Abstract
The broiler breeder industry is facing a problem with fertility, hatchability, and the number of broiler breeder eggs produced per hen. The supplementation of trace minerals such as zinc, manganese, and copper in broiler breeder diets has been previously linked to an increase in eggshell quality, which may lead to increased fertility, hatchability, and chick quality. The objective of this study was to explore the effect of amino-acid complexed mineral supplementation on eggshell quality, fertility, hatchability, chick quality, and shell translucency. Translucency score was tested to determine if it would impact other eggshell quality parameters. To evaluate this, zinc, manganese, and copper were added into the water of commercial broiler breeder houses at the levels of 40, 40, and 7 ppm, respectively, for 10 wk. This was conducted on 4 farms with one house receiving mineral supplementation (T) and another house on the same farm receiving no supplementation (C). A total of 5,120 eggs were collected after 10 wk and analyzed for translucency score (TS1-3; low-high), eggshell lightness (L* score), thickness, and breaking strength. Up to 60 infertile eggs per farm underwent microbial analysis to determine bacteria and E. coli prevalence on d 18 of incubation. All data was analyzed using PROC-GLM or chi-square with P ≤ 0.05; means were separated by Tukey's when appropriate. The results showed that mineral supplementation increased the L* score from 74.9 (C) to 75.1 (T) (P = 0.0245), shell thickness from 0.451 mm to 0.455 mm (P = 0.0402), TS1 eggs by 1.05%, TS2 eggs by 5.86%, and decreased TS3 eggs by 6.91% (P < 0.0001). Shell thickness differed (P < 0.0001) between all levels of translucency. Significance was also found for L* score (P < 0.0001) between TS3 eggs (74.2) when compared to TS1 (74.8) and TS2 (75.2) eggs. Improvements in fertility (83.0% C vs. 86.1% T) and hatchability (74.3% C vs. 76.8% T) were not statistically significant. It can be concluded that amino-acid complexed mineral supplementation improves eggshell quality, particularly shell translucency score, leading to potential improvements in commercial breeder operations.
Key words: broiler breeder, translucency score, eggshell quality, trace mineral supplementation, fertility
INTRODUCTION
The growing population is creating a strain on current resources and production systems across the world. The poultry industry must continuously evolve to withstand the demand for their products. Within recent years, the industry has been struggling with fertility rates and hatchability in broiler breeders. Fertility has been linked to both the hen and rooster genetics, making improvements an unique challenge, while hatchability is heavily influenced by the hen thus allowing for easier improvement (Wolc et al., 2009; Wolc et al., 2010). These relationships between performance traits and sex improve our approach to more effective solutions to the current breeder challenges. One of great importance is the influence that breeder hens have on eggshell quality.
Holst et al. (1932) discovered eggshell mottling, also called translucency, and determined that this trait was linked to an increased moisture content within the eggshell. White Leghorn eggs with a high translucency score have been found to have a significantly stronger egg shell and a numerically thicker eggshell compared to less translucent eggs (Wang et al., 2017). Additionally, a more translucent egg has been linked to increased levels of bacterial penetration and microcracks (Bain et al., 2006; Chousalkar et al., 2010). The observations from these studies gave varying results, with Wang et al.’s study finding that more translucent eggs had better eggshell quality, and Chousalkar et al.’s study finding that more translucent eggs were more likely to be contaminated with bacteria. Due to these differing results with different breeds, it would be beneficial to determine how translucency score can affect all parameters associated with eggshell quality in the modern broiler breeder hen.
Differences in eggshell quality have been found in eggs that differ in eggshell color, thickness, weight, and age (Roque and Soares, 1994; Ingram et al., 2008; King'ori et al., 2011; and Orellana et al., 2023). Ingram et al. (2008) found that eggshell lightness (L* score) measurements were most similar to the overall eggshell color, and it alone was beneficial to use to determine eggshell quality. When looking at the effects of eggshell color on quality, regardless of age, a darker color eggshell will lead to an increased eggshell thickness, hatchability, and egg weight and a decreased embryonic mortality rate (Orellana et al., 2023). However, it has been previously found that eggshell color is more directly related to the stress levels of the bird, and may not be a good predictor of eggshell quality on its own (Mertens et al., 2010). Thus it is important to get a better understanding of how eggshell color relates to other eggshell quality parameters before using it alone as an eggshell quality predicting parameter. Eggshell thickness has a significant effect on hatchability, embryonic mortality, and infertility. Eggs with thicker eggshells have been found to have a higher hatchability and lower mortality and infertility when compared to eggs with thinner eggshells (Roque and Soares, 1994).
Trace minerals play a crucial role in the development of the eggshell (Araujo et al., 2019). A deficiency of trace minerals in the broiler breeder diet, although not common, have been linked to decreased egg production and eggshell quality and an increase in embryonic mortality. The majority of these trace minerals will also be deposited into the yolk to aid in the development of the embryo (Araujo et al., 2019). Zinc (Zn) is an essential cofactor for many different enzymes, such as carbonic anhydrase which is vital for the deposition of calcium into the eggshell (Nys et al., 2004). Manganese (Mn) is vital in the development of mucopolysaccharides that make up the organic matrix of the eggshell (Swiatkiewicz and Koreleski, 2008). Lysyl oxidase, which is essential in converting lysine to isodesomine and desmosine (amino acids that are present in the eggshell membrane), has a component that is made of copper (Cu) (Akagawa et al., 1999). The importance of the use of these minerals within the diet of broiler breeders has already been proven; however, it would be beneficial to determine if the supplementation of these minerals in their organic form could increase the eggshell quality. Organically sourced trace minerals have a higher bioavailability in the gastrointestinal tract of the bird when compared to their inorganic forms (Bao and Choct, 2009; Araujo et al., 2019; van den Brand et al., 2023). Previously, the supplementation of trace minerals in their organic form to Cobb 500 breeders showed an increase in both eggshell and chick quality (Araujo et al., 2019). Therefore, we hypothesize that the supplementation of organic trace minerals in the hen's water source would positively impact the eggshell quality of laid eggs as well as the chick quality of the hatched eggs.
