Simple Summary
The traditional three-tier grading system for endometrioid endometrial cancer has been replaced in research—and recently in clinical practise—by a binary system that combines grade 1 and grade 2 tumours under the low-grade umbrella. This simplification attempts to overcome diagnostic clinicopathological challenges but overlooks the unique nature of grade 2 endometrioid endometrial tumours. The aim of the present study is to investigate the unique prognostic characteristics and recurrence patterns of grade 2 endometrioid endometrial cancers and elucidate where the prognosis of grade 2 tumours stands in the spectrum between grade 1 and grade 3 cancers.
Keywords: G2, grade 2, low grade, moderately differentiated, endometrioid endometrial cancer
Abstract
Background: Although grade is a well-recognised prognostic factor for endometrioid endometrial cancer (EEC), in more studies grade 1 (G1) and grade 2 (G2) EEC are combined and compared together with grade 3 (G3) tumours. The aim of our study is to separately investigate the outcomes, prognostic factors and recurrence patterns of G2 EEC and whether the differentiation between G1 and G2 EEC is clinically useful. Methods: we retrospectively reviewed 523 patients with EEC treated with primary surgery over a decade (March 2010–January 2020) at Oxford University Hospitals NHS Trust, focusing on those with G2 disease. Results: Patients with G2 EEC had worse 5-year cancer-specific survival (93.3% vs. 98.5%, p < 0.01) compared to patients with G1 EEC, but a favourable prognosis compared to G3 EEG, both in terms of disease-free survival (91.6 vs. 83.8%, p = 0.04) and cancer-specific survival (93.3% vs. 78.5%, p < 0.01). Both stage and grade are independent risk factors for cancer-specific mortality in EEC. Cervical stromal involvement, parametrial involvement and distant metastatic disease are all independent risk factors for cancer-related mortality in G2 ECC. Only 12.5% of recurrences of G2 EEC were diagnosed with examination in routine follow up in asymptomatic patients. Conclusions: our results suggest that the grading system should continue to differentiate G1 EEC and G2 EEC for better prognosis interpretation.
1. Introduction
In 2020, 417,000 women were diagnosed and 97,000 died of uterine corpus cancer, the sixth most common cancer in women [1]. Almost 80% of endometrial cancers are endometrioid endometrial carcinomas (EECs), which are traditionally characterised as grade 1 (G1), or well differentiated; grade 2 (G2), or moderately differentiated; and grade 3 (G3), or poorly differentiated, according to architectural features and the extent of nuclear atypia [2].
Current grading criteria, according to the WHO Classification of Tumours, are primarily based on architectural features but also consider cytologic atypia [3]. Histological grade has always been one of the widely recognised prognostic factors for EEC [4]. However, many clinicians combine G1 and G2 tumours to a single low-grade category to increase inter- and intra-observer agreement and the reproducibility of diagnosis [5]. This binary tumour grading system has been adopted by FIGO [6] and ESMO/ESTRO/ESP [7].
The switch from the traditional three-tier to the binary grading system was not imposed by the prognostic similarities between G1 and G2 but was a result of the diagnostic challenge of distinguishing between G1 and G2. The wide use of the binary grading system is the main reason for the paucity of evidence about the prognosis of G2 EEC. The aim of the present study is to investigate the prognostic characteristics and recurrence patterns of G2 ECC and elucidate where the prognosis of G2 tumours stands in the spectrum between G1 and G3 EEC.
2. Materials and Methods
Our retrospective study included all patients with EEC treated at Churchill Cancer Centre and John Radcliffe Women’s Centre, Oxford, between March 2010 and January 2020. The following exclusion criteria were implemented: a non-endometrioid histology, the presence of synchronous malignancy, primary non-surgical management, and suboptimal follow up (patient with less than 2 years follow up) [8].
Demographic, treatment, and survival data were retrieved from electronic and hard copy records of patients for a service evaluation project on endometrial cancer. The relevant protocol was registered (registration number 5832) [9] and approved by the Institutional Review Board of Oxford University Hospitals NSH Trust (ID: 5832, Ref:06/01/2020-SUWON-Soleymani-2). The data collected were anonymized and all patients had signed a relevant consent form before surgery. The study design complies with the Helsinki Declaration, the Committee on Publication Ethics guidelines [10] and the Reporting of studies Conducted using Observational Routinely collected health Data (RECORD) Statement validated by the Enhancing the Quality and Transparency of Health Research Network [11,12].
