Abstract
Aims
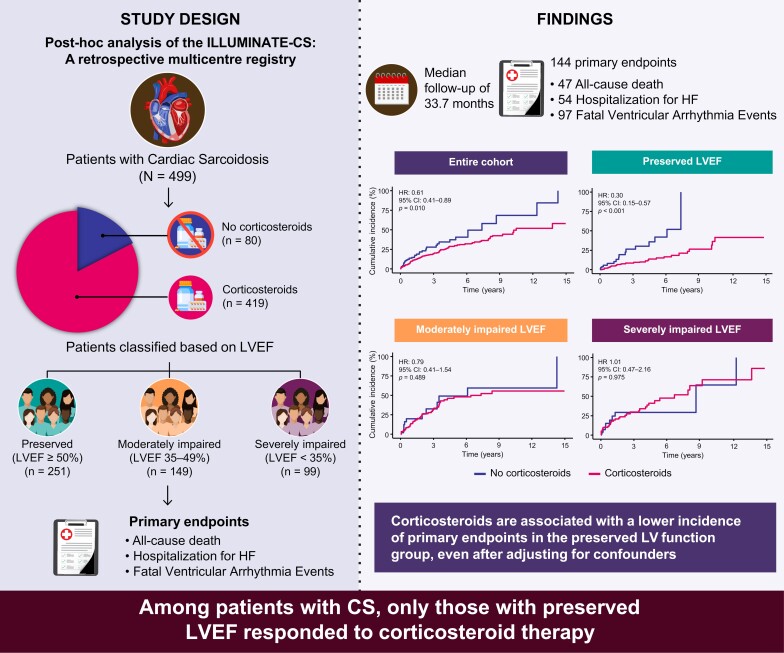
This study aimed to investigate the relationship between corticosteroid therapy and long-term outcomes in patients with cardiac sarcoidosis, stratified by left ventricular ejection fraction (LVEF) at diagnosis.
Methods and results
This study conducted a post hoc analysis of the ILLUstration of the Management and prognosIs of JapaNese PATiEnts with Cardiac Sarcoidosis, a retrospective multicentre registry. Cardiac sarcoidosis was diagnosed based on the 2016 Japanese Circulation Society and 2014 Heart Rhythm Society criteria. The primary endpoint was a composite of all-cause death, hospitalization for heart failure, and fatal ventricular arrhythmia events. Patients were divided into three groups based on LVEF: preserved LVEF (≥50%, n = 251), moderately impaired LVEF (LVEF, 35–49%; n = 149), and severely impaired LVEF (<35%, n = 99). Among 499 patients with cardiac sarcoidosis (mean age: 61.6 ± 11.4 years, male: 36.1%), 419 (84.0%) were treated with corticosteroids after diagnosis. During a median follow-up of 33.7 months (interquartile range, 16.8–62.7 months), 144 primary endpoints (28.9%) occurred. Corticosteroid therapy was associated with better prognosis when assessed in terms of primary endpoint in the entire cohort [hazard ratio (HR) 0.61, 95% confidence interval (CI) 0.41–0.89, P = 0.010]. When stratified by LVEF, corticosteroid therapy was significantly associated with a lower incidence of primary endpoints in the preserved LVEF group (HR, 0.30; 95% CI, 0.15–0.57, P < 0.001), but not in the moderately and severely impaired LVEF groups. This association remained robust, even after adjusting for confounders.
Conclusion
In this large cohort of cardiac sarcoidosis, corticosteroid therapy was associated with a lower incidence of long-term outcomes only in patients with preserved LVEF at diagnosis.
Clinical Trial Registration
UMIN000034974.
Keywords: Cardiac sarcoidosis, Corticosteroid, Left ventricular ejection fraction, Prognosis
Graphical abstract
Graphical Abstract.
CI, confidence interval; HF, heart failure; HR, hazard ratio; ILLUMINATE-CS, ILLUstration of the Management and prognosIs of JapaNese pATiEnts with Cardiac Sarcoidosis; LV, left ventricular; LVEF, left ventricular ejection fraction.
Introduction
Sarcoidosis is a multisystem inflammatory disorder characterized by non-caseating granulomas in multiple tissues and organs. Cardiac involvement in sarcoidosis plays a pivotal role as a prognostic factor, affecting ∼5% of patients with sarcoidosis.1 The primary clinical presentations of cardiac sarcoidosis are conduction abnormalities, ventricular arrhythmias including sudden death, and heart failure (HF). Corticosteroids are used as the first-line treatment for cardiac sarcoidosis to attenuate inflammation, with the goal of preventing fibrosis and cardiac dysfunction and reducing ventricular arrhythmias burden.2–5 However, the efficacy and significance of corticosteroid therapy in cardiac sarcoidosis remain unclear.2,6
As cardiac sarcoidosis is an inflammatory disease, persistent inflammation is assumed to contribute to left ventricular (LV) dysfunction and disease progression.7,8 The administration of corticosteroids before the onset of LV dysfunction may control inflammation, prevent the development of LV dysfunction, and improve prognosis. However, data on the relationship between corticosteroid administration and long-term outcomes according to the LV function at diagnosis are scarce. In particular, most studies have not included corticosteroid-naive patients; if they did, the numbers were limited.2
In this study, we evaluated whether corticosteroid therapy affected the prognosis of patients with cardiac sarcoidosis-stratified LV function. To this end, using one of the largest cohorts of cardiac sarcoidosis reported to date, the ILLUstration of the Management and prognosIs of JapaNese PATiEnts with Cardiac Sarcoidosis (ILLUMINATE-CS), which included a substantial number of corticosteroid-naive patients, we investigated the long-term prognosis of patients treated with and without corticosteroids in each group stratified by LV ejection fraction (LVEF), which is recognized as a useful indicator of LV function in cardiac sarcoidosis.9–11
Methods
Study design
This was a post hoc analysis of the ILLUMINATE-CS study and its detailed design has been previously described.12 Briefly, the ILLUMINATE-CS is a multicentre retrospective registry used to evaluate the clinical characteristics and outcomes of patients with cardiac sarcoidosis. Patients with cardiac sarcoidosis who were first diagnosed between 2001 and 2017 at 33 hospitals in Japan enroled in the registry. cardiac sarcoidosis was defined based on the criteria outlined in the Heart Rhythm Society (HRS) consensus statement or the Japanese Circulation Society (JCS) guidelines.13,14 The ethics committee of each participating study site approved the study protocol. This study was conducted in accordance with the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. All participants were notified of their enrolment in the ILLUMINATE-CS study and informed that they would be free to opt-out anytime.
