Abstract
TFIIS is a transcription elongation factor for RNA polymerase II (pol II), which can suppress ribonucleotide misincorporation. We reconstituted transcription complexes in a highly purified pol II system on adenovirus Major-Late promoter constructs. We noted that these complexes have a high propensity for read-through upon GTP omission. Read-through occurred during the early stages at all registers analyzed. Addition of TFIIS reversed read-through of productive elongation complexes, which indicated that it was due to misincorporation. However, before register 13 transcription complexes were insensitive to TFIIS. These findings are discussed with respect to the structural models for pol II and we propose that TFIIS action is linked to the RNA:DNA hybrid.
INTRODUCTION
Regulation of transcription is the most common mechanism to selectively express eukaryotic genes. Each step of the RNA polymerase II (pol II) transcription cycle is subjected to regulation, which illustrates the versatility of this process. The first step involves pol II pre-initiation complex (PIC) assembly requiring assistance of basal transcription initiation factors (reviewed in 1–3). ATP hydrolysis by the basal factor TFIIH marks the activation step, which is accompanied by open complex formation. The activated state requires continuous ATP hydrolysis as addition of ATPγS, a non-hydrolyzable ATP analog, results in reversion to the closed complex (4,5). RNA synthesis starts with an abortive phase during which multiple short RNA products can be formed. These short RNAs are not stably bound to the transcription complex. After formation of three phosphodiester bonds the open complex is no longer ATPγS sensitive (5) and the 4-nt RNA product remains associated with the transcription complex (6). This transition has been called escape commitment by Kugel and Goodrich (6). Stable ternary complexes are formed after register 11, when the transcription cycle enters the productive elongation phase (5). Elongating pol II enzymes can stall upon encountering elongation blocks imposed by proteins binding to the template and by intrinsic DNA structures. In some cases transient stalling of pol II complexes converts to stable transcriptional arrest. Arrest is accompanied by a structural change within the transcription complex involving disengagement of the 3′-end of the RNA transcript from the active site and backtracking of the pol II enzyme along the DNA template (7–9).
Transcription elongation factor TFIIS can reactivate arrested complexes by stimulating exonucleolytic cleavage of the nascent RNA, which realigns the 3′-end in register with the catalytic center (reviewed in 10,11). Released cleavage products range from 2 to 14 nt and this may relate to the extent of backtracking (9,12). The reactivation process involves direct interactions of TFIIS with pol II. Most likely, the RPB1 and RPB6 subunits provide the surfaces for this interaction (13,14). Structural analysis of yeast TFIIS indicated that its three-helix bundle domain II (region 130–240) mediates pol II binding. Intact domain III (residues 240–309) harboring a zinc-ribbon structure is required for the RNA cleavage function of the pol II/TFIIS complex (15). Interestingly, domain I (the first 130 amino acids) is homologous to transcription proteins elongin A and CRSP. It forms a four-helix bundle structure (16), but is dispensible for both in vivo and in vitro TFIIS activity (17,18). Binding experiments using crude extracts indicated that domain I is required for the holo-enzyme form of pol II but not for core pol II (19).
Besides the rescue of transcriptional arrest, TFIIS was also shown to induce proofreading activity of pol II. The isolated enzyme has a relatively high misincorporation rate during elongation (estimated 1: 103–105; 20). Hawley and colleagues (21) applied a protocol, which forced pol II to incorporate IMP during productive elongation. Product analysis showed that inclusion of TFIIS severely suppressed IMP incorporation. They also showed that upon TFIIS addition, a mismatched ribonucleoside is efficiently removed from the 3′-end of nascent RNA. Similar findings were made using dumbbell templates, which were used to force misincorporation (22).
The mechanisms of TFIIS-mediated rescue of arrested complexes and of TFIIS-induced proofreading are probably related. It is unlikely that TFIIS itself performs the exonucleolytic cleavage reaction. It seems that this activity resides within the pol II enzyme itself and becomes exposed after association with TFIIS (10,20). While the exact cleavage mechanism is not completely understood, the recently described structural models for elongating pol II shed some light on this (23–25). The models revealed an inverted funnel leading via pore 1 to the catalytical center. It was proposed that this funnel not only allows the entry of ribonucleotide substrates, but also that the 3′-end of the nascent RNA from arrested complexes is extruded through pore 1 into the funnel (23,24). The funnel probably harbors the TFIIS binding site (23,25). Possibly, pol II binding via domain II brings domain III of TFIIS in close proximity to pore 1 at the end of the inverted funnel. Domain III could induce allosteric changes in the catalytic center, which stimulate exonucleolytic cleavage upon DNA:RNA mismatch or backtracking of arrested complexes. This model is supported by a study by Reines and co-workers (26), which showed that TFIIS could be cross-linked at a low efficiency to the 3′-end of RNA.
In this paper we investigate TFIIS action during the early steps of the transcription cycle in reconstituted in vitro transcription assays. This was motivated by our observations that omitting GTP from our reactions did not result in a homogenous stalling of transcription complexes at G residues in the template (5,8). Rather, precise stalling of transcription initiation complexes required the presence of a RNA chain terminator (3′-O-methyl guanine triphosphate in our case). In the presence of natural substrates significant amounts of read-through products were observed and this already occurred at NTP concentrations just above the apparent Km of the RNA polymerization reaction. In the following set of experiments we address the question of whether misincorporation occurs at all registers during early initiation of transcription and whether TFIIS can reverse this in all cases. This is investigated in our pol II transcription system reconstituted with recombinant or highly purified transcription factors. We find that read-through rates are remarkably high at all stages of initiation. TFIIS can efficiently prevent read-through of productive transcription complexes. In contrast, pol II complexes in the abortive mode of RNA synthesis are largely refractory to TFIIS action. These results support the model that fundamental conformational changes occur within pol II transcription complexes after initiation when they proceed to productive RNA synthesis. These changes seem to affect multiple aspects of the pol II transcription process.