The initial treatment of all the patients of the study was surgery (laparoscopic or open hysterectomy and bilateral salpingo-oophorectomy). Pelvic lymphadenectomy, omental biopsy and adjuvant treatment was offered to all except from low-risk patients [13]. Follow up was conducted with clinical examination at three-month intervals for the first year after treatment, four-month intervals for the second year and annually thereafter [13]. Grade was confirmed in the final histology as follows: G1: less than 5% of a nonsquamous or nonmorular solid growth pattern; G2: 6–50% of a nonsquamous or nonmorular solid growth pattern; and G3: greater than 50% of a nonsquamous or nonmorular solid growth pattern [14]. Excessive nuclear atypia raised the grade from G1 and G2 to G2 and G3, respectively [14].
Patients’ comorbidities were summarised using the Age-Adjusted Charlson Comorbidity score (AAC score) [15] and patients were divided according to their fitness in three groups (0–1, 2–3 and >3). We have also retrieved data regarding FIGO 2009 Stage [16], the depth of myometrial invasion, cervical stromal involvement, serosal breaching, adnexal, parametrial and pelvic lymph node involvement, the presence of distant metastases, and the presence of lymphovascular invasion (LVSI). All cases were classified according to the ESGO-ESTRO-ESP risk stratification model [7]. Finally, we collected data regarding surgical approach, pelvic lymphadenectomy, and the administration of adjuvant treatment.
Statistical analysis was performed using IBM©SPSS Statistics 22.0. and statistical significance was considered for p < 0.05. Kaplan–Meier Curves and a log-rank test were used to calculate and compare survival rates. The contribution of potential risk factors to relapse and mortality for G2 endometrioid endometrial cancer was assessed using univariate and multivariate Cox proportional hazards analysis.
3. Results
Out of the 523 patients with endometrioid endometrial cancer of our cohort, 238 (45.5%) were G1, 189 (36.1%) G2 and 96 (18.4%) were high grade.
Patients with G2 EEC had a mean age of 67.29 years (range 26–91 years) and mean BMI of 32.83kg/m2 (range 16–70.9). Of those patients, 92.5% had a laparoscopic surgical staging, but only 35.4% had pelvic lymphadenectomy/sampling, with an average of 14.25 removed nodes (range 1–25). In total, 85.8% of patients with G2 EEC received the indicated (where indicated) adjuvant treatment, whereas 14.2% of patients did not, either because they were not fit, or because they declined. Most of the patients (83.1%) with G2 EEC were diagnosed with stage I or stage II disease (early stage) and in almost two thirds (66.1%) there was lymphovascular invasion present on final histology. Demographic data, treatment details and the clinicopathological characteristics of G2 EEC are summarised in Table 1.
Table 1.
Demographic data, treatment details and clinicopathological characteristics of patients with G2 EEC.
| N (%) | Recurrence (% of Each Subgroup) | Cancer-Specific Mortality (% of Each Subgroup) | |
|---|---|---|---|
| Age | |||
| <65 | 73 (38.6) | 6 (8.2) | 7 (9.6) |
