Abstract
Transfection of cis-element double-stranded oligonucleotides, referred to as decoy ODNs, has been reported to be a powerful tool that provides a new class of antigene strategies for gene therapy. However, one of the major limitations of the decoy approach is the rapid degradation of phosphodiester oligonucleotides by intracellular nucleases. To date, several DNA analogs have been employed to overcome this issue, but insufficient efficacy and/or specificity have limited their in vivo usefulness. In this paper we have investigated the use of conformationally restricted nucleotides in the design of decoy molecules for nuclear transcription factor κB (NF-κB). Starting from a synthetic double-stranded oligonucleotide, containing the κB consensus binding sequence, we designed a panel of decoy molecules modified to various extents and at various positions with locked nucleic acids (LNAs). Our results indicate that the addition of terminal LNA bases, outside the κB sequence, to generate LNA–DNA–LNA co-polymers was sufficient to confer appreciable protection towards nuclease digestion, without interfering with transcription factor binding. Conversely, insertion of LNA substitutions in the context of the κB-binding site resulted in further increased stability, but caused a loss of affinity of NF-κB for the target sequence. However, our results also indicate that this latter effect was apparently dependent not only on the extent but also on strand positioning of the internal LNA substitutions. This observation is of great importance since it provides evidence for the possibility of tuning DNA–LNA duplexes with internal LNAs into decoy agents with improved features in terms of biological stability and inhibitory effect.
INTRODUCTION
In recent years, oligonucleotides (ODNs) have received considerable attention since they provide a rational way to design sequence-specific ligands of nucleic acids or DNA-binding regulatory proteins as tools for selective regulation of gene expression. This is of particular interest in developing new pharmaceutical interventions to treat diseases characterized by aberrant activation and expression of genes whose products are involved in the initiation and progression of pathogenesis. In particular, as altered activation of transcription factors has become a better understood component of many pathways of disease pathogenesis, including cancer, viral infection and chronic inflammatory diseases, the development of molecular strategies targeting transcription-activating proteins has emerged as an attractive field of investigation (1). In this context, transfection of cis-element double-stranded ODNs, referred to as decoy ODNs, has been reported to be a powerful tool that provides a new class of antigene strategies for gene therapy (2–4). Once delivered to cells, synthetic double-stranded ODNs bearing the consensus binding sequence of a specific transcription factor are specifically recognized and bound by the target factor. Interaction with the decoy results in both an inability of the protein to subsequently bind to the promoter regions of target genes and in the removal of bound trans-factor from the endogenous cis-element (5–7). The final result is a significant reduction in or even a blockade of transcriptional activation.
Although ODN-based strategies appear very simple from a theoretical point of view, their practical application to biological systems has clearly outlined several problems, including the rapid degradation of phosphodiester ODNs by serum and intracellular nucleases (both endonucleases and exonucleases) (5,8,9). For this reason, in the past decade, significant effort has been expended by synthetic chemists to develop nuclease-resistant ODNs. The most commonly employed modification is the replacement of a non-bridging oxygen in phosphate linkages with a sulphur to form phosphorothioate-modified ODNs. These molecules are highly resistant to degradation by nucleases (10), exhibit increased cellular uptake (11) and retain the ability to form sequence-specific duplexes, although with reduced stability relative to unmodified ODNs (12). However, largely because of their polyanionic nature, phosphorothioates cause non-specific protein binding, with consequent sequence-independent effects that limit many applications (13–15).
More recently, DNA analogs with a pseudo-peptide backbone composed of N-(2-aminoethyl)glycine units (peptide nucleic acids or PNAs) have been explored as potential agents for decoy approaches (16). PNAs are, in fact, ideal candidates as decoy molecules due to their ability to form very stable duplexes with complementary DNA and PNA sequences (17) and to their stability when exposed to DNases and proteinases (18). Unfortunately, DNA-binding proteins, such as nuclear transcription factor κB (NF-κB), exhibit a markedly low binding efficiency to PNA oligomers. In vitro experiments, performed with PNA decoy molecules carrying NF-κB-specific cis-elements, have clearly demonstrated that NF-κB p52 is able to recognize only DNA/PNA hybrids generating, in addition, molecular complexes with very low stability (16).
Interestingly, conformational restriction has been successfully applied in recent years to the design of high affinity ODNs. Among nucleoside analogs containing bi- and tri-cyclic carbohydrate moieties, of particular interest are the locked nucleic acids (LNAs), which contain an extra 2′-O,4′-C-methylene bridge added to the ribose ring (19–21). In fact, ODNs containing this modification have shown hitherto unprecedented helical thermal stability when hybridized to complementary DNA or RNA, as shown by the significant increase in melting temperatures compared with unmodified duplexes (19,20,22–25). In general, the thermal stability of a LNA/DNA duplex is increased by between 4.0 and 9.3°C per modified base in the ODN. Furthermore, LNA ODNs can be synthesized using conventional phosphoroamidite chemistry, allowing automated synthesis of pure as well as mixed ODNs containing both LNA and DNA monomers (19–21). These features, together with the demonstration that LNAs are stable towards 3′-exonucleolytic degradation (26) and LNA–DNA co-polymers are not readily degraded in blood serum and cell extracts (19,26), prompted us to evaluate LNAs in the design of decoy molecules. In fact, while LNA ODNs have proved very efficient as antisense molecules (26), no information is available to date on the possible use of LNA decoys able to interact with DNA-binding proteins such as transcription factors. Starting from a synthetic double-stranded 20mer ODN containing two copies of the κB consensus binding sequence present in the PRDII domain of the interferon-β (IFN-β) promoter, we designed a panel of decoy molecules modified to various extents with LNAs. Furthermore, LNA substitutions were positioned outside or inside and outside the PRDII elements in order to confer potential resistance towards exonuclease and endonuclease digestion. Importantly, since benefits in terms of nuclease resistance may often be negated by a decrease in the binding affinity of the transcription factor, modified ODNs were further studied for their ability to interact and form specific complexes with NF-κB proteins by electrophoretic mobility shift assay. Results presented in the present paper suggest that LNAs could really represent a step ahead in pursuing structural modifications intended to enhance biological stability and inhibitory effects of decoy agents.
