Simple Summary
Our study evaluates how well anti-PD-1 immunotherapy works for melanoma patients in routine clinical practice, as previous clinical trials may not fully reflect daily outcomes. We focused on patients who had melanoma surgically removed and then received anti-PD-1 therapy to prevent the cancer from returning. By reviewing data from 245 patients from several centers, we confirmed that real-world survival rates are lower than those reported in trials and identified factors that may influence recurrence, such as the location of the original primary tumor or delays in initiating therapy after surgery. This highlights the importance of real-world studies and provides insight into current needs to improve patient outcomes.
Keywords: melanoma, adjuvant immunotherapy, survival outcomes, prognostic factors, patterns of relapse
Abstract
Background: Anti-PD-1-based immunotherapy has improved outcomes in stage IIB to IV resected melanoma patients in clinical trials. However, little is known about real-world outcomes, prognostic factors and patterns of relapse. Methods: This is a retrospective multicenter observational study including patients with resected melanoma treated with subsequent anti-PD-1-based adjuvant immunotherapy. Data on clinical and demographic characteristics, delivered treatment, prognostic factors, time and pattern of relapse were collected. Results: We included 245 patients from eight centers; 4% of patients were at stage IIB-C, 80% at stage IIIA-D and 16% at stage IV. Recurrence-free survival (RFS) rates at 18 and 36 months were 60% and 48%, respectively, with a median RFS of 33.7 months. Prognostic factors associated with recurrence were melanoma primary site (HR 2.64, 95% CI 1.15–6.01) and starting adjuvant therapy more than 12 weeks after the last resection (HR 1.68, 95% CI 1.13–2.5); presence of serious immune-related adverse events was associated with better RFS (HR 0.4, 95% CI 0.19–0.87). Early relapses accounted for 63% of the total recurrences, with a higher number of metastatic sites (18%); in contrast, late relapses presented more frequently with brain metastases (20%). Conclusions: In our patients with resected melanoma who underwent anti-PD-1-based adjuvant immunotherapy, survival outcomes were worse than those reported in clinical trials. Primary melanoma site and time interval between the last resection and the start of adjuvant therapy were associated with survival.
1. Introduction
Cutaneous melanoma accounts for 325,000 new diagnoses worldwide, with age-standardized incidence rates ranging from 14 (dark-skinned populations) to 42 (fair-skinned populations) per 100,000 person-years [1]. The prognosis of patients diagnosed with advanced melanoma has dramatically improved since the introduction of anti-PD-1-based immunotherapy and targeted therapy with BRAF and MEK inhibitors for BRAF-mutated patients [2,3,4,5].
These treatments have also improved outcomes after complete resection in the adjuvant setting. Previously, ipilimumab improved recurrence-free survival (RFS), distant metastasis-free survival (DMFS) and overall survival (OS) compared with placebo in stage III, although 7-year RFS rate was 39.2% [6]. Subsequently, nivolumab showed better RFS and DMFS with less toxicity compared with ipilimumab in stage III, while pembrolizumab improved RFS and DMFS compared with placebo [7,8,9,10,11,12]. More recently, both pembrolizumab and nivolumab improved RFS compared with placebo in stage II, although longer follow-up is needed to determine other outcomes [13,14]. Last, the combination of ipilimumab and nivolumab has also shown superior efficacy in stage IV but not in stage III [15,16]. Regarding targeted therapy, dabrafenib and trametinib also improved RFS and DMFS compared to placebo in BRAF-mutated stage III melanoma [17].
Despite these advances, recurrence rates in clinical trials are far from ideal, with 50% of patients experimenting recurrences 5–7 years [9,12]. Moreover, a review of real-world studies suggests that this rate may be higher, indicating discrepancies between real-world practice and the outcomes observed in clinical trials [18].
Several real-world studies have reported on survival outcomes and treatment of early relapse in patients with resected stage III melanoma treated with adjuvant anti-PD-1 monotherapy [18,19,20]. However, there is limited evidence regarding prognostic factors and patterns of relapse. Additionally, little is known about patients in real-world settings who have stage IIB-IIC or IV melanoma treated with anti-PD-1-based combination immunotherapy.
This study aims to describe post-treatment and survival outcomes for melanoma patients who have undergone complete resection followed by adjuvant immunotherapy in a real-world setting. Additionally, it also seeks to evaluate prognostic factors influencing these outcomes and to describe patterns of relapse observed in this patient population.
2. Materials and Methods
2.1. Study Design
This is an observational retrospective multicenter study that collected data from 8 different Spanish sites. The study protocol was approved by the institutional ethics review board, and all patient data were anonymized upon entry into the database.
Eligible patients were adults (18 years or older), with a histologically confirmed melanoma with resection of all known disease lesions, and had received at least one dose of adjuvant anti-PD-1-based therapy (either as monotherapy or in combination with another immunotherapy). Patients with less than nine months of follow-up post-treatment initiation or with uveal melanoma were excluded from the study.
Patients were enrolled consecutively from local registries if they met all the inclusion criteria and no exclusion criteria. The sample size was not restricted due to the exploratory nature of the study.
2.2. Outcomes and Asessments
Collected data included patient demographics and disease characteristics at the start of adjuvant therapy, details of the treatment regimen and its timing, observed toxicities, pattern of relapse and survival outcomes. Adverse events were graded using the Common Terminology of Cancer Adverse Events (CTCAE) criteria, version 5.0.
