Abstract
Background
The extent of the performance and utility of scores for the risk of cardiovascular disease (CVD) in persons with type 1 diabetes (T1DM) largely remains unclear.
Objective
The purpose of this study was to synthesize data on the performance of CVD risk scores in people living with T1DM.
Methods
This study is a systematic review and meta-analysis. PubMed and EMBASE were searched through December 31, 2023. The included studies: 1) were retrospective, prospective, or cross-sectional in design; 2) included persons with T1DM; 3) assessed CVD outcomes; and 4) had data on at least on CVD risk score. Measures of calibration and discrimination qualitatively summarized. Measures of discrimination were combined using random-effects models stratified by type of risk model.
Results
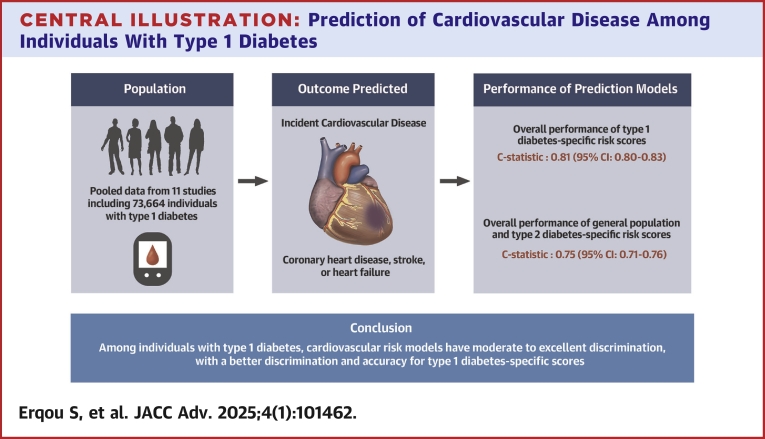
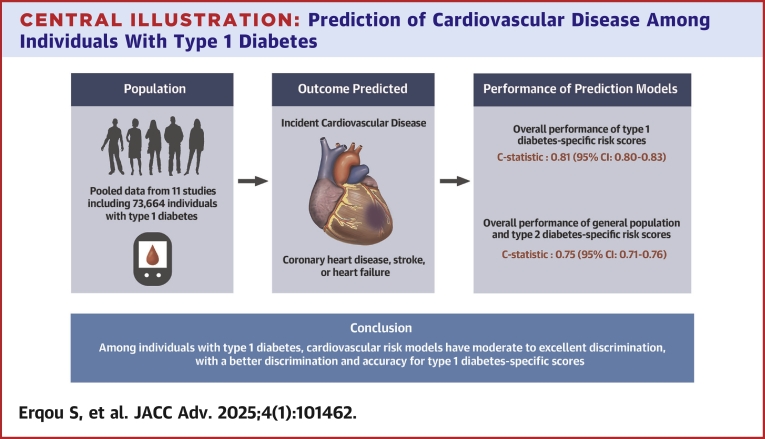
In a meta-analysis of observational studies of CVD risk scores in T1DM individuals, including 11 studies and 73,664 participants (mean age of 34 years, mainly White individuals and male [55%]), we evaluated 12 CVD risk prediction models (7 T1DM-specific, 1 type 2 diabetes–specific, and 4 general population models). Most risk scores had a moderate to excellent discrimination (C-statistic: 0.73-0.85) and predicted CVD risk well when compared to actual clinical events. CVD risk scores specifically developed in T1DM individuals exhibited a higher discriminative performance—pooled C-statistic of 0.81 vs 0.75 for risk scores developed in the general population or those with type 2 diabetes and also showed a better calibration.
Conclusions
Among individuals with T1DM, CVD risk models had a moderate to excellent discrimination, with a better discrimination and accuracy for T1DM-specific scores.
Key words: cardiovascular risk, epidemiology, risk prediction, risk scores, type 1 diabetes
Central Illustration
Among individual with type 1 diabetes (T1DM), cardiovascular disease (CVD) is the leading cause of death.1, 2, 3 In individuals with T1DM, CVD happens at least a decade or more earlier than among individuals in general population or those with type 2 diabetes (T2DM).2 Indeed, T1DM is generally diagnosed at younger ages than T2DM, with the time of exposure to diabetes-related CVD risk factors being much longer.4 Furthermore, risk factors for CVD seem to operate differently in T1DM than in T2DM, including a more profound effect of hyperglycemia,1,4,5 a higher risk among women,6 a much higher risk of some of the CVD outcomes (eg; heart failure),7,8 all suggesting a difference in the pathophysiology of CVD in T1DM.
Although patients with T1DM are at increased risk of developing CVD, they are currently undertreated,9,10 with low rates of treatment for hypertension, dyslipidemia, and microalbuminuria.11,12 A factor that limits preventing or delaying CVD disease in T1DM is the delayed identification and management of the risk factors. In current guidelines, there are no formal recommendations on using a specific risk tool for predicting the future risk of CVD among those with T1DM.13 Thus, there are no risk scores used on a regular basis in clinical practice for assessing the risk of CVD in T1DM. The 2013 revised American College of Cardiology/American Heart Association pooled cohort equations 14 and the more recent American Heart Association prediction equation for CVD incorporating cardiovascular kidney metabolic health 15 are not applicable to individuals with T1DM. The development of these risk tools did not specifically include these individuals nor the age range within which patients with T1DM generally fall. Furthermore, risk scores developed for individuals with T2DM, such as the UKDPS Risk Engine,16 may underestimate the risk of CVD among individuals with T1DM. Indeed, most of the recommendations for managing CVD risk among people with T1DM are extrapolated from data obtained in people with T2DM.17
A number of studies have reported on the performance of risk scores for predicting CVD developed specifically among individuals with T1DM. These studies have presented variables results and are limited by relatively small sample size.
There is a lack of clarity on the estimated performance of risk scores for predicting CVD among individuals with T1DM. The existing data on the development and assessment of CVD risk scores in people with T1DM have not been adequately synthesized. Therefore, we conducted a systematic review and meta-analysis of all available evidence on the performance of risk scores for the prediction of CVD among people with T1DM. Our overarching goal is to guide clinicians and people with type 1 diabetes to prevent CVD.
Methods
Data sources and search strategy
We searched PubMed and EMBASE from inception up to December 2023. The reference lists of identified studies were manually scanned and cited references were screened through the ISI Web of Knowledge database for additional eligible studies.