| ≥65 | 116 (61.4) | 10 (8.6) | 11 (9.5) |
| AACCS | |||
| 0–1 | 43 (22.8) | 4 (9.3) | 6 (14) |
| 2–3 | 93 (49.2) | 2 (2.2) | 3 (3.2) |
| >3 | 53 (28) | 10 (18.9) | 9 (17) |
| Surgical approach | |||
| Laparoscopy | 147 (92.5) | 12 (8.2) | 14 (9.5) |
| Laparotomy | 12 (7.5) | 2 (16.7) | 2 (16.7) |
| Pelvic lymph node dissection | |||
| No | 122 (64.6) | 14 (11.5) | 15 (12.3) |
| Yes | 67 (35.4) | 2 (3) | 3 (4.5) |
| Administration of indicated adjuvant treatment | |||
| No | 23 (14.2) | 4 (17.4) | 4 (14.4) |
| Yes | 139 (85.8) | 11 (7.9) | 13 (9.4) |
| FIGO Stage | |||
| IA | 91 (48.1) | 3 (3.3) | 2 (2.2) |
| ΙΒ | 54 (28.6) | 9 (16.7) | 7 (13) |
| ΙΙ | 12 (6.3) | 0 (0) | 0 (0) |
| ΙΙΙA | 12 (6.3) | 2 (16.7) | 2 (16.7) |
| ΙΙΙΒ | 14 (7.4) | 2 (14.3) | 4 (28.6) |
| ΙΙΙC1 | 3 (1.6) | 0 (0) | 0 (0) |
| IIIC2 | 1 (0.5) | 0 (0) | 1 (100) |
| IVB | 2 (1.1) | 0 (0) | 2 (100) |
| Stage category | |||
| Early (I–II) | 157 (83.1) | 12 (7.6) | 9 (5.7) |
| Advanced (III–IV) | 32 (16.9) | 4 (12.5) | 9 (28.1) |
| Myometrial invasion | |||
| <50% | 104 (55) | 5 (4.8) | 6 (5.8) |
| ≥50% | 85 (45) | 11 (12.9) | 12 (14.1) |
| Cervical stroma involvement | |||
| No | 159 (84.1) | 15 (9.4) | 14 (8.8) |
| Yes | 30 (15.9) | 1 (3.3) | 4 (13.3) |
| Adnexal involvement | |||
| No | 182 (96.3) | 15 (8.2) | 17 (9.3) |
| Yes | 7 (3.7) | 1 (14.3) | 1 (14.3) |
| Serosal breach | |||
| No | 180 (95.2) | 15 (8.3) | 16 (8.9) |
| Yes | 9 (4.8) | 1 (11.1) | 2 (22.2) |
| Parametrial involvement | |||
| No | 174 (92.1) | 14 (8) | 13 (7.5) |
| Yes | 15 (7.9) | 2 (13.3) | 5 (33.3) |
| Pelvic lymph node involvement | |||
| No | 185 (97.9) | 16 (8.6) | 17 (9.2) |
| Yes | 4 (2.1) | 0 | 1 (25) |
| Distant metastases | |||
| No | 187 (98.9) | 16 (8.6) | 16 (8.6) |
| Yes | 2 (1.1) | 0 | 2 (100) |
| LVSI | |||
| No | 125 (66.1) | 7 (5.6) | 8 (6.4) |
| Yes | 64 (33.9) | 9 (14.1) | 10 (15.6) |
| ESGO-ESTRO-ESP Risk stratification | |||
| Low | 76 (40.2) | 3 (3.9) | 2 (2.6) |
| Intermediate | 33 (17.5) | 3 (9.1) | 3 (9.1) |
| High-intermediate | 48 (25.4) | 6 (12.5) | 4 (8.3) |
| High | 32 (16.9) | 4 (12.5) | 9 (28.1) |
Overall, 5-year and cancer-specific survival for G2 EEC was 88% (mean 131.54 months; 95% CI 125.37–137.71) and 93.3% (mean 139.37 months; 95% CI 134.25–144.49) respectively. Recurrence rate was 8.5% and 5-year disease-free survival was 91.6% (mean 139.95 months; 95% CI 134.76–145.15).
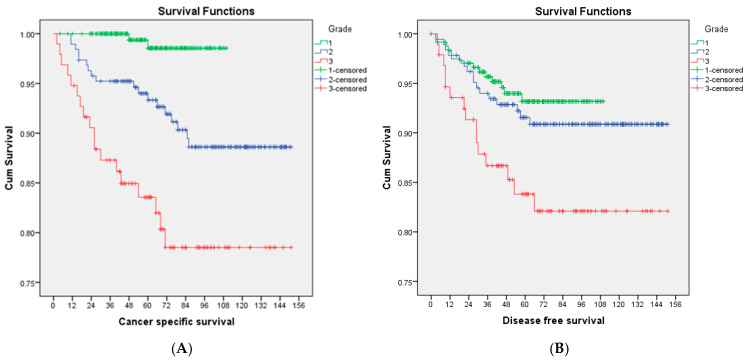
Patients with G2 EEC had comparable 5-year disease-free survival rates (91.6% vs. 93.2%, p = 0.44), but worse 5-year cancer-specific survival (93.3% vs. 98.5%, p < 0.01) compared to patients with G1 EEC. On the other hand, G2 EEC has a favourable prognosis compared to G3 EEC, both in terms of disease-free survival (91.6 vs. 83.8%, p = 0.04) and cancer-specific survival (93.3% vs. 83.6%, p < 0.01) (Figure 1). Multivariable Cox analysis showed that only stage is an independent risk factor for recurrence and that both grade and stage are independent risk factors for cancer-specific mortality in endometrioid endometrial cancer after adjusting for LVSI stage and grade.
