Simple Summary
Spinal meningiomas are among the most frequent primary intradural extramedullary tumors, predominantly affecting women and the thoracic spine. While generally benign, these tumors require surgical intervention to achieve gross total resection and prevent recurrence. The Simpson grading system, originally developed for intracranial meningiomas, remains a pivotal factor in predicting recurrence risk, though its applicability to spinal meningiomas has been debated. This study combines a mono-institutional case series with a systematic review and meta-analysis to evaluate the prognostic significance of Simpson grading in spinal meningioma surgery. By analyzing recurrence rates and surgical outcomes, this work aims to provide evidence-based insights into optimal management strategies for this challenging pathology.
Keywords: spinal meningioma, simpson grade, systematic review, meta-analysis, meningioma recurrence, intradural extramedullary tumor
Abstract
Background: Although its validity has recently been questioned since its introduction, the Simpson grade has remained one of the most relevant factors in estimating the recurrence risk of intracranial meningiomas. This study aims to assess its role in spinal meningiomas through a retrospective analysis of a mono-institutional surgical series and literature meta-analysis. Methods: We conducted a systematic review and meta-analysis of the literature from 1980 to 2023, complemented by a mono-institutional series of 74 patients treated at “Santa Maria delle Grazie” hospital. Demographic, clinical, neuroradiological, pathological, surgical, and outcome data of case series were analyzed. For the meta-analysis, studies were selected based on predefined inclusion criteria, and a fixed-effects model was used to synthesize data due to assumed homogeneity among included studies. Statistical analyses included odds ratios (OR) for recurrence risk and assessment of publication bias using Peter’s test. Results: Mono-institutional sample included 74 patients, most of whom were women (85%) with a median age of 61.9 years. The thoracic spine was the most common tumor location (81%). Recurrences occurred in patients with Simpson grade II and III resections. The meta-analysis involved 2142 patients from 25 studies and revealed a significantly higher recurrence rate for Simpson grades III–V compared to grades I–II (OR 0.10; CI95 0.06–0.16). Additionally, Simpson grade II had a higher recurrence risk than grade I (OR 0.42; CI95 0.20–0.90). Conclusions: The Simpson grading remains a valid predictor of recurrence also for spinal meningiomas. Our findings revealed a significant increase in recurrence rate with higher Simpson grades. These results support the need to strive for Simpson grade I resection when feasible.
1. Introduction
Meningiomas are generally benign, slow-growing tumors that arise from arachnoid cap cells, although some lesions may be atypical and malignant. Available records in the literature claim spinal meningiomas represent 1.2 to 12% of all meningiomas and 25 to 45% of all intradural spinal tumors [1,2,3]. These lesions are described as intradural extramedullary tumors and mainly occur at the thoracic level in women aged between 50 and 80 [3]. The standard of care is the safe and precise surgical resection of the lesion, with satisfactory functional recovery and preservation of spinal stability [4]. The primary goal of surgery is complete tumor excision, typically Simpson grades I or II, along with spinal cord decompression [4,5]. Recent studies suggest that Simpson II, the current gold standard in spinal meningioma surgery, is associated with a significantly higher rate of symptomatic recurrences requiring reoperation [6,7]. Furthermore, accredited studies report that a higher Simpson grade is an independent increased risk factor for recurrence of spinal meningioma [8]. However, solid evidence is lacking in the literature regarding the objective risk of recurrence of spinal meningiomas following higher Simpson resection grades [8,9]. Introduced in 1957 to estimate the recurrence risk of intracranial meningiomas based on the extent of resection [10], nowadays, the Simpson grading still remains one of the most relevant predicting factors of recurrence [2,11,12,13], although its validity has been recently questioned by several authors [14,15,16,17]. As the extent of resection is a modifiable risk factor in terms of surgical aggressivity, it is very important to verify its validity in order to tailor the strategy of treatment for each patient.
In this setting, our question was as follows: more than half a century after its introduction, considering the refinements of the surgical techniques and pre- and intraoperative tools, as well as the increasing role of the molecular markers and related targeted therapies, is Simpson grading still valid as predicting factor of recurrence for spinal meningiomas? Can we plan the decision-making process of management according to it? The present study aims to answer these questions. Therefore, we investigated the existing evidence in the literature of the last four decades on the relevance of the Simpson grading system for spinal meningiomas and the weighted risk of recurrence for each Simpson grade through a detailed and comprehensive literature meta-analysis and a retrospective analysis of a mono-institutional surgical series of spinal meningiomas.
2. Methods
2.1. Study Setting
The study was conducted as a systematic review and meta-analysis of the literature from 1980 to 2023, using strict inclusion criteria and solid statistical methodology. An institutional series of 74 patients was described and included in the study. The analyses studied the weighted risk of recurrence among patients who received Simpson grade III or more against Simpson I and II, as well as the risk of recurrence between resection grades I and II.
2.2. Definition of the Institutional Cohort
Medical record data of patients with histological diagnosis of spinal meningiomas and operated on at the “Santa Maria delle Grazie” hospital between May 2006 and January 2023 were retrospectively reviewed.
The inclusion criteria were patients aged 18 years old or older who were operated on for spinal meningioma at first diagnosis, a reliable surgical Simpson grading system (Table 1), pre-and 6-month postoperative contrasted-enhanced spinal MRI availability, and a follow-up of at least one year.
Table 1.
Simpson grading system on meningioma resection.
| Simpson Grade | Definition |
|---|---|
| I | Macroscopically complete tumor resection with removal of affected dura and underlying bone |
| II | Macroscopically complete tumor resection with coagulation of affected dura only |
| III | Macroscopically complete tumor resection without removal of affected dura or underlying bone |
| IV | Subtotal tumor resection |
| V | Decompression with or without biopsy |
All patients underwent detailed standard neurological examination at admission and after surgery by a neurosurgeon and pre- and postoperative (3 months after surgery) contrast-enhanced spinal MRI. All surgeries were performed through a standard posterior midline approach with laminectomy extended to include the rostral and caudal limits of the tumor, and the dura was opened on the midline. For Simpson grade I, the tumor dural attachment was resected and the dural defect was reconstructed with an artificial dural substitute. For Simpson grade II, the tumor dural attachment was coagulated along the extension of the dural tail sign at the beginning of the procedure for the deafferentation of the tumor and at the end after using the ultrasonic aspirator. The Simpson grade was defined based on the intraoperative view.
The histology was defined according to the 2021 WHO classification of tumors of the Central Nervous System. Patients’ baseline characteristics are listed in Table 2.
Table 2.
Institutional case series consisting of 74 consecutive spinal meningiomas, from May 2006 to January 2023.
| No. of Patients * | 74 (100) |
|---|---|
| Sex * | |
| Male | 11 (15) |
| Female | 63 (85) |
| Age † (years) | 61.92 (±13.94; 24–84) |
| Segment * | |
| Cervical (C2–C7) | 14 (19) |
| High thoracic (T1–T4) | 12 (16) |
| Middle thoracic (T5–T8) | 14 (19) |
| Lower thoracic (T9–T12) | 34 (46) |
| Lumbosacral (L1-S1) | 0 |
| Dural implant * | |
| Anterior | 33 (45) |
| Lateral | 22 (30) |
| Posterior | 19 (25) |
| Symptom duration before surgery † (Years) | 5.21 (±3.72; 0.12–12) |
| Exordium signs and symptoms * | |
| Pyramidal signs | 30 (40) |
| Radicular band pain | 27 (36) |
| Ataxia or gait impairment | 6 (8) |
| Hyposthenia | 5 (6) |
| Incidentally found | 6 (8) |
| Operative time † (mins) | 293 min (±69.58) |
| Intraoperative blood loss † (Hb g/dL) | 1.32 (±0.95) |
| Postoperative degency † (days) | 293 (±69.58) |
| Histopathology * | |
| Psammomatous | 38 (51) |
| Transitional | 31 (42) |
| Microcystic | 3 (4) |
| Syncytial | 1 (1) |
| Fibroblastic | 1 (1) |
| Atypical | 1 (1) |
| ASA † | 2.31 (±0.58) |
| Calcific lesions * | 21 (28) |
| Simpson resection grade * | |
| I | 16 (22) |
| II | 43 (58) |
| III | 12 (16) |
| IV | 3 (4) |
| V | 0 |
| Recurrencies * | 2 (3) |
| Follow-up † (months) | 92.43 (±56.68; 1–15 years) |
* Categorical variables are expressed as raw frequencies (%). † Qualitative variables are expressed as mean (±SD); range.