MATERIALS AND METHODS
Oligonucleotides and LNAs
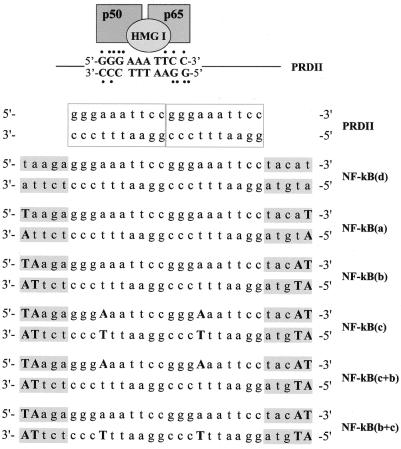
Upper strand and reverse complement phosphodiester ODNs (20mers), corresponding to two copies of the NF-κB-binding sequence found in the PRDII domain of the IFN-β promoter, were purchased from Sigma Genosys (Cambridge, UK) (Fig. 1). ODNs containing LNAs were purchased from Proligo LLC (Boulder, CO). In this case the PRDII sequence was capped at both ends with unrelated extra sequences 5 nt long to generate a 30mer ODN (Fig. 1). The extra sequences were selected in order to avoid self- and inter-strand hybridization involving two complementary LNA bases, which would generate complexes with high stability. One or two nucleotides were replaced with LNAs at both the 5′- and 3′-terminal ends [NF-κB(a) and NF-κB(b)] (Fig. 1). Alternatively, additional nucleotides of the internal PRDII sequences were substituted with LNAs as shown in Figure 1 [NF-κB(c)]. In this paper, all the ODNs containing LNAs are referred to as LNA ODNs. A 30mer phosphodiester ODN with the same sequence was purchased from Sigma Genosys (Cambridge, UK) and used as a control [NF-κB(d)] (Fig. 1).
Figure 1.
Schematic representation of the PRDII κB-binding consensus sequence and of the derived LNA ODNs. Schematic representation of the PRDII κB element showing binding of the NF-κB p50/p65 heterodimer in the outer GC-rich sequence and of HMG I proteins in the core AT-rich domain. Both NF-κB and HMG I can separately bind to the PRDII sequence. In the context of the IFN-β promoter, HMG I functions as an architectural factor facilitating the assembly of transcriptionally active nucleoprotein complexes (40,41). Dots represent hydrogen bonds mediating base-specific contacts between p50 and p65 subunits and the κB site, according to evidence obtained in crystallographic studies (39). LNA ODNs were synthesized on the basis of the κB sequence contained in the PRDII domain of the IFN-β promoter which has been extended at both terminal ends with unrelated extra sequences of 5 nt (in gray). LNA substitutions are indicated in bold upper case letters.
Annealing and 32P-labeling
Stock solutions of DNA and LNA ODNs were made in TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0) and stored at –80°C. Complementary DNA and LNA oligomers were hybridized in TE buffer to generate DNA/DNA [NF-κB(d) and PRDII] and LNA/LNA [NF-κB(a), NF-κB(b) and NF-κB(c)] duplexes or mixed hybrids containing two LNAs at the terminal ends of both strands and internal LNAs only in one strand [NF-κB(c+b) and NF-κB(b+c)], as shown in Figure 1. Annealing was performed in a thermal cycler according to the following temperature profile: 5 min at 100°C, followed by a slow decrease from 100 to 37°C over 60 min and from 37 to 4°C over 30 min.
Double-stranded DNA and LNA ODNs were 5′-end-labeled using T4 polynucleotide kinase (T4 PNK, EC 2.7.1.78). Briefly, 2 pmol ODN was incubated in a final volume of 20 µl with 8 U T4 PNK (Roche Diagnostics, Mannheim, Germany), 60 µCi [γ-32P]ATP (Perkin Elmer Life Sciences, Boston, MA), 50 mM Tris–HCl, 10 mM MgCl2, 0.1 mM EDTA, 5 mM dithiothreitol (DTT), 0.1 mM spermine, pH 8.2, at 37°C for 15 min. Unincorporated nucleotides were removed by chromatography through a Sephadex G-25 spin column equilibrated in TEN buffer (10 mM Tris–HCl, pH 7.5, 1 mM EDTA, 100 mM NaCl). The efficiency of 32P incorporation into LNA ODNs was 3-fold lower than that obtained, under the same experimental conditions, for the corresponding phosphodiester ODN NF-κB(d) [∼5300 c.p.m./pmol for the LNA ODNs versus 16 000 c.p.m./pmol for NF-κB(d)]. Analysis of the radiolabeled products by non-denaturing polyacrylamide gel electrophoresis, followed by radiography of the gel, clearly demonstrated that most of the radioactivity counted was associated with double-stranded species, indicating that the efficiency of T4 PNK to catalyze the transfer of the phosphate group of ATP to the 5′-hydroxylated terminus of an ODN could be impaired, but not completely abrogated, by the presence of terminally positioned LNAs (not shown).
Cell culture and nuclear extract preparation
NIH 3T3 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum at 37°C in a humidified 5% CO2 incubator. Cells were plated at a density of 5 × 105 cells/60 mm diameter culture dish the day before stimulation.
Nuclear extracts were prepared from NIH 3T3 cells stimulated with 10 ng/ml tumor necrosis factor-α (TNF-α) (Boehringer Mannheim Biochemia, Mannheim, Germany) for 30 min at 37°C in the presence of 5% CO2. After stimulation, cells were extensively washed with cold phosphate-buffered saline and scraped from the dishes with cold buffer A [10 mM HEPES–KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, 0.2 mM EDTA, 10 µg/ml leupeptin, 10 µg/ml pepstatin, 4 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), 1 mM NaF, 1mM Na3VO4 and 0.1% Nonidet-P40]. The cell suspension was then chilled on ice for 10 min before centrifugation at 10 000 g. The resultant nuclear pellet was resuspended in cold buffer B (20 mM HEPES–KOH, pH 7.9, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 1 mM DTT, 0.2 mM EDTA, 10 µg/ml leupeptin, 10 µg/ml pepstatin, 4 mM AEBSF, 1 mM NaF and 1 mM Na3VO4) and incubated on ice for 20 min before being centrifuged at 10 000 g. Nuclear extract supernatant was collected, diluted 1:5 in buffer C (20 mM HEPES–KOH, pH 7.9, 20% glycerol, 50 mM KCl, 1 mM DTT, 0.2 mM EDTA and 1 mM AEBSF) and stored at –80°C.
