Simple Summary
Pathogenic and likely pathogenic germline variants in the BRCA1 and BRCA2 genes play a pivotal role in breast cancer development and progression and can determine the optimal risk-reducing strategies and personalized case management for the carriers of such variants. Our study aimed to evaluate the carrier status in a group of 58 patients who were referred to our center for genetic testing of the two genes, as well as establish a set of correlations between their genotypes and their clinical–pathological features. The study revealed that 15.5% of the patients harbored pathogenic variants in either of the two genes and that carriers of the BRCA1 pathogenic variants manifested a more aggressive tumor phenotype. These findings provide valuable insights that could be useful for the improvement in current national screening strategies and consolidate genetic testing as a valuable instrument in the personalized management of breast cancer.
Keywords: BRCA1, BRCA2, breast cancer, genetic susceptibility
Abstract
Background: Conditions associated with BRCA1/2 pathogenic (PVs) or likely pathogenic variants (LPVs) are often severe. The early detection of carrier status is ideal, as it provides options for effective case management. Materials and Methods: The study involved 58 patients with a personal and familial history of breast cancer (BC) who underwent genetic testing at the Regional Centre for Medical Genetics Dolj over a three-year period. An immunohistochemical panel (HER2, ER, PR, and Ki-67) was used to define the molecular subtypes of breast tumors. The AmpliSeq for Illumina BRCA Panel was used to evaluate germline variants in the BRCA1 and BRCA2 genes in patients with BC. The χ2 test and Fisher’s exact test were used to compare the different parameters studied. Results: Our findings revealed that 15.5% of the patients carried either BRCA1 or BRCA2 PVs or LPVs. BRCA1 carriers had aggressive tumors whereas BRCA2 carriers had rather low-grade tumors. Conclusions: The study revealed that PVs in both BRCA genes have a significant frequency among BC patients in our region, and BRCA1 carriers tend to develop more aggressive tumors than carriers of BRCA2 PVs and patients with no germline PVs in either of the two genes. These observations could provide new epidemiologic data for this disease in our region and contribute further to the development of national screening strategies.
1. Introduction
In 2020, approximately 2.3 million new BC cases were reported; this represents 11.7% of all cancer cases [1,2]. BC is the fifth leading cause of cancer-related deaths worldwide, with 685,000 deaths [1,2] despite the recent advances in personalized cancer therapy such as oncogene targeting, CAR-T, or gene therapy [3]. It is projected that by 2040, the incidence of newly diagnosed cases will rise to approximately 33.8% [1]. BC has surpassed lung cancer as the primary contributor to the rising global incidence of cancer in women, accounting for one in four cancer cases and one in six cancer-related deaths in women.
In Romania, BC is diagnosed annually in approximately 10,000 women and is responsible for approximately 3300 deaths among females [4]. Additionally, there has been a consistent upward trend recorded of malignant breast tumors in women, rising from 56,251 in 2013 to 73,021 in 2020 [5,6,7]. While the reported incidence rate of BC in Romania is approximately two times lower than that of the European Union, the mortality rate is closer to the European average (30.7%000). In 2020, in the southwestern region of the country, the mortality rate due to malignant breast tumors was around 23%000 in Dolj county, reaching approximately 27%000. Despite regional differences, the lower survival rates may be attributed to late-stage diagnosis as a common occurrence in Romania [8].
Complex BC etiology and pathogeny contribute to these concerning statistics. Susceptibility to this condition can be influenced by modifiable and non-modifiable risk factors, including genetic factors. Gene expression analysis using microarrays allowed for the classification of breast tumors into molecular subtypes which first displayed an obvious specificity in their gene expression markers and were then identified using immunohistochemistry; they include the luminal A, luminal B, Her2-positive, and triple-negative subtypes [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. They proved to reveal significant differences in their grade and prognosis (prediction of disease-free survival and overall survival), which resulted in the designing of clearly different therapeutic strategies for each subtype. Thus, the luminal A subtype is usually a low-grade proliferation that benefits from endocrine therapy and has a highly favorable prognosis. The luminal B subtype is moderately differentiated, with differentiated therapy algorithms depending on the Her2 positivity and with an intermediate prognosis. The Her2-positive subtype encompasses high-grade tumors with poor prognosis which still benefit from targeted therapy. Finally, the triple-negative subtype includes high-grade tumors with morphological, molecular, and clinical heterogeneity, as well as with the worst prognosis, requiring complex, combined therapeutic algorithms [24,25,26,27,28,29,30,31,32].
In addition, gene expression analysis identified variations in genes such as BRCA1 and BRCA2, known tumor suppressor genes with a role in DNA repair, or in the genes that interact with BRCA1 and BRCA2 [33]. A variation which can be inherited is called a germline variation; on the other hand, somatic variations can be acquired in isolated tissues due to a combination of genetic, environmental, and lifestyle factors. One of the processes through which epithelial malignancies progress to a higher phenotype is type 3 epithelial–mesenchymal transition, a process highlighted in many tumors, including kidney, bladder, and even breast proliferations [34,35,36]. In breast tumors, this higher phenotype defines the basal-like cells of triple-negative tumors [34].
Germline variants in the BRCA1 and BRCA2 genes have significant implications, as they are associated with a higher likelihood of developing certain types of BC. For BRCA1 carriers, the estimated risk of developing BC is 60% for one breast and 83% for both breasts. Similarly, for BRCA2 carriers, the cumulative risk is estimated at 55% for one breast and 62% for both breasts [4,37,38,39]. They are also associated with a higher likelihood of developing particular types of BC. For instance, BRCA1 is tightly linked with many of the molecules involved in the epithelial–mesenchymal transition process, with this relationship being able to determine the appearance of aggressive tumor phenotypes like triple-negative variants [40,41,42]. Moreover, the presence of pathogenic or likely pathogenic variants in the BRCA1 and BRCA2 genes is strongly associated not only with breast tumors but also with ovarian (OC), prostatic, and pancreatic cancers. The mutational patterns observed in these genes include frameshift variants, nonsense and missense mutations that disrupt protein function, splice site mutations leading to protein truncation, and large rearrangements [43,44,45].
Lastly, both germline and somatic gene mutations are also related to the therapeutical step in the management of BC. The studies carried out over time have shown, on the one hand, that particular germline gene mutations could benefit from particular therapeutic schemes. For instance, in tumors with pathogenic BRCA1 mutations associated with hormone receptor positivity, hormone therapy (tamoxifen and aromatase inhibitors) delays tumor progression, reduces risk significantly, and prevents the onset of contralateral tumors [46,47]. In turn, in hormone receptor-deficient tumors, the combination PARP inhibitors and chemotherapy/immunotherapy can increase the killing effect of BRCA1 germline mutation [48,49]. Finally, another interesting observation of the researchers was that, in mutated BRCA1/2 tumors, secondary mutations of BRCA1/2 were identified that were associated with an acquired resistance to previously efficient drugs [50,51,52,53].
Taking the above issues into consideration, through the present study, we aimed to assess the mutational status of the BRCA1 and BRCA2 genes in a cohort of individuals with malignant breast tumors in the southwest region of Romania.
2. Materials and Methods
The study group consisted of 58 patients, with a personal history of BC, who were referred to the Regional Centre for Medical Genetics (RCMG) Dolj, Craiova, Romania, for genetic investigation and counseling during a three-year period. The inclusion criteria for the study group were female patients with genetic testing for germline PVs or LPVs in the two BRCA genes.
The initial group of cases was divided into two main groups according to the results of genetic investigation, namely BRCA non-carriers and BRCA carriers. Secondly, the BRCA carrier group was divided, according to the mutation type, into BRCA1 carriers and BRCA2 carriers.
The selection criteria for genetic testing followed the guidelines and standards established by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) [45,54]. These criteria encompassed the specific indications and risk parameters related to oncological conditions.
Collected data included age, personal and family history, clinical phenotypes, diagnostic procedure, histogenetic type of the lesions, histopathological (HP) diagnosis (if carried out), and molecular classification of lesions (if carried out).
The appropriate pre- and post-test genetic counseling was offered to all subjects, adhering to best practice protocols.
Data concerning breast lesions were obtained by clinical examination, biopsies, and surgical procedures with histopathological examination.
For the histopathological assessment, tissue samples were processed using the classical HP technique (formalin fixation and paraffin embedding). The hematoxylin–eosin stain was used for the histopathological assessment, which was performed in accordance with the last WHO classification of breast tumors [55]. The immunohistochemical three-stage indirect Avidin–Biotin Peroxidase complex method was used for the molecular classification of lesions which was carried out in accordance with the works of Perou et al. [9] and Sørlie et al. [10,11,12], as updated by Tsang et al. [24]. The antibodies used and their significance are listed in Table 1.
Table 1.
