Abstract
Background
Chronic active Epstein-Barr virus (CAEBV) colitis is a rare disease with clinical and endoscopic manifestations very similar to those of inflammatory bowel disease (IBD). In clinical practice, it is easy to be misdiagnosed and mistreated, leading to poor clinical outcomes.
Case presentation
We report a case of a 56-year-old Chinese woman who presented with 6 years of intermittent severe diarrhea, fever, and abdominal pain. Ulcerative colitis was initially suspected. The patient’s clinical symptoms were partially relieved after treatment with mesalazine and probiotics. However, the symptoms were repeated and improved after supportive and symptomatic treatment each time. Colonoscopy revealed multiple mucosal erosion and edema in the colon, EBV-encoded small RNA 1/2 in situ hybridization in the pathological tissue of the colon was positive (20/HP), and EBVDNA in the peripheral blood was positive. CAEBV colitis was diagnosed. The patient was given dexamethasone and acyclovir, and she was improved after treatment. Unfortunately, she was discharged without outpatient follow-up, and similar symptoms recurred one year later, with similar colonoscopy and pathological examinations. Symptoms were relieved after dexamethasone treatment.
Conclusion
This case highlights the diagnostic challenges posed by nonspecific clinical manifestations of CAEBV colitis, which should be included as a differential diagnosis in patients with recurrent diarrhea and fever to avoid misdiagnosis.
Keywords: Chronic active Epstein-Barr virus, Chronic diarrhea, Inflammatory bowel disease, Misdiagnosis
Background
Chronic active Epstein-Barr virus (CAEBV) is a chronic disease caused by Epstein-Barr virus (EBV) infection and it is most common in East Asia. CAEBV is characterized by persistent or intermittent infectious mononucleosis-like symptoms, including fever, lymphadenopathy, and hepatosplenomegaly [1] CAEBV is a rare cause of chronic diarrhea and abdominal pain [1] Common causes of chronic diarrhea include irritable bowel syndrome, inflammatory bowel disease (IBD), celiac disease, and microscopic colitis [2]. CAEBV colitis is easily misdiagnosed as IBD because of its nonspecific clinical manifestations and ability to mimick IBD. Here, we describe a case of a female without any underlying disease, who experienced 6 years of recurrent diarrhea and fever, was initially misdiagnosed with ulcerative colitis (UC), and was eventually diagnosed with CAEBV colitis.
Case presentation
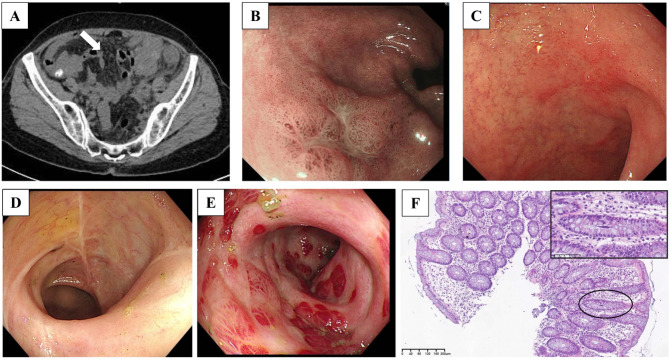
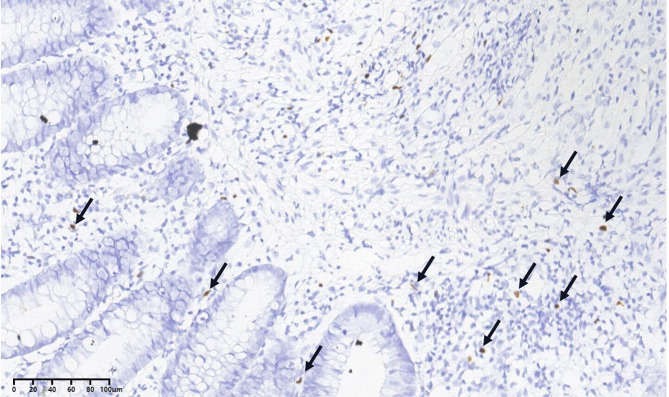
A 56-year-old Chinese female presented with a 6-year history of intermittent diarrhea, fever and abdominal pain. She denied any history of immune system disorders or family history, and she did not use glucocorticoids or immunosuppressive agents. Approximately 6 years ago, the patient started experiencing recurrent diarrhea (approximately 10 bowel movements per day), abdominal pain and episodes of moderate-grade fever without an obvious cause. She denied nausea, vomiting, tenesmus, melena or hematochezia. She was admitted to a local hospital and was suspected to have UC. She was treated with mesalazine and probiotics, which resulted in limited clinical improvement and was discontinued after 3 weeks. After that, severe diarrhea occurred approximately once or twice