Electrophoretic mobility shift assay (EMSA) and supershift
Nuclear extracts (3 µg) were preincubated with 5 µg double-stranded non-specific DNA competitor poly(dI–dC) (Amersham Pharmacia Biotech, Piscataway, NJ) for 10 min on ice in the presence of 20 mM HEPES–KOH, pH 7.9, 0.1 M KCl, 5% glycerol, 0.2 mM EDTA, 0.2 mM EGTA and 1 mM DTT. Binding activity was assessed by incubating the reaction mixture with the indicated 32P-end-labeled probe to a final concentration of 1.2 nM for 20 min on ice. Protein–DNA complexes were then separated on 5% native polyacrylamide gels (29:1 cross-linked) in Tris–glycine buffer (25 mM Tris base, 192 mM glycine). In competition experiments, increasing concentrations of competitor ODN, either a phosphodiester or a LNA-modified duplex as specified, were incubated with the nuclear extract for 15 min before adding the radiolabeled PRDII probe. Detection and quantification of NF-κB–DNA complex formation was performed in a GS-250 Molecular Imager (Bio-Rad Laboratories, Milan, Italy). Specificity of the NF-κB–DNA complex detected was assessed by supershift analysis, using polyclonal subunit-specific antisera against p50 and p65 (Rel A) (Santa Cruz Biotechnology, Santa Cruz, CA). In this assay, antisera (2 µg) were incubated with nuclear extract for 20 min at room temperature prior to addition of the radiolabeled probe.
Stability assays of double-stranded LNA ODNs
Double-stranded DNA and LNA ODNs (2.7 µM) were incubated either with 10 U/ml DNase I (EC 3.1.21.1) (Roche Diagnostics) in 50 mM Tris–HCl, pH 7.5, 1 mM MgCl2 and 0.1 mg/ml bovine serum albumin at 20°C or with 10 U/ml BAL-31 nuclease (New England Biolabs, Hitchin, UK) in a reaction buffer consisting of 0.6 M NaCl, 20 mM Tris–HCl, pH 8.0, 12 mM CaCl2, 12 mM MgCl2 and 1 mM EDTA at 30°C. Aliquots of the reaction mixture corresponding to 250 ng double-stranded ODN were removed at different incubation times, quenched in 4 mM EDTA or 20 mM EGTA in order to stop DNase I and BAL-31 activity, respectively, and submitted to non-denaturing electrophoretic separation in 2.5% (w/v) agarose gels and ethidium bromide staining. Degradation of the decoy molecules was determined by densitometric calculation using Molecular Analyst (Bio-Rad) imaging software. The volume density of the band corresponding to double-stranded molecules was calculated in each lane with correction for the background. Results are presented as percentage recovery with respect to the relative time zero value.
RESULTS
Design of decoy molecules containing LNA substitutions
Double-stranded decoy ODNs, corresponding to the κB site contained in the PRDII domain of the IFN-β promoter, were synthesized to selectively target NF-κB. This choice was essentially based on the widely recognized role of NF-κB in a number of human diseases (27,28) and on the bulk of literature describing the successful blockade of this transcription factor by decoy strategies (2,4,29–33). All the experiments were performed using a 30mer double-stranded ODN containing two PRDII elements in tandem (10 bp each), capped with unrelated extra sequences of 5 nt at both terminal ends (Fig. 1, compare NF-κB probes with PRDII probe). These extra sequences were added to the putative PRDII regions to allow insertion of LNAs at the 3′ and 5′ termini of both strands in order to confer protection from nuclease digestion and to minimize interference with the binding of NF-κB [Fig. 1, NF-κB(a) and NF-κB(b)]. Additional LNA modifications were introduced in the PRDII elements to generate duplexes containing, besides terminal LNAs, also internal LNA substitutions involving complementary nucleotides in both strands [Fig. 1, NF-κB(c)] or nucleotides in only one strand [Fig. 1, NF-κB(c+b) and NF-κB(b+c)]. In replacing internal nucleotides with LNAs we took into consideration the published solution structure of an ODN containing LNA bases bound to unmodified complementary DNA which provides evidence of a change in buckle so as to create a transition from B-type to A-type DNA in the neighborhood of LNA modification (34–36). This is an important observation because the ability of LNA nucleotides to affect conformation of the duplex can also be expected to heavily alter the binding affinity of the transcription factor. To minimize this effect, we decided to introduce internal LNA substitutions in the PRDII AT-rich DNA sequence. In fact, X-ray crystallographic studies have shown that in NF-κB–DNA complexes, the protein mainly interacts with the GC-rich outer regions of the site, and all base contacts are made in the major groove (37–39). This leaves open the minor groove of the κB site, where high mobility group proteins I (HMG I) are believed to interact mostly with the AT-rich sequence of PRDII, by functioning as architectural factors able to potentiate the transcriptional activation of NF-κB (40,41) (Fig. 1).
Double-stranded LNA ODNs display higher resistance to exonuclease and endonuclease digestion with respect to DNA ODNs
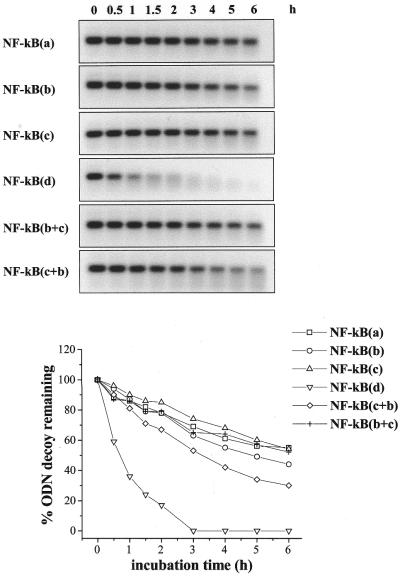
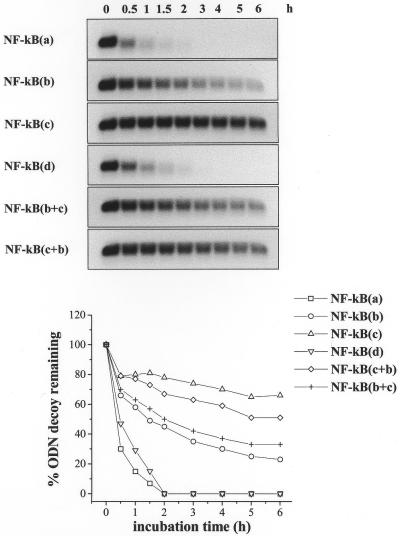
As a first step, we tested susceptibility to nuclease degradation of double-stranded LNA decoys in comparison with the corresponding phosphodiester molecule NF-κB(d). Under our experimental conditions NF-κB(d) was completely degraded after 3 h incubation with DNase I, a double-strand-specific endonuclease (Fig. 2). In contrast, the presence of one or two terminal LNAs [NF-κB(a) and NF-κB(b)] was sufficient to markedly increase ODN stability (Fig. 2). In fact, at the same incubation time, <35% of LNA ODNs were degraded (Fig. 2). No significant improvements in terms of resistance to endonuclease digestion were observed in the presence of internally positioned LNA modifications [NF-κB(c), Fig. 2]. Similar results were also obtained with the mixed hybrids NF-κB(c+b) and NF-κB(b+c), with the first being the most unstable of the LNA ODNs (Fig. 2).