Antibodies used in the study.
| Antibody | M/P | Clone | Source | Specificity | Significance | Dilution |
|---|---|---|---|---|---|---|
| ER | M | 1D5 | DAKO | Semiquantitative ER nuclear detection in BC | +—≥1% | 1:50 |
| PR | M | PgR636 | DAKO | Semiquantitative PR nuclear detection in BC. | +—≥20% | 1:50 |
| HER2 | M | 4B5 | DAKO | Membrane expression HER2 in BC | +—>10% | 1:500 |
| Ki-67 | M | MIB-1 | DAKO | Nuclear protein that is associated with and may be necessary for cellular proliferation Cellular marker for proliferation (Ki-67 Index) |
Low—<14% | 1:10 |
| Moderate—<20% | ||||||
| High—>20% |
Legend: BC = Breast cancer; ER = Estrogen Receptor; HER-2 = Receptor tyrosine kinase erbB-2; Ki-67 = Antigen Kiel 67; PR = Progesterone Receptor; M = Monoclonal; P = Polyclonal.
Index case germline genetic testing was performed on EDTA venous blood using next-generation sequencing (NGS). The Ampliseq for Illumina (Illumina, Inc., San Diego, CA, USA) BRCA panel with Illumina Rapid Capture library preparation kit was used. Paired end 2 × 150 bp reads on the Illumina NetSeq550 IVD sequencing platform with at least median 100× coverage were mapped to GRCh37 using the iGenomes resource bundle, and pushed through the nf-core/sarek 2.7.1 pipeline. Variants with a depth of over 20× were considered for diagnosis. Additionally, coverage of the BRCA1 and BRCA2 genes was manually investigated. Situationally, capillary sequencing was used to obtain the full coverage of exons in the genes of interest. Targeted testing for the identified variants among family members of the index cases was performed using the ABI3730 capillary sequencing platform from Applied Biosystems™ (Thermo Fisher Scientific Inc., Waltham, MA, USA). The MutationSurveyor® DNA Variant Analysis Software v.5 (Softgenetics, State College, PA, USA) was used for data analysis.
We identified the pathogenic/likely pathogenic variants and classified them according to the ACMG guidelines [56,57]. This classification is applicable to variants in all Mendelian genes and comprises a five-tier system of classification for variants relevant to Mendelian disease. The germline variants identified were annotated using ENSEMBL variant effect predictor (VEP) [58], with several plugins for predictive scores; online aggregate databases such as OMIM [59], ClinVar [60], Varsome [61] were also consulted. Segregation data, where available, were used for ACMG-compliant variant classification [56,57].
3. Results
The patients with a modified status of the BRCA genes represented less than 20% (15.5% precisely) of the entire group of patients tested.
The clinical pathological findings of the patients are summarized in Table 2.
Table 2.
Clinical pathological profiles of studied cases, segregated by mutational status in the two BRCA genes.
| Groups | Non-Carriers | BRCA Carriers | Total | ||||
|---|---|---|---|---|---|---|---|
| Variables | Cases | 49 | 9 |
58
100% |
|||
| 85.5% | 15.5% | ||||||
| Age | ≤40 years | 7 | 2 |
χ2 Test “p” value 0.6495 |
|||
| >40 years | 39 | 7 | |||||
| NOS | 3 | 0 | |||||
| Mean | 51.6 | 50.3 | |||||
| Two-sample t-test ”p” value = 0.788 | |||||||
|
Family history for
breast cancer |
Yes | 19 | 5 | 0.3475 | |||
| No | 30 | 4 | |||||
| Diagnostic procedure | CL | 18 | 1 | 0.1086 | |||
| CL + BIO | 8 | 4 | |||||
| OP + HP | 23 | 4 | |||||
| Histogenetic type | NOS | 16 | 1 | 0.187 | |||
| F-C Ch | 5 | 0 | |||||
| M | 28 | 8 | |||||
|
Histopathological
diagnosis |
NCO | 18 | 1 | 0.494 | |||
| NN | 3 | 0 | |||||
| Malignancies | DCIS | 28 | 1 | 8 | 0 | ||
| IDC | 19 | 7 | |||||
| ILC | 5 | 1 | |||||
| Mixed | 2 | 0 | |||||
| MUC | 1 | 0 | |||||
|
Molecular
classification |
NCO | 23 | 2 | 0.0034 | |||
| L-A | 12 | 1 | |||||
| L-B | 7 | 1 | |||||
| HER2+ | 5 | 0 | |||||
| T-N | 2 | 5 | |||||
Legend: BIO = Biopsy; CL = Clinical diagnosis; DCIS = Ductal Carcinoma In Situ; IDC = Invasive Ductal Carcinoma; F-C Chs = Fibro-cystic changes; HP = Histopathology; ILC = Invasive Lobular Carcinoma; L-A = Luminal A type; L-B = Luminal B type; NCO = Not Carried Out; T-N = Triple-Negative; NCO = Not Carried Out; NN = Non-Neoplastic lesions; NOS = Not Otherwise Specified; M = Malignancies; Mixed = IDC+ ILC; MUC = Mucinous; OP = Surgical procedure.
3.1. Age
A comparative analysis of the patients’ ages in the two subgroups revealed some small differences between the non-carriers and carriers; the mean age of the former was higher, with one year more than that of the latter, and, consequently, more patients belonging to the carrier group were younger than 40 years of age (Table 2). In other words, patients carrying tumors with BRCA mutations were discovered a bit earlier than those without mutations.
3.2. Family History
The presence of BC in the family histories of the investigated patients was different in the two subgroups. On the one hand, almost two-thirds of the patients with no BRCA PVs had no relatives (grandmother, mother, aunt, sister, cousin, daughter) with BC, whereas more than half of the carrier patients had relatives with breast malignancies. On the other hand, BRCA PVs were present in almost 21% of patients with a family history of BC but in only 11.7% of patients without a family history of BC. However, the statistical tests did not validate these differences as significant (Table 2).
3.3. Diagnostic Procedure
Considering the type of diagnostic procedure used to determine the patients that were suitable for further molecular assessment, three distinct categories were observed. The first category consisted of 21 patients that carried out the molecular investigation following a clinical examination which revealed a mammary nodule. Most of these patients were BRCA non-carriers, except for one 28-year-old patient who expressed a BRCA1 mutation. The second category included only 10 patients who were referred to the Regional Centre for Medical Genetics after a clinical examination which revealed a mammary nodule followed by a guided biopsy of the nodular lesion. Almost two-thirds of these cases were BRCA non-carriers, with the rest of the cases proving to be BRCA carriers and representing almost half of the patients with a BRCA mutation. It should be noted, however, that the histopathological result of the biopsies revealed a malignant proliferation within the clinically detected nodule/nodules in all these cases. The third category included patients with an established diagnosis of breast malignancy; most of them proved to be non-carriers but the rest of them represented the other almost half of the cases harboring BRCA mutations.
The group of non-carriers mainly included either patients that underwent a surgical procedure and were diagnosed with a type of BC or patients with a clinical diagnosis of a mammary nodule. In turn, the group of carriers included mostly patients with an established histopathological diagnosis either by biopsy or after a surgical procedure. These differences were, however, not validated as significant from the statistical point of view (Table 2).
3.4. Histogenetic Types
There were differences between the two groups concerning the determination of the histogenetic type of mammary lesions. Thus, almost one-third of the non-carrier cases had no specification of the histogenic type of nodular lesions and that was because all these cases belonged to the group of patients with a clinical examination only. In 10% of the non-carrier cases, the supposed diagnosis was of fibro-cystic changes. However, from a histopathological point of view, more than half of the cases were diagnosed as malignancies of the mammary gland.
In turn, with one exception, all carrier cases were diagnosed as breast carcinomas. However, these differences were not validated from the statistical point of view as significant (Table 2).
3.5. Histopathological Diagnosis
Moving beyond the assessment of histogenetic type, HP profile determination showed different situations in the two groups of patients. In more than one-third of the non-carrier cases, HP evaluation did not follow the genetic testing, as patients were only concerned with assessing their risk of developing BC. Further, in less than 10% of the cases, the histological examination revealed only fibro-cystic changes in the mammary parenchyma. All the other cases were malignant epithelial proliferations. Except for one case of mucinous carcinoma, as well as one case of “in situ” proliferation, all the other tumors proved to be invasive carcinomas that were mainly of the ductal type.
Almost the same situation was observed in the group with BRCA PVs, where, with the exception of one case without HP evaluation, all the cases turned out to be invasive carcinomas and almost all of the ductal type.
