Abstract
Objective
To demonstrate the usefulness of ultrasonography in detecting knee ossification centers in infants with permanent congenital hypothyroidism (PCH).
Methods
From 2011 to 2021, all infants with PCH referred for thyroid ultrasound also underwent left knee ultrasound and radiography on the same day. Knee radiographs were compared with knee sonograms. Two pediatric radiologists reviewed the consensus knee radiographs and sonograms to identify femoral and tibial epiphyseal ossification centers (presence/absence). The concordance between ultrasonography and radiography was assessed. Another radiologist conducted a second late review to evaluate interobserver agreement.
Results
We identified 125 patients (65 girls, 60 boys) with a mean age of 24 days (5 days–5 months). On scintigraphy, the thyroid was in place in 66.4%, ectopic in 24%, and absent in 9.6% of patients. The femoral center was observed in 108 patients (86.4%) via sonography and 106 patients (84.8%) via radiography. The tibial center was observed in 84 patients (67.2%) via sonography and radiography. Both femoral and tibial centers were present on sonography and radiography in 84 patients (67.2%). A single nucleus was present in 24 patients (19.2%) on sonography and 22 patients (17.6%) on radiography; it corresponded to the femoral center in all patients. The concordance between ultrasonography and radiography was 99% and 100%, respectively, for the detection of the femoral and tibial centers. Interobserver agreement was substantial to almost perfect for both ultrasonography and radiography.
Conclusion
Ultrasonography is as effective as radiography in detecting knee ossification centers in PCH. It can be performed at the same time as thyroid examination, in place of radiography.
Keywords: congenital hypothyroidism, epiphyseal cartilage, knee joint, ossification, physiologic, radiography, ultrasound
Short abstract
Access the CME test here and search by article title.
Abbreviations
- ALARA
as low as reasonably achievable
- PCH
permanent congenital hypothyroidism
- PACS
picture archiving and communication system
Congenital hypothyroidism is a syndrome of thyroid insufficiency that occurs from birth. It affects approximately 1 in 3000–4000 newborns and predominates in girls. 1 It can be permanent or transient, primary (thyroid dysfunction) or secondary (hypothalamic–pituitary dysfunction). The most common form of permanent congenital hypothyroidism (PCH) is primary hypothyroidism. In approximately half of the cases, PCH results from thyroid dysgenesis, that is, abnormal development of the thyroid gland corresponding to a gland ectopy, a gland absence (true athyreosis) or more rarely, to gland hypoplasia. In the other half of cases, PCH is secondary to impaired thyroid hormone production or dyshormonogenesis. If not treated early, PCH causes growth retardation, delayed bone maturation, and, most importantly, delayed cerebral development, with a risk of intellectual disability. 2 , 3 PCH is thus the most common preventable cause of mental retardation. 4
The clinical diagnosis of congenital hypothyroidism is difficult at birth, as symptoms are subtle and unspecific; therefore, systematic neonatal screening is usually performed when possible. When the test (also known as the heel prick test) is positive, a blood sample is taken to measure thyroid hormones and look for congenital hypothyroidism. If the diagnosis is confirmed, early treatment is initiated. To determine the cause of congenital hypothyroidism, further tests, including thyroid ultrasound (morphological gland studies) and/or thyroid scintigraphy (functional gland studies), are needed. Both techniques are recommended by international experts. 5 , 6 Thyroid ultrasound examination is designed to detect the presence of thyroid tissue, whether normal or ectopic, to distinguish a rudimentary gland from a normal one and to detect a possible goiter. Thyroid scintigraphy is used to distinguish between athyreosis (absence of tracer uptake) and thyroid ectopy (ectopic tracer uptake) and is coupled with a perchlorate discharge test when the gland is in a normal position to search for iodine‐related disorders.
