Summary
The One Health approach musters growing concerns about antimicrobial resistance due to the increased use of antibiotics in healthcare and agriculture, with all of its consequences for human, livestock, and environmental health. In this perspective, we explore the current knowledge on how interactions at different levels of biological organization, from genetic to ecological interactions, affect the evolution of antimicrobial resistance. We discuss their role in different contexts, from natural systems with weak selection, to human-influenced environments that impose a strong pressure toward antimicrobial resistance evolution. We emphasize the need for an eco-evolutionary approach within the One Health framework and highlight the importance of horizontal gene transfer and microbiome interactions for increased understanding of the emergence and spread of antimicrobial resistance.
Subject areas: Health sciences, Biological sciences, Microbiology, Evolutionary biology
Graphical abstract
Health sciences; Biological sciences; Microbiology; Evolutionary biology
Introduction
The advent of antimicrobials has vastly benefited the healthcare and agricultural systems by providing an effective means for combating microbial infections in humans and livestock, as well as for protecting crops from a wide diversity of pests.1 Unfortunately, the (over)use of antimicrobials in these settings has concurrently led to the large-scale evolution of antimicrobial resistance among microbes, strongly impacting the effectiveness of antimicrobial treatments with worldwide repercussions.2,3,4,5 The trade of agricultural products and human travel further contribute to the spread of these antimicrobial-resistant microbes across the globe.4
The emergence, selection, and spread of antimicrobial-resistant microbes threaten the health of livestock and human patients, who may suffer from prolonged infections, resulting in higher mortality rates.6 Additionally, the presence of antimicrobials in the environment can potentially lead to microbiome shifts in important aquatic and terrestrial habitats, potentially disrupting ecosystem functions.7 This multifaceted problem of antimicrobial resistance is therefore approached in a collaborative effort, using a One Health approach, leveraging the interconnection between human, animal, and environmental health.8
To fully grasp the role of antimicrobial resistance in the One Health framework, we must recognize that the functionality of microbes is part of a complex interplay of genes, hosts, populations, communities, and environments. Humans, animals, and the wider environment exert different selective pressures on the microbes and the antimicrobial resistance genes they may carry. In this context, we position antimicrobial resistance as a property of bacterial host cells, where antimicrobial resistance genes (ARGs) are disseminated through both vertical transmission (VGT) via clonal expansion and horizontal gene transfer (HGT) within and between populations and communities9,10 (Figure 1). HGT is an essential adaptive force in evolution where the movement of heritable genetic information is transferred horizontally between individuals who may or may not be related.11 This genetic exchange between bacteria occurs in various ways, including transformation, transduction, and conjugation.12 HGT is, therefore, a key mechanism that interconnects microorganisms across environments. Here, we underscore the significance of viewing antimicrobial resistance through the lens of microbial community ecology and evolution, where species interactions influence HGT. We will focus on the emergence and subsequent spread of antimicrobial resistance via HGT, particularly via conjugation. This is explored at various levels of biological organization and selection, from controlled laboratory microcosms to natural systems where ARGs and antimicrobial resistance naturally occur under weak antimicrobial selection and anthropogenically impacted environments where strong antimicrobial selection drives the spread of antimicrobial resistance. We underline the importance of HGT as a natural process that fosters connectivity among organisms across all ecosystems, shedding light on the significance of microbiome interactions in combating antimicrobial resistance.
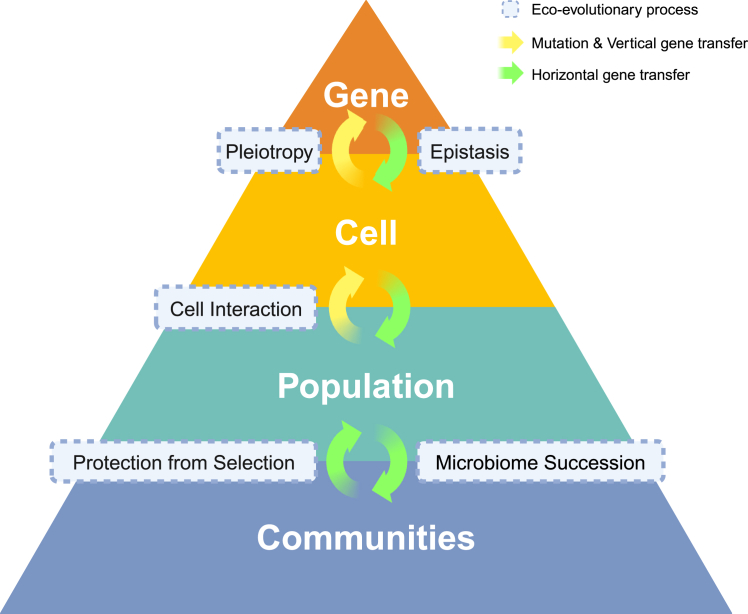
Figure 1.
Horizontal gene transfer at different levels of organization, from genes to communities
To grasp the importance of the dissemination and transmission of antimicrobial resistance in the context of One Health, it is crucial to address the role of HGT in disseminating ARGs across several levels of organization. HGT is affected by interactions at the genetic, cell, population, and community levels. At the genetic level, antimicrobial resistance can evolve via mutations in the chromosome and transfer vertically, from parent to offspring (yellow arrows), as well as by the acquisition of plasmids carrying antimicrobial resistance genes through HGT (green arrows). At the cell level, antimicrobial resistance enables survival and proliferation under antimicrobial selection, yet the genetic background of the bacterial host (epistasis) and pleiotropy can affect the level of resistance. At the population and community level, microbial interactions can affect resistance and conjugation efficiency. Additionally, at the community level, HGT can contribute to microbiome stability and succession.
Origin and selection of antimicrobial resistance across levels of biological organization
Antimicrobial resistance can be encoded on the chromosome or extrachromosomal mobile genetic elements (MGEs) carried by the bacterial hosts. MGEs are segments of DNA that mediate the movement of DNA within genomes (intracellular mobility) or between bacterial cells (intercellular mobility).13 Conjugative plasmids are a subset of MGEs and are responsible for many outbreaks of antimicrobial resistance, especially when appropriate infection control measures are breached in hospital settings.14,15 Antimicrobial resistance genes are often located on other MGEs, such as integrons, transposons, or between insertion sequences.16 Such MGEs can move within and between genomes,17 bacterial host species, or cross-species barriers.18,19,20 A prominent example of this is the case of colistin resistance in animals and humans. Colistin is a polymyxin antimicrobial with broad-spectrum activity against Gram-negative bacteria. The resistance, previously found to be conferred by chromosomal mutations, was found to be encoded by the mcr-1 gene, which was mobilized by an ISApl1 transposon to a plasmid transmitted by conjugation and maintained in Enterobacteriaceae such as Klebsiella pneumoniae and Escherichia coli, as well as Pseudomonas aeruginosa.21,22 The different levels of organization of the genetic architecture, from mutations to genes to (parts of) the genetic backbone of bacteria or the type of MGE, may impact the spread and selective benefit of antimicrobial resistance.
Genetic and environmental interactions mediate fitness costs and benefits associated with antimicrobial resistance
Both the presence of MGE and antimicrobial resistance are common phenomena in the microbial world, playing a crucial role in the evolution of microorganisms.23,24,25 Yet, for an antimicrobial resistance trait to be selected, the gene(s) encoding antimicrobial resistance must be functionally embedded and provide a benefit in the genetic background and environment of the bacterial host species.26 This is illustrated by the globally distributed epidemic plasmid pOXA-4827 with a broad host range of replication, which encodes for enzymes that can hydrolyze β-lactam antibiotics28 and last-resort carbapenems.29 Recent studies have shown that pOXA-48 has species and strain-specific variability in plasmid-associated antimicrobial resistance levels and conjugation dynamics,30 with Klebsiella spp. strains showing higher pOXA-48-mediated AMR and conjugation frequencies than E. coli strains.31 This shows that the genetic background of the bacterial host affects the phenotypic level of resistance and conjugation efficiency of plasmids containing antimicrobial resistance genes.