Figure 2.
Susceptibility to DNase I degradation of LNA-modified ODNs. LNA-modified [NF-κB(a), (b), (c), (c+b) and (b+c)] and control phosphodiester [NF-κB(d)] decoy molecules were incubated for different lengths of time, as indicated, with 0.5 U/ml DNase I and then submitted to electrophoretic separation on 2.5% (w/v) agarose gels. Detection and quantitation of the ethidium bromide stained bands were performed in a Molecular Analyst. Volume densities of the bands are expressed as percent ODN decoy remaining with respect to the relative time zero value and are shown as line graphs.
Stability towards exonucleolytic degradation was assessed using BAL-31 nuclease, which degrades both the 3′ and 5′ termini of duplex DNA without generating internal scissions. The results in Figure 3 clearly show that with the exception of NF-κB(a), which was degraded at the same rate as NF-κB(d), all the LNA ODNs displayed higher stability with respect to the corresponding phosphodiester molecule (Fig. 3). The most significant stabilization was achieved with internal LNAs, but only when they were present on both strands [NF-κB(c)] (Fig. 3). In contrast, the mixed hybrids NF-κB(c+b) and NF-κB(b+c) exhibited decay kinetics more similar to those observed for NF-κB(c) and NF-κB(b), respectively (Fig. 3), suggesting that positioning of internal LNA bases in the sense or in the antisense strand has important effects in terms of resistance to BAL-31 nuclease action.
Figure 3.
Susceptibility to BAL-31 nuclease degradation of LNA-modified ODNs. LNA-modified [NF-κB(a), (b), (c), (c+b) and (b+c)] and control phosphodiester [NF-κB(d)] decoy molecules were incubated for different lengths of time, as indicated, with 0.5 U/ml BAL-31 and then submitted to electrophoretic separation on 2.5% (w/v) agarose gels. Detection and quantitation of the ethidium bromide stained bands were performed in a Molecular Analyst. Volume densities of the bands are expressed as percent ODN decoy remaining with respect to the relative time zero value and are shown as line graphs.
NF-κB specifically binds to double-stranded ODNs containing LNA modifications
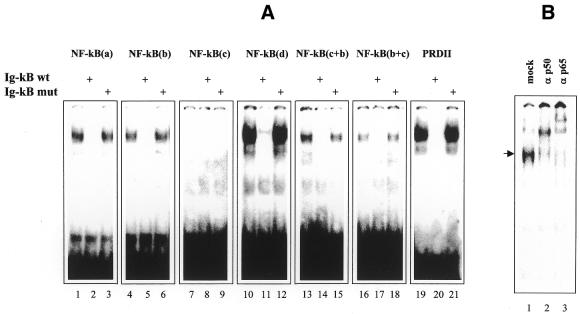
The binding of NF-κB to 32P-labeled DNA and LNA duplexes was analyzed by EMSA, using nuclear extracts from TNF-stimulated cells as a source of NF-κB. A single protein–DNA complex migrating with the same electrophoretic mobility was observed with all probes (Fig. 4A, lanes 1, 4, 10, 13, 16 and 19), with the exception of NF-κB(c), for which we could not detect any shifted complex, even after longer exposure of the gel (Fig. 4A, lane 7). Furthermore, addition of a 60 M excess of a DNA probe, encompassing the immunoglobulin light chain NF-κB (Ig-κB) element (5′-TCAACAGAGGGGACTTTCCGAGAGGCC-3′), demonstrated that the complex detected with both DNA and LNA probes was specific for the NF-κB site, as determined by the complete disappearance of the radiographic signal (Fig. 4A, lanes 2, 5, 11, 14, 17 and 20). In addition, mutagenesis of the Ig-κB consensus sequence (5′-TCAACAGAGCTCACTTTATGAGAGGCC-3′) completely prevented competition for the binding of NF-κB to the probes, confirming the specificity of complex formation (Fig. 4A, lanes 3, 6, 12, 15, 18 and 21). Further demonstration that the complexes detected in bandshift assays contained members of the Rel/NF-κB family of proteins was obtained by supershift experiments. As shown in Figure 4, preincubation of the nuclear extracts with specific antibodies against p50 and p65 resulted in complete up-shift of the retarded band (Fig. 4B, compare lane 1 with 2 and 3), indicating that both subunits were part of the protein–PRDII complex. The same up-shifting was observed using radiolabeled probes consisting of LNA ODNs or of the corresponding phosphodiester ODN NF-κB(d) (not shown).
Figure 4.
Direct binding of NF-κB to radiolabeled LNA-modified and phosphodiester probes and supershift analysis of the TNF-induced protein–PRDII complex. (A) Nuclear extracts obtained from TNF-stimulated NIH 3T3 cells were incubated with radiolabeled NF-κB(a) (lanes 1–3), NF-κB(b) (lanes 4–6), NF-κB(c) (lanes 7–9), NF-κB(d) (lanes 10–12), NF-κB(c+b) (lanes 13–15), NF-κB (b+c) (lanes 16–18) and PRDII (lanes 19–21) probes and analyzed by EMSA. Specificity of binding was assessed by competition with a cold wild-type Ig-κB probe (Ig-κB wt) (lanes 2, 5, 8, 11, 14, 17 and 20) or a mutated form of Ig-κB (Ig-κB mut) which ablates NF-κB binding (lanes 3, 6, 9, 12, 15, 18 and 21). Due to the different specific activities of the probes (see Materials and Methods) the data are only qualitative and not quantitative. (B) Nuclear extracts prepared from TNF-stimulated NIH 3T3 cells were incubated with the PRDII probe in the absence (lane 1) or presence of the indicated NF-κB subunit-specific antisera (lanes 2 and 3). The resulting complexes were resolved on a 5% non-denaturing gel and detected in a Molecular Imager.