Electronic search used key search terms related to T1DM, CVD, and risk prediction. The search terms are shown in the Supplemental Appendix. The inclusion criteria included the following: 1) patients with a diagnosis of T1DM; 2) retrospective or prospective studies with cardiovascular outcomes (eg; MI, CAD, CVD); and 3) available data on a minimum of one cardiac risk score (eg, Steno Risk score, Swedish Risk score).
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.18
Data extraction
We extracted prespecified information in duplicate from the publications using standardized forms (performed by AS and MH). The extracted study-level information included study location, study period (years), design, size, proportion of men, race/ethnicity, average of participants (mean or median), proportion with hypertension, proportion of smokers, proportion on lipid-lowering medications, proportion on blood pressure lowering medications, and the mean values of glycosylated hemoglobin (HbA1C), blood pressure, total cholesterol, LDL-cholesterol, triglycerides, and duration of diabetes.
For each CVD risk prediction model, we extracted the following parameters if available: type of risk model (T1DM-specific, T2DM-specific, general population), outcomes assessed, follow-up period, N cases/events, event rate per 1,000 person-years, measures of discrimination (eg, C-statistic, C-index), measures of model calibration (eg, Hosmer-Lemeshow [HL] test ratio of expected to observed outcomes), any direct comparisons with one or more additional models including measures of reclassification—net reclassification index to evaluate whether a new model adds value in correctly reclassifying individuals compared to an existing model, integrated discrimination improvement, and information on validation. A model discrimination indicates its ability to correctly identifying those who are actually at a high risk of developing a cardiovascular disease and separate them from those who are actually at low risk of future CVD.19 A model calibration indicates the ability of a risk model to rank order individuals’ risks.19 The reclassification indices generally indicated the proportion of individuals reclassified from 1 risk stratum (based on a first model) to a different risk stratum (based on a different model with additional variables compared with the first model).19
We resolved discrepancies by consensus, adjudicated by 2 additional reviewers (S.E. and J.B.E.T.).
Quality of reporting and risk of bias in included studies
We assessed the quality of studies included in the review using the Transparent Reporting of a Multivariable Prediction model for Individual Prognosis or Diagnosis (TRIPOD) checklist.20 A number of the included studies fulfilled several important requirements for reporting of risk prediction models highlighted in the TRIPOD checklist, except for actions taken for blinding assessment of the outcome or predictor variables, which none of the studies reported. Supplemental Table 1 describes the evaluation of included studies using the TRIPOD checklist.
We assessed the risk of bias in included studies using the Newcastle-Ottawa Quality Assessment Scale for cohort studies.21 This scale is derived by assigning points to 3 aspects of study design with a maximum total of 10 points: selection of study participants (maximum 5 points), comparability of study groups (maximum 2 points), and ascertainment of the outcome of interest (maximum 3 points). The 3 components of the scoring system related to assessment of the nonexposed cohort and its comparability with the exposed cohort were not relevant to the studies assessing performance of risk scores, making the maximum possible total points 7 (instead of 10) in this review.
Data synthesis
The characteristics of the studies (eg number of participants, % males, % White individuals, % Black participants) are presented in tables and summarized as the range of values. We report the weighted average of these study-level characteristics weighted by the appropriate denominators (N). We took mean and median values as equivalent approximations of central tendencies (average value). Measures of calibration, including expected to observed ratios, and measures of discrimination are presented in tables and qualitatively summarized. Whenever possible, we combined discrimination measures stratified by type of risk model across studies, using random-effects model. We considered area under receiver operator curve reported as C-statistic and Harrel’s C-index are equivalent. The individual studies were weighted by the study size. Where confidence intervals of C-index or C-statistic were reported, we calculated the standard error as the difference between the upper and lower limits divided by 3.92 (2∗1.96). Where neither the standard error nor the confidence intervals of the estimates were reported, we assigned the standard error from another study included in this review that has the closest number of cases as the study missing this value. Such an imputation was necessary for 5 studies lacking the standard error of the estimates of the performance of risk models.22, 23, 24, 25, 26
We conducted sensitivity analysis including the following: 1) pooling the C-statistics using analytical weight based on the number of CVD cases observed in each study; and 2) performing a logit transformation of the C-statistic measures before pooling them across the studies. We report pooled estimates and 95% confidence intervals.
All analyses were performed using Stata software (Stata Corp v15). We reported the findings according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.18
Role of the funding source and ethical approval
The funder had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Our study is a meta-analysis of published studies, so it did not require any approval by the Johns Hopkins University Institutional Review Board.
Results
Characteristics of studies
The study selection process generated a total of 3,438 studies based on titles, abstracts, and full texts (Supplemental Figure 1). Of these, we included 10 publications, representing data from 11 independent studies of people with T1DM in the meta-analyses.22, 23, 24, 25, 26, 27, 28, 29, 30, 31
The characteristics of the included studies are shown in Table 1. The studies were conducted predominantly in Europe, with 2 studies from US, and 1 study from Australia. The size of the studies ranged widely from 84 to 33,183 individuals with T1DM (median 1973, total 73,664). In all studies, the ascertainment of T1DM was made based on clinical records; no study used data on antibodies. The average age of participants ranged between 28 and 50 years (weighted average of 34 years) across the studies. The proportions of males ranged between 35% and 60% (weighted average 55%). The participants were predominantly of European ancestry (White individuals), although data on race were largely not reported. The mean HbA1C ranged from 7.8% to 9.1% (weighted average 8.3%), mean diabetes duration ranged from 11.5 to 22.7 years (weighted average 15.3 years), mean body mass index count ranged from 23.4 to 26.5 kg/m2 (weighted average 25.2 kg/m2), mean SBP ranged from 113 to 137 mm Hg (weighted average 125 mm Hg), mean total-cholesterol ranged from 4.6 to 5.2 mmol/L (weighted average 5.9 mmol/L), mean LDL-cholesterol ranged from 2.4 to 3.2 mmol/L (weighted average 2.9 mmol/L), mean HDL-cholesterol ranged from 1.2 to 1.7 mmol/L (weighted average 1.6 mmol/L), and mean triglycerides ranged from 0.7 to 1.3 mmol/L (weighted average 1.0 mmol/L). The proportion of current smokers ranged from 12% to 64% (weighted average 32%), that of those using hypertension medications from 5% to 40% (weighted average 25%), and that of those using lipid-lowering medications ranged from 8% to 41% (weighted average 22%).
Table 1.