3.6. Molecular Classification
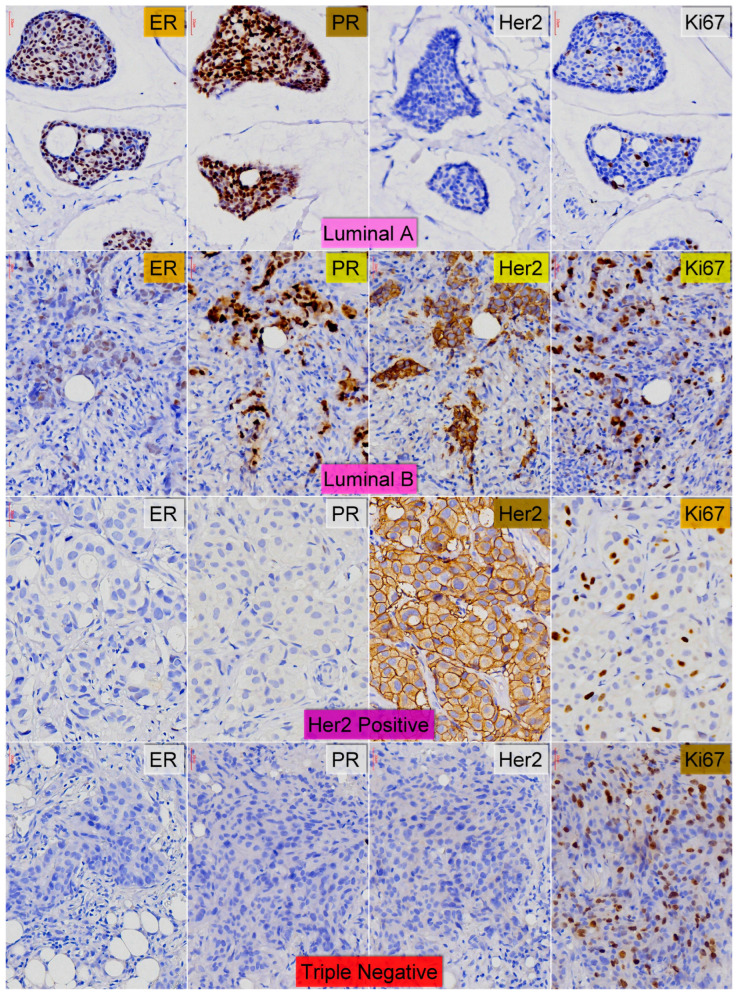
Immunohistochemical assays for the molecular classification of breast lesions, the only parameter crucial for the design of a therapeutical strategy, were not carried out in all the patients. In the non-carrier group, eighteen patients only had a clinical examination, following which they referred to the RCMG for genetic testing; the other five patients, after a clinical examination, had a sample of breast tissue taken which was not subjected to immunohistochemical assays after the standard histological examination. In more than half of the rest of the cases in this group, immunohistochemical assessments revealed that many of the tumors (12 cases—46%) were luminal A-type carcinomas, followed by luminal B-type carcinomas (14.3%) and Her2-positive tumors (10.2%). In the BRCA carrier group, more than half of the tumors (five cases—55%) were triple-negative tumors but only two of these had a high Ki67 index. Two of the cases were luminal-type malignancies and, in two cases, immunohistochemical assays were not carried out (Table 2 and Figure 1).
Figure 1.
Molecular types of assessed breast carcinomas—Line I—Luminal A type (ER ≥ 1%; PR ≥ 20%; HER2 ≤ 10%; Ki-67 < 14%); Line II—Luminal B type+HER2+ (ER ≥ 1%; PR variable; HER2 > 10%; Ki-67 > 20%); Line III—HER2 enriched (ER < 1%; PR < 20%; HER2 > 10%; Ki-67 > 20%); Line IV—Triple-Negative (ER < 1%; PR < 20%; HER2 ≤ 10%; Ki-67 > 30%); Ob-X40 (all pictures).
This striking difference between the two groups was validated as highly significant by the statistical tests (p value of χ2 test was 0.007).
3.7. Assessment of BRCA Variants
Deleterious germline BRCA1 or BRCA2 variants (pathogenic (P)/likely pathogenic (LP) variants) were identified in 9 out of 58 (15.5%) patients (Table 2). Variants of uncertain significance (VUS) were not found in our cohort. BRCA1/2-related BC had similar pathological characteristics with regards to the sporadic tumors in terms of histological grade and lymph node involvement. Table 3 presents the pathogenic or likely pathogenic BRCA1/BRCA2 variants that we identified in our study group.
Table 3.
Characterization of reported BRCA variants.
| Gene | Cases | Gene Variant | Variant Type | ACMG Score | Associated Phenotype |
Relevant Literature |
|---|---|---|---|---|---|---|
| BRCA1 | 3 |
NM_007300.4:c.5329dup p.(Gln1777ProfsTer74) |
Duplication frameshift |
Pathogenic (PVS1, PP5, PM2) |
TN | [4,62,63,64,65] |
| L-B | ||||||
| NCO | ||||||
| BRCA1 | 2 |
NM_007300.4: c.843_846del p.(Ser282TyrfsTer15) l |
Deletion, frameshift | Pathogenic (PVS1, PP5, PM2) |
TN | [66,67,68] |
| TN | ||||||
| BRCA1 | 1 |
NM_007300.4: c.5093_5096del p.(Thr1698IlefsTer2) |
Deletion, frameshift | Pathogenic (PVS1, PP5, PM2) |
TN | [63,69,70] |
| BRCA2 | 1 |
NM_000059.4: c.2471T>G p.(Leu824Ter) |
Substitution, Missense | Pathogenic (PVS1, PP5, PM2) |
TN | [71,72] |
| BRCA2 | 1 |
NM_000059.4: c.5576_5579del (p.Ile1859fs) |
Deletion, frameshift | Pathogenic (PVS1, PP5, PM2) |
L-A | [73,74,75,76,77] |
| BRCA2 | 1 |
NM_000059.3: c.8331+1G>Ap.? |
Substitution, Missense splice site, intron 18 |
Pathogenic (PVS1, PP5, PM2) |
L-A | [78,79,80,81] |
Legend: BC = Breast carcinoma; L-A = Luminal A type BC; L-B = Luminal B type BC; NCO = Not Carried Out; T-N = Triple-Negative BC.
The BRCA1 variant NM_007300.4:c.5329dup was found in three females, aged 58, 45, and 28. The 58-year-old female was diagnosed at age 49 and underwent a radical mastectomy for left BC at the same age. She has a daughter with BC. This frameshift null variant introduces an early termination signal, potentially producing a truncated protein or leading to the absence of a protein through a mechanism known as nonsense-mediated decay (NMD). Both scenarios are recognized pathways to disease [78]. The impacted exon affects a single functional domain, specifically the ‘BRCT 2’ domain as denoted by UniProt’s BRCA1_HUMAN protein annotation. Within this exon, 98 pathogenic variants have been reported, while the area that would be truncated by the mutation contains 317 pathogenic variants.
Another BRCA1 variant, NM_007300:c.843_846delCTCA, introduces an early termination signal, also producing NMD. c843_846del was found in our study in two female patients, with onset at the ages of 35 and 47, respectively. In both cases, the invasive ductal carcinoma was PR, ER, and HER2 negative. Only one of the cases had a positive family history of tumors; her sister had ovarian cancer, her mother gastric cancer, and her brother bladder cancer.
We observed the BRCA1 variant NM_007300.4:c.5093_5096del in a 63-year-old patient with BC and a negative family history. This frameshift null variant is anticipated to lead to nonsense-mediated mRNA decay (NMD). The loss-of-function effect is a well-established cause of disease, supported by 3443 previously reported pathogenic loss-of-function variants for this gene. The affected exon interacts with a specific functional domain, namely the ‘BRCT 1’ domain, as identified in the UniProt entry for the human BRCA1 protein. Within this particular exon, there are 72 known pathogenic variants, and the region that would be truncated due to this frameshift contains 624 pathogenic variants.
The BRCA2 variant NM_000059.4:c.2471T>G was identified in a 61 year-old patient that was diagnosed with bilateral breast ductal carcinoma at the age of 44. Her family has a rich clinical history, with her mother and daughter having BC and her sister being diagnosed with ovarian cancer.
BRCA2 NM_000059.4:c.5576_5579delTTAA has been cited in breast and ovarian cancer cases [74,75,77]. The variant has not been reported in general population-based databases [82]. The deletion of four nucleotides leads to a frameshift that likely causes a truncated protein or a complete absence of the transcript. We identified the PV in a 42-year-old woman without a history of breast or ovarian neoplasia.
The BRCA2 variant NM_000059.3:c.8331+1G>A was identified in a 52-year-old patient that was diagnosed with bilateral BC at the ages of 39, with lobular carcinoma in the right breast, and 47, with ductal carcinoma in the left breast; both were PR and ER positive and HER2 negative.
3.8. Clinical and Pathological Differences Between the Two Carrier Subgroups
We further analyzed both groups of BRCA PV carriers to see if they showed different clinical–morphological profiles (Table 4). The patients carrying BRCA1 PVs represented two-thirds of the entire group of carriers.
Table 4.
