Abstract
Tetrastigma hemsleyanum (TH) has attracted much attention for its heat clearing and detoxification effects, but whether it can become an effective feed supplement in chickens remains unclear. Herein, a total of 120 male Jinhua yellow chickens (two-mth-old) were randomly divided into into four groups (CON, TH-L, TH-M, and TH-H) for a 56-day feeding trial to explore its effects on growth performance and underlying mechanism. Results revealed that dietary TH notably increased the average daily growth (ADG), and decreased the average daily feed intake (ADFI) and feed conversion ratio (FCR) in TH-H group during 29-56 days. Meanwhile, dietary TH improved the development of duodenum and notably increased the contents of essential amino acids and flavor amino acids, while the serum oxidation stress index as well as abdominal fat deposition were not affected in Jinhua yellow chickens. Additionally, TH supplementation notably increased gut microbiota richness, then selectively increased the colonization of potential probiotics and the microbial abundance associated to amino acid synthesis and metabolic pathways in duodenum. Furthermore, qPCR analysis results preliminarily verified that dietary TH not only enhanced intestinal amino acids (rBAT and EAAT3) and peptides (PepT1 and APN) transport but also alleviated the inflammation (IL-1β, IL-6 and IFN-γ), thus thereby improved intestinal development and growth performance in Jinhua yellow chickens. These findings demonstrated that TH is a feed additive that can improve growth performance, muscle amino acids composition and intestinal development.
Keywords: Tetrastigma hemsleyanum, feed additive, growth performance, intestinal nutritional transport, Jinhua yellow chickens
Introduction
Traditional Chinese herbal medicine (TCM) has been a practice of natural medicine that employs various parts and extracts of plants for therapeutic purposes (Jun et al., 2021; Jin et al., 2023; Liao et al., 2023). TCM, in particular, has emerged as a promising supplementary therapy and feed addition due to its natural constituents, therapeutic characteristics, and wide-ranging functions (Liu et al., 2023a; Liu et al., 2024; Tu et al., 2023). TCM is often considered as a naturally adaptable medicine with few hazardous side effects, limited resistance potential, and low drug residual levels. Chinese herbal feed additives (CHFA) are animal feed additives that incorporate components of TCM. Studies have shown that they can improve animal health and performance, reduce disease risks, and even prevent infections from certain viruses (Abdallah et al., 2019; Song et al., 2023; Wan et al., 2024). Moreover, CHFA is inexpensive, making it an appealing option for farmers in need of feed additives. Additionally, CHFA is an environmentally beneficial alternative to conventional animal feed additives, helping to reduce waste generated during the animal feed production process. Currently, the abuse and excessive use of some feed additives that did not meet the basic requirements of safety, effective, and non-polluting to the environment may lead to food safety incidents and environmental pollution. Long-term or excessive use of medicinal additives even may lead to the development of drug resistance in pathogens within animals, posing difficulties for human antibiotic treatment. Therefore, China has made a strategic decision to "completely ban the use of antibiotics" in feed by the year 2020. Consequently, there is a burgeoning interest in the application of CHFA in poultry breeding.
Tetrastigma hemsleyanum Diels et Gilg (abbreviated as TH), also known as Sanyeqing in China, was a widely used folk Chinese traditional herbal remedy. The entire plant, including the tubers, has been shown to be particularly beneficial in terms of anti-inflammatory, analgesic, antipyretic, anticancer, and antiviral properties (Han et al., 2023; Suhail et al., 2021; Zhai et al., 2022; Zhou et al., 2022). Because of their health-promoting properties, the tuber and leaf sections have been commonly used as a nutritional supplement, vitamin, soup, or tea. Multiple authorities in Zhejiang province issued a fresh list of "Zhebawei" in 2017, including TH. It was abundantly cultivated in Zhejiang province's mountainous areas, with high quality and a large market demand. Especially in recent years, TH has unique curative effect in the treatment of febrile convulsions, colitis, acute and chronic hepatitis and other diseases, known as '' plant antibiotics '' (Feng et al., 2024; Xiao et al., 2023). In our previous study, both the roots and leaves of TH exhibit multiple pharmacological activities (Lou et al., 2021; Wei et al., 2022; Wang et al., 2023). Network pharmacology analysis and experimental data revealed that TH leaf extract has anti-inflammatory properties by preserving Th17/Treg immunological homeostasis (Lou et al., 2021). TH may serve as an anticancer drug by inhibiting cell cycle checkpoints in CDK6-driven malignancies (Wei et al., 2022). Gut microbiota and transcriptome profiling demonstrated that aqueous extract of TH leaves protects mice from ulcerative colitis (Wang et al., 2023). Therefore, TH has become a highly valuable and significant species in Zhejiang due to its therapeutic benefits and commercial significance. TH has the potential to become a qualified Chinese herbal additive due to its several pharmacological benefits, which include anti-tumor, anti-inflammatory, anti-oxidation, antipyretic and analgesic properties, and liver protection. In the realm of poultry production, the use of feed additives is paramount for enhancing animal health, productivity, and the overall efficiency. These include the need for sustainable and cost-effective solutions, the quest for alternatives to antibiotics in light of increasing antibiotic resistance, and the pursuit of additives. TH has the characteristics of safety, low toxicity and low residue, which meets the requirements of green sustainable development. In addition, the stems and leaves of TH are not traditionally used for medicine. Therefore, the development of TH as a potential feed additive can reduce waste and achieve high-value utilization of resources.
Although researchers have been combining nutrients appropriately or adding feed additives that improve the quality of livestock and poultry, such as active polysaccharides (Cai et al., 2022), antioxidants (Guo et al., 2020), organic salts (Mion et al., 2022), traditional Chinese medicine extract (Li et al., 2021), probiotics (Khan et al., 2020). However, only the roots of TH are used as medicinal parts for traditional Chinese medicine processing, if its above-ground parts such as stems and leaves are not fully utilized, resulting in severe resource waste. Furthermore, there has been no application of the non-medicinal parts of TH in livestock and poultry breeding feed, nor has there been any exploration of the effects of stem and leaf powder of TH on chickens. In this study, we investigated the effects of 1.25-5g/kg TH supplementation on growth performance, muscle amino acids composition, intestinal morphology as well as gut microbiota in Jinhua yellow-feathered chickens. Our aim was to demonstrate the effect and underlying mechanism of TH in broilers, with the hope of promoting its development and application.
Material and methods
Experiment design
A total of 120 two-mth-old Jinhua yellow chickens were randomly assigned to four groups (3 replicates of 10 each group) for a 56-day feeding trial. The control group (CON) received a basal diet, while the treated groups were given a baseline diet with 1.25 g/kg (TH-L), 2.50 g/kg (TH-M), and 5.00 g/kg (TH-H) TH. TH was acquired from Ningbo Shengwang Biotechnology Co. Ltd. and substituted for limestone in the diet. The chickens were fed ad libitum and had unlimited access to water during the trial. Heaters controlled and maintained the room temperature, keeping it reasonably steady. The birds were handled in accordance with the guidelines of the Animal Care and Use Committee of Zhejiang A&F University (Protocol Number ZAFUAC202457).
Sample collection and growth performance
Feed consumption was monitored throughout the study period for each replicate, and mortality was quickly recorded to adjust the FCR. The rearing cycle of broilers was generally 50-60 days, and the detection time points in subsequent trials were consistent with previous studies (Abdallah et al., 2019). Chickens were weighed in duplicates at 0, 28, and 56 days, and the ADFI, body weight (BW), ADG, and FCR for each replicate were then computed. At 56 days into the feeding study, 10 randomly selected chickens from each replication were weighed and killed following a 12 h feed deprivation. A portion of the liver, abdominal fat, and mid-segment of the duodenum were fixed in 10 % buffered formalin for 24 h at 25°C before being embedded in paraffin. Cecal digesta was taken, frozen in liquid nitrogen, and stored at -80 °C. Serum samples were collected, separated by centrifugation and kept at -80°C for subsequent analysis. Breast muscle was extracted, immediately frozen in liquid nitrogen, and transferred to the laboratory in a dry-ice pack before being kept at -20°C for examination of basic muscle composition and amino acid profile.
Serum lipid determination and histological analysis
Serum triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), malondialdehyde (MDA), total antioxidant capacity (T-AOC), and total superoxide dismutase (T-SOD) were detected using test kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's instructions. Tissue samples (Liver, abdominal fat, duodenum) were sectioned and stained with hematoxylin-eosin (HE) staining. The relevant indicators of the abdominal fat cell and the intestinal morphology were counted and analyzed by Image-pro plus 6.0.
16s rRNA sequencing and gut microbiota analysis
Six intestinal content samples were randomly selected from each group for 16s rRNAs sequencing and analysis. Total genomic DNA was extracted using the TGuide S96 Magnetic Soil/Stool DNA Kit (Tiangen). The quality and quantity of the extracted DNA were examined using electrophoresis on a 1.8 % agarose gel, and its purity and concentration were evaluated using the NanoDrop 2000. The hypervariable V3-V4 region of the bacterial 16S rRNA gene were amplified using the primer pairs 338F and 806R. The amplicon library was paired-end sequenced on an Illumina novaseq 6000. The extraction of genomic DNA, the amplification of 16S rRNA V3-V4 region, the library construction, and the sequencing were performed and completed by Beijing Biomarker Technologies Co., Ltd.