Characteristics of Includes Studies
| Zgibor et al, 2006 22 | Zgibor et al, 201023 | Davis et al, 201027 | Cederholm et al, 2011 24 | Soedamah et al, 201425 | Vistisen et al, 201628 | Llaurado et al, 201729 | Boscari et al, 202026 | Mcgurnaghan, et al, 202130 | Tecce et al, 202231 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subset | NA | Deriv. | Valid. | NA | Deriv. | Valid. | Deriv. | Valid. | Valid. | Valid. | Deriv. | Valid. | NA | NA | Deriv. | Valid. | NA |
| Study period | 1986-2001 | 1986-2001 | 1989-1999 | 1993-2006 | 2002-2007 | 2003-2007 | 1989-1999 | 1986-2001 | 1994-2009 | 2000-2002 | 2001-2013 | 2001-2013 | NR | 2013-2014 | 2008-2018 | 2002-2013 | 2002-2019 |
| Study design | PC | PC | RC | PC | RC | RC | PC | PC | PC | PC | PC | PC | X-Sec | RC | RC | RC | RC |
| Study Place | PA | PA | Europe | Australia | Sweden | Sweden | Europe | PA | Finland | CO | Denmark | Denmark | Spain | Italy | Scotland | Sweden | Italy |
| N | 537 | 603 | 2,328 | 117 | 3,661 | 4,484 | 1973 | 554 | 2,999 | 590 | 4,306 | 2,118 | 84 | 223 | 27,527 | 33,183 | 456 |
| Cohort | EDC | EDC | EURODIAB PCS | Fremantle Diabetes Study | Swedish National Diabetes Register | Swedish National Diabetes Register | EURODIAB PCS | EDC Original | FinnDiane | CACTI | Steno Diabetes Center | Funen Diabetes Database | _ | Italian Popn | Scottish Care Information-Diabetes_ | Swedish National Diabetes Register _ | Italian popn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of male | 49.9 | 50.4 | 48.4 | 59.8 | 55.6 | 54.9 | 52.0 | 49.0 | 51.0 | 45.0 | 54.0 | 57.7 | 50.0 | 34.5 | 57.0 | 54.6 | 50.0 |
| Average age, y | 27.3 | 27.2 | 32.2 | 44 | 44.6 | 44.6 | 30.3 | 28.0 | 37.3 | 36.3 | 42.2 | 44.2 | 50.1 | 43.0 | 35.0 | 31.9 | 30.8 |
| Diabetes duration | 19.1 | 19.0 | 14.3 | 12.5 | 28.0 | 28.1 | 11.5 | 18.6 | 19.0 | 22.7 | 16.6 | 13.6 | 19.0 | 22.0 | 13.0 | 16.9 | 14.2 |
| A1C | 10.2 | 10.4 | - | 8.6 | 7.9 | 8.1 | 8.3 | 9.1 | 8.4 | 8.0 | 8.4 | 8.1 | 7.9 | 7.8 | 8.7 | 8.0 | 7.92 |
| % smoking | - | 22.2 | - | 26.3 | 14.8 | 13.4 | 31.0 | 20.0 | 26.0 | 12.0 | 64.1 | 29.4 | 31 | 16.1 | 53.0 | 12.6 | 37.91 |
| Average SBP, mm Hg | 113.0 | 112.9 | 120.5 | 137.0 | 130.0 | 130 | 118.0 | 113 | 132.0 | 117.0 | 132.2 | 130.1 | 126.4 | 119.0 | 127.0 | 122.0 | 119.65 |
| BMI, kg/m2 | - | 23.6 | 23.5 | 26.5 | 25.4 | 25.4 | 23.4 | 23.8 | 25.1 | 26.3 | 24.8 | 25.5 | 26.0 | 24.8 | 26.0 | 24.6 | 24.5 |
| TC, mg/dL | 4.9 | 5.09 | 5.3 | 5.1 | 5.0 | 4.9 | 5.2 | 4.9 | 4.9 | 4.6 | 4.9 | 4.8 | 4.6 | - | NR | NR | 4.6 |
| HDL-C, mg/dL | 1.4 | 1.4 | 1.5 | 1.2 | 1.6 | 1.7 | 1.5 | 1.4 | 1.3 | 1.5 | 1.7 | 1.7 | 1.7 | 1.7 | NR | NR | 1.6 |
| LDL-C, mg/dL | NR | 3.0 | 3.4 | - | 2.9 | 2.8 | 3.2 | 3.0 | 3.1 | 2.6 | 2.7 | 2.6 | 2.4 | 2.5 | NR | NR | 2.6 |
| TG, mg/dL | 1.2 | 1.3 | 1.2 | 1.2 | 0.9 | 0.9 | 1.0 | 0.9 | 1.0 | 1.0 | 0.7 | 0.8 | NR | NR | 0.8 | ||
| % on lipid meds | NR | NR | NR | 7.7 | 27.2 | 27.2 | NR | NR | NR | NR | 9.8 | 41 | - | 35.4 | 34.6 | 12.6 | NR |
| % on BP meds | NR | NR | NR | 26.5 | 37.7 | 37.8 | 5.0 | 8.0 | 32.0 | 34.0 | 28.8 | 40.4 | 19.2 | 28.9 | 21.3 | 13.14 |
A1C = glycosylated hemoglobin; BMI = body mass index; BP = blood pressure; Deriv. = Derivation; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; NR = not reported; PA = Pennsylvania; PC = prospective cohort; Popn = Population; RC = retrospective cohort; SBP = systolic blood pressure; TC = total cholesterol; % = percentage; X-Sec = cross-sectional.
Performance of risk scores for prediction of cardiovascular disease
Together, the studies reported data on the performance of 12 CVD risk prediction models (7 T1DM-specific, 1 T2DM-specific, and 4 general population models) CVD risk prediction models: Steno T1DM risk engine,28 Swedish T1DM risk score,24 ASCVD Risk Equation, Fremantle T1DM Risk Equation,27 silent myocardial infarction (SMI) Risk Model,30 EURODIAB risk score,25 Epidemiology of Diabetes Complication (EDC) risk model (also known as Pittsburgh CHD in T1DM risk model),22 Scottish Care Information (SCI)-Diabetes CVD risk score, United Kingdom Prospective Diabetes Study (UKPDS) Risk Engine,16 Framingham Risk Score (FRS), Joint Societies ASCVD risk score, QRISK3, and the ESC 2019 Risk Classification (Tables 1 and 2).22, 23, 24, 25, 26, 27, 28, 29, 30, 31 The variables included in each of the examined risk models and the corresponding outcomes assessed are shown in Supplemental Table 2. Overall, the CVD event rates ranged from 3 to 27 per 1000-person years across the studies (weighted average 14 per 1000-person years). Only a few studies performed a comprehensive assessment of model prediction including model calibration and model discrimination (Supplemental Table 2).