The carriage of MGEs, such as plasmids, may lead to fitness costs, thus leading to negative selection in the absence of antimicrobials. In such a case, antimicrobial resistance can be diminished in the population when the selective agent is not present anymore, e.g., after antimicrobial treatment is halted.32,33,34,35 This fitness cost may also depend on the genetic background of the bacterial isolate carrying resistance, such as in the case of pOXA-48 plasmid in K. pneumoniae and E. coli.31 Moreover, numerous studies have shown that genomic compensatory mutations, as well as transcriptional changes, can alleviate the costs associated with antimicrobial resistance plasmid carriage,32,36 allowing for the persistence of the antimicrobial trait in the population long after exposure to the antimicrobial has ceased. Another way to overcome this negative selection is by the transfer of “silent antimicrobial resistance genes” that encode a plastic phenotype, conferring the phenotypic trait of antimicrobial resistance,37 e.g., efflux pumps that may be activated in some environments but not in others.38,39 This may, in fact, be adaptive if resistance is costly and the antimicrobial is present only in some environments.
Pleiotropy and linkage can affect the selection for antimicrobial resistance
Antimicrobial resistance can also evolve in the absence of antimicrobial exposure, and adaptation to specific environments can result in decreased susceptibility to a number of different antimicrobial classes.40 For instance, mutations improving growth rate under specific conditions, e.g., the rpoB gene, have pleiotropic effects that alter bacterial physiology and coincidentally lead to antibiotic resistance.40,41,42,43
Antimicrobial resistance genes can also spread by hitch-hiking, along with other, beneficial traits, either via genetic linkage on plasmids (e.g., linked to pathogenicity islands44 or by linkage with a beneficial bacterial chromosomal genetic background). Several recent studies show a convergence of virulence or other growth-enabling factors related to antimicrobial resistance, particularly in lineages causing disease in humans. Plasmids encoding such virulence or antimicrobial-resistance traits may alter gene-expression levels in bacterial host cells45 in the human reservoir,46,47 which may only favor growth and survival in that particular host. This suggests that such antimicrobial resistance-carrying bacterial lineages may be specialists in humans or animals and that HGT or vertical transfer to other environments, such as soil or water, may not necessarily enhance the spread of resistance,48 whereas generalist lineages, plasmids, or lineage-plasmid associations may actually contribute to an increase in resistance dissemination.49
The spread of antimicrobial resistant bacteria and the rate of horizontal and vertical transfer of antimicrobial resistance genes in bacterial lineages is thus determined by a combination of several factors and properties: the genetic makeup and physiology of the bacterial host species, the interaction of the bacterial host with the (mobile) genetic element,50 the niche that they inhabit or are transferred to, and the ecological interactions with other bacteria in those niches51,52 (Figure 1).
Community interactions affect horizontal gene transfer and antimicrobial resistance phenotype
Given that bacteria generally coexist in polymicrobial communities, biotic and abiotic interactions within communities are critical to understanding how bacteria respond to antimicrobial exposure and evolve antimicrobial resistance, which can have important clinical, ecological, and environmental consequences.53,54 In this context, the horizontal transmission of genes can play a pivotal role in facilitating the transfer of genetic traits across the microbiome.55 Within these complex microbial ecosystems, HGT is involved in shaping the genetic diversity and adaptation of microbial communities and, hence, in controlling the public health problem of antimicrobial resistance.56
Community interactions can alter the efficacy of antimicrobials, the selective pressures, and the evolutionary responses of bacteria via metabolic cross-feeding,57,58,59 quorum sensing,60 or other density-dependent interactions,61 e.g., a population of bacterial cells at a sufficiently high density can survive an antibiotic treatment at doses that are lethal to a low-density population. Such collective tolerance or resistance can lead to less susceptible phenotypes in mixed populations of resistant and sensitive bacteria.62 A prominent example of collective resistance is the secretion of β-lactamase enzymes by individual bacteria that can provide passive resistance to whole bacterial populations.63,64 The inactivation of β-lactam antibiotics by resistant cells is, in fact, a cooperative behavior that enables sensitive cells to survive antibiotic treatment in the population.65,66 Similarly, it has been demonstrated that in a drug-sensitive E. coli population, a small number of resistant mutants can improve the survival of the population’s less resistant cells, partly by indole production. Indole is a signaling molecule generated by actively growing cells, which enhances the survival of the whole population in stressful environments, such as in the presence of antibiotics.67
Species in a polymicrobial environment can also benefit from these collective effects. For example, E. coli strains harboring the pOXA-48 plasmid can detoxify the environment for other species in the presence of piperacillin and tazobactam, a penicillin beta-lactam antibiotic and a beta-lactamase inhibitor. Such detoxification processes can alter the microbial community structure by enabling increased growth of community members, leading to more interspecies variation at degraded antibiotic concentrations.68 Overall, these mechanisms of collective resistance are increasingly contributing to clinical resistance.69,70
Biofilms are another prominent example of structured microbial communities that protect sensitive cells from the antibiotics in the environment.71 Bacterial communication in such biofilms (e.g., via quorum sensing) can lead to changes in gene expression, potentially enhancing virulence and the altered acquisition of ARGs by HGT.60,72,73 Additionally, the lateral transduction of ARGs has been shown to play a role in such structures.74 Biofilm formation can, therefore, promote HGT and provide a means by which bacterial communities can collectively resist the action of antimicrobials.
Community interactions affect conjugation efficiency
Community interactions can also directly affect the conjugation efficiency. In microbial communities, the ability of plasmids to move horizontally can be impacted by community composition and diversity. Studies have revealed that the diversity of bacteria can restrict the horizontal transfer of plasmids and the efficiency at which plasmids are transferred between co-infected host cells that carry different conjugative plasmids. This could be due to a “dilution effect,” whereby living alongside less proficient host species reduces the expected infection risk for a focal host species.75 Community interactions may also promote conjugation at low bacterial densities. For instance, Enterococcal species have been shown to increase the conjugation rate of pOXA-48 between uropathogenic E. coli.30
Even though antibiotics can promote the rate of MGE acquisition under particular (e.g., stressful) circumstances,76,77 the presence of antibiotics may also reduce the frequency of conjugation by reducing the population sizes of those populations that donate or receive the plasmid, potentially negating the effect of positive selection for the transconjugant.78 On the other hand, resistant populations may also benefit from competitive release, which is the case if most of its competitors have been killed by antibiotics.79 The nature of the (a)biotic environment can thus promote or inhibit the spread of ARGs in bacterial communities.
Community interactions affect plasmid loss and persistence
Community interactions can determine the costs and persistence of conjugative plasmids. For instance, interspecific competition can lead to the increased carriage cost of the plasmid, resulting in plasmid loss in a microbial community.80 As mentioned above, fitness costs of plasmid carriage are, in theory, a barrier to HGT; however, when specific genetic conflicts cause such costs, they may be ameliorated by single compensatory mutations, as discussed above,32 thus enabling the long-term maintenance of plasmids in bacterial genomes.81 However, when the rate of plasmid loss in a multi-species community is promoted by conjugation inhibition, plasmid-mediated antibiotic resistance between members of microbial communities decreases, hence supporting the reversal of antibiotic resistance.82 Thus, plasmid loss critically affects the level of plasmid maintenance in the population.
