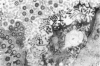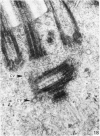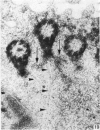Abstract
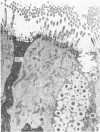
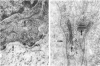
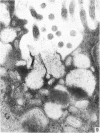
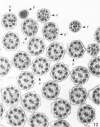
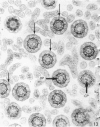
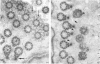
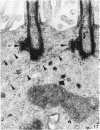
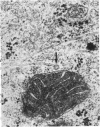
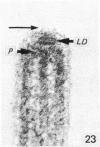
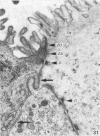
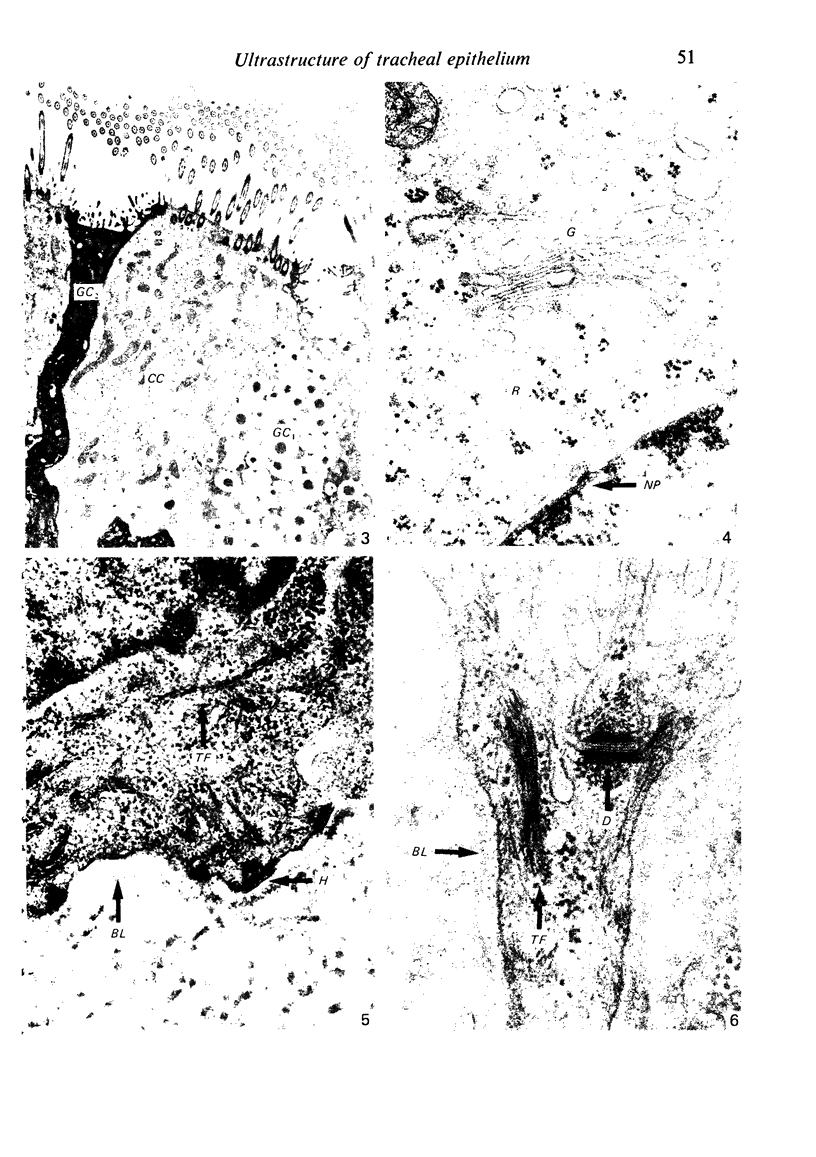
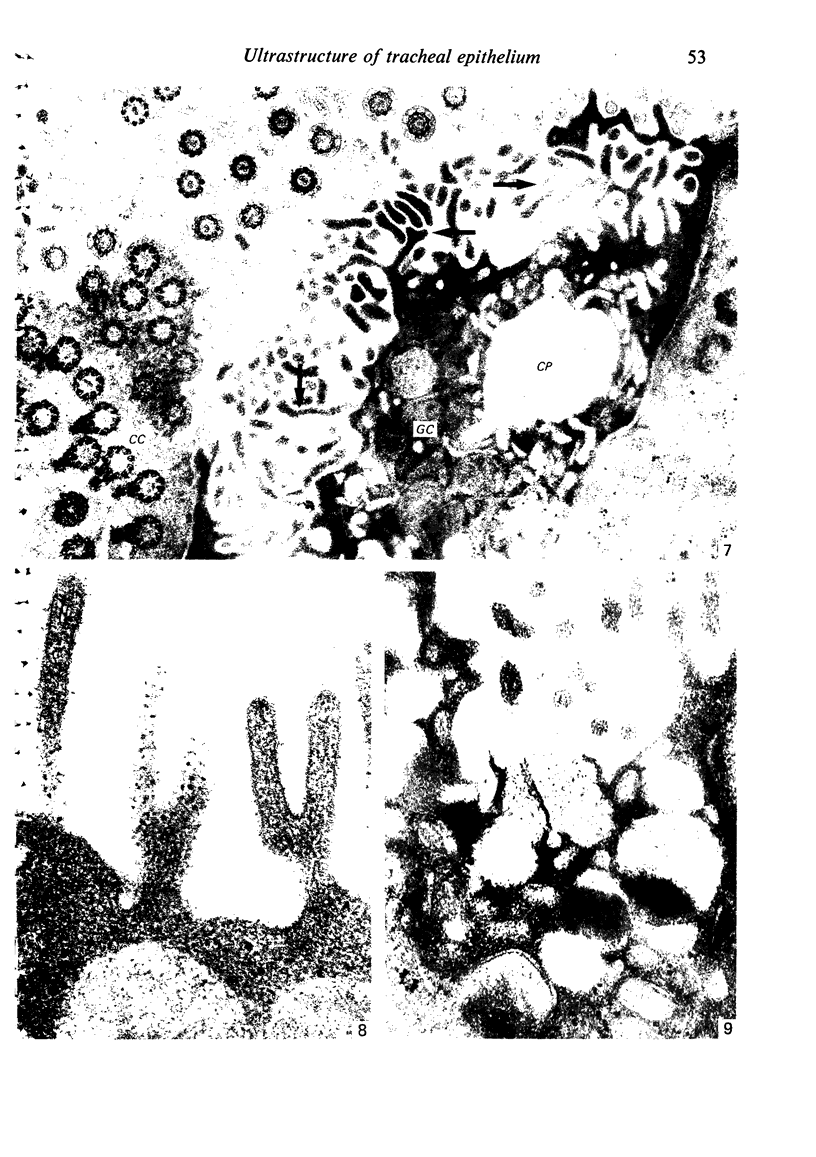
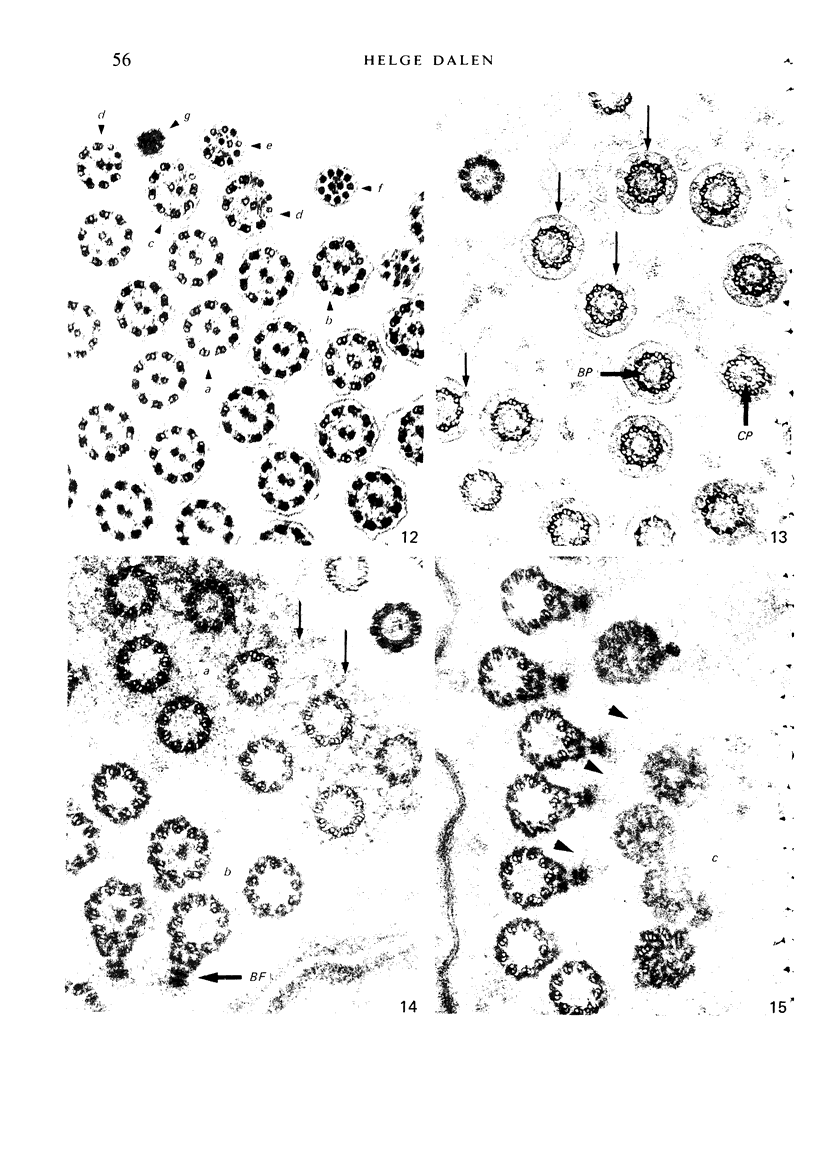
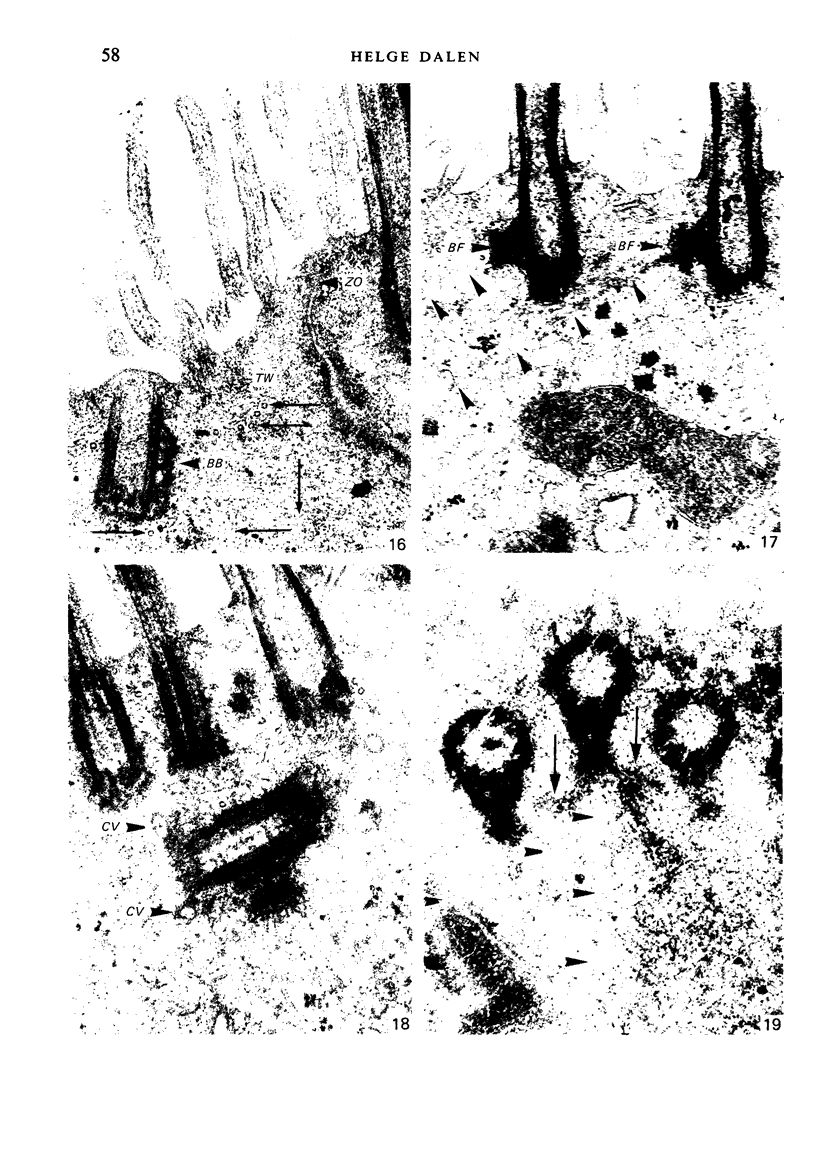
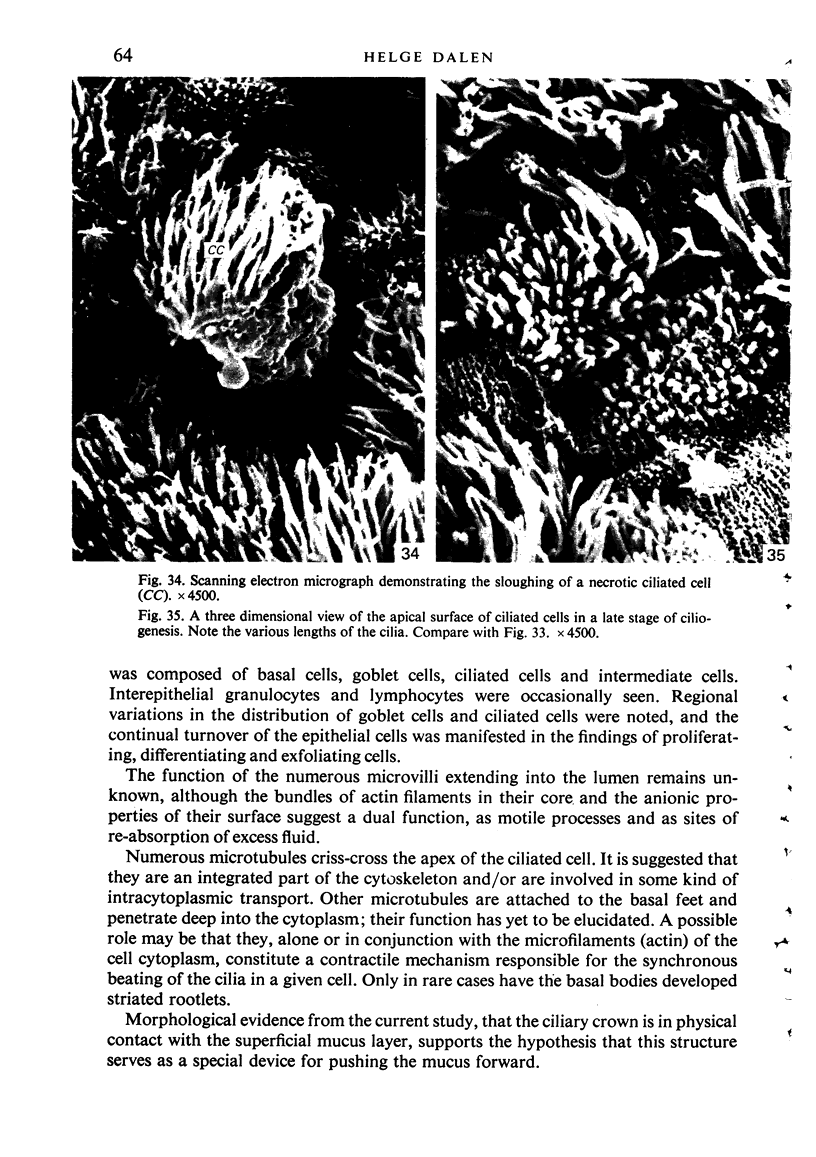
The ultrastructure of the normal guinea-pig tracheal mucosa has been characterised by transmission and scanning electron microscopy. The pseudostratified epithelium was composed of basal cells, goblet cells, ciliated cells and intermediate cells. Interepithelial granulocytes and lymphocytes were occasionally seen. Regional variations in the distribution of goblet cells and ciliated cells were noted, and the continual turnover of the epithelial cells was manifested in the findings of proliferating, differentiating and exfoliating cells. The function of the numerous microvilli extending into the lumen remains unknown, although the bundles of actin filaments in their core and the anionic properties of their surface suggest a dual function, as motile processes and as sites of re-absorption of excess fluid. Numerous microtubules