To complement thyroid imaging techniques, a frontal knee radiograph is recommended to look for delayed bone maturation, that is, absence of visibility of the epiphyseal ossification centers of the distal femur and/or proximal tibia. 5 , 6 The absence of these ossification centers in a newborn at term is indicative of intrauterine hypothyroidism and, consequently, of severe PCH with a poorer neurological prognosis. 2 In our institution, frontal radiographs of the left knee are traditionally performed on infants with PCH on the same day as thyroid ultrasound. From January 2011, we decided to use ultrasound to explore the left knee of infants with PCH referred for thyroid ultrasound examination.
As ultrasonography is able to detect epiphyseal ossification centers of long bones and radiography is an ionizing examination, the purpose of this study was, under the ALARA (as low as reasonably achievable) principle for minimizing radiation exposure, to compare knee ultrasonography with knee radiography in the detection of femoral and tibial ossification centers in infants with PCH.
Materials and Methods
Ethical Considerations
This retrospective study was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the ethics research board of our institution. The requirement for informed consent was waived because of the retrospective nature of the study.
Objective
The primary objective of this study was to evaluate and record the presence or absence (categorical data) of femoral and/or tibial ossification centers in infants suffering from PCH.
Study Population
The population consisted of infants with a confirmed diagnosis of PCH who were referred to the imaging department of our institution between January 2011 and December 2021. The diagnosis of PCH was made by our pediatric endocrinologist colleagues based on clinical (if present) and laboratory abnormalities. As part of their PCH work‐up, infants were referred for a thyroid sonographic examination and a left knee radiograph. During this period, additional examination of the left knee via ultrasound was also performed. Medical records were reviewed for patient sex, age at knee imaging, and cause of PCH. Infants who did not undergo sonography or radiography of the left knee on the same day were excluded. The picture archiving and communication system (PACS) search and extraction of data were performed by 1 pediatric senior radiologist (D.R.) with 11 years of experience in pediatric radiology. Patient clinical data were recorded via Microsoft Excel spreadsheets. Another pediatric junior radiologist (C.C.) with 2 years of experience in pediatric radiology ensured that there were no errors before starting the data analysis.
Ultrasonography
Thyroid and knee examinations were performed via GE (Logiq E9, USA) and Canon (Aplio 500, Japan) ultrasound units equipped with hockey stick‐type linear transducers operating at 6.1–18 MHz (Logiq E9, L8–18i‐D probe; Aplio 500, 14L7 probe). Sonograms were obtained by 3 different radiologists (F.A., D.R., and H.L.) with 45, 11, and 4 years of experience in pediatric radiology.
During the ultrasound examination, the infant was placed in a supine position, with the head hyperextended on a rolled towel for better assessment of the thyroid gland. Once the thyroid examination had been completed, the knee examination was performed on the left side, with the knee placed in extension. The transducer was first placed sagittally in the midline over the anterior aspect of the knee and then gradually moved up from medial to lateral and from proximal to distal until the cartilaginous epiphyses of the distal femur and proximal tibia were fully explored (Figure 1). Ultrasound images were then automatically transferred to the healthcare system and picture archiving and communication system (IntelliSpace PACS 4.4, Philips Healthcare Informatics, Foster, CA, USA) of our institution. The knee examination took an average of 2 minutes of additional examination time for each patient. Just after or before the ultrasound examination, an anteroposterior radiograph of the left knee was taken by a technician in the imaging department and transferred to the PACS.
Figure 1.
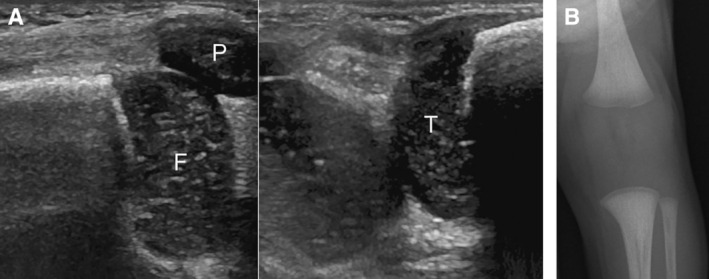
The absence of knee epiphyseal ossification centers on sonography and radiography in a 12‐day‐old female patient with PCH. A, Sagittal sonograms showing no epiphyseal ossification center within the cartilaginous epiphyses of the femur and tibia. F, femur; T, tibia; P, patella. B, Corresponding anterior–posterior radiograph of the knee.