2.3. Literature Search Strategy
A systematic review was performed from January 1980 to May 2023. This study was not registered on PROSPERO or other similar databases. However, the study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure methodological rigor and transparency.
PubMed, EMBASE, Cochrane Library, Web of Science, and Google Scholar electronic online databases were searched for studies describing the surgical outcomes of operated spinal meningiomas to identify articles regarding an association between the Simpson grade of resection and the recurrence of spinal meningiomas. Keywords included “Spinal meningioma”, “Adult spinal surgery”, “Intradural extramedullary tumor”, “Laminectomy”, “Simpson grade”, and “Extent of resection”. The synonyms of Medical Subject Headings (MESH) terms and Boolean operators “AND” or “OR” were also used to search.
The search strategy was unrestricted by study design or publication date but included only English-language keywords. Additional articles were identified via citation searching. The titles and abstracts of all records were screened by G.C. and S.C. Two researchers (G.C. and S.C.) evaluated the level of evidence of observational studies using the Melnyk and Fineout-Overholt system [18], and another Author (A.B.) further resolved any conflict in data analysis and studies enrollment. The search strategy is listed in Table 3.
Table 3.
The search strategy of the network meta-analysis. Data regarding the histology, the involved spinal level, the clinical and anthropometric parameters of the patients, the Simpson grade of resection, and the recurrence rates were collected from full texts when available. G.C. and S.C. performed data extraction.
| Frame | Mesh Terms | Search | Inclusion Criteria | Exclusion Criteria | Sources |
|---|---|---|---|---|---|
| P (patients, participants, population) | #1 “Spinal meningioma” #2 “Adult spinal surgery” OR “Intradural extramedullary tumor” |
#1 AND #2 AND #3 AND #4 AND #5 |
Published in peer-review journals | Irrelevant title or abstract Irrelevant full text Editorial, reviews, meta-analysis Studies with less than 20 subjects Experimental/nonhuman studies Mean follow up less than one year |
Databases (PubMed, Cochrane Library, ClinicalTrials.gov, Web of Science, and Scopus) |
| I (intervention) | #3 “Laminectomy” | English language | |||
| C (comparator) | #4 “Simpson I” OR “Simpson II” OR “Simpson III, IV, V” | Randomized controlled trials, non-randomized observational studies, case series | |||
| O (outcome) | #5 “Recurrence” OR “Reoperation” OR “Relapsed spinal meningioma” | Accurately described sample characteristics, Simpson resection grade, stratified recurrences, histopathological diagnosis | Reference list | ||
| T (time) | The search period: 1980 until December 2023 |
2.4. Selection Criteria
Two independent researchers (G.C. and S.C.) screened the literature according to titles and abstracts. After excluding irrelevant studies, the remaining abstracts were read for inclusion. Subsequently, a selection of articles was made, for which odds ratio (OR) calculations and prevalence analysis could be carried out based on the following inclusion and exclusion criteria: histological diagnosis of Spinal Meningioma, operated patients, unambiguous data related to patients’ Simpson resection grade for spinal meningiomas (Table 1), lesion’s recurrences after at least six months from the surgery, and one year stated follow-up minimum. Inclusion criteria for the type of studies were as follows: case series (CS) reporting at least twenty patients, retrospective (RCoh) or prospective cohort studies (PCoh), and case–control (CC) studies; reviews, systematic reviews, and meta-analyses, not describing a proper case series, were investigated for references.
Exclusion criteria included articles not clearly stating a follow-up time or the follow-up time being <1 year, patients lost at follow-up >20%, unknown or inaccurate data, multiple reports or repeated literature on the same population (in case of published data, we only included the most significant and conspicuous population), no data on control or relapse-related influencing factors, not reported Simpson resection grade, low-quality studies, the level of evidence being <6.
The majority of spinal meningiomas were classified as WHO grade I, reflecting their benign nature. Atypical (grade II) and malignant (grade III) lesions were rare and significantly impacted recurrence risk.
In the institutional cohort, tumor location and extension were recorded and considered in the analysis. However, due to inconsistent reporting across the reviewed studies, these factors could not be universally included in the meta-analysis.
While some studies included patients treated before 1980, all were published after 1980 and adhered to the predefined inclusion criteria, ensuring methodological consistency.
Recurrence was defined as tumor relapse for Simpson grades I and II, and lesion volume doubled for Simpson III, IV, and V, requiring reoperation at least six months after the first surgery. Therefore, those patients were the scope of our study. Table 4 lists the criteria and definitions used in the current meta-analysis to group complications reported among the eligible studies.
Table 4.
Criteria and definitions used in the current meta-analysis for grouping of complications reported among the eligible studies.
| Event(s) | Definition |
|---|---|
| Recurrence | Tumor relapse for Simpson grades I and II, and lesion volume doubled for Simpson III and IV, requiring reoperation. |
| Reintervention | Surgical intervention on the operated patient, due to a relapse of the tumor, on the same level as the previous surgery. |
| No recurrence | No visible lesion at the operated level, at the last follow-up. |
| Simpson grading scale | Postoperative surgical scale to assess the extent of surgical resection in meningioma. Grade I: Complete removal, including resection of the underlying bone and associated dura. Grade II: Complete removal with coagulation of dural attachment. Grade III: Complete removal without resection of dura or coagulation. Grade IV: Subtotal resection. Grade V: Simple decompression with or without biopsy. |
2.5. Data Extraction
Two researchers (G.C. and S.C.) independently extracted relevant data from eligible studies. The extracted variables included essential characteristics of the studies (Author, publication year, country of study, follow-up time, sample size, sex distribution), number of patients, number of recurrence-free patients, first surgery Simpson grade, age at surgery, level involved, and histological diagnosis. The data were incorporated into Excel (Microsoft Corporation, Redmond, WA, USA; Version 2016).
2.6. Objectives
The study aimed to investigate the prognostic value of the Simpson grading for spinal meningiomas and the acceptableness of Simpson II resection for spinal meningiomas. Studies enrolled in the different statistical tests are described in Table 5.
Table 5.