Table 2.
Outcome Measured, Event Rate, and Risk Prediction Parameters for Studies Reporting Discrimination And/or Calibration Measures
| Cohort | Sex/Deriv. vs Valid. | Male % |
Average Age, y | Composite Outcome | Risk Model | Average Follow-Up, y | No. of Cases | Rate per 1,000 py | Calibration |
E:0 Ratio | C-Statistic (95% CI) Result | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Result | ||||||||||||
| Zgibor, 200622 | EDC | All | 49.9 | 27.3 | Fatal CHD, nonfatal MI | UKPDS | 11.2 | 36 | 6.8 | H-L | 324.1 (P < 0.0001) | NR | 0.76 |
| Zgibor, 200622 | EDC | Male | NR | NR | Fatal CHD, nonfatal MI, Q waves | FRS | 11.2 | 20 | 7.5 | H-L | 310.3 (P < 0.0001) | NR | 0.77 |
| Zgibor, 200622 | EDC | Female | NR | NR | Fatal CHD, nonfatal MI, Q waves | FRS | 11.2 | 16 | 6.1 | H-L | 6,873.9 (P < 0.0001) | NR | 0.87 |
| Zgibor, 201023 | EDC Male | Male/Deriv. | 100 | 27.1 | CHD-related death, fatal/nonfatal MI, Q-waves on EKG | EDCa | 8.0 | 26 | 10.7 | H-L | NR | Graph | 0.84 |
| Zgibor, 201023 | EDC Female | Female/Deriv. | 0 | 27.3 | CHD-related death, fatal/nonfatal MI, Q-waves on EKG | EDCa | 8.0 | 20 | 8.36 | H-L | NR | Graph | 0.89 |
| Zgibor, 201023 | Eurodiab PCS Male |
Male/Deriv. | 100 | 32.1 | CHD-related death, fatal/nonfatal MI, Q-waves on EKG | EDCa | 7.0 | 26 | 3.248 | H-L | NR | Graph | 0.77 |
| Zgibor, 201023 | Eurodiab PCS Female |
Female/Deriv. | 0 | 32.2 | CHD-related death, fatal/nonfatal MI, Q-waves on EKG | EDCa | 7.0 | 27 | 3.252 | H-L | NR | 0.78 | |
| Davis, 201027 | FDS | All | 59.8 | 44 | MI, CVA, Cardiac death, CVA death, sudden death | Fremantle T1D Risk Equation | 5.0 | 6 | 10.5 | H-L | (P = 0.67) | NR | 0.84 |
| Cederholm, 201124 | SNDR - Der | All/Deriv. | 55.6 | 44.6 | CAD, CVA | Swedish T1D Risk Score | 4.9 | 197 | 11.1 | Modified H-L |
0.1 (P = 0.90) | 1.00 | 0.83 |
| Cederholm, 201124 | SNDR - Der | Male/Deriv. | NR | CAD, CVA | Swedish T1D Risk Score | 4.9 | 120 | NR | Modified H-L |
0.6 (P = 0.90) | 0.96 | 0.84 | |
| Cederholm, 201124 | SNDR - Der | Female/Deriv. | NR | CAD, CVA | Swedish T1D Risk Score | 4.9 | 77 | NR | Modified H-L |
3.6 (P = 0.30) | 1.05 | 0.83 | |
| Cederholm, 201124 | SNDR-Val | All/Valid. | NR | 44.6 | CAD, CVA | Swedish T1D Risk Score | 3.9 | 201 | NR | Modified H-L |
0.2 (P = 0.90) | 0.94 | 0.80 |
| Cederholm, 201124 | SNDR-Val | Male/Valid. | NR | CAD, CVA | Swedish T1D Risk Score | 3.9 | 118 | NR | Modified H-L | 0.1 (P = 0.90) | 0.94 | 0.77 | |
| Cederholm, 201124 | SNDR-Val | Female/Valid. | NR | CAD, CVA | Swedish T1D Risk Score | 3.9 | 83 | NR | Modified H-L | 0.1 (P = 0.90) | 0.93 | 0.83 | |
| Soedamah, 201425 | Eurodiab-PCS | All/Deriv. | 52 | 30.3 | CHD/CVA/ESRD/Amputation/Blindness/All-cause Death | EURODIAB | 7.4 | 95 | 6.51 | calibration plots | NR | NR | 0.74 |
| Soedamah, 201425 | EDC-O | All/Valid. | 49 | 28 | CHD/CVA/ESRD/Amputation/Blindness/All-cause Death/nonfatal MI/major Q-waves/fatal and nonfatal CVA | EURODIAB | 8.1 | 98 | 21.83 | calibration plots | NR | NR | 0.79 |
| Soedamah, 201425 | FinnDiane | All/Valid. | 51 | 37.3 | CHD, CVA, ESRD, Amputation, Blindness, All-cause Death | EURODIAB | 7.5 | 315 | 14.0 | calibration plots | NR | NR | 0.82 |
| Soedamah, 201425 | CACTI | All/Valid. | 45 | 36.3 | CHD, CVA, ESRD, Amputation, Blindness, All-cause Death | EURODIAB | 7.3 | 42 | 9.9 | calibration plots | NR | NR | 0.73 |
| Vistisen, 201628 | Steno | All/Deriv. | 54 | 42.2 | CHD, CVA, PVD, HF | Steno T1D Risk Score | 6.8 | 793 | 27.2 | H-L | 12.14 (P = 0.136) | NR | 0.826 (0.807-0.845) |