On the other hand, there can be situations where the presence of antibiotics in the environment has only marginal effects on plasmid loss and persistence. If plasmids provide fitness benefits or do not provide costs to community members, they can persist in the absence of conjugation. This is the case for plasmid pOXA-48 in the human gut microbiota,31 but also in or on other hosts such as the phytosphere,83 and in earthworms.84 Even under non-selective conditions, such as in the absence of antibiotics, community-level persistence of plasmids is sustained as long as plasmid fitness benefits exist in multiple phylotypes.85 This is exemplified by biofilms, where cells are already metabolically inactive, and growth rates are low,86 they can act as plasmid reserves, even without antibiotics.87,88 Overall, interactions between the members in polymicrobial communities can have important effects on the acquisition and spread of antimicrobial resistance by altered selective pressure and changes in the conjugation and plasmid loss rates. An interplay of these factors is, therefore, thought to be responsible for the spread of antimicrobial resistance in the microbiome.89
Antimicrobial resistance genes under weak selective pressure in natural environments: The example of soil microbial communities
Although the use of antibiotics in natural environments, such as in clinical and veterinary settings, has accelerated the spread of antimicrobial resistance, antibiotic-producing genes, as well as those that confer resistance, existed in the environment long before the application of antimicrobials for human purposes, as they are part of the natural microbial warfare.90 Soils, for instance, harbor a large diversity of microbial species and represent an important environmental source for antibiotic discovery: vancomycin was found in soil-dwelling bacteria that use this antibiotic to defend against other microbes91; streptomycin, used to treat tuberculosis and other bacterial infections, is produced by the soil bacterium Streptomyces griseus.92 These antibiotics are natural components of chemical warfare regulating microbial interactions, which depends on the balance between antibiotic production and antimicrobial resistance. Studies have shown that soil bacteria harbor a wide range of ARGs, including those that confer resistance to multiple classes of antibiotics,93 confirming that the mechanisms that underlie antimicrobial resistance are ancient and predate the use of antibiotics by the human population.94
Microbial warfare is especially important in soil communities where microbial species are less motile and subjected to interactions in soil aggregates (Figure 2). In fact, the ability to synthesize or resist antimicrobials is widely considered an important driver of microbiome community assembly during soil development. By studying the functionality of the soil microbiome along a primary succession gradient, Dini-Andreote et al.23 showed that genes associated with antibiotic resistance and antibiotic production were linked to ecological trade-offs. In salt marshes, it has been shown that these genes are enriched in mature, well-developed soils. In contrast, young soils were primarily enriched in genes associated with cell motility, which is more relevant in the early stages due to the daily flooding of the salt marshes.23 The natural occurrence of antimicrobial resistance in soil microbiomes, including resistance to a wide range of antibiotics used in clinical settings and other antimicrobials produced by soil microorganisms, has important consequences for the One Health approach. Yet, the exposure to the naturally produced antimicrobials that contribute to the structuring of soil communities likely leads to relatively local and weak selective pressures on the microbial populations when compared to selection imposed by the overuse of antibiotics by the clinical or veterinary sector, as discussed later in discussion.
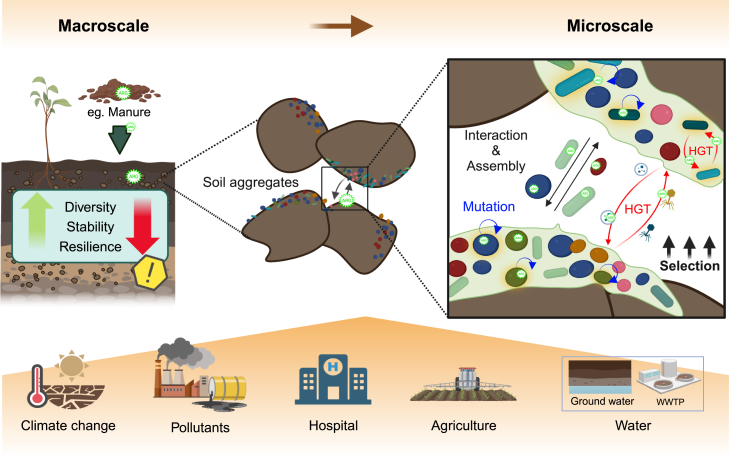
Figure 2.
Soil as a source of ARG and antimicrobial resistance
Soil represents a natural source of antimicrobial resistance, as antibiotics are an important component of chemical warfare between microbes. At the micro-scale, the soil microbiomes are found in micro-aggregates,95 forming biofilms that protect against predation, desiccation, and antibiotic exposure while improving nutrient and oxygen availability and providing a niche for HGT.96 At the macro scale, the soil microbiomes are subjected to various selective pressures, such as climate change, pollutants, agriculture, and the introduction of contaminated water.
Anthropocentric disturbances lead to increased selective pressures for antimicrobial resistance
Human activities can cause significant disturbances to the soil microbiome and exert selective pressures that impact its diversity, stability, and resilience (Figure 2). Although the presence of antimicrobials in natural communities may result in weak selection, anthropogenic disturbances can easily strengthen selection. For instance, exposure creates a selective pressure that favors the survival of bacteria carrying natural ARGs,97 affecting the soil microbiome at both the micro and macro scales. At the micro-scale, the soil microbiome often occurs in biofilms inside aggregates, being subjected to different evolutionary processes, including a higher rate of HGT than planktonic populations when facing selection98 (Figure 2). At the macro scale, anthropogenic disturbances in soil and water,99 such as exposure to antibiotics and other biocides, can lead to the co-selection of antimicrobial resistance due to co-resistance or cross-resistance. The latter occurs when resistance to one class of antimicrobials confers resistance to other classes that target the same cellular process.100
Co-resistance occurs when resistance genes for two or more antimicrobial agents are located on the same MGE, such as a plasmid or transposon. For instance, bacteria harbor resistance or tolerance genes toward both antibiotics and other compounds, such as biocides and metals,101 on the same MGE. If two or more resistance mechanisms are genetically linked on the same MGE, selecting one of these resistances in a particular context favors the spread of the other resistance types simultaneously102 via co-selection.103 This increases the likelihood of co-selecting ARGs by pesticides,104 biocides, and metals. Regarding the latter, human activities such as mining could have contributed to the spread of these elements as heavy metals such as copper, zinc, and arsenic, commonly used in ancient mining, can co-select for ARGs105 due to the physical proximity of heavy metal resistance genes and ARGs on mobile genetic elements such as plasmids and multi-resistance integrons.106 Heavy-metal contaminated soils are enriched in efflux pump genes encoded on MGEs, leading to an increased richness of antibiotic resistance and antibiotic-resistant bacteria in those environments.107 This co-selection is evident in bacteria present within wastewater treatment plants108 but can also be found in agriculture. According to a study conducted by Heydari et al., antibiotic and heavy metal resistance genes were more common in soil bacteria from agricultural rather than non-agricultural areas. The study also found a significant correlation between heavy metals and antibiotic resistance in soil bacteria.109 Regardless of the type, co-selection can lead to the spread of antibiotic resistance among bacterial populations in one specific environment or across environmental reservoirs, highlighting the importance of addressing co-selection and co-resistance in the context of One Health.