Profiles of the two subgroups of BRCA carriers.
| Groups | BRCA1 Carriers | BRCA2 Carriers | Total | |
|---|---|---|---|---|
| Cases | 6 | 3 |
9
100% |
|
| 66.7% | 33.3% | |||
| Age | ≤40 years | 2 | 0 |
χ2 Test “p” value 0.257 |
| >40 years | 4 | 3 | ||
| Family history for breast cancer | Yes | 4 | 1 |
χ2 Test “p” value 0.3428 |
| No | 2 | 2 | ||
|
Diagnostic
procedure |
CL | 1 | 0 |
χ2 Test “p” value 0.569 |
| CL + BIO | 3 | 1 | ||
| OP + HP | 2 | 2 | ||
|
Histogenetic
type |
NOS | 1 | 0 |
Fisher’s exact test
1 |
| F-C Ch | 0 | 0 | ||
| M | 5 | 3 | ||
| Histopathological diagnosis | NCO | 1 | 0 |
Fisher’s exact test 0.583 |
| NN | 0 | 0 | ||
| DCIS | 0 | 0 | ||
| IDC | 5 | 2 | ||
| ILC | 0 | 1 | ||
|
Molecular
classification |
NCO | 1 | 0 |
χ2 Test “p” value 0.1009 |
| L-A | 0 | 2 | ||
| L-B | 1 | 0 | ||
| HER2+ | 0 | 0 | ||
| T-N | 4 | 1 | ||
Legend: BIO = Biopsy; CL = Clinical diagnosis; DCIS = Ductal Carcinoma In Situ; F-C Chs = Fibro-cystic changes; HP = Histopathology; IDC = Invasive Ductal Carcinoma; ILC = Invasive Lobular Carcinoma; L-A = Luminal A type; L-B = Luminal B type; M = Malignancies; NCO = Not Carried Out; NN = Non-Neoplastic lesions; NOS = Not Otherwise Specified; OP = Surgical procedure; T-N = Triple-Negative.
Age. The comparative analysis of the patients’ ages in the two subgroups revealed that one-third of the BRCA1 carriers were younger than 40 years whereas all the BRCA2 carriers were older than 40 years (Table 4).
Family history of breast cancer. We observed that two-thirds of the BRCA1 carriers had breast malignancies in their family history, whereas two-thirds of BRCA2 carriers had no breast malignancies in their family history.
Diagnostic procedure. The tumors of BRCA1 carriers were diagnosed more frequently by clinical examination and biopsy while the tumors of BRCA2 carriers were diagnosed more often by a surgical procedure followed by histopathological examination.
Histogenetic type. Tumors examined were practically malignancies in both subgroups of carriers.
Histopathological diagnosis. All the tumors investigated were invasive, but the tumors with BRCA1 PVs were all ductal-type proliferations, whereas one of the three tumors with BRCA2 PVs was of lobular type.
Molecular classification. Except for one case, which lacked immunohistochemical evaluation, nearly all the other tumors (two-thirds) in the subgroup of BRCA1 carriers were of the triple-negative subtype. In turn, two-thirds of the subgroup of BRCA2 carriers had luminal A-type tumors (estrogen receptor positive and low Ki67 index).
However, all these differences were not pronounced enough for the statistical tests to validate them.
3.9. Corelations Between Molecular Phenotypes and Genetic Variants
Another step in our analysis was to check if there was any correlation between the molecular profile and the presence or absence of different variants of BRCA mutations. In this respect, we took into consideration only those cases with two investigations performed. Thus, almost half of the non-carrier patients had luminal A-type tumors and, to a lesser extent, luminal B-type tumors (26.9%). Poorly differentiated tumors (Her2-positive and triple-negative subtypes) were present in the same percentage, with more than twice the prevalence of Her2-positive malignancies.
A total of 80% of the BRCA1 carriers had triple-negative tumors, whereas two-thirds of the BRCA2 carriers had luminal A tumors.
We can summarize that, in our study, non-carrier patients had the lowest aggressive tumors, while BRCA1 carriers had the most aggressive tumors. BRCA2 carriers were placed in an intermediate position (Table 5).
Table 5.
Comparison between molecular phenotypes defined by the immuno-histochemical assays and genetic variants.
| BRCA Tested Groups | Non-Carriers | BRCA1 Carriers | BRCA2 Carriers | χ2 Test “p” Value | |
|---|---|---|---|---|---|
|
Molecular
classification |
L-A | 12 | 0 | 2 | 0.0138 |
| L-B | 7 | 1 | 0 | ||
| HER2+ | 5 | 0 | 0 | ||
| T-N | 2 | 4 | 1 | ||
Legend: BIO = Biopsy; L-A = Luminal A type; L-B = Luminal B type; NCO = Not Carried Out; T-N = Triple-Negative.
4. Discussions
Human BRCA1 and BRCA2 genes encode proteins that are crucial for the repair of double-stranded DNA breaks through homologous recombination [69]. Germline mutations in these genes lead to impaired DNA repair, resulting in genomic instability and susceptibility to cancer, notably breast and ovarian cancers.
Mutations in these genes can be inherited, leading to a significantly higher risk of developing cancer in carriers. Routine molecular profiling for deleterious germline variants in BRCA1 and BRCA2 has become an integral component of the diagnostic and therapeutic approach to hereditary breast and ovarian neoplasms. Individuals with a family history of breast or ovarian cancer are often encouraged to consider testing.
The penetrance of BRCA mutations is high, meaning that individuals carrying a deleterious mutation have a significantly increased lifetime risk of developing cancer compared to the general population. For instance, women with a BRCA1 mutation have a lifetime risk of 55–72% of developing BC and 39–44% of developing ovarian cancer [83].
4.1. Carrier Profiles of the Two BRCA Genes in Our Study
Each of the BRCA PV carrier groups revealed a distinct profile. Thus, in our study, the BRCA1 carriers were mostly over 40 years old, usually with a family history of BC. They were often diagnosed with a breast lesion by a clinical examination coupled with a biopsy. The breast lesion was almost always malignant, namely an invasive carcinoma that was either ductal or lobular and was usually a triple-negative tumor.
The BRCA2 carriers were all older than 40 years, with no family history of BC. They were often diagnosed with a breast lesion by a clinical examination coupled with a surgical procedure followed by a histopathological examination. The breast lesion was always malignant, namely an invasive carcinoma that was either ductal or lobular and was usually a luminal A-type tumor. These differences between the two subgroups of BRCA carriers, together with the differences described previously between the BRCA carriers and non-carriers, are in concordance with the literature data, even though many of the important tested correlations (e.g., age, family history, histopathological diagnosis) were only trend-like in our study.
This means that, in general, BRCA carriers are more prone to developing BC than BRCA-negative patients; when they do develop BC, the tumors are of a higher grade and more aggressive, with a higher recurrence risk score and a worse survival rate. Further, BRCA1 carriers are more likely to develop more aggressive BCs, with a worse prognosis and at an earlier age than BRCA2 carriers [39,40,42,66,67,84,85,86,87,88,89,90,91,92]. The fact that our observations only have trend-like values can be explained by the small size of the study group, this limit being determined by the fact that our center is a very new one, and the study is one of the first attempts to present our activities.
4.2. BRCA1 and BRCA2 Mutational Status in Romania
There have been several studies carried out in Romania to evaluate the mutational status of BRCA1/2 in the context of both BC [66,93,94,95,96] and combined breast and ovarian cancer [4,6,7]. The study groups consisted mostly of patients from the northwestern [4,66,93,94,95] and northeastern [6,7] regions of the country, and only one from the southern/southeastern region [96]. Throughout these studies, BRCA1 variants were more prevalent than BRCA2, as was the case in our group. Some of the studies, although performed on larger groups and on patients with BC or OC, only identified four variants of uncertain significance (VUS), two of which were in BRCA1 and the other two in BRCA2 [4,66,94]. We did not report any VUS in our study mainly due to the smaller sample size given the more restrictive inclusion criteria.
The most frequently found variants were the c.3607C>T (p.Arg1203Ter) in the BRCA1 gene, and c.9371A>T (p.Asn3124Ile) in the BRCA2 gene, respectively. Interestingly, the c.3607C>T (p.Arg1203Ter) variant was not found within our study, although it has a high frequency in the northwestern and northeastern regions of Romania [4,66,94,95] and has also been frequently reported in the southern and southeastern regions of the European continent [97].
Also, BRCA2 c.9371A>T was not reported in our group despite it being previously reported in the Romanian population. This variant was reported in twelve cases in one study, indicating that it is a prevalent mutation among Romanian breast and ovarian cancer patients [4]. Additionally, another report highlighted it as the most common pathogenic variant described in the Romanian population, with seven cases of BC and six cases of ovarian cancer [94]. Moreover, a study in 2022 that included 250 women with BC and 240 with ovarian cancer undergoing germline molecular testing showed c.9371A>T to be one of the most common variants identified for BRCA2 [95].