| Vistisen, 201628 | Funen | All/Valid. | 57.7 | 44.2 | CHD, CVA, PVD, HF | Steno T1D Risk Score | 6.6 | 243 | 17.8 | H-L | 10.9 (P = 0.207) | NR | 0.803 (0.767-0.839) |
| Vistisen, 201628 | Steno | All | 54 | 42.2 | CHD, CVA, PVD, HF | Swedish T1D Risk Score | 6.8 | 793 | 27.2 | H-L | 430.4 (P < 0.001) | NR | 0.794 (0.772-0.816) |
| Vistisen, 2016 28 | Funen | All | 57.7 | 44.2 | CHD, CVA, PVD, HF | Swedish T1D Risk Score | 6.6 | 243 | 17.8 | H-L | 206.9 (P < 0.001) | NR | 0.78 (0.745-0.815) |
| Vistisen, 201628 | Steno | All | 54 | 42.2 | CHD, CVA, PVD, HF | UKPDS Risk Engine | 6.8 | 793 | 27.2 | H-L | 711.8 (P < 0.001) | NR | 0.766 (0.743-0.789) |
| Vistisen, 201628 | Funen | All | 57.7 | 44.2 | CHD, CVA, PVD, HF | UKPDS Risk Engine | 6.6 | 243 | 17.8 | H-L | 210.9 (P < 0.001) | NR | 0.737 (0.699-0.775) |
| Vistisen, 201628 | Steno | All | 54 | 42.2 | CHD, CVA, PVD, HF | ASCVD Risk Equation | 6.8 | 793 | 27.2 | H-L | 402.5 (P < 0.001) | NR | 0.748 (0.724-0.771) |
| Vistisen, 201628 | Funen | All | 57.7 | 44.2 | CHD, CVA, PVD, HF | ASCVD Risk Equation | 6.6 | 243 | 17.8 | H-L | 130.3 (P < 0.001) | NR | 0.748 (0.713-0.789) |
| Llaurado, 201729 | NR | All | 50 | 50.1 | SMI via perfusion stress | SMI Risk Model | NA | 10 | NA | NR | NR | NR | 0.833 (0.692-0.974) |
| Llaurado, 201729 | NR | All | 50 | 50.1 | SMI via perfusion stress | FRS | NA | 10 | NA | NR | NR | NR | 0.688 (0.545-0.83) |
| Llaurado, 201729 | NR | All | 50 | 50.1 | SMI via perfusion stress | UKPDS Risk Engine | NA | 10 | NA | NR | NR | NR | 0.559 (0.424-0.693) |
| Llaurado, 201729 | NR | All | 50 | 50.1 | SMI via perfusion stress | EDCa | NA | 10 | NA | NR | NR | NR | 0.558 (0.352-0.763) |
| Boscari, 202026 | NR | All | 34.5 | 43 | Fatal and nonfatal events of IHD, ischemic stroke, HF, and PAD | Steno T1D Risk Score | 4.7 | 3 | 2.9 | NR | NR | 0.95 | |
| Mcgurnaghan, 202130 | SCI-Diabetes | All | 57 | 35 | MI/Stroke/UA/TIA/PVD or CAD/CVD/PAD revasc or major amputation or ACS | SCI-Diabetes CVD Risk Score | 10 | 2,790 | 14 | H-L | (P = 0.70) | NR | 0.82 (0.81-0.83) |
| Mcgurnaghan, 202130 | SCI-Diabetes | All | 57 | 35 | MI/Stroke/UA/TIA/PVD or CAD/CVD/PAD/ACS | Steno T1D Risk Score | 10 | 2,790 | 14 | H-L | NR | 1.27 | 0.82 (0.81-0.83) |
| Mcgurnaghan, 202130 | SCI-Diabetes | All | 57 | 35 | MI/Stroke/UA/TIA/PVD or CAD/CVD/PAD/ACS | QRISK3 | 10 | 2,790 | 14 | H-L | NR | 0.72 | 0.75 (0.74-0.76) |
| Mcgurnaghan, 202130 | SNDR | All | 54.6 | 31.9 | MI/Stroke/UA/TIA/PVD or CAD/CVD/PAD/ACS | SCI-Diabetes CVD Risk Score | 8.6 | 3,262 | 12.88 | H-L | NR | NR | 0.85 (0.84-0.86) |
| Tecce, 202231 | NR | All | 50 | 30.8 | MI, CABG, stenting, extra coronary bypass, stroke, PAD, major amputations, | Steno T1D Risk Score | 8.5 | 24 | NR | NR | NR | NR | NR |
| Tecce, 202231 | NR | All | 50 | 30.8 | MI, CABG, stenting, extra coronary bypass, stroke, PAD, major amputations, | ESC 2019 Risk Classification | 8.5 | 24 | NR | NR | NR | NR | NR |
AUC = area under receiver operator curve; CABG = coronary artery bypass graft; CAD = coronary heart disease, CHD = coronary heart disease; CVD = cardiovascular disease; CVA = cerebrovascular disease, ESRD = end stage renal disease; IHD = ischemic heart disease; H-L = Hosmer–Lemeshow test of goodness of fit; MI = myocardial infarction; NA = not available; NR = not reported; PAD = peripheral arterial disease; PCI = percutaneous coronary intervention; SMI = silent myocardial infarction; TIA = transient ischemic attack.
EDC also known as Pittsburgh CHD in T1DM risk model.