Figure 1.
(A) Cancer-specific survival and (B) Disease-free survival of G1 EEC (green), G2 EEC (blue) and G3 EEC (red) in months.
Cox proportional hazard ratio analysis including multiple variables (AACCS, pelvic lymphadenectomy, adjuvant treatment, depth of myometrial invasion, cervical stromal invasion, adnexal involvement, serosal breach, parametrial invasion, pelvic lymph node involvement, distant metastases, and LVSI) showed that none of the above are independent risk factors for recurrence for G2 EEC. On the other hand, cervical stromal involvement (OR = 10.10, p = 0.04), parametrial involvement (OR = 40.88, p < 0.01) and distant metastatic disease (OR = 547.44, p < 0.01) are all independent risk factors for cancer-specific mortality in moderately differentiated endometrioid endometrial cancers.
In total, 43.8% of recurrences occurred within two years and 68.8% within three years of initial treatment. One recurrence was diagnosed after discharge from the 5-year regular follow up (6.3%). The median disease-free survival among patients with recurrence was 27 months and the median survival after recurrence 63 months.
Disease recurrence was diagnosed in three asymptomatic patients; in two of them during a routine follow up examination (12.5% of recurrences) and in one as an incidental finding on a CT scan that was carried out for other indications. In total, 13 patients (81.3%) presented with symptoms before the diagnosis of recurrence, with the most common symptom vaginal bleeding and discharge, seen in 6 cases (37.5% of recurrences). Single-site recurrence was diagnosed in 5 patients (31.3% of recurrences) and single vaginal vault recurrence in three cases (18.8% of recurrences), whereas more than half the recurrent cases (56.3%) had extra-pelvic disease at initial presentation. Recurrence characteristics are summarised in Table 2.
Table 2.
Cases of recurrence of G2 EEC and their characteristics.
| Stage | LVSI | Symptoms | Site | Treatment | Disease-Free Survival (Months) | Survival After Recurrence (Months) |
|---|---|---|---|---|---|---|
| IA | Absent | Vaginal bleeding | Vaginal vault + pelvic LN | BSC | 29 | 14 |
| IA | Absent | Persistent cough | Lungs | HT | 42 | 33 |
| IA | Absent | 1st—asymptomatic (found on examination) 2nd—shortness of breath |
1st—inguinal LN 2nd—ediastinal LN |
1st SE 2nd BSC |
57 | 29 |
| IB | Present | Abdominal pain | Lungs + LN (supraclavicular, para-aortic, pelvic) | BSC | 8 | 3 |
| IB | Present | Diabetic complications + diarrhoea | Paracardiac LN + peritoneum + omentum + sigmoid | HT | 11 | 10 |
| IB | Absent | Persistent cough | lungs + mediastinal LN + adrenal | BSC | 21 | 3 |
| IB | Absent | Persistent discharge | Vaginal vault | Chemotherapy | 3 | 24 |
| IB | Present | Vaginal bleeding | Vaginal vault + pelvic LN (recurrence as G3 ECC) | Chemotherapy | 23 | 43 |
| IB | Absent | Fatigue and reduced appetite | Peritoneum—malignant ascites + LN (retrocaval, paracardiac) | BSC | 63 | 8 |
| IB | Present | 1st—vaginal bleeding 2nd—shortness of breath |
1st—vaginal vault 2nd—lungs |
1st SE 2nd CT |
27 | 58 |
| IB | Present | Asymptomatic (incidental finding on CT scan for other reason) | Peritoneal nodularity and ascites (small volume disease) + umbilical nodule | RT + HT | 31 | 78 |
| IB | Present | Vaginal bleeding | Vaginal vault + pelvic tumour | SE + HT | 27 | 107 |
| IIIA | Present | Diarrhoea | Pelvic + paraaortic LN | RT | 18 | 42 |
| IIIA | Absent | Pelvic pain | Lungs + bones | RT + HT | 55 | 24 |
| IIIB | Present | Asymptomatic (found on examination) | Vaginal vault + pelvic LN | HT | 9 | 5 |
| IIIB | Present | Vaginal bleeding | 1st—vaginal vault 2nd—pelvic LN + bones |
1st SE 2nd BSC |
37 | 14 |
LN = lymph nodes; RT = radiotherapy; CT = chemotherapy; HT = hormonotherapy; BSC = best supportive care; SE = surgical excision.