MATERIALS AND METHODS
Materials
Nucleotides were obtained from Roche Molecular Biochemicals. Radioactive nucleotides were obtained from NEN/DuPont. Restriction enzymes, Klenow DNA polymerase and calf intestine phosphatase were purchased from Pharmacia. The DNA constructs were verified using the ABI Prism Big Dye™ Terminator Cycle Sequencing Ready Reaction Kit in the GeneAmp PCR System 2400 and analyzed with the ABI Prism 310 Genetic Analyzer (all PE Applied Biosystems). The Qiaquick gel extraction kit was purchased from Qiagen. Quantification by PhosphorImager analysis was performed on a Storm 820 gel scanner from Molecular Dynamics using ImageQuaNT software version 4.2.
Purification of the transcription factors
Recombinant histidine-tagged human TBP, recombinant human TFIIB and recombinant human TFIIE were expressed in Escherichia coli and purified as described previously (27,28). Recombinant human ΔTFIIS protein (residues 130–301) was a gift from C. Kane (UC Berkeley). Full-length his-tagged human TFIIS (pET22a-HisTFIIS expression plasmid, a gift from C. Kane) was expressed in E.coli and purified by chromatography on Ni-NTA and MonoS columns. In short, peak fractions from the Ni-NTA column were adjusted to buffer A (20 mM HEPES–KOH pH 8.05, 20% glycerol, 1 mM EDTA, 0.5 mM PMSF and 1 mM DTT) plus 40 mM KCl. After loading on a MonoS HR10/10 column, bound his-TFIIS protein was eluted by a linear gradient of 40–470 mM KCl in buffer A. The protein eluted from the MonoS column at 220–240 mM KCl and was homogenous as indicated by Coomassie staining of protein gels.
Recombinant TFIIF (human RAP74/rat RAP30) was purified from baculovirus-infected insect cells as described previously (28). Pol II was affinity purified from calf thymus using the 8WG16 antibody (28) and TFIIH was purified from HeLa cell extracts (8) as described.
Preparations of TBP, TFIIB, TFIIE and TFIIF were homogeneous and TFIIH and pol II preparations are highly purified as judged from silver-stained protein gels. None of the purified proteins contained detectable nuclease, topoisomerase or other basal factor activity as shown by direct assays.
Template DNA
The adenovirus Major-Late (AdML) promoter constructs were derived from pDN-AdMLmut (29). The pDN-AdML+3G, +4G, +6G, +9G, +10G and +11G plasmids have been described previously (30) as well as the pDN-AdML+17G, +19G, +21G, +23G and +25G plasmids (8). The pDN-AdML+7G plasmid was constructed by inserting the appropriate oligonucleotides into the BssHII–XbaI-digested pDN-AdML+6G plasmid. The pDN-AdML+13G plasmid was constructed by inserting the appropriate oligonucleotides into the BssHII–XbaI-digested pDN-AdML+19G plasmid. All constructions were verified by DNA sequence analysis. For transcription assays, promoter DNA fragments (282–292 bp) were isolated from the appropriate pDN-AdMLmut derivative by restriction enzyme digestion with PvuII. The template fragments were recovered from preparative 2% agarose gels using the Qiaquick gel extraction kit.
Transcription assays
Template DNA fragments (5 ng of the PvuII fragments) were incubated at 30°C for 30 min with 25 ng TBP, 25 ng TFIIB, 80 ng TFIIF, 40 ng pol II, 30 ng TFIIE, saturating amounts of TFIIH and varying amounts of human TFIIS or ΔTFIIS (as indicated in the figure legends) in a 20 µl reaction that contained 12 mM HEPES–KOH pH 7.9, 60 mM KCl, 5 mM MgCl2, 3 mM (NH4)2SO4, 0.6 mM EDTA, 0.7 mM DTT and 90 µg/ml BSA. After transcription complex assembly, the nucleotides were added to the concentrations indicated in the figure legends and incubated at 30°C for the indicated time. Reactions analyzing products <5 nt were heat inactivated (3 min at 95°C), treated with 5 U of calf intestine phosphatase for 30 min at 37°C and were loaded directly on the gel. The other reactions were stopped and processed including an ethanol-precipitation step as described previously (31). RNA products were analyzed by electrophoresis on 21% polyacrylamide (acrylamide:bisacrylamide 19:2)–7 M urea gels and visualized by autoradiography on Fuji SuperRX films.
RESULTS
Establishment of the reconstituted basal pol II transcription system
In order to analyze the effect of TFIIS on the catalytic properties of pol II complexes during the early phase of transcription we devised the following protocol. PICs were assembled on AdML promoter templates with basal transcription factors from recombinant sources (TBP, TFIIB, TFIIE, TFIIF, TFIIS and ΔTFIIS), HeLa cells (TFIIH) or calf thymus (pol II). The AdML templates were modified by insertion of G residues at specific positions in the non-template strand in order to allow stalling of pol II complexes between register 3 and 25 by omission of GTP (Fig. 1). After PIC assembly nucleotides (ATP, UTP and CTP) were added at concentrations two to four times their respective Km, which was determined experimentally in this system (data not shown). In these reactions the activity of two different forms of human TFIIS protein were analyzed: full-length protein or a deletion variant lacking domain I (the first 129 residues).