Extent and positioning of LNA bases are important determinants for efficient binding of NF-κB to modified oligonucleotide duplexes
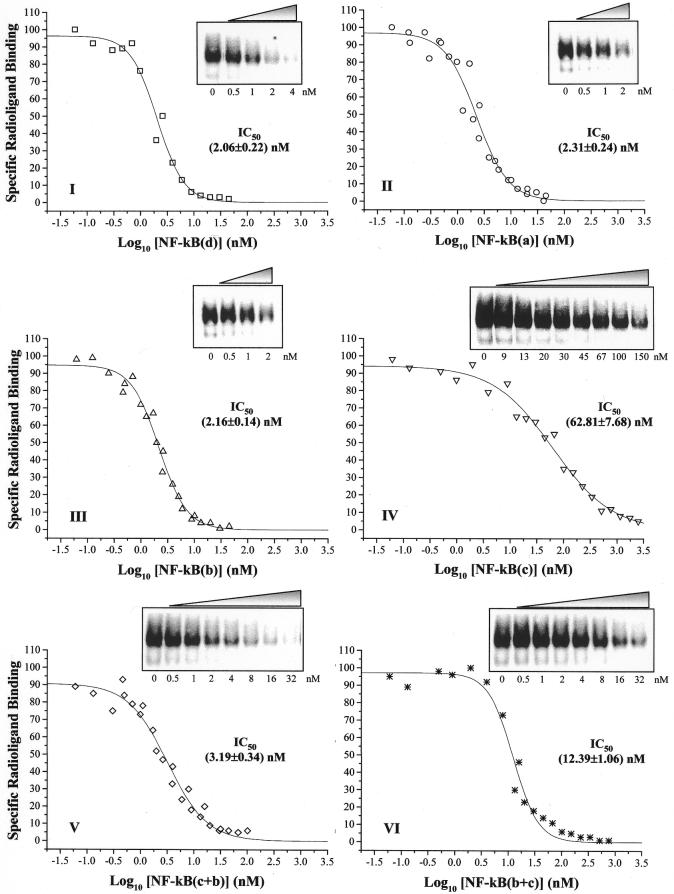
NF-κB binding affinity for decoy LNA ODNs was evaluated by gel shift competition experiments. Binding assays were performed by incubating crude nuclear extracts with increasing concentrations of unlabeled LNA ODNs together with a fixed amount of the [32P]PRDII probe, so that the radiolabeled DNA duplex was in excess over the amount of NF-κB present in the incubation mixture. Results of a typical competition experiment are shown in Figure 5, where a progressive reduction in the radiographic signal, corresponding to the NF-κB–PRDII complex, was observed upon addition of the indicated competitors. These data suggest that decoy LNA ODNs are recognized by NF-κB, confirming previous evidence of direct binding of NF-κB to radiolabeled LNA probes (Fig. 4). However, these results clearly indicated that not all the competitors had the same efficiency to compete for binding of NF-κB to the radioactive probe. For this reason, the IC50 value of each LNA ODN was calculated from quantitation of titration experiments by fitting the data to a dose–response curve (Fig. 5I–VI). ODNs with LNA substitutions outside the binding consensus sequence for NF-κB [NF-κB(a) and NF-κB(b)] displayed not significantly different IC50 values with respect to NF-κB(d) (Fig. 5, compare II and III with I). Surprisingly, the duplex containing LNA modifications also in the internal PRDII elements [NF-κB(c)] acted as an inhibitor, despite being less efficient with respect to NF-κB(d), as determined by a 30-fold increase in the IC50 value (Fig. 5IV versus I). This low efficiency could explain the reason why we did not observe direct binding to the radiolabeled NF-κB(c) ODN in bandshift assays (Fig. 4A, lane 7), where the concentration and specific activity of the probe were probably too low to allow observation and detection of NF-κB binding. Interesting results were obtained with mixed hybrids NF-κB(c+b) and NF-κB(b+c), which exhibited IC50 values 1.5- and 6-fold higher, respectively, than that calculated for NF-κB(d) (Fig. 5, compare V and VI with I). These data suggest that the presence of internal LNAs in only one strand results in a moderate interference with transcription factor binding, with the best NF-κB binding efficiency observed when LNA substitutions were positioned in the sense strand [NF-κB(c+b)] rather than in the antisense strand [NF-κB(b+c)].
Figure 5.
Competition of LNA and phosphodiester ODNs for binding of NF-κB to radiolabeled PRDII probe. Competition experiments were performed by incubating crude nuclear extracts together with the [32P]PRDII probe (1.2 nM) and the indicated unlabeled competitor. NF-κB–PRDII complex formation in the presence of increasing competitor concentrations was analyzed by EMSA and quantitated in a Molecular Imager. Data were expressed as percent binding relative to the level of NF-κB–radioligand complex formation in the absence of the competitor. IC50 values for each type of competitor were estimated by plotting these data as a function of log10 of the competitor concentration (nM) and fitting to a dose-response curve. Curve fitting was performed with Origin 4.1 software. Fifty percent inhibitory concentrations (nM) ± SE are indicated.
DISCUSSION
A growing number of transcription factors have been identified that regulate changes in gene expression during pathogenesis of a wide range of disorders (1). A recent method to antagonize the function of these proteins is the decoy approach, which involves the use of synthetic double-stranded ODNs able to compete for binding of the protein to the authentic cis-elements (5–7). Although the mechanism of action of transcription factor decoy is distinct from the antisense strategy, the same critical parameters exist in that a decoy agent must be nuclease resistant, be taken up by cells and have sequence-specific effects (42). Advances in the synthesis of DNA now provide an exciting new chemical approach to overcome these issues. To date, several ODNs with a modified phosphodiester backbone, such as phosphorothioates and PNAs, have been widely studied as antisense and antigene reagents, however, neither of them has yet proved able to fulfill all the requirements. For this reason, novel ODN analogs and mimics are therefore high priority goals in medicinal chemistry.
In this paper we have investigated the possible use of conformationally restricted nucleotides (LNA) with a 2′-O,4′-C-methylene bridge in the design of decoy agents for transcription factor κB. Results presented here demonstrate that unlike DNA duplexes, double-stranded LNA–DNA–LNA co-polymers were not readily degraded by nucleases. Numerous studies have shown that end capping of an antisense oligomer with a short stretch of nuclease-resistant derivatives increases its lifetime (43–45). However, while the degradation of single-stranded oligonucleotides and their analogs has been studied extensively because of their potential importance as antisense agents, little is known about degradation of double-stranded ODNs and their analogs. To our knowledge, only a few papers have investigated the susceptibility to nuclease degradation of modified double-stranded ODNs, although it has been demonstrated that single-stranded and double-stranded DNA molecules are differentially degraded in human serum and in cellular extracts (9). Under our experimental conditions, addition of one LNA base both at the 5′ and 3′ termini of a double-stranded ODN containing the NF-κB consensus binding sequence was sufficient to confer the maximum magnitude of resistance to DNase I endonuclease degradation (Fig. 2). In contrast, at least two terminal LNA bases per strand were required to confer protection from exonuclease digestion, as demonstrated by a more rapid degradation of NF-κB(a) versus NF-κB(b) when they were incubated with BAL-31 (Fig. 3). Resistance to DNase I degradation of NF-κB(a) and NF-κB(b) is of particular interest since this nuclease is known to recognize certain helical parameters of its respective DNA substrate, such as groove width and flexibility (46,47). For this reason, perturbation of the molecular interactions between DNase I and the target DNA suggests that LNA–LNA base pairing at the terminal ends is probably sufficient to induce changes in the ODN phosphate backbone geometry. Interestingly, this LNA-induced structural modification does not apparently produce effects on the interactions between transcription factor κB and LNA–DNA–LNA co-polymers. In fact, these molecules exhibited high binding specificity and affinity, being able to form stable complexes with NF-κB in NIH 3T3 nuclear extracts (Fig. 4), and competed for binding of NF-κB to the PRDII probe with the same efficiency as the corresponding DNA duplex NF-κB(d) (Fig. 5). Thus, these molecules seem to offer an attractive set of properties not exhibited by either fully or partially modified phosphorothioate ODNs or by fully modified PNA oligomers, which have been demonstrated to be poorly specific (15) or insufficiently able to interact and form stable complexes with target transcription factors (16), respectively.