4. Discussion
Our study showed a clear prognostic difference between G1, G2 and G3 endometrioid endometrial cancer, with G2 between G1 and G3 tumours in terms of 5-year cancer-specific survival (G1—98.5%, G2—93.3%, G3—83.6%; p < 0.05). Multivariable Cox analysis confirmed that the above difference is independent of LVSI and stage. Our findings are similar to another study which analysed 800 patients with endometrioid endometrial cancer and concluded that 5-year disease-specific survival rates for G1, G2 and G3 EEC were 97%, 94% and 76%, respectively (p < 0.001) [17]. A recent large cohort of 1630 patients with endometrioid endometrial cancer from Sweden confirmed the significant prognostic differences among low-grade endometrial cancers in terms of 5-year overall [94.5% (95% CI: 92.6–96.3) for G1 EEC vs. 84.9% (95% CI: 82.3–87.6) for G2 EEC] and net survival [104.4% (95% CI: 102.1–106.7) for G1EC and 95.9% (95% CI: 92.7–99.3) for G2EC]. In the same study, the recurrence rate is 3.8%, 11.3% and 12.8% for G1, G2 and G3 EEC (p < 0.001), respectively [18].
On the other hand, other studies failed to demonstrate any prognostic difference between G1 EEC and G2 EEC. In a cohort of 253 patients with endometrial cancer from the Netherlands, there was no difference in 5-year cancer-specific survival between G1 and G2 (92 vs. 95%), whereas a high grade was found to be a significant adverse prognostic factor (p < 0.001) [19]. However, in that study, the sample was small and possibly insufficient to detect subtle outcome differences. Investigators of another cohort of 776 patients with endometrioid endometrial cancer also failed to find any statistically significant differences between G1 and G2 EEC on survival indicators, despite the significant variations in major pathological parameters between the groups, but cancer-specific survival was not assessed [20].
A binary staging system is more reproducible, with less inter-observer variability compared to the ternary FIGO grading system [5,17,19]. Although combining G1 and G2 in one category overcomes the diagnostic challenges, our data suggest that the above practise compromises the prognostication and increases the heterogeneity within the low-grade group. The global implementation of molecular profiling is expected to overcome the limitations of grading and identify distinct prognostic subgroups within low-grade tumours [21,22]. However, some studies challenge the routine molecular profiling in patients with low-grade EEC, and stress the importance of accurate tumour grading and selective profiling for these patients [23].
To our knowledge, our study is the first to focus on the prognostic features of G2 EEC. We did not find any independent risk factor for recurrence, possibly because of our small sample and the low recurrence rate. On the other hand, our data suggest that cervical involvement, parametrial involvement and distant metastatic disease are all independent risk factors for cancer-related mortality in G2 EEC. Similarly, cervical stromal invasion is an independent pathological risk factor for cancer-specific mortality in high-grade endometrioid endometrial cancer [9].
The significance of cervical stromal and parametrial involvement in survival for G2 endometrial cancer highlights the importance of accurate pre-operative staging and proper surgical management. Although three-dimensional transvaginal ultrasound (3D-TVUS) and magnetic resonance imaging (MRI) are equally effective for the evaluation of cervical stromal invasion in patients with endometrial cancer [24], MRI remains the gold standard for the evaluation of both cervical and parametrial involvement [25]. Accurate pre-operative staging allows for optimal surgical planning. Radical hysterectomy does not improve outcome in patients with cervical involvement [26], but it can be considered in order to achieve complete surgical resection in patients with cervical or parametrial involvement [14]. Finally, although laparoscopic staging appears safe even in cases with cervical involvement [27,28], it is very important to take all the necessary measures in order to minimise uterine manipulation [29].