Improvements in the eggshell quality, fertility, and hatchability of broiler breeder hens would maximize the production of broiler chicks without increasing the number of breeders. The objectives of this study were to explore the effects of amino-acid complexed mineral supplementation on translucency scores, eggshell quality characteristics, and breeder reproductive performance, as well as the effect of translucenct score on eggshell quality characteristics.
MATERIALS AND METHODS
Four commercial farms in the southeastern United States containing Ross 708 broiler breeder hens and YP males were selected based on hen age. Two houses per farm were utilized for this study; one house was labeled as the test house (T) and received water supplementation of 40, 40, and 7 ppm of Zn, Mn, and Cu, respectively on top of dietary levels of trace mineral supplementation. Minerals were supplemented through the water rather than the feed due to the logistics of a commercial operation and the challenges of delivering multiple feeds to the same farm. Supplementation was administered for 10 wk before the egg collection period. In contrast, the other was labeled as the control house (C) and did not receive any mineral supplementation via the water lines.
A total of 5,120 eggs were utilized for this study. 1,280 eggs per farm were collected daily (640 from each house) for 4 consecutive days from farms with hens aged 39-, 40-, 40-, and 44-wk. Eggs were stored in a commercial hatchery egg room for 4 d before utilization for this study. The protocol used in this study was approved by the Mississippi State University IACUC board (number 22-399).
Eggshell Quality Parameters
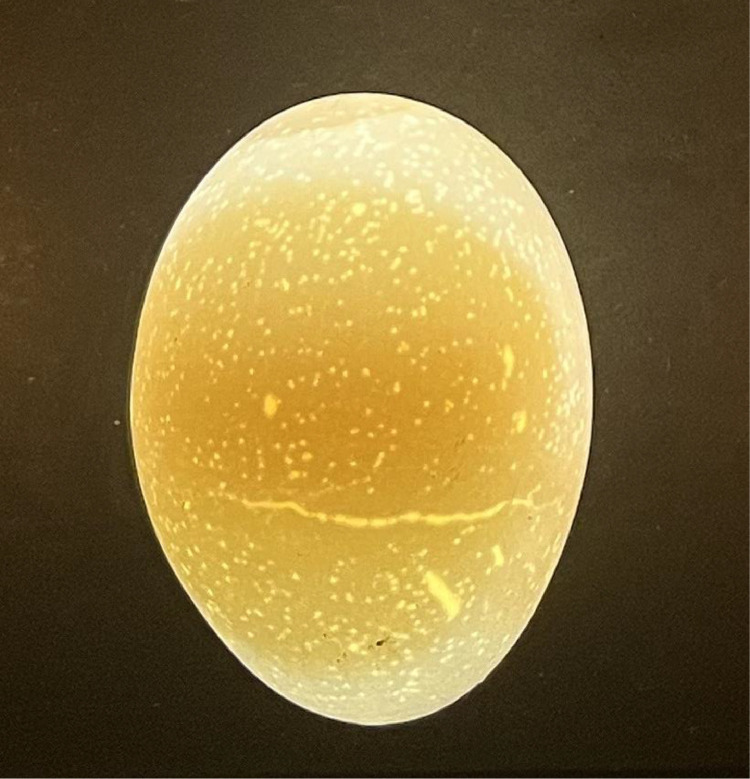
On the day of collection, eggs were individually numbered 1 to 640 for each of the houses. All 640 eggs from each treatment underwent analysis for shell lightness, translucency, microcracks (Figure 1), and shell thickness. Eggshell lightness was measured using an electronic colorimeter (Nix Color Sensor Pro 2) to determine individual egg L* scores. Translucency was measured using the Zinpro BlueBox (Zinpro Corporation) and eggs were scored on a scale of 1 to 3 (1: few to no translucency spots, 2: several and small translucency spots, and 3: many and large translucency spots; Figure 2). The BlueBox was also used to determine the amount of microcracks, if any, that each egg had. Thickness measurements were gathered using an ultrasonic thickness gauge (Orka Food Technology Precision Ultrasonic Eggshell Thickness Gauge). In addition to the above 4 measurements, the first 100 eggs (1–100) from each house underwent eggshell-breaking strength measurements. Eggshell-breaking strength was measured using an eggshell force reader (Orka Food Technology). From the eggs utilized for breaking strength, 10 composite yolk samples consisting of 3 yolks per sample, and 6 composite shell samples consisting of 5 shells per sample per house were sent to an outside laboratory (yolk samples: Midwest Laboratories; shell samples: Cumberland Valley Analytical Services) for wet chemistry mineral analysis. Once all samples were collected, eggs were then transported to Mississippi State University Poultry Research Farm to undergo incubation.
Figure 1.
Example of an egg with microcracks seen in the lower half of the eggshell.
Figure 2.
Example of eggs with each translucency score. The egg on the left has a translucency score of 2, the egg in the middle has a translucency score of 3, and the egg on the right has a translucency score of 1.
Incubation
Eggs were first weighed in sequential sets of 90 to determine the average egg weight at set. Eggs were placed in 4 identical single-stage incubators (Natureform, NMC 1080). Each incubator had eggs from one farm, and eggs were separated within the incubator only by their treatment. Eggs were allowed to incubate for 21 d at 37.5°C and 55% humidity with eggs being turned every hour.