General characteristics of eligible studies. * The value is expressed in months. † Level of evidence according to Melnyk & Fineout-Overholt 2023. ¥ For studies not separately mentioning Simpson grades I and II.
| Author | Year | Country | Study Design | Recruitment Period | Patients | Simpson I | Simpson II | Simpson III, IV, V | Mean Follow Up * | Max Follow Up * | Level of Evidence † | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| From | To | Sample | Sex (F-M) |
Pts | Ev | Pts | Ev | Pts | Ev | |||||||
| Levy et al. [19] | 1982 | USA | CS | 1946 | 1982 | 97 | 78-19 | 55 | 0 | 25 | 2 | 7 | 2 | 84 | 336 | 6 |
| Solero et al. [20] | 1989 | Italy | CS | 1954 | 1983 | 174 | 143-31 | 25 | 0 | 125 | 11 | 6 | 1 | 48 | 384 | 6 |
| Bostrom et al. [21] | 2008 | Germany | R/Coh | 1990 | 2005 | 61 | 50-11 | 5 | 0 | 56 | 5 | - | - | 31.3 | 120 | 4 |
| Nakamura et al. [6] | 2012 | Japan | CS | 1983 | 2006 | 68 | 56-12 | 43 | 0 | 25 | 4 | 6 | 6 | 145.2 | 264 | 6 |
| Kim et al. [22] | 2016 | South Korea | CS | 1989 | 2003 | 55 | 40-15 | 21 | 0 | 20 | 1 | 25 | 9 | 45 | 156 | 6 |
| Maiti et al. [23] | 2016 | USA | CS | 2001 | 2015 | 38 | 31-7 | 2 | 0 | 35 | 4 | - | - | 51.2 | 82 | 6 |
| Raco et al. [24] | 2017 | Italy | R/Coh | 1976 | 2011 | 173 | 138-35 | 52 | 1 | 119 | 9 | 2 | 0 | 50.8 | 120 | 4 |
| Gilard et al. [25] | 2018 | France | CS | 2009 | 2016 | 87 | 70-17 | 5 | 0 | 76 | 6 | 6 | 2 | 92.4 | 300 | 6 |
| Kwee et al. [26] | 2020 | Netherlands | R/Coh | 1989 | 2018 | 166 | 139-27 | 33 | 2 | 96 | 8 | 18 | 5 | 12 | 276 | 4 |
| Kobayashi et al. [1] | 2021 | Japan | R/Coh | 1998 | 2018 | 116 | 94-22 | 29 | 1 | 79 | 4 | 8 | 6 | 84.8 | 252 | 4 |
| Our study | 2024 | Italy | R/Coh | 2006 | 2023 | 74 | 63-11 | 16 | 0 | 43 | 2 | 15 | 1 | 92.4 | 180 | 4 |
| Simpson I, II ¥ | Simpson III, IV, V | |||||||||||||||
| Klekamp et al. [27] | 1999 | Germany | R/Coh | 1977 | 1998 | 130 | 93-24 | - | - | 115 | 34 | 15 | 10 | 20 | 156 | 4 |
| Gottfried et al. [28] | 2003 | USA | CS | 1992 | 2002 | 25 | 19-6 | - | - | 23 | 0 | 2 | 1 | 23 | 64 | 6 |
| Schaller et al. [29] | 2005 | Germany | R/Coh | 1980 | 1995 | 33 | 30-3 | - | - | 28 | 1 | 5 | 0 | 96 | 180 | 4 |
| Setzer et al. [30] | 2007 | USA | R/Coh | 1999 | 2007 | 80 | 58-22 | - | - | 75 | 5 | 5 | 3 | 43.5 | 142.7 | 4 |
| Yoon et al. [31] | 2007 | South Korea | CS | 1970 | 2005 | 38 | 31-7 | - | - | 32 | 0 | 6 | 5 | 73 | 223 | 6 |
| Sandalcioglu et al. [32] | 2008 | Germany | CS | 1990 | 2006 | 131 | 114-17 | - | - | 127 | 3 | 4 | 1 | 61 | 116 | 6 |
| Postalci et al. [33] | 2011 | Turkey | CS | 1995 | 2009 | 46 | 33-13 | - | - | 38 | 0 | 8 | 8 | 60 | 60 | 6 |
| Maiuri et al. [11] | 2011 | Italy | R/Coh | 1986 | 2008 | 117 | 87-30 | - | - | 111 | 2 | 6 | 2 | 144 | 276 | 4 |
| Arima et al. [3] | 2014 | Japan | CS | 2007 | 2014 | 23 | 15-8 | - | - | 18 | 2 | 5 | 2 | 32.1 | 84 | 6 |
| Lonjon et al. [34] | 2016 | UK | CS | 2004 | 2014 | 22 | 16-7 | - | - | 14 | 1 | 9 | 3 | 40 | 146 | 6 |
| Schwake et al. [35] | 2018 | Germany | CS | 1996 | 2016 | 84 | 53-31 | - | - | 66 | 0 | 18 | 1 | 19 | 40 | 6 |
| Baro et al. [36] | 2021 | Italy | R/Coh | 2011 | 2018 | 90 | 75-15 | - | - | 78 | 1 | 12 | 1 | 17 | 75 | 4 |
| Pettersson-Segerlind et al. [8] | 2021 | Sweden-Denmark | R/Coh | 2005 | 2017 | 129 | 106-23 | - | - | 92 | 2 | 37 | 4 | 98.4 | 192 | 4 |
| Tominaga et al. [37] | 2021 | Japan | CS | 1992 | 2010 | 29 | 22-7 | - | - | 18 | 0 | 11 | 3 | 132 | 160.5 | 6 |
| Jesse et al. [38] | 2022 | Switzerland | CS | 2009 | 2021 | 86 | 75-11 | - | - | 61 | 1 | 25 | 2 | 29.8 | 162.6 | 6 |
2.7. Statistical Analysis
After selecting articles suitable for the analysis, articles were classified into two groups. The first group included the studies explicitly mentioning the recurrence rates for Simpson grades I and II and Simpson grades III, IV, and V. These were analyzed to compare the recurrence rates, prevalence, and Odds Ratio between the two cohorts. The second group, a subset of the first one, included studies explicitly mentioning the recurrence rates for Simpson grade I and Simpson grade II to compare the recurrence rates, prevalence, and Odds Ratio between these two cohorts of patients. The proportions of recurrences among each Simpson grade were calculated for each study (prevalence per study). Odds Ratio (OR) values assessed categorical variables with 95% confidence intervals (CIs) and p-values. A restricted maximum likelihood (REML) “Fixed effect model” tests were adopted for meta-analysis. Cochran Q and I2 tests were adopted to determine whether the population under study deviated significantly from the general prevalence. An I2 value of less than 40% was defined as homogeneous. Peter’s test was used to detect possible publication bias. Meta-analyses were conducted for each of the two groups. All statistical tests were two-tailed. All statistical analyses were performed using GraphPad Prism (Insight Partners, New York, NY, USA; Version 10.1.2).