Imaging Assessment
Radiography is considered the gold standard for detecting epiphyseal ossification centers of the knee. An epiphyseal ossification center was defined as present or absent on the basis of ultrasound and radiographic findings. When present on ultrasonography, the nucleus was visible as a rounded or arciform hyperechoic image associated with posterior acoustic shadowing, surrounded by the nonossified epiphysis. The latter appeared as hypoechoic cartilage containing hyperechoic spots. The femoral center was observed more proximally than the tibial center was.
Data were extracted from the PACS by 1 author (H.L.) who created 2 anonymized files, one corresponding to knee radiographs and the other to ultrasound images. The senior radiologist (D.R.) and the junior radiologist (C.C.) conducted together a consensus‐based review of ultrasound images and radiographs. During the review process on the PACS, the radiologists were blinded to the radiographic findings when assessing the ultrasound findings and vice versa for each patient. After an interval of 1 year, another senior radiologist (N.B.) conducted a second review of the knee examinations and their reports on the same PACS to evaluate interobserver agreement.
Statistical Analysis
The concordance of ultrasonography and radiography in detecting femoral and tibial epiphyseal ossification centers in infants with PCH was evaluated via contingency tables and by calculating Cohen's kappa coefficient. We also calculated Cohen's kappa coefficient (K) to assess interobserver agreement in the detection of femoral and tibial epiphyseal ossification centers via radiographs and sonograms.
Results
We identified 125 infants with a clinical diagnosis of PCH who underwent an ultrasound examination and a radiograph of the left knee on the same day. There were 65 girls and 60 boys. The mean age was 24 days (range, 5 days–5 months). The mean gestational age was 39 weeks (range, 35–42 weeks). A total of 110 (88%) patients were full‐term infants. The mean adjusted age at time of imaging based on gestational age was 14 days (range, 0–147 days).
Most of our cases of PCH (n = 121, 96.8%) were identified by neonatal screening. In 4 patients (3.2%), PCH was associated with a Down syndrome or trisomy 21 (n = 2), a Goldenhar syndrome (n = 1), or a Pendred syndrome (n = 1).
On scintigraphy, the thyroid gland was present and normally located in 83 patients (66.4%), ectopically located in 30 patients (24%) and absent in 12 patients (9.6%). On ultrasonography, the thyroid was in situ in 85 patients (68%), ectopic in 12 patients (9.6%), and absent in 28 patients (22.4%). The results are summarized in Table 1. Using scintigraphy as the reference examination for determining PCH etiology, a comparison of sonographic and radiographic findings (presence or absence of the femoral and tibial epiphyseal ossification centers) is shown in Table 2.
Table 1.
Results of Thyroid Imaging Techniques
| Thyroid Gland | Sonography | Scintigraphy |
|---|---|---|
| In situ | 85/125 (68%) | 83/125 (66.4%) |
| Ectopic | 12/125 (9.6%) | 30/125 (24%) |
| Absent | 28/125 (22.4%) | 12/125 (9.6%) |
Table 2.
Detection of Epiphyseal Ossification Centers With Ultrasonography and Radiography Depending on PCH Etiology
| Thyroid | Ultrasonography | Radiography | ||||
|---|---|---|---|---|---|---|
| F + T, n | F, n | Absent, n | F + T, n | F, n | Absent, n | |
| In situ (n = 83) | 70 | 11 | 2 | 70 | 11 | 2 |
| Ectopic (n = 30) | 13 | 9 | 8 | 12 | 8 | 10 |
| Absent (n = 12) | 2 | 4 | 6 | 2 | 3 | 7 |
F + T, presence of both femoral and tibial epiphyseal centers; F, presence of the femoral epiphyseal center alone.