Locoregional relapses were defined as those occurring at the previous primary surgical site, regional skin, subcutaneous tissue or lymph nodes, while distant relapses were defined as those occurring at any other location, even if detected at a previously resected metastatic site.
Recurrences were classified as early relapse (primary resistance) if they occurred during adjuvant treatment or within 12 weeks after treatment completion, in accordance with the Society for Immunotherapy in Cancer (SITC) criteria [21].
Survival times were calculated from the start date of therapy and the occurrence of the following events: recurrence-free survival (RFS), defined by the date of first recurrence or death; distant metastasis-free survival (DMFS), marked by the first distant recurrence or death; time to next treatment (TTNT), defined by the date of initiation of the next systemic therapy for melanoma or death; and overall survival (OS), defined by the date of death. If a patient’s date of death was not documented, survival times were censored at the last recorded date the patient was known to be alive.
2.3. Statistical Analysis
Descriptive statistics were used to assess baseline demographics characteristics, disease-related variables and treatment-related data. For continuous variables, results are reported as median with ranges, while categorical measures are summarized by patient counts and percentages.
The Kaplan–Meier method was employed to estimate median survival times (with 95% confidence intervals, CI) for RFS, DMFS, TTNT and OS, as well as to generate survival curves. Survival rates at 18 and 36 months (with 95% CI) were calculated using an actuarial method. The reverse Kaplan–Meier method was used to determine the median follow-up time and descriptive statistics were used to report the minimum follow-up duration and range.
The log-rank test was used to compare survival times based on different clinical subgroups, and the single-variable Cox proportional hazards regression method was used to calculate univariate hazard ratio (HR). For time variables where median survival was not reached, subgroup comparisons using the log-rank test and HR were considered informative if each subgroup had 10% or more of events. For clinical subgroups with a significant association with survival, a multivariate Cox regression analysis was conducted. A time-dependent Cox regression analysis was conducted to evaluate the relationship between toxicity and survival, adjusting potential biases due to different treatment exposure.
A significance threshold of p < 0.05 was set for all analyses, which were conducted using SPSS (PAWS statistical software), version 29.0.
3. Results
3.1. Patients and Treatment Characteristics
A total of 245 patients were included in the study from 1 July 2017 to 30 June 2022. Baseline characteristics are summarized in Table 1. The median age was 59 years, with 61% of patients under the age of 65, and 58% were male. The majority of patients (82%) had a cutaneous melanoma primary site, and 44% of the patients presented BRAF V600 mutations. According to the 8th edition of the AJCC-TNM classification, 4% of patients were at stage IIB-C, 80% at stage IIIA-D and 16% at stage IV.
Table 1.
Baseline patients and disease characteristics.
| Patients (N: 245) | |
|---|---|
| N (%)/Median [Range] | |
| Sex | |
| - Male | 142 (58%) |
| - Female | 103 (42%) |
| Age (years old) | 59 [19–84] |
| - <65 | 150 (61%) |
| - 139 | 66 (27%) |
| - ≥75 | 29 (12%) |
| Primary site | |
| - Cutaneous melanoma | 202 (82%) |
| - Acral melanoma | 21 (9%) |
| - Mucosal melanoma | 7 (3%) |
| - Unknown primary | 15 (6%) |
| BRAF status | |
| - BRAF wild type | 116 (47%) |
| - BRAF V600 mutation | 108 (44%) |
| - Unknown | 21 (9%) |
| Breslow index (mm) | 3.7 [0–296] |
| - <0.8 | 20 (8%) |
| - 0.8–2 | 32 (13%) |
| - 5 | 44 (18%) |
| - 6 | 31 (13%) |
| - >4 | 103 (42%) |
| - Unknown | 15 (6%) |
| Ulceration | |
| - Absent | 87 (35%) |
| - Present | 136 (56%) |
| - Unknown | 22 (9%) |
| LDH (IU/dL) | 186 [105–545] |
| - <ULN | 111 (45%) |
| - ULN—2xULN | 99 (40%) |
| - 2xULN–5xULN | 29 (12%) |
| - >5xULN | 6 (3%) |
| Mitosis (1/mm2) | |
| - <1 | 31 (13%) |
| - 11 | 118 (48%) |
| - ≥10 | 66 (27%) |
| - Unknown | 30 (12%) |
| Stage (8th AJCC edition) | |
| - IIB | 4 (2%) |
| - IIC | 5 (2%) |
| - IIIA | 15 (6%) |
| - IIIB | 47 (19%) |
| - IIIC | 120 (49%) |
| - IIID | 14 (6%) |
| - IV | 40 (16%) |
AJCC: American Joint Committee on Cancer; IU: international units; LDH: lactate dehydrogenase; mm: millimeters; N: number of patients; ULN: upper limit of normality.
Treatment details are summarized in Table 2. The majority of patients (87%) received immune checkpoint inhibitor (ICPI) monotherapy, with nivolumab being the most common (74%). The median duration of treatment was 11.7 months [0.7–13.1 months]. In 78% of patients, adjuvant therapy began within 12 weeks of their last surgical resection. Reasons for treatment discontinuation were treatment completion (53%), relapse (32%) and toxicity (12%).
Table 2.