criss-cross the apex of the ciliated cell. It is suggested that they are an integrated part of the cytoskeleton and/or are involved in some kind of intracytoplasmic transport. Other microtubules are attached to the basal feet and penetrate deep into the cytoplasm; their function has yet to be elucidated. A possible role may be that they, alone or in conjunction with the microfilaments (actin) of the cell cytoplasm, constitute a contractile mechanism responsible for the synchronous beating of the cilia in a given cell. Only in rare cases have the basal bodies developed striated rootlets. Morphological evidence from the current study, that the ciliary crown is in physical contact with the superficial mucus layer, supports the hypothesis that this structure serves as a special device for pushing the mucus forward.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Biochemical and cytochemical evidence for ATPase activity in basal bodies isolated from oviduct. J Cell Biol. 1977 Aug;74(2):547–560. doi: 10.1083/jcb.74.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Hein C. E. Distribution of anionic sites on the oviduct ciliary membrane. J Cell Biol. 1977 Feb;72(2):482–492. doi: 10.1083/jcb.72.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G. The three-dimensional structure of the basal body from the rhesus monkey oviduct. J Cell Biol. 1972 Aug;54(2):246–265. doi: 10.1083/jcb.54.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becci P. J., McDowell E. M., Trump B. F. The respiratory epithelium. II. Hamster trachea, bronchus, and bronchioles. J Natl Cancer Inst. 1978 Aug;61(2):551–561. [PubMed] [Google Scholar]