Data collection of clinical variables and outcomes
Baseline characteristics, including clinical comorbidities, blood test data, and cardiovascular imaging findings, were obtained during the initial cardiac sarcoidosis diagnostic process. In this study, the corticosteroid therapy group included patients who received corticosteroids at any time prior to the first occurrence of the primary endpoint. Patients who did not receive corticosteroids prior to the occurrence of the primary endpoint or who started corticosteroid therapy only after the primary endpoint had already occurred were classified into the no corticosteroid group. This approach was applied to other immunosuppressive agent groups. Corticosteroid therapy was typically initiated in cases of severe ventricular arrhythmia, such as atrioventricular (AV) block and ventricular tachycardia, local wall motion abnormalities, or decreased cardiac function. The decision to initiate therapy was at the discretion of the attending physician and was guided by the presence of inflammation or other clinical risk factors. Corticosteroid therapy was typically avoided in patients with known contraindications, including systemic infections, uncontrolled diabetes, and severe cardiovascular conditions. The echocardiographic findings at baseline and during the follow-up period were collected from both groups. Echocardiography performed closest to and before the diagnosis of cardiac sarcoidosis was used as the baseline echocardiogram, whereas the last echocardiography performed within 15 years after diagnosis during the follow-up period was considered the final follow-up echocardiographic result. In the corticosteroid-treated group, an echocardiogram obtained after the maintenance phase of immunosuppressive therapy (defined as three or more repeated administrations of corticosteroids at the same dose) within 15 years after diagnosis was used as the final follow-up echocardiogram. Left ventricular ejection fraction was determined using the modified Simpson method. All outcomes were obtained from medical records, direct contact, or telephone interviews with the referring physician at the study site. The primary endpoint was a composite of all-cause death, hospitalization for HF, and fatal ventricular arrhythmia events. Fatal ventricular arrhythmia events were defined as a combination of sudden cardiac death and documented ventricular fibrillation, sustained ventricular tachycardia lasting >30 s, or appropriate implantable cardioverter-defibrillator therapy. The causes of death, including sudden cardiac death and HF hospitalization, were defined according to the definitions recently proposed by the Heart Failure Collaboratory and Academic Research Consortium.15
To evaluate the association between corticosteroid therapy and outcomes based on LV function, patients were divided into three LV function groups based on LVEF at diagnosis: preserved LVEF (LVEF ≥ 50%), moderately impaired LVEF (LVEF, 35–49%), and severely impaired LVEF (LVEF < 35%).10
Statistical analysis
Categorical variables are presented as numbers (percentages), and continuous variables are shown as the mean ± standard deviation for normally distributed variables or as the median [interquartile range (IQR)] for non-normally distributed variables. Student’s t-test or the Mann–Whitney U-test for continuous variables, and chi-squared or Fisher’s exact test for categorical variables, were used for comparisons between groups, as appropriate. Statistical significance was defined as a two-sided P-value of <0.05. Cox regression analysis was used to assess potential variables associated with the endpoint. Since the time interval between diagnosis and introduction of corticosteroids varied among corticosteroid-treated patients, we analysed corticosteroid therapy as a time-varying covariate to avoid immortal time bias. In this analysis, all patients were initially considered corticosteroid-unexposed until corticosteroid initiation. We also plotted the cumulative incidence of events using Simon and Makuch’s method, which modified the Kaplan–Meier survival curves to account for time-varying exposures.16 The P-value for interaction was calculated to assess the heterogeneity in the treatment effect for the primary endpoint across LVEF subgroups using an interaction analysis. We included an interaction term between the LVEF subgroups and corticosteroid therapy in a Cox proportional hazards regression model. Cox proportional hazards regression was used to evaluate differences in the cumulative incidence curves. The association between corticosteroid therapy and primary outcomes was evaluated using multivariate Cox proportional hazards regression analysis, adjusted for age, sex, and pertinent covariates. Covariates for the multivariate analysis were selected based on factors known to significantly impact the prognosis of cardiac sarcoidosis and the effectiveness of corticosteroid therapy.6,12,17 The covariates included age, sex, the severity of HF, LV function, the presence of arrhythmias, markers of myocardial inflammation, and interventions after diagnosis. Device implantation and ventricular tachycardia ablation were treated as time-varying covariates. Additionally, the sensitivity analysis was conducted using factors that were significantly associated with the primary endpoint in previous studies from the same cohort.12 The factors included the log-transformed B-type natriuretic peptide (BNP) level, LVEF, a history of sustained ventricular tachycardia or ventricular fibrillation, and ablation for ventricular tachycardia after diagnosis. Univariate Fine–Gray competing-risks regression models were used to assess the effects of corticosteroids on fatal ventricular arrhythmia events and HF hospitalization, considering death as a competing event. Proportional hazard assumption violations were evaluated by visually inspecting complementary log–log plots and Schoenfeld residuals. Paired t-tests were used to compare the LVEF at the final follow-up with that at baseline. Changes in LVEF with and without corticosteroid therapy were evaluated using analysis of covariance (ANCOVA), adjusting for the time interval between baseline and final follow-up echocardiography and LVEF at diagnosis. We used multiple imputations to address the missing data. Incomplete variables were imputed under a fully conditional specification using chained equations under the assumption of missing data at random. We created and analysed 1000 multiply imputed datasets using the ‘mice’ package version 3.14.0 in R (https://www.r-project.org/) and combined the parameter estimates using Rubin’s rules. All statistical analyses were performed using EZR version 1.60 (https://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html) and R version 4.2.0.
Results
Characteristics of the patient
Of the 512 patients initially enroled in the ILLUMINATE-CS, 13 were excluded because of missing baseline LVEF data, leaving a final cohort of 499 patients for analysis. Among this cohort, 419 individuals (84.0%) underwent corticosteroid therapy, with an average initial dosage of 30.3 ± 5.4 mg/day and a maintenance dosage of 6.9 ± 3.5 mg/day. Supplementary material online, Figure S1 shows the time distribution from diagnosis to corticosteroid initiation. The median interval between diagnosis and initiation of corticosteroids was 21 days (IQR: 4–46 days), and 62.1% of patients received corticosteroids within 30 days of cardiac sarcoidosis diagnosis. While 19 patients (3.8%) received a regimen of corticosteroids combined with other immunosuppressive agents, none of them received other immunosuppressive agents without also receiving corticosteroids. Among the 435 patients for whom the date of echocardiography was available, 431 (99.1%) underwent echocardiography within 12 months prior to diagnosis.