The BRCA1 c.843_846delCTCA has been reported in the Romanian population, with the following two cases identified: one in a patient with BC and one in a patient with ovarian cancer [66]. These occurrences suggest the presence of this mutation among the Romanian population affected by these cancers; however, the data do not specify an incidence rate in the general population.
BRCA1 c.5329dup is one of the more common BRCA1 PVs observed in Romanian patients with breast and ovarian cancer. In a study that evaluated women of Romanian ethnicity, this mutation was identified 17 times among patients with these cancers. It was the second most prevalent variant after the c.3607C>T mutation in BRCA1. This suggests that NM_007300.4:c.5329dup is a significant PV within the Romanian population with breast and ovarian cancer. Although it is described as a founder mutation in some populations [94], an earlier study in the northeastern region of Romania suggested that it would not have a recurrent or founder effect in our country [7], with further studies being required on larger patient groups to sustain or infirm this hypothesis.
BRCA1 c.5093_5096del is a rare occurrence in the Romanian population, being reported in two patients within two different studies [94,95]. However, it has been reported in the literature in three patients within a Tunisian population with early-onset hereditary breast and ovarian cancer syndrome (HBOC) [69] and other patients in the Middle Eastern, North African, and South European countries [97].
We are reporting the BRCA2 c.2471T>G variant for the first time in Romania as it has not been reported in any of the existing studies within our country, probably due to the low addressability of genetic screening tests in the population. There are also only a few publications in the specialized literature where it has been mentioned, the most notable of which being a 2018 worldwide study on 29,700 families that harbor BRCA mutations [67].
The BRCA2 c.8331+1G>A intronic splice-site variant is also novel within the Romanian population and is one of the few that has frequently been reported in male BC patients [79,80,81].
The c.5576_5579del (p.Ile1859fs) variant in the BRCA2 gene is only reported once in the Romanian specialized literature in one patient with BC in the northwestern region of the country [95].
These findings suggest that the variants reported in the current study are significant variants in the context of Romanian BC cases.
4.3. BRCA1 and BRCA2 Testing and Strategies
There are several benefits of the genetic screening of BRCA variants, including the following: (1) early detection through enhanced monitoring and surveillance follow-up plans in carriers; (2) preventive surgical intervention in carriers at high risk; and (3) establishment of therapeutic strategy. Recent randomized phase III trials have demonstrated the efficacy of therapeutic regimens involving platinum salts and PARP inhibitors specifically targeting certain germline mutations in patients with advanced BC [93,98].
Determining BRCA mutational status in patients with breast or ovarian malignancies can profoundly influence clinical decision-making, impacting both disease-free survival and overall prognosis. In recognition of its pivotal role, multiple international and national scientific consortia have promulgated various clinical management guidelines. These delineate both the surgical interventions and chemotherapeutic regimens optimized for prophylactic measures and therapeutic modalities, contingent upon the specific BRCA mutational profile.
Case management protocols for BRCA-associated BC syndrome are formulated based on a comprehensive understanding of the precocious manifestation of the disease, the augmented susceptibility to ovarian malignancies, and the propensity for male mammary carcinogenesis in those with a BRCA1/2 PV. Emphasizing the imperative nature of early detection in individuals harboring the BRCA PV, it is quintessential for the timely identification of neoplastic transformations.
Individuals with a strong family history suggestive of HBOC are the primary candidates for genetic testing. This includes families with multiple cases of early-onset breast or ovarian cancer, bilateral BC, male BC, or combinations of other BRCA-associated cancers. Once a PV is identified in an index case, the cascade testing of at-risk relatives is essential for identifying other carriers who may benefit from enhanced surveillance or risk-reducing strategies.
In individuals with a hereditary predisposition to breast and ovarian cancer, specifically carriers of the BRCA1/2 PVs/LPVs, early and intensive screening is of paramount importance due to the early age of disease onset. Protocols recommend breast awareness training from 18 years old, clinical breast examinations bi-annually from 25 years old, and tailored imaging schedules often starting in the mid-twenties, particularly with an MRI which has shown higher sensitivity compared to mammography. Data suggest that mammography might not be as effective in younger women due to factors like breast tissue density and rapidly growing tumors. MRI not only detects early-stage tumors with higher sensitivity but also reduces the radiation exposure risks associated with mammography. For optimal cancer risk management, both mammography and MRI are crucial, especially given the increasing evidence of MRI’s sensitivity in detecting tumors in BRCA carriers [66,67,68].
However, the specific intervals and imaging modalities remain a subject of ongoing research. Post-test counseling should extensively discuss risk-reducing surgical options and their implications. Ovarian cancer screening in high-risk women suggests potential earlier detection but definitive survival impacts are still under investigation. Men with the BRCA variants are also advised to start undergoing breast and prostate cancer screenings from specific ages. Ultimately, comprehensive BRCA screening and surveillance, combined with ongoing research, are critical to effectively manage and detect cancers early in individuals with a higher genetic risk.
The accessibility and coverage of BRCA testing are subject to healthcare policies and can vary depending on geographical location and healthcare systems, necessitating the consideration of healthcare equity in implementing testing strategies. While there has been a consistent rise in BC incidence in Romania over recent years, BRCA mutation testing remains largely inaccessible to medical professionals. As stated above, the RCMG Dolj is part of a recently formed national public network of regional medical genetics centers. Even though these centers have been established in the main administrative regions of the country, the addressability and accessibility of genetic screening tests are poor mainly due to increased costs in spite of the low income rates, especially in the rural areas, as well as the lack of national programs and government financing that could partially or totally subsidize these costs. Also, patients in the rural areas are more likely to be referred to municipal hospitals rather than regional ones, where the RCMGs are set in, for logistical and financial reasons. As a result, the range of BRCA1 and BRCA2 mutations in both sporadic and familial BC cases within our population is not fully characterized. This study contributes significant findings regarding a cohort of Romanian patients from the southwestern part of the country who underwent NGS BRCA1/2 panel testing, among the few reported to date [66,93,94,95].
The scientific rationale for BRCA1 and BRCA2 testing is multifaceted, incorporating genetic epidemiology, molecular oncology, molecular pathology, guideline-driven clinical practice, therapeutic advancements, and ethical considerations. The decision for testing should be individualized, considering the person’s risk, family history, and preference, and should be conducted within a framework of comprehensive genetic counseling and informed consent.
Well-designed longitudinal outcome studies are also needed to clarify the prognostic outlook for patients with BC harboring germline BRCA PVs/LPVs at all disease stages [99] and establish how these would affect tumor microenvironment and potential novel immune therapies and treatment protocols using the latest tools in sequencing technologies such as targeted single-cell sequencing or long-read sequencing expanded by broader functional studies [100,101].
5. Conclusions
Our study, although conducted on a reduced number of cases coming from the southwestern region of the country, revealed that a significant percentage of the tested tumors carried pathogenic germline variants. The spectrum and frequencies of the germline variants in the BRCA1/2 genes mirrored those described in the literature, and the BRCA1 pathogenic variants were associated with the aggressive phenotypes of malignant proliferations. Therefore, BRCA1/2 testing or broader genetic panels could be more efficiently popularized and implemented in cost-effective screening and risk-reducing strategies, contributing to the genetic epidemiology of breast cancer, enforcing its management both at a regional and national level, and providing optimal therapeutic options for patients harboring germline PVs, given the current availability of personalized therapy for these variants such as PARP inhibitors.
Acknowledgments
This work is supported by the Craiova University of Medicine and Pharmacy, Internal Grant no. 23/516/54/16.05.2022. Genetic testing was funded through the National Health Program (XIII Programul national de sanitate a femeii si copilului 2.3. Prevenirea bolilor genetice prin diagostic pre-si postnatal) and POCU ProGeneRare-SMIS code 108073.
Author Contributions
Conceptualization, R.M.P., A.-L.R., and I.S.; methodology, R.M.P., I.G., A.M.A., Ș.D., A.B., and A.M.; software, A.-L.R., I.G., A.M., G.-C.C., A.G., and C.V.L.; validation, G.-C.C., C.V.L., M.S., and F.B.; investigation, R.M.P., G.-C.C., C.V.L., M.S., I.G., A.M.A., Ș.D., A.B., C.S.M., and A.M.; resources, R.M.P., A.-L.R., and I.S.; data curation, A.M.A., I.G., G.-C.C., C.V.L., M.S., and F.B.; writing—original draft preparation, R.M.P., A.M.A., and I.G.; writing—review and editing, R.M.P., A.-L.R., and I.S.; supervision, M.S., F.B., and I.S.; project administration, R.M.P., A.-L.R., and I.S.; funding acquisition, R.M.P., A.-L.R., and I.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Emergency County Hospital of Craiova (approval number 14274/29.03.2022).