Figure 1.
DNA sequence of the non-template strand of the pDN-AdML+nG plasmids. Indicated in bold are the natural transcription start site (also depicted by the broken arrow) and the first three G residues in the RNA chain. Transcribed sequences are depicted in capitals and template sequences outside the indicated region are identical.
Selective suppression of extended RNA products by TFIIS
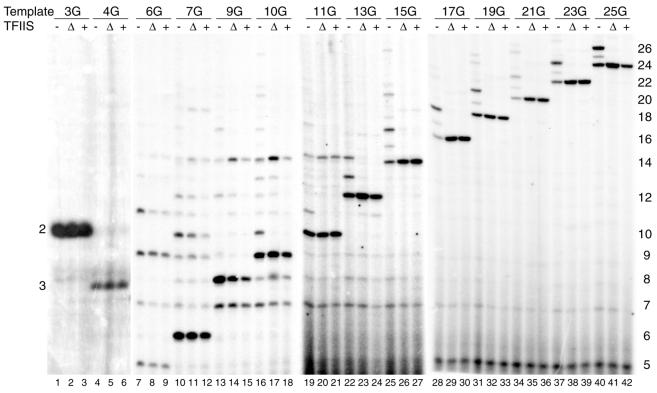
Figure 2 shows that the predicted RNA products are formed on the different templates. Importantly, in the absence of TFIIS, larger than predicted RNAs are also observed on all templates (for the AdML+3G and AdML+4G only visible upon longer exposure; 30). In some instances the predicted RNA constitutes only a minority of the RNA products (lanes 7, 25, 28, 34 and 37). Inspection of the sizes and relative intensities of the larger RNAs indicates that transcription predominantly stops before the second G residue in most cases. For example, prominent bands are the 14-nt products on the AdML+7G to AdML+13G templates (lanes 10, 13, 16, 19 and 22) and the 16-, 18-, 20-, 22-, 24- and 26-nt products on the AdML+15G, AdML+17G, AdML+19G, AdML+21G, AdML+23G and AdML+25G templates, respectively (lanes 25, 28, 31, 34, 37 and 40). It is unlikely that these larger RNAs result from low levels of GTP contamination. First, they can be reverted to the predicted size by addition of TFIIS (see below). In case a contaminating GTP would have been incorporated, addition of TFIIS would have no effect. Secondly, they are observed irrespective of the batch of ultrapure nucleoside triphosphates. Also, it has been shown that uridine misincorporation opposite to deoxyguanosine is rather efficient (22). This suggests that the larger than predicted RNAs are read-through products resulting from misincorporation of ribonucleosides by pol II.
Figure 2.
Analysis of the RNA products formed with the mutant AdML promoter templates in absence and presence of ΔTFIIS or full-length TFIIS. Transcription initiation complexes were assembled for 30 min on AdML+3G (lanes 1–3), AdML+4G (lanes 4–6), AdML+6G (lanes 7–9), AdML+7G (lanes 10–12), AdML+9G (lanes 13–15), AdML+10G (lanes 16–18), AdML+11G (lanes 19–21), AdML+13G (lanes 22–24), AdML+15G (lanes 25–27), AdML+17G (lanes 28–30), AdML+19G (lanes 31–33), AdML+21G (lanes 34–36), AdML+23G (lanes 37–39) or AdML+25G (lanes 40–42) templates as described in the Materials and Methods. Reactions 1, 4, 7, 10, 13, 16, 19, 22, 25, 28, 31, 34, 37 and 40 received no TFIIS while reactions 2, 5, 8, 11, 14, 17, 20, 23, 26, 29, 32, 35, 38 and 41 contained 40 nM ΔTFIIS protein and reactions 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, 39 and 42 contained 40 nM full-length TFIIS protein. Nucleotide triphosphates (60 µM ATP, 10 µM UTP and 2 µM [α-32P]CTP) were added for 15 min. Subsequently, RNA products were processed and analyzed by denaturing polyacrylamide gel electrophoresis as described in Materials and Methods. The length of the RNA products is indicated on the left (for lanes 1–6) and right of the figure.
Inclusion of TFIIS activity during PIC assembly dramatically reduces the appearance of read-through RNAs on templates AdML+13G to AdML+25G (Fig. 2, lanes 22–42). In contrast, TFIIS addition has a more limited effect on transcription from the AdML+3G to AdML+11G templates. For example, the 7- and 9-nt read-through RNAs formed on the AdML+7G template are not affected and the 10- and 12-nt products are only partially reduced, which is best observed in ΔTFIIS reaction (Fig. 2, compare lane 10 with lanes 11 and 12). In almost all cases TFIIS efficiently suppresses the formation of read-through RNA products >14 nt (for example, Fig. 2, compare lane 16 with lanes 17 and 18). Interestingly, in case of the AdML+9G, AdML+10G and AdML+11G the 14-nt product increases in intensity upon TFIIS addition (Fig. 2, lanes 13–21). The reason for this may be the trimming down of large RNAs to 14 nt, which is the position of the second G residue in these templates. This also suggests that misincorporation at the position of the first G residue does not lead to further exonucleolytic cleavage in these cases. TFIIS trimming of read-through products results in the release of small RNAs (10). It is important to note that in order to suppress background resulting from non-incorporated CTP in reactions using AdML+6G to AdML+25G (lanes 7–42) we have to include an ethanol-precipitation step. RNAs <5 nt are lost in this precipitation step and therefore are not visible. Reactions with AdML+3G and AdML+4G (lanes 1–6) yield high levels of abortive RNAs (30) and in these cases no ethanol precipitation is required. In the experiment of Figure 2 and in other experiments (data not shown) we do not observe significant functional differences between full-length human TFIIS and the ΔTFIIS derivative, which lacks the N-terminal third (residues 1–129). Therefore, only results with full-length human TFIIS are shown in subsequent experiments.