- Breeze R. G., Wheeldon E. B. The cells of the pulmonary airways. Am Rev Respir Dis. 1977 Oct;116(4):705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- Breipohl W., Herberhold C., Kerschek R. Die Oberflächenmorphologie des Trachealepithels männlicher Albinoratten. Eine reflektionsrasterelektronenmikroskopische Studie. Arch Otorhinolaryngol. 1975 Dec 30;211(4):267–281. doi: 10.1007/BF00456347. [DOI] [PubMed] [Google Scholar]
- Brunser O., Luft H. J. Fine structure of the apex of absorptive cell from rat small intestine. J Ultrastruct Res. 1970 May;31(3):291–311. doi: 10.1016/s0022-5320(70)90133-4. [DOI] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Hu S. S. Ultrastructural studies of hamster tracheal epithelium in vivo and in vitro. J Ultrastruct Res. 1980 Jan;70(1):70–81. doi: 10.1016/s0022-5320(80)90023-4. [DOI] [PubMed] [Google Scholar]
- Cireli E. Elektronenmikroskopische Analyse der prä- und postnatalen Differenzierung des Epithels der oberen Luftwege der Ratte. Z Mikrosk Anat Forsch. 1966;74(2):132–178. [PubMed] [Google Scholar]
- Dahlgren S. E., Dalen H., Dalhamm T. Ultrastructural observations on chemically induced inflammation in guinea pig trachea. Virchows Arch B Cell Pathol. 1972;11(3):211–223. doi: 10.1007/BF02889400. [DOI] [PubMed] [Google Scholar]
- Dalen H. An ultrastructural study of primary cilia, abnormal cilia and ciliary knobs from the ciliated cells of the guinea-pig trachea. Cell Tissue Res. 1981;220(4):685–697. doi: 10.1007/BF00210455. [DOI] [PubMed] [Google Scholar]