LC-MS/MS non-target and amino acids target metabolic analysis
Muscle samples were used for determining the untargeted metabolites and the targeted amino acids based on the LC-MS/MS platform. Biological samples were placed in a lyophilizer for vacuum freeze-drying and then grind to powder with a grinder. Weigh 100 mg of powder and dissolve it in 1.2 ml of 70 % methanol extract, and then vortex once every 30 minutes, each time lasting for 30 sec, a total of 6 times. Place the sample in the refrigerator at 4 ℃ overnight. After centrifugation, the supernatant was aspirated and filtered with microporous filter membrane and stored in the injection bottle for LC-MS/MS analysis. Principal component analysis and Spearman correlation analysis were used to assess the samples' repeatability. The selected substances are checked for classification and route information in the KEGG, HMDB and lipidmaps databases. To execute OPLS-DA modeling, the R language package ropls was utilized. The model's dependability was verified by 200 permutation tests (FC>1, P < 0.05, and VIP>1). The hypergeometric distribution test was used to determine the relevance of different metabolites in KEGG pathway enrichment.
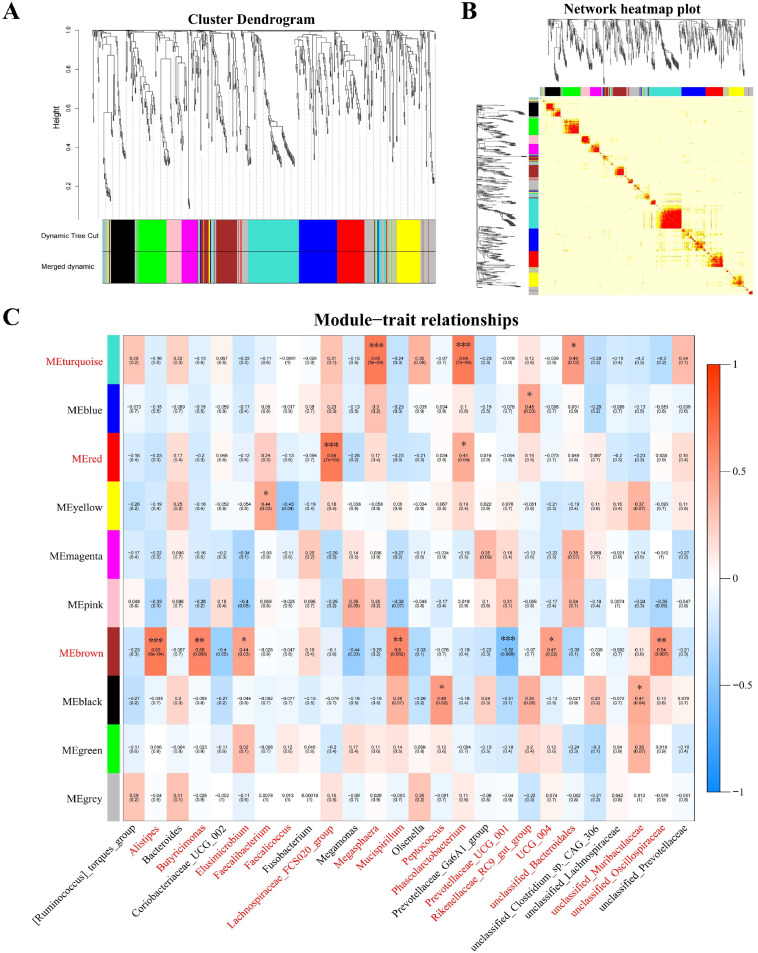
Integration of metabolome data and gut microbiota by WGCNA
To better clarify relationships of the data between the muscle metabolites (Module) and bacterial genera (Concerned trait), weighted gene coexpression network analysis (WGCNA) analysis was performed using the default parameters in the free online data analysis tool-BMKCloud Platform (https://international.biocloud.net). The network heatmap plot, the module heatmap and the trait-related modules were constructed by using WGCNA. To identify bacterial genera associated with the muscle metabolism, the low-expressed metabolites (SD<0.5) were filtered out. The Pearson correlation coefficient between metabolic modules and bacterial genera was then calculated using module-trait association analysis, after which the relevant and significant bacterial genera were selected for further investigation. The statistical significance threshold of the correlation of the modules and the bacterial genera was set at P < 0.05.
RNA isolation and real-time quantitative PCR analysis
Total RNA was extracted using FastPure Tissue Total RNA Isolation Kit V2 (Vazyme, China) and then reverse transcribed into cDNA using TransScript II One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen, China). The RNA and cDNA were stored at -80 ℃ for a long time. The cDNA template was diluted to the appropriate concentration. Real-time PCR was performed using Taq Pro Universal SYBR qPCR Master Mix (Vazyme, China) on the QuantStudio 3 Real-Time PCR system (Thermo Fisher Scientific Inc., CA, USA). GAPDH was used as the internal reference genes. All primer sequences are listed in Supplemental Table S2. For each 20 μL PCR reaction, 1 μL of the cDNA, 10 μL of Taq Pro Universal SYBR qPCR Master Mix, 8.2 μL of water, and 0.4 μL of the forward and reverse primer at a 10 μM stock concentration were added. The PCR conditions consisted of the following: 95°C for 3 minutes for denaturation; 95 °C for 10 s for annealing; and 60°C for 30 s for extension, for 40 cycles. Each experiment was repeated three times. The primers used are listed in Supplementary Table S1.
Statistical analysis
Data were collected and tabulated individually for each treatment, and the normality test was performed first. The results were statistically analyzed using GraphPad Prism 8.0, STAMP 2.1.3, or R 2.15.0. Spearman's correlations between key bacterial abundance and muscle amino acids were calculated and visualized using R 2.15.0. Statistical differences between more than two groups were assessed using one-way ANOVA and Tukey's multiple comparison posttests. The unpaired two-tailed t-test was used to assess differences between the two groups.
Results
Effect of dietary TH on growth performance
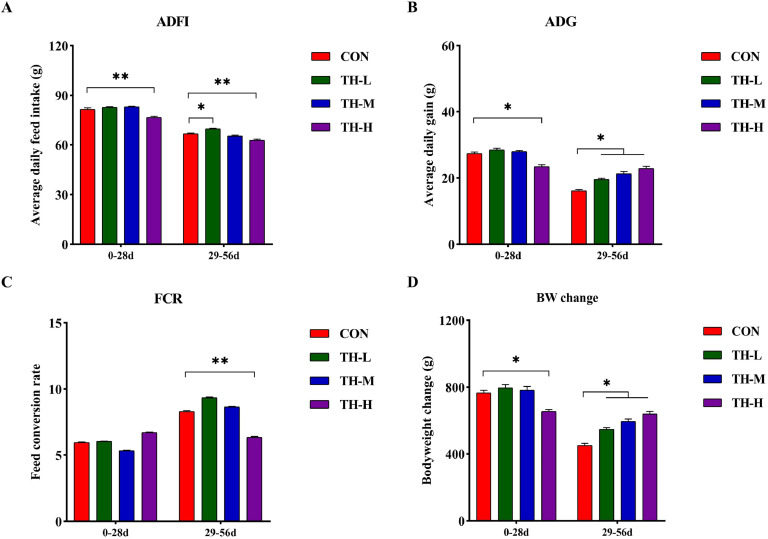
The feeding cycle was divided into two phases: early (0-28 days) and late (29-56 days) for statistical analysis. During the early phase, there were no significant variations in growth performance between the CON and TH-treat groups, with the exception of the TH-H group. However, obvious alterations in ADFI, ADG and FCR occurred during the late phase (Fig. 1). Interestingly, the ADG increased considerably (P < 0.05) in all TH-treated groups compare with the CON group (Fig. 1B). Compared to the CON group, the ADFI and FCR in all TH-treated groups steadily reduced as the TH additive dosage increased (Fig. 1A, 1C). All detected performance indicators, particularly in the TH-H group, significantly improved at the late phase (Fig. 1). At 29-56 days of the feeding trial, the BW of chickens rose in a dose-dependent manner with the addition of TH (Fig. 1D). During the whole feeding trial, all detected performance indicators significantly improved in the TH-H group compared with the CON group (P < 0.05) (Fig. S1). These results indicated that dietary TH can improve growth performance of chickens, particularly at the late phase of the feeding trial in the TH-H group.
Fig. 1.
Effect of dietary TH on growth performance in the feeding trial. (A) Average daily feed intake (ADFI). (B) Average daily feed intake (ADG). (C) Feed conversion rate (FCR). (D) Body weight (BW) change. Data are expressed as mean ± SEM, n = 6. Asterisk indicates significant differences according to the unpaired t-test (*P < 0.05, **P < 0.01).