In conclusion, the results of Figure 2 indicate that TFIIS can only suppress formation of longer RNAs when pol II complexes have transcribed beyond register 9. Efficient TFIIS suppression is obtained when transcription proceeds to register 12. This coincides with the transition from abortive to productive transcription (5). However, the correlation is not very strict as indicated by TFIIS sensitivity of the 10-nt RNA formed on AdML+9G (lanes 16 and 17) and TFIIS insensitivity of the 12-nt RNA formed on AdML+7G (lanes 10 and 11).
Characteristics of read-through RNA formation
We decided to further investigate the nature of the longer RNA products. If they are genuine read-through products one expects that increasing the concentrations of nucleotide triphosphates in the reaction would stimulate their formation. On the other hand increasing the amount of TFIIS is expected to counter this effect.
These predictions were tested by increasing the amount of TFIIS protein or ATP and UTP to concentrations that are at least 100-fold higher than their Km. The concentration of CTP was not varied as it provides the radiolabel. It is not possible to achieve 100-fold higher CTP concentrations with the same specific activity. As expected Figure 3A shows that increasing ATP concentrations in reactions with the AdML+11G template stimulates the formation of larger RNAs at the expense of the 10-nt product. Most abundant are RNA products, which are one residue shorter than the next G residue to be incorporated: 14-, 16-, 19-, 24- and 27-nt RNAs (lanes 1–4). This indicates that transcription stalls each time it encounters a G residue and strongly suggests that these larger RNAs result from misincorporation events at the preceding G residue. Exceptions to this are the 11- and 20-nt RNA products. It has been shown that misincorporations inhibit the addition of the next nucleotide (21). Accumulation of the 11- and 20-nt RNAs is consistent with misincorporation of an adenine residue at registers 11 and 20 and we did not characterize these extended RNAs further. The occurrence of the 15- and 17-nt products can be explained similarly, but it is unclear why they accumulate to lower levels. When the ATP concentration is increased in the presence of the standard amount of TFIIS, formation of read-through RNAs is also stimulated (lanes 5–8). This effect can be counteracted by increasing the TFIIS concentration (lanes 8–11). In fact, at the highest dose tested (1.2 µM) TFIIS can reverse read-through very efficiently and the expected 10-nt product re-appears (compare lanes 4 and 11). This analysis also shows that contaminating GTP cannot be responsible for the read-through products, because such products would not be susceptible to TFIIS.
Figure 3.
Titration of nucleotides and TFIIS protein in transcription reactions using the AdML+11G template. (A) Titration of ATP and TFIIS. Transcription initiation complexes were assembled in absence or presence of varying concentrations of full-length TFIIS protein as indicated at the top of the figure. Reactions 1–4 received no TFIIS. Reactions 5–8 received 40 nM TFIIS. Reactions 9–11 received 120, 400 and 1200 nM TFIIS, respectively. Nucleotide triphosphates (10 µM UTP, 2 µM [α-32P]CTP and varying concentrations of ATP as indicated at the top of the figure) were added for 15 min. (B) Titration of UTP and TFIIS. Transcription initiation complexes were assembled in absence or presence of varying concentrations of full-length TFIIS proteins as indicated at the top of the figure. Reactions 1–4 received no TFIIS. Reactions 5–8 received 40 nM TFIIS. Reactions 9–11 received 120, 400 and 1200 nM of TFIIS, respectively. Nucleotide triphosphates (60 µM ATP, 2 µM [α-32P]CTP and varying concentrations of UTP as indicated at the top of the figure) were added for 15 min. All reactions were processed and analyzed by denaturing polyacrylamide gel electrophoresis as described in Materials and Methods. The length of the RNA products is indicated on the left of the figure parts and the sequence of the non-template strand of pDNAdML+11G is indicated on the right of the figure parts with the G residues shown in bold.
Similar results were obtained when the UTP concentration was increased (Fig. 3B). Raising the UTP concentration to 300 µM stimulates formation of read-through products ending one residue before the next G residue (again with the exception of the 11-, 15- and 20-nt products). Formation of read-through products can be completely suppressed by increasing the TFIIS concentration to 0.4 µM (compare lanes 4 and 10). We note that the comparison of Figure 3A and B suggests that ATP is more efficient than UTP in promoting read-through. This may be related to the specific misincorporation event: AMP instead of GMP versus UMP instead of GMP. Consistent with this is that Hawley and colleagues (21) showed that ATP misincorporation is rather efficient.
Together, the experiments of Figure 3 support the conclusion that the larger RNA products result from read-through by misincorporation. Although read-through readily occurs in the reconstituted system at low nucleotide levels, it is greatly enhanced by increasing nucleotide concentrations. In this case, high doses of TFIIS are needed to revert read-through. At these concentrations (0.4–1.2 µM) TFIIS is in large excess over transcription complexes (<4 nM). This indicates that suppression of read-through by TFIIS is a dynamic process and also that TFIIS is not quantitatively bound to transcription complexes under the conditions of these reactions.
TFIIS does not reduce the rate of RNA product formation
Models for transcript elongation by RNA polymerase indicate that at each register the enzyme decides between pausing and nucleotide addition (reviewed in 32). Part of the suppressive effect of TFIIS on read-through could be due to a reduction in the overall polymerization rate. It has been suggested that TFIIS can slow the production of long transcripts in vitro (21). This raises the possibility that the lack of effect on transcription complexes in the abortive phase may be explained by a TFIIS-induced reduction in the formation rate of productive, but not of abortive RNAs.