The epiphyseal ossification center of the femur was observed in 108 patients (86.4%) via sonography and 106 patients (84.8%) via radiography. The epiphyseal ossification center of the tibia was observed in 84 patients (67.2%) via sonography and radiography. Both epiphyseal ossification centers were present in 84 patients (67.2%) on sonography and radiography (Figure 2). A single ossification center was present in 24 patients (19.2%) on sonography and 22 patients (17.6%) on radiography (Figure 3). In all the patients, this single ossification center corresponded to the femoral nucleus on both sonography and radiography (Figure 3). The epiphyseal ossification center of the tibia was never seen alone, either on ultrasonography or radiographically. The results are also summarized in Table 3.
Figure 2.
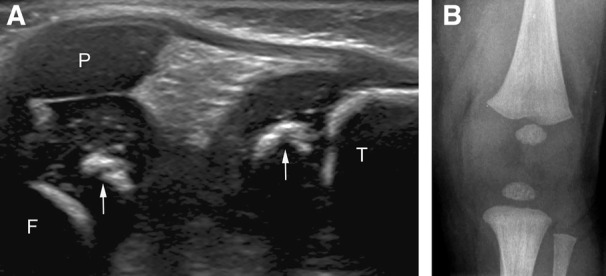
The presence of both femoral and tibial epiphyseal ossification centers on sonography and radiography in a 26‐day‐old female patient. A, Sagittal sonogram showing femoral and tibial epiphyseal ossification centers (arrows). F, femoral metaphysis; T, tibial metaphysis; P, patella. B, Corresponding radiograph of the knee.
Figure 3.
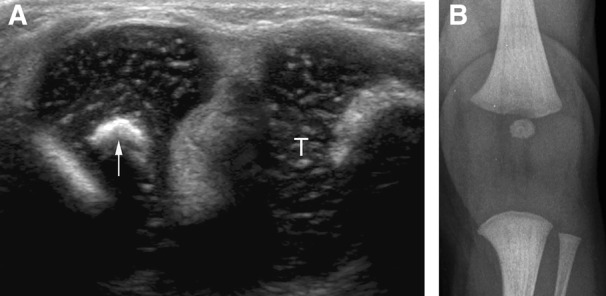
Presence of the femoral epiphyseal ossification center alone on sonography and radiography in a 10‐day‐old female patient. A, Sagittal sonogram showing the femoral center (arrow). T, tibia. B, Corresponding radiograph of the knee.
Table 3.
Detection of Epiphyseal Ossification Centers via Ultrasonography and Radiography
| Ossification Center | Sonography | Radiography |
|---|---|---|
| Femur | 108/125 (86.4%) | 106/125 (84.8%) |
| Tibia | 84/125 (67.2%) | 84/125 (67.2%) |
| Both femur and tibia | 84/125 (67.2%) | 84/125 (67.2%) |
| Femur alone | 24/125 (19.2%) | 22/125 (17.6%) |
| Tibia alone | 0/125 (0%) | 0/125 (0%) |
The epiphyseal ossification center of the femur was observed via ultrasonography, but it was not visible via radiography in 2 patients (1.6%) (Figure 4). Each time the epiphyseal ossification center of the tibia was observed via ultrasonography, it was visible via radiography (100%).
Figure 4.