On d 18 of incubation, eggs were weighed in the same set of 90 eggs to determine the moisture loss during incubation. Moisture loss was calculated using the following formula:
All eggs were then candled to determine fertility, status of embryonic development, or if they were contaminated or cracked. Fertility was calculated using the following formula:
Eggs containing viable embryos were then transferred into hatching baskets and placed back in the incubator.
On d 21 of incubation, hatching baskets were removed from the incubators. Chicks and unhatched eggs were separated to determine hatchability and hatch of fertile percentages. Hatchability and hatch of fertile percentages were calculated using the following formulas:
*number of chicks includes dead chicks when pulled from incubator
Chicks were weighed to determine average chick weight by treatment. Live chicks were then scored based on: healed vs. unhealed navels as well as red hocks vs normal hocks. Of the good-quality chicks, 12 chicks from each treatment were randomly selected, euthanized and necropsied to collect yolk sac swabs to determine total bacterial prevalence. Yolk sac swabs were added to 50mL test tubes containing 10 mL of buffered peptone water (BPW; Difco) and then 100 μL plated in duplicates on Plate Count Agar (PCA; Difco) and MacConkey Agar (MA; Oxoid). Plates were allowed to incubate at 37°C for 24 h and then checked for prevalence of bacteria.
Microbial Analysis
On d 18, of the infertile eggs determined during candling, up to 20 eggs per translucency score (up to 60 per farm) were collected to undergo microbial analysis. The variance in eggs chosen per farm were due to the availability of the infertile eggs of each score (Table 1). Eggs were placed in sterile 24 oz Whirl-Pak bags and rinsed with 50 mL of BPW. The egg was then removed and rinsed with 70% ethanol to kill bacteria remaining on the shell and broken open. The contents were then poured into a sterile 7 oz Whirl-Pak bag and 50 mL of BPW was added and the bag was mixed thoroughly. From this bag, 100 μL of each sample were then streaked onto plates containing PCA in duplicate. Plates and Whirl-Pak bags were then incubated at 37°C for 24 h. After this period, PCA plates were removed and checked for the prevalence of bacteria. Additionally, after incubation, the bags were removed, and 100 μL of each sample was plated onto MA. These plates were allowed to incubate at 37°C for 24 h. Afterward, plates were removed and checked for the prevalence of pink colonies, which is indicative of Escherichia coli (E. coli).
Table 1.
Breakdown of infertile eggs chosen to undergo microbial analysis on d 18.
| Farm A: 60 eggs total | |||
|---|---|---|---|
| Control | TS 1: 9 | TS 2: 10 | TS 3: 10 |
| Test | TS 1: 11 | TS 2: 10 | TS 3: 10 |
| Farm B: 48 eggs total | |||
| Control | TS 1: 2 | TS 2: 10 | TS 3: 13 |
| Test | TS 1: 5 | TS 2: 10 | TS 3: 8 |
| Farm C: 54 eggs total | |||
| Control | TS 1: 7 | TS 2: 10 | TS 3: 10 |
| Test | TS 1: 7 | TS 2: 10 | TS 3: 10 |
| Farm D: 49 eggs total | |||
| Control | TS 1: 9 | TS 2: 10 | TS 3: 10 |
| Test | TS 1: 2 | TS 2: 10 | TS 3: 8 |
Statistical Analysis
All statistical analyses were performed utilizing SAS (Version 9.4, SAS Institute Inc., Cary, NC). Significance was set at P ≤ 0.05 for all analyses. Data was analyzed concerning the treatment (with or without mineral supplementation), regardless of farm/age. By analyzing the data this way, it limits the effect of age by each treatment having an equal amount of eggs per treatment.
Percent data was arcsin transformed using the following equation, where s represents the percent data:
This data as well as other eggshell quality parameters, egg weight at set and transfer, and chick weight were then analyzed using a PROC GLM one-way ANOVA and when appropriate means were separated utilizing Tukey's HSD Post Hoc Test. Chi-square tests were used to determine significance for infertility, embryonic mortality, contaminated eggs, microbial analysis, swab analysis, and chick quality parameters, and when appropriate, a Kruskal–Wallis test was used to separate means.
The effects of translucency score and trace mineral supplementation were analyzed for variables on an egg basis (thickness, breaking strength, L* score, microcracks, infertility, and embryonic mortality). Only the effects of trace mineral supplementation were analyzed for variables that were on a farm or group basis (egg weights, chick weights, moisture loss, chick quality, etc.).
RESULTS
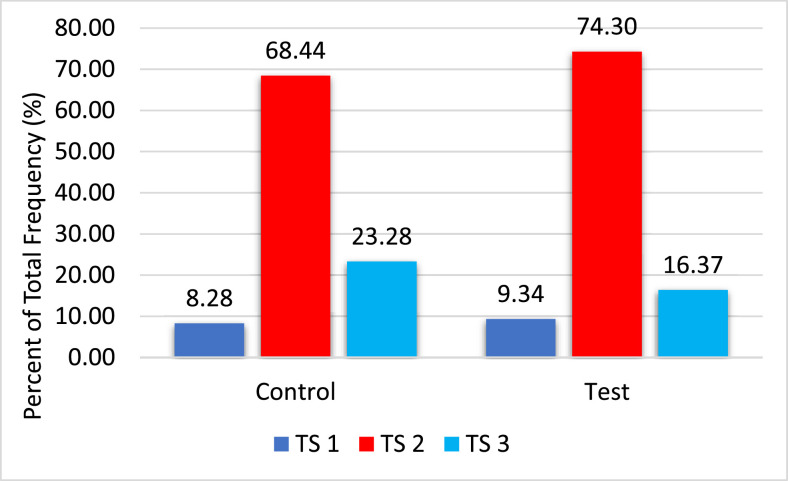
When comparing the 2 treatments for eggshell quality parameters, a significant difference was found for thickness (P = 0.049), translucency score (P < 0.001), and L* score (P = 0.025; Table 2). The test houses had an increased shell thickness and L* score (resulting in a lughter colored egg), with a lower translucency score. The distribution of translucency scores for both treatments is shown in Figure 3. No significant difference was observed for breaking strength or percent of microcracks between the 2 treatments (Table 2, Table 3). No differences were observed when comparing the yolk and shell mineral concentrations between the 2 treatments (Table 4).