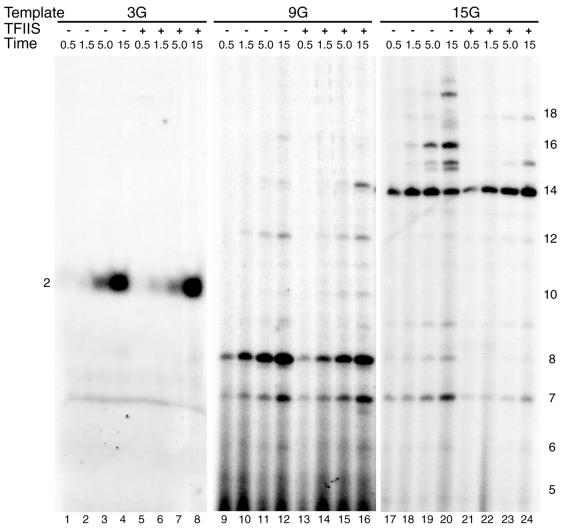
To test this idea, we performed time-course experiments comparing the formation of 2-, 8- and 14-nt RNAs and their accompanying read-through products in the presence and absence of TFIIS. Figure 4 shows that the addition of TFIIS has no effect on the kinetics of RNA product formation from transcription complexes in the abortive phase or in the productive phase of transcription (compare lanes 1–4 with 5–8, lanes 9–12 with 13–16 and lanes 17–20 with 21–24). As before, distribution of RNA products from abortive complexes is not altered by inclusion of TFIIS. The only exception is the TFIIS-dependent appearance of the 14-nt RNA (Fig. 4, lane 16). As mentioned above, this product probably accumulates as a result of trimming down of RNAs of 16 nt and larger. As expected, TFIIS efficiently suppresses read-through by the productively transcribing AdML+15G complex (Fig. 4, compare lanes 17–20 with 21–24). We note the presence of a 7-nt product in the reactions with templates AdML+9G and AdML+15G. This RNA was also observed (to a lesser extent) in Figure 2. Its appearance may result from pausing at register 7, which was recently noted by Pal and Luse (33). It is also interesting to note that formation of the 16-nt product resulting from one misincorporation event (specifically at register 15) is relatively slow compared with formation of the bona fide 14-nt product, which is completed after 1.5 min (Fig. 4, lanes 17–20). This agrees with the conclusion that misincorporation by pol II is a slow event (21). The experiment of Figure 4 indicates that the lack of TFIIS on read-through RNAs formed during the abortive phase is not due to a TFIIS effect on the rate of the polymerization reaction.
Figure 4.
TFIIS does not alter the kinetics of synthesis of small RNA products. Transcription initiation complexes were assembled in absence or presence of full-length TFIIS on the mutant AdML templates indicated at the top of the figure. Reactions 1–4, 9–12 and 17–20 received no TFIIS, while reactions 5–8, 13–16 and 21–24 received 40 nM full-length TFIIS protein. Initiation complexes were assembled for 30 min with AdML+3G (lanes 1–8), AdML+9G (lanes 9–16) and AdML+15G (lanes 17–24). Nucleotide triphosphates (60 µM ATP, 10 µM UTP, 2 µM [α-32P]CTP) were added for the times indicated at the top of the figure (0.5, 1.5, 5 or 15 min). Subsequently, RNA products were processed and analyzed by denaturing polyacrylamide gel electrophoresis as described in Materials and Methods. The length of the RNA products is indicated on the left (for lanes 1–8) and right of the figure.
Several groups found that addition of TFIIS after nucleotide incorporation still reversed misincorporation from productively transcribing complexes (9,21). Therefore, we decided to investigate whether the time of TFIIS addition had an effect on the suppression of read-through RNAs. Figure 5 shows that in our system we obtained identical results. Figure 5 compares the effect of TFIIS on RNA synthesis from the abortive AdML+6G and the productive AdML+15G complexes. For the productive complex, the TFIIS effect is observed when the protein is added during pre-incubation (lane 6), at the time of nucleotide addition (lane 7) or 5 min after nucleotide addition (lane 8). In all cases the transcription reaction proceeded for 15 min in total. In contrast to the productive complexes, TFIIS has no significant effect on RNA synthesis from the abortive AdML+6G complex under all conditions (lanes 1–4). Thus, the timing of TFIIS addition does not seem to be important for the induction of the exonucleolytic cleavage.
Figure 5.

TFIIS also functions after read-through products have been formed. Transcription initiation complexes were assembled in absence or presence of 40 nM full-length TFIIS on mutant AdML templates as indicated at the top of the figure. Reactions 1 and 5 received no TFIIS, reactions 2 and 6 received TFIIS during the pre-incubation, reactions 3 and 7 received TFIIS together with the nucleotide triphosphates and reactions 4 and 8 received TFIIS 5 min after the addition of nucleotide triphosphates. Initiation complexes were assembled with AdML+6G (lanes 1–4) and AdML+15G (lanes 5–8). The nucleotide triphosphates (60 µM ATP, 10 µM UTP, 2 µM [α-32P]CTP) were added for 15 min. Subsequently, RNA products were processed and analyzed by denaturing polyacrylamide gel electrophoresis as described in Materials and Methods. The lengths of the RNA products are indicated on the left of the figure.