Treatment characteristics.
| Patients | |
|---|---|
| N (%)/Median [Range] | |
| ICPI Treatment | |
| - Anti-PD-1 monotherapy | 214 (87%) |
| - Nivolumab | 182 (74%) |
| - Pembrolizumab | 23 (9%) |
| - Other | 9(4%) |
| - Anti-PD-1-based combination | 31 (13%) |
| - Ipilimumab–Nivolumab | 15 (6%) |
| - Other combination | 16 (7%) |
| Time on treatment (months) | 11.7 [0.7–13.1] |
| Interval from last surgery initiation of adjuvant treatment | |
| <12 weeks | 192 (78%) |
| >12 weeks | 53 (22%) |
| End of treatment | |
| - Completion | 129 (53%) |
| - Toxicity | 30 (12%) |
| - Relapse | 79 (32%) |
| - Ongoing | 2 (1%) |
| - Unknown | 5 (2%) |
ICPI: immune checkpoint inhibitor; PD-1: programmed death-1; N: number of patients.
3.2. Survival and Prognostic Factors
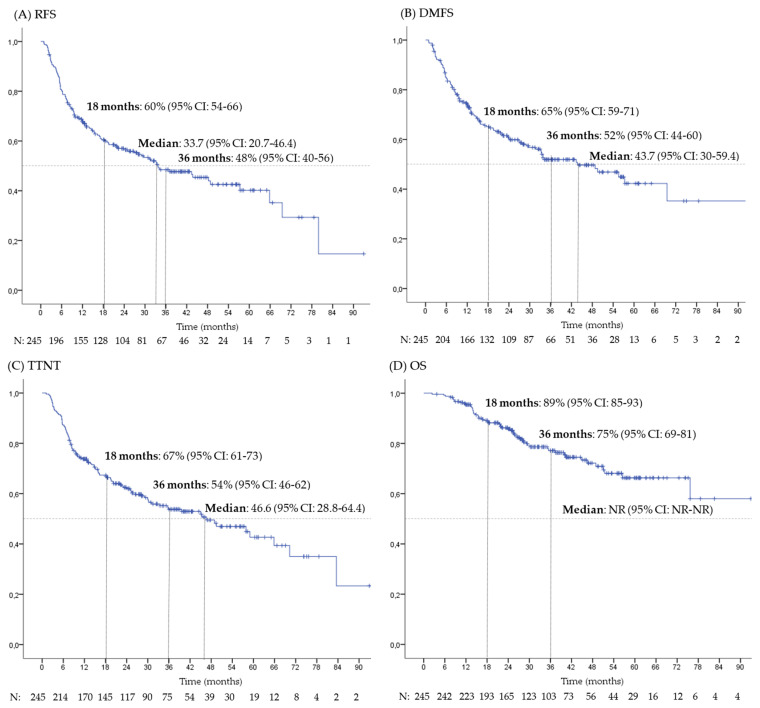
The median follow-up period was 38.4 months (range 2–139, 95% CI: 35.7–41), with a minimum follow-up of 9 months for censored patients. Kaplan–Meier survival curves for RFS, DMFS, TTNT and OS are shown in Figure 1.
Figure 1.
Survival outcomes. Kaplan–Meier curves for (A) recurrence-free survival (RFS); (B) distant metastasis-free survival (DMFS); (C) time to next treatment (TTNT); (D) overall survival (OS). Median times are reported in months. CI: confidence interval; NR: not reached.
At data cut-off, 123 patients (50%) had presented recurrence. The median RFS was 33.7 months (95% CI: 20.7–46.4). Actuarial RFS rates at 18 and 36 months were 60% (95% CI: 54–66%) and 48% (95% CI: 40–56%), respectively. The median RFS according to different subgroups is presented in Table 3. In the univariate analysis, the primary site of melanoma, disease stage and interval from last resection to the start of adjuvant treatment were identified as predictors of RFS. In the multivariate analysis, a significant association was observed between RFS and both primary site of melanoma and time interval from last resection to initiation of adjuvant treatment.
Table 3.
Recurrence-free survival (RFS): median RFS and univariate and multivariate analysis according to patient and disease characteristics.