Almost half of the recurrences (43.8%) in our cohort of G2 EEC were diagnosed within two years and more than two thirds (68.8%) were within three years of treatment. Those figures are in accordance with the literature [30] and justify the need for increased vigilance for three years.
Our data suggest that for G2 EEC, the recurrence risk is relatively low for all risk groups (3.9%, 9.1%, 12.5% and 12.5% for the Low, Intermediate, High-Intermediate and High risk group, respectively). In addition, only 16.7% of recurrences among Low and Intermediate risk cases and only 10% of recurrences among High-Intermediate and High risk patients were diagnosed during examination at follow up in asymptomatic patients. Hence, clinical examination is of limited value in the early diagnosis of recurrence of G2 EEC before clinical manifestation. Moreover, 81.3% of all G2 EEC recurrences presented with symptoms and there is evidence that in symptomatic patients, diagnosis might be delayed when a routine follow up appointment is scheduled [31].
The British Society of Gynaecological Oncology (BSGE) suggests patient-initiated follow up (PIFU) for Low and Intermediate risk cancers and clinic or telephone follow up for at least 2 (and up to 5) years for High-Intermediate and High risk cases. However, the abovementioned findings suggest that PIFU can be safely expanded to all G2 endometrial cancers. Both women treated for endometrial cancer and health care providers are mostly supportive of PIFU [32], whereas traditional follow up strategies do not meet cancer patients’ needs [33]. Finally, PIFU is expected to reduce waiting times and waiting lists due to a net reduction in follow up appointments (21,653 appointments for endometrial cancer in 2022–2023 in the UK) [34].
5. Conclusions
In conclusion, our study suggests that G2 EEC is a distinct entity, and thus the grading system should continue to differentiate G1 EEC and G2 EEC for better prognosis interpretation. Cervical and parametrial involvement are independent risk factors for cancer-specific mortality for G2 EEC. Finally, the recurrence pattern suggests that PIFU is a cost-effective alternative for the follow up of patients with G2 EEC.
Author Contributions
Conceptualization, A.Z., F.F., S.A. and H.S.M.; methodology, A.Z., A.K., S.L.S. and H.S.M.; formal analysis, A.Z., C.P. and H.S.M.; data curation, A.K., N.S., A.S., S.D. and M.A.; writing—original draft preparation, A.Z., A.K. and C.P.; writing—review and editing, A.D., F.F., S.L.S., S.D., M.A., S.K., S.A. and H.S.M.; supervision, S.K. and H.S.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Oxford University Hospitals NSH Trust (ID: 5832, Ref:06/01/2020-SUWON-Soleymani-2).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are unavailable due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd J.H. Revised FIGO staging for gynaecological cancer. Br. J. Obstet. Gynaecol. 1989;96:889–892. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Female Genital Tumours, 5th ed. Volume 4 IARC Press; Lyon, France: 2020. Classification of Tumours Editorial Board. [Google Scholar]
- 4.Abeler V.M., Kjorstad K.E., Berle E. Carcinoma of the endometrium in Norway: A histopathological and prognostic survey of a total population. Int. J. Gynecol. Cancer. 1992;2:9–22. doi: 10.1046/j.1525-1438.1992.02010009.x. [DOI] [PubMed] [Google Scholar]
- 5.Sagae S., Saito T., Satoh M., Ikeda T., Kimura S., Mori M., Sato N., Kudo R. The Reproducibility of a Binary Tumor Grading System for Uterine Endometrial Endometrioid Carcinoma, Compared with FIGO System and Nuclear Grading. Oncology. 2004;67:344–350. doi: 10.1159/000082917. [DOI] [PubMed] [Google Scholar]
- 6.Berek J.S., Matias-Guiu X., Creutzberg C., Fotopoulou C., Gaffney D., Kehoe S., Lindemann K., Mutch D., Concin N., Endometrial Cancer Staging Subcommittee, FIGO Women’s Cancer Committee FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Cancer. 2023;162:383–394. doi: 10.1002/ijgo.14923. [DOI] [Google Scholar]