- Dentler W. L. Microtubule-membrane interactions in cilia and flagella. Int Rev Cytol. 1981;72:1–47. doi: 10.1016/s0074-7696(08)61193-6. [DOI] [PubMed] [Google Scholar]
- Dirksen E. R., Satir P. Ciliary activity in the mouse oviduct as studied by transmission and scanning electron microscopy. Tissue Cell. 1972;4(3):389–403. doi: 10.1016/s0040-8166(72)80017-x. [DOI] [PubMed] [Google Scholar]
- FAWCETT D. W. SURFACE SPECIALIZATIONS OF ABSORBING CELLS. J Histochem Cytochem. 1965 Feb;13:75–91. doi: 10.1177/13.2.75. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Agee C. C., Cameron A. M. Differential distribution of ciliated epithelial cells in the trachea of hamsters: implications for studies of pathogenesis. J Infect Dis. 1977 Jan;135(1):9–19. doi: 10.1093/infdis/135.1.9. [DOI] [PubMed] [Google Scholar]
- Gordon R. E., Lane B. P., Miller F. Identification of contractile proteins in basal bodies of ciliated tracheal epithelial cells. J Histochem Cytochem. 1980 Nov;28(11):1189–1197. doi: 10.1177/28.11.7000888. [DOI] [PubMed] [Google Scholar]
- Greenwood M. F., Holland P. The mammalian respiratory tract surface. A scanning electron microscopic study. Lab Invest. 1972 Sep;27(3):296–304. [PubMed] [Google Scholar]
- Hansell M. M., Moretti R. L. Ultrastructure of the mouse tracheal epithelium. J Morphol. 1969 Jun;128(2):159–169. doi: 10.1002/jmor.1051280203. [DOI] [PubMed] [Google Scholar]
- Inoue S., Hogg J. C. Freeze-etch study of the tracheal epithelium of normal guinea pigs with particular reference to intercellular junctions. J Ultrastruct Res. 1977 Oct;61(1):89–99. doi: 10.1016/s0022-5320(77)90008-9. [DOI] [PubMed] [Google Scholar]