DISCUSSION
In this study we find that pol II has a remarkably high rate of misincorporation, which results in the formation of read-through products. These observations were made at low nucleotide concentrations in reconstituted reactions measuring basal pol II transcription. Read-through can occur at all registers during the early phases of transcription. We show that addition of the transcription elongation factor TFIIS reverses read-through of productive transcription complexes. In contrast, read-through products formed during the early phase of transcription are largely resistant to TFIIS.
Pal and Luse recently described transcript slippage during transcription initiation from the AdML promoter (33). This probably results in the insertion of CU-dinucleotides around the pause site at register 7. These observations were made with ApC-primed transcription complexes assembled in crude nuclear extracts. Some of our RNAs formed with standard nucleotides may be explained by slippage. In particular, the 7- and 9-nt products formed on the AdML+6G and AdML+7G templates (Fig. 2) could result from this phenomenon. However, it is unlikely that slippage is responsible for the other larger RNAs as pausing at register 7 is not very efficient in our reconstituted basal transcription system (Figs 2 and 4). Moreover, the larger RNAs formed by productive complexes can be suppressed and reverted by inclusion of TFIIS, which induces trimming of RNAs from the 3′-end. Whereas slippage results in extension by 2-nt increments (33), most of the extended products in our study stop before or at the encoded G residue. Furthermore, transcript slippage is most prominent in ApC-primed reactions and much weaker with standard nucleotides as used in our study (33).
Our observations suggest that errors in the 5′-end of RNAs caused by misincorporation or transcript slippage may also occur unnoticed in vivo. In fact, an early study analyzing the 5′-ends of polyoma virus transcripts showed a microheterogeneity, which was explained by template slippage (34). Slippage involves DNA:RNA mismatching at some stage and this is apparently not corrected. Lack of requirement for TFIIS-dependent correction during the early phases of pol II transcription may be rationalized in different ways. First of all, it may not be important as the extreme 5′-end of mRNAs rarely carry essential genetic information. Secondly, release of short RNA products during the abortive phase already constitutes a pathway to discard RNAs containing mismatches. It has been shown that decreasing the stability of the initial RNA:DNA hybrid increases abortive initiation (35). Thirdly, a TFIIS-induced extensive backtracking of abortive complexes would result in complete digestion and loss of the nascent RNA. This would require a new initiation event. In such a situation it would be difficult for the polymerase to find the transcription start site. Supporting the latter, we observed previously that high GTP concentrations during forced abortive transcription favor an (repeated) initiation event downstream of the natural start site (5). In this respect it is also interesting to note that Luse and colleagues observed extensive backtracking of specific complexes from registers 23 or 27, but backtracking halted at register 10 (9).
TFIIS is the only transcription elongation factor that can activate arrested pol II complexes (reviewed in 10,11). Most probably, this reactivation results from stimulation of the intrinsic exonucleolytic activity of pol II. This activity releases small oligo-ribonucleotides from the 3′-end of the nascent RNA (36). This action is believed to re-align the 3′-end of the RNA with the catalytic center of pol II. We assume that TFIIS-induced cleavage is responsible for the suppression of read-through observed in this study. Although we do not directly demonstrate TFIIS-dependent cleavage products, we have detected such cleavage products in our system previously (8).
Several explanations exist for the inability of TFIIS to suppress formation of read-through RNAs during the very early phase of transcription. A trivial explanation is that short read-through RNAs are released from abortive complexes before TFIIS can have its effect. This is very unlikely as gel filtration experiments showed that RNAs >4 (6) or 7 nt (9) are stably bound in ternary pol II complexes. Moreover, we find that even a 14-nt RNA can escape TFIIS suppression (Figs 2 and 3). Hawley and co-workers (21) demonstrated that TFIIS removes misincorporations during productive transcription, which is inherently fast. We observed that cleavage of preformed read-through products is very rapid and is completed within 15 s after TFIIS addition (data not shown). Furthermore, it is reasonable to assume that TFIIS can bind to abortive transcription complexes. First, TFIIS binds free pol II molecules with a high affinity (Kd ~50 nM; 15). Secondly, the TFIIS-interaction surface of pol II maps to the inverted funnel structure (23), and this funnel is accessible both in the free and elongating form of pol II (23,24). Thirdly, it is unlikely that basal transcription factors obstruct TFIIS interaction as it was shown that (full-length) TFIIS also efficiently binds the holo-form of pol II harboring several basal transcription factors (19).
We propose that insensitivity to TFIIS-induced cleavage is intrinsic to the early stage of pol II transcription and related to the path of the nascent RNA in the pol II transcription complex. Several observations suggest that backtracking of pol II transcription complexes results in extrusion of the 3′-end of the nascent RNA through pore 1 and the structural analysis of pol II indicates that TFIIS binds in the inverted funnel leading to pore 1 (see Introduction). We propose that backtracking, which would lead to extrusion of the 3′-end of the RNA, is not very efficient during the early phase of pol II transcription. This may relate to the dimensions of the RNA:DNA hybrid channel and properties of the RNA-exit channel. The RNA:DNA hybrid channel can accommodate 8–9 bp (24). Possibly, efficient backtracking requires a completely filled RNA:DNA channel. Previous experiments with E.coli RNA polymerase indicated that the length of the RNA:DNA hybrid can influence stability and processivity of the elongation complex (37). In a sense our proposal bears similarities to the recently proposed ‘DNA scrunching’ model for the single-subunit T7 RNA polymerase (38). This model invokes that topological strain is introduced by packing of an increasing amount of template DNA into T7 RNA polymerase. This strain would be employed to generate momentum to release RNA polymerase–promoter contacts and, thus, to allow the transition to elongation. Possibly, such filling of the RNA:DNA hybrid channel is required before a mismatched 3′-end of the RNA can be extruded through pore 1 into the funnel. Experiments by Reines and co-workers (26) showed that in arrested complexes TFIIS could be cross-linked to the 3′-end of the RNA. Based on our model one would predict that TFIIS could only be cross-linked to GTP-deprived complexes in the productive mode and not to abortive complexes.
