Abstract
Purpose
To investigate the rare obstetric emergency with no specific treatments called acute fatty liver of pregnancy. The primary objective was to evaluate association of adverse perinatal outcomes with blood components transfusion. While the secondary objective focused on further establishing the predictive risk factors for adverse perinatal outcomes.
Participants and Methods
This retrospective cohort study included patients, who diagnosed with acute fatty liver of pregnancy without hepatic/malignant diseases in Qilu Hospital of Shandong University over 12-year period (collected 2007–2019, aged 20–41years). Chi-square test was used to explore the relevance between blood transfusion therapy and adverse perinatal outcomes. Meanwhile, logistic regression analysis was performed to identify predictive risk factors.
Results
Of 146 patients, 26 (26/146, 17.8%) received prenatal blood transfusions. These patients had reduced gestational ages and exhibited more severe clinical symptoms. The association between blood transfusion and adverse maternal outcomes yielded a P value of 0.044, while the association with fetal outcomes was highly significant (P<0.001). Multivariate logistic regression analysis identified seven high-risk factors for maternal outcomes and six for fetal outcomes, all demonstrating strong discriminatory capacity.
Conclusion
Blood component transfusion may serve as a marker of disease severity. Prompt identification of patients with high-risk factors is crucial to improve maternal and fetal outcomes.
Keywords: maternal-fetal medicine, blood transfusion therapy, adverse maternal and fetal outcomes, predictive risk factors
Plain Language Summary
This retrospective study evaluated 146 patients with acute fatty liver of pregnancy and 172 fetuses to determine the effectiveness of blood transfusion therapy and identify predictive risk factors for adverse outcomes. Dr. Li’s and Zhang’s team found that patients who received blood transfusions exhibited more severe symptoms. Seven high-risk factors for maternal outcomes and six for fetal outcomes were identified. Blood transfusion therapy may act as an early indicator of disease severity, requiring immediate intervention for both mothers and fetuses with high-risk factors.
Introduction
Acute fatty liver of pregnancy (AFLP) is a rare yet potentially life-threatening condition unique to pregnancy. Its estimated incidence ranges between 1 in 900 and 1 in 20,000 deliveries.1–3 Despite advances in obstetric care reducing mortality rates,4 AFLP continues to cause significant adverse perinatal outcomes for both mothers and fetuses.
AFLP is characterized by the accumulation of microvascular fat droplets in hepatocytes, leading to liver injury or failure.5 The liver plays a critical role in synthesizing coagulation factors,6 and patients with liver disease frequently suffer from coagulation disorders, increasing their risk of severe bleeding and related complications.3 Traditionally, transfusions of fresh frozen plasma and other blood components have been used to address these coagulation issues.7 However, emerging evidence suggests that blood component transfusion carries potential risks, including adverse reactions.8,9 We are facing the same condition, the precise relationship between blood transfusion and perinatal outcomes remains unclear, particularly in pregnancy-related liver diseases like AFLP.
Given the rarity of pregnancy-related liver diseases, the complexity of pregnancy, and the frequent need for cesarean delivery in AFLP cases, blood transfusion is often considered a necessary intervention.10 This therapy is widely used to manage coagulopathy associated with AFLP, yet its impact on maternal and fetal outcomes remains uncertain. Therefore, this retrospective cohort study was designed to investigate the association between blood component transfusion and adverse perinatal outcomes, aiming to improve the scientific understanding and clinical management of AFLP.
Materials and Methods
Diagnosis
All patients were diagnosed using the Swansea criteria11 and the AFLP-triad,12 which includes clinical manifestations, abnormal laboratory findings, common complications and accessory examinations (Table 1). Diagnosis required at least three of these criteria.
Table 1.