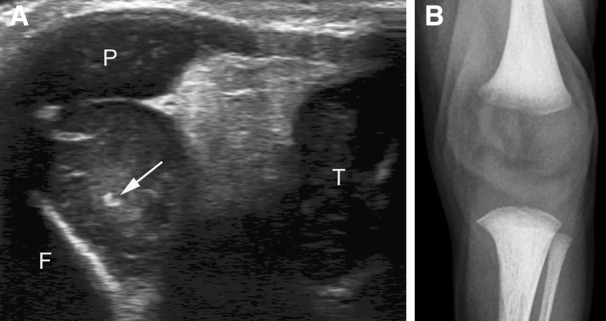
Discordance between sonography and radiography in an 11‐day‐old female patient. A, Sagittal sonogram showing an incipient epiphyseal ossification center of the femur (arrow). F, femoral metaphysis; T, tibia; P, patella. B, On the corresponding radiograph of the knee, the femoral center is absent.
The concordance between radiography and sonography was 99% for the detection of the femoral ossification center (Table 4) and 100% for the detection of the tibial ossification center (Table 5).
Table 4.
Detection of the Femoral Epiphyseal Ossification Center: Comparison Between Ultrasonography and Radiography
| Femoral Center | Radiography | |||
|---|---|---|---|---|
| Presence | Absence | Total | ||
| Sonography | Presence | 106 | 2 | 108 |
| Absence | 0 | 17 | 17 | |
| Total | 106 | 19 | 125 | |
| K = Cohen's kappa coefficient | K = 0.94 (0.84–1.00) | |||
Table 5.
Detection of the Tibial Epiphyseal Ossification Center: Comparison Between Ultrasonography and Radiography
| Radiography | ||||
|---|---|---|---|---|
| Tibial center | Presence | Absence | Total | |
| Sonography | Presence | 84 | 0 | 84 |
| Absence | 0 | 41 | 41 | |
| Total | 84 | 41 | 125 | |
| K = Cohen's kappa coefficient | K = 1 | |||
The interobserver analysis revealed substantial to almost perfect agreement regarding the detection of femoral and tibial ossification centers with ultrasonography (femur, K = 0.87: 0.71–1.00; tibia, K = 0.93: 0.85–1.00) and radiography (femur, K = 0.96: 0.89–1.00; tibia, K = 0.98: 0.94–1.00). The results are summarized in Table 6. However, some discrepancies were noted between the first and second readings (Table 6): except in 1 case, all the reading discrepancies were related to the very small size of the ossification center, which was dot‐like both on sonography (Figure 4) and radiography. In the last case, the tibial ossification center was considered absent on the first reading (no center visible on sonographic images), but it was considered present on the second reading (no center visible on images, but its presence was entered by mistake in the final report).
Table 6.
Agreement Between Readers
| Ossification Center | Femur—US | Tibia—US | Femur—XR | Tibia—XR |
|---|---|---|---|---|
| First reading | 111 | 85 | 108 | 86 |
| Second reading | 114 | 85 | 109 | 87 |
| Discrepancies | 3 (2.4%) | 4 (3.2%) | 1 (0.8%) | 1 (0.8%) |
| K (95% CI) | 0.87 (0.71–1.00) | 0.93 (0.85–1.00) | 0.96 (0.89–1.00) | 0.98 (0.94–1.00) |
K, Cohen's kappa coefficient; US, ultrasonography; XR, radiography.
Discussion
The secondary ossification center of the distal femur, also known as Beclard's point, appears around the 36th week of gestation, whereas the secondary ossification center of the proximal tibia, also known as Todd's point, appears later around the 38th week of gestation. This explains why the tibial ossification center was never observed alone in our study, either via radiography or sonography. Both the femoral and tibial centers are normally present in newborns at term. If not, their absence indicates a nonspecific delay in bone maturation, as observed among other diagnoses, in infants suffering from congenital hypothyroidism. In this case, delayed bone maturation reflects severe hypothyroidism that begins antenatally, and the absence of knee ossification centers on radiography is correlated with impaired neurocognitive and neurosensory development, 2 which may require closer follow‐up during infancy. Therefore, it is now advisable to carry out frontal radiographs of the knee (usually the left knee by convention) in newborns with PCH. 5 , 6
Until 2010, as recommended by international experts 5 , 6 and as requested by our pediatrician colleagues, we routinely performed frontal radiographs of the left knee in newborns with PCH. Even though the dose delivered remains minimal (~0.2 μSv effective radiation dose per exposure according to our calculations on a child body phantom), adhering to the ALARA principle, we decided from January 2011 to explore the left knee by ultrasound at the same time as the thyroid ultrasound examination. The additional examination time for the knee is minimal, no special positioning of the child is needed, and anterior scans of the left knee are easy to obtain. However, to the best of our knowledge, no other study in infants with PCH has compared ultrasonography and radiography in the detection of epiphyseal ossification centers of the knee. Owing to the proven performance of ultrasonography compared with radiography in their detection, we have no longer been performing knee radiographs in our department since January 2022, and this has facilitated the management of infants suffering from PCH.