In conclusion, we favor the hypothesis that the geometric constraints of a fully occupied RNA:DNA hybrid channel is linked to backtracking and TFIIS-induced exonucleolytic cleavage. This explains the lack of TFIIS effect on misincorporation during the early phase of transcription. However, it is also possible that there is no link between the RNA:DNA hybrid channel and backtracking and that other factors are responsible for differences in the geometry of the pore and/or catalytic center between abortive and productive complexes. Pol II undergoes an isomerization step upon transcription elongation and this may alter the geometry of the catalytic center such that TFIIS can now induce exonucleolytic cleavage. Although backtracking by pol II would occur at all stages, the interaction of TFIIS with the extruded 3′-end of the nascent RNA would have no functional consequences. In this scenario one would expect that TFIIS could be cross-linked at all stages of the transcription cycle. Besides cross-linking experiments, structural determinations of pol II/TFIIS complexes could also resolve this issue. In either case the experiments of our paper lend further support for significant structural differences between pol II transcription complexes in the initiation and elongation mode of transcription.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Dr C. Kane (UC Berkeley) for providing the human TFIIS expression constructs. We also thank Drs R. Kornberg and P. Cramer (Stanford) and members of the Timmers’ Laboratory for stimulating discussions. We thank Marcin Klejman, Lloyd Pereira and Bas Winkler for critical review of the manuscript. U.F. was supported by a long-term fellowship from EMBO. Grants from the Human Frontier Science Program Organization (RG196/98) and from the Netherlands Organization for Scientific Research (NWO; Pionier 900-98-142) to H.Th.M.T. have supported this work.
REFERENCES
- 1.Roeder R.G. (1996) The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci., 21, 327–335. [PubMed] [Google Scholar]
- 2.Orphanides G., Lagrange,T. and Reinberg,D. (1996) The general transcription factors of RNA polymerase II. Genes Dev., 10, 2657–2683. [DOI] [PubMed] [Google Scholar]
- 3.Dvir A., Conaway,J.W. and Conaway,R.C. (2001) Mechanism of transcription initiation and promoter escape by RNA polymerase II. Curr. Opin. Genet. Dev., 11, 209–214. [DOI] [PubMed] [Google Scholar]
- 4.Conaway R.C. and Conaway,J.W. (1988) ATP activates transcription initiation from promoters by RNA polymerase II in a reversible step prior to RNA synthesis. J. Biol. Chem., 263, 2962–2968. [PubMed] [Google Scholar]
- 5.Holstege F.C.P., Fiedler,U. and Timmers,H.T.M. (1997) Three transitions in the RNA polymerase II-transcription complex during initiation. EMBO J., 16, 7468–7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kugel J.F. and Goodrich,J.A. (2002) Translocation after synthesis of a four-nucleotide RNA commits RNA polymerase II to promoter escape. Mol. Cell. Biol., 22, 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samkurashvili I. and Luse,D. (1996) Translocation and transcriptional arrest during transcript elongation by RNA polymerase II. J. Biol. Chem., 271, 23495–23505. [DOI] [PubMed] [Google Scholar]
- 8.Fiedler U. and Timmers,H.T.M. (2001) Analysis of the open region of RNA polymerase II transcription complexes in the early phase of elongation. Nucleic Acids Res., 29, 2706–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal M., McKean,D. and Luse,D.S. (2001) Promoter clearance by RNA polymerase II is an extended multistep process strongly affected by sequence. Mol. Cell. Biol., 21, 5815–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wind M. and Reines,D. (2000) Transcription elongation factor SII. Bioessays, 22, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conaway J.W., Shilatifard,A., Dvir,A. and Conaway,R.C. (2000) Control of elongation by RNA polymerase II. Trends Biochem. Sci., 25, 375–380. [DOI] [PubMed] [Google Scholar]
- 12.Izban M.G. and Luse,D.S. (1993) The increment of SII-facilitated transcript cleavage varies dramatically between elongation competent and incompetent RNA polymerase II ternary complexes. J. Biol. Chem., 268, 12874–12885. [PubMed] [Google Scholar]
- 13.Archambault J., Lacroute,F., Ruet,A. and Friesen,J.D. (1992) Genetic interaction between transcription elongation factor TFIIS and RNA polymerase II. Mol. Cell. Biol., 12, 4142–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishiguro A., Nogi,Y., Hisatake,K., Muramatsu,M. and Ishihama,A. (2000) The Rpb6 subunit of fission yeast RNA polymerase II is a contact target of the transcription elongation factor TFIIS. Mol. Cell. Biol., 20, 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awrey D.E., Shimasaki,N., Koth,C., Weilbaecher,R., Olmsted,V., Kazanis,S., Shan,X., Arello,J., Arrowsmith,C.H., Kane,C.M. and Edwards,A.M. (1998) Yeast transcription elongation factor (TFIIS), structure and function. J. Biol. Chem., 273, 22595–22605. [DOI] [PubMed] [Google Scholar]