| RFS | Univariate HR | p-Value * | Multivariate HR | |
|---|---|---|---|---|
| Median (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Sex | ||||
| - Male | 37.1 (22.9–51.4) | Reference | ||
| - Female | 26.9–14.2–39.5) | 1.13 (0.79–1.62) | 0.49 | |
| Age (years old) | 0.39 | |||
| - <65 | 33.4 (25.7–41) | Reference | ||
| - 65–74 | 79.9 (79.9-NR) | 0.96 (0.66–1.39) | 0.76 | |
| - ≥75 | 15.4 (0–37.4) | 1.47 (0.9–2.4) | 0.12 | |
| Primary site of melanoma | 0.01 | 2.64 (1.15–6.01) p = 0.02 |
||
| - Cutaneous melanoma | 43.4 (26.1–60.7) | Reference | ||
| - Acral melanoma | 14.5 (6.3–22.6) | 1.72 (0.96–3.08) | 0.07 | |
| - Mucosal melanoma | 9.2 (1.8–16.6) | 3.15 (1.37–7.25) | <0.01 | |
| - Unknown origin | 28 (6.2–49.7) | 1.39 (0.7–2.76) | 0.35 | |
| BRAF status | ||||
| - BRAF wild type | 33.2 (23.18–43.2) | Reference | ||
| - BRAF V600 mutant | 19.1 (3.1–35) | 1.3 (0.9–1.84) | 0.16 | |
| Breslow index (mm) | 0.81 | |||
| - <0.8 | NR (NR-NR) | Reference | ||
| - 0.8–2 | 43.4 (43.4-NR) | 1.01 (0.91–2.15) | 0.81 | |
| - 5 | 33.8 (16.6–51) | 1.25 (0.66–2.39) | 0.5 | |
| - 6 | 24 (0-NR) | 1.32 (0.65–2.68) | 0.44 | |
| - >4 | 37.1 (15.4–58.9) | 1.08 (0.6–1.93) | 0.8 | |
| Ulceration | ||||
| - Absent | 57.3 (32.7–81.9) | Reference | ||
| - Present | 31.2 (19.9–42.5) | 1.29 (0.87–1.92) | 0.21 | |
| LDH | 0.47 | |||
| - <ULN | 43.7 (26.6–60.8) | Reference | ||
| - ULN—2xULN | 19.5 (11.5–27.5) | 1.37 (0.94–2) | 0.11 | |
| - 2xULN–5xULN | 79.9 (49.2–110.7) | 0.53 (0.27–1.06) | 0.07 | |
| - >5xULN | 29.7 (16.1–43.3) | 1.15 (0.79–1.66) | 0.46 | |
| Mitosis (/mm2) | 0.96 | |||
| - <1 | 48.8 (48.7-NR) | Reference | ||
| - 11 | 37.1 (14.8–59.5) | 0.93 (0.52–1.64) | 0.79 | |
| - ≥10 | 33.8(19.1–48.4) | 0.95 (0.51–1.77) | 0.87 | |
| Stage (8th AJCC edition) | 1.5 (1.01–2.37) | 0.04 | 1.5 (0.98–2.33) p = 0.06 |
|
| - IIB-IIC | NR (NR-NR) | Reference | ||
| - IIIA-IIIB | 48.3 (29.8–66.7) | |||
| - IIIA | 43.4 (6.2–80.6) | 2.66 (0.32–22.14) | 0.37 | |
| - IIIB | 48.3 (24.7–71.8) | 4.89 (0.65–36.51) | 0.12 | |
| - IIIC-IIIID | 23.9 (11.7–36.2) | |||
| - IIIC | 24 (11.2–36.7) | 7.06 (0.98–50.86) | 0.05 | |
| - IIID | 19.1 (5.7–42.7) | 7.01 (0.88–55.57) | 0.06 | |
| - IV | 31.7 (20.6–42.7) | 6.74 (0.91–49.77) | 0.06 | |
| Interval from last resection to initiation of adjuvant treatment | ||||
| <12 weeks | 43.7 (31.5–55.9) | Reference | 1.68 (1.13–2.5) p = 0.01 |
|
| >12 weeks | 14.7 (8.1–21.3) | 1.64 (1.11–2.44) | 0.01 |
* p-values <0.05 are marked in bold. AJCC: American Joint Committee on Cancer; CI: confidence interval; HR: hazard ratio; IU: international units; LDH: lactate dehydrogenase; mm: millimeters; N: number of patients; NR: not reached; mRFS: median recurrence free survival; ULN: upper limit of normality.
At the time of data cut-off, 111 patients (45%) had developed distant metastases. The median DMFS was 43.7 months (95% CI: 30–59.4). Actuarial DMFS rates at 18 and 36 months were 65% (95% CI: 59–71%) and 52% (95% CI: 44–60%), respectively. The median DMFS according to different subgroups is presented in Supplementary Table S1. Primary site of melanoma was the only factor associated with DMFS.
At data cut-off, 113 patients (46%) had initiated additional systemic treatment with a median TTNT of 46.6 months (95% CI: 28.8–64.4).
Additionally, at data cut-off, fifty-six patients (23%) had died due to melanoma while three patients (1%) patients had died of unrelated causes. The median OS was not reached. Actuarial OS rates at 18 and 36 months were 89% (95% CI: 85–93%) and 75% (95% CI: 69–81%), respectively. The primary site of melanoma, the presence of ulceration and the stage of the disease were identified as predictors of OS. In the multivariate analysis, a significant association was found between OS and primary site of melanoma (Supplementary Table S2).
3.3. Toxicities
A summary of the observed toxicities is provided in Table 4. Serious adverse events (Grade 3 or higher) occurred in 11% of patients with no treatment-related deaths. Persistent toxicities were documented in 12% of cases, most commonly hypothyroidism (5%).
Table 4.
Immune-related adverse events (irAEs) from adjuvant anti-PD-1 therapy in patients with resected melanoma.
| Any Grade | Grade 3–4 | |
|---|---|---|
| N (%) | N (%) | |
| - No toxicity | 135 (55%) | 218 (89%) |
| - Any toxicity | 110 (45%) | 27 (11%) |
| Toxicity * | ||
| - Cutaneous toxicity | 76 (31%) | 1 (<1%) |
| - Hypothyroidism | 12 (5%) | 0 |
| - Colitis | 10 (4%) | 4 (2%) |
| - Arthritis | 9 (4%) | 1 (<1%) |
| - Hepatic toxicity | 8 (3%) | 7 (3%) |
| - Pneumonitis | 8 (3%) | 2 (1%) |
| - Nephritis | 4 (2%) | 3 (1%) |
| - Hypophysitis | 1 (<1%) | 1 (<1%) |
| - Pancreatic toxicity | 1 (<1%) | 1 (<1%) |
| - Adrenal insufficiency | 1 (<1%) | 1 (<1%) |
| - Gastritis | 1 (<1%) | 1 (<1%) |
| - Myositis | 1 (<1%) | 1 (<1%) |
| - Myocarditis | 1 (<1%) | 1 (<1%) |
| - Myelitis | 1 (<1%) | 1 (<1%) |
* Patients may have more than one toxicity. N: number of patients.