The baseline characteristics of the patients with and without corticosteroid use are summarized in Table 1. Patients receiving corticosteroids show a high proportion of myocardial uptake on 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) scans.
Table 1.
Clinical characteristics of the study population with and without corticosteroids
| Overall (n = 499) | No corticosteroids (n = 80) | Corticosteroids (n = 419) | P-valuea | |
|---|---|---|---|---|
| Age (years) | 61.6 ± 11.4 | 62.8 ± 14.0 | 61.3 ± 10.8 | 0.286 |
| Male sex, n (%) | 180 (36.1) | 34 (42.5) | 146 (34.8) | 0.191 |
| NYHA III/IV, n (%) | 61 (12.8) | 12 (16.4) | 49 (12.2) | 0.314 |
| Medical history | ||||
| Hypertension, n (%) | 177 (37.3) | 30 (41.7) | 147 (36.5) | 0.402 |
| Diabetes mellitus, n (%) | 129 (27.3) | 21 (29.6) | 108 (26.9) | 0.636 |
| Dyslipidaemia, n (%) | 75 (16) | 13 (18.1) | 62 (15.6) | 0.597 |
| Coronary artery disease, n (%) | 24 (5.1) | 7 (9.6) | 17 (4.2) | 0.054 |
| HF admission, n (%) | 98 (20.5) | 17 (23.3) | 81 (20) | 0.515 |
| Ventricular tachycardia or ventricular fibrillation, n (%) | 74 (15.6) | 13 (18.1) | 61 (15.1) | 0.529 |
| Non-sustained ventricular tachycardia, n (%) | 103 (22.1) | 14 (20) | 89 (22.4) | 0.653 |
| High-degree AV block, n (%) | 210 (43.8) | 30 (41.7) | 180 (44.2) | 0.687 |
| Atrial fibrillation, n (%) | 47 (10.0) | 10 (14.1) | 37 (9.3) | 0.216 |
| Laboratory parameters | ||||
| Creatinine (mg/dL) | 0.78 [0.66–0.96] | 0.80 [0.66–1.07] | 0.78 [0.66–0.95] | 0.354 |
| eGFR (mL/min/1.73 m2) | 64.1 [52.9–76.3] | 59.1 [48.9–71.3] | 65.3 [53.7–76.8] | 0.357 |
| BNP (pg/mL) | 123 [53–321] | 107 [57–480] | 124 [52–320] | 0.790 |
| Echocardiographic parameters | ||||
| LVEF (%) | 50 [37–61] | 49 [36–61] | 50 [38–61] | 0.360 |
| LVDD (mm) | 52 [46–59] | 52 [46–59] | 52 [46–59] | 0.952 |
| 67Ga scintigraphy | ||||
| Patients underwent 67Ga scintigraphy, n (%) | 256 (51.3) | 42 (52.5) | 214 (51.1) | 0.815 |
| 67Ga uptake in patients undergoing 67Ga scintigraphy, n (%) | 95 (37.0) | 11 (26.8) | 84 (38.9) | 0.143 |
| CMR | ||||
| Patients who underwent CMR, n (%) | 308 (61.7) | 53 (66.3) | 255 (60.9) | 0.363 |
| LGE-positive in patients undergoing CMR, n (%) | 279 (91.8) | 48 (88.9) | 231 (92.4) | 0.394 |
| FDG-PET | ||||
| Patients who underwent FDG-PET, n (%) | 341 (68.3) | 51 (63.8) | 290 (69.2) | 0.336 |
| FDG uptake in patients undergoing FDG-PET, n (%) | 321 (95.0) | 42 (84.0) | 279 (96.9) | <0.001 |
| Isolated cardiac sarcoidosis | ||||
| Histological, n (%) | 23 (4.6) | 4 (5.0) | 19 (4.5) | 0.857 |
| Clinical, n (%) | 87 (19.4) | 15 (18.8) | 82 (19.6) | 0.865 |
| Histological/clinical, n (%) | 120 (24.0) | 19 (23.8) | 101 (24.1) | 0.946 |
| Concomitant medications | ||||
| ACEi/ARB, n (%) | 249 (50.6) | 45 (58.4) | 204 (49.2) | 0.135 |
| Beta-blocker, n (%) | 200 (40.7) | 37 (48.1) | 163 (39.4) | 0.155 |
| MRA, n (%) | 92 (18.9) | 15 (19.7) | 77 (18.7) | 0.838 |
| Amiodarone, n (%) | 50 (10.2) | 11 (14.3) | 39 (9.5) | 0.203 |
| Device implantation | ||||
| Pacemaker/CRT-P, n (%) | 136 (27.9) | 21 (26.9) | 115 (28.0) | 0.839 |
| ICD/CRT-D, n (%) | 54 (11.3) | 10 (13.2) | 44 (11.0) | 0.587 |
| Other immunosuppressive agents (before a primary endpoint) | ||||
| Methotrexate, n (%) | 15 (3.0) | 0 (0) | 15 (3.6) | 0.145 |
| Cyclosporine, n (%) | 1 (0.2) | 0 (0) | 1 (0.2) | 1.000 |
| Cyclophosphamide, n (%) | 1 (0.2) | 0 (0) | 1 (0.2) | 1.000 |
| Azathioprine, n (%) | 2 (0.4) | 0 (0) | 2 (0.5) | 1.000 |
Data are presented as median (IQR) or mean ± standard deviation for continuous variables, and as n (%) for categorical variables.
ACEi/ARB, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker; AV, atrioventricular; BNP, B-type natriuretic peptide; CMR, cardiac magnetic resonance imaging; CRT-P, cardiac resynchronization therapy pacemaker; eGFR, estimated glomerular filtration rate; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; HF, heart failure; ICD/CRT-D, implantable cardioverter defibrillator/cardiac resynchronization therapy defibrillator; LAD, left atrial diameter; LGE, late gadolinium enhancement; LVDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; MRA, mineralocorticoid receptor antagonist.
aThe P-value refers to the comparison between patients treated with corticosteroids and those not treated with corticosteroids.