Informed Consent Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Emergency County Hospital of Craiova (approval number 14274/29 March 2022).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
The article publishing fees were funded by the University of Medicine and Pharmacy of Craiova, Romania.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA Cancer J. Clin. 2024;74:12–49. doi: 10.3322/caac.21820. Erratum in CA Cancer J. Clin. 2024, 74, 203. [DOI] [PubMed] [Google Scholar]
- 3.Sonkin D., Thomas A., Teicher B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024;286:18–24. doi: 10.1016/j.cancergen.2024.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidra R., Ciuleanu T.E., Nemeș A., Pascu O., Heroiu A.M., Antone N., Vidrean A.I., Oprean C.M., Pop L.A., Berindan-Neagoe I., et al. Spectrum of BRCA1/2 Mutations in Romanian Breast and Ovarian Cancer Patients. Int. J. Environ. Res. Public Health. 2022;19:4314. doi: 10.3390/ijerph19074314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucu M.A., Matei E., Galan A., Ursu C., Dima C., Georgescu D. Raport Național privind Starea de Sănătate a Populației României, 2020. [(accessed on 22 December 2024)];2021 ISSN2559-2610, ISSN-L2559-2610, București. Available online: https://insp.gov.ro/download/cnepss/stare-de-sanatate/rapoarte_si_studii_despre_starea_de_sanatate/starea_de_sanatate/starea_de_sanatate/RAPORTUL-NATIONAL-AL-STARII-DE-SANATATE-A-POPULATIEI-%25E2%2580%2593-2020.pdf.
- 6.Negura L., Uhrhammer N., Negura A., Artenie V., Carasevici E., Bignon Y.J. Complete BRCA mutation screening in breast and ovarian cancer predisposition families from a North-Eastern Romanian population. Fam. Cancer. 2010;9:519–523. doi: 10.1007/s10689-010-9361-6. [DOI] [PubMed] [Google Scholar]
- 7.Negură L., Duşa C.P., Balmuş M.I., Azoicăi D., Negură A.M., Marinca M.V., Miron L. BRCA1 5382insC founder mutation has not a significative recurrent presence in Northeastern Romanian cancer patients. Rom. J. Morphol. Embryol. 2015;56:379–385. [PubMed] [Google Scholar]
- 8.Mortalitatea Prin Cancer La Sân În România-36%. Asociaţiile De Pacienţi Cer Program De Screening. Mediafax.ro; Bucharest, Romania: 2015. [Google Scholar]
- 9.Perou C.M., Sørlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 10.Sørlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sørlie T., Tibshirani R., Parker J., Hastie T., Marron J.S., Nobel A., Deng S., Johnsen H., Pesich R., Geisler S., et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sørlie T. Molecular portraits of breast cancer: Tumour subtypes as distinct disease entities. Eur. J. Cancer. 2004;40:2667–2675. doi: 10.1016/j.ejca.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Herschkowitz J.I., Simin K., Weigman V.J., Mikaelian I., Usary J., Hu Z., Rasmussen K.E., Jones L.P., Assefnia S., Chandrasekharan S., et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prat A., Parker J.S., Karginova O., Fan C., Livasy C., Herschkowitz J.I., He X., Perou C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eroles P., Bosch A., Pérez-Fidalgo J.A., Lluch A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat. Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Leidy J., Khan A., Kandil D. Basal-like breast cancer: Update on clinicopathologic, immunohistochemical, and molecular features. Arch. Pathol. Lab. Med. 2014;138:37–43. doi: 10.5858/arpa.2012-0439-RA. [DOI] [PubMed] [Google Scholar]
- 17.Lam S.W., Jimenez C.R., Boven E. Breast cancer classification by proteomic technologies: Current state of knowledge. Cancer Treat. Rev. 2014;40:129–138. doi: 10.1016/j.ctrv.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Dai X., Li Y., Bai Z., Tang X.Q. Molecular portraits revealing the heterogeneity of breast tumor subtypes defined using immunohistochemistry markers. Sci. Rep. 2015;5:14499. doi: 10.1038/srep14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai X., Xiang L., Li T., Bai Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer. 2016;7:1281–1294. doi: 10.7150/jca.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y., Wang Y., Kiani M.F., Wang B. Classification, Treatment Strategy, and Associated Drug Resistance in Breast Cancer. Clin. Breast Cancer. 2016;16:335–343. doi: 10.1016/j.clbc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Pourteimoor V., Mohammadi-Yeganeh S., Paryan M. Breast cancer classification and prognostication through diverse systems along with recent emerging findings in this respect; the dawn of new perspectives in the clinical applications. Tumour. Biol. 2016;37:14479–14499. doi: 10.1007/s13277-016-5349-7. [DOI] [PubMed] [Google Scholar]
- 22.Fusco N., Geyer F.C., De Filippo M.R., Martelotto L.G., Ng C.K., Piscuoglio S., Guerini-Rocco E., Schultheis A.M., Fuhrmann L., Wang L., et al. Genetic events in the progression of adenoid cystic carcinoma of the breast to high-grade triple-negative breast cancer. Mod. Pathol. 2016;29:1292–1305. doi: 10.1038/modpathol.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt M., Thomssen C., Untch M. Intrinsic Subtypes of Primary Breast Cancer--Gene Expression Analysis. Oncol. Res. Treat. 2016;39:102–110. doi: 10.1159/000444409. [DOI] [PubMed] [Google Scholar]
- 24.Tsang J.Y.S., Tse G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020;27:27–35. doi: 10.1097/PAP.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y., Spezia M., Huang S., Yuan C., Zeng Z., Zhang L., Ji X., Liu W., Huang B., Luo W., et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106. doi: 10.1016/j.gendis.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fragomeni S.M., Sciallis A., Jeruss J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018;27:95–120. doi: 10.1016/j.soc.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashmi A.A., Aijaz S., Khan S.M., Mahboob R., Irfan M., Zafar N.I., Nisar M., Siddiqui M., Edhi M.M., Faridi N., et al. Prognostic parameters of luminal A and luminal B intrinsic breast cancer subtypes of Pakistani patients. World J. Surg. Oncol. 2018;16:1. doi: 10.1186/s12957-017-1299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llombart-Cussac A., Cortés J., Paré L., Galván P., Bermejo B., Martínez N., Vidal M., Pernas S., López R., Muñoz M., et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): An open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 2017;18:545–554. doi: 10.1016/S1470-2045(17)30021-9. [DOI] [PubMed] [Google Scholar]
- 29.Russnes H.G., Lingjærde O.C., Børresen-Dale A.L., Caldas C. Breast Cancer Molecular Stratification: From Intrinsic Subtypes to Integrative Clusters. Am. J. Pathol. 2017;187:2152–2162. doi: 10.1016/j.ajpath.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Yam C., Mani S.A., Moulder S.L. Targeting the Molecular Subtypes of Triple Negative Breast Cancer: Understanding the Diversity to Progress the Field. Oncologist. 2017;22:1086–1093. doi: 10.1634/theoncologist.2017-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocca A., Farolfi A., Maltoni R., Carretta E., Melegari E., Ferrario C., Cecconetto L., Sarti S., Schirone A., Fedeli A., et al. Efficacy of endocrine therapy in relation to progesterone receptor and Ki67 expression in advanced breast cancer. Breast Cancer Res. Treat. 2015;152:57–65. doi: 10.1007/s10549-015-3423-2. [DOI] [PubMed] [Google Scholar]
- 32.Goldhirsch A., Winer E.P., Coates A.S., Gelber R.D., Piccart-Gebhart M., Thürlimann B., Senn H.J., Panel members Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Łukasiewicz S., Czeczelewski M., Forma A., Baj J., Sitarz R., Stanisławek A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers. 2021;13:4287. doi: 10.3390/cancers13174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarrió D., Rodriguez-Pinilla S.M., Hardisson D., Cano A., Moreno-Bueno G., Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 35.Stepan A.E., Mărgăritescu C., Stoica L.E., Stepan M.D., Simionescu C.E. Clear cell renal cell carcinomas-epithelial and mes-enchymal immunophenotype. Rom. J. Morphol. Embryol. 2018;59:1189–1194. [PubMed] [Google Scholar]
- 36.Stepan A.E., Ciurea R.N., Drăgoescu P.O., Florescu M.M., Stepan M.D. Immunoexpression of transcription factors in urothe-lial bladder carcinomas. Rom. J. Morphol. Embryol. 2017;58:863–869. [PubMed] [Google Scholar]
- 37.Kobayashi H., Ohno S., Sasaki Y., Matsuura M. Hereditary breast and ovarian cancer susceptibility genes (review) Oncol. Rep. 2013;30:1019–1029. doi: 10.3892/or.2013.2541. [DOI] [PubMed] [Google Scholar]