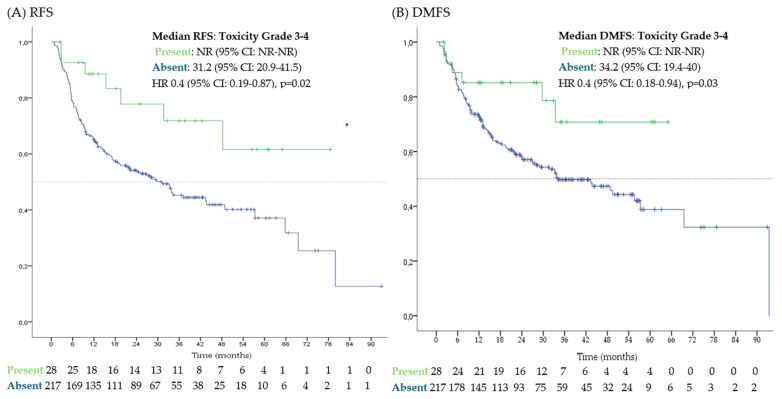
The presence of serious immune-related adverse events (irAEs) (grade 3 or 4) was also associated with longer RFS (median not reached [NR; 95% CI NR-NR] vs. 31.2 months [95% CI 20.9–41.5] if absence of irAEs, HR 0.4 [95% CI: 0.19–0.87]) and DMFS (median NR [95% CI NR-NR] vs. 34.2 months [95% CI 19.4–40] if absence of irAES, HR 0.4 [95% 0.18–0.94]). Figure 2 presents survival curves of patients with and without serious irAEs. No differences on RFS or DMFS were observed between different immunotherapies (p = 0.06) or between the use of monotherapy vs. combination (p = 0.22).
Figure 2.
Survival outcomes according to the presence or absence of severe adverse events. Kaplan–Meier curves for (A) recurrence-free survival (RFS); (B) distant metastasis-free survival (DMFS). CI: confidence interval; HR: hazard ratio; NR: not reached.
3.4. Patterns of Relapse
Among the 123 patients who experienced recurrence, 29 (24%) had locoregional relapse, 70 (57%) had systemic relapse and 24 (19%) had both locoregional and systemic relapse. The most common distant metastatic sites were distant lymph nodes (74 patients, 60%) and soft tissue (46 patients, 37%), while liver and brain metastases were present in 14 (11%) and 18 (15%) patients, respectively. The number of metastatic sites was less than three in 104 patients (85%).
Early relapse was observed in 78 patients (32% of all patients, 63% of patients with recurrence). Patterns of relapse according to time of relapse are shown in Table 5.
Table 5.
Melanoma recurrence patterns according to time and site of relapse after adjuvant therapy.
| All Patients with Relapse | Primary Resistance/Early Relapse | Late Relapse | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| N = 123 | N = 78 | N = 45 | |
| Location of relapse | |||
| - Locoregional relapse | 29 (24%) | 18 (23%) | 11 (24%) |
| - Systemic relapse only | 70 (57%) | 40 (51%) | 30 (66%) |
| - Locoregional and systemic | 24 (19%) | 20 (26%) | 4 (9%) |
| Systemic relapse: affected organs * | |||
| - Distant lymph nodes | 74 (60%) | 49 (63%) | 25 (55%) |
| - Soft tissues | 46 (37%) | 34 (43%) | 12 (26%) |
| - Bone | 21 (17%) | 12 (15%) | 9 (20%) |
| - Lung and pleura | 35 (28%) | 20 (26%) | 15 (33%) |
| - Liver | 14 (11%) | 12 (15%) | 2 (4%) |
| - Brain | 18 (15%) | 7 (9%) | 11 (24%) |
| - Others ** | 10 (8%) | 8 (10%) | 2 (4%) |
| Stage at recurrence | |||
| - M0 | 29 (24%) | 15 (19%) | 11 (24%) |
| - M1a | 50 (41%) | 39 (50%) | 14 (31%) |
| - M1b | 12 (10%) | 4 (5%) | 8 (18%) |
| - M1c | 16 (13%) | 13 (17%) | 3 (7%) |
| - M1d | 16 (13%) | 7 (9%) | 9 (20%) |
| Number of metastatic sites | |||
| - Less than 3 | 104 (85%) | 64 (82%) | 40 (89%) |
| - 3 or more | 19 (15%) | 14 (18%) | 5 (11%) |
* Patients may present more than one affected organ. ** Including peritoneal, adrenal and splenic metastases. N: number of patients.
4. Discussion
The current retrospective multicenter observational study of patients treated with adjuvant immunotherapy for resected melanoma reports lower 18- and 36-month RFS, DMFS and OS rates and shorter median RFS and DMFS than those reported in clinical trials [7,8,9,10,11,12]. Negative prognostic factors for RFS were time from last resection to the start of adjuvant therapy (>12 weeks) and primary site of melanoma (especially mucosal melanoma). Serious immune-related adverse event (irAE) rates were consistent with those observed in clinical trials, and their presence were also associated with better RFS and DMFS. Additionally, early relapses presented with a higher number of metastatic sites than late relapses, although late relapses were more likely to present with brain metastases.