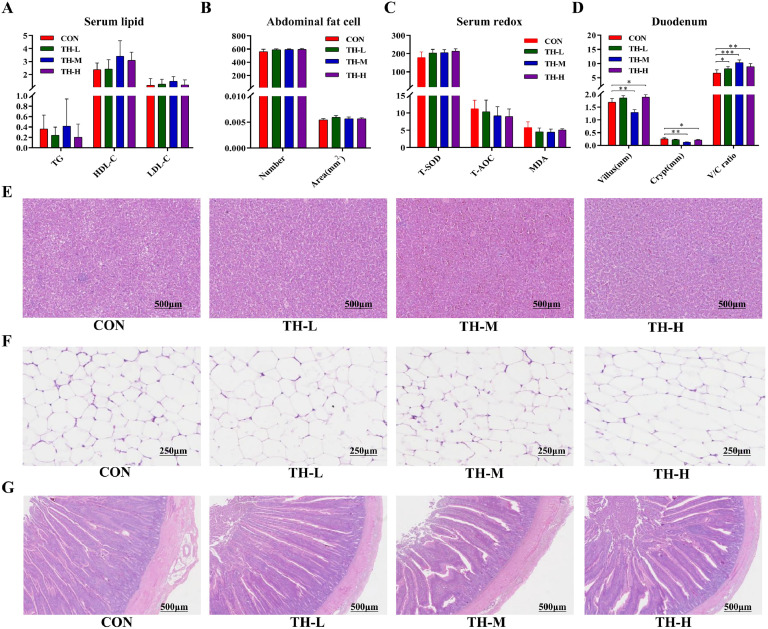
Effect of dietary TH on serum parameters, fat deposition and intestinal morphology
All TH-treated groups had similar TG, HDL-C, and LDL-C levels to the CON group (Fig. 2A). The adipocyte size in the TH-treated groups was unaltered compared to the CON group (Fig. 2B, 2F), indicating that dietary TH had no significant effect on abdominal fat formation. Hepatic morphology tests revealed no obvious alterations in hepatic fat accumulation (Fig. 2B). There were no substantial changes between adipose cells. There were no significant variations in T-SOD, T-AOC, and MDA content across the groups compared to the CON group (Fig. 2C), demonstrating that dietary TH had no noticeable effect on redox balance. In addition, no noticeable alterations were found in hepatic fat accumulation (Fig. 2E). In terms of intestinal development, the TH-L and TH-H groups had significantly higher villus heights (P < 0.01) compared to the CON group. In the TH-L and TH-H groups, the villus height to crypt depth ratio (V/C ratio) in the duodenum notably increased by 18.41 % (P < 0.05) and 25.52 % (P < 0.01) compared to the CON group (Fig. 2D, 2G). In the TH-M group, the villus height and crypt depth were 23.70 % (P < 0.01) and 51.66 % (P < 0.01) lower than in the CON group, respectively. The TH-M group significantly improved their V/C ratio in the duodenum, with a 35.88 % rise (P < 0.001) (Fig. 2D, 2G), exceeding all other groups.
Fig. 2.
Effect of dietary TH on serum lipid, fat deposition, redox proxies and intestinal morphology of chickens. (A) The content of TG, HDL-C, and LDL-C. (B) Numbers and area of abdominal fat cell. (C) Antioxidant index. (D) Villus height, crypt depth, and V/C ratio of duodenum. (E) HE staining sections of liver. (F) HE staining sections of abdominal fat. (G) HE staining of duodenum. Data are expressed as mean ± SEM, n = 6. Asterisk indicates significant differences according to the unpaired t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
Effect of dietary th on muscle amino acids profile and gut microbiota
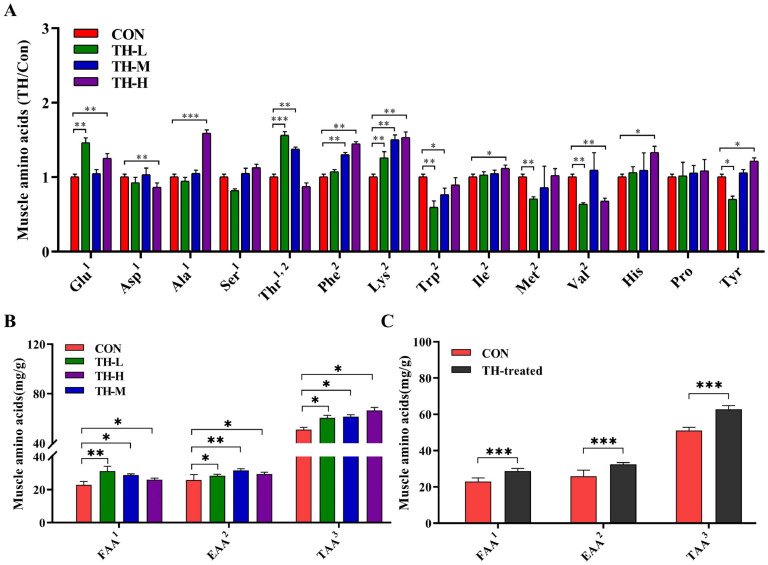
The muscle amino acids in TH-treated groups differed significantly from the CON group, particularly flavor amino acids (FAA) and essential amino acids (EAA) (Fig. 3). Compared to the CON group, the TH-H group showed significant increases in Glu, Ala, Tyr, Phe, Lys, and His (P < 0.05), while the TH-L and TH-M groups exhibited notable enhancements in Glu, Thr, Lys, and in Thr, Phe, Lys, respectively (P < 0.05) (Fig. 3A). All of the TH-treated groups had higher levels of Glu, Pro, Phe, Lys, and Ile than the CON group (Fig. 3A). Compared to the CON group, the TAA content in TH-L, TH-M and TH-H groups significantly increased (P < 0.05) in a dose-dependent manner. Meanwhile, the FAA content and EAA content in the TH-L, TH-M and TH-H groups also significantly increased (P < 0.05), respectively (Fig. 3B). In summary, the FAA, EAA and TAA content in the TH-treated groups notably (P < 0.001) increased by 15.64 %, 15.49 %, and 13.71 % (Fig. 3C). Dietary TH supplement significantly raised the FAA, EAA, and TAA levels of muscle in Jinhua yellow chickens.
Fig. 3.
Amino acids levels of muscle in the CON and TH-treated groups. (A) Amino acids levels of muscle in the CON, TH-L, TH-M and TH-H groups. (B) FAA, EAA and TAA in the CON, TH-L, TH-M and TH-H groups. (C) FAA, EAA and TAA in the CON group and TH-treated group. 1FAA, flavor amino acids, (Glu, Asp, Ala, Ser, Thr); 2EAA, essential amino acids (Thr, Phe, Lys, Trp, Ile, Met, Val); 3TAA, total amino acids. Data are expressed as mean ± SEM, n = 6. Asterisk indicates significant differences according to the unpaired t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
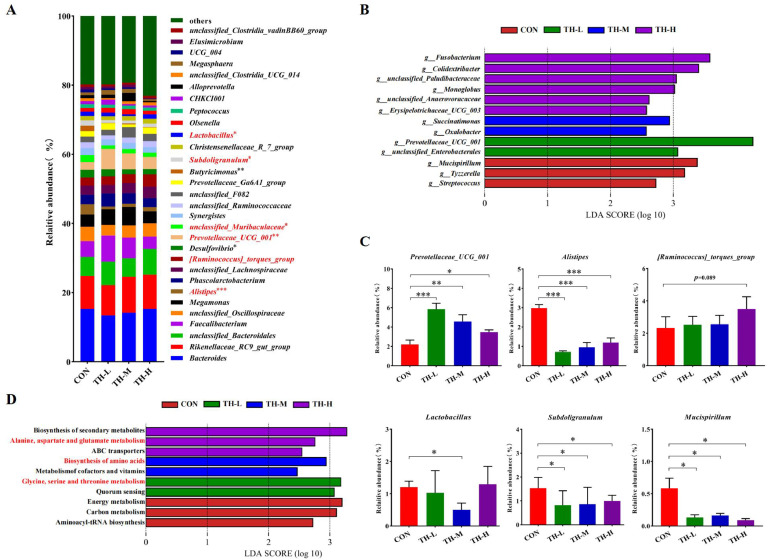
A total of 1561 OTUs were obtained, and 93, 87, 85, and 97 specific OTUs were identified as unique to the CON, TH-L, TH-M, and TH-H groups, respectively (Fig. S2A). The ACE (bacterial richness) and Chao1 of the α-diversity index in the TH-H group were notably increased (P < 0.05) in comparison with the CON group. However, no significant difference was observed in the Simpson index (bacterial diversity and evenness) and the Shannon index among all groups (Fig. S2B). As shown in Fig. S2C, the gut microbiota was mostly consisted of seven phyla in the CON and TH-treated groups, with the relative abundance of the phylum Deferribacterota changing significantly. At the family level, a notable decline in the families Deferribacteraceae and Streptococcaceae was observed in the TH-treated groups with the increase of supplementation level. Conversely, the relative abundance of the families Prevotellaceae, Paludibacteraceae and Monoglobaceae exhibited a marked increase (Fig. S2D, 2E).