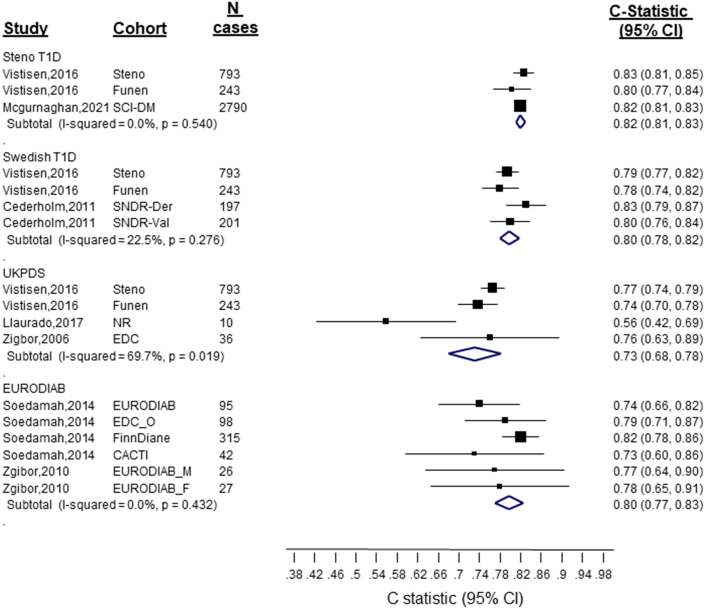
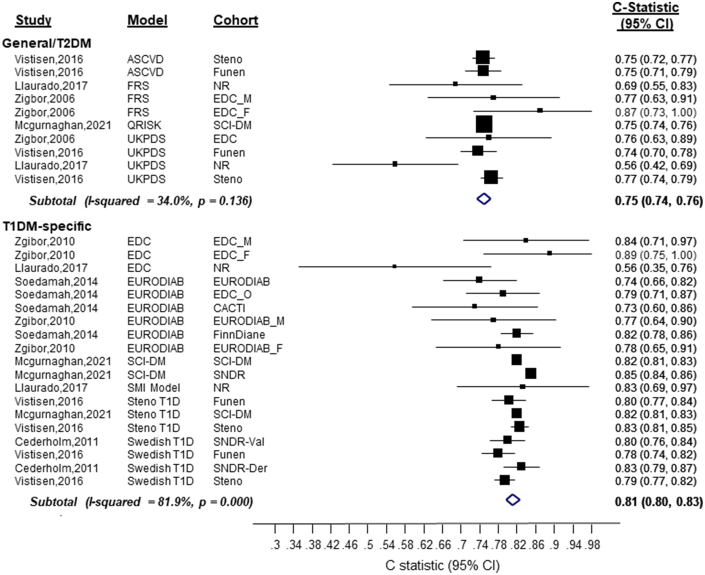
Generally, the models performed moderately or excellently in discriminating,19 with most studies reporting a C statistic (80% the reported estimates) that ranged between 0.73 and 0.85 (Central Illustration, Table 2, Figure 1). The discrimination ability of the T1DM-specific risk models was generally higher than that of the T2DM and general population risk models (Central Illustration, Figures 1 and 2). Specifically, the pooled C-index for the Steno T1DM, Swedish T1DM, EURODIAB, and UKPDS models were as follows: 0.82 (95% CI: 0.81-0.83), 0.80 (95% CI: 0.78-0.82), 0.80 (95% CI: 0.77-0.83), and 0.73 (95% CI: 0.68-0.78), respectively. The pooled C-index of all T1DM-specific risk models (C-statistic: 0.81 [0.80-0.83]) was higher than the pooled C-index of all risk models developed in the general population or among people with T2DM (C-statistic: vs 0.75 [0.74-0.76]) (Figure 2). The sensitivity analyses that included are: 1) pooling the C-statistics using analytical weight based on the number of CVD cases observed in each study (Supplemental Figure 2); or 2) performing a logit transforming the C-statistic measures before pooling them across the studies (Supplemental Figure 3), yielded similar results.
Central Illustration.
Prediction of Cardiovascular Disease Among Individuals With Type 1 Diabetes
Figure 1.
Discrimination of Cardiovascular Disease Risk Scores in People With Type 1 Diabetes
Figure 2.
Discrimination of Cardiovascular Disease Risk Scores in People With T1DM by Model Type (Developed in the General Population/T2DM Individuals vs T1DM-Specific Populations)
In terms of model calibration, the prediction models developed in T1DM cohorts showed good model fit with closely matching observed and expected tests and a nonsignificant H-L test. On the other hand, UKPDS Risk Engine and the general population models were poorly calibrated with actual event rates (observed, O) being significantly higher than predicted event rates (expected, E), with O/E >1 and highly significant H-L test with large chi-square values (Table 2).
In terms of model validation, the authors of Steno T1D Risk score, Swedish T1DM Risk Score, EURODIAB, EDC, and SCI-Diabetes based risk model reported validation studies based on split sample (Swedish T1DM Risk Score) or external validation in one or more T1DM cohorts other than the derivation cohort, which gave comparable results (Table 2).
Data on subgroup analyses of model performance by sex were limited by the number of studies reporting these values but suggested these may also be important factors to consider (Table 2). None of the studies performed an assessment of the comparative performance of several models in the same population with T1DM, using reclassification indices.
The evaluation of the quality of studies in terms of risk of bias using the Newcastle-Ottawa Scale showed that the studies were overall low risk for bias (Supplemental Table 3). None of the examined CVD risk score was studied for its effect on patient management and outcomes in T1DM.
Discussion
In a meta-analysis of observational studies assessing the performance of CVD risk scores among individuals with T1DM, including 11 separate studies involving 73,664 young, predominantly male and White participants, we found that most of the 12 CVD risk scores evaluated had a moderate to excellent discriminatory performance. The models specifically developed in people with T1DM tended to have a higher discriminative ability (which is to say, was more likely to correctly identify individuals who do and do not have a CVD event) and more importantly exhibited a better calibration (ie the ability to accurately predict the risk) than the risk scores initially developed among people with T2DM (eg; UKPDS Risk Engine) or the general population.
That scores for T2DM or the general population exhibited poorer performance suggests that recommendations for managing CVD risk among those with T1DM should not be based on scores developed from individuals with T2DM, as is currently the case.17 The inappropriate reliance of current guidelines for CVD risk on studies conducted in individuals with T2DM is partially explained by the lack of clinical trial evidence on the CVD benefits of lowering CVD risk factors other than HbA1C32 and LDL-cholesterol33 in individuals with T1DM.34 Importantly, these guidelines do not seem to account for the steep gradient of risk associated with most risk factors, even in the seemingly “normal” range (for example; blood pressure35 and triglycerides36) among individuals with T1DM. This suggests that the targets or thresholds for interventions should perhaps be lower than those used in individuals with T2DM or the general population. Using T1DM-specific risk scores can help address these issues, as this could help capture the totality of the risk associated with all risk factors and thus increase the number of high-risk individuals who will benefit from therapies. The results of this rigorous systematic review and meta-analysis on the performance of CVD risk prediction models among individuals with T1DM provides clinicians with much needed data to make informed decisions in the primary prevention of CVD in their T1DM patients.
A limited number of studies have assessed CVD risk prediction score among individuals with T1DM models. The risk scores that were specifically developed among T1DM tended to predict CV risk well, while the ones that were not initially or specifically developed for those with T1DM-specific underpredicted cardiovascular risk. These findings are consistent with current understanding that CVD risk associated with T1DM is driven by factors that are different from that of those in action in people with T2DM or in the general population besides. Some of the T1DM-specific factors may not be adequately captured by general population risk models. Indeed, people with T1DM and T2DM represent 2 very different phenotypes with respect to age at onset, diabetes duration, onset of kidney disease, and lifetime glycemic load.4 Other T1DM-specific factors include for example immunological factors.37 Our study is the first-of-its-kind to synthesize available data on the performance of CVD risk scores in people with T1DM, yielding the most comprehensive and largest assessment on this topic to date. The review provided information on 12 different risk prediction scores, which is more than what has been previously reported by any prior single study. Indeed, our systematic review examined scores across different populations including both men and women across age groups, which improved the statistical power to detect smaller effects.