Hormone synthesis disorders were more common (66.4%) than thyroid dysgenesis (33.6%) in our study. In agreement with the literature, scintigraphy was also more effective than ultrasonography in detecting an ectopic gland (24% versus 9.6%), even with the use of color/power Doppler mode, which easily identifies hypervascular thyroid tissue. 7 Indeed, the ectopic thyroid can be located anywhere along the path of its normal embryological descent from the tongue base to the thyroid cartilage, and ultrasonography cannot always detect lingual and sublingual ectopy. According to Wasniewska et al, 2 athyreosis is associated with more severe thyroid function impairment and consequently, with more delayed bone maturation, as reflected by the absence of knee ossification centers on radiography. In our study, femoral and tibial ossification centers were absent in 6 cases/12 (50%) on ultrasonography and in 7 cases/12 (58.3%) on radiography in infants without a thyroid gland, but both ossification centers were also absent in 8 cases/12 (66.7%) and 10 cases/12 (83.3%) on ultrasonography and radiography, respectively, in infants with an ectopic gland. However, infants suffering from athyreosis represented less than 10% of our study population.
In children with PCH, epiphyseal ossification centers of the knee may also exhibit morphological abnormalities, such as a small size, irregular contours, and bony fragmentation (also known as “epiphyseal dysgenesis”), over time. Some authors 8 have measured the largest and smallest diameters of each ossification center when present and compared the mean combined (femoral plus tibial) epiphyseal diameter, as described by Von Harnack, 9 with normal reference values 10 and thyroid hormone levels. The mean epiphyseal size was less than normal in 70% of their cases for the distal femoral nucleus and in 65% of their cases for the proximal tibial nucleus, whereas a combined epiphyseal size of 7 mm or less was significantly correlated with lower T4 levels. 8 However, reference values 10 in infants born at term are rarely used in practice, and epiphyseal ossification centers may be physiologically fragmented or exhibit irregular contours. 11 In our study, we opted for binary scoring (presence versus absence of ossification centers) in accordance with the recommendations of European and international experts of PCH concerning knee radiography.
The present study demonstrated that ultrasonography is as effective as radiography for detecting epiphyseal ossification centers of the knee. In 2 patients, sonography was even more effective than radiography in detecting the distal femoral nucleus. This finding was not surprising. When performing an ultrasound examination in infants around 1 month of life to look for congenital hip dislocation, it is not uncommon to observe within the cartilaginous femoral head the proximal femoral nucleus as a small hyperechoic dot, even though it is not yet visible on radiography. In fact, the secondary ossification center of the proximal femur starts to ossify (and become radiographically visible) at 5 months of age, 12 although this may vary slightly from one child to another.
Our results show that interobserver agreement was substantial to almost perfect 13 for the detection of knee ossification centers with ultrasonography. Few discrepancies (femur, n = 3; tibia, n = 4) were recorded; however, these discrepancies were associated mainly with punctiform ossification centers. A nonossified epiphysis is demonstrated on sonography as a hypoechogenic structure containing multiple echogenic spots related to the presence of vessels within the epiphyseal cartilage anlage. When the ossification nucleus has just appeared as a millimetric dot, it may be difficult on static images to distinguish it from the rest of the cartilaginous epiphysis. In practice, however, the dynamic nature of ultrasonography makes it easier to observe the difference between a punctiform ossification center and the intrinsic vasculature of the cartilaginous epiphysis.