- 16.Booth V., Koth,C.M., Edwards,A.M. and Arrowsmith,C.H. (2000) Structure of a conserved domain common to the transcription factors TFIIS, elongin A and CRSP70. J. Biol. Chem., 275, 31266–31268. [DOI] [PubMed] [Google Scholar]
- 17.Cipres-Palacin G. and Kane,C.M. (1994) Cleavage of the nascent transcript induced by TFIIS is insufficient to promote read-through of intrinsic blocks to elongation by RNA polymerase II. Proc. Natl Acad. Sci. USA, 91, 8087–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakanishi T., Shimoaraiso,M., Kubo,T. and Natori,S. (1995) Structure-function relationship of yeast S-II in terms of stimulation of RNA polymerase II, arrest relief and suppression of 6-azauracil sensitivity. J. Biol. Chem., 270, 8991–8995. [DOI] [PubMed] [Google Scholar]
- 19.Pan G., Aso,T. and Greenblatt,J. (1997) Interaction of elongation factors TFIIS and elongin A with a human RNA polymerase II holoenzyme capable of promoter-specific initiation and responsive to transcriptional activators. J. Biol. Chem., 272, 24563–24571. [DOI] [PubMed] [Google Scholar]
- 20.Uptain S.M., Kane,C.M. and Chamberlin,M.J. (1997) Basic mechanisms of transcript elongation and its regulation. Annu. Rev. Biochem., 66, 117–172. [DOI] [PubMed] [Google Scholar]
- 21.Thomas M.J., Platas,A.A. and Hawley,D.K. (1998) Transcriptional fidelity and proofreading by RNA polymerase II. Cell, 93, 627–637. [DOI] [PubMed] [Google Scholar]
- 22.Jeon C. and Agarwal,K. (1996) Fidelity of RNA polymerase II transcription controlled by elongation factor TFIIS. Proc. Natl Acad. Sci. USA, 93, 13677–13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer P., Bushnell,D.A., Fu,J., Gnatt,A.L., Maier-Davis,B., Thompson,N.E., Burgess,R.R., Edwards,A.M., David,P.R. and Kornberg,R.D. (2000) Architecture of RNA polymerase II and implications for the transcription mechanism. Science, 288, 640–649. [DOI] [PubMed] [Google Scholar]
- 24.Gnatt A.L., Cramer,P., Fu,J., Bushnell,D.A. and Kornberg,R.D. (2001) Structural basis of transcription: an RNA polymerase II elongation complex at 3.3 Å resolution. Science, 292, 1876–1882.11313499 [Google Scholar]
- 25.Cramer P., Bushnell,D.A. and Kornberg,R.D. (2001) Structural basis of transcription: RNA polymerase at 2.8 Å resolution. Science, 292, 1863–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell W., Bartholomew,B. and Reines,D. (1996) Elongation factor SII contacts the 3′-end of RNA in the RNA polymerase II elongation complex. J. Biol. Chem., 271, 22301–22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmers H.T.M. (1994) Transcription initiation by RNA polymerase II does not require hydrolysis of the β–γ phosphoanhydride bond of ATP. EMBO J., 13, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holstege F.C.P., Tantin,D., Carey,M., van der Vliet,P.C. and Timmers,H.T.M. (1995) The requirement for the basal transcription factor IIE is determined by the helical stability of promoter DNA. EMBO J., 14, 810–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holstege F.C.P., van der Vliet,P.C. and Timmers,H.T.M. (1996) Opening of an RNA polymerase II promoter occurs in two distinct steps and requires the basal transcription factors IIE and IIH. EMBO J., 15, 1666–1677. [PMC free article] [PubMed] [Google Scholar]
- 30.Holstege F.C.P. and Timmers,H.T.M. (1997) Analysis of open complex formation during RNA polymerase II transcription initiation using heteroduplex templates and potassium permanganate probing. Methods, 12, 203–211. [DOI] [PubMed] [Google Scholar]
- 31.Timmers H.T.M. and Sharp,P.A. (1991) The mammalian TFIID protein is present in two functionally distinct complexes. Genes Dev., 5, 1946–1956. [DOI] [PubMed] [Google Scholar]
- 32.von Hippel P.H., Bear,D.G., Morgan,W.D. and McSwiggen,J.A. (1984) Protein-nucleic acid interactions in transcription: a molecular analysis. Annu. Rev. Biochem., 53, 389–446. [DOI] [PubMed] [Google Scholar]
- 33.Pal M. and Luse,D.S. (2002) Strong natural pausing by RNA polymerase II within 10 bases of transcription start may result in repeated slippage and reextension of the nascent RNA. Mol. Cell. Biol., 22, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowie A., Jat,P. and Kamen,R. (1982) Determination of sequences at the capped 5′ ends of polyoma virus early region transcripts synthesizes in vivo and in vitro demonstrates an unusual microheterogeneity. J. Mol. Biol., 159, 225–255. [DOI] [PubMed] [Google Scholar]
- 35.Keene R.G. and Luse,D.S. (1999) Initially transcribed sequences strongly affect the extent of abortive initiation by RNA polymerase II. J. Biol. Chem., 274, 11526–11534. [DOI] [PubMed] [Google Scholar]
- 36.Izban M.G. and Luse,D.S. (1992) The RNA polymerase II ternary complex cleaves the nascent transcript in a 3′–5′ direction in the presence of elongation factor SII. Genes Dev., 6, 1342–1356. [DOI] [PubMed] [Google Scholar]
- 37.Sidorenkov I., Komissarova,N. and Kashlev,M. (1998) Crucial role of the RNA:DNA hybrid in the processivity of transcription. Mol. Cell, 2, 55–64. [DOI] [PubMed] [Google Scholar]
- 38.Brieba L.G. and Sousa,R. (2001) T7 promoter release mediated by DNA scrunching. EMBO J., 20, 6826–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]