Our inferior survival results (compared to those in clinical trials) are in line with the recently reported 18-month RFS data in patients with stage III melanoma treated with adjuvant pembrolizumab in real-world setting [18]. Additionally, our study also provides information about DMFS, OS and 36-month RFS rates, which were also lower than those reported from clinical trials. Those findings support the relevance of real-world data to study the outcomes in an everyday practice population together with clinical trials.
One possible explanation for those lower outcomes may be the higher incidence of negative prognostic factors in real-world data studies compared with clinical trials. Interestingly, similar 12-month RFS rates have been reported when patients with similar characteristics to clinical trials are included [22]. In addition, similar OS to that reported in the Checkmate-238 trial has been reported in a real-world population, although RFS and DMFS data are lacking and the median follow-up for the real-world cohort of patients was 25.5 months, and data on OS data may not be mature enough [20].
The patients included in the present study have a higher median age than those of clinical trials and also include resected stage IV and mucosal melanoma in similar proportions to Checkmate-238, while these populations were excluded in the Keynote-054 trial [7,10]. In addition, this study included 22% of patients who started adjuvant therapy more than 12 weeks after their last resection, who were also excluded from clinical trials [7,10]. We did not confirm that stage was an independent prognostic factor, and the median RFS was similar in stage IV to that of the overall population, as observed in Checkmate-238 [10], but recent studies suggest the benefit of more aggressive treatment by adding ipilimumab to nivolumab in resected stage IV melanoma [16]. Our findings confirm the negative prognostic role of mucosal melanoma and suggest another subgroup of patients who may be candidates for treatment intensification with immunotherapy combinations or newer approaches, which should be explored in future clinical trials. Our analysis also evaluates the negative impact of delaying the start of therapy beyond 12 weeks after last resection, pointing out that efforts should be made in patient workflows to avoid delays in surgical recovery, oncologist referrals and start of therapy. In contrast, the presence of serious irAEs, although slightly lower than those reported in the clinical trials (14% in both Keynote-054 and Checkmate-238 [7,10]), was also associated with better RFS and DMFS, consistent with previous reports [23]. This evidence on prognostic factors may be relevant to adapt follow-up and define stratification factors in future studies.
Systemic relapse rates were similar to those previously reported, although early relapses were lower because more late relapses were detected with longer follow-up [19]. We also observed a higher incidence of brain metastases in patients with late recurrence, compared to early recurrences, emphasizing the importance of brain imaging in the follow-up of these patients.
This study has certain limitations inherent to its observational and retrospective nature, including selection bias, missing data and potential underreporting of relevant variables. In addition, follow-up studies were conducted according to clinical practice of each center and there may be differences. Patient follow-up, although longer than in previous real-world studies in this setting, might not be enough to properly assess OS outcomes and late recurrence patterns; also, the sample size may be small to adequately assess some prognostic factors. Last, the population may be heterogenous as it includes patients with stage IIB-C to IV, cutaneous and non-cutaneous melanoma, and treated with both anti-PD-1-based monotherapy and combinations. Despite these limitations, our study included a more representative population of clinical practice than clinical trials in terms of patient characteristics and treatment administration, and therefore complements other data from adjuvant clinical trials, but without the usual restrictions of a clinical trial. In addition, our results are consistent with previous real-world studies that provide further evidence on different patient subgroups and/or with longer follow-up.
5. Conclusions
The results of our real-world study showed that patients with resected melanoma treated with adjuvant anti-PD-1-based immunotherapy exhibited inferior outcomes in terms of recurrence-free survival (RFS), distant metastasis-free survival (DMFS) and overall survival (OS) compared to those observed in clinical trials, as reported in previous real-world data reports. The initiation of adjuvant therapy more than 12 weeks after the last resection and the presence of mucosal melanoma were associated with an increased risk of recurrence. Early relapses were more frequent than late relapses, although a higher incidence of brain metastases was observed in late relapses. These results support the need to investigate real-world data in parallel to clinical trials, and suggest that in patients with poor prognostic factors, new combination strategies should be investigated, in addition to closer follow-up.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers17010143/s1, Supplementary Table S1. Distance metastases-free survival (DMFS): median survival time and univariate analysis* according to different clinical subgroups. Supplementary Table S2. Overall survival (OS): median survival time and univariate/multivariate analysis according to different clinical subgroups.
Author Contributions
Conceptualization, S.M.-R., M.A.M.-P., A.B. and M.M.; methodology, S.M.-R. and M.M.; software, S.M.-R. and M.M.; validation, S.M.-R., M.A.M.-P., A.B. and M.M.; formal analysis, S.M.-R. and M.M.; data curation, all authors; writing—original draft preparation, S.M.-R. and M.M.; writing—review and editing, all authors; visualization, all authors; supervision, M.M.; project administration, S.M.-R. and M.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Hospital de la Santa Creu I Sant Pau (protocol code IIBSP-MMI-2022–115, date of approval 15 March 2024).