Our study had a few limitations. First, the study was a retrospective analysis, and our study population was heterogeneous, with small sample sizes in each subgroup of infants with PCH (thyroid in situ, ectopic thyroid, absent thyroid). Additional studies with larger sample sizes are warranted to compare these subgroups in terms of bone maturation retardation. Second, we did not measure the size of the epiphyseal ossification centers of the knee via radiography or ultrasonography but chose to assess only their presence or absence, following expert recommendations for knee radiography in infants with PCH.
Conclusion
In infants suffering from PCH, knee ultrasonography is as reliable as knee radiography in confirming the presence or absence of secondary ossification centers of the distal femur and/or proximal tibia. The absence of these ossification centers indicates delayed bone maturation and, consequently, more severe PCH with a greater risk of neurodevelopmental disorders. Knee ultrasound examination can be performed at the same time as thyroid ultrasound examination: it is easy to perform, does not significantly increase examination time, and avoids radiography, in line with the ALARA principle.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Castanet M, Polak B‐PC, Lyonnet S, Czernichow P, Léger J. Nineteen years of national screening for congenital hypothyroidism: familial caes with thyroid dysgenesis suggest the involvement of genetic factors. J Clin Endocrinol Metab 2001; 86:2009–2014. [DOI] [PubMed] [Google Scholar]
- 2. Wasniewska M, De Luca F, Cassio A, et al. In congenital hypothyroidism bone maturation at birth may be a predictive factor of psychomotor development during the first year of life irrespective of other variables related to treatment. Eur J Endocrinol 2003; 149:1–6. [DOI] [PubMed] [Google Scholar]
- 3. Léger J, Barroque B, Norton J. Influence of severity of congenital hypothyroidism and adequacy of treatment on school achievement in young adolescents: a population‐based cohort study. Acta Paediatr 2001; 90:1249–1256. [DOI] [PubMed] [Google Scholar]
- 4. Agrawal P, Philip R, Saran S, et al. Congenital hypothyroidism. Indian J Endocrinol Metab 2015; 19:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Léger J, Olivieri A, Donaldson M, et al. European Society for Pediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab 2014; 99:363–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Trosenburg P, Stoupa A, Léger J, et al. Congenital hypothyroidism: a 2020–2021 consensus guidelines update—an ENDO—European reference network initiative endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021; 31:387–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marinovic D, Garel C, Czernichow P, Léger J. Ultrasonographic assessment of the ectopic thyroid tissue in children with congenital hypothyroidism. Pediatr Radiol 2004; 34:109–113. [DOI] [PubMed] [Google Scholar]
- 8. Newland CJ, Swift PG, Lamont AC. Congenital hypothyroidism—correlation between radiographic appearances of the knee epiphyses and biochemical data. Postgrad Med J 1991; 67:553–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Von Harnack GA. Das übertragene, untergewichtige neugeborene. Monatsschr Kinderheilkd 1960; 108:412–415.13711647 [Google Scholar]
- 10. Senecal J, Grosse MC, Vincent A, Simon J, Lefreche JN. Maturation osseuse du fœtus et du nouveau‐né. Arch Fr Pediatr 1977; 34:424–438. [PubMed] [Google Scholar]
- 11. Augusto ACL, Goes PCK, Flores DV, et al. Imaging review of normal and abnormal skeletal maturation. Radiographics 2022; 42:861–879. [DOI] [PubMed] [Google Scholar]
- 12. Schaefer M, Black S, Scheuer L. The lower limb. Juvenile Osteology: A Laboratory and Field Manual. San Diego, CA: Elsevier Academic Press; 2009:255‐295. [Google Scholar]
- 13. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