Anthropocentric disturbances lead to altered microbial network interactions in soil
The impact of human activities results in disturbances that directly affect the diversity and resilience of the microbiome, as shown in Figure 2. These disturbances can create a cycle where microbial populations carrying antimicrobial resistance genes become more abundant due to external pressures. This can lead to the acquisition of additional antimicrobial resistance genes by the soil microbiome through HGT, particularly for genes on mobile genetic elements with high transfer rates.110 Such stressful conditions have also been shown to increase the rate of HGT.111
Ultimately, the loss of microbial diversity, especially in the context of anthropogenic influences, which are usually mono-sourced and drastic, imposes a limit on the capacity of the community to resist or be resilient, i.e., to return to the original stage after disturbance.112 For instance, soil contamination with polycyclic aromatic hydrocarbons (PAHs) leads to the selection of PAH-degrading bacterial populations,113 reducing the overall diversity of the soil microbiome. As a consequence, this community is likely more sensitive to antibiotic spills114 or other strong selection pressures, as compounded perturbations reduce microbiome resilience.115 Under the presence of a combination of exacerbating anthropogenic factors, such as antimicrobial agents, contaminants, and pesticides, the local soil microbiome and the natural environmental factors are drastically affected, with important consequences for the microbial network and soil health.116 Finally, the presence of resistance genes can also affect soil stability. However, the extent of their impact on community dynamics depends on factors such as ecological interactions within the communities and the rate of mobility of the ARGs within the network. For instance, increasing ecological stability was influenced by the presence of ARGs in the focal communities, whereas intense competition causes decreasing stability.117
Transmission of antimicrobial resistance across environmental reservoirs
Antimicrobial resistance in the One Health context spans several levels of organization, from individual populations to communities, but it also flows through different environmental reservoirs. For instance, ARGs can spread through different systems, linking components such as water systems and the food industry (Figure 3). Wastewater treatment plants (WWTPs) receive wastewater containing various pollutants, including antibiotics and chemicals from different sources, representing both a source of ARGs and a conducive environment for spreading antimicrobial resistance. Raw and treated wastewater carries more antibiotic-resistant bacteria than surface water, requiring advanced treatment processes.118 For instance, chemical disinfection only marginally affected the composition of the phage communities, including human fecal related phages and those tested positive for harboring ARGs, suggesting the potential to facilitate the HGT of ARGs.119 Once in the WWTP, phages can infect the local bacteria, promoting the transfer of genetic material between bacteria during the lytic cycle.120 Alternatively, ARGs of phage origin can also be spread through conjugation if inserted in transposable elements or conjugative plasmids, further contributing to the spread of ARGs. Untreated WWTPs represent important hotspots of HGT and ARG dissemination, which can spread further into the environment if wastewater is used for the irrigation of agricultural areas. In that case, ARG-enriched water is likely to facilitate the movement of these genes into the soil and plant microbiome.121
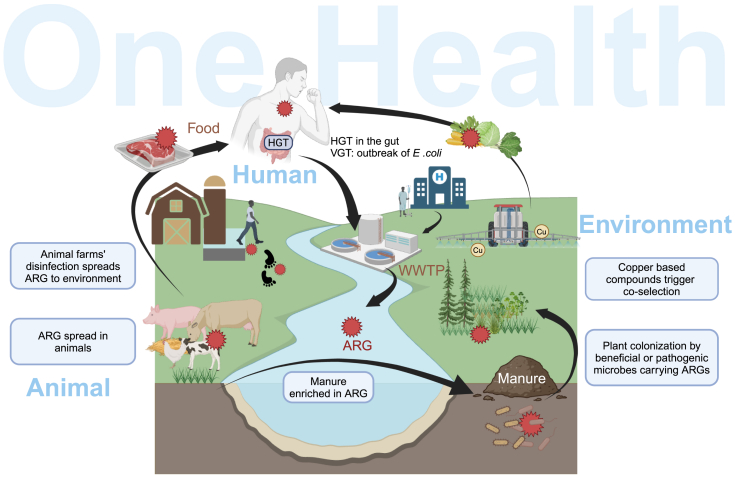
Figure 3.
ARGs in One Health - dissemination and selection across environments and reservoirs
Humans, animals, and the environment are intertwined components of One Health, contributing to the spread of antimicrobial resistance through different routes. For instance, the use of antibiotics in clinical settings and animal husbandry contributes to the selection of ARGs that further spread via waterways and in WWTPs. Likewise, the use of biocides, such as copper and disinfectants, in agriculture and animal husbandry might contribute to the co-selection of ARGs. The use of wastewater for irrigation, or ARG-enriched manure, can further promote the spread of ARGs in soils and crops. Finally, residues of antimicrobials or other biocides in food products can lead to stronger selective pressure in the human gut, promoting the spread of ARGs via clonal expansion (VGT) or through HGT.
The application of manure from animals treated with antibiotics to the soil can also increase the dissemination of ARGs in agriculture. In animal husbandry, the widespread use of antibiotics for growth-promoting and preventing diseases contributes to the spread of these genes. Up to 30–90% of administered antibiotics are excreted through urine and feces, which leads to the accumulation of residual antibiotics in the manure. ARGs that reach the soil can spread to other soil organisms. For instance, the abundance of certain bacteria associated with the soil fauna gut is associated with the concentrations of soil pollutants and is closely related to the abundance of ARGs.122 The residuesphere, the area between decaying plant material and soil, was shown to be a hotspot for bacterial conjugation. Furthermore, the presence of fungi increased bacterial colonization and facilitated the transfer of genes, including ARGs, through conjugation.123 These examples highlight how inter- and intra-kingdom interactions might facilitate HGT, from animal gut to plant microbiome via rhizosphere soil,124 increasing the probability of distributing ARGs from bacteria to human pathogens.125 While examining the presence of ARGs in distinct reservoirs is essential, the preceding examples underscore the importance of understanding the specific processes through which ARGs spread among microbial populations both within and across these reservoirs. It is therefore crucial to identify potential transmission routes and interactions between components, as well as to determine the selective pressures and barriers to adaptation that may restrict spillover and, consequently, mitigate the burden of antimicrobial resistance.
Conclusion
The relevance of antimicrobial resistance as a response to the increased use of antibiotics in clinical or agricultural settings has direct consequences for environmental health, being at the core of the One Health context. In this perspective piece, we explored the ecological and evolutionary constraints on HGT and antimicrobial resistance at different levels of biological organization, from mutations to genes to linkage to genetic backgrounds, and the diverse effects on microbial community ecology from confined laboratory settings to the larger natural environments. These levels of biological organization affect the costs and benefits associated with HGT and antimicrobial resistance. Hence, we propose that One Health should be studied from a system perspective, as genetic and ecological interactions can alter the efficacy of antibiotics as well as the spread of antimicrobial resistance via HGT, either by imposing genetic constraints or by affecting the selective pressures.
From a community perspective, we showed that several eco-evolutionary drivers interact, influencing the emergence and spread of antimicrobial resistance. The evidence that species interactions affect conjugation rates emphasizes the need to revise antimicrobial resistance and HGT as a community rather than a population trait. Importantly, this should be addressed in the context of the costs of maintaining the antimicrobial resistance genes, for instance, when those are located in plasmids, but also considering their potential to persist in natural communities as they provide fitness to different members of the community, even under non-selective conditions. Exploring how ecological interactions affect antimicrobial resistance spread in the microbiome might help predict its spread via HGT72 and design strategies that would limit the selective pressure and hence, the emergence and spread of antibiotic resistance.
In this perspective, we also discussed how the environmental component of One Health, which is often neglected in studies on antimicrobial resistance, can contribute to antimicrobial resistance, even under weak selective pressure. As natural components of microbial warfare, ARGs are a constant in environments such as soils, where co-selection can lead to the spread of antibiotic resistance among bacterial populations. Anthropogenic disturbances or even overuse of chemicals such as pesticides in agriculture can have effects on increasing the likelihood of co-selecting ARGs,104 as well as in changing species dynamics and promoting a reduction in soil microbial diversity, generating communities that are less resilient to environmental perturbations. Finally, we argue that understanding antimicrobial resistance in the One Health context involves not only tracing its presence or transmission across different One Health reservoirs but also determining the selective pressures and barriers that may amplify or restrict the spread of resistance to clinically and environmentally relevant microbes.
Acknowledgments
MB was supported by the Faculty of Science and Engineering - Adaptive Life PhD Scholarship from the University of Groningen, awarded by GELIFES. SM was supported by the China Scholarship Council (CSC).
Declaration of interests
The authors declare no competing interests.