- Inoue S., Hogg J. C. Intercellular junctions of the tracheal epithelium in guinea pigs. Lab Invest. 1974 Jul;31(1):68–74. [PubMed] [Google Scholar]
- Ito S. The enteric surface coat on cat intestinal microvilli. J Cell Biol. 1965 Dec;27(3):475–491. doi: 10.1083/jcb.27.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. New observations of rat airway epithelium: a quantitative and electron microscopic study. J Anat. 1975 Nov;120(Pt 2):295–320. [PMC free article] [PubMed] [Google Scholar]
- Kilburn K. H. A hypothesis for pulmonary clearance and its implications. Am Rev Respir Dis. 1968 Sep;98(3):449–463. doi: 10.1164/arrd.1968.98.3.449. [DOI] [PubMed] [Google Scholar]
- Kuhn C., 3rd, Engleman W. The structure of the tips of mammalian respiratory cilia. Cell Tissue Res. 1978 Jan 31;186(3):491–498. doi: 10.1007/BF00224937. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M. L., Lane B. P., Gordon R. E., Drummond E. Ultrastructure of rat tracheal epithelium. Lung. 1979;156(4):223–236. doi: 10.1007/BF02714016. [DOI] [PubMed] [Google Scholar]
- Matsuda K. Studies on mucous flow of the trachea and scanning electron microscopic studies of mucous membrane. Nihon Geka Hokan. 1978 Nov 1;47(6):643–672. [PubMed] [Google Scholar]
- Mooseker M. S., Tilney L. G. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol. 1975 Dec;67(3):725–743. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood J. T., Anderson R. G. Evidence that adhesive sites on the tips of oviduct cilia membranes are required for ovum pickup in situ. Biol Reprod. 1980 Nov;23(4):788–791. doi: 10.1095/biolreprod23.4.788. [DOI] [PubMed] [Google Scholar]