During the preparation of this manuscript, Romanelli et al. published preliminary results indicating that a PNA–DNA–PNA chimera mimicking the κB site displays the ability to resist enzymatic degradation and to interact with NF-κB (48). Our results demonstrate that the same effects can be obtained using LNAs instead of PNAs, with several advantages. In fact, while PNAs and LNAs share some similarities, there are also important differences that, in our opinion, make LNAs more suitable tools for the design of decoy molecules. First, LNAs have unprecedented binding affinities for complementary sequences, forming duplexes with greater stability compared with PNAs (49). Second, PNAs are uncharged and also have low solubility, whereas LNAs have a normally charged phosphate backbone, so they are readily soluble in water (49) and can be delivered into cells using standard protocols employing cationic lipids (26). In addition, by virtue of their structural resemblance to natural nucleic acid monomers, LNAs are expected not to affect interactions of the transcription factor with the DNA backbone, which have been demonstrated to be important for stabilization of the protein–DNA complex and which are lost with uncharged PNAs (16). In this regard, it should be noted that the binding of NF-κB to DNA, beyond base-specific interactions, is strengthened by extensive contacts with the deoxyribose phosphate backbone (37–39). Third, LNAs are assembled using standard synthesis techniques that allow LNA bases to be easily interspersed among DNA, thus permitting the properties of the LNA-containing oligomers to be fine tuned in order to optimize nuclease resistance without markedly compromising transcription factor binding (49). Our results in this regard clearly indicate that a significant increase in resistance to nuclease digestion can be attained by positioning further LNA substitutions in the context of the PRDII element by modifying complementary nucleotides in both strands [NF-κB(c)] (Figs 2 and 3). In fact, only slight improvements in terms of stability were obtained when internal LNAs were introduced in only one strand [NF-κB(c+b) and NF-κB(b+c)] (Figs 2 and 3). However, while LNA substitutions involving both strands resulted in a weaker affinity of NF-κB for its target sequence [NF-κB(c); Fig. 5], internal LNA modifications in one strand had more limited effects on NF-κB binding [NF-κB(c+b) and NF-κB(b+c); Fig. 5], with some differences related to their positioning (see below). Thus, these results suggest that LNAs might induce changes in the molecular structure of the κB-binding sequence which are proportional to the extent of modification, leading to a different degree of perturbation of the interactions with NF-κB. This hypothesis is corroborated by NMR studies which have determined the solution structure of an ODN, containing one internal LNA, hybridized to complementary DNA (34). Results presented by Nielsen et al. clearly indicate that by virtue of its C3′-endo conformation the LNA introduces a higher population of the N-type conformations of the neighboring unmodified nucleotides on the same strand, resulting in a local change in the phosphate backbone geometry (34,35). Based on our evidence, it might be speculated that such architectural differences apparently disturb the interaction between NF-κB and its consensus sequence. However, it is worth noting that, besides extent, positioning of the LNA modification also contributes to this effect. This is evident from the observed higher affinity of NF-κB for PRDII elements containing LNAs in the sense strand [NF-κB (c+b)] rather than in the antisense strand [NF-κB(b+c)]. This result can be explained by the observation that structural modifications conferred by the introduction of a LNA base are apparently propagated to adjacent unmodified nucleotides in the C3′ direction, with the first nucleotide following the LNA containing an appreciable fraction of N-type conformation and some of the other nucleotides with a non-negligable fraction of this conformation (34). As a consequence, it could be assumed that the LNA in the sense strand of NF-κB(c+b) might affect the conformation of the AT-rich sequence of PRDII in the minor groove (Fig. 1). Crystallographic studies of NF-κB bound to DNA have shown that this region does not make base-specific contacts with NF-κB (37–39), thus explaining the modest decrease in the binding affinity of NF-κB to the NF-κB(c+b) probe (Fig. 5). Conversely, the LNA in the antisense strand of NF-κB(b+c) might modify the conformation of the GC-rich region of PRDII, which makes extensive base-specific contacts with the transcription factor (Fig. 1) (37–39), inducing a more evident loss of affinity of NF-κB for its binding element (Fig. 5).
In conclusion, the results reported herein suggest that LNA modification represents a valuable tool in designing decoys with improved features in terms of potential stability and activity. This has been clearly demonstrated for LNA–DNA–LNA co-polymers, encouraging further experiments focused on the possible use of these molecules as decoy agents in in vivo systems. In addition, the finding that internal LNA modification of both strands leads to a further improvement in ODN biostability [NF-κB(c)] appears to be of great interest. In fact, although less efficient in binding NF-κB, this decoy molecule has been shown to compete with a DNA probe in the nanomolar range of concentrations. This evidence does not necessarily exclude its possible in vivo decoy activity. Furthermore, data obtained with the mixed hybrids NF-κB(c+b) and NF-κB(b+c) suggest a role of the intra-strand positioning on NF-κB binding, thus opening up the possibility of minimizing perturbation of the interaction between NF-κB and LNA–DNA duplexes with internal LNAs, such as NF-κB(c), by more convenient substitutions in the κB sequence.
Acknowledgments
ACKNOWLEDGEMENTS
The authors would like to thank Proligo LLC for helpful suggestions in the design of the decoys. This study was supported by MURST-CNR, 1.27/12/1997 no. 449 and COFIN-MIUR PRIN 2001.