3. Results
3.1. Demographic and Clinical Features of the Institutional Cohort
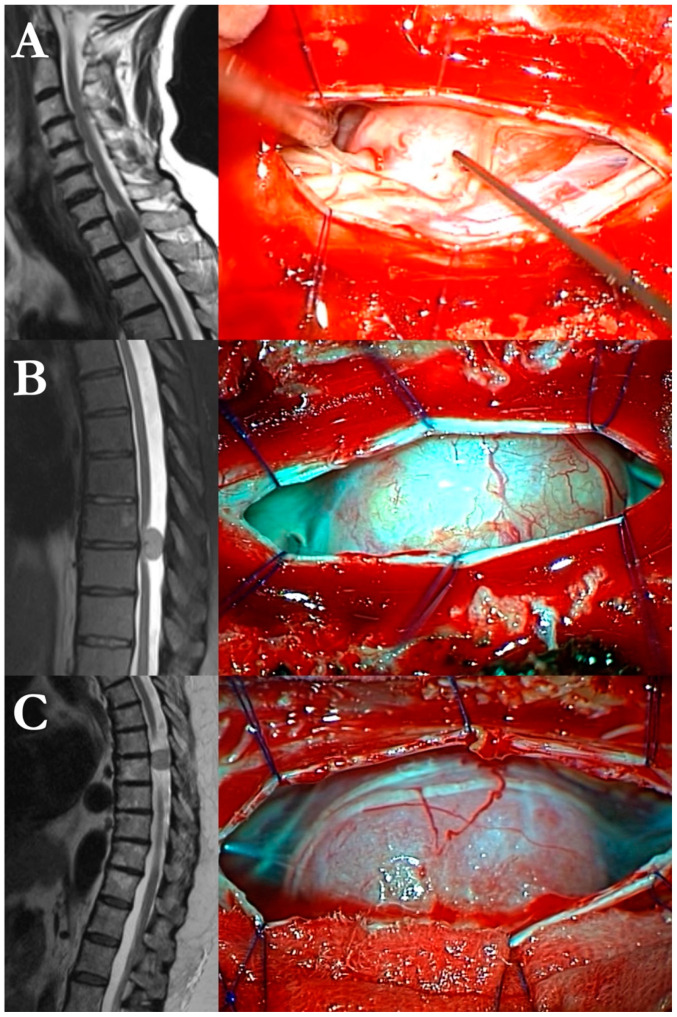
According to the inclusion criteria, 74 consecutive patients were enrolled in the study. Among them, 63 (85.1%) were women, and 11 (14.9%) were men, with a median age of 61.92 (±13.94; range 24–84). The mean time to treatment (interval between the clinical onset and the operative procedure) was 5.21 years (±3.72; 0.12–12). The main presenting signs and symptoms were pyramidal (n = 30/74, 40%), followed by thoracic radicular pain (n = 27/74, 36%), ataxia or gait impairment (n = 6/74, 8%), hyposthenia (n = 5/74, 6%); six lesions were incidentally found on MRI scans for low back pain. The mean operative time was 293 min (±69.58), the mean ASA was 2.31 (±0.58), mean blood loss was 1.32 g/dL Hb points (±0.95). Tumors were most located in the thoracic spine (n = 60/74, 80%), followed by cervical location (n = 14, 20%). The dural tumoral base of attachment was anterior in 33 patients (45%), lateral in 22 (30%), and posterior in 19 (25%). The histopathological diagnoses were distributed as follows: 38 were psammomatous lesions (51%), 31 were transitional (42%), 3 were microcystic (4%), 1 was atypical (1%), 1 syncytial (1%), and 1 fibroblastic (1%). Three illustrative cases are exposed in Figure 1.
Figure 1.
Three illustrative cases from our series of 74 spinal meningiomas: left, preoperative T1-weighted MRI scans; right, intraoperative images. (A) T1 transitional meningioma with anterior growth. (B) T7–T8 psammomatous/calcific meningioma with posterolateral extension. (C) T7 fibroblastic meningioma with right lateral extension.
There was only one case of WHO grade II tumor, whereas the remaining 73 lesions were WHO grade I. The Simpson grade was so distributed: 16 patients received Simpson grade I, 43 grade II, 12 grade III, and 3 Simpson grade IV. Recurrences were observed in patients who underwent Simpson grade II and III resections: the first occurred four years after Simpson grade II, and another one two years after Simpson grade III. The mean follow-up was 92.43 months (±56.68; 1–15 years). Sample baseline characteristics are listed in Table 2.
3.2. Systematic Review
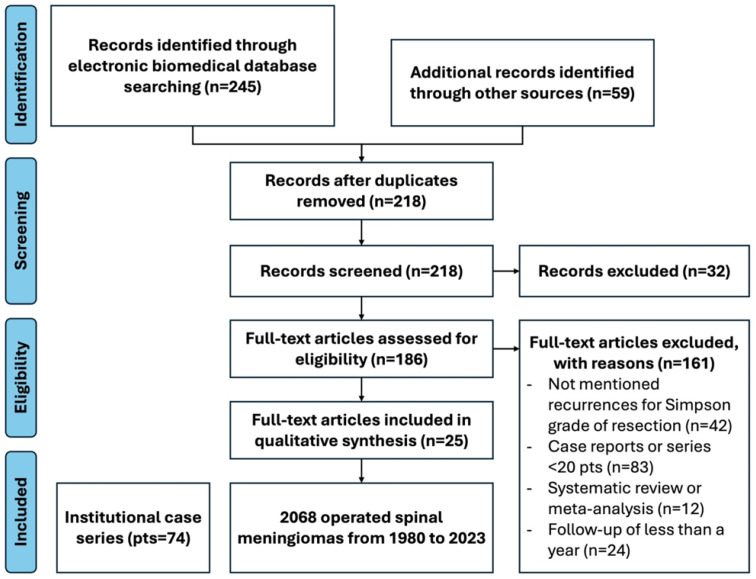
Figure 2 shows the PRISMA flowchart for the study selection process at different stages.
Figure 2.
PRISMA flowchart for study selection.
A total of 304 records were identified. Among them, 245 were found by searching electronic biomedical databases, and 59 studies were manually retrieved and cross-referenced with the corresponding reference lists of identified articles. English-language full text was unavailable for 32 records; hence, these were excluded. After removing 64 duplicates, 186 abstracts were screened for pertinence and methodology. Finally, 25 full-text articles were included in the systematic review and meta-analysis: 10 were case series, and 9 were retrospective cohort studies. The included studies and our institutional case series included 2142 individual patients operated for spinal meningioma.
3.3. Meta-Analysis
The study characteristics are presented in Table 5. Twenty-five articles were selected as suitable for the odds, ORs, and prevalence analyses. A total of 2142 spinal meningioma patients from 1980 to 2023 were included. Regarding the variables, 23 studies (94%) mentioned the respective recurrence rates for Simpson resection grade I and II and Simpson resection grade III, IV, and V, and 10 studies explicitly mentioned the recurrence rates for Simpson I and Simpson II. When comparing the studies, substantial homoscedasticity was shown between the enrolled samples (Cochran Q 20.59; df = 18; I2 = 17.47%). Therefore, a fixed-effect model was chosen for the meta-analysis. In the analysis of all the patients, the proportion of female patients (n 1718; 80.2%) was hugely higher than males (n 424; 19.7%) (Cochran Q 73.74; df = 18; I2 = 76.9); the mean age was 59.93 years (SD 14.99) (Cochran Q 23.60; df = 18; I2 = 27.96); the thoracic segment mainly was involved (n 1548; 72.3%), followed by the cervical segment (n 418; 19.5%), and lumbar (n 176; 7.9%) (Cochran Q 45.74; df = 18; I2 = 62.83%); the mean postoperative follow-up was 64.57 months (SD 31.19) (Cochran Q 75.75; df = 18; I2 = 72.29%).
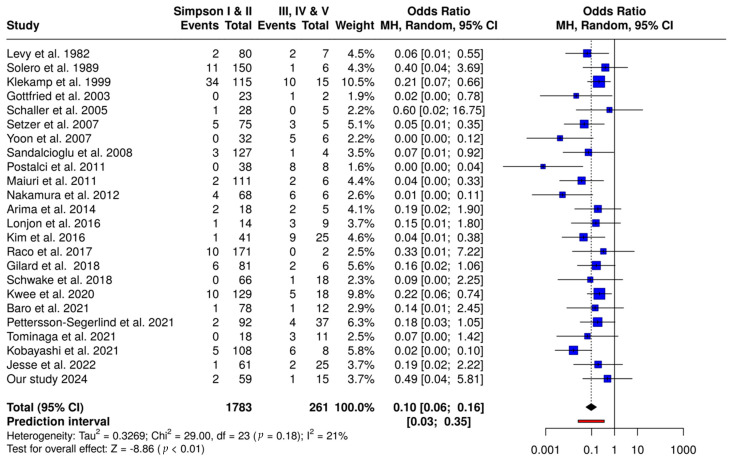
Based on the fixed-effects model, the meta-analysis revealed a considerably greater recurrence prevalence in Simpson III, IV, and V compared with patients who received Simpson grades I and II (OR 0.10; CI95 0.06–0.16). Figure 3 illustrates the results of this analysis.