The top 30 genera identified among the CON and TH-treated groups were similar. However, the relative abundances of Alistipes, Desulfovibrio, an unclassified genus of Muribaculaceae family, Synergistes, Butyricimonas and Subdoligranulum were significantly (P < 0.05) affected by TH supplementation (Fig. 4A). LEfSe analysis (P < 0.05, LDA > 2.0) was performed to discriminate the differences on the community composition between all groups (Fig. 4B). A total of 13 significant differential bacterial genera were identified in the CON and TH-treated groups. Mucispirillum, Tyzzerella and Streptococcus were found to be enriched in the CON group. The TH-L group was enriched with Prevotellaceae_UCG_001 and an unclassified genus of Enterobacterales family, whereas the TH-M group was differentially enriched with Succinatimonas and Oxalobacter. The most enriched differential bacterial genera were identified in TH-H group, including Fusobacterium, Colidextribacter, an unclassified genus of Paludibacteraceae family, Monoglobus, an unclassified genus of Anaerovoracaceae family and Erysipelotrichaceae_UCG_003. TH supplementation enhanced the colonization of the beneficial bacteria (Prevotellaceae_UCG_001, and [Ruminococcus]_torques_group) and reduced the abundance of harmful bacteria (Mucispirillum, SuccinatimonasZ, and Monoglobus), thereby indicating the positive effects of TH on gut microbiota.
Fig. 4.
Dietary TH altered the composition of gut microbiota at the genus level in Jinhua yellow chickens. (A) Relative abundance of gut microbiota at the genus level (top30 genera were shown). (B) LEfSe analysis of gut microbiota between the treatments at the genus level. (C) STAMP reveal the differences of main microbial functions predicted by PICRUSt at level 2 between the CON group and TH-treated groups. (D) Relative abundance of differential gut microbiota between the treatments at the genus level. Data are expressed as means ± SEM, n = 6. Asterisk indicates significant differences according to the unpaired t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
According to the results of Fig. 4A, 4B, and 4C, we found out 6 specific genera (Prevotellaceae_UCG_001, [Ruminococcus]_torques_group, Alistipes, Lactobacillus, Subdoligranulum, and Mucispirillum) that were notably altered by TH supplementation. Statistical analysis showed that the relative abundances of intestinal probiotics Prevotellaceae_UCG_001 (P < 0.05) and [Ruminococcus]_torques_group increased with the addition of TH, whereas the relative abundances of genera Subdoligranulum (P < 0.05), conditional pathogenic bacteria Alistipes (P < 0.001) and Mucispirillum (P < 0.05) were decreased (Fig. 4C). LEfSe analysis (P < 0.05, LDA > 2.0) was also applied to discriminate the microbial functions of gut microbiota predicted by PICRUSt among all groups (Fig. 4D). The CON group showed an enrichment of genes belonging to pathways of energy metabolism, carbon metabolism and aminoacyl-tRNA biosynthesis. The metabolism pathways assigned into the biosynthesis of amino acid, such as glycine, serine and threonine, were differentially enriched in the TH-L and TH-M group. The amino acid metabolism and carbohydrate biosynthesis-related pathways, such as the biosynthesis of secondary metabolites, alanine, aspartate and glutamate metabolism, and ABC transporters, were enriched in the TH-H group (Fig. 4D).
WGCNA analysis indicated that dietary TH affected muscle metabolism Via altered gut microbiota
In order to evaluate the metabolite variations with dietary TH supplement, the analysis of metabolites in broilers was performed using muscle samples collected from various TH-treated groups. The metabolic data were evaluated using principal component analysis (PCA) (Fig. S3A) and orthogonal partial least squares discriminant analysis (OPLS-DA) (Fig. S3B) in CON and TH-treated groups. PCA and OPLS-DA revealed the comprehensive distinctions in the metabolite profiles between the CON group and TH-treated groups, which suggested that metabolic profiling of muscle samples changed significantly with the supplement of TH (Fig. S3C).
In total, a total of 1,512 metabolites (1136 and 376 metabolites at positive and negative model, respectively) were obtained in muscle samples. At the superclass level, lipids and lipidlike molecules (33.72 %), organic acids and derivatives (23.56 %), organoheterocyclic compounds (13.86 %), and organic oxygen compounds (10.16 %) were predominant in muscle samples (Fig. S4A). All identified metabolites were annotated using the KEGG database, and then more than half of the top 20 annotation information are concentrated in “amino acid metabolism and lipid metabolism” (Fig. S4B). To preliminarily visualize the abundance and difference in distribution of metabolites among the CON and TH-treated groups, heatmap clustering analysis was performed on the top 50 differential metabolites (DEMs) in muscle samples (Fig. S3C).
As depicted in Fig. 5, WGCNA was performed to identify metabolic modules clustering to investigate the correlation of bacterial genera and muscle metabolites. Our results showed that a soft threshold power of 9 was the lowest power with a scale-free topology model fit index of 0.80 and relatively high mean connectivity (Fig. S5). For the muscle metabolic profile, ten modules labeled with different colors were detected using the clustering algorithm (Fig. 5A, 5B). The heatmap of module-traits relationships indicated that 16 related genera had significant correlation with six modules (P < 0.05), mainly including the ‘MEbrown’, ‘MEturquoise’ and ‘MEred’ modules (Fig. 5C). We observed that the ‘brown’ module had the most significant and strongest correlation with five genera (|r|≥0.52, P < 0.01), including Alistipes, Butyricimonas, Mucispirillum, unclassified_Oscillospiraceae, and Prevotellaceae_UCG_001. In addition, Alistipes, Mucispirillum, and Prevotellaceae_UCG_001 were significantly up/downregulated after the addition of TH (Fig. 4C), which were indicated by their distribution in Fig. 5C. In addition, the ‘turquoise’ module had the highest correlation with Phascolarctobacterium (r = 0.64, P < 0.001) and Megasphaera (r=0.65, P < 0.001), while the ‘red’ module had the highest correlation with Lachnospiraceae_FCS020_group (r=0.64, P < 0.001). These results suggested that 16 bacteria genera were closely related related to muscle metabolism after dietary TH supplement, including the differential bacteria Alistipes, Mucispirillum, and Prevotellaceae_UCG_001.
Fig. 5.
Construction of correlated co-expression networks between muscle metabolites and bacterial genera by using WGCNA. (A) Gene clustering dendrogram with adjacency-based dissimilarity, together with assigned module colors. (B) Construction of module heatmap. (C) Construction of the relationships between modules and traits. Red represents a positive correlation, and blue represents a negative correlation. Significantly associated bacterial genera are labeled in red color. Significant correlations are marked by *P < 0.05, **P < 0.01, ***P < 0.001.
Gut microbiota alteration was closely associated with muscle amino acids synthesis and metabolism
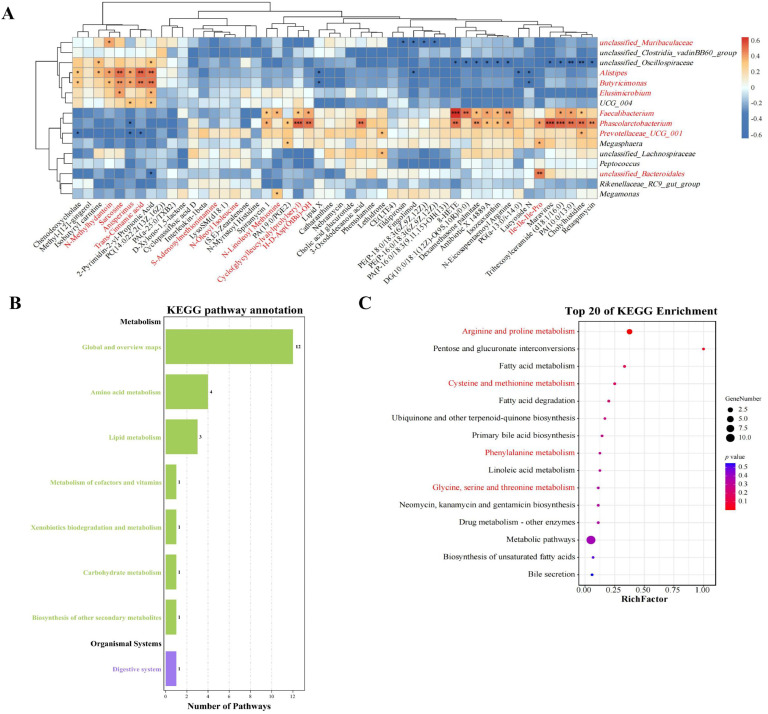
The 16 related genera selected by WGCNA were analyzed for pearson correlation analysis with the top 50 differential metabolites in muscle samples (Fig. 6A). Pearson correlation coefficients showed that 33 differential metabolites significantly (P < 0.05) related to the above 16 bacterial genera were annotated through the enrichment analysis. Interestingly, we found that ten of these metabolites were enriched in the pathways related to amino acid synthesis and metabolism (Fig. 6B, 6C, Fig. S6 and Table S3). Among them, the most abundant metabolite, Trans-Cinnamic acid, was downregulated in TH-treated group and enriched to the phenylalanine metabolism pathway. N-Methylhydantoin was enriched to the Arginine and proline metabolism pathway, whereas the Sarcosine was enriched to the pathway of Glycine, serine and threonine metabolism. Five of these metabolites (Anisperimus, N-Oleoyl Isoleucine, Cyclo(glycylleucylvalylprolylseryl)), Ile-Ile-Ile-Pro and H-D-Asp (OtBu)-OH) belonged to “amino acids, peptides, and analogs”, which were significantly (P < 0.05) associated with multiple bacterial genera in the TH-treated groups. Faecalibacterium and Phascolarctobacterium were positively associated with H-D-Asp (OtBu)-OH and Cyclo(glycylleucylvalylprolylseryl), but negatively associated with Anisperimus. Alistipes were positively associated with Trans-Cinnamic acid, Anisperimus, N-Methylhydantoin and Sarcosine, while Prevotellaceae_UCG_001 was negatively associated with Anisperimus and Trans-Cinnamic acid. Moreover, Phascolarctobacterium and unclassified_Bacteroidales were positively associated with Ile-Ile-Ile-Pro and Megamonas and Faecalibacterium was positively associated with N-Linoleoyl Methionine. These results indicated that the synthesis and metabolism of the above muscle amino acids were closely associated with intestinal differential bacteria regulated by TH supplementation.