REFERENCES
- 1.Papavassiliou A.G. (1998) Transcription-factor-modulating agents: precision and selectivity in drug design. Mol. Med. Today, 4, 358–366. [DOI] [PubMed] [Google Scholar]
- 2.Morishita R., Higaki,J., Tomita,N. and Ogihara,T. (1998) Application of transcription factor “decoy” strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ. Res., 82, 1023–1028. [DOI] [PubMed] [Google Scholar]
- 3.Cho-Chung Y.S., Park,Y.G. and Lee,Y.N. (1999) Oligonucleotides as transcription factor decoys. Curr. Opin. Mol. Ther., 1, 386–392. [PubMed] [Google Scholar]
- 4.Mann M.J. and Dzau,V.J. (2000) Therapeutic applications of transcription factor decoy oligonucleotides. J. Clin. Invest., 106, 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielinska A., Shivdasani,R.A., Zhang,L. and Nabel,G.J. (1990) Regulation of gene expression with double-stranded phosphorothioate oligonucleotides. Science, 250, 997–1000. [DOI] [PubMed] [Google Scholar]
- 6.Morishita R., Gibbons,G.H., Horiuchi,M., Ellison,K.E., Nakajima,M., Zhang,L., Kaneda,Y., Ogihara,T. and Dzau,V.J. (1995) A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc. Natl Acad. Sci. USA, 92, 5855–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morishita R., Sugimoto,T., Aoki,M., Kida,I., Tomita,N., Moriguchi,A., Maeda,K., Sawa,Y., Kaneda,Y., Higaki,J. and Ogihara,T. (1997) In vivo transfection of cis element “decoy” against nuclear factor -kB binding site prevents myocardial infarction. Nature Med., 3, 894–899. [DOI] [PubMed] [Google Scholar]
- 8.Uhlmann E. and Peyman,A. (1990) Antisense oligonucleotides: a new therapeutic principle. Chem. Rev., 90, 543–584. [Google Scholar]
- 9.Chu B.C.F. and Orgel,L.E. (1992) The stability of different forms of double-stranded decoy DNA in serum and nuclear extracts. Nucleic Acids Res., 20, 5857–5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein C.A., Subasinghe,C., Shinozuka,K. and Cohen,J.S. (1988) Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res., 16, 3209–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Q., Matson,S., Herrera,C.J., Fisher,E., Yu,H. and Krieg,A.M. (1993) Comparison of cellular binding and uptake of antisense phosphodiester, phosphorothioate and mixed phosphorothioate and methylphosphonate oligonucleotides. Antisense Res. Dev., 3, 53–66. [DOI] [PubMed] [Google Scholar]
- 12.Kibler-Herzog L., Zon,G., Uznanski,B., Whittier,G. and Wilson,W.D. (1991) Duplex stabilities of phosphorothioate, methylphosphonate and RNA analogs of two DNA 14-mers. Nucleic Acids Res., 19, 2979–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao W.Y., Han,F.S., Storm,C., Egan,W. and Cheng,Y.C. (1992) Phosphorothioate oligonucleotides are inhibitors of human DNA polymerases and RNase H: implications for antisense technology. Mol. Pharmacol., 41, 223–229. [PubMed] [Google Scholar]
- 14.Yaswen P., Stampfer,M.R., Ghosh,K. and Cohen,J.S. (1993) Effects of sequence of thioated oligonucleotides on cultured human mammary epithelial cells. Antisense Res. Dev., 3, 67–77. [DOI] [PubMed] [Google Scholar]
- 15.Brown D.A., Kang,S.H., Gryaznov,S.M., DeDionisio,L., Heidenreich,O., Sullivan,S., Xu,X. and Nerenberg,M.I. (1994) Effect of phosphorothioate modification of oligodeoxynucleotides on specific protein binding. J. Biol. Chem., 269, 26801–26805. [PubMed] [Google Scholar]
- 16.Mischiati C., Borgatti,M., Bianchi,N., Rutigliano,C., Tomassetti,M., Feriotto,G. and Gambari,R. (1999) Interaction of the human NF-kB p52 transcription factor with DNA–PNA hybrids mimicking the NF-kB binding sites of the human immunodeficiency virus type 1 promoter. J. Biol. Chem., 274, 33114–33122. [DOI] [PubMed] [Google Scholar]
- 17.Egholm M., Buchardt,O., Christensen,L., Behrens,C., Freier,S.M., Driver,D.A., Berg,R.H., Kim,S.K., Norden,B. and Nielsen,P.E. (1993) PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature, 365, 566–568. [DOI] [PubMed] [Google Scholar]
- 18.Demidov V.V., Potaman,V.N., Frank-Kamenetsk,M.D., Egholm,M., Buchard,O., Sonnichsen,S.H. and Nielsen,P.E. (1994) Stability of peptide nucleic acids in human serum and cellular extracts. Biochem. Pharmacol., 48, 1310–1313. [DOI] [PubMed] [Google Scholar]
- 19.Singh S.K., Nielsen,P., Koshkin,A. and Wengel,J. (1998) LNA (locked nucleic acids): synthesis and high-affinity nucleic acid recognition. Chem. Commun., 455–456. [Google Scholar]
- 20.Koshkin A., Singh,S.K., Nielsen,P., Rajwanshi,V.K., Kumar,R., Meldgaard,M., Olsen,C.E. and Wengel,J. (1998) LNA (locked nucleic acids): synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation and unprecedented nucleic acid recognition. Tetrahedron, 54, 3607–3630. [Google Scholar]
- 21.Wengel J. (1999) Synthesis of 3′-C-and 4′-C-branched oligodeoxynucleotides and the development of locked nucleic acid (LNA). Acc. Chem. Res., 32, 301–310. [Google Scholar]
- 22.Singh S.K. and Wengel,J. (1998) Universality of LNA-mediated high-affinity nucleic acid recognition. Chem. Commun., 1247–1248. [Google Scholar]
- 23.Obika S., Nanbu,D., Hari,Y., Morio,J.A.K., Doi,T. and Imanishi,T. (1998) Stability and structural features of the duplexes containing nucleoside analogues with a fixed N-type conformation, 2′-O,4′-C-methyleneribonucleosides. Tetrahedron Lett., 39, 5401–5404. [Google Scholar]
- 24.Pfundheller H.M. and Wengel,J. (1999) Oligonucleotides containing 4′-C-aminomethyl-2′-modified thymidines show increased binding affinity towards DNA and RNA. Bioorg. Med. Chem. Lett., 9, 2667–2672. [DOI] [PubMed] [Google Scholar]