Figure 3.
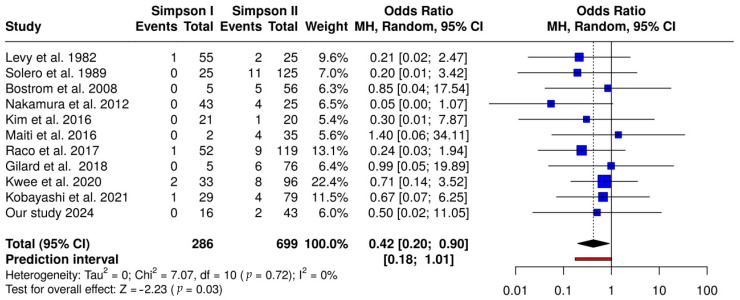
Forest plot comparing Simpson I vs. Simpson II resections. This plot presents a meta-analysis of recurrence rates for patients with Simpson grade I versus Simpson grade II resections. Each study’s odds ratio (OR) and 95% confidence interval (CI) are shown, with the overall OR calculated using a Mantel–Haenszel random-effects model. The pooled OR is 0.42 (95% CI: 0.20–0.90), indicating a statistically significant advantage for Simpson grade I resections in reducing recurrence rates (p < 0.05). The heterogeneity assessment shows no significant variability among studies (I2 = 0%), supporting the consistency of effect sizes across studies [1,3,6,8,12,19,20,22,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
A further analysis of the subset of studies separately mentioning recurrences for Simpson grades I and II showed a significant prevalence of recurrence in Simpson II-treated spinal meningiomas compared to Simpson grade I procedures (OR 0.42; CI95 0.20–0.90). Figure 4 illustrates the results of this analysis.
Figure 4.
Forest plot comparing Simpson I and II vs. Simpson III, IV, and V resections. This plot illustrates a meta-analysis comparing recurrence rates for combined Simpson grades I and II versus Simpson grades III, IV, and V resections. Individual study ORs and 95% CIs are provided, with an overall pooled OR of 0.10 (95% CI: 0.06–0.16), suggesting a substantial reduction in recurrence risk for grades I and II compared to grades III, IV, and V (p < 0.01). Significant heterogeneity was detected (I2 = 21%), indicating variability in effect sizes across studies [1,6,19,20,21,22,23,24,25,26].
3.4. Sensibility Analysis
A leave-one-out sensitivity analysis was conducted to evaluate the stability and robustness of the meta-analysis findings. By systematically removing each study from the dataset, the pooled odds ratios (ORs) were recalculated to identify any single study exerting a disproportionate influence on the overall results. The analysis demonstrated consistent pooled ORs across iterations, with minimal variation observed when individual studies were excluded. This stability in OR values suggests that the conclusions drawn from the meta-analysis are robust and not driven by any single study. Furthermore, the lack of significant fluctuation in the I-squared values across leave-one-out iterations indicates that heterogeneity was not excessively influenced by individual studies. (Table 6 and Table 7).
Table 6.
Leave-one-out sensitivity analysis for Simpson I and II vs. Simpson III, IV, V. This table summarizes the leave-one-out sensitivity analysis for the comparison between Simpson I and II versus Simpson III, IV, and V resections. Each row displays the pooled OR and 95% CI after systematically excluding one study. The results show consistent pooled ORs around 6.415, indicating the robustness of the meta-analysis findings. Heterogeneity remained low (I2 < 40%) throughout, underscoring the consistency of effect sizes across the included studies.
| Excluded_Study | Pooled_Log_Or | Pooled_Or | Ci_Low | Ci_High | Pooled_Se | I_Squared |
|---|---|---|---|---|---|---|
| Levy et al. [19] | 1.859 | 6.415 | 4.067 | 10.119 | 0.233 | <40% |
| Solero et al. [20] | 1.905 | 6.721 | 4.254 | 10.619 | 0.233 | <40% |
| Klekamp et al. [27] | 1.858 | 6.409 | 3.943 | 10.417 | 0.248 | <40% |
| Gottfried et al. [28] | 1.999 | 7.378 | 4.598 | 11.84 | 0.241 | <40% |
| Schaller et al. [29] | 1.874 | 6.516 | 4.141 | 10.253 | 0.231 | <40% |
| Yoon et al. [31] | 1.823 | 6.19 | 3.921 | 9.771 | 0.233 | <40% |
| Setzer et al. [30] | 1.905 | 6.721 | 4.254 | 10.619 | 0.233 | <40% |
| Sandalcioglu et al. [32] | 1.851 | 6.366 | 4.037 | 10.037 | 0.232 | <40% |
| Postalci et al. [33] | 1.797 | 6.032 | 3.824 | 9.516 | 0.233 | <40% |
| Maiuri et al. [11] | 1.8 | 6.047 | 3.81 | 9.599 | 0.236 | <40% |
| Nakamura et al. [6] | 1.776 | 5.907 | 3.735 | 9.341 | 0.234 | <40% |
| Arima et al. [3] | 1.883 | 6.572 | 4.162 | 10.377 | 0.233 | <40% |
| Raco et al. [24] | 1.861 | 6.432 | 4.055 | 10.203 | 0.235 | <40% |
| Kwee et al. [26] | 1.979 | 7.235 | 4.46 | 11.738 | 0.247 | <40% |
| Pettersson-Segerlind et al. [8] | 1.888 | 6.604 | 4.153 | 10.5 | 0.237 | <40% |
| Tominaga et al. [37] | 1.905 | 6.721 | 4.262 | 10.599 | 0.232 | <40% |
| Kobayashi et al. [1] | 1.909 | 6.743 | 4.29 | 10.601 | 0.231 | <40% |
| Jesse et al. [38] | 1.883 | 6.571 | 4.165 | 10.367 | 0.233 | <40% |
| Our study | 1.887 | 6.597 | 4.19 | 10.386 | 0.232 | <40% |
Table 7.
Leave-one-out sensitivity analysis for Simpson I vs. Simpson II. This table presents the results of a leave-one-out sensitivity analysis, showing the pooled odds ratio (OR) and 95% confidence intervals (CI) after excluding each study from the Simpson I vs. Simpson II meta-analysis. The stability of the pooled OR, which remains around 2.169, suggests that no single study disproportionately influences the overall results. Heterogeneity was consistently low, with I2 < 40%, indicating uniformity in effect sizes across studies.
| Excluded_Study | Pooled_Log_Or | Pooled_Or | Ci_Low | Ci_High | Pooled_Se | I_Squared |
|---|---|---|---|---|---|---|
| Levy et al. [19] | 0.774 | 2.169 | 0.938 | 5.016 | 0.428 | <40% |
| Solero et al. [20] | 0.687 | 1.988 | 0.833 | 4.747 | 0.444 | <40% |
| Bostrom et al. [21] | 0.81 | 2.247 | 0.953 | 5.299 | 0.438 | <40% |
| Nakamura et al. [6] | 0.459 | 1.582 | 0.666 | 3.757 | 0.441 | <40% |
| Raco et al. [24] | 0.898 | 2.454 | 1.041 | 5.786 | 0.438 | <40% |
| Kwee et al. [26] | 1.114 | 3.047 | 1.22 | 7.609 | 0.467 | <40% |
| Kobayashi et al. [1] | 0.982 | 2.67 | 1.155 | 6.17 | 0.427 | <40% |
| Our study | 0.872 | 2.391 | 1.033 | 5.534 | 0.428 | <40% |
3.5. Publication Bias
Publication bias was evaluated using Peter’s test, which examines the relationship between effect sizes and their standard errors. The test yielded a coefficient of −0.2311 for the standard error (SE-logOR) with a p-value of 0.799. Given that this p-value is substantially higher than the conventional threshold of 0.05, there is no significant indication of publication bias in the meta-analysis. This result suggests that the effect sizes observed are likely representative and not skewed by selective publication.