References
- 1.Ryan M. OECD; 2019. Evaluating the economic benefits and costs of antimicrobial use in food-producing animals (OECD) [Google Scholar]
- 2.Adebisi Y.A. Balancing the risks and benefits of antibiotic use in a globalized world: the ethics of antimicrobial resistance. Glob. Health. 2023;19:27. doi: 10.1186/s12992-023-00930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne A.J., Chipeta M.G., Haines-Woodhouse G., Kumaran E.P.A., Hamadani B.H.K., Zaraa S., Henry N.J., Deshpande A., Reiner R.C., Day N.P.J., et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet. Health. 2021;5:e893–e904. doi: 10.1016/S2542-5196(21)00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laxminarayan R., Duse A., Wattal C., Zaidi A.K.M., Wertheim H.F.L., Sumpradit N., Vlieghe E., Hara G.L., Gould I.M., Goossens H., et al. Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 5.Woolhouse M., Ward M., Van Bunnik B., Farrar J. Antimicrobial resistance in humans, livestock and the wider environment. Phil. Trans. Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashbolt N.J., Amézquita A., Backhaus T., Borriello P., Brandt K.K., Collignon P., Coors A., Finley R., Gaze W.H., Heberer T., et al. Human Health Risk Assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environ. Health Perspect. 2013;121:993–1001. doi: 10.1289/ehp.1206316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polianciuc S.I., Gurzău A.E., Kiss B., Ştefan M.G., Loghin F. Antibiotics in the environment: causes and consequences. Med. Pharm. Rep. 2020;93:231–240. doi: 10.15386/mpr-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackenzie J.S., Jeggo M. The One Health approach—why is it so important? Trop. Med. Infect. 2019;4:88. doi: 10.3390/tropicalmed4020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker S., Thomson N., Weill F.-X., Holt K.E. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Science. 2018;360:733–738. doi: 10.1126/science.aar3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meek R.W., Vyas H., Piddock L.J.V. Nonmedical uses of antibiotics: time to restrict their use? PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall J.P.J., Brockhurst M.A., Harrison E. Sampling the mobile gene pool: innovation via horizontal gene transfer in bacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuya E.Y., Lowy F.D. Antimicrobial-resistant bacteria in the community setting. Nat. Rev. Microbiol. 2006;4:36–45. doi: 10.1038/nrmicro1325. [DOI] [PubMed] [Google Scholar]
- 13.Frost L.S., Leplae R., Summers A.O., Toussaint A. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 2005;3:722–732. doi: 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- 14.MacLean R.C., San Millan A. The evolution of antibiotic resistance. Science. 2019;365:1082–1083. doi: 10.1126/science.aax3879. [DOI] [PubMed] [Google Scholar]
- 15.Samaha-Kfoury J.N., Araj G.F. Recent developments in β lactamases and extended spectrum β lactamases. BMJ. 2003;327:1209–1213. doi: 10.1136/bmj.327.7425.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillings M.R. Integrons: past, present, and future. Microbiol. Mol. Biol. Rev. 2014;78:257–277. doi: 10.1128/MMBR.00056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Partridge S.R., Kwong S.M., Firth N., Jensen S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018;31 doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Che Y., Yang Y., Xu X., Břinda K., Polz M.F., Hanage W.P., Zhang T. Conjugative plasmids interact with insertion sequences to shape the horizontal transfer of antimicrobial resistance genes. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2008731118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sastre-Dominguez J., DelaFuente J., Toribio-Celestino L., Herencias C., Herrador-Gomez P., Costas C., Hernandez-Garcia M., Canton R., Rodriguez-Beltran J., Santos-Lopez A., et al. Plasmid-encoded insertion sequences promote rapid adaptation in clinical enterobacteria. Nature Ecology ∖& Evolution. 2024;8:2097–2112. doi: 10.1038/s41559-024-02523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acman M., Wang R., Van Dorp L., Shaw L.P., Wang Q., Luhmann N., Yin Y., Sun S., Chen H., Wang H., Balloux F. Role of mobile genetic elements in the global dissemination of the carbapenem resistance gene bla NDM. Nat. Commun. 2022;13:1131. doi: 10.1038/s41467-022-28819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y.-Y., Wang Y., Walsh T.R., Yi L.-X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet. Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang R., van Dorp L., Shaw L.P., Bradley P., Wang Q., Wang X., Jin L., Zhang Q., Liu Y., Rieux A., et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018;9:1179. doi: 10.1038/s41467-018-03205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dini-Andreote F., van Elsas J.D., Olff H., Salles J.F. Dispersal-competition tradeoff in microbiomes in the quest for land colonization. Sci. Rep. 2018;8:9451. doi: 10.1038/s41598-018-27783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Li R., Hou J., Sun X., Wang Y., Li L., Yang F., Yao Y., An Y. Mobile genetic elements affect the dissemination of antibiotic resistance genes (ARGs) of clinical importance in the environment. Environ. Res. 2024;243 doi: 10.1016/j.envres.2023.117801. [DOI] [PubMed] [Google Scholar]
- 25.van Dijk B., Buffard P., Farr A.D., Giersdorf F., Meijer J., Dutilh B.E., Rainey P.B. Identifying and tracking mobile elements in evolving compost communities yields insights into the nanobiome. ISME Commun. 2023;3:90. doi: 10.1038/s43705-023-00294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coluzzi C., Guillemet M., Mazzamurro F., Touchon M., Godfroid M., Achaz G., Glaser P., Rocha E.P.C. Chance favors the prepared genomes: Horizontal Transfer shapes the emergence of antibiotic resistance mutations in core genes. Mol. Biol. Evol. 2023;40:msad217. doi: 10.1093/molbev/msad217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carattoli A. Plasmids and the spread of resistance. Int. J. Med. Microbiol. 2013;303:298–304. doi: 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Poirel L., Bonnin R.A., Nordmann P. Genetic features of the widespread plasmid coding for the Carbapenemase OXA-48. Antimicrob. Agents Chemother. 2012;56:559–562. doi: 10.1128/AAC.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2016;3:15–21. doi: 10.1177/2049936115621709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bustamante M., Koopman F., Martens J., Brons J.K., DelaFuente J., Kuipers O.P., Van Doorn S., De Vos M.G.J. Community context influences the conjugation efficiency of E. coli. bioRxiv. 2024 doi: 10.1101/2024.01.30.577951. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso-del Valle A., León-Sampedro R., Rodríguez-Beltrán J., DelaFuente J., Hernández-García M., Ruiz-Garbajosa P., Cantón R., Peña-Miller R., San Millán A. Variability of plasmid fitness effects contributes to plasmid persistence in bacterial communities. Nat. Commun. 2021;12:2653. doi: 10.1038/s41467-021-22849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson D.I., Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat. Rev. Microbiol. 2010;8:260–271. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 33.Bell G., MacLean C. The search for ‘evolution-proof’ antibiotics. Trends Microbiol. 2018;26:471–483. doi: 10.1016/j.tim.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Schulz Zur Wiesch P., Engelstädter J., Bonhoeffer S. Compensation of fitness costs and reversibility of antibiotic resistance mutations. Antimicrob. Agents Chemother. 2010;54:2085–2095. doi: 10.1128/AAC.01460-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogwill T., MacLean R.C. The genetic basis of the fitness costs of antimicrobial resistance: a meta-analysis approach. Evol. Appl. 2015;8:284–295. doi: 10.1111/eva.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckner M.M.C., Saw H.T.H., Osagie R.N., McNally A., Ricci V., Wand M.E., Woodford N., Ivens A., Webber M.A., Piddock L.J.V. Clinically relevant plasmid-host interactions indicate that transcriptional and not genomic modifications ameliorate fitness costs of Klebsiella pneumoniae Carbapenemase-carrying plasmids. mBio. 2018;9 doi: 10.1128/mBio.02303-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deekshit V.K., Srikumar S. ‘To be, or not to be’—The dilemma of ‘silent’ antimicrobial resistance genes in bacteria. J. Appl. Microbiol. 2022;133:2902–2914. doi: 10.1111/jam.15738. [DOI] [PubMed] [Google Scholar]