Table 2.
Effects of trace mineral supplementation on eggshell quality parameters.
| Parameter | Without mineral supplementation | With mineral supplementation | SEM | P-values |
|---|---|---|---|---|
| Thickness (mm) | 0.451b | 0.454a | 0.0009 | 0.049 |
| Translucency score | 2.15a | 2.07b | 0.0103 | <0.001 |
| L* score | 74.85b | 75.12a | 0.0842 | 0.025 |
| Breaking strength (kg) | 4.376 | 4.405 | 0.0378 | 0.59 |
Differing superscripts within rows represent statistically significant differences (P < 0.05).
Figure 3.
Distribution of translucency scores (TS) for mineral supplementation.
Table 3.
Treatment and translucency score effects on percent of microcracks.
| Percent of microcracks | |
|---|---|
| Without mineral supplementation | 6.95a |
| With mineral supplementation | 5.74b |
| P-value | 0.08 |
| Translucency score 1 | 5.99 |
| Translucency score 2 | 6.10 |
| Translucency score 3 | 7.39 |
| P-value | 0.31 |
Table 4.
Effects of trace mineral supplementation on yolk and shell mineral content.
| Yolk analysis |
Shell analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mineral | Without mineral supplementation |
With mineral supplementation |
SEM | P-value | Mineral | Without mineral supplementation |
With mineral supplementation |
SEM | P-value |
| Zinc (ppm) | 43.7350 | 43.1025 | 0.5916 | 0.45 | Zinc (ppm) | 6.7083 | 6.5417 | 0.5826 | 0.84 |
| Phosphorus (%) | 0.6507 | 0.6458 | 0.0072 | 0.63 | Phosphorus (%) | 0.1246 | 0.1196 | 0.0037 | 0.35 |
| Manganese (ppm) | 1.3775 | 1.3575 | 0.0328 | 0.67 | Manganese (ppm) | 1.5833 | 1.4167 | 0.2974 | 0.69 |
| Magnesium (%) | 0.01025 | 0.01000 | 0.0002 | 0.32 | Magnesium (%) | 0.3896 | 0.3767 | 0.0064 | 0.16 |
| Calcium (%) | 0.1675 | 0.1635 | 0.0022 | 0.20 | Calcium (%) | 37.1238 | 36.3775 | 0.5062 | 0.30 |
| Copper (ppm) | 1.5450 | 1.5425 | 0.0250 | 0.94 | Copper (ppm) | 4.7083 | 4.3333 | 0.5436 | 0.63 |
| Sodium (%) | 0.05475 | 0.05475 | 0.0010 | 0.55 | Sodium (%) | 0.1517 | 0.1500 | 0.0020 | 0.55 |
| Potassium (%) | 0.1370 | 0.1328 | 0.0016 | 0.06 | Potassium (%) | 0.2967 | 0.2796 | 0.0103 | 0.25 |
| Iron (ppm) | 74.7975 | 72.6900 | 1.3447 | 0.27 | Iron (ppm) | 25.0000 | 19.4583 | 2.6095 | 0.14 |
| Sulfur1 (%) | 0.1925 | 0.1923 | 0.0017 | 0.92 | |||||
Sulfur concentrations were not measured in the shell.
Comparing the different translucency scores for eggshell quality parameters, a significant difference was observed for thickness and L* score (both P < 0.001; Table 5). Eggs with a translucency score of 1 were found to be the thinnest, whereas eggs with a translucency score of 3 were found to be the thickest. A different pattern was found for L* scores, with eggs with a translucency score of 2 having the lightest eggshells and eggs with a translucency score of 3 having the darkest eggshells. Again, no significant differences were observed between breaking strength and percent of microcracks between the translucency scores (Table 3, Table 5).
Table 5.
Effects of translucency score on eggshell quality parameters.
| Parameter | Translucency score |
SEM | P-values | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Thickness (mm) | 0.440c | 0.451b | 0.462a | 0.0013 | <0.001 |
| L* Score | 74.78b | 75.23a | 74.18c | 0.1343 | <0.001 |
| Breaking Strength (kg) | 4.239 | 4.397 | 4.429 | 0.0599 | 0.195 |
Differing superscripts within rows represent statistically significant differences (P < 0.05).
The percentage of infertile eggs significantly decreased in the test houses when compared to the control houses (P = 0.004; Table 6). However, there was no significant difference found between any stage of embryonic mortality or contaminated eggs. A significant difference was observed between the egg weight at set between the 2 treatments (P = 0.0189; Table 7). Along with this, a significant difference was observed between the egg weights at transfer (P = 0.044; Table 7). The test houses had lower weights for both egg weights at set and transfer. No significant difference was found for the moisture loss percentage, chick weight, chick quality, or number of dead chicks (Table 6, Table 7). There was no significant difference seen between the fertility, hatchability, and hatch of fertile percentages of the 2 treatments, however, there was a numerical increase in the average fertility and hatchability percentages in the test houses (Table 7). When looking at embryonic mortality and infertility compared to translucency score, no significant difference was found between any parameter (Table 8).
Table 6.