4. Discussion
While meningiomas are the most common primary intracranial tumors of the central nervous system, spinal meningiomas are the most common primary spinal tumors [39].
Nevertheless, while many different demographic, clinical, neuroradiological, pathological, and therapeutic risk factors of recurrence have extensively been investigated for intracranial meningiomas [2], fewer data are available on meningiomas located in the spine. In this setting, factors associated with higher recurrence rates for spinal meningiomas are male sex [1,23], younger age [1], extradural extension in young patients [4], arachnoid scarring [27], ventral implant [27], Ki67 and arachnoid invasion [40], and higher WHO grade [30].
Regarding the role of the extent of resection according to Simpson grading as a predictor of recurrence in spinal meningiomas, there is no unanimous consent in the pertinent literature with contrasting results, with some studies reporting no difference [1,40,41] and others reporting a higher recurrence rate associated with Simpson grade II versus grade I [1,37].
The results of the present meta-analysis revealed a considerably higher recurrence rate in Simpson grades III, IV, and V compared with patients who received Simpson grades I and II (OR 9.789; CI95 6.087–15.743) (Figure 3). Furthermore, a significant prevalence of recurrence in Simpson II-treated spinal meningiomas compared to Simpson grade I procedures (OR 2.271; CI95 1.018–5.068).
Functional outcomes following surgical resection of spinal meningiomas were seldom reported in the analyzed literature, limiting comprehensive comparisons. Among the studies reviewed, Simpson grades I and II consistently demonstrated superior functional outcomes compared to grades III, IV, and V [28]. Patients undergoing Simpson grade I resections exhibited the most favorable recovery, with minimal long-term deficits [6]. While Simpson grade II resections provided good postoperative recovery, outcomes were slightly less favorable [6]. Grades III and IV, characterized by subtotal resection, were associated with poorer functional outcomes and persistent neurological deficits [8].
Surgery represents the first line of treatment, with the goal of a gross total (Simpson grade I—macroscopically complete removal of the lesion including its dural attachment and any abnormal bone) and en-bloc tumor resection while preserving/restoring or arresting the deterioration of the neurological functions and without harming the spinal instability, to avoid or decrease the rate of recurrence [42,43]. Nevertheless, this purpose is not always achievable due to the anatomical relationship of the tumor with a high functional structure like the spinal cord; therefore, a Simpson grade II (macroscopically complete removal of the lesion and coagulation of its dural attachment) is an acceptable alternative. Particularly, tumors with ventral attachment represent a technical challenge for Simpson grade I. In addition, the risk of postoperative complications associated with Simpson grade I, including neurological complications, pseudomeningoceles, and CSF leak, should be considered. In this scenario, Saito et al. [44]. developed an alternative technique in which after tumor exeresis, only the inner dural layer is resected, preserving the outer dural layer, to avoid the technical difficulties in the dural reconstruction and the postoperative complications associated.
Therefore, even if the resection of the pathological dura (Simpson I) is associated with a lower risk of recurrence compared to its coagulation and preservation (Simpson II), the strategy of treatment should be carefully balanced between pros and cons and several features correlated to the pathology and the pathology should be taken into account [39]. Remembering the mantra “Primum non nocere”, for ventral spinal meningiomas in elderly symptomatic patients with short life expectancy, the tumor debulking associated with dural coagulation is advisable.
In spinal meningiomas, achieving Simpson grade I resection is often limited by anatomical constraints and the risk of postoperative instability, particularly in ventral lesions. As a result, Simpson grade II resection frequently represents the most viable balance between safety and recurrence risk.
In symptomatic young or adult patients with ventral tumoral implants, a Simpson grade II aiming clinical symptoms and signs resolution, with the option of a re-operation if symptoms will occur over the years, represents a valid option, mainly when intraoperative biopsy detects a WHO grade I tumor.
While Simpson grading provides a valuable framework, WHO grading remains a critical factor influencing outcomes. Grade I lesions showed excellent prognosis, but grades II and III were associated with significantly higher recurrence rates, highlighting the need for tailored surgical and postoperative strategies.
The surgical procedure is tailored case by case according to the patient and pathology features, and involves a midline posterior approach to the spinal canal, through laminectomy or hemilaminectomy, followed by a midline linear dural incision and tumor resection, respecting the spinal cord or exerting gentle traction to gain the tumor access [37]. Intraoperative neurophysiological monitoring is mandatory in this surgery since it changes the functional postoperative outcome dramatically [38]. Techniques for meningioma resection vary depending on the dural attachment of the meningioma and the consistency and extent of the tumor [31]. Intuitively, the extent of resection of the meningioma depends mainly on the dural attachment [7]. For posterior meningiomas, a Simpson I is more likely to be achieved; for ventrally attached lesions, removing the involved dura mater is challenging [45]. Conversely, the procedure is also challenging for lesions extruding laterally into the conjugation foramina. The calcific consistency is a further issue [44]. However, in the literature, spinal meningiomas with posterior dural attachment are described as more easily resectable along with their dural implant, configuring a Simpson I grade [46]. On the other hand, for ventral meningiomas, a Simpson grade II resection has been proposed as acceptable [5]. In the literature, the recurrence rate for microsurgically operated spinal meningiomas varies between 1.3 and 14.7% [21], with disparities due to gender [27], age [8], dural attachment [33], WHO grade [26], and degree of surgical resection [32].
This study demonstrated the standard of care for spinal meningiomas should be Simpson grade I, where achievable, since this was statistically correlated with a significant decrease in recurrence risk. This study represents the first significant evidence of the validity of Simpson grading for spinal meningiomas, after more than 50 years since its adoption for meningioma surgery.
A limitation of this study was that it did not consider further variables for analysis, such as dural attachment, histology, WHO grade, and patient age, in multivariate analysis. Regrettably, no studies describing recurrence rates are available for these variables among studies enrolled in this meta-analysis.
The paucity of data about functional recovery across the included studies represents a significant limitation. Most literature prioritized recurrence and tumor control, underscoring the need for future studies to systematically document neurological outcomes and quality of life to better guide surgical management.
Tumor location and extension are critical factors influencing Simpson grade and surgical outcomes. While these parameters were included in our institutional cohort, their inconsistent reporting across the included studies limited their integration into the meta-analysis, representing a notable limitation.
Another limitation is the lack of data on the timing of relapses for the different Simpson grades. Thereafter, a limitation concerns the low number of studies reporting Simpson grade I and II separately, given the common association with gross total resection of spinal meningioma. A large-scale prospective study should be performed to provide robust evidence about the predictive factors for recurrence in spinal meningiomas.
A large-scale prospective study should be performed to provide robust evidence about the predictive factors for recurrence in spinal meningiomas.