- 38.Jeannot K., Sobel M.L., El Garch F., Poole K., Plésiat P. Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J. Bacteriol. 2005;187:5341–5346. doi: 10.1128/JB.187.15.5341-5346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whittle E.E., McNeil H.E., Trampari E., Webber M., Overton T.W., Blair J.M.A. Efflux impacts intracellular accumulation only in actively growing bacterial cells. mBio. 2021;12 doi: 10.1128/mBio.02608-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knöppel A., Näsvall J., Andersson D.I. Evolution of antibiotic resistance without antibiotic exposure. Antimicrobial agents and chemotherapy. 2017;61 doi: 10.1128/AAC.01495-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandis G., Wrande M., Liljas L., Hughes D. Fitness-compensatory mutations in rifampicin-resistant RNA polymerase. Mol. Microbiol. 2012;85:142–151. doi: 10.1111/j.1365-2958.2012.08099.x. [DOI] [PubMed] [Google Scholar]
- 42.Paulander W., Maisnier-Patin S., Andersson D.I. The fitness cost of streptomycin resistance depends on rpsL mutation, carbon source and RpoS ($∖sigma$S) Genetics. 2009;183:539–546. doi: 10.1534/genetics.109.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodríguez-Verdugo A., Gaut B.S., Tenaillon O. Evolution of Escherichia coli rifampicin resistance in an antibiotic-free environment during thermal stress. BMC Evol. Biol. 2013;13:50. doi: 10.1186/1471-2148-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt H., Hensel M. Pathogenicity islands in bacterial pathogenesis. Clin. Microbiol. Rev. 2004;17:14–56. doi: 10.1128/CMR.17.1.14-56.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall R.J., Snaith A.E., Thomas M.J.N., Brockhurst M.A., McNally A. Multidrug resistance plasmids commonly reprogram the expression of metabolic genes in Escherichia coli. mSystems. 2024;9 doi: 10.1128/msystems.01193-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biggel M., Moons P., Nguyen M.N., Goossens H., Van Puyvelde S. Convergence of virulence and antimicrobial resistance in increasingly prevalent Escherichia coli ST131 papGII+ sublineages. Commun. Biol. 2022;5 doi: 10.1038/s42003-022-03660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connor C.H., Zucoloto A.Z., Munnoch J.T., Yu I.-L., Corander J., Hoskisson P.A., McDonald B., McNally A. Multidrug-resistant E. coli encoding high genetic diversity in carbohydrate metabolism genes displace commensal E. coli from the intestinal tract. PLoS Biol. 2023;21 doi: 10.1371/journal.pbio.3002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calland J.K., Haukka K., Kpordze S.W., Brusah A., Corbella M., Merla C., Samuelsen Ø., Feil E.J., Sassera D., Karikari A.B., et al. Population structure and antimicrobial resistance among Klebsiella isolates sampled from human, animal, and environmental sources in Ghana: a cross-sectional genomic One Health study. Lancet. Microbe. 2023;4:e943–e952. doi: 10.1016/S2666-5247(23)00208-2. [DOI] [PubMed] [Google Scholar]
- 49.Matlock W., Lipworth S., Chau K.K., AbuOun M., Barker L., Kavanagh J., Andersson M., Oakley S., Morgan M., Crook D.W., et al. Enterobacterales plasmid sharing amongst human bloodstream infections, livestock, wastewater, and waterway niches in Oxfordshire, UK. eLife. 2023;12 doi: 10.7554/eLife.85302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benz F., Hall A.R. Host-specific plasmid evolution explains the variable spread of clinical antibiotic-resistance plasmids. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2212147120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bottery M.J., Matthews J.L., Wood A.J., Johansen H.K., Pitchford J.W., Friman V.-P. Inter-species interactions alter antibiotic efficacy in bacterial communities. The ISME journal. 2022;16:812–821. doi: 10.1038/s41396-021-01130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klümper U., Recker M., Zhang L., Yin X., Zhang T., Buckling A., Gaze W.H. Selection for antimicrobial resistance is reduced when embedded in a natural microbial community. The ISME journal. 2019;13:2927–2937. doi: 10.1038/s41396-019-0483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bottery M.J., Pitchford J.W., Friman V.-P. Ecology and evolution of antimicrobial resistance in bacterial communities. The ISME Journal. 2021;15:939–948. doi: 10.1038/s41396-020-00832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nair R.R., Andersson D.I. Interspecies interaction reduces selection for antibiotic resistance in Escherichia coli. Commun. Biol. 2023;6 doi: 10.1038/s42003-023-04716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jain R., Rivera M.C., Moore J.E., Lake J.A. Horizontal gene transfer accelerates genome innovation and evolution. Mol. Biol. Evol. 2003;20:1598–1602. doi: 10.1093/molbev/msg154. [DOI] [PubMed] [Google Scholar]
- 56.Smets B.F., Barkay T. Horizontal gene transfer: perspectives at a crossroads of scientific disciplines. Nat. Rev. Microbiol. 2005;3:675–678. doi: 10.1038/nrmicro1253. [DOI] [PubMed] [Google Scholar]
- 57.Lara E.G., van der Windt I., Molenaar D., de Vos M.G.J., Melkonian C. Using functional annotations to study pairwise interactions in urinary tract infection communities. Genes (Basel) 2021;12:1221. doi: 10.3390/genes12081221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melkonian C., Seidl M.F., van der Hooft J.J.J., de Vos M.G.J. Metabolic interactions shape a community’s phenotype. Trends Microbiol. 2022;30:609–611. doi: 10.1016/j.tim.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Yu J.S.L., Correia-Melo C., Zorrilla F., Herrera-Dominguez L., Wu M.Y., Hartl J., Campbell K., Blasche S., Kreidl M., Egger A.-S., et al. Microbial communities form rich extracellular metabolomes that foster metabolic interactions and promote drug tolerance. Nat. Microbiol. 2022;7:542–555. doi: 10.1038/s41564-022-01072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fux C.A., Costerton J.W., Stewart P.S., Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Karslake J., Maltas J., Brumm P., Wood K.B. Population density modulates drug inhibition and gives rise to potential bistability of treatment outcomes for bacterial infections. PLoS Comput. Biol. 2016;12 doi: 10.1371/journal.pcbi.1005098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meredith H.R., Srimani J.K., Lee A.J., Lopatkin A.J., You L. Collective antibiotic tolerance: mechanisms, dynamics and intervention. Nat. Chem. Biol. 2015;11:182–188. doi: 10.1038/nchembio.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorg R.A., Lin L., van Doorn G.S., Sorg M., Olson J., Nizet V., Veening J.-W. Collective resistance in microbial communities by intracellular antibiotic deactivation. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.2000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yurtsev E.A., Chao H.X., Datta M.S., Artemova T., Gore J. Bacterial cheating drives the population dynamics of cooperative antibiotic resistance plasmids. Mol. Syst. Biol. 2013;9:683. doi: 10.1038/msb.