- 25.Christensen U., Jacobsen,N., Rajwanshi,V.K., Wengel,J. and Koch,T. (2001) Stopped-flow kinetics of locked nucleic acid (LNA)–oligonucleotide duplex formation: studies of LNA–DNA and DNA–DNA interactions. Biochem. J., 354, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahlestedt C., Salmi,P., Good,L., Kela,J., Johnsson,T., Hokfelt,T., Broberger,C., Porreca,F., Lai,J., Ren,K., Ossipov,M., Koshkin,A., Jakobsen,N., Skouv,J., Oerum,H., Jacobsen,M.H. and Wengel,J. (2000) Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc. Natl Acad. Sci. USA, 97, 5633–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkins N.D. (2000) The Rel/NF-kB family: friend and foe. Trends Biochem. Sci., 25, 434–440. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin A.S. (2001) Series introduction: the transcription factor NF-kB and human diseases. J. Clin. Invest., 107, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma H.W., Perez,J.R., Higgins-Sochaski,K., Hsiao,R. and Narayanan,R. (1996) Transcription factor decoy approach to decifer the role of NF-kB in oncogenesis. Anticancer Res., 16, 61–69. [PubMed] [Google Scholar]
- 30.Miagkov A.V., Kovalenko,D.V., Brown,C.E., Didsbury,J.R., Cogswell,J.P., Stimpson,S.A., Baldwin,A.S. and Makarov,S.S. (1998) NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc. Natl Acad. Sci. USA, 95, 13859–13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushita H., Morishita,R., Nata,T., Aoki,M., Nakagami,H., Taniyama,Y., Yamamoto,K., Higaki,J., Yasufumi,K. and Ogihara,T. (2000) Hypoxia-induced endothelial apoptosis through nuclear factor-kappaB (NF-kappaB)-mediated bcl-2 suppression: in vivo evidence of the importance of NF-kappaB in endothelial cell regulation. Circ. Res., 86, 974–981. [DOI] [PubMed] [Google Scholar]
- 32.Cooper J.A., Parks,J.M., Carcelen,R., Kahlon,S.S., Sheffield,M. and Culbreth,R. (2000) Attenuation of interleukin-8 production by inhibiting nuclear factor-kappaB translocation using decoy oligonucleotides. Biochem. Pharmacol., 59, 605–613. [DOI] [PubMed] [Google Scholar]
- 33.Tomita N., Morishita,R., Tomita,S., Gibbons,G.H., Zhang,L., Horiuchi,M., Kaneda,Y., Higaki,J., Ogihara,T. and Dzau,V.J. (2000) Transcription factor decoy for NFkappaB inhibits TNF-alpha-induced cytokine and adhesion molecule expression in vivo. Gene Ther., 7, 1326–1332. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen C.B., Singh,S.K., Wengel,J. and Jacobsen,J.P. (1999) The solution structure of a locked nucleic acid (LNA) hybridized to DNA. J. Biomol. Struct. Dyn., 17, 175–191. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen K.E., Singh,S.K., Wengel,J. and Jacobsen,J.P. (2000) Solution structure of an LNA hybridized to DNA: NMR study of the d(CT(L)GCT(L)T(L)CT(L)GC):d(GCAGAAGCAG) duplex containing four locked nucleotides. Bioconjug. Chem., 11, 228–238. [DOI] [PubMed] [Google Scholar]
- 36.Petersen M., Nielsen,C.B., Nielsen,K.E., Jensen,G.A., Bondensgaard,K., Singh,S.K., Rajwanshi,V.K., Koshkin,A.A., Dahl,B.M., Wengel,J. and Jacobsen,J.P. (2000) The conformations of locked nucleic acids (LNA). J. Mol. Recognit., 13, 44–53. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh G., van Duyne,G., Ghosh,S. and Sigler,P.B. (1995) Structure of NF-kappa B p50 homodimer bound to a kB site. Nature, 373, 303–310. [DOI] [PubMed] [Google Scholar]
- 38.Muller C.W., Rey,F.A., Sodeoka,M., Verdine,G.L. and Harrison,S.C. (1995) Structure of the NF-kappa B p50 homodimer bound to DNA. Nature, 373, 311–317. [DOI] [PubMed] [Google Scholar]
- 39.Chen F.E., Huang,D.B., Chen,Y.Q. and Ghosh,G. (1998) Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature, 391, 410–413. [DOI] [PubMed] [Google Scholar]
- 40.Thanos D. and Maniatis,T. (1992) The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell, 71, 777–789. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani F., Covaceuszach,S., Rustighi,A., Sgarra,R., Heath,C., Goodwin,G.H. and Manfioletti,G. (1998) NF-κB mediated transcriptional activation is enhanced by the architectural factor HMGI-C. Nucleic Acids Res., 26, 1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein C.A. and Cheng,Y.-C. (1993) Antisense oligonucleotides as therapeutic agents. Is the bullet really magical? Science, 261, 1004–1011. [DOI] [PubMed] [Google Scholar]
- 43.Shaw J.P., Kent,K., Bird,J., Fishback,J. and Froehler,B. (1991) Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res., 19, 747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uhlman E., Ryte,A. and Peyman,A. (1997) Studies on the mechanism of stabilization of partially phosphorothioated oligonucleotides against nucleolytic degradation. Antisense Nucleic Acid Drug Dev., 7, 345–350. [DOI] [PubMed] [Google Scholar]
- 45.Pandolfi D., Rauzi,F. and Capobianco,M.L. (1999) Evaluation of different types of end-capping modifications on the stability of oligonucleotides towards 3′- and 5′-exonucleases. Nucl. Nucl., 18, 2051–2069. [DOI] [PubMed] [Google Scholar]
- 46.Suck D. and Oefner,C. (1986) Structure of Dnase I at 2.0 Å resolution suggests a mechanism for binding to and cutting DNA. Nature, 321, 620–625. [DOI] [PubMed] [Google Scholar]
- 47.Suck D. (1997) DNA recognition by structure-selective nucleases. Biopolymers, 44, 405–421. [DOI] [PubMed] [Google Scholar]
- 48.Romanelli A., Pedone,C., Saviano,M., Bianchi,N., Borgatti,M., Mischiati,C. and Gambari R. (2001) Molecular interactions between nuclear factor-kB (NF-kB) transcription factors and PNA-DNA chimera mimicking NF-kB binding sites. Eur. J. Biochem., 268, 6066–6075. [DOI] [PubMed] [Google Scholar]
- 49.Braasch D.A. and Corey,D.R. (2000) Locked nucleic acids (LNA): fine-tuning the recognition of DNA and RNA. Chem. Biol., 55, 1–7. [DOI] [PubMed] [Google Scholar]