Supplementary material online, Table S1 shows the baseline characteristics categorised based on LV function at diagnosis. The three LV function groups found no significant differences in the initial and maintenance corticosteroid dosages or time intervals from diagnosis to corticosteroid initiation; all these were similar when using other immunosuppressive agents. The prevalence of isolated cardiac sarcoidosis was higher in the moderately and severely impaired LV function groups than in the preserved LV function group. Patients with severely impaired LV function had a history of HF hospitalization, ventricular tachycardia or ventricular fibrillation, non-sustained ventricular tachycardia, severe HF symptoms, elevated BNP levels, and renal dysfunction. They were administered angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker (ACEi/ARBs), beta-adrenergic receptor blockers, mineralocorticoid receptor antagonist (MRAs), and amiodarone. Additionally, the prevalence of implantable cardioverter defibrillators or cardiac resynchronization therapy defibrillators was higher in these patients.
Supplementary material online, Table S2 outlines the characteristics of patients with preserved LVEF, comparing those who received corticosteroid therapy with those who did not. In the preserved LVEF group, patients who underwent corticosteroid therapy showed a higher prevalence of positive FDG-PET and gallium scintigraphy findings.
Prognosis of cardiac sarcoidosis patients with or without corticosteroid therapy
During a median follow-up of 33.7 months (IQR: 16.8–62.7 months), 144 (28.9%) primary endpoints were observed (47 all-cause mortalities, 97 fatal ventricular arrhythmia events, and 54 HF-related hospitalizations).
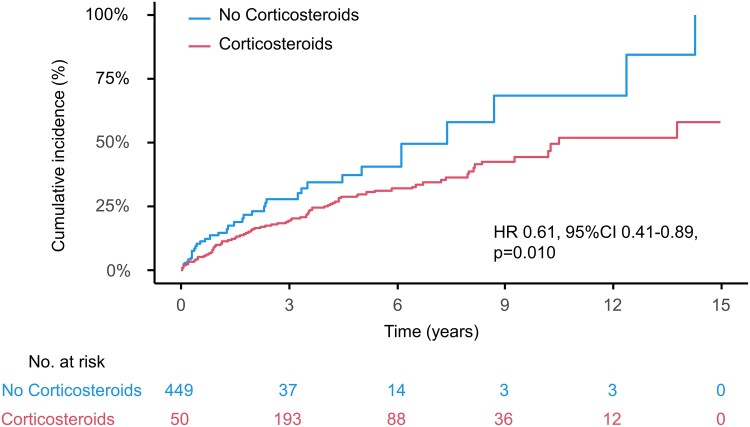
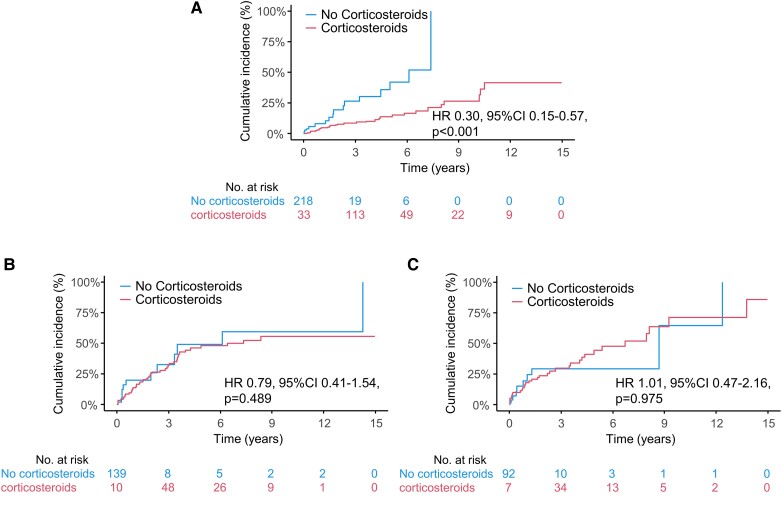
In the entire cohort, corticosteroid therapy was associated with better prognosis assessed as the primary endpoint [hazard ratio (HR) 0.61, 95% confidence interval (CI) 0.41–0.89, P = 0.010] (Figure 1). Figure 2 shows the cumulative incidence curves illustrating the relationship between corticosteroid therapy and the occurrence of the primary endpoint in the three LV function groups. The curves show that corticosteroid therapy was significantly associated with a lower incidence of the primary endpoint in the preserved LVEF group (HR, 0.30; 95% CI, 0.15–0.57, P < 0.001). In contrast, this association was not observed in the remaining two impaired LVEF groups (HR, 0.79; 95% CI, 0.41–1.53, P = 0.489, and HR, 1.01; 95% CI, 0.47–2.16, P = 0.975, respectively). Supplementary material online, Table S3 shows the relationship between corticosteroid therapy and clinical endpoints in each LVEF group based on univariate Cox regression analysis. Corticosteroid administration was associated with improvements in the entire cohort’s primary endpoint and hospitalization for HF. When divided by LVEF at diagnosis, corticosteroid therapy was associated with a decreased incidence of all-cause mortality, fatal ventricular arrhythmia events, and HF hospitalization (HR 0.16, 95% CI 0.03–0.71, P = 0.016; HR 0.42, 95% CI 0.2–0.9, P = 0.026; and HR 0.17, 95% CI 0.06–0.47, P < 0.001, respectively) only in the preserved LVEF group, with similar associations observed for primary endpoint. However, no association has been observed in patients with moderately or severely impaired LV function. The associations established between the primary endpoint and corticosteroid use remained robust in the multivariate analysis, even after adjusting for previously reported prognostic factors related to HF severity, cardiac function, arrhythmia, post-diagnosis intervention, and evidence of myocardial inflammation (Table 2). Additionally, a further sensitivity analysis was conducted using factors that were significantly associated with the primary endpoint in previous studies from the same cohort. This analysis demonstrated that corticosteroid therapy was significantly associated with a reduced risk of the primary endpoint in the preserved LV function group (HR 0.19, 95% CI 0.07–0.49, P < 0.001).
Figure 1.