Informed Consent Statement
Informed consent was obtained in accordance with the research protocol of the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
All the conflicts of interest declared are outside the present manuscript. SMR has received payment as a speaker from Pfizer, AZ, Pierre-Fabre, Sanofi, BMS, Takeda, Novartis and Lilly and has received support for attending meetings and/or travel from Roche, Merck, Sanofi, Novartis, MSD, Pierre Fabre, Lilly, BMS and Pfizer. MMP has received support for attending meetings and/or travel from Novartis. EMC has received consulting fees from BMS, MSD, Regeneron, Immunocore and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS, MSD, Novartis, Pierre-Fabre, Regeneron, Sanofi, Sun Pharma, Immunocore, Menari, support for attending meetings and/or travel from MSD and Pierre Fabre. ARST declares no conflict of interests. FA declares no conflict of interests. AA has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Almirall, Biontech, BMS, MSD, Novartis, Pierre-Fabre and Roche and support for attending meetings and/or travel from BMS, MSD, Novartis, Pierre-Fabre and Roche. MO declares no conflict of interests. JML has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astella, BMS, MSD, Novartis, Pierre-Fabre, Pfizer, Roche and Sanofi, support for attending meetings and/or travel from BMS, MSD, Novartis, Pierre-Fabre, Pfizer, Roche, Ipsen and Merck, and participation on a data safety monitoring board or advisory board from BMS, Trialing Health, Roche, Highlight therapeutics, Novartis, Sanofi, Pierre-Fabre. LFM declares no conflict of interests. RL has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis and MSD, and has received support for attending meetings and/or travel from BMS, MSD, Novartis and Pierre-Fabre. MQV has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AZ, Novartis, MSD, BMS, GSK, Pierre-Fabre and Sanofi, support for attending meetings and/or travel from AZ, MSD, GSK, Pierre-Fabre and Novartis, and participation on a data safety monitoring board or advisory board from AZ, Pierre-Fabre, MSD, GSK, Sanofi, Novartis, AbbVie and Immunocore. MN has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and/or travel from Pierre-Fabre and Novartis. JV has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS, Pierre-Fabre, Merck, Amgen, Novartis and MSD, and support for attending meetings and/or travel from Merck and Pierre-Fabre. DMP declares no conflict of interests. AB has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS, Pfizer, Sanofi, Pierre Fabre, Takeda, Johnson and Johnson and AZ, payment for expert testimony from AZ and BMS, support for attending meetings and/or travel from BMS, MSD, Pfizer Pierre-Fabre and Roche, and taken part in an advisory board from Roche and Sanofi. MM reports receiving grants from Roche and Astra Zeneca, taking part as invited speaker for Roche, MSD, Pfizer, Sanofi, Pierre Fabre, Novartis, Takeda and Astra Zeneca, and receiving support for attending meetings and/or travel from Astra Zeneca, MSD and Sanofi.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Arnold M., Singh D., Laversanne M., Vignat J., Vaccarella S., Meheus F., Cust A.E., de Vries E., Whiteman D.C., Bray F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022;158:495–503. doi: 10.1001/jamadermatol.2022.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C., Ribas A., Schachter J., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C.M., Lotem M., et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–1251. doi: 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 3.Robert C., Grob J.J., Stroyakovskiy D., Karaszewska B., Hauschild A., Levchenko E., Chiarion Sileni V., Schachter J., Garbe C., Bondarenko I., et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019;381:626–636. doi: 10.1056/NEJMoa1904059. [DOI] [PubMed] [Google Scholar]
- 4.Dummer R., Flaherty K.T., Robert C., Arance A., de Groot J.W.B., Garbe C., Gogas H.J., Gutzmer R., Krajsová I., Liszkay G., et al. COLUMBUS 5-Year Update: A Randomized, Open-Label, Phase III Trial of Encorafenib Plus Binimetinib Versus Vemurafenib or Encorafenib in Patients With BRAF V600–Mutant Melanoma. J. Clin. Oncol. 2022;40:4178–4188. doi: 10.1200/JCO.21.02659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok J.D., Chiarion-Sileni V., Rutkowski P., Cowey C.L., Schadendorf D., Wagstaff J., Queirolo P., Dummer R., Butler M.O., Hill A.G., et al. Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2024;392:11–22. doi: 10.1056/NEJMoa2407417. [DOI] [PubMed] [Google Scholar]
- 6.Eggermont A.M.M., Chiarion-Sileni V., Grob J.J., Dummer R., Wolchok J.D., Schmidt H., Hamid O., Robert C., Ascierto P.A., Richards J.M., et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: Long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur. J. Cancer. 2019;119:1–10. doi: 10.1016/j.ejca.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont A.M.M., Blank C.U., Mandala M., Long G.V., Atkinson V., Dalle S., Haydon A., Lichinitser M., Khattak A., Carlino M.S., et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont A.M.M., Blank C.U., Mandalà M., Long G.V., Atkinson V.G., Dalle S., Haydon A.M., Meshcheryakov A., Khattak A., Carlino M.S., et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:643–654. doi: 10.1016/S1470-2045(21)00065-6. [DOI] [PubMed] [Google Scholar]