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frost I., Smith W.P.J., Mitri S., Millan A.S., Davit Y., Osborne J.M., Pitt-Francis J.M., MacLean R.C., Foster K.R. Cooperation, competition and antibiotic resistance in bacterial colonies. The ISME journal. 2018;12:1582–1593. doi: 10.1038/s41396-018-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medaney F., Dimitriu T., Ellis R.J., Raymond B. Live to cheat another day: bacterial dormancy facilitates the social exploitation of β-lactamases. The ISME journal. 2016;10:778–787. doi: 10.1038/ismej.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee H.H., Molla M.N., Cantor C.R., Collins J.J. Bacterial charity work leads to population-wide resistance. Nature. 2010;467:82–85. doi: 10.1038/nature09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pathak A., Angst D.C., León-Sampedro R., Hall A.R. Antibiotic-degrading resistance changes bacterial community structure via species-specific responses. The ISME Journal. 2023;17:1495–1503. doi: 10.1038/s41396-023-01465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bush K. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr. Opin. Microbiol. 2010;13:558–564. doi: 10.1016/j.mib.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Poole K. Resistance to $∖beta$-lactam antibiotics. Cell. Mol. Life Sci. 2004;61:2200–2223. doi: 10.1007/s00018-004-4060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H.Y., Prentice E.L., Webber M.A. Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2024;2:27. doi: 10.1038/s44259-024-00046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cooper R.M., Tsimring L., Hasty J. Inter-species population dynamics enhance microbial horizontal gene transfer and spread of antibiotic resistance. eLife. 2017;6 doi: 10.7554/eLife.25950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mah T.-F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012;7:1061–1072. doi: 10.2217/fmb.12.76. [DOI] [PubMed] [Google Scholar]
- 74.Abe K., Nomura N., Suzuki S. Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020;96:fiaa031. doi: 10.1093/femsec/fiaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kottara A., Carrilero L., Harrison E., Hall J.P.J., Brockhurst M.A. The dilution effect limits plasmid horizontal transmission in multispecies bacterial communities. Microbiology. 2021;167 doi: 10.1099/mic.0.001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beaber J.W., Hochhut B., Waldor M.K. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature. 2004;427:72–74. doi: 10.1038/nature02241. [DOI] [PubMed] [Google Scholar]
- 77.Guerin É., Cambray G., Sanchez-Alberola N., Campoy S., Erill I., Da Re S., Gonzalez-Zorn B., Barbé J., Ploy M.-C., Mazel D. The SOS response controls integron recombination. Science. 2009;324 doi: 10.1126/science.1172914. [DOI] [PubMed] [Google Scholar]
- 78.Lopatkin A.J., Huang S., Smith R.P., Srimani J.K., Sysoeva T.A., Bewick S., Karig D.K., You L. Antibiotics as a selective driver for conjugation dynamics. Nat. Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Varga J.J., Zhao C.Y., Davis J.D., Hao Y., Farrell J.M., Gurney J.R., Voit E., Brown S.P. Antibiotics drive expansion of rare pathogens in a chronic infection microbiome model. mSphere. 2022;7 doi: 10.1128/msphere.00318-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sünderhauf D., Klümper U., Gaze W.H., Westra E.R., van Houte S. Interspecific competition can drive plasmid loss from a focal species in a microbial community. ISME J. 2023;17:1–9. doi: 10.1038/s41396-023-01487-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall J.P.J., Wright R.C.T., Harrison E., Muddiman K.J., Wood A.J., Paterson S., Brockhurst M.A. Plasmid fitness costs are caused by specific genetic conflicts enabling resolution by compensatory mutation. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lopatkin A.J., Meredith H.R., Srimani J.K., Pfeiffer C., Durrett R., You L. Persistence and reversal of plasmid-mediated antibiotic resistance. Nat. Commun. 2017;8:1689. doi: 10.1038/s41467-017-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Elsas J.D., Turner S., Bailey M.J. Horizontal gene transfer in the phytosphere. New Phytol. 2003;157:525–537. doi: 10.1046/j.1469-8137.2003.00697.x. [DOI] [PubMed] [Google Scholar]
- 84.Daane L.L., Molina J.A., Berry E.C., Sadowsky M.J. Influence of earthworm activity on gene transfer from Pseudomonas fluorescens to indigenous soil bacteria. Appl. Environ. Microbiol. 1996;62:515–521. doi: 10.1128/aem.62.2.515-521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li L., Dechesne A., Madsen J.S., Nesme J., Sørensen S.J., Smets B.F. Plasmids persist in a microbial community by providing fitness benefit to multiple phylotypes. The ISME journal. 2020;14:1170–1181. doi: 10.1038/s41396-020-0596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trampari E., Holden E.R., Wickham G.J., Ravi A., Martins L.d.O., Savva G.M., Webber M.A. Exposure of Salmonella biofilms to antibiotic concentrations rapidly selects resistance with collateral tradeoffs. npj Biofilms Microbiomes. 2021;7 doi: 10.1038/s41522-020-00178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Metzger G.A., Ridenhour B.J., France M., Gliniewicz K., Millstein J., Settles M.L., Forney L.J., Stalder T., Top E.M. Biofilms preserve the transmissibility of a multi-drug resistance plasmid. npj Biofilms Microbiomes. 2022;8 doi: 10.1038/s41522-022-00357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Røder H.L., Trivedi U., Russel J., Kragh K.N., Herschend J., Thalsø-Madsen I., Tolker-Nielsen T., Bjarnsholt T., Burmølle M., Madsen J.S. Biofilms can act as plasmid reserves in the absence of plasmid specific selection. npj Biofilms Microbiomes. 2021;7:1–6. doi: 10.1038/s41522-021-00249-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duxbury S.J.N., Alderliesten J.B., Zwart M.P., Stegeman A., Fischer E.A.J., de Visser J.A.G.M. Chicken gut microbiome members limit the spread of an antimicrobial resistance plasmid in Escherichia coli. Proc. Biol. Sci. 2021;288 doi: 10.1098/rspb.2021.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noor Z.Z., Rabiu Z., Sani M.H.M., Samad A.F.A., Kamaroddin M.F.A., Perez M.F., Dib J.R., Fatima H., Sinha R., Khare S.K., Zakaria Z.A. A review of bacterial antibiotic resistance genes and their removal strategies from wastewater. Curr. Pollut. Rep. 2021;7:494–509. [Google Scholar]
- 91.Dasgupta A. In: Advances in Clinical Chemistry. Makowski G.S., editor. Elsevier; 2012. Chapter 3 - Advances in antibiotic measurement; pp. 75–104. [DOI] [PubMed] [Google Scholar]
- 92.El-Naggar N.E.-A. In: Microbial Cell Factories Engineering for Production of Biomolecules. Singh V., editor. Academic Press; 2021. Streptomyces-based cell factories for production of biomolecules and bioactive metabolites; pp. 183–234. [Google Scholar]
- 93.Delgado-Baquerizo M., Hu H.-W., Maestre F.T., Guerra C.A., Eisenhauer N., Eldridge D.J., Zhu Y.-G., Chen Q.-L., Trivedi P., Du S., et al. The global distribution and environmental drivers of the soil antibiotic resistome. Microbiome. 2022;10:219. doi: 10.1186/s40168-022-01405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Costa V.M., King C.E., Kalan L., Morar M., Sung W.W.L., Schwarz C., Froese D., Zazula G., Calmels F., Debruyne R., et al. Antibiotic resistance is ancient. Nature. 2011;477:457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 95.Nunan N. The microbial habitat in soil: Scale, heterogeneity and functional consequences. J. Plant Nutr. Soil Sci. 2017;180:425–429. doi: 10.1002/jpln.201700184. [DOI] [Google Scholar]
- 96.Sørensen S.J., Bailey M., Hansen L.H., Kroer N., Wuertz S. Studying plasmid horizontal transfer in situ: a critical review. Nat. Rev. Microbiol. 2005;3:700–710. doi: 10.1038/nrmicro1232. [DOI] [PubMed] [Google Scholar]