Cumulative incidence curves for primary endpoint in patients with and without corticosteroid therapy in entire cohort. Corticosteroids showed significant association with better prognosis in terms of primary endpoint in entire cohort. CI, confidence interval; HR, hazard ratio.
Figure 2.
Cumulative incidence curves for primary endpoint in patients with and without corticosteroid therapy stratified by left ventricular ejection fraction at diagnosis. Patients were divided into three subgroups based on left ventricular ejection fraction at diagnosis for analysis; (A) Preserved left ventricular ejection fraction (left ventricular ejection fraction≥ 50%), (B) Moderately impaired left ventricular ejection fraction (left ventricular ejection fraction 35–49%), (C) Severely impaired left ventricular ejection fraction (left ventricular ejection fraction < 35%). CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction.
Table 2.
Association between corticosteroids and primary endpoint in patients with preserved left ventricular function (multivariate analysis)
| HR (95% CI) | P-value | |
|---|---|---|
| Unadjusted | 0.30 (0.15–0.57) | <0.001 |
| Model 1 | 0.31 (0.16–0.60) | <0.001 |
| Model 2 | 0.24 (0.10–0.59) | 0.002 |
| Model 3 | 0.32 (0.16–0.63) | 0.001 |
| Model 4 | 0.32 (0.15–0.68) | 0.003 |
| Model 5 | 0.22 (0.10–0.46) | <0.001 |
| Model 6 | 0.17 (0.03–0.91) | 0.038 |
Model 1: Adjusted for age and sex.
Model 2: Model 1 + severity of heart failure [NYHA functional Class (III or IV) and log BNP].
Model 3: Model 1 + left ventricular function variables (LVEF and LVDD).
Model 4: Model 1 + arrhythmia (ventricular tachycardia or ventricular fibrillation, non-sustained ventricular tachycardia and atrial fibrillation).
Model 5: Model 1 + intervention after diagnosis (device implantation and ventricular tachycardia ablation as time-varying covariates).
Model 6: Model 1 + myocardial inflammation (Uptake of 67Ga and 18F-FDG uptake).
BNP, B-type natriuretic peptide; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; LVDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
The interaction analysis revealed a significant interaction between corticosteroid therapy and LVEF subgroups (P = 0.028).
Effect of corticosteroid therapy on left ventricular function
Table 3 displays changes in LVEF from baseline to final follow-up, with patients grouped based on corticosteroid therapy and baseline LV function. While LVEF decreased significantly in patients with preserved LVEF, an elevation in LVEF was observed in patients with moderately or severely impaired LVEF, irrespective of the administration of corticosteroids. Subsequent ANCOVA analysis showed that corticosteroid therapy tended to mitigate LVEF reduction in patients with preserved LVEF, while it did not affect LVEF changes in patients with moderately impaired LVEF. Interestingly, in patients with severely impaired LVEF, corticosteroid therapy tended to hinder LVEF improvement.
Table 3.
Change in LVEF from diagnosis to final follow-up
| Initial LVEF | Corticosteroid therapy | n | LVEF at diagnosis | LVEF at final follow-up | Effect of corticosteroids | ||
|---|---|---|---|---|---|---|---|
| Paired t-test | ANCOVA | ||||||
| P-value | Estimate | P-value | |||||
| Preserved (LVEF ≥ 50%) | No corticosteroids | 30 | 62.5 ± 7.7 | 55.6 ± 12.5 | 0.009 | 3.806 | 0.090 |
| Corticosteroids | 221 | 62.0 ± 7.9 | 59.0 ± 10.0 | <0.001 | |||
| Moderately impaired (LVEF 35–49%) | No corticosteroids | 14 | 42.0 ± 4.0 | 42.8 ± 10.5 | 0.011 | 1.541 | 0.670 |
| Corticosteroids | 135 | 41.9 ± 4.3 | 44.0 ± 11.5 | 0.042 | |||
| Severely impaired (LVEF < 35%) | No corticosteroids | 16 | 27.3 ± 4.9 | 41.1 ± 15.7 | 0.011 | −7.141 | 0.070 |
| Corticosteroids | 83 | 25.8 ± 6.4 | 33.2 ± 12.2 | <0.001 | |||
Data are mean ± SD. LVEF, left ventricular ejection fraction; ANCOVA, analysis of covariance.
The LVEF at final follow-up was missing for 52 out of 499 patients (10.4%), and the time interval between baseline and final follow-up echocardiography was missing for 105 out of 499 patients (21.0%). Missing data were handled using multiple imputations.
Discussion
This study investigated the effects of corticosteroid therapy in patients with cardiac sarcoidosis stratified according to LV function at the time of diagnosis. The post hoc analysis of the ILLUMINATE-CS yielded three main findings. First, when categorized by LV function, corticosteroid therapy was associated with lower incidence of long-term prognosis only in patients with preserved LV function. Second, corticosteroid therapy related to a beneficial trend in changes in LV function only in patients with cardiac sarcoidosis who had preserved LV function. Finally, we first described the natural course of LVEF in patients with cardiac sarcoidosis who were not treated with corticosteroids. These data suggest that corticosteroid therapy might be more beneficial in patients with cardiac sarcoidosis and preserved LVEF than in those with impaired LVEF. Our findings provide novel insights into corticosteroid therapy, suggesting the importance of corticosteroid initiation in the early stages of cardiac sarcoidosis.
Corticosteroid therapy in patients with cardiac sarcoidosis in Japan
In our study, more than four-fifths of the patients received corticosteroid treatment. The proportion of patients who received corticosteroids and the initial and maintenance doses of corticosteroids in this study were consistent with those described in earlier studies conducted in Japan.11,18 The administered corticosteroid dose was closely aligned with the initial dose of 30 mg/day (0.5 mg/kg), and the maintenance dose ranged from 5 to 10 mg/day, as recommended by the JCS.14 As shown in previous studies, patients treated with corticosteroids had a higher proportion of myocardial inflammation than those who were not.18 These results may reflect that, in routine clinical practice, corticosteroids were initiated after confirmation of myocardial inflammation, as recommended by the HRS consensus statement.13 The time from diagnosis to corticosteroid initiation was ∼3 weeks in our study. While data on the optimal timing of initiation are limited, Padala et al.19 reported that corticosteroids were initiated within 30 days in 77% of patients with cardiac sarcoidosis, underscoring the importance of early initiation. In our study, the proportion of patients who received corticosteroids within 30 days of diagnosis was slightly lower than that reported previously, suggesting that treatment was initiated sufficiently early.