Fig. 6.
Correlation analysis and KEGG enrichment between muscle differential metabolites and related genera. (A) Pearson correlation analysis between different microbiota genera and muscle metabolites. (B) KEGG annotation of muscle differential metabolites in CON and TH-treated groups. (C) Top 20 pathways of KEGG enrichment of muscle differential metabolites in CON and TH-treated groups. Yellow represents a positive correlation, and blue represents a negative correlation. Significant correlations are marked by *P < 0.05, **P < 0.01, ***P < 0.001.
Correlation of gut microbiota composition with amino acids profile, intestinal development and growth performanc
Considering the notable changes in amino acids composition of the TH-treated groups and the pathway enrichment analysis of muscle metabolites, pearson correlation analysis was performed to investigate the correlations between the changes of gut microbiota at the genus level and production performance, intestinal development and muscle amino acids composition in all the groups. Two genera were positively (P < 0.05) associated with growth performance parameter (ADG), implying a positive correlation with productive performance. Conversely, four genera were correlated (P < 0.05) with decreased serum ADG and FCR levels, suggesting that these genera were negatively correlated with growth performance (Fig. S7A). Notably, these increased genera in the TH-treated groups including Prevotellaceae_UCG_001 and Phascolarctobacterium were significantly, positively (P < 0.05) correlated with increased ADG level, whicle the abundances of decreased genera (Bacteroides and unclassified_Oscillospiraceae) were significantly (P < 0.05), negatively associated with increased ADG level. Moreover, two genera abundant in the TH-treated groups included Prevotellaceae_Ga6A1_group and unclassified_Clostridium_sp._CAG_306 (Fig. S7B) were positively (P < 0.05) associated with increased villus height (VH), implying a positive correlation with intestinal development.
A total of 7 genera were positively (P < 0.05) correlated with increased amino acid content, whereas 6 genera showed a negative (P < 0.05) relationship (Fig. S7A). These genera in the TH-treated groups included Elusimicrobium, Faecalibacterium, Megasphaera, unclassified_Oscillospiraceae and unclassified_Muribaculaceae were positively (P < 0.05) correlated with increases in muscle amino acids content. However, the abundances of Prevotellaceae_UCG_001, unclassified_Bacteroidales, Megamonas and Phascolarctobacterium were significantly (P < 0.05), negatively associated with the increased muscle amino acid content. The results showed that most of these genera associated with increased amino acid levels overlap with those that affect chicken metabolism, such as Prevotellaceae_UCG_001, Elusimicrobium, Faecalibacterium and Phascolarctobacterium, etc. These findings suggested that these genera regulated by dietary TH not only altered the muscle amino acid content and metabolites but also affected the intestinal development, thus further improve the production performance.
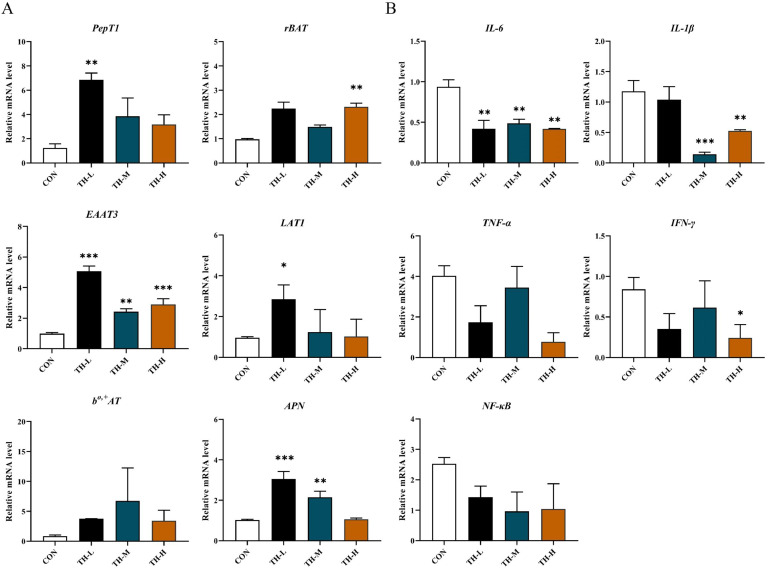
Dietary TH exerted beneficial effects on intestinal amino acid and peptide transport and inflammation
To further verify the positive effect of dietary TH supplementation on the gut of broilers, the quantitative real-time PCR analysis was performed on the intestinal nutrient transporter and enzyme mRNA in broilers between the CON and TH-treated groups, including the peptide transporter PepT1, 4 amino acid transporters (rBAT, bo,+AT, LAT1, and EAAT3), and a digestive enzyme (aminopeptidase N, APN). Studies have shown that the entire plant of TH, including the tubers, has anti-inflammatory properties (Feng et al., 2024; ; Xiao et al., 2023; Zhou et al., 2022). Therefore, the potential contribution of TH to inflammation was analyzed, and then the mRNA levels of 5 common inflammatory factors (IL-1β, IL-6, TNF-α, NF-κB and IFN-γ) in broilers were detected.
As shown in Fig. 7A, the mRNA level of PepT1 in the TH-treated group was up-regulated compared with the control group, especially in TH-L group (P < 0.01). Moreover, the mRNA level of EAAT3 (P < 0.01) in the TH-treated group was significantly increased, and other amino acid transport key factors rBAT, bo,+AT, and LAT1 were also up-regulated in the TH-treated group (Fig. 7A). Similarly, compared with the control group, the mRNA level of APN was up-regulated in the TH-treated group, especially in TH-L group (P < 0.001) and TH-M group (P < 0.01) (Fig. 7A), which could facilitate the hydrolysis of the polypeptides into amino acids and supplies amino acids and peptides to the enterocytes for absorption. In conclusion, TH supplementation could enhance the transport of amino acids and peptides, promote the nutrient absorption of the intestine, and then improve intestinal health. Meanwhile, the pro-inflammatory factor IL-6 (P < 0.01), IL-1β (P < 0.01), and IFN-γ (P < 0.05) were significantly decreased in the TH-H group after dietary TH supplement (Fig. 7B). The mRNA expressions of IL-6, IL-1β, TNF-α, IFN-γ and NF-κB were reduced in the TH-treated group compared with the control group. These results indicate that dietary TH (TH-H group) can alleviate inflammation in broilers, just like previous studies demonstrated (Xiao et al., 2023; Feng et al., 2024).
Fig. 7.
Dietary TH improved intestinal development and inflammation in jinhua yellow chickens. (A) Gene expression of the intestinal nutrient transport key factors in chickens. (B) Gene expression of IL-6, IL-1β, TNF-α, IFN-γ and NF-κB inflammatory factors in chickens. All data were expressed as means ± SEM, n = 6. Asterisk indicates significant differences according to the unpaired t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
Herbal medicine has been increasingly gaining attention due to its characteristics of high efficiency, low toxicity, no drug resistance, and environmental friendliness (Ao and Kim, 2020; Meng et al., 2023). Recently, relevant research has found that herbal feed additives have a certain enhancing effect on the growth performance of livestock and poultry (Cai et al., 2022; Song et al., 2022; Wang et al., 2021). However, there are few reports concerning the use of non-medicinal components of herbs. Studies have shown that the stems and leaves of TH have similar biological activities to its roots (Bai et al., 2022; Ji et al., 2021; Lin et al., 2023). Thus, the effects of TH stem and leaf powder as a feed additive on the performance of broilers were evaluated in this study. During 0-28 days, only ADFI and ADG significantly decreased in TH-H group. It is speculated that broilers may require a certain period to adapt to high-dose TH. Interestingly, dietary TH notably increased the improved all detected performance indicators of broilers in the late feeding phase and the whole feeding trial, especially in the TH-H group. Therefore, the stem and leaf powder of TH might be a candidate feed additive for broilers.