Our results have important clinical implications as the risk of CVD among T1DM is generally understated or overlooked.9, 10, 11, 12 The findings of a good performance of the T1DM-specific risks score suggest that these models may be used to guide clinicians and health policy makers in assessing CVD risk in people with T1DM. Our results will help to raise awareness about elevated CVD risk among people with T1DM and the need for intensifying primary prevention strategies, as well as stimulate future studies on risk prediction models in this high-risk population. There is a need for an analysis of the effect of the use of the examined CVD risk scores on patient outcomes, a neglected but highly important area. Thus far, no study has assessed the impact of applying the examined CVD prediction model in routine clinical management of individuals with T1DM and ultimately on CVD outcomes. Such an evaluation will facilitate an effective use of these CVD risk score for managing T1DM. Indeed, the current recommendations for managing CVD risk among individuals with T1DM do not incorporate any risk assessment tool.13,17 An extensive validation of the existing T1DM-specific risk models will facilitate their incorporation in clinical guidelines.
Study limitations
The limitations of the study merit further consideration. First, the diagnosis of T1DM was based on physician-reported diagnosis from the medical records and did not include auto-antibody or C-peptide assessment, which are more specific than sensitive. However, prior studies have shown that identifying T1DM using administrative records is valid.38 Second, no data existed on the calibration of the various models, the comparative performance between different models, and thus the reclassification ability of the models. Few studies comprehensive assessed the model’s prediction ability, including both model calibration and model discrimination. Third, there were few studies for each individual risk prediction model compiled in the analysis. Fourth, there was substantial heterogeneity across the studies, with only a few studies contributing data for each risk score, limiting the interpretation of the findings. Most risk scores did not include data on time during which glucose levels remained in range (time-in-range), imaging parameters, lifestyle factors (physical activity or diet), or on biomarkers. Another important predictor of cardiovascular ouctomes that was not always included in the T1DM risk scores is the renal function. This consideration is important, especially in the context of emerging cardiometabolic therapies that are also protective of the kidney. Only limited data were available for non-White populations. For some of the studies, we had to impute the standard error of the estimates of performance of the risk prediction models. The number and design of the included studies limited our ability to conduct relevant subgroups analyses by sex, race/ethnicity, presence or absence of relevant comorbidities.
The limitations of existing studies would need to be addressed in future studies, which would use large-scale data on T1DM, include a more racial or ethnically-mixed populations, simultaneously assess a comprehensive set of CVD risk prediction models, use a larger set of risk prediction measures, and perform head-to-head comparison of the models’ ability to improve CVD risk assessment in T1DM individuals. Performing more external validation studies will also prove useful for models developed in small cohorts. The current evidence could be complemented by modelling studies of CVD outcomes that leverage data from studies such as Diabetes Interventions and Complications Study (DCCT/EDIC) that has an interventional component.39,40
Conclusions
Our systematic review and meta-analysis included CVD prediction models for risk estimation among people with T1DM, which had a moderate to excellent discrimination in their ability to predict higher versus lower CVD risk. In particular, and perhaps as expected, models developed specifically in cohorts comprised exclusively of individuals with T1DM had better performance metrics. Further studies are required that address whether implementing these models into clinical care improves the quality of care and delays or prevents cardiovascular disease.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The performance of CVD disease risk prediction models (T1DM-specific or not) among people with T1DM is unknown. In a systematic review and meta-analysis of 11 observational studies involving 73,664 individuals with T1DM and 12 CVD risk scores, most CVD risk scores had a moderate to excellent discriminative performance, with a better discriminative ability for T1DM-specific scores versus other populations risk scores. T1DM-specific models predicted CVD events with greater accuracy.
TRANSLATIONAL OUTLOOK: The results of this study support the potential utility of CVD risk scores in routine clinical practice for preventing CVD in people with T1DM.
Funding support and author disclosure
Dr Tcheugui was supported by NIH/NHLBI grant K23 HL153774. Dr Erqou is supported by Department of Veterans Affairs, Providence VA Medical Center and Lifespan Cardiovascular Institute. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, figures, and search terms please see the online version of this paper.
Supplementary data
References
- 1.Lind M., Svensson A.-M., Kosiborod M., et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 2.Bjornstad P., Donaghue K.C., Maahs D.M. Macrovascular disease and risk factors in youth with type 1 diabetes: time to be more attentive to treatment? Lancet Diabetes Endocrinol. 2018;6(10):809–820. doi: 10.1016/S2213-8587(18)30035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P., Nathan D.M., Abraham K., et al. Report of the national heart, lung, and blood institute-national Institute of diabetes and digestive and kidney diseases working group on cardiovascular complications of type 1 diabetes mellitus. Circulation. 2005;111(25):3489–3493. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- 4.Rosengren A., Dikaiou P. Cardiovascular outcomes in type 1 and type 2 diabetes. Diabetologia. 2023;66(3):425–437. doi: 10.1007/s00125-022-05857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juutilainen A., Lehto S., Rönnemaa T., Pyörälä K., Laakso M. Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care. 2008;31(4):714–719. doi: 10.2337/dc07-2124. [DOI] [PubMed] [Google Scholar]
- 6.Huxley R.R., Peters S.A.E., Mishra G.D., Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(3):198–206. doi: 10.1016/S2213-8587(14)70248-7. [DOI] [PubMed] [Google Scholar]
- 7.Ohkuma T., Komorita Y., Peters S.A.E., Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia. 2019;62(9):1550–1560. doi: 10.1007/s00125-019-4926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAllister D.A., Read S.H., Kerssens J., et al. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018;138(24):2774–2786. doi: 10.1161/CIRCULATIONAHA.118.034986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah V.N., Grimsmann J.M., Foster N.C., et al. Undertreatment of cardiovascular risk factors in the type 1 diabetes exchange clinic network (United States) and the prospective diabetes follow-up (Germany/Austria) registries. Diabetes Obes Metabol. 2020;22(9):1577–1585. doi: 10.1111/dom.14069. [DOI] [PubMed] [Google Scholar]