Impact of corticosteroid therapy on long-term prognosis
Since cardiac sarcoidosis is an inflammatory disease, corticosteroid therapy can plausibly improve the prognosis; however, the data are too limited to draw conclusions. A previous study showed that patients with corticosteroids had a higher survival rate than those without treatment, although the untreated group of patients was an autopsy series.9 A more recent study compared 67 corticosteroid-treated patients with 16 untreated patients.18 The authors found an association between corticosteroid therapy and improved prognosis in a composite endpoint, including all-cause mortality, HF-related hospitalization, and symptomatic arrhythmias, but not all-cause mortality or symptomatic arrhythmias. The results for the entire cohort were consistent with these findings. When stratified by LVEF, we found an association between corticosteroid therapy and long-term prognosis for all pre-specified endpoints in patients with preserved LVEF; however, no such association was found in patients with moderately or severely impaired LVEF. There are two main possibilities for why corticosteroid therapy was not effective in patients with low LVEF. First, there is a possibility that other conditions were unexpectedly included. The patients with impaired LV function had a higher prevalence of isolated cardiac sarcoidosis. It is possible that some patients clinically diagnosed with isolated cardiac sarcoidosis actually had other underlying conditions, such as lymphocytic myocarditis, giant cell myocarditis, or dilated cardiomyopathy, which may not respond to corticosteroids in the same way as sarcoidosis.14,20 Secondly, in patients with low LVEF, the myocardium may have already undergone extensive fibrosis due to prolonged inflammation, leaving little viable myocardium to respond to therapy. In such cases, the predominant pathology is irreversible scarring, and corticosteroids are unlikely to improve cardiac function. On the other hand, in a study by Kato et al.21 examining the prognosis with and without corticosteroids in 20 patients with AV block and preserved LV function, there were no deaths in the corticosteroid-treated group; however, 2 of 13 patients in the non-treated group died. Additionally, the corticosteroid-treated group exhibited a higher AV block recovery rate and fewer ventricular tachycardia occurrences than the non-treated group. These results support our finding of a favourable response to corticosteroid therapy in patients with cardiac sarcoidosis and preserved LVEF. A limitation of previous studies is that many did not include patients who did not take corticosteroids, and even if they were included, the number of patients was too small. The present study was larger and included a relatively large number of steroid-naive patients. Our results reinforce and extend the findings of earlier studies.
Effect of corticosteroid therapy on left ventricular function
The effect of corticosteroid therapy on LV function remains unclear. Several studies have investigated the changes in LVEF in patients receiving corticosteroid treatment. A recent study focusing on the effect of corticosteroids on LV function in cardiac sarcoidosis reported that LVEF was maintained in corticosteroid-treated patients with LVEF of ≧50%.22
A systematic review involving 194 corticosteroid-treated patients in 9 studies reported no significant change in LVEF in patients with normal LV function at baseline. Our study comprising 221 cardiac sarcoidosis patients with preserved LVEF treated relatively uniformly compared with a systematic review showed a significant reduction in LVEF, even after corticosteroid administration.2
The effect of corticosteroid therapy on cardiac function in patients with moderately and severely impaired LV function has been controversial.2 One study from Japan reported an enhancement in LVEF in patients with cardiac sarcoidosis and initially moderate LV dysfunction.23 Additionally, a Finnish nationwide study revealed LVEF improvement from 27.9 ± 4.1% to 34.1 ± 8.3% in the 22 corticosteroid-treated patients with severely impaired LV function.10 Similarly, we found a significant improvement in LVEF in corticosteroid-treated patients with moderately and severely impaired LV function.
Most previous studies did not include corticosteroid-untreated patients, so the natural course of LVEF changes remains unclear. One study reported LVEF decline in 13 patients without corticosteroids despite initially exhibiting normal LVEF.21 Similarly, our study found a significant LVEF reduction in patients with preserved baseline LVEF without corticosteroids. Importantly, compared with the non-treatment group, LVEF decline tended to be attenuated in the treatment group. For moderate LVEF impairment, Nagai et al.2,18 reported a decrease in LVEF from 32.5 to 18.5% in corticosteroid-treated patients. Conversely, our study showed significant LVEF improvement in patients despite corticosteroid absence, while the corticosteroid administration did not affect the change in LVEF. No study has investigated the natural LVEF trajectory changes in patients with cardiac sarcoidosis and severely impaired LVEF at baseline. Our results indicate that the LVEF increased even when patients were not treated with corticosteroids. Interestingly, improvement in LV function tended to be greater in patients not taking corticosteroids than in those taking corticosteroids. As previously mentioned, isolated cardiac sarcoidosis was more frequently observed in patients with reduced LVEF, and it is possible that these cases included conditions resembling dilated cardiomyopathy. In this study, patients with impaired LV function had a higher rate of standard HF medication use compared with those with preserved LVEF. Supplementary material online, Table S4 shows that while the utilization of standard HF therapies, including ACEi/ARBs, beta-blockers, and MRAs, was higher in patients with LVEF < 50%, there were no significant differences in the use of these medications between those treated with corticosteroids and those who were not. This suggests that the observed improvement in LV function in patients with impaired LVEF is likely driven by the HF therapies themselves, rather than corticosteroid treatment. Consequently, standard therapies for HF, such as beta-blockers and renin–angiotensin system inhibitors, may have contributed to the reversal of LV remodelling in cardiac sarcoidosis patients with impaired LVEF.
Clinical implications
Corticosteroids have widely recognized adverse effects, particularly with long-term use, such as infections, diabetes, weight gain, and osteoporosis.18,24 Therefore, unnecessary administration should be avoided with optimal intentions. Our results suggest that avoiding corticosteroid administration in patients with a less favourable LVEF spectrum can reduce side effects.
Previous studies have emphasized the importance of initiating corticosteroids early in patients with cardiac sarcoidosis, prior to the development of myocardial scarring and LVEF reduction.6,19 Supplementary material online, Figure S2 illustrates the cumulative incidence curves of the six groups subdivided by the combination of corticosteroids and LV function. Based on our study results, corticosteroids positively affect long-term prognosis only when baseline LVEF is preserved.