a year, and symptoms improved with supportive and symptomatic treatment each time. Five days prior, she was admitted to our hospital due to recurrent diarrhea, fever (39 °C) and abdominal pain. On physical examination at admission, her vital signs were normal. Abdominal examination revealed no swelling of the liver or spleen, abdominal tenderness or rebound pain. Stool examination revealed a greenish petal with mucus, large numbers of white blood cells and pus cells, and undetected clostridium difficile toxin and yeast-like fungus. Routine blood tests revealed a normal white blood cell count of 5.26 × 109/L, a lymphocyte count of 1.34 × 109/L, a hemoglobin concentration of 129 g/L and a platelet count of 194 × 109/L. Blood inflammatory marker levels were slightly elevated, with 42.36 pg/ml interleukin 6 and 15.50 mg/L C-reactive protein (CRP). Her procalcitonin level was normal at 0.03 ng/mL. Flow cytometry revealed a slightly decreased CD4 + T-cell count of 227 cells/µl, a CD8 + T-cell count of 109 cells/µl and a B-cell count of 167 cells/µl. Liver and kidney functions were normal. Serum tests for human immunodeficiency virus were negative. The immunoglobulin levels were normal, and extractable nuclear antigen antibodies and antinuclear antibodies were negative. Multiple stool cultures were negative. The EBV deoxyribonucleic acid (DNA) load in peripheral blood was 4.93 × 102 copies/mL, and a greater EBV DNA load was also detected in pharyngeal secretions (6.34 × 104 copies/mL). Her abdominal computerized tomography (CT) scan revealed thickening and swelling of the descending colon, sigmoid colon, and upper rectal wall (Fig. 1A, white arrow) and enlarged adjacent mesenteric lymph nodes. Endoscopic examination of the gastrointestinal tract revealed chronic nonatrophic gastritis with erosions (Fig. 1B and C), multiple scars in the distal ileum and right half of the colon, mucosal hyperemia and swelling with erosion in the descending colon and sigmoid colon (Fig. 1D and E). Pathology of the colonic mucosal biopsy revealed chronic inflammatory activity in the mucosa and focal microtissue hyperplasia with lymphocyte infiltration (Fig. 1F). Metagenomic next-generation sequencing analysis of fresh colon tissue revealed 1252 sequence reads matching EBV and eight sequence reads matching human betaherpesvirus 6B. EBV-encoded small RNA (EBER) 1/2 in situ hybridization (ISH) was detected in colonic tissues (20/HP) (Fig. 2, arrow). In addition, there was no evidence of clonal T-cell receptor gene amplification peaks. CAEBV colitis was diagnosed. Dexamethasone (5 mg/day) and acyclovir were given. Her daily episodes of diarrhea gradually decreased, and her temperature returned to normal. After one week, she was discharged. Follow-up in the hematology department and infectious disease department was recommended.
Fig. 1.
Abdominal computerized tomography (CT) scan indicating thickening of the colon wall (panel A, white arrow) and enlarged adjacent mesenteric lymph nodes. Endoscopy revealed chronic nonatrophic gastritis with erosions (Panels B and C), multiple colonic scars, edema and mucosal erosion (Panels D and 1E). H&E staining revealed chronic inflammation, erosion, and focal fibrous hyperplasia with lymphocyte infiltration in gastric and colon biopsies (panel F). Abbreviations: H&E, hematoxylin and eosin
Fig. 2.