- 7.Concin N., Matias-Guiu X., Vergote I., Cibula D., Mirza M.R., Marnitz S., Ledermann J., Bosse T., Chargari C., Fagotti A., et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer. 2021;31:12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 8.Zouridis A., Kashif A., Pappa C., Zarrindej K., Rencher J., Smyth S.L., Sadeghi N., Sattar A., Damato S., Abdalla M., et al. 554 Where does the prognosis of grade 2 endometrioid endometrial cancer stands in the spectrum between low and high grade endometrioid tumours? In Proceedings of the ESGO 2024 Congress, Barcelona, Spain, 7–10 March 2024; [Google Scholar]
- 9.Zouridis A., Zarrindej K., Rencher J., Pappa C., Kashif A., Smyth S., Sadeghi N., Sattar A., Damato S., Ferrari F., et al. The Prognostic Characteristics and Recurrence Patterns of High Grade Endometrioid Endometrial Cancer: A Large Retrospective Analysis of a Tertiary Center. J. Clin. Med. 2023;12:3141. doi: 10.3390/jcm12093141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COPE: Committee on Publication Ethics. [(accessed on 1 February 2024)]. Available online: https://publicationethics.org/
- 11.EQUATOR Enhancing the QUAlity and Transparency of Health Research. [(accessed on 1 February 2024)]. Available online: https://www.equator-network.org/
- 12.Benchimol E., Smeeth L., Guttmann A., Harron K., Moher D., Petersen I., Sorensen H., Elm E., Langan S. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;10:12. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison J., Balega J., Buckley L., Clamp A., Crosbie E., Drew Y., Durrant L., Forrest J., Fotopoulou C., Gajjar K., et al. British Gynaecological Cancer Society (BGCS) uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022;270:50–89. doi: 10.1016/j.ejogrb.2021.11.423. [DOI] [PubMed] [Google Scholar]
- 14.Koskas M., Amant F., Mirza M., Creutzberg C. Cancer of the corpus uteri: 2021 update. Int. J. Gynecol. Obstet. 2021;155((Suppl. 1)):45–60. doi: 10.1002/ijgo.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Donato V., D’Oria O., Giannini A., Bogani G., Fischetti M., Santangelo G., Tomao F., Palaia I., Perniola G., Muzii L., et al. Age-Adjusted Charlson Comorbidity Index Predicts Survival in Endometrial Cancer Patients. Gynecol. Obstet. Investig. 2022;87:191–199. doi: 10.1159/000525405. [DOI] [PubMed] [Google Scholar]
- 16.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynecol. Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Scholten A.N., Smit V.T.H.B.M., Beerman H., Van Putten W.L.J., Creutzberg C.L. Prognostic significance and interobserver variability of histologic grading systems for endometrial carcinoma. Cancer. 2004;100:764–772. doi: 10.1002/cncr.20040. [DOI] [PubMed] [Google Scholar]
- 18.Åkesson Å., Adok C., Dahm-Kähler P. Recurrence and survival in endometrioid endometrial cancer—A population-based cohort study. Gynecol. Oncol. 2023;168:127–134. doi: 10.1016/j.ygyno.2022.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Scholten A.N., Creutzberg C.L., Noordijk E.M., Smit V.T.H.B.M. Long-term outcome in endometrial carcinoma favors a two- instead of a three-tiered grading system. Int. J. Radiat. Oncol. 2002;52:1067–1074. doi: 10.1016/S0360-3016(01)02710-9. [DOI] [PubMed] [Google Scholar]
- 20.Khatib G., Gulec U.K., Guzel A.B., Bagir E., Paydas S., Vardar M.A. Prognosis Trend of Grade 2 Endometrioid Endometrial Carcinoma: Toward Grade 1 or 3? Pathol. Oncol. Res. 2020;26:2351–2356. doi: 10.1007/s12253-020-00836-w. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson A., Vermij L., Kramer C.J.H., Jobsen J.J., Jürgemlienk-Schulz I., Lutgens L., Mens J., Haverkort M.A.D., Slot A., Naut R.A., et al. Clinical Behavior and Molecular Landscape of Stage I p53-Abnormal Low-Grade Endometrioid Endometrial Carcinomas. Clin. Cancer Res. 2023;29:4949–4957. doi: 10.1158/1078-0432.CCR-23-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puppo A., Fraternali Orcioni G., Clignon V., Musizzano Y., Zavattero C.A., Vocino Trucco G., Benazzo G.M., Vizzielli G., Restaino S., Mariuzzi L., et al. Where Morphological and Molecular Classifications Meet: The Role of p53 Immunohistochemistry in the Prognosis of Low-Risk Endometrial Carcinoma (GLAMOUR Study) Cancers. 