Dietary TH had no toxic and side effects on serum lipid, serum glucose, and liver and kidney function indexes of broilers (Fig. 2 and Table S4). Moreover, the H&E staining results revealed that dietary TH has no detectable effect on abdominal fat deposition. Therefore, the increased body weight of broilers in the TH-treated groups was not a result of the increase of body fat deposition. These results indicate that TH supplementation had no adverse effects on the blood lipid and internal organs of broilers. In addition, dietary TH also did not affect its oxidation-reduction equilibrium of broilers. Intestinal morphology parameters, including villus height, crypt depth, and V/C ratio, are crucial indicators of intestinal health, which influence nutrient digestion and absorption (Liao et al., 2021; Pham et al., 2020). Longer villus, lower crypt depth, and higher V/C ratio led to higher mucosal surface area, resulting in an enhanced digestive capacity in broilers (Zhang et al., 2022a; Zhang et al., 2022b). The H&E staining results of the duodenum showed that dietary TH tended to increase the villus height and increased the V/C ratio in the TH-H group. Notably, dietary TH significantly decreased the crypt depth and increased the V/C ratio in the TH-M group, resulted in a higher apparent metabolic rate of feed nutrients, decreased FCR, and increased ADG in broilers. These results demonstrated that dietary TH supplementation effectively improves the intestinal development of broilers, which may partially explain the improvement of growth performance.
In the present study, dietary TH significantly increased the ACE and Chao1 diversity index, indicating higher richness in TH-treated groups. We observed that dietary TH selectively manipulated gut microbiota, including stimulating the colonization of the beneficial bacteria Prevotellaceae_UCG_001, [Ruminococcus]_torques_group, and Succinivibrionaceae, and suppressing the abundance of Mucispirillum. Prevotellaceae are commonly recognized as a beneficial bacteria and potential probiotics associated with a healthy plant-based diet, which aids in the breakdown of proteins and carbohydrates within humans and animals (Liang et al., 2021; Ugural and Akyol, 2022). Previous studies suggested that Prevotellaceae_UCG_001 can be a potential probiotic, which is beneficial to reduce intestinal pH and promote calcium absorption in laying hens (Wang et al., 2022b). Moreover, the family or genus Succinivibrionaceae were positively correlated with internal carbon metabolism, and feed digestion and absorption in poultry (Honerlagen et al., 2023; Toghyani et al., 2017). Conversely, Mucispirillum was reported as an opportunistic pathogen that may exacerbate inflammation, whose variation in abundance was often associated with the developmental progression of intestinal inflammation (Cheng et al., 2023; Liu et al., 2023b). Evidence provided by our previous studies revealed that the therapeutic effect of T. hemsleyanum might be regulated by inhibiting the inflammation reaction in mice and the mechanism might be related to regulating the inflammation signaling pathway (Ji et al., 2024; Wang et al., 2022a). This may precisely explain the significant decrease of the family or genus Mucispirillum after TH feed addition. The promoted colonization of these certainly beneficial bacteria by TH supplementation was directly responsible for the improvements of intestinal development and feed nutrient utilization of broilers.
The EAA content is regarded as an important nutritive value index in meat production, and FAA play an important role in the formation of meat flavor (Deng et al., 2022; Wang et al., 2022c). The EAA content increased notably in the TH-treated groups, implying that dietary TH promoted the muscle nutritive values of broilers. Meanwhile, the FAA content (Glu, Asp, Ala, Ser, and Thr) in the TH-treated groups also notably increased and accounted for approximate 40 % in TAA. Furthermore, the relative quantity of desirable amino acids possesses an umami (Glu and Asp) or sweet (Ala, Ser, and Thr) taste affecting the taste of chicken (Kim et al., 2023; Yan et al., 2018). FAA not only contribute to basic tastes (sweet and umami) but also act as precursors to more intricate flavors, thereby playing a crucial role in the development of the meat's overall flavor profile. Therefore, the overall flavor of the breast muscle in the TH-treated groups was inevitable to be enhanced. PICRUSt function prediction of gut microbiota further demonstrated that a significant higher capacity of amino acid biosynthesis and metabolism was observed in the TH-treated groups. This phenomenon may be associated with the increased amino acids derived from gut microbiota modulation. Coincidentally, WGCNA analysis confirmed that multiple metabolites closely positively associated with gut microbiota are precisely involved in amino acid synthesis and metabolic pathways. Pearson's correlation analysis further corroborated that the genera that increased in the TH-treated groups had a significant positive correlation with the elevated levels of amino acids, while the genera that decreased showed an inverse relationship. This was consistent with a previous study that the enhancement of breast meat flavor was concomitant with the modulation of gut microbiota (Yang et al., 2022). These findings indicated that the changes of gut microbiota by TH supplementation may contribute to the utilization and deposition of feed amino acid, resulting in muscle composition alteration and better overall flavor. The beneficial bacteria (Prevotellaceae_UCG_001 and Prevotellaceae_Ga6A1_group) were closely associated with the increase of ADG and villus height, wheras the decreased abundance of genera (Bacteroides, unclassified_Oscillospiraceae, and Fusobacterium) showed negative correlation with the ADG and FCR. Dietary TH may alter amino acid composition via regulating the specific gut bacteria described above, which ultimately contributed to the growth performance of broilers.
The intestine is the main digestive organ and the key site for nutrient absorption (Taylor et al., 2021). It is well known that the uptake of amino acids is mediated by the action of the peptide transporter PepT1 and a number of different amino acid transporters (Hu et al., 2023; Wang et al., 2017). Thus, we observed that the expression of PepT1, EAAT3, rBAT and APN were significantly increased. The up-regulation of PepT1 will accelerate the transport of oligopeptides and free amino acids (Yu et al., 2021). The up-regulation of EAAT3, rBAT, bo,+AT and LAT1 will accelerate the Na+-dependent transport with various specificity ion types of amino acids, including the neutral, anionic, and cationic amino acids (Yi et al., 2019). As a digestive enzyme that cleaves amino acids from the N-terminal end of peptides (Farsa et al., 2022; Liu et al., 2020), the up-regulation of APN would provide a constant supply of substrates for the amino acid transporters. Inflammation is a vital component of the body's immune defense, acting as a shield against various external irritants. In this study, dietary TH decreased five inflammatory factors (IL-1, IL-6, TNF-α, NF-κB and IFN-γ) in all TH-treated groups, and significantly down-regulated three pro-inflammatory cytokines (IL-6, IL-1β and IFN-γ) in TH-H group. Based on the above results, dietary TH can improve the intestinal development and alleviate the inflammation in broilers.
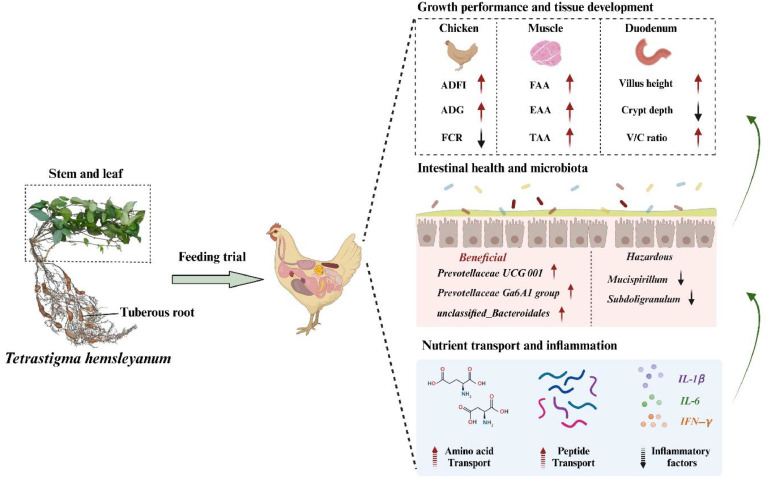
In conclusion, the present study revealed that dietary TH at 1.25-5 g/kg increased the ADFI and ADG, and decreased the FCR in Jinhua yellow chickens, which filled a knowledge gap regarding the effects of various feed addition levels of TH. This study observed a significant increase in growth performance and muscle amino acids by TH supplementation, providing new insight into the beneficial effect of TH stem and leaf powder on broilers. Furthermore, the alteration of gut microbiota, nutrient transport manipulated and inflammation by dietary TH significantly improved intestinal development and health. Therefore, dietary TH not only directly improved the intestinal development, muscle amino acids composition, but also significantly enhanced the better performance in broilers (Fig. 8). In summary, TH may be a Chinese medicine feed additive that can improve growth performance, muscle amino acids composition and intestinal health for broilers.
Fig. 8.