EBV-encoded small RNA (EBER) 1/2 in situ hybridization in colonic tissues (20/HP) (arrows)
She was not followed up regularly after discharge. A year later, she was admitted to the local hospital again with recurrent diarrhea, abdominal pain, fever, and blood in her stool. Colonoscopy also revealed multiple scars, obvious congestion and edema of the mucosa, and scattered patchy ulcers. Biopsy of the ascending colon lesions revealed mucosal surface erosion, inflammatory necrotic exudates, reduced intestinal crypts, submucosal bleeding, and lymphocyte and plasma cell infiltration. Immunohistochemistry was not performed. Dexamethasone was given, and her condition improved, and she was discharged. Postdischarge hematology outpatient follow-up was again recommended.
Discussion and Conclusions
CAEBV enteritis is a rare cause of diarrhea in immunocompetent individuals and is often misdiagnosed as IBD [3]. The clinical, laboratory, and colonoscopy findings of CAEBV enteritis and IBD are very similar, posing significant challenges in clinical differentiation [4]. Diarrhea and abdominal pain are common symptoms associated with both CAEBV involvement of the GI tract and IBD; however, systemic symptoms such as fever, liver/spleen enlargement and lymphadenopathy are more common in patients with CAEBV enteritis [4, 5]. The erythrocyte sedimentation rate (ESR), CRP level and EBV antibody level are increased in patients with CAEBV enteritis and IBD, but the serum EBVDNA and ferritin levels are significantly increased in patients with CAEBV enteritis, and the serum EBV DNA load is more than 105 copies/mL in most patients [4, 5].
Endoscopy revealed multiple forms of inflammation and ulceration in the colon and small intestine or mucosal damage in patients with CAEBV enteritis. Ulcer morphologies are generally small, shallow, irregularly shaped, and scattered, and large and profound ulcers can also be observed [4, 5]. Colonoscopy during the hospitalization of the patient in our hospital revealed colonic congestion, swelling and erosion, accompanied by multiple scar-like changes, and no ulcers. However, multiple ulcers were found during the latest colonoscopy at a local hospital, which may be related to the severity of the disease, duration and response to treatment. A previous case of CAEBV enteritis, which had been misdiagnosed as CD, also showed scarring changes in the jejunum and ileum after IBD treatment [6].
The histological manifestations of CAEBV enteritis are mainly mucosal lymphoplasmic cell infiltration and focal crypt structure disturbance [4], but this can also occur in ulcerative colitis [7]. CAEBV enteritis may also present with Crohn’s disease-like features, such as transmural inflammation, fissure-like ulcers, and lymphoid aggregation in the intestinal wall [8], but without granuloma or chronic connective tissue changes. Patients with IBDs may also experience EBV reactivation due to immunosuppressant use, which may lead to a delayed diagnosis of CAEBV enteritis [9]. UC with EBV infection was the main differential diagnosis in this case. The histological manifestations of UC are nonspecific, such as crypt deformation, basal plasmacytosis, epitheliosis, and goblet cell depletion [10], and differ from the histological lymphocytosis of our patient. At present, the best marker for detecting EBV infection in lesions is EBER-ISH. The presence of EBER-positive lymphocytes in infected intestinal tissue is crucial for determining the diagnosis of CAEBV enteritis [1]. However, the percentage of EBER-positive cells per high-power field defining the criteria for EBV infection has still not been established [1], with thresholds ranging from 10 to 20% according to previous studies. Overall, single evidence such as EBER-positive cells or a high EBV DNA load in peripheral blood is not sufficient to diagnose CAEBV, which should be considered on the basis of patient symptoms, laboratory tests, endoscopy results, and histopathological findings.