In managing cardiac sarcoidosis, early initiation of corticosteroids, before the manifestation of myocardial damage, evokes the adage ‘a stitch in time saves nine’. The importance of screening for cardiac sarcoidosis has been underscored because of its association with a poor prognosis. However, the significance of identifying cardiac sarcoidosis before the decline of LVEF is poorly understood. This study emphasizes the need to screen for cardiac sarcoidosis in patients with a preserved LVEF.
Study limitations
This study had several limitations. First, the limitations of this study are inherent in its real-world, multicentre, retrospective observational design. It is important to acknowledge that the decision to initiate corticosteroid therapy was based on clinical judgment, which may introduce a selection bias. Patients with more severe disease or a higher perceived risk of progression were more likely to receive corticosteroids, potentially affecting the comparability between the corticosteroid and non-corticosteroid groups. Although we adjusted for key clinical variables in our analysis, residual confounding may still exist, and this should be considered when interpreting the results. Approximately one-quarter of the patients had missing final follow-up echocardiographic data after the maintenance phase of immunosuppressive therapy, which could have led to selection bias, even though multiple imputations were performed. Second, the relatively short and widely divergent follow-up period may limit our ability to adequately evaluate the effects of persistent corticosteroid therapy on long-term outcomes. Third, the study did not specifically capture detailed information on the discontinuation of corticosteroid therapy, which limits our ability to fully assess the impact of treatment duration and discontinuation on patient outcomes. Fourth, due to the changes in the diagnostic criteria for cardiac sarcoidosis over time, some patients diagnosed earlier did not meet the current criteria and were excluded from the analysis, which may introduce selection bias. However, we retrospectively confirmed that all enrolled patients meet the current criteria, ensuring diagnostic consistency. Fifth, detailed data on guideline-directed HF medical therapy following enrolment were unavailable, which may have influenced the prognostic outcomes. Sixth, our cohort consisted primarily of Japanese individuals, and the representation of other races was limited. Seventh, considering the rarity of cardiac sarcoidosis, despite an adequate sample size, the limited number of events affected the statistical power of the comparative analysis of time-to-event data. Therefore, we addressed this issue using multiple models of multivariate Cox proportional hazards regression analyses. Eighth, echocardiography was conducted by trained and experienced clinicians or technician, ensuring a high level of initial assessment. However, the images were not re-evaluated for this study, which could introduce variability in the evaluation of LV function and impact data consistency. Ninth, the actual indications for the use of corticosteroids partially overlap with the primary outcome, which may have affected the results. In this study, some patients who were not receiving corticosteroid treatment at the time of diagnosis started corticosteroid after the outcome occurred. Since these patients were censored from the study after starting corticosteroid, the results may be biased. From an intuitive point of view, this bias is likely to be more prevalent in patients with preserved ejection fraction, where the indication for corticosteroid use is considered to be less clear at first. However, when we assessed the 20 patients who started corticosteroids after the outcome occurred, there was no significant difference in LV function (see Supplementary material online, Table S5) indicating that the impact of this bias is minimal in our study.
Conclusions
In this large cardiac sarcoidosis cohort study, corticosteroid therapy was associated with lower incidence of long-term outcomes only in patients with preserved LV function at diagnosis. However, due to the observational nature of this study, future randomized controlled trials are necessary to better understand the impact of corticosteroid therapy on long-term outcomes in patients with cardiac sarcoidosis.
Supplementary Material
Contributor Information
Takatsugu Segawa, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, 2-2 Yamada-oka, Osaka 565-0871, Japan.
Tatsunori Taniguchi, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, 2-2 Yamada-oka, Osaka 565-0871, Japan.
Takeru Nabeta, Department of Cardiovascular Medicine, Kitasato University School of Medicine, 1-15-1, Kitazato, Minami-ku, Sagamihara 252-0374, Japan.
Yoshihisa Naruse, Division of Cardiology, Internal Medicine III, Hamamatsu University School of Medicine, 20-1 Handayama, Chuo-ku, Hamamatsu, Japan.
Takeshi Kitai, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, 6-1 Kishibe-Shimmachi, Suita, Osaka 564-8565, Japan.
Kenji Yoshioka, Department of Cardiology, Kameda Medical Center, 929 Higashi-cho, Chiba 296-8602, Japan.
Hidekazu Tanaka, Division of Cardiovascular Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine, 7-5-2, Kusunoki-Cho, Chuo-Ku, Kobe 650-0017, Japan.
Takahiro Okumura, Department of Cardiology, Nagoya University Graduate School of Medicine, 65 Tsuruma-cho, Showa-ku, Nagoya 466-8560, Japan.
Yuichi Baba, Department of Cardiology and Geriatrics, Kochi Medical School, Kochi University, 185-1 Kohasu, Oko-cho, Nankoku 783-8505, Japan.
Yuya Matsue, Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-8421, Japan.
Yasushi Sakata, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, 2-2 Yamada-oka, Osaka 565-0871, Japan.
Lead author biography

Takatsugu Segawa is a PhD candidate at the Osaka University Graduate School of Medicine, in Osaka, Japan. He is a board-certified cardiologist and was engaged in the management of critically ill patients, including those undergoing heart transplantation and fulminant myocarditis treatment. Currently, he is conducting research on inflammatory heart diseases, focusing on myocarditis. His work specifically explores immune PET imaging and the development of novel therapies aimed at improving diagnostic accuracy and therapeutic strategies for myocarditis.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
This work was supported by Novartis Pharma Research Grants.