- 38.Mavaddat N., Peock S., Frost D., Ellis S., Platte R., Fineberg E., Evans D.G., Izatt L., Eeles R.A., Adlard J., et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 2013;105:812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 39.Baretta Z., Mocellin S., Goldin E., Olopade O.I., Huo D. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine. 2016;95:e4975. doi: 10.1097/MD.0000000000004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armstrong N., Ryder S., Forbes C., Ross J., Quek R.G. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin. Epidemiol. 2019;11:543–561. doi: 10.2147/CLEP.S206949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengodan S.K., KH S., Nadhan R., Srinivas P. Regulation of epithelial to mesenchymal transition by BRCA1 in breast cancer. Crit. Rev. Oncol. Hematol. 2018;123:74–82. doi: 10.1016/j.critrevonc.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Atchley D.P., Albarracin C.T., Lopez A., Valero V., Amos C.I., Gonzalez-Angulo A.M., Hortobagyi G.N., Arun B.K. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J. Clin. Oncol. 2008;26:4282–4288. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narod S.A., Salmena L. BRCA1 and BRCA2 mutations and breast cancer. Discov. Med. 2011;12:445–453. [PubMed] [Google Scholar]
- 44.Walsh T., Casadei S., Coats K.H., Swisher E., Stray S.M., Higgins J., Roach K.C., Mandell J., Lee M.K., Ciernikova S., et al. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA. 2006;295:1379–1388. doi: 10.1001/jama.295.12.1379. [DOI] [PubMed] [Google Scholar]
- 45.Balmaña J., Díez O., Castiglione M. BRCA in breast cancer: ESMO clinical recommendations. Ann. Oncol. 2009;20((Suppl. S4)):19–20. doi: 10.1093/annonc/mdp116. [DOI] [PubMed] [Google Scholar]
- 46.Nemati Shafaee M., Goutsouliak K., Lin H., Bevers T.B., Gutierrez-Barrera A., Bondy M., Arun B. Aromatase inhibitors and contralateral breast cancer in BRCA mutation carriers. Breast Cancer Res Treat. 2022;196:143–152. doi: 10.1007/s10549-022-06688-z. [DOI] [PubMed] [Google Scholar]
- 47.Narod S.A., Brunet J.S., Ghadirian P., Robson M., Heimdal K., Neuhausen S.L., Stoppa-Lyonnet D., Lerman C., Pasini B., de los Rios P., et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: A case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet. 2000;356:1876–1881. doi: 10.1016/S0140-6736(00)03258-X. [DOI] [PubMed] [Google Scholar]
- 48.Domchek S.M., Postel-Vinay S., Im S.A., Park Y.H., Delord J.P., Italiano A., Alexandre J., You B., Bastian S., Krebs M.G., et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21:1155–1164. doi: 10.1016/S1470-2045(20)30324-7. [DOI] [PubMed] [Google Scholar]
- 49.Mateo J., Lord C.J., Serra V., Tutt A., Balmaña J., Castroviejo-Bermejo M., Cruz C., Oaknin A., Kaye S.B., de Bono J.S. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019;30:1437–1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhillon K.K., Swisher E.M., Taniguchi T. Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci. 2011;102:663–669. doi: 10.1111/j.1349-7006.2010.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakai W., Swisher E.M., Jacquemont C., Chandramohan K.V., Couch F.J., Langdon S.P., Wurz K., Higgins J., Villegas E., Taniguchi T. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69:6381–6386. doi: 10.1158/0008-5472.CAN-09-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swisher E.M., Sakai W., Karlan B.Y., Wurz K., Urban N., Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards S.L., Brough R., Lord C.J., Natrajan R., Vatcheva R., Levine D.A., Boyd J., Reis-Filho J.S., Ashworth A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 54.Sessa C., Balmaña J., Bober S.L., Cardoso M.J., Colombo N., Curigliano G., Domchek S.M., Evans D.G., Fischerova D., Harbeck N., et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann. Oncol. 2023;34:33–47. doi: 10.1016/j.annonc.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 55.WHO Classification of Tumours Editorial Board . WHO Classification of Tumours. 5th ed. Volume 2. IARC Publications; Lyon, France: 2019. Breast Tumours. [Google Scholar]
- 56.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masson E., Zou W.B., Génin E., Cooper D.N., Le Gac G., Fichou Y., Pu N., Rebours V., Férec C., Liao Z., et al. Expanding ACMG variant classification guidelines into a general framework. Hum. Genom. 2022;16:31. doi: 10.1186/s40246-022-00407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hunt S.E., Moore B., Amode R.M., Armean I.M., Lemos D., Mushtaq A., Parton A., Schuilenburg H., Szpak M., Thormann A. Annotating and prioritizing genomic variants using the ensembl variant effect predictor—A tutorial. Hum. Mutat. 2021;43:986–997. doi: 10.1002/humu.24298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.OMIM. [(accessed on 22 December 2024)]. Available online: http://www.omim.org.
- 60.ClinVar. [(accessed on 22 December 2024)]; Available online: https://www.ncbi.nlm.nih.gov/clinvar/
- 61.Kopanos C., Tsiolkas V., Kouris A., Chapple C.E., Albarca Aguilera M., Meyer R., Massouras A. Varsome: The human genomic variant search engine. Bioinformatics. 2018;35:1978–1980. doi: 10.1093/bioinformatics/bty897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes R., Soares B.L., Felicio P.S., Michelli R., Netto C.B.O., Alemar B., Ashton-Prolla P., Palmero E.I., Moreira M.Â.M. Haplotypic characterization of BRCA1 c.5266dupC, the prevailing mutation in Brazilian hereditary breast/ovarian cancer. Genet. Mol. Biol. 2020;43:e20190072. doi: 10.1590//1678-4685-gmb-2019-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grzymski J.J., Elhanan G., Morales Rosado J.A., Smith E., Schlauch K.A., Read R., Rowan C., Slotnick N., Dabe S., Metcalf W.J., et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat. Med. 2020;26:1235–1239. doi: 10.1038/s41591-020-0982-5. [DOI] [PubMed] [Google Scholar]
- 64.Dorling L., Carvalho S., Allen J., González-Neira A., Luccarini C., Wahlström C., Pooley K.A., Parsons M.T., Fortuno C., Wang Q., et al. Breast Cancer Risk Genes-Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021;384:428–439. doi: 10.1056/NEJMoa1913948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shani H., Bernstein-Molho R., Laitman Y., Netzer I., Friedman E. Double heterozygosity for TP53 and BRCA1 mutations: Clinical implications in populations with founder mutations. Breast Cancer Res. Treat. 2021;186:259–263. doi: 10.1007/s10549-020-06084-5. [DOI] [PubMed] [Google Scholar]
- 66.Goidescu I.G., Caracostea G., Eniu D.T., Stamatian F.V. Prevalence of deleterious mutations among patients with breast cancer referred for multigene panel testing in a Romanian population. Clujul. Med. 2018;91:157–165. doi: 10.15386/cjmed-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rebbeck T.R., Friebel T.M., Friedman E., Hamann U., Huo D., Kwong A., Olah E., Olopade O.I., Solano A.R., Teo S.H., et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum. Mutat. 2018;39:593–620. doi: 10.1002/humu.23406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heramb C., Wangensteen T., Grindedal E.M., Ariansen S.L., Lothe S., Heimdal K.R., Mæhle L. BRCA1 and BRCA2 mutation spectrum-an update on mutation distribution in a large cancer genetics clinic in Norway. Hered Cancer Clin. Pract. 2018;16:3. doi: 10.1186/s13053-017-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ben Ayed-Guerfali D., Ben Kridis-Rejab W., Ammous-Boukhris N., Ayadi W., Charfi S., Khanfir A., Sellami-Boudawara T., Frikha M., Daoud J., Mokdad-Gargouri R. Novel and recurrent BRCA1/BRCA2 germline mutations in patients with breast/ovarian cancer: A series from the south of Tunisia. J. Transl. Med. 2021;19:108. doi: 10.1186/s12967-021-02772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Talhouet S., Peron J., Vuilleumier A., Friedlaender A., Viassolo V., Ayme A., Bodmer A., Treilleux I., Lang N., Tille J.C., et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci. Rep. 2020;10:7073. doi: 10.1038/s41598-020-63759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borg A., Haile R.W., Malone K.E., Capanu M., Diep A., Törngren T., Teraoka S., Begg C.B., Thomas D.C., Concannon P., et al. Characterization of BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance in unilateral and bilateral breast cancer: The WECARE study. Hum. Mutat. 2010;31:E1200–E1240. doi: 10.1002/humu.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lecarpentier J., Noguès C., Mouret-Fourme E., Gauthier-Villars M., Lasset C., Fricker J.P., Caron O., Stoppa-Lyonnet D., Berthet P., Faivre L., et al. Variation in breast cancer risk associated with factors related to pregnancies according to truncating mutation location, in the French National BRCA1 and BRCA2 mutations carrier cohort (GENEPSO) Breast Cancer Res. 2012;14:R99. doi: 10.1186/bcr3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blay P., Santamaría I., Pitiot A.S., Luque M., Alvarado M.G., Lastra A., Fernández Y., Paredes A., Freije J.M., Balbín M. Mutational analysis of BRCA1 and BRCA2 in hereditary breast and ovarian cancer families from Asturias (Northern Spain) BMC Cancer. 2013;13:243. doi: 10.1186/1471-2407-13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foster K.A., Harrington P., Kerr J., Russell P., DiCioccio R.A., Scott I.V., Jacobs I., Chenevix-Trench G., Ponder B.A., Gayther S.A. Somatic and germline mutations of the BRCA2 gene in sporadic ovarian cancer. Cancer Res. 1996;56:3622–3625. [PubMed] [Google Scholar]