Effects of trace mineral supplementation on d 18 parameters and chick quality.
| Parameter | Without mineral supplementation | With mineral supplementation | P-values |
|---|---|---|---|
| Infertile (%) | 16.95a | 13.81b | 0.004 |
| Early dead (%) | 2.62 | 2.43 | 0.69 |
| Mid dead (%) | 0.65 | 0.61 | 0.84 |
| Late dead (%) | 4.30 | 5.13 | 0.20 |
| Contaminated (%) | 0.47 | 0.70 | 0.32 |
| Presence of red hocks (%) | 22.61 | 22.44 | 0.91 |
| Presence of unhealed navels (%) | 19.51 | 21.76 | 0.11 |
| Dead chicks (%) | 0.57 | 1.04 | 0.13 |
| Total embryonic mortality (%) | 7.57 | 8.16 | 0.47 |
Differing superscripts within rows represent statistically significant differences (P < 0.05).
Table 7.
Trace mineral supplementation effects on fertility, hatchability, hatch of fertile, egg weight at set, egg weight at transfer, moisture loss, and chick weight.
| Parameter | Without mineral supplementation | With mineral supplementation | SEM | P-values |
|---|---|---|---|---|
| Average egg weight at set (g) | 65.68a | 65.33b | 0.0989 | 0.019 |
| Average egg weight at transfer (g) | 59.36a | 58.97b | 0.0921 | 0.044 |
| Moisture loss (%) | 9.61 | 9.74 | 0.0022 | 0.44 |
| Average chick weight (g) | 44.73 | 44.32 | 0.1614 | 0.82 |
| Fertility (%) | 82.98 | 86.10 | 0.0344 | 0.38 |
| Hatchability (%) | 74.28 | 76.77 | 0.0283 | 0.48 |
| Hatch of fertile (%) | 89.50 | 89.16 | 0.0116 | 0.75 |
Differing superscripts within rows represent statistically significant differences (P < 0.05).
Table 8.
Effects of translucency score on d 18 parameter.
| Parameter | Translucency score |
P-values | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Infertile (%) | 16.58 | 14.85 | 16.79 | 0.31 |
| Early dead (%) | 2.89 | 2.60 | 2.05 | 0.60 |
| Mid dead (%) | 1.05 | 0.55 | 0.72 | 0.47 |
| Late dead (%) | 3.16 | 4.91 | 4.71 | 0.32 |
| Contaminated (%) | 0.53 | 0.46 | 1.09 | 0.10 |
| Total embryonic mortality (%) | 7.11 | 8.06 | 7.49 | 0.73 |
A significant difference was observed for total aerobic bacterial prevalence in the contents between the treatments (P < 0.001; Table 9), with no significance found on the shell. The test houses had 33.36% less aerobic bacteria present in the contents. No significant difference was observed for the prevalence of E. coli in the eggs’ contents between the 2 treatments; however, there was a significant difference between the 2 treatments of E. coli prevalence on the shell of the egg, (P = 0.040; Table 9), with the control houses having 13.56% less E. coli present on the shell. There was no significant difference in yolk sac swabs of hatched chicks between the 2 treatments for the prevalence of aerobic bacteria or E. coli.
Table 9.
Effects of trace mineral supplementation on bacterial prevalence on eggshells and in egg contents.
| Parameter | Without mineral supplementation | With mineral supplementation | P-value |
|---|---|---|---|
| Total aerobic bacterial prevalence – contents (%) | 76.36a | 43.00b | <0.001 |
| Total aerobic bacterial prevalence – Shell (%) | 68.18 | 65.00 | 0.63 |
| Escherichia coli prevalence – contents (%) | 44.55 | 50.51 | 0.39 |
| Escherichia coli prevalence – Shell (%) | 28.44b | 42.00a | 0.040 |
| Yolk Sac Swab – total aerobic bacterial prevalence (%) | 66.67 | 62.50 | 0.67 |
| Yolk Sac Swab – Escherichia coli prevalence (%) | 45.83 | 58.33 | 0.22 |
Differing superscripts within rows represent statistically significant differences (P < 0.05).
A significant difference was observed between the translucency score and total aerobic bacterial prevalence on the shell, with translucency score 3 eggs having the highest percentage and scores 1 and 2 having statistically similar percentage present. (Table 10). No significance was observed between translucency score and aerobic bacterial prevalence in the contents of the shell or E. coli prevalence on the shell or in the contents.
Table 10.
Effects of eggshell translucency score on bacterial prevalence on eggshells and in egg contents.
| Parameter | Translucency score |
P-values | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Total aerobic bacterial prevalence – contents (%) | 55.77 | 62.50 | 61.54 | 0.72 |
| Total aerobic bacterial prevalence – shell (%) | 65.38ab | 57.50a | 76.92b | 0.034 |
| Escherichia coli prevalence – contents (%) | 47.06 | 45.00 | 50.00 | 0.81 |
| Escherichia coli prevalence – shell (%) | 25.49 | 31.25 | 44.87 | 0.05 |
*Signifies statistical differences within rows (P < 0.05).
DISCUSSION
An increase in shell thickness was observed for the test houses when compared to the control. This data agrees with the findings of Araujo et al. (2019) observing that the inclusion of organic trace minerals significantly increased shell thickness. However, van den Brand et al. (2023) found that the utilization of organic-sourced trace minerals in the diet decreased the eggshell thickness compared to inorganic trace minerals. The differing results from these authors could be caused by the differing genetic lines used in these studies; Araujo et al. (2019) utilized birds from a Cobb 500 line whereas van den Brand et al (2023) utilized birds from a Ross 308 line. Although we observed an increase in eggshell thickness, we found that there was no significant difference between the breaking strength of the 2 treatments. This disagrees with Araujo et al. (2019) who found that regardless of hen age, eggshell breaking strength was increased in the organically sourced mineral-fed hens when compared to the inorganically sourced. We hypothesize that the difference between the literature and our findings could be caused by the differing genetic lines used as well as our birds being fed the organic minerals as a supplementation. In contrast, the other study fed the organic minerals in place of inorganic minerals.