- 9.Eggermont A.M., Kicinski M., Blank C.U., Mandala M., Long G.V., Atkinson V., Dalle S., Haydon A., Meshcheryakov A., Khattak A., et al. Seven-Year Analysis of Adjuvant Pembrolizumab versus Placebo in Stage III Melanoma in the EORTC1325/KEYNOTE-054 Trial. Eur. J. Cancer. 2024;211:114327. doi: 10.1016/j.ejca.2024.114327. [DOI] [PubMed] [Google Scholar]
- 10.Weber J., Mandala M., Del Vecchio M., Gogas H.J., Arance A.M., Cowey C.L., Dalle S., Schenker M., Chiarion-Sileni V., Marquez-Rodas I., et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 11.Ascierto P.A., Vecchio M.D., Mandalá M., Gogas H., Arance A.M., Dalle S., Cowey C.L., Schenker M., Grob J.-J., Chiarion-Sileni V., et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:1465–1477. doi: 10.1016/S1470-2045(20)30494-0. [DOI] [PubMed] [Google Scholar]
- 12.Larkin J., Del Vecchio M., Mandalá M., Gogas H., Arance Fernandez A.M., Dalle S., Cowey C.L., Schenker M., Grob J.-J., Chiarion-Sileni V., et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results from CheckMate 238. Clin. Cancer Res. 2023;29:3352–3361. doi: 10.1158/1078-0432.CCR-22-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luke J.J., Rutkowski P., Queirolo P., Vecchio M.D., Mackiewicz J., Chiarion-Sileni V., Merino L.d.l.C., Khattak M.A., Schadendorf D., Long G.V., et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718–1729. doi: 10.1016/S0140-6736(22)00562-1. [DOI] [PubMed] [Google Scholar]
- 14.Kirkwood J.M., Del Vecchio M., Weber J., Hoeller C., Grob J.J., Mohr P., Loquai C., Dutriaux C., Chiarion-Sileni V., Mackiewicz J., et al. Adjuvant nivolumab in resected stage IIB/C melanoma: Primary results from the randomized, phase 3 CheckMate 76K trial. Nat. Med. 2023;29:2835–2843. doi: 10.1038/s41591-023-02583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber J.S., Schadendorf D., Del Vecchio M., Larkin J., Atkinson V., Schenker M., Pigozzo J., Gogas H., Dalle S., Meyer N., et al. Adjuvant Therapy of Nivolumab Combined With Ipilimumab Versus Nivolumab Alone in Patients With Resected Stage IIIB-D or Stage IV Melanoma (CheckMate 915) J. Clin. Oncol. 2022;41:517–527. doi: 10.1200/JCO.22.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livingstone E., Zimmer L., Hassel J.C., Fluck M., Eigentler T.K., Loquai C., Haferkamp S., Gutzmer R., Meier F., Mohr P., et al. Adjuvant nivolumab plus ipilimumab or nivolumab alone versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): Final results of a randomised, double-blind, phase 2 trial. Lancet. 2022;400:1117–1129. doi: 10.1016/S0140-6736(22)01654-3. [DOI] [PubMed] [Google Scholar]
- 17.Long G.V., Hauschild A., Santinami M., Kirkwood J.M., Atkinson V., Mandala M., Merelli B., Sileni V.C., Nyakas M., Haydon A., et al. Final Results for Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2024;391:1709–1720. doi: 10.1056/NEJMoa2404139. [DOI] [PubMed] [Google Scholar]
- 18.Weichenthal M., Mangana J., Gavrilova I., Lugowska I., Shalamanova G.K., Kandolf L., Chiarion-Sileni V., Mohr P., Karanikolova T.S., Teterycz P., et al. Adjuvant Use of Pembrolizumab for Stage III Melanoma in a Real-World Setting in Europe. Cancers. 2024;16:3558. doi: 10.3390/cancers16213558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen C.N., Shoushtari A.N., Chauhan D., Palmieri D.J., Lee B., Rohaan M.W., Mangana J., Atkinson V., Zaman F., Young A., et al. Management of early melanoma recurrence despite adjuvant anti-PD-1 antibody therapy☆. Ann. Oncol. 2020;31:1075–1082. doi: 10.1016/j.annonc.2020.04.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moser J.C., Bhatia S., Amin A., Pavlick A.C., Betts K.A., Du E.X., Poretta T., Shelley K., Srinivasan S., Sakkal L.A., et al. Clinical outcomes of adjuvant nivolumab in resected stage III melanoma: Comparison of CheckMate 238 trial and real-world data. Cancer Immunol. Immunother. CII. 2024;73:116. doi: 10.1007/s00262-024-03697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kluger H.M., Tawbi H.A., Ascierto M.L., Bowden M., Callahan M.K., Cha E., Chen H.X., Drake C.G., Feltquate D.M., Ferris R.L., et al. Defining tumor resistance to PD-1 pathway blockade: Recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J. Immunother. Cancer. 2020;8:e000398. doi: 10.1136/jitc-2019-000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Meza M.M., Ismail R.K., Rauwerdink D., van Not O.J., van Breeschoten J., Blokx W.A.M., de Boer A., van Dartel M., Hilarius D.L., Ellebaek E., et al. Adjuvant treatment for melanoma in clinical practice—Trial versus reality. Eur. J. Cancer. 2021;158:234–245. doi: 10.1016/j.ejca.2021.08.044. [DOI] [PubMed] [Google Scholar]
- 23.Eggermont A.M.M., Kicinski M., Blank C.U., Mandala M., Long G.V., Atkinson V., Dalle S., Haydon A., Khattak A., Carlino M.S., et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020;6:519–527. doi: 10.1001/jamaoncol.2019.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.