References
- 1. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol 2016;68:411–421. [DOI] [PubMed] [Google Scholar]
- 2. Fazelpour S, Sadek MM, Nery PB, Beanlands RS, Tzemos N, Toma M, Birnie DH. Corticosteroid and immunosuppressant therapy for cardiac sarcoidosis: a systematic review. J Am Heart Assoc 2021;10:e021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenthal DG, Parwani P, Murray TO, Petek BJ, Benn BS, De Marco T, Gerstenfeld EP, Janmohamed M, Klein L, Lee BK, Moss JD, Scheinman MM, Hsia HH, Selby V, Koth LL, Pampaloni MH, Zikherman J, Vedantham V. Long-term corticosteroid-sparing immunosuppression for cardiac sarcoidosis. J Am Heart Assoc 2019;8:e010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Circulation 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 5. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, de Chillou C, Eckardt L, Friede T, Haugaa KH, Hocini M, Lambiase PD, Marijon E, Merino JL, Peichl P, Priori SG, Reichlin T, Schulz-Menger J, Sticherling C, Tzeis S, Verstrael A, Volterrani M; ESC Scientific Document Group . 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2022;43:3997–4126.36017572 [Google Scholar]
- 6. Stievenart J, Guenno GL, Ruivard M, Rieu V, André M, Grobost V. Cardiac sarcoidosis: systematic review of literature on corticosteroid and immunosuppressive therapies. Eur Respir J 2021;59:2100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah HH, Zehra SA, Shahrukh A, Waseem R, Hussain T, Hussain MS, Batool F, Jaffer M. Cardiac sarcoidosis: a comprehensive review of risk factors, pathogenesis, diagnosis, clinical manifestations, and treatment strategies. Front Cardiovasc Med 2023;10:1156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilotra NA, Griffin JM, Pavlovic N, Houston BA, Chasler J, Goetz C, Chrispin J, Sharp M, Kasper EK, Chen ES, Blankstein R, Cooper LT, Joyce E, Sheikh FH. Sarcoidosis-related cardiomyopathy: current knowledge, challenges, and future perspectives state-of-the-art review. J Card Fail 2022;28:113–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yazaki Y, Isobe M, Hiroe M, Morimoto S-I, Hiramitsu S, Nakano T, Izumi T, Sekiguchi M. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001;88:1006–1010. [DOI] [PubMed] [Google Scholar]
- 10. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, Kokkonen J, Pelkonen M, Pietilä-Effati P, Utrianen S, Kupari M. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015;131:624–632. [DOI] [PubMed] [Google Scholar]
- 11. Kusano K, Ishibashi K, Noda T, Nakajima K, Nakasuka K, Terasaki S, Hattori Y, Nagayama T, Mori K, Takaya Y, Miyamoto K, Nagase S, Aiba T, Yasuda S, Kitakaze M, Kamakura S, Yazaki Y, Morimoto SI, Isobe M, Terasaki F. Prognosis and outcomes of clinically diagnosed cardiac sarcoidosis without positive endomyocardial biopsy findings. JACC Asia 2021;1:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nabeta T, Kitai T, Naruse Y, Taniguchi T, Yoshioka K, Tanaka H, Okumura T, Sato S, Baba Y, Kida K, Tamaki Y, Matsumoto S, Matsue Y. Risk stratification of patients with cardiac sarcoidosis: the ILLUMINATE-CS registry. Eur Heart J 2022;43:3450–3459. [DOI] [PubMed] [Google Scholar]
- 13. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1304–1323. [DOI] [PubMed] [Google Scholar]
- 14. Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, Inomata T, Ishibashi-Ueda H, Eishi Y, Kitakaze M, Kusano K, Sakata Y, Shijubo N, Tsuchida A, Tsutsui H, Nakajima T, Nakatani S, Horii T, Yazaki Y, Yamaguchi E, Yamaguchi T, Ide T, Okamura H, Kato Y, Goya M, Sakakibara M, Soejima K, Nagai T, Nakamura H, Noda T, Hasegawa T, Morita H, Ohe T, Kihara Y, Saito Y, Sugiyama Y, Morimoto SI, Yamashina A; Japanese Circulation Society Joint Working Group . JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis―digest version. Circ J 2019;83:2329–2388. [DOI] [PubMed] [Google Scholar]
- 15. Abraham WT, Psotka MA, Fiuzat M, Filippatos G, Lindenfeld J, Mehran R, Ambardekar AV, Carson PE, Jacob R, Januzzi JL Jr, Konstam MA, Krucoff MW, Lewis EF, Piccini JP, Solomon SD, Stockbridge N, Teerlink JR, Unger EF, Zeitler EP, Anker SD, O’Connor CM. Standardized definitions for evaluation of heart failure therapies: scientific expert panel from the Heart Failure Collaboratory and Academic Research Consortium. Eur J Heart Fail 2020;22:2175–2186. [DOI] [PubMed] [Google Scholar]
- 16. Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med 1984;3:35–44. [DOI] [PubMed] [Google Scholar]
- 17. Lehtonen J, Uusitalo V, Pöyhönen P, Mäyränpää MI, Kupari M. Cardiac sarcoidosis: phenotypes, diagnosis, treatment, and prognosis. Eur Heart J 2023;44:1495–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagai T, Nagano N, Sugano Y, Asaumi Y, Aiba T, Kanzaki H, Kusano K, Noguchi T, Yasuda S, Ogawa H, Anzai T. Effect of corticosteroid therapy on long-term clinical outcome and left ventricular function in patients with cardiac sarcoidosis. Circ J 2015;79:1593–1600. [DOI] [PubMed] [Google Scholar]
- 19. Padala SK, Peaslee S, Sidhu MS, Steckman DA, Judson MA. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. Int J Cardiol 2017;227:565–570. [DOI] [PubMed] [Google Scholar]
- 20. Giblin GT, Murphy L, Stewart GC, Desai AS, Di Carli MF, Blankstein R, Givertz MM, Tedrow UB, Sauer WH, Hunninghake GM, Dellaripa PF, Divakaran S, Lakdawala NK. Cardiac sarcoidosis: when and how to treat inflammation. Card Fail Rev 2021;7:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kato Y, Morimoto S, Uemura A, Hiramitsu S, Ito T, Hishida H. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis 2003;20:133–137. [PubMed] [Google Scholar]
- 22. Wand AL, Pavlovic N, Duvall C, Rosen NS, Chasler J, Griffin JM, Okada DR, Jefferson A, Chrispin J, Tandri H, Mathai SC, Sharp M, Chen ES, Kasper EK, Hays AG, Gilotra NA. Effect of corticosteroids on left ventricular function in patients with cardiac sarcoidosis. Am J Cardiol 2022;177:108–115. [DOI] [PubMed] [Google Scholar]
- 23. Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol 2005;95:143–146. [DOI] [PubMed] [Google Scholar]
- 24. Gallegos C, Oikonomou EK, Grimshaw A, Gulati M, Young BD, Miller EJ. Non-steroidal treatment of cardiac sarcoidosis: a systematic review. Int J Cardiol Heart Vasc 2021;34:100782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.