoid misadministrations during intracavitary high dose rate brachytherapy for carcinoma of the cervix. Med Phys. 1999;26(5):768–770. doi: 10.1118/1.598598. [DOI] [PubMed] [Google Scholar]
- 42.Inoue T, Inoue T, Hori S, Ozawa R, Hata K, Kawanabe K. A dose monitoring system in high dose rate intracavitary remote afterloading therapy of carcinoma of the uterine cervix using semi-conductor dosimeter. Strahlentherapie, [online] 1980; 156(10), pp.703–707. Available at: https://pubmed.ncbi.nlm.nih.gov/7434377/ [Accessed 31 Aug. 2023]. [PubMed]
- 43.Andersen C.E., Nielsen S.K., Lindegaard J.C., Tanderup K. Time-resolved in vivo luminescence dosimetry for online error detection in pulsed dose-rate brachytherapy. Med Phys. 2009;36(11):5033–5043. doi: 10.1118/1.3238102. [DOI] [PubMed] [Google Scholar]
- 44.Fonseca G.P., Landry G., Reniers B., Hoffmann A., Rubo R.A., Antunes P.C.G., et al. The contribution from transit dose for192Ir HDR brachytherapy treatments. Phys Med Biology/Physics Med Biol. 2014;59(7):1831–1844. doi: 10.1088/0031-9155/59/7/1831. [DOI] [PubMed] [Google Scholar]
- 45.Tanderup K., Nesvacil N., Pötter R., Kirisits C. Uncertainties in image guided adaptive cervix cancer brachytherapy: Impact on planning and prescription. Radiother Oncol. 2013;107(1):1–5. doi: 10.1016/j.radonc.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Ecker S., Kirisits C., Schmid M., Knoth J., Heilemann G., De Leeuw A., et al. EviGUIDE - a tool for evidence-based decision making in image-guided adaptive brachytherapy for cervical cancer. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiol Oncol [online] 2023;186 doi: 10.1016/j.radonc.2023.109748. [DOI] [PubMed] [Google Scholar]
- 47.Georgiades F., Kouriefs C., Makanjuola J., Grange P. Trans-urethral bladder suture in female patients: Not a tour de force but a quick and realistic answer to complex situations. Urologia. 2021;89(2):231–234. doi: 10.1177/03915603211001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johansen J., Kertzscher G., Jørgensen E., Rylander S., Bentzen L., Hokland S.B., et al. Dwell time verification in brachytherapy based on time resolved in vivo dosimetry. Phys Med. 2019;60:156–161. doi: 10.1016/j.ejmp.2019.03.031. [DOI] [PubMed] [Google Scholar]
- 49.Debnath S.B.C., Ferre M., Tonneau D., Fauquet C., Tallet A., Goncalves A., et al. High resolution small-scale inorganic scintillator detector: HDR brachytherapy application. Med Phys. 2021;48(4):1485–1496. doi: 10.1002/mp.14727. [DOI] [PubMed] [Google Scholar]
- 50.Fonseca G.P., Johansen J.G., Smith R.L., Beaulieu L., Beddar S., Kertzscher G., et al. In vivo dosimetry in brachytherapy: Requirements and future directions for research, development, and clinical practice. Phys Imaging Radiat Oncol. 2020;16:1–11. doi: 10.1016/j.phro.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reniers B., Landry G., Eichner R., Hallil A., Verhaegen F. In vivodosimetry for gynaecological brachytherapy using a novel position sensitive radiation detector: Feasibility study. Med Phys. 2012;39(4):1925–1935. doi: 10.1118/1.3693049. [DOI] [PubMed] [Google Scholar]
- 52.Towards Safer Radiotherapy 3 Contents. (n.d.). Available at: https://www.rcr.ac.uk/system/files/publication/field_publication_files/Towards_saferRT_final.pdf.
- 53.Toita T., Kodaira T., Shinoda A., Uno T., Akino Y., Mitsumori M., et al. Patterns of Radiotherapy Practice for Patients With Cervical Cancer (1999–2001): Patterns of Care Study in Japan. International Journal of Radiation Oncology*Biology*Physics. 2008;70(3):788–794. doi: 10.1016/j.ijrobp.2007.10.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.