The L* scores in the test house improved significantly compared to the control houses. This finding cannot be directly linked to the supplementation of Zn, Mn, and Cu. Previous research is not clear whether increased hen age causes an increase or a decrease in the lightness of the eggshell (Odabasi et al., 2007; Ingram et al., 2008; Bi et al., 2018). However, it has been found that eggshell color is directly related to the stress levels of the bird (Mertens et al., 2010). The stress levels or the differing ages of the birds used in this study could be the result of this increase in L* scores.
A significant difference in translucency score was observed in the test house, which could be a result of the supplemented trace minerals serving as cofactors to enzymes that aid in the assembly and organization of eggshell membranes, yielding an improvedshell ultrastructure (Chousalkar et al., 2010; Richards et al., 2010; Araujo et al., 2019). However, van den Brand et al. (2023) observed no difference in translucency score between organic and inorganic trace mineral source. The differences observed could be linked to the location that our study and the van den Brand study were completed in. Our study was completed in the United States using a standard commercial diet, versus the other study being completed in the Netherlands utilizing their standard commercial diet. In the current experiment, we hypothesize that the supplementation of organically sourced trace minerals led to an improvement in shell membrane development and construction of the shell ultrastructure causing the improved translucency score.
We found no significant differences in yolk and shell mineral content. Avila et al. (2023) observed a statistical increase in the concentration of Mn found in whole eggs and yolks of 40, 50, and 60-wk-old broiler breeders fed organic minerals or a blend of organic and inorganic minerals. We hypothesize the differences between this study and our results could be due to the hens in the Avila et al. (2023) study being fed these differing mineral sources for over 30 wk compared to ours being supplemented in the water for 10 wk.
Interestingly, eggs with a translucency score of 1 were found to be thinner and darker than eggs with a translucency score of 3. Previous researchers found similar results when looking at translucency as well as looking at translucenct vs opaque eggs (Wang et al., 2017; Orellana et al., 2023). Differences within the ultrastructural composition, mainly the shell membrane, of the eggshell in translucent and non-translucent eggs could be the reason these differences are seen (Choulsakar et al. 2010; Kroetz Neto et al., 2024). Translucent eggs were found to have good-quality mammillary caps that are extensively attached to the shell membrane of the eggshell; however, these eggs are found to be less dense due to differences in shell matrix fibers causing a weaving pattern to appear, allowing for a higher light transmittance through the eggshell (Chousalkar et al., 2010; Olkowski et al., 2015). Eggs with a translucency score of 1 overall have a more consistent arrangement of their mammillae and calcite minerals, which could explain their thinner eggshells. Although a significant difference was seen in the lightness of the shell for each translucency score, this could be explained by the differing ages of the hens utilized for this study. Eggshell color has been shown to be directly linked to the age of the hen at lay; with eggshell color getting darker as the bird ages (Ingram et al., 2008). In this study, an increase in breaking strength was seen, although not significant, between translucency score 1 and translucency score 3. This finding contradicts a previous finding that translucent eggs have a lower breaking strength than normal eggs (Garlich et al., 1975). The difference between our findings and the earlier finding could be the changes made in genetics over the past fifty years, however, to our knowledge no other study has looked at the effects of translucency score on breaking strength.
No significance was observed for any embryonic mortality parameter. A previous finding by Noetzold et al. (2022) also found no difference in embryonic mortality parameters when comparing different inclusions of inorganic and organically sourced trace minerals. However, Favero et al., (2013) observed a significant increase in embryonic mortality when replacing part of the inclusion of inorganic trace minerals with organically sourced trace minerals.
At set, eggs supplemented with trace minerals weighed significantly less than eggs without supplementation. Interestingly, this difference in weight was 0.35 g and may be contributed to the limited egg weight values collected during this study (30 egg weight values from each treatment group). A similar finding was found for egg weight at transfer, where the test house eggs had a significantly lower transfer weight than eggs from the control houses. These findings contradict findings found by van den Brand et al. (2023) who found that eggs from hens 55 to 57 wk of age fed an organic source of trace minerals had no difference in egg set or transfer weight than eggs from hens fed an inorganic source of trace minerals. However, in hens aged 34 to 36 wk of age in the same study, it was found that eggs from the organic source trace mineral-fed hens weighed significantly less at both set and transfer. This means that there could be an age effect that skews the significance that was found for these 2 parameters in the current study; this significance could also be caused by the supplementation of additional trace minerals rather than the use of organically-sourced over inorganically-sourced minerals. Despite the significance observed for egg weights at both set and transfer, no significance was observed for moisture loss percentage between the 2 treatments. This finding agrees with van den Brand et al.’s (2023) who found that there was no significance in moisture loss between inorganic or organic mineral sources between either hen age group.
There was no significance seen for the fertility, hatchability, or hatch of fertile percentages. However, there was a numerical difference in fertility (82.98% C vs. 86.10% T) and hatchability (74.32% C vs. 76.77% T). This finding disagrees with a previous finding where fertility and hatchability were decreased when comparing a normal commercial diet with a diet containing organically sourced trace minerals (Wang et al., 2019). However, it does agree with a finding by Avila et al. (2023), who found that the utilization of organic trace minerals and a mix of organic and inorganic trace minerals lead to a numerical increase in fertility, hatchability, and hatch of fertile. The differences between our results and those found by Wang et al. (2019) may be due to that study being completed in laying hens, whereas our study was completed in broiler breeders. Whereas the similarity between the Avila et al. (2023) study and our study can be linked to the use of broiler breeder hens during both studies.