Dietary TH exerted beneficial effects on growth performance, gut microbiota, nutrient transport and inflammation in Jinhua yellow chickens.
CRediT authorship contribution statement
Chao Lu: Conceptualization, Data curation, Formal analysis, Writing – original draft. Yun Xiang: Conceptualization, Funding acquisition, Writing – review & editing. Kewei Xu: Data curation, Formal analysis. Fengrui Gao: Data curation, Formal analysis. Shaofeng Zhu: Data curation, Formal analysis. Fangfang Lou: Project administration. Lu Liu: Project administration. Xin Peng: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgments
This work was financially supported by Agricultural key projects Funded by Jinhua Science and Technology Bureau (2021-2-008), Ningbo Natural Science Foundation (2024J252), the Major Science and Technology Projects of Breeding New Varieties of Agriculture in Zhejiang Province (2021C02074), the Ningbo Top Medical and Health Research Program (No.2022030309), and Co construction Key Laboratory for Kidney Essence Deficiency of National and Zhejiang Provincial Administration of Traditional Chinese Medicine (GZY-ZJ-SY-2306).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2024.104652.
Appendix. Supplementary materials
References
- Abdallah A., Zhang P., Zhong Q., Sun Z. Application of traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug. Metab. 2019;20:54–64. doi: 10.2174/1389200219666180523102920. [DOI] [PubMed] [Google Scholar]
- Ao X., Kim I. Effects of grape seed extract on performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poult. Sci. 2020;99:2078–2086. doi: 10.1016/j.psj.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Gu Y., Liu S., Jiang L., Han M., Geng D. Flavonoids metabolism and physiological response to ultraviolet treatments in Tetrastigma hemsleyanum. Diels et Gilg. Front. Plant. Sci. 2022;13 doi: 10.3389/fpls.2022.926197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G., Mao N., Gu P., Zhu T., He J., Peng S., Yang Y., Liu Z., Hu Y., Wang D. Effects of alhagi honey polysaccharides as feed supplement on intestine function and microbiome, immune function, and growth performance in chicken. Int. J. Mol. Sci. 2022;23:14332. doi: 10.3390/ijms232214332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Lei H., Xie C., Chen J., Yi X., Zhao F., Yuan Y., Chen P., He J., Luo C. B lymphocyte development in the bursa of fabricius of young broilers is influenced by the gut microbiota. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.04799-22. e0479922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Liu R., Li C., Xu X., Zhou G. Meat quality and flavor compounds of soft-boiled chickens: effect of Chinese yellow-feathered chicken breed and slaughter age. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsa O., Ballayová V., Žáčková R., Kollar P., Kauerová T., Zubáč P. Aminopeptidase N inhibitors as pointers for overcoming antitumor treatment resistance. Int. J. Mol. Sci. 2022;23:9813. doi: 10.3390/ijms23179813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Ye W., Feng L. Bioactives and metabolites of Tetrastigma hemsleyanum root extract alleviate DSS-induced ulcerative colitis by targeting the SYK protein in the B cell receptor signaling pathway. J. Ethnopharmacol. 2024;322 doi: 10.1016/j.jep.2023.117563. [DOI] [PubMed] [Google Scholar]
- Guo Q., Li F., Duan Y., Wen C., Wang W., Zhang L., Huang R., Yin Y. Oxidative stress, nutritional antioxidants and beyond. Sci. China. Life. Sci. 2020;63:866–874. doi: 10.1007/s11427-019-9591-5. [DOI] [PubMed] [Google Scholar]
- Han B., Zhai Y., Li X., Zhao H., Sun C., Zeng Y., Zhang W., Lu J., Kai G. Total flavonoids of Tetrastigma hemsleyanum Diels et Gilg inhibits colorectal tumor growth by modulating gut microbiota and metabolites. Food. Chem. 2023;410 doi: 10.1016/j.foodchem.2022.135361. [DOI] [PubMed] [Google Scholar]
- Honerlagen H., Reyer H., Abou-Soliman I., Segelke D., Ponsuksili S., Trakooljul N., Reinsch N., Kuhla B., Wimmers K. Microbial signature inferred from genomic breeding selection on milk urea concentration and its relation to proxies of nitrogen-utilization efficiency in Holsteins. J. Dairy. Sci. 2023;106:4682–4697. doi: 10.3168/jds.2022-22935. [DOI] [PubMed] [Google Scholar]
- Hu Y., Huang Y., Wang C., Zhang W., Qu Y., Li D., Wu W., Gao F., Zhu L., Wu B. The organic zinc with moderate chelation strength enhances the expression of related transporters in the jejunum and ileum of broilers. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T., Ji W.W., Wang J., Chen H.J., Peng X., Cheng K.J., Qiu D., Yang W.J. A comprehensive review on traditional uses, chemical compositions, pharmacology properties and toxicology of Tetrastigma hemsleyanum. J. Ethnopharmacol. 2021;264 doi: 10.1016/j.jep.2020.113247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Zhu H., Xing B., Chu C., Ji T., Ge W., Wang J., Peng X. Tetrastigma hemsleyanum suppresses neuroinflammation in febrile seizures rats via regulating PKC-δ/caspase-1 signaling pathway. J. Ethnopharmacol. 2024;318 doi: 10.1016/j.jep.2023.116912. [DOI] [PubMed] [Google Scholar]
- Jin D., Wang J., Xue J., Zhao Y., Yan G., Li X., Wang X. Contribution of Chinese herbal medicine in the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2023;37:1015–1035. doi: 10.1002/ptr.7669. [DOI] [PubMed] [Google Scholar]
- Jun P., Rahmat E., Han C., Yang C., Kang Y. Traditional Chinese medicine and traditional Indonesian medicine: a comparative review of herbal medicines restricted in pregnancy. Chin. J. Integr. Med. 2021;27:794–800. doi: 10.1007/s11655-021-3487-7. [DOI] [PubMed] [Google Scholar]
- Khan S., Moore R.J., Stanley D., Chousalkar K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microb. 2020;86 doi: 10.1128/AEM.00600-20. e00600-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Cho E., Munyaneza J.P., Ediriweera T.K., Cha J., Jin D., Cho S., Lee J.H. Genome-wide association study for the free amino acid and nucleotide components of breast meat in an F2 crossbred chicken population. J. Anim. Sci. Technol. 2023;65:57. doi: 10.5187/jast.2022.e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Lin Z., Lu Z., Ying Z. Effects of a traditional Chinese medicine formula containing the Coix seed and Lotus seed on the intestinal morphology and microbiota of local piglets. AMB Express. 2021;11:1–15. doi: 10.1186/s13568-021-01318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Kou S., Chen C., Raza S.H.A., Wang S., Ma X., Zhang W.J., Nie C. Effects of Clostridium butyricum on growth performance, metabonomics and intestinal microbial differences of weaned piglets. BMC. Microbiol. 2021;21:1–16. doi: 10.1186/s12866-021-02143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H., Chen H., Livneh H., Huang H., Lai N., Lu M., Yeh C., Tsai T. Integration of Chinese herbal medicine into routine care was related to lower risk of chronic kidney disease in patients with rheumatoid arthritis: a population-based nested case–control study in Taiwan. J. Multidiscip. Healthc. 2023;16:1191–1201. doi: 10.2147/JMDH.S400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L., Li J., Li J., Huang Y., Wu Y. Effects of Astragalus polysaccharides on intestinal morphology and intestinal immune cells of Muscovy ducklings infected with Muscovy duck reovirus. Poult. Sci. 2021;100:64–72. doi: 10.1016/j.psj.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Lv Y., Mao Z., Chen X., Chen Y., Zhu B., Yu Y., Ding Z., Zhou F. Polysaccharides from Tetrastigma Hemsleyanum Diels et Gilg ameliorated inflammatory bowel disease by rebuilding the intestinal mucosal barrier and inhibiting inflammation through the SCFA-GPR41/43 signaling pathway. Int. J. Biol. Macromol. 2023;250 doi: 10.1016/j.ijbiomac.2023.126167. [DOI] [PubMed] [Google Scholar]
- Liu B., Ma R., Yang Q., Yang Y., Fang Y., Sun Z., Song D. Effects of traditional chinese herbal feed additive on production performance, egg quality, antioxidant capacity, immunity and intestinal health of laying hens. Animals. 2023;13:2510. doi: 10.3390/ani13152510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Mo W., Cao W., Jia G., Zhao H., Chen X., Wu C., Zhang R., Wang J. Digestive abilities, amino acid transporter expression, and metabolism in the intestines of piglets fed with spermine. J. Food. Biochem. 2020;44:e13167. doi: 10.1111/jfbc.13167. [DOI] [PubMed] [Google Scholar]