CAEBV is a potentially life-threatening illness and some patients may develop lymphoma/overt leukemia. The efficacy of antiviral therapy is uncertain. Acyclovir may play a role in the early stage of primary infection, and ganciclovir combined with inducers of EBV protein kinase expression in latently infected cells (such as bortezomib) may lead to cell death. Although there is no significant evidence or guidelines to recommend antiviral therapy, acyclovir was not contraindicated in this patient, so we tried it. Owing to financial difficulties, she was discharged after her condition improved. Because she was not followed up after discharge, EBVDNA and colonoscopy could not be performed again. Immunosuppressive therapy (corticosteroids, cyclosporin, or azathioprine) only temporarily relieves symptoms; immunomodulatory therapy/T-cell targeted therapy strategies, including interferon-α, hydroxyurea, and lenalidomide, are not successful; and chemotherapy only reduces the severity of CAEBV disease and does not achieve complete remission. Only hematopoietic stem cell transplantation is considered curative [1, 11]. Our patient was initially suspected to have UC and was treated with mesalazine for 3 weeks. The reason for the alleviation of the diarrhea symptoms might be related to the ability of mesalazine to inhibit inflammatory responses and regulate the intestinal flora. This patient generally experiences a relatively small number of recurrent severe diarrhea episodes annually, possibly because she is not significantly immunocompromised.
In conclusion, this case highlights that CAEBV enteritis should be considered in the differential diagnosis of patients with recurrent diarrhea and fever. EBER-ISH of the lesion is helpful in diagnosing CAEBV. Early diagnosis, appropriate therapeutic interventions and effective long-term management should be implemented to improve the clinical prognosis.
Acknowledgements
We are grateful for Wenyan Zhang, West China Hospital, Sichuan University for the analysis of pathological tissue.
Abbreviations
- CAEBV
Chronic active Epstein-Barr virus
- CD
Crohn’s disease
- CRP
C-reactive protein
- CT
Computed tomography
- DNA
Deoxyribonucleic acid
- EBER
EBV-encoded small RNA
- EBV
Epstein-Barr virus
- IBD
Inflammatory bowel disease
- ISH
In situ hybridization
- UC
Ulcerative colitis
Author contributions
J.Q. designed the study. M.L. collected and interpreted the clinical data and drafted the manuscript. J.Q. modified the manuscript and finally approved the version to be published. All authors reviewed the manuscript.
Funding
This work was supported by the 1·3·5 project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University [grant number 2021HXFH032].
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The investigation was approved by the Ethics Committee of West China Hospital, Sichuan University.
Consent for publication
The authors declare that written informed consent has been obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kawada JI, Ito Y, Ohshima K, et al. Updated guidelines for chronic active Epstein–Barr virus disease. Int J Hematol. 2023;118(5):568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgers K, Lindberg B, Bevis ZJ. Chronic diarrhea in adults: evaluation and Differential diagnosis. Am Fam Physician. 2020;101(8):472–80. [PubMed] [Google Scholar]
- 3.Zhou Y, Zhang Y, Zhao H, et al. EBV-associated lymphoproliferative disorder involving the gastrointestinal tract which mimic IBD in immunocompetent patients: case reports and literature review. Int J Colorectal Dis. 2019;34(11):1989–93. [DOI] [PubMed] [Google Scholar]
- 4.Liu R, Wang M, Zhang L, et al. The clinicopathologic features of chronic active Epstein–Barr virus infective enteritis. Mod Pathol. 2019;32(3):387–95. [DOI] [PubMed] [Google Scholar]
- 5.Xu W, Jiang X, Chen J, et al. Chronic active Epstein–Barr virus infection involving gastrointestinal tract mimicking inflammatory bowel disease. BMC Gastroenterol. 2020;20(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aihara Y, Moriya K, Shimozato N, et al. Chronic active EBV infection in refractory enteritis with longitudinal ulcers with a cobblestone appearance: an autopsied case report. BMC Gastroenterol. 2021;21(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133(5):1670–89. [DOI] [PubMed] [Google Scholar]
- 8.Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based Consensus on the diagnosis and management of Crohn’s Disease 2016: part 1: diagnosis and Medical Management. J Crohns Colitis. 2017;11(1):3–25. [DOI] [PubMed] [Google Scholar]
- 9.Lopes S, Andrade P, Conde S, et al. Looking into enteric virome in patients with IBD: defining Guilty or Innocence? Inflamm Bowel Dis. 2017;23(8):1278–84. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6(1):74. [DOI] [PubMed] [Google Scholar]
- 11.Bollard CM, Cohen JI. How I treat T-cell chronic active Epstein–Barr virus disease. Blood. 2018;131(26):2899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.