Chick weight was found to be similar between the 2 treatments; with the chicks from the test houses weighing only slightly less, which may be attributed to the lighter egg weights observed in this experiment. However, other researchers have found that the use of organically sourced trace minerals led to differing results. Araujo et al. (2019) found that chicks from a hen fed a diet containing organic trace minerals were significantly lighter than chicks from a hen fed a diet containing inorganic trace minerals; while de Arruda Roque et al. (2022) found that chicks from a hen fed organic trace minerals were significantly heavier. In terms of chick quality, in the current experiment, no difference was observed in the percentage of red hocks or unhealed navels between the 2 treatments. Noetzold et al. (2022) observed an improvement in chick length and leg scores from hens fed a diet supplemented with organically sourced trace minerals only. Contradicting findings between the current research and other researchers evaluating hens fed organic trace mineral sources and their effect on progeny highlight the need for further research. Factors including, but not limited to, hen age, duration and application method (feed or water) of supplementation, breed, and dietary composition should be evaluated to determine effects on chick quality.
A significant difference was found between the treatments for total aerobic bacterial prevalence in the contents of the eggs as well as the E. coli prevalence on the eggshell. To our knowledge, there have been no studies reported looking at trace mineral supplementation or source on microbial prevalence of the eggshell or egg contents. Based on our results, it can be hypothesized that the differing ultrastructural arrangement of the eggshells between the 2 treatments could be the reason for the decreased aerobic bacterial prevalence within the shell contents. However, the significant increase in E. coli prevalence on the eggshell in the test treatment yields the need for futher research that enumerates the bacterial load to gain a better understanding of how trace mineral supplementation affects microbial loads.
Translucency score only had a significant effect on the aerobic bacteria prevalence on the eggshell; where TS2 had the lowest percentage of prevalence and TS3 had the highest percentage of prevalence. Chousalkar et al. (2010) found that eggs that were penetrated with bacteria (study used S. infantis and E. coli strains) tend to have a higher translucency score than eggs that are not penetrated. We did not find a similar significant difference in penetration rate, as there was no significant difference between translucency score and aerobic bacterial or E. coli penetration rate. The difference between this study and Chousalkar et al.’s study is that this study only utilized the bacteria already found on/in eggshells during incubation, rather than being inoculated with known concentrations of bacteria.
In conclusion, the supplementation of organically sourced trace minerals to commercial breeder hens can improve the eggshell quality, fertility, and hatchability. Along with this, translucency score can be regarded as an effective predicting parameter for different eggshell quality parameters such as eggshell thickness and L* scores. Utilizing both of these factors in broiler breeders could positively improve the performance of commercial broiler breeder hens.
Contradicting findings between the current research and other researchers evaluating hens fed organic trace mineral sources and their effect on progeny highlight the need for further research. Further research is also needed to determine how different translucency scores affect bird productivity; as well as the effect of different storage lengths and temperatures on translucency scores. The effect of translucency score and trace mineral supplementation on different broiler genetic lines should be determined as well. Finally, the effects of amino-acid complexed trace mineral supplementation when given in the feed rather than the water should be explored to more accurately represent the commercial supplementation of trace minerals in the poultry industry. The improvements observed in the current study warrant continued research to further support and confirm the effects of supplementation of organically sourced compared to inorganically sourced trace minerals added in broiler breeder diets.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank Zinpro Corporation for the financial and technical support during this study. We would also like to thank the owners of the breeder farms as well as the commercial company and hatchery personnel for allowing us to conduct this research and for their contributions.
REFERENCES
- Akagawa M., Wako Y., Suyama K. Lysyl oxidase coupled with catalase in egg shell membrane. Biochem. Biophys. Acta. 1999;1434:151–160. doi: 10.1016/s0167-4838(99)00169-7. [DOI] [PubMed] [Google Scholar]
- Araújo C.S.S., Hermes R.G.H., Bittencourt L.C., Silva C.C., Araújo L.F., Granghelli C.A., Pelissari P.H., Roque F.A., Leite B.G.S. Different dietary trace mineral sources for broiler breeders and their progenies. Poult. Sci. 2019;98:4716–4721. doi: 10.3382/ps/pez182. [DOI] [PubMed] [Google Scholar]
- de Arruda Roque F., Chen J., Araujo R.B., Murcio A.L., de Souza Leite B.G., Dias Tanaka M.T., Granghelli C.A., Pelissari P.H., Bueno Carvalho R.S., Torres D., Vázquez-Añón M., Hancock D., Soares da Silva Araujo C., Araujo L.F. Maternal supplementation of different trace mineral sources on broiler breeder production and progeny growth and gut health. Front. Phys. 2022;13 doi: 10.3389/fphys.2022.948378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila L.P., Sweeney K.M., Roux M., Buresh R.E., White D.L., Kim W.K., Wilson J.L. Evaluation of industry strategies to supply dietary chelated trace minerals (Zn, Mn, and Cu) and their impact on broiler breeder hen reproductive performance, egg quality, and early offspring performance. J. Appl. Poult. Res. 2023;32 [Google Scholar]