- 75.Schneegans S.M., Rosenberger A., Engel U., Sander M., Emons G., Shoukier M. Validation of three BRCA1/2 mutation-carrier probability models Myriad, BRCAPRO and BOADICEA in a population-based series of 183 German families. Fam. Cancer. 2012;11:181–188. doi: 10.1007/s10689-011-9498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang J.H., Lee J.E., Kwon M.J., Ki C.S., Kim J.W., Nam S.J., Yang J.H. Spectra of BRCA1 and BRCA2 mutations in Korean patients with breast cancer: The importance of whole-gene sequencing. J. Hum. Genet. 2012;57:212–215. doi: 10.1038/jhg.2011.139. [DOI] [PubMed] [Google Scholar]
- 77.George J., Alsop K., Etemadmoghadam D., Hondow H., Mikeska T., Dobrovic A., de Fazio A., Smyth G.K., Levine D.A., Mitchell G., et al. Nonequivalent gene expression and copy number alterations in high-grade serous ovarian cancers with BRCA1 and BRCA2 mutations. Clin. Cancer Res. 2013;19:3474–3484. doi: 10.1158/1078-0432.CCR-13-0066. [DOI] [PubMed] [Google Scholar]
- 78.Chirita-Emandi A., Andreescu N., Popa C., Mihailescu A., Riza A.L., Plesea R., Ioana M., Arghirescu S., Puiu M. Biallelic variants in BRCA1 gene cause a recognizable phenotype within chromosomal instability syndromes reframed as BRCA1 deficiency. J. Med. Genet. 2021;58:648–652. doi: 10.1136/jmedgenet-2020-107198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gelli E., Colombo M., Pinto A.M., De Vecchi G., Foglia C., Amitrano S., Morbidoni V., Imperatore V., Manoukian S., Baldassarri M., et al. Usefulness and Limitations of Comprehensive Characterization of mRNA Splicing Profiles in the Definition of the Clinical Relevance of BRCA1/2 Variants of Uncertain Significance. Cancers. 2019;11:295. doi: 10.3390/cancers11030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fraile-Bethencourt E., Díez-Gómez B., Velásquez-Zapata V., Acedo A., Sanz D.J., Velasco E.A. Functional classification of DNA variants by hybrid minigenes: Identification of 30 spliceogenic variants of BRCA2 exons 17 and 18. PLoS Genet. 2017;13:e1006691. doi: 10.1371/journal.pgen.1006691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pritzlaff M., Summerour P., McFarland R., Li S., Reineke P., Dolinsky J.S., Goldgar D.E., Shimelis H., Couch F.J., Chao E.C., et al. Male breast cancer in a multi-gene panel testing cohort: Insights and unexpected results. Breast Cancer Res. Treat. 2017;161:575–586. doi: 10.1007/s10549-016-4085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.gnomAD. [(accessed on 22 December 2024)]. Available online: https://gnomad.broadinstitute.org/
- 83.Antoniou A., Pharoah P.D., Narod S., Risch H.A., Eyfjord J.E., Hopper J.L., Loman N., Olsson H., Johannsson O., Borg A., et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Casaubon J.T., Kashyap S., Regan J.P. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. [(accessed on 22 December 2024)]. BRCA1 and BRCA2 Mutations. [Updated 23 July 2023] Available online: https://www.ncbi.nlm.nih.gov/books/NBK470239/ [Google Scholar]
- 85.Fu X., Tan W., Song Q., Pei H., Li J. BRCA1 and Breast Cancer: Molecular Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2022;10:813457. doi: 10.3389/fcell.2022.813457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Layman R.M., Lin H., Gutierrez Barrera A.M., Karuturi M.S., Yam C., Arun B.K. Clinical outcomes and Oncotype DX Breast Recurrence Score® in early-stage BRCA-associated hormone receptor-positive breast cancer. Cancer Med. 2022;11:1474–1483. doi: 10.1002/cam4.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sønderstrup I.M.H., Jensen M.R., Ejlertsen B., Eriksen J.O., Gerdes A.M., Kruse T.A., Larsen M.J., Thomassen M., Lænkholm A.V. Subtypes in BRCA-mutated breast cancer. Hum. Pathol. 2019;84:192–201. doi: 10.1016/j.humpath.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Copson E.R., Maishman T.C., Tapper W.J., Cutress R.I., Greville-Heygate S., Altman D.G., Eccles B., Gerty S., Durcan L.T., Jones L., et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018;19:169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krammer J., Pinker-Domenig K., Robson M.E., Gönen M., Bernard-Davila B., Morris E.A., Mangino D.A., Jochelson M.S. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2017;163:565–571. doi: 10.1007/s10549-017-4198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malone K.E., Daling J.R., Doody D.R., Hsu L., Bernstein L., Coates R.J., Marchbanks P.A., Simon M.S., McDonald J.A., Norman S.A., et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–82308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 91.Foulkes W.D., Metcalfe K., Sun P., Hanna W.M., Lynch H.T., Ghadirian P., Tung N., Olopade O.I., Weber B.L., McLennan J., et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: The influence of age, grade, and histological type. Clin. Cancer Res. 2004;10:2029–2034. doi: 10.1158/1078-0432.CCR-03-1061. [DOI] [PubMed] [Google Scholar]
- 92.Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. Erratum in Nature 1996, 379, 749. [DOI] [PubMed] [Google Scholar]
- 93.Catana A., Apostu A.P., Antemie R.G. Multi gene panel testing for hereditary breast cancer-is it ready to be used? Med. Pharm. Rep. 2019;92:220–225. doi: 10.15386/mpr-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goidescu I.G., Nemeti G., Surcel M., Caracostea G., Florian A.R., Cruciat G., Staicu A., Muresan D., Goidescu C., Pintican R., et al. Spectrum of High-Risk Mutations among Breast Cancer Patients Referred for Multigene Panel Testing in a Romanian Population. Cancers. 2023;15:1895. doi: 10.3390/cancers15061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cătană A., Trifa A.P., Achimas-Cadariu P.A., Bolba-Morar G., Lisencu C., Kutasi E., Chelaru V.F., Muntean M., Martin D.L., Antone N.Z., et al. Hereditary Breast Cancer in Romania—Molecular Particularities and Genetic Counseling Challenges in an Eastern European Country. Biomedicines. 2023;11:1386. doi: 10.3390/biomedicines11051386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burcoş T., Cimponeriu D., Ion D.A., Spandole S., Apostol P., Toma M., Radu I., Popa I., Stanilescu S., Popa E. Analysis of several BRCA1 and BRCA2 mutations in a hospital-based series of unselected breast cancer cases. Chirurgia. 2013;108:468–472. [PubMed] [Google Scholar]
- 97.Laitman Y., Friebel T.M., Yannoukakos D., Fostira F., Konstantopoulou I., Figlioli G., Bonanni B., Manoukian S., Zuradelli M., Tondini C., et al. The spectrum of BRCA1 and BRCA2 pathogenic sequence variants in Middle Eastern, North African, and South European countries. Hum. Mutat. 2019;40:e1–e23. doi: 10.1002/humu.23842. [DOI] [PubMed] [Google Scholar]
- 98.Forbes C., Fayter D., de Kock S., Quek R.G. A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, genetic counseling, and treatment of BRCA-mutated breast cancer. Cancer Manag. Res. 2019;11:2321–2337. doi: 10.2147/CMAR.S189627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arun B., Couch F.J., Abraham J., Tung N., Fasching P.A. BRCA-mutated breast cancer: The unmet need, challenges and therapeutic benefits of genetic testing. Br. J. Cancer. 2024;131:1400–1414. doi: 10.1038/s41416-024-02827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu H., Dong A., Rasteh A.M., Wang P., Weng J. Identification of the novel exhausted T cell CD8 + markers in breast cancer. Sci. Rep. 2024;14:19142. doi: 10.1038/s41598-024-70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li R., Huang Y., Liu H., Dilger J.P., Lin J. Abstract 2162: Comparing volatile and intravenous anesthetics in a mouse model of breast cancer metastasis. Cancer Res. 2018;78((Suppl. S13)):2162. doi: 10.1158/1538-7445.AM2018-2162. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.