2024;16:1088. doi: 10.3390/cancers16061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrede S.W., Kasius J., Bulten J., Teerenstra S., Huvila J., Colas E., Gil-Moreno A., Boll D., Vos M.C., Altena A.M., et al. Relevance of Molecular Profiling in Patients With Low-Grade Endometrial Cancer. JAMA Netw. Open. 2022;5:e2247372. doi: 10.1001/jamanetworkopen.2022.47372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spagnol G., Noventa M., Bonaldo G., Marchetti M., Vitagliano A., Laganà A.S., Cavallin F., Scioscia M., Saccardi T., Tozzi R. Three-dimensional transvaginal ultrasound vs magnetic resonance imaging for preoperative staging of deep myometrial and cervical invasion in patients with endometrial cancer: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2022;60:604–611. doi: 10.1002/uog.24967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otero-García M.M., Mesa-Álvarez A., Nikolic O., Blanco-Lobato P., Basta-Nikolic M., De Llano-Ortega R.M., Paredes-Velázquez L., Nikolic N., Szewczyk-Bieda M. Role of MRI in staging and follow-up of endometrial and cervical cancer: Pitfalls and mimickers. Insights Imaging. 2019;10:19. doi: 10.1186/s13244-019-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barquet-Muñoz S.A., Cantú-de-León D., Bandala-Jacques A., González-Enciso A., Isla-Ortiz D., Prada D., Herrera L.A., Salcedo-Hernández R.A. What is the impact of radical hysterectomy on endometrial cancer with cervical involvement? World J. Surg. Oncol. 2020;18:101. doi: 10.1186/s12957-020-01876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zouridis A., Kehoe S.T., Soleymani Majd H. Should laparoscopy be revisited in the management of stage II endometrial cancer in the post-LACC era? Minerva Obstet. Gynecol. 2023;75:553–558. doi: 10.23736/S2724-606X.23.05258-2. [DOI] [PubMed] [Google Scholar]
- 28.Abel M.K., Chan J.K., Chow S., Darcy K., Tian C., Kapp D.S., Mann A.K., Liao C.I. Trends and survival outcomes of robotic, laparoscopic, and open surgery for stage II uterine cancer. Int. J. Gynecol. Cancer. 2020;30:1347–1355. doi: 10.1136/ijgc-2020-001646. [DOI] [PubMed] [Google Scholar]
- 29.Padilla-Iserte P., Lago V., Tauste C., Díaz-Feijoo B., Gil-Moreno A., Oliver R., Coronado P., Martín-Salamanca M.B., Pantoja-Garrido M., Marcos-Sanmartin J., et al. Impact of uterine manipulator on oncological outcome in endometrial cancer surgery. Am. J. Obs. Gynecol. 2021;224:65.e1–65.e11. doi: 10.1016/j.ajog.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Fung-Kee-Fung M., Dodge J., Elit L., Lukka H., Chambers A., Oliver T. Follow-up after primary therapy for endometrial cancer: A systematic review. Gynecol. Oncol. 2006;101:520–529. doi: 10.1016/j.ygyno.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Nordin A.J., The National Group Of Gynaecology Nssg Leads Mode of detection of recurrent gynecological malignancy: Does routine follow-up delay diagnosis and treatment? Int. J. Gynecol. Cancer. 2006;16:1746–1748. doi: 10.1136/ijgc-00009577-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Dretzke J., Lorenc A., Adriano A., Herd C., Mehanna H., Nankivell P., Moore D.J., PETNECK2 Research Team Systematic review of patients’ and healthcare professionals’ views on patient-initiated follow-up in treated cancer patients. Cancer Med. 2023;12:16531–16547. doi: 10.1002/cam4.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperling C., Sandager M., Jensen H., Knudsen J.L. Current organisation of follow-up does not meet cancer patients’ needs. Dan. Med. J. 2014;61:A4855. [PubMed] [Google Scholar]
- 34.Department of Health NHS Hospital Outpatient Activity 2022–2023. [(accessed on 21 September 2024)]. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-outpatient-activity/2022-23.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are unavailable due to privacy restrictions.