This study represents strong evidence about the prognostic role of the Simpson grading system in spinal meningiomas. Whenever possible, the dural margins and overlying bone should be resected together with the spinal meningioma in surgery to minimize the recurrence rates of spinal meningiomas in the long term.
5. Conclusions
This systematic review and meta-analysis revealed the validity of Simpson’s grading to predict the recurrence rate according to the extent of resection in spinal meningiomas; therefore, it represents useful information for driving the decision-making process of management strategy. Surgery is the first line of treatment with the goal of maximal safe tumor resection (Simpson grade I) while preserving/restoring or arresting deterioration of neurological functions; nevertheless, when the risk of postoperative complications is high, especially for tumors with ventral tumoral dural attachment, the Simpson grade II aiming clinical resolution is a valid alternative, with the option of multiple reoperations over the years if symptoms will occur. The results of this study suggest that the standard of care for spinal meningiomas should be reconsidered, given the significant predictive value of the Simpson grading system for spinal meningiomas.
Author Contributions
G.C.: conceptualization, methodology, investigation, data curation, formal analysis, writing—original draft, and supervision; S.C.: data curation, formal analysis, investigation, methodology, writing—review and editing; V.C., C.M., and M.R.S.: investigation, methodology, project administration, resources, supervision, and validation; S.D.C.: data curation, formal analysis, project administration, and supervision; L.S.: data curation, formal analysis, methodology, project administration, and validation; A.B.: data curation, formal analysis, funding acquisition, investigation, methodology, and supervision; R.d.F.: data curation, formal analysis, investigation, methodology, supervision, and validation. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kobayashi K., Ando K., Matsumoto T., Sato K., Kato F., Kanemura T., Yoshihara H., Sakai Y., Hirasawa A., Nakashima H. Clinical features and prognostic factors in spinal meningioma surgery from a multicenter study. Sci. Rep. 2021;11:11630. doi: 10.1038/s41598-021-91225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corvino S., Altieri R., La Rocca G., Piazza A., Corazzelli G., Palmiero C., Mariniello G., Maiuri F., Elefante A., de Divitiis O. Topographic Patterns of Intracranial Meningioma Recurrences—Systematic Review with Clinical Implication. Cancers. 2024;16:2267. doi: 10.3390/cancers16122267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arima H., Takami T., Yamagata T., Naito K., Abe J., Shimokawa N., Ohata K. Surgical management of spinal meningiomas: A retrospective case analysis based on preoperative surgical grade. Surg. Neurol. Int. 2014;5:S333. doi: 10.4103/2152-7806.139642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen-Gadol A.A., Zikel O.M., Koch C.A., Scheithauer B.W., Krauss W.E. Spinal meningiomas in patients younger than 50 years of age: A 21-year experience. J. Neurosurg. Spine. 2003;98:258–263. doi: 10.3171/spi.2003.98.3.0258. [DOI] [PubMed] [Google Scholar]
- 5.Voldřich R., Netuka D., Beneš V. Spinal meningiomas: Is Simpson grade II resection radical enough? Acta Neurochir. 2020;162:1401–1408. doi: 10.1007/s00701-020-04280-2. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura M., Tsuji O., Fujiyoshi K., Hosogane N., Watanabe K., Tsuji T., Ishii K., Toyama Y., Chiba K., Matsumoto M. Long-term surgical outcomes of spinal meningiomas. Spine. 2012;37:E617–E623. doi: 10.1097/BRS.0b013e31824167f1. [DOI] [PubMed] [Google Scholar]
- 7.Yamamuro K., Seichi A., Kimura A., Kikkawa I., Kojima M., Inoue H., Hoshino Y. Histological investigation of resected dura mater attached to spinal meningioma. Spine. 2012;37:E1398–E1401. doi: 10.1097/BRS.0b013e318268c419. [DOI] [PubMed] [Google Scholar]
- 8.Pettersson-Segerlind J., Fletcher-Sandersjöö A., Tatter C., Burström G., Persson O., Förander P., Mathiesen T., Bartek J., Jr., Edström E., Elmi-Terander A. Long-term follow-up and predictors of functional outcome after surgery for spinal meningiomas: A population-based cohort study. Cancers. 2021;13:3244. doi: 10.3390/cancers13133244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz T.H., McDermott M.W. The Simpson grade: Abandon the scale but preserve the message. J. Neurosurg. 2020;135:488–495. doi: 10.3171/2020.6.JNS201904. [DOI] [PubMed] [Google Scholar]
- 10.SIMPSON D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiuri F., De Caro M.D.B., de Divitiis O., Vergara P., Mariniello G. Spinal meningiomas: Age-related features. Clin. Neurol. Neurosurg. 2011;113:34–38. doi: 10.1016/j.clineuro.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Maiuri F., Corvino S., Corazzelli G., Berardinelli J., Di Crescenzo R.M., De Caro M.D.B. Time to Recurrence of Intracranial Meningiomas from a Monoinstitutional Surgical Series. World Neurosurg. 2024;185:e612–e619. doi: 10.1016/j.wneu.2024.02.087. [DOI] [PubMed] [Google Scholar]
- 13.Maiuri F., Corvino S., Corazzelli G., Del Basso De Caro M. Single versus multiple reoperations for recurrent intracranial meningiomas. J. Neuro-Oncol. 2024;168:527–535. doi: 10.1007/s11060-024-04673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chotai S., Schwartz T.H. The Simpson grading: Is it still valid? Cancers. 2022;14:2007. doi: 10.3390/cancers14082007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gousias K., Schramm J., Simon M. The Simpson grading revisited: Aggressive surgery and its place in modern meningioma management. J. Neurosurg. 2016;125:551–560. doi: 10.3171/2015.9.JNS15754. [DOI] [PubMed] [Google Scholar]
- 16.Simon M., Gousias K. Grading meningioma resections: The Simpson classification and beyond. Acta Neurochir. 2024;166:28. doi: 10.1007/s00701-024-05910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behling F., Fodi C., Hoffmann E., Renovanz M., Skardelly M., Tabatabai G., Schittenhelm J., Honegger J., Tatagiba M. The role of Simpson grading in meningiomas after integration of the updated WHO classification and adjuvant radiotherapy. Neurosurg. Rev. 2021;44:2329–2336. doi: 10.1007/s10143-020-01428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melnyk B.M., Fineout-Overholt E., Giggleman M., Choy K. A test of the ARCC© model improves implementation of evidence-based practice, healthcare culture, and patient outcomes. Worldviews Evid.-Based Nurs. 2017;14:5–9. doi: 10.1111/wvn.12188. [DOI] [PubMed] [Google Scholar]
- 19.Levy W.J., Bay J., Dohn D. Spinal cord meningioma. J. Neurosurg. 1982;57:804–812. doi: 10.3171/jns.1982.57.6.0804. [DOI] [PubMed] [Google Scholar]