- Liu M., Chen R., Wang T., Ding Y., Zhang Y., Huang G., Huang J., Qu Q., Lv W., Guo S. Dietary Chinese herbal mixture supplementation improves production performance by regulating reproductive hormones, antioxidant capacity, immunity, and intestinal health of broiler breeders. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Lei X., Li J., Zhong Y., Tan D., Zhang Q., Kong Z. Effects of fermented Andrographis paniculata on growth performance, carcass traits, immune function, and intestinal health in Muscovy ducks. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou T., Ji T., Peng X., Ji W., Yuan L., Wang J., Li S.m., Zhang S., Shi Q. Extract from tetrastigma hemsleyanum leaf alleviates Pseudomonas aeruginosa lung infection: network pharmacology analysis and experimental evidence. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.587850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W.S., Zou Q., Xiao Y., Ma W., Zhang J., Wang T., Li D. Growth performance and cecal microbiota of broiler chicks as affected by drinking water disinfection and/or herbal extract blend supplementation. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion B., Van Winters B., King K., Spricigo J., Ogilvie L., Guan L., DeVries T., McBride B., LeBlanc S., Steele M. Effects of replacing inorganic salts of trace minerals with organic trace minerals in pre-and postpartum diets on feeding behavior, rumen fermentation, and performance of dairy cows. J. Dairy. Sci. 2022;105:6693–6709. doi: 10.3168/jds.2022-21908. [DOI] [PubMed] [Google Scholar]
- Pham V.H., Kan L., Huang J., Geng Y., Zhen W., Guo Y., Abbas W., Wang Z. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J. Anim. Sci. Biotechnol. 2020;11:1–18. doi: 10.1186/s40104-019-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B., Li P., Yan S., Liu Y., Gao M., Lv H., Lv Z., Guo Y. Effects of dietary astragalus polysaccharide supplementation on the Th17/treg balance and the gut microbiota of broiler chickens challenged with necrotic enteritis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.781934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Zou Z., Chen X., Tan J., Liu L., Wei Q., Xiong P., Song Q., Chen J., Su W. Effects of traditional Chinese herbal feed supplement on growth performance, immunity, antioxidant levels, and intestinal health in chickens: a study on Ningdu yellow chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhail M., Khan M.S., Ahmad A., Zughaibi T.A., Husain F.M., Rehman M.T., Tabrez S. Flavonoids and PI3K/Akt/mTOR signaling cascade: a potential crosstalk in anticancer treatment. Curr. Med. Chem. 2021;28:8083–8097. doi: 10.2174/0929867328666210804091548. [DOI] [PubMed] [Google Scholar]
- Taylor S.R., Ramsamooj S., Liang R.J., Katti A., Pozovskiy R., Vasan N., Hwang S.K., Nahiyaan N., Francoeur N.J., Schatoff E.M. Dietary fructose improves intestinal cell survival and nutrient absorption. Nature. 2021;597:263–267. doi: 10.1038/s41586-021-03827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toghyani M., Girish C., Wu S., Iji P., Swick R. Effect of elevated dietary amino acid levels in high canola meal diets on productive traits and cecal microbiota population of broiler chickens in a pair-feeding study. Poult. Sci. 2017;96:1268–1279. doi: 10.3382/ps/pew388. [DOI] [PubMed] [Google Scholar]
- Tu W., Zhang W., Wang H., Zhang Y., Huang J., Li B., Li X., Tan Y., Wu X. Effects of Chinese herbal feed additives on the sperm quality and reproductive capacity in breeding boars. Front. Vet. Sci. 2023;10 doi: 10.3389/fvets.2023.1231833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugural A., Akyol A. Can pseudocereals modulate microbiota by functioning as probiotics or prebiotics? Crit. Rev. Food. Sci. 2022;62:1725–1739. doi: 10.1080/10408398.2020.1846493. [DOI] [PubMed] [Google Scholar]
- Wan L., Huang Q., Li C., Yu H., Tan G., Wei S., El-Sappah A.H., Sooranna S., Zhang K., Pan L. Integrated metabolome and transcriptome analysis identifies candidate genes involved in triterpenoid saponin biosynthesis in leaves of Centella asiatica (L.) Urban. Front. Plant. Sci. 2024;14 doi: 10.3389/fpls.2023.1295186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Lin Y., Zhou M., Fu S., Zhu B., Chen Y., Ding Z., Zhou F. Polysaccharides from Tetrastigma Hemsleyanum Diels et Gilg attenuate LPS-induced acute lung injury by modulating TLR4/COX-2/NF-κb signaling pathway. Biomed. Pharmacother. 2022;155 doi: 10.1016/j.biopha.2022.113755. [DOI] [PubMed] [Google Scholar]
- Wang C., Liu S., Xie X., Tan Z. Regulation profile of the intestinal peptide transporter 1 (PepT1) Drug. des. dev. Ther. 2017;11:3511–3517. doi: 10.2147/DDDT.S151725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cao W., Ji T., Zhao M., Liu T., Wu J., Feng F., Zhou A., Peng X. Gut microbiota and transcriptome profiling revealed the protective effect of aqueous extract of Tetrastigma hemsleyanum leaves on ulcerative colitis in mice. Curr. Res. Food. Sci. 2023;6 doi: 10.1016/j.crfs.2022.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ding L., Wei H., Jiang C., Yan Q., Hu C., Jia G., Zhou Y., Henkin Z., Degen A. Astragalus membranaceus root supplementation improves average daily gain, rumen fermentation, serum immunity and antioxidant indices of Tibetan sheep. Animal. 2021;15 doi: 10.1016/j.animal.2020.100061. [DOI] [PubMed] [Google Scholar]
- Wang X., Wu X., Cong X., Ren J., Li J., Zhu J., Dai M., Hrabchenko N., Du Y., Qi J. The functional role of fecal microbiota transplantation on Salmonella Enteritidis infection in chicks. Vet. Microbiol. 2022;269 doi: 10.1016/j.vetmic.2022.109449. [DOI] [PubMed] [Google Scholar]
- Wang Y., Tuccillo F., Lampi A.M., Knaapila A., Pulkkinen M., Kariluoto S., Coda R., Edelmann M., Jouppila K., Sandell M. Flavor challenges in extruded plant-based meat alternatives: a review. Compr. Rev. Food. Sci. F. 2022;21:2898–2929. doi: 10.1111/1541-4337.12964. [DOI] [PubMed] [Google Scholar]
- Wei C., Zhao Y., Ji T., Sun Y., Cai X., Peng X. Cyclin-dependent kinase 6 identified as the target protein in the antitumor activity of tetrastigma hemsleyanum. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.865409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Xiong H., Deng Z., Peng X., Cheng K., Zhang H., Jiang L., Sun Y. Tetrastigma hemsleyanum leaf extracts ameliorate NAFLD in mice with low-grade colitis via the gut–liver axis. Food. Funct. 2023;14:500–515. doi: 10.1039/d2fo03028d. [DOI] [PubMed] [Google Scholar]
- Yan J., Liu P., Xu L., Huan H., Zhou W., Xu X., Shi Z. Effects of exogenous inosine monophosphate on growth performance, flavor compounds, enzyme activity, and gene expression of muscle tissues in chicken. Poult. Sci. 2018;97:1229–1237. doi: 10.3382/ps/pex415. [DOI] [PubMed] [Google Scholar]
- Yang C., Qiu M., Zhang Z., Song X., Yang L., Xiong X., Hu C., Pen H., Chen J., Xia B. Galacto-oligosaccharides and xylo-oligosaccharides affect meat flavor by altering the cecal microbiome, metabolome, and transcriptome of chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Yang G., Xiong Y., Wu Q., Xiao H., Wen X., Yang X., Wang L., Jiang Z. Integrated metabolomic and proteomics profiling reveals the promotion of Lactobacillus reuteri LR1 on amino acid metabolism in the gut–liver axis of weaned pigs. Food. Funct. 2019;10:7387–7396. doi: 10.1039/c9fo01781j. [DOI] [PubMed] [Google Scholar]
- Yu C., Zhang J., Zhang H., Chen Y., Wang C., Zhang L., Ding L., Wang T., Yang Z. Influence of trans-anethole on the nutrient digestibility and intestinal barrier function in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y., Sun J., Sun C., Zhao H., Li X., Yao J., Su J., Xu X., Xu X., Hu J. Total flavonoids from the dried root of Tetrastigma hemsleyanum Diels et Gilg inhibit colorectal cancer growth through PI3K/AKT/mTOR signaling pathway. Phytother. Res. 2022;36:4263–4277. doi: 10.1002/ptr.7561. [DOI] [PubMed] [Google Scholar]
- Zhang L., Hong Y., Liao Y., Tian K., Sun H., Liu X., Tang Y., Hassanin A.A., Abdelnour S.A., Suthikrai W. Dietary Lasia spinosa thw. Improves growth performance in broilers. Front. Nutr. 2022;8 doi: 10.3389/fnut.2021.775223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Mahmood T., Tang Z., Wu Y., Yuan J. Effects of naturally oxidized corn oil on inflammatory reaction and intestinal health of broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Lu Y., Sun T., Sun L., Wang B., Lu J., Li Z., Zhu B., Huang S., Ding Z. Antitumor effects of polysaccharides from Tetrastigma hemsleyanum Diels et Gilg via regulation of intestinal flora and enhancing immunomodulatory effects in vivo. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.