- Norwood J. T., Hein C. E., Halbert S. A., Anderson R. G. Polycationic macromolecules inhibit cilia-mediated ovum transport in the rabbit oviduct. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4413–4416. doi: 10.1073/pnas.75.9.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack R. J., Al-Ugaily L. H., Morris G. The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscope study. J Anat. 1981 Jan;132(Pt 1):71–84. [PMC free article] [PubMed] [Google Scholar]
- Pack R. J., Al-Ugaily L. H., Morris G., Widdicombe J. G. The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res. 1980;208(1):65–84. doi: 10.1007/BF00234174. [DOI] [PubMed] [Google Scholar]
- Pavelka M., Ronge H. R., Stockinger G. Vergleichende Untersuchungen am Trachealepithel verschiedener Säuger. Acta Anat (Basel) 1976;94(2):262–282. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODIN J., DALHAMN T. Electron microscopy of the tracheal ciliated mucosa in rat. Z Zellforsch Mikrosk Anat. 1956;44(4):345–412. doi: 10.1007/BF00345847. [DOI] [PubMed] [Google Scholar]
- Reverdin N., Gabbiani G., Kapanci Y. Actin in tracheo-bronchial ciliated epithelial cells. Experientia. 1975 Nov 15;31(11):1348–1350. doi: 10.1007/BF01945820. [DOI] [PubMed] [Google Scholar]
- Richter-Reichhelm H. B., Emura M., Althoff J. Scanning electron microscopical investigations on the respiratory epithelium of the Syrian golden hamster. I. Postnatal differentiation. Zentralbl Bakteriol Mikrobiol Hyg B. 1980 Sep;171(4-5):424–432. [PubMed] [Google Scholar]