- 20.Solero C.L., Fornari M., Giombini S., Lasio G., Oliveri G., Cimino C., Pluchino F. Spinal meningiomas: Review of 174 operated cases. Neurosurgery. 1989;25:153–160. doi: 10.1227/00006123-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Boström A., Bürgel U., Reinacher P., Krings T., Rohde V., Gilsbach J.M., Hans F.-J. A less invasive surgical concept for the resection of spinal meningiomas. Acta Neurochir. 2008;150:551–556. doi: 10.1007/s00701-008-1514-0. [DOI] [PubMed] [Google Scholar]
- 22.Kim C.H., Chung C.K., Lee S.-H., Jahng T.-A., Hyun S.-J., Kim K.-J., Yoon S.H., Kim E.-S., Eoh W., Kim H.-J. Long-term recurrence rates after the removal of spinal meningiomas in relation to Simpson grades. Eur. Spine J. 2016;25:4025–4032. doi: 10.1007/s00586-015-4306-2. [DOI] [PubMed] [Google Scholar]
- 23.Maiti T.K., Bir S.C., Patra D.P., Kalakoti P., Guthikonda B., Nanda A. Spinal meningiomas: Clinicoradiological factors predicting recurrence and functional outcome. Neurosurg. Focus. 2016;41:E6. doi: 10.3171/2016.5.FOCUS16163. [DOI] [PubMed] [Google Scholar]
- 24.Raco A., Pesce A., Toccaceli G., Domenicucci M., Miscusi M., Delfini R. Factors leading to a poor functional outcome in spinal meningioma surgery: Remarks on 173 cases. Neurosurgery. 2017;80:602–609. doi: 10.1093/neuros/nyw092. [DOI] [PubMed] [Google Scholar]
- 25.Gilard V., Goia A., Ferracci F.-X., Marguet F., Magne N., Langlois O., Perez A., Derrey S. Spinal meningioma and factors predictive of post-operative deterioration. J. Neuro-Oncol. 2018;140:49–54. doi: 10.1007/s11060-018-2929-y. [DOI] [PubMed] [Google Scholar]
- 26.Kwee L., Harhangi B., Ponne G., Kros J., Dirven C., Dammers R. Spinal meningiomas: Treatment outcome and long-term follow-up. Clin. Neurol. Neurosurg. 2020;198:106238. doi: 10.1016/j.clineuro.2020.106238. [DOI] [PubMed] [Google Scholar]
- 27.Klekamp J., Samii M. Surgical results for spinal meningiomas. Surg. Neurol. 1999;52:552–562. doi: 10.1016/S0090-3019(99)00153-6. [DOI] [PubMed] [Google Scholar]
- 28.Gottfried O.N., Gluf W., Quinones-Hinojosa A., Kan P., Schmidt M.H. Spinal meningiomas: Surgical management and outcome. Neurosurg. Focus. 2003;14:1–7. doi: 10.3171/foc.2003.14.6.2. [DOI] [PubMed] [Google Scholar]
- 29.Schaller B. Spinal meningioma: Relationship between histological subtypes and surgical outcome? J. Neuro-Oncol. 2005;75:157–161. doi: 10.1007/s11060-005-1469-4. [DOI] [PubMed] [Google Scholar]
- 30.Setzer M., Vatter H., Marquardt G., Seifert V., Vrionis F.D. Management of spinal meningiomas: Surgical results and a review of the literature. Neurosurg. Focus. 2007;23:E14. doi: 10.3171/FOC-07/10/E14. [DOI] [PubMed] [Google Scholar]
- 31.Yoon S.H., Chung C.K., Jahng T.A. Surgical outcome of spinal canal meningiomas. J. Korean Neurosurg. Soc. 2007;42:300. doi: 10.3340/jkns.2007.42.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandalcioglu I.E., Hunold A., Müller O., Bassiouni H., Stolke D., Asgari S. Spinal meningiomas: Critical review of 131 surgically treated patients. Eur. Spine J. 2008;17:1035–1041. doi: 10.1007/s00586-008-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Postalci L., Tugcu B., Gungor A., Guclu G. Spinal meningiomas: Recurrence in ventrally located individuals on long-term follow-up; a review of 46 operated cases. Turk. Neurosurg. 2011;21:449–453. doi: 10.5137/1019-5149.JTN.3518-10.2. [DOI] [PubMed] [Google Scholar]
- 34.Lonjon N., Russo V., Barbarisi M., Choi D., Allibone J., Casey A. Spinal cervical meningiomas: The challenge posed by ventral location. World Neurosurg. 2016;89:464–473. doi: 10.1016/j.wneu.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Schwake M., Adeli A., Sporns P., Ewelt C., Schmitz T., Sicking J., Hess K., Spille D.C., Paulus W., Stummer W. Spinal meningiomas–Risks and potential of an increasing age at the time of surgery. J. Clin. Neurosci. 2018;57:86–92. doi: 10.1016/j.jocn.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Baro V., Moiraghi A., Carlucci V., Paun L., Anglani M., Ermani M., Saladino A., Chioffi F., d’Avella D., Landi A. Spinal meningiomas: Influence of cord compression and radiological features on preoperative functional Status and outcome. Cancers. 2021;13:4183. doi: 10.3390/cancers13164183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tominaga H., Kawamura I., Ijiri K., Yone K., Taniguchi N. Surgical results of the resection of spinal meningioma with the inner layer of dura more than 10 years after surgery. Sci. Rep. 2021;11:4050. doi: 10.1038/s41598-021-83712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jesse C.M., Alvarez Abut P., Wermelinger J., Raabe A., Schär R.T., Seidel K. Functional outcome in spinal meningioma surgery and use of intraoperative neurophysiological monitoring. Cancers. 2022;14:3989. doi: 10.3390/cancers14163989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elsamadicy A.A., Reeves B.C., Craft S., Sherman J.J., Koo A.B., Sayeed S., Sarkozy M., Kolb L., Lo S.-F.L., Shin J.H. A current review of spinal meningiomas: Epidemiology, clinical presentation and management. J. Neuro-Oncol. 2023;161:395–404. doi: 10.1007/s11060-023-04238-1. [DOI] [PubMed] [Google Scholar]
- 40.Maiuri F., Del Basso De Caro M., de Divitiis O., Guadagno E., Mariniello G. Recurrence of spinal meningiomas: Analysis of the risk factors. Br. J. Neurosurg. 2019;34:569–574. doi: 10.1080/02688697.2019.1638886. [DOI] [PubMed] [Google Scholar]
- 41.Barber S.M., Konakondla S., Nakhla J., Fridley J.S., Xia J., Oyelese A.A., Telfeian A.E., Gokaslan Z.L. Oncologic benefits of dural resection in spinal meningiomas: A meta-analysis of Simpson grades and recurrence rates. J. Neurosurg. Spine. 2019;32:441–451. doi: 10.3171/2019.8.SPINE19859. [DOI] [PubMed] [Google Scholar]
- 42.Goldbrunner R., Stavrinou P., Jenkinson M.D., Sahm F., Mawrin C., Weber D.C., Preusser M., Minniti G., Lund-Johansen M., Lefranc F., et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021;23:1821–1834. doi: 10.1093/neuonc/noab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wach J., Banat M., Schuss P., Güresir E., Vatter H., Scorzin J. Age at diagnosis and baseline myelomalacia sign predict functional outcome after spinal meningioma surgery. Front. Surg. 2021;8:682930. doi: 10.3389/fsurg.2021.682930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito T., Arizono T., Maeda T., Terada K., Iwamoto Y. A novel technique for surgical resection of spinal meningioma. Spine (Phila Pa 1976) 2001;26:1805–1808. doi: 10.1097/00007632-200108150-00017. [DOI] [PubMed] [Google Scholar]
- 45.Takami T., Naito K., Yamagata T., Yoshimura M., Arima H., Ohata K. Posterolateral approach for spinal intradural meningioma with ventral attachment. J. Craniovertebral Junction Spine. 2015;6:173–178. doi: 10.4103/0974-8237.167862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gezen F., Kahraman S., Çanakci Z., Bedük A. Review of 36 cases of spinal cord meningioma. Spine. 2000;25:727–731. doi: 10.1097/00007632-200003150-00013. [DOI] [PubMed] [Google Scholar]