- 97.Wang F., Han W., Chen S., Dong W., Qiao M., Hu C., Liu B. Fifteen-year application of manure and chemical fertilizers differently impacts soil ARGs and microbial community structure. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai P., Sun X., Wu Y., Gao C., Mortimer M., Holden P.A., Redmile-Gordon M., Huang Q. Soil biofilms: microbial interactions, challenges, and advanced techniques for ex-situ characterization. Soil Ecol. Lett. 2019;1:85–93. [Google Scholar]
- 99.Leão I., Khalifa L., Gallois N., Vaz-Moreira I., Klümper U., Youdkes D., Palmony S., Dagai L., Berendonk T.U., Merlin C., et al. Microbiome and resistome profiles along a sewage-effluent-reservoir trajectory underline the role of natural attenuation in wastewater stabilization reservoirs. Applied and Environmental Microbiology. 2023;89 doi: 10.1128/aem.00170-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wales A.D., Davies R.H. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics. 2015;4:567–604. doi: 10.3390/antibiotics4040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hughes V.M., Datta N. Conjugative plasmids in bacteria of the ‘pre-antibiotic’ era. Nature. 1983;302:725–726. doi: 10.1038/302725a0. [DOI] [PubMed] [Google Scholar]
- 102.Cantón R., Ruiz-Garbajosa P. Co-resistance: an opportunity for the bacteria and resistance genes. Curr. Opin. Pharmacol. 2011;11:477–485. doi: 10.1016/j.coph.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 103.Murray L.M., Hayes A., Snape J., Kasprzyk-Hordern B., Gaze W.H., Murray A.K. Co-selection for antibiotic resistance by environmental contaminants. npj Antimicrob. Resist. 2024;2:9. [Google Scholar]
- 104.Xing Y., Wu S., Men Y. Exposure to environmental levels of pesticides stimulates and diversifies evolution in Escherichia coli toward higher antibiotic resistance. Environ. Sci. Technol. 2020;54:8770–8778. doi: 10.1021/acs.est.0c01155. [DOI] [PubMed] [Google Scholar]
- 105.Singh C.K., Sodhi K.K., Shree P., Nitin V. Heavy Metals as Catalysts in the Evolution of Antimicrobial Resistance and the Mechanisms Underpinning Co-selection. Curr. Microbiol. 2024;81:148. doi: 10.1007/s00284-024-03648-2. [DOI] [PubMed] [Google Scholar]
- 106.Chen J., Li J., Zhang H., Shi W., Liu Y. Bacterial Heavy-Metal and Antibiotic Resistance Genes in a Copper Tailing Dam Area in Northern China. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun M., Ye M., Wu J., Feng Y., Shen F., Tian D., Liu K., Hu F., Li H., Jiang X., et al. Impact of bioaccessible pyrene on the abundance of antibiotic resistance genes during Sphingobium sp.- and sophorolipid-enhanced bioremediation in soil. J. Hazard. Mater. 2015;300:121–128. doi: 10.1016/j.jhazmat.2015.06.065. [DOI] [PubMed] [Google Scholar]
- 108.McMillan E.A., Gupta S.K., Williams L.E., Jové T., Hiott L.M., Woodley T.A., Barrett J.B., Jackson C.R., Wasilenko J.L., Simmons M., et al. Antimicrobial resistance genes, cassettes, and plasmids present in Salmonella enterica associated with United States food animals. Frontiers in microbiology. 2019;10 doi: 10.3389/fmicb.2019.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heydari A., Kim N.D., Horswell J., Gielen G., Siggins A., Taylor M., Bromhead C., Palmer B.R. Co-Selection of Heavy Metal and Antibiotic Resistance in Soil Bacteria from Agricultural Soils in New Zealand. Sustainability. 2022;14:1790. doi: 10.3390/su14031790. [DOI] [Google Scholar]
- 110.Cury J., Oliveira P.H., de la Cruz F., Rocha E.P.C. Host range and genetic plasticity explain the coexistence of integrative and extrachromosomal mobile genetic elements. Mol. Biol. Evol. 2018;35:2230–2239. doi: 10.1093/molbev/msy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aminov R.I. Horizontal Gene Exchange in environmental microbiota. Front. Microbiol. 2011;2:158. doi: 10.3389/fmicb.2011.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klümper U., Gionchetta G., Catão E., Bellanger X., Dielacher I., Elena A.X., Fang P., Galazka S., Goryluk-Salmonowicz A., Kneis D., et al. Environmental microbiome diversity and stability is a barrier to antimicrobial resistance gene accumulation. Commun. Biol. 2024;7:706. doi: 10.1038/s42003-024-06338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maurya A.P., Rajkumari J., Pandey P. Enrichment of antibiotic resistance genes (ARGs) in polyaromatic hydrocarbon–contaminated soils: a major challenge for environmental health. Environmental Science and Pollution Research. 2021;28:12178–12189. doi: 10.1007/s11356-020-12171-3. [DOI] [PubMed] [Google Scholar]
- 114.Cunningham C.J., Kuyukina M.S., Ivshina I.B., Konev A.I., Peshkur T.A., Knapp C.W. Potential risks of antibiotic resistant bacteria and genes in bioremediation of petroleum hydrocarbon contaminated soils. Environ. Sci. Process. Impacts. 2020;22:1110–1124. doi: 10.1039/c9em00606k. [DOI] [PubMed] [Google Scholar]
- 115.Jurburg S.D., Nunes I., Brejnrod A., Jacquiod S., Priemé A., Sørensen S.J., Van Elsas J.D., Salles J.F. Legacy effects on the recovery of soil bacterial communities from extreme temperature perturbation. Front. Microbiol. 2017;8:1832. doi: 10.3389/fmicb.2017.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barlow J., Lennox G.D., Ferreira J., Berenguer E., Lees A.C., Mac Nally R., Thomson J.R., Ferraz S.F.d.B., Louzada J., Oliveira V.H.F., et al. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature. 2016;535:144–147. doi: 10.1038/nature18326. [DOI] [PubMed] [Google Scholar]
- 117.Coyte K.Z., Stevenson C., Knight C.G., Harrison E., Hall J.P.J., Brockhurst M.A. Horizontal gene transfer and ecological interactions jointly control microbiome stability. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Uluseker C., Kaster K.M., Thorsen K., Basiry D., Shobana S., Jain M., Kumar G., Kommedal R., Pala-Ozkok I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.717809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sabatino R., Sbaffi T., Sivalingam P., Corno G., Fontaneto D., Di Cesare A. Bacteriophages limitedly contribute to the antimicrobial resistome of microbial communities in wastewater treatment plants. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.01101-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pfeifer E., Bonnin R.A., Rocha E.P.C. Phage-Plasmids Spread Antibiotic Resistance Genes through Infection and Lysogenic Conversion. mBio. 2022;13 doi: 10.1128/mbio.01851-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li T., Xu J., Zhao X., Zhang Q., Zhu T., Fan D., Liu J. Impacts of irrigation with treated livestock wastewater on the accumulation characteristic of ARGs in the farmland soil: a case study in Hohhot, China. Environ. Geochem. Health. 2024;46:26. doi: 10.1007/s10653-023-01811-5. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Q., Zhang Z., Lu T., Yu Y., Penuelas J., Zhu Y.-G., Qian H. Gammaproteobacteria, a core taxon in the guts of soil fauna, are potential responders to environmental concentrations of soil pollutants. Microbiome. 2021;9:196. doi: 10.1186/s40168-021-01150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sengeløv G., Kowalchuk G.A., Sørensen S.J. Influence of fungal-bacterial interactions on bacterial conjugation in the residuesphere. FEMS Microbiol. Ecol. 2000;31:39–45. doi: 10.1016/s0168-6496(99)00079-3. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y., Zhou J., Wu J., Hua Q., Bao C. Distribution and transfer of antibiotic resistance genes in different soil–plant systems. Environmental Science and Pollution Research. 2022;29:59159–59172. doi: 10.1007/s11356-021-17465-8. [DOI] [PubMed] [Google Scholar]
- 125.Emamalipour M., Seidi K., Zununi Vahed S., Jahanban-Esfahlan A., Jaymand M., Majdi H., Amoozgar Z., Chitkushev L.T., Javaheri T., Jahanban-Esfahlan R., Zare P. Horizontal gene transfer: from evolutionary flexibility to disease progression. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]