- Bain M.M., MacLeod N., Thomson R., Hancock J.W. Microcracks in eggs. Poult. Sci. 2006;85:2001–2008. doi: 10.1093/ps/85.11.2001. [DOI] [PubMed] [Google Scholar]
- Bao Y.M., Choct M. Trace mineral nutrition for broiler chickens and prospects of application of organically complexed trace minerals: a review. Anim. Prod. Sci. 2009;49:269–282. [Google Scholar]
- Bi H., Liu Z., Sun C., Li G., Wu G., Shi F., Liu A., Yang N. Brown eggshell fading with layer ageing: dynamic change in the content of protoporphyrin IX. Poult. Sci. 2018;97:1948–1953. doi: 10.3382/ps/pey044. [DOI] [PubMed] [Google Scholar]
- Chousalkar K.K., Flynn P., Sutherland M., Roberts J.R., Cheetham B.F. Recovery of Salmonella and Escherichia coli from commercial egg shells and effect of translucency on bacterial penetration in eggs. Int. J. Food Micro. 2010;142:207–213. doi: 10.1016/j.ijfoodmicro.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Favero A., Viera S.L., Angel C.R., Bess F., Cemin H.S., Ward T.L. Reproductive performance of Cobb 500 breeder hens fed diet supplemented with zinc, manganese, and copper from inorganic and amino acid-complexed sources. J. Appl. Poult. Res. 2013;22:80–91. doi: 10.3382/ps.2012-02670. [DOI] [PubMed] [Google Scholar]
- Garlich J.D., Parkhurst C.R., Ball H.R. The Comparison of Rough, Normal, and Translucent egg shells with respect to shell strength and calcification. Poult. Sci. 1975;54:1574–1580. [Google Scholar]
- Holst W.F., Almquist H.J., Lorenz F.W. A study of shell texture of the hen's egg. Poult. Sci. 1932;11:144–149. [Google Scholar]
- Ingram D.R., Hatten L.F., III, Homan K.D. A study on the relationship between eggshell color and eggshell quality in commercial broiler breeders. Int. J. Poult. Sci. 2008;7:700–703. [Google Scholar]
- King'ori A.M. Review of the factors that influence egg fertility and hatchability in poultry. Int. J. Poult. Sci. 2011;10:483–492. [Google Scholar]
- Kroetz Neto F., Barbosa B.B., Novaes G.A., Blank M.H., Fireman A.K.A.T., Junior A.B., Pereira R.J.G. Eggshell translucency: its relationship with specific gravity, and eggshell color and its influence on broiler egg weight loss, hatchability, and embryonic mortalities. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.103528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens K., Vaesen I., Loffel J., Kemps B., Kamers B., Perianu C., Zoons J., Darius P., Decuypere E., De Baerdemaeker J., De Ketelaere B. The transmission color value: a novel egg quality measure for recording shell color used for monitoring the stress and health status of a brown layer flock. Poult. Sci. 2010;89:609–617. doi: 10.3382/ps.2009-00261. [DOI] [PubMed] [Google Scholar]
- Noetzold T.L., Vieira S.L., Xavier B.B., Olabarriaga Y.J., Fireman A.K. Supplemental effects of amino acid-complexed trace minerals on broiler breeder hen performance. Anim. Feed Sci. and Tech. 2022;290 [Google Scholar]
- Nys Y., Gautron J., Garcia-Ruiz J.M., Hincke M.T. Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. C.R. Palevol. 2004;3:549–562. [Google Scholar]
- Odabasi A.Z., Miles R.D., Balaban M.O., Portier K.M. Changes in brown eggshell color as the hen ages. Poult. Sci. 2007;86:356–363. doi: 10.1093/ps/86.2.356. [DOI] [PubMed] [Google Scholar]
- Olkowski A.A., Nain S., Laarveld B., Wojnarowicz C. Changes in eggshell structure and predeposition of broiler to health problems: is there a common pathophysiology? Br. Poult. Sci. 2015;56:267–274. doi: 10.1080/00071668.2015.1008995. [DOI] [PubMed] [Google Scholar]
- Orellana L., Neves D., Krehling J., Burin R., Soster P., Almeida L., Urrutia A., Munoz L., Escobar C., Bailey M., Chaves-Cordoba B., Williams C., Rebollo M., Macklin K. Effect of translucency and eggshell color on broiler breeder egg hatchability and hatch chick weight. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.D., Zhao J., Harrel R.J., Atwell C.A., Dibner J.J. Trace mineral nutrition in poultry and swine. Asian-Australas. J. Anim. Sci. 2010;23:1527–1534. [Google Scholar]
- Roque L., Soares M.C. Effects of eggshell quality and broiler breeder age on hatchability. Poult. Sci. 1994;73:1838–1845. doi: 10.3382/ps.0731838. [DOI] [PubMed] [Google Scholar]
- Swiatkiewicz S., Koreleski J. The effect of zinc and manganese source in the diet for laying hens on eggshell and bones quality. Vet. Medic. 2008;10:555–563. [Google Scholar]
- van den Brand H., Hubers T., van den Anker I., Torres C.A., Frehen E., Ooms M., Arts J., Laurennssen B.F.A., Heetkamp M.J.W., Kemp B., Molenaar R. Effects of trace minerals source in the broiler breeder diet and eggshell translucency on embryonic development of the offspring. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Li Y., Liu L., Liu J., Bao M., Yang N., Zhuo-Cheng H., Ning Z. Traits of eggshells and shell membranes of translucent eggs. Poult. Sci. 2017;96:351–358. doi: 10.3382/ps/pew328. [DOI] [PubMed] [Google Scholar]
- Wang G., Liu L.J., Tao W.J., Xiao Z.P., Pei X., Liu B.J., Wang M.Q., Lin G., Ao T.Y. Effects of replacing inorganic trace minerals with organic trace minerals on the production performance, blood profiles, and antioxidant status of broiler breeders. Poult. Sci. 2019;98:2888–2895. doi: 10.3382/ps/pez035. [DOI] [PubMed] [Google Scholar]
- Wolc A., White I.M., Olori V.E., Hill W.G. Inheritance of fertility in broiler chickens. Genet. Sel. Evol. 2009;41:1–9. doi: 10.1186/1297-9686-41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolc A., White I.M.S., Hill W.G., Olori V.E. Inheritance of hatchability in broiler chickens and its relationship to egg quality traits. Poult. Sci. 2010;89:2334–2340. doi: 10.3382/ps.2009-00614. [DOI] [PubMed] [Google Scholar]