- 10.Varkevisser R.D.M., Birnie E., Vollenbrock C.E., et al. Cardiovascular risk management in people with type 1 diabetes: performance using three guidelines. BMJ Open Diabetes Res Care. 2022;10(4) doi: 10.1136/bmjdrc-2022-002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood J.R., Miller K.M., Maahs D.M., et al. Most youth with type 1 diabetes in the T1D exchange clinic registry do not meet American diabetes association or international society for pediatric and adolescent diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035–2037. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels M., DuBose S.N., Maahs D.M., et al. Factors associated with microalbuminuria in 7,549 children and adolescents with type 1 diabetes in the T1D Exchange clinic registry. Diabetes Care. 2013;36(9):2639–2645. doi: 10.2337/dc12-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Ferranti S.D., de Boer I.H., Fonseca V., et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014;130(13):1110–1130. doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 14.Goff D.C., Lloyd-Jones D.M., Bennett G., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan S.S., Coresh J., Pencina M.J., et al. Novel prediction equations for absolute risk assessment of total cardiovascular disease incorporating cardiovascular-kidney-metabolic health: a scientific statement from the American heart association. Circulation. 2023;148(24):1982–2004. doi: 10.1161/CIR.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 16.Stevens R.J., Kothari V., Adler A.I., Stratton I.M., Holman R.R. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56) Clin Sci. 2001;101(6):671–679. [PubMed] [Google Scholar]
- 17.American Diabetes Association Professional Practice Committee 10. Cardiovascular disease and risk management: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179–S218. doi: 10.2337/dc24-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lloyd-Jones D.M. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121(15):1768–1777. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 20.Collins G.S., Reitsma J.B., Altman D.G., Moons K.G.M. Transparent reporting of a multivariable prediction model for individual Prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 21.Lo C.K.-L., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zgibor J.C., Piatt G.A., Ruppert K., Orchard T.J., Roberts M.S. Deficiencies of cardiovascular risk prediction models for type 1 diabetes. Diabetes Care. 2006;29(8):1860–1865. doi: 10.2337/dc06-0290. [DOI] [PubMed] [Google Scholar]
- 23.Zgibor J.C., Ruppert K., Orchard T.J., et al. Development of a coronary heart disease risk prediction model for type 1 diabetes: the Pittsburgh CHD in Type 1 Diabetes Risk Model. Diabetes Res Clin Pract. 2010;88(3):314–321. doi: 10.1016/j.diabres.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cederholm J., Eeg-Olofsson K., Eliasson B., Zethelius B., Gudbjörnsdottir S. A new model for 5-year risk of cardiovascular disease in Type 1 diabetes; from the Swedish National Diabetes Register (NDR) Diabet Med. 2011;28(10):1213–1220. doi: 10.1111/j.1464-5491.2011.03342.x. [DOI] [PubMed] [Google Scholar]
- 25.Soedamah-Muthu S.S., Vergouwe Y., Costacou T., et al. Predicting major outcomes in type 1 diabetes: a model development and validation study. Diabetologia. 2014;57(11):2304–2314. doi: 10.1007/s00125-014-3358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boscari F., Morieri M.L., Amato A.M.L., et al. Performance of the Steno type 1 risk engine for cardiovascular disease prediction in Italian patients with type 1 diabetes. Nutr Metabol Cardiovasc Dis. 2020;30(10):1813–1819. doi: 10.1016/j.numecd.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Davis W.A., Davis T.M.E. Cardiovascular risk prediction in adults with type 1 diabetes: the Fremantle Diabetes Study. Diabetes Res Clin Pract. 2010;90(3):e75–e78. doi: 10.1016/j.diabres.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Vistisen D., Andersen G.S., Hansen C.S., et al. Prediction of first cardiovascular disease event in type 1 diabetes mellitus: the Steno type 1 risk engine. Circulation. 2016;133(11):1058–1066. doi: 10.1161/CIRCULATIONAHA.115.018844. [DOI] [PubMed] [Google Scholar]
- 29.Llauradó G., Cano A., Hernández C., et al. Type 1 diabetes: developing the first risk-estimation model for predicting silent myocardial ischemia. The potential role of insulin resistance. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGurnaghan S.J., McKeigue P.M., Read S.H., et al. Development and validation of a cardiovascular risk prediction model in type 1 diabetes. Diabetologia. 2021;64(9):2001–2011. doi: 10.1007/s00125-021-05478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tecce N., Masulli M., Palmisano L., et al. Performance of 2019 ESC risk classification and the Steno type 1 risk engine in predicting cardiovascular events in adults with type 1 diabetes: a retrospective study. Diabetes Res Clin Pract. 2022;190 doi: 10.1016/j.diabres.2022.110001. [DOI] [PubMed] [Google Scholar]
- 32.Nathan D.M., Cleary P.A., Backlund J.-Y.C., et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins R., Armitage J., Parish S., Sleigh P., Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 34.Manrique-Acevedo C., Hirsch I.B., Eckel R.H. Prevention of cardiovascular disease in type 1 diabetes. N Engl J Med. 2024;390(13):1207–1217. doi: 10.1056/NEJMra2311526. [DOI] [PubMed] [Google Scholar]
- 35.Guo J., Brooks M.M., Muldoon M.F., Naimi A.I., Orchard T.J., Costacou T. Optimal blood pressure thresholds for minimal coronary artery disease risk in type 1 diabetes. Diabetes Care. 2019;42(9):1692–1699. doi: 10.2337/dc19-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolonen N., Forsblom C., Mäkinen V.-P., et al. Different lipid variables predict incident coronary artery disease in patients with type 1 diabetes with or without diabetic nephropathy: the FinnDiane study. Diabetes Care. 2014;37(8):2374–2382. doi: 10.2337/dc13-2873. [DOI] [PubMed] [Google Scholar]
- 37.Sousa G.R., Pober D., Galderisi A., et al. Glycemic control, cardiac autoimmunity, and long-term risk of cardiovascular disease in type 1 diabetes mellitus. Circulation. 2019;139(6):730–743. doi: 10.1161/CIRCULATIONAHA.118.036068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisman A., Tu K., Young J., et al. Validation of a type 1 diabetes algorithm using electronic medical records and administrative healthcare data to study the population incidence and prevalence of type 1 diabetes in Ontario, Canada. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mount Hood 4 Modeling Group Computer modeling of diabetes and its complications: a report on the fourth mount hood challenge meeting. Diabetes Care. 2007;30(6):1638–1646. doi: 10.2337/dc07-9919. [DOI] [PubMed] [Google Scholar]
- 40.Miller R.G., Costacou T., Orchard T.J. Risk factor modeling for cardiovascular disease in type 1 diabetes in the Pittsburgh Epidemiology of diabetes complications (EDC) study: a comparison with the diabetes control and complications trial/epidemiology of diabetes interventions and complication. Diabetes. 2019;68(2):409–419. doi: 10.2337/db18-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.