- Satir P. Structural basis of ciliary movement. Environ Health Perspect. 1980 Apr;35:77–82. doi: 10.1289/ehp.803577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolich J. J., Stratford B. F., Maloney J. E., Ritchie B. C. Postnatal development of the epithelium of larynx and trachea in the rat: scanning electron microscopy. J Anat. 1977 Dec;124(Pt 3):657–673. [PMC free article] [PubMed] [Google Scholar]
- Stockinger L. Ultrastruktur und Histophysiologie des Respirationstraktes. Mikroskopie. 1970 Jul;26(3):83–98. [PubMed] [Google Scholar]
- Summers K. The role of flagellar structures in motility. Biochim Biophys Acta. 1975 Aug 15;416(2):153–168. doi: 10.1016/0304-4173(75)90005-1. [DOI] [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner F. D., Mitchell D. R. Dynein: the mechanochemical coupling adenosine triphosphatase of microtubule-based sliding filament mechanisms. Int Rev Cytol. 1980;66:1–43. doi: 10.1016/s0074-7696(08)61970-1. [DOI] [PubMed] [Google Scholar]
- Warner F. D., Satir P. The structural basis of ciliary bend formation. Radial spoke positional changes accompanying microtubule sliding. J Cell Biol. 1974 Oct;63(1):35–63. doi: 10.1083/jcb.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates G. T., Wu T. Y., Johnson R. E., Cheung A. T., Frand C. L. A theoretical and experimental study on tracheal muco-ciliary transport. Biorheology. 1980;17(1-2):151–162. doi: 10.3233/bir-1980-171-216. [DOI] [PubMed] [Google Scholar]