Diagnostic Criteria
| (a) Swansea criteria | |
| Clinical manifestations | Vomiting |
| Abdominal pain | |
| Polydipsia/polyuria | |
| Encephalopathy | |
| Abnormal laboratory findings | Elevated bilirubin |
| Hypoglycaemia | |
| Elevated urate | |
| Elevated transaminases | |
| Elevated ammonia | |
| Common complications | Leucocytosis |
| Renal impairment | |
| Coagulopathy | |
| Accessory examinations | Microvesicular steatosis on liver biopsy |
| Ascites or bright liver on ultrasound scan | |
| (b) AFLP-triad | |
| Symptoms | Nausea/vomiting |
| Jaundice | |
| Epigastric pain | |
| Laboratory findings | Renal dysfunction |
| Coagulopathy | |
| Liver function abnormalities | |
| Low glycemia | |
| Common complications | Renal failure |
| Coagulopathy | |
| Ascites | |
| Encephalopathy | |
Patient Information
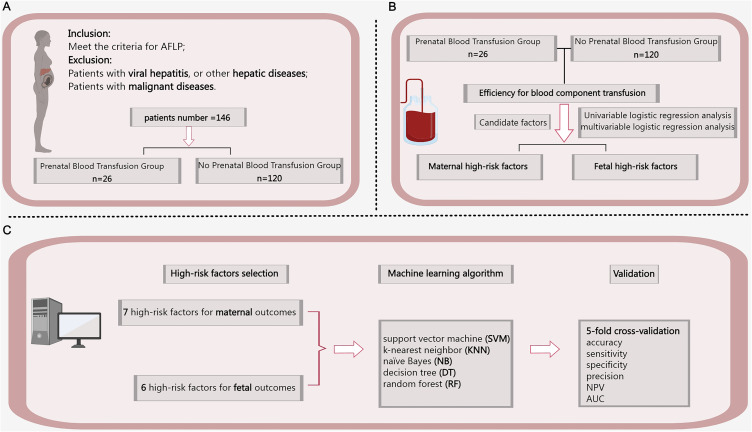
The research procedure is summarized in Figure 1. Medical records were used to collect patient information on gestational age (GA), clinical manifestations, etc (Table 2), with detailed abnormal laboratory results provided in Table S1.
Figure 1.
Flow chart of the study. (A) Dataset; (B) Analysis of blood component effectiveness and high-risk factors; (C) High-risk factors validation based on machine learning. Bold font represents significant data.
Abbreviations: AFLP, acute fatty liver of pregnancy; SVM, Support vector machine; KNN, K-nearest neighbor; NB, Naïve Bayes; DT, Decision tree; RF, Random forest; NPV, negative predictive value; and AUC, area under the receiver operating characteristic curve.
Table 2.
Baseline Characteristics of Patients
| Total (n=146) | PBCT (n=26) | No PBCT (n=120) | P * | |
|---|---|---|---|---|
| Age at delivery (years) | 27 (20–41) | 26 (21–39) | 27 (20–41) | 0.805 |
| Gestational age (weeks) | 36.6 (26.4–41) | 35.6 (26.4–38.7) | 36.7 (28–41) | 0.028 |
| Birth weight (g) | 2500 (1000–5600) | 2500 (1000–3600) | 2500 (1200–5600) | 0.934 |
| Parity | 0.976 | |||
| 0 | 67 (45.9) | 13 (50.0) | 54 (45.0) | |
| ≥1 | 79 (54.1) | 13 (50.0) | 66 (55.0) | |
| Mode of delivery | 0.238 | |||
| Vaginal delivery | 18 (12.3) | 5 (19.2) | 13 (10.8) | |
| Cesarean | 128 (87.7) | 21 (80.8) | 107 (89.2) | |
| Number of babies | 0.579 | |||
| 1 | 124 (84.9) | 23 (88.5) | 101 (84.2) | |
| ≥2 | 22 (15.1) | 3 (11.5) | 19 (15.8) | |
| Clinical manifestation | ||||
| Nausea | 61 (41.8) | 11 (42.3) | 50 (41.7) | 0.952 |
| Vomiting | 59 (40.4) | 11 (42.3) | 48 (40.0) | 0.828 |
| Jaundice | 46 (31.5) | 13 (50.0) | 33 (27.5) | 0.025 |
| Vaginal bleeding/discharge | 45 (30.8) | 6 (23.1) | 39 (32.5) | 0.345 |
| Abdominal pain | 41 (28.1) | 6 (23.1) | 35 (29.2) | 0.531 |
| Dizzy | 18 (12.3) | 7 (26.9) | 11 (9.2) | 0.013 |
| Acid reflux | 11 (7.5) | 0 (0.0) | 11 (9.2) | 0.108 |
| Complication | ||||
| Gestational hypertension | 22 (15.1) | 1 (3.8) | 21 (17.5) | 0.078 |
| Gestational diabetes mellitus | 10 (6.8) | 0 (0.0) | 10 (8.3) | 0.127 |
| Hospital stay duration | 9 (1–54) | 8 (1–35) | 9 (1–54) | 0.096 |
Notes: Bold font represents significant data. Values are median (range) or n (%). * The values are calculated by the chi-square test or nonparametric rank-sum test.
Abbreviation: PBCT, prenatal blood component transfusion.
Inclusion and Exclusion Criteria
Patients who delivered at Qilu Hospital between January 2007 and December 2019 (12-year period, aged 20–41 years) were included if diagnosed with AFLP based on standard criteria, along with their fetuses. Patients with viral hepatitis, other hepatic diseases, or malignancies were excluded. The study also controlled for factors like pre-eclampsia, diabetes, and infections that can affect maternal/fetal outcomes. This research ultimately included 146 patients and 172 fetuses.
Groups
Patients were divided into two groups based on whether they received prenatal blood component transfusion (PBCT). Other treatments were provided based on individual conditions.
Outcome Measures
Maternal outcomes included disseminated intravascular coagulation (DIC), postpartum hemorrhage (PPH), and maternal death. Fetal outcomes included fetal growth restriction, Apgar score≤7, low birth weight (<2500g), intrauterine fetal distress, intrauterine fetal death, and neonatal death. Any occurrence of adverse events was considered as the outcome. DIC diagnosis was based on clinical manifestations, evaluated using guidelines from the Italian Society for Haemostasis and Thrombosis (SISET).13
Statistical Analyses
To compare baseline characteristics between groups, chi-square tests were used for categorical variables, and nonparametric rank-sum tests for continuous variables. P<0.05 were considered statistically significant. Chi-square tests were also used to assess associations between blood transfusions and adverse perinatal outcomes, followed by logistic regression analysis to further explore the relationship.
High-risk factors associated with adverse perinatal outcomes were identified using logistic regression analysis. Receiver operating characteristic (ROC) curves were employed to calculate the Youden Index (YI, YI=sensitivity+specificity-1),14 and univariate logistic regression analysis was conducted to screen clinical features. Variables with P-values<0.15 were included in the multivariate logistic regression analysis, with the area under the ROC curve (AUC) used to evaluate factor discrimination. AUC values approaching 0.80 indicated good discrimination.15 The results are represented as odds ratio (OR), 95%confidence interval (CI) and P value.
Machine learning (ML) techniques, including support vector machine, k-nearest neighbor, Naïve Bayes, decision tree, and random forest, were applied to verify the predictive accuracy of high-risk factors. Five-fold cross validation is a technique for evaluating model performance and adjusting parameters by dividing the dataset into 5 equal parts, taking turns training the model with 4 parts as the training set and evaluating model performance with the remaining parts as the testing set. It was used to assess model performance, with results evaluated based on sensitivity, specificity, accuracy, and standard deviation of AUCs.
IBM SPSS Statistics (version 25.0), GraphPad Prism (version 8.0.1), and Python (version 3.7.9) were utilized for statistical analysis.
Results
Patient Information
This study included 146 patients and 172 fetuses. Table 2 presents the baseline characteristics. The median maternal age at delivery was 27 years (range 20–41 years), and the median GA was 36.6 weeks (range 26.4–41 weeks). Most patients (124/146, 84.9%) had singleton pregnancies, while 15.1% (22/146, 15.1%) had multiple pregnancies, including twins, triplets, and quadruplet. Of the fetuses, 102 cases (59.3%, 102/172) were male; the other 70 cases (40.7%, 70/172) were female. Birth weights ranged from 1000g to 5600g. Among the patients, 45.9% (67/146) were primigravida, and 54.1% (79/146) were multigravida. Meanwhile, 128 patients (87.7%, 128/146) delivered via cesarean section, 18 patients (12.3%, 18/146) delivered via transvaginal method.
Table 2 also described seven most common clinical manifestations and their occurrence rates, 87% of patients experienced at least one of them. Common clinical manifestations included nausea (41.8%, 61/146), vomiting (40.4%, 59/146), jaundice (31.5%, 46/146), and vaginal bleeding/discharge (30.8%, 45/146).
Laboratory abnormalities and detailed adverse perinatal outcomes are provided in Tables S1 and S2, respectively. Maternal adverse events were observed in 28.1% (41/146) of patients, including DIC (17.8%, 26/146), PPH (17.8%, 26/146), and maternal death (5.5%, 8/146). Fetal adverse outcomes occurred in 52.9% (91/172) of fetuses, with low birth weight (39.5%, 68/172) and intrauterine fetal distress (23.3%, 40/172) being the most common; followed by low Apgar score (14.0%, 24/172), fetal growth restriction (1.7%, 3/172), intrauterine fetal death (4.7%, 8/172) and neonatal death (5.2%, 9/172).
Comparison of Baseline and Laboratory Characteristics
Patients were divided into the PBCT group (17.8%, 26/146) and the no PBCT group (82.2%, 120/146) (Table 2). The PBCT group had significantly shorter GA (median 35.6 weeks vs 36.7 weeks, P=0.028) and more severe symptoms, including jaundice (P=0.025) and dizziness (P=0.013). Laboratory results in Table S1 revealed prolonged prothrombin time (PT) (P=0.041), activated partial thromboplastin time (APTT) (P=0.034), and lower fibrinogen levels (P=0.014) in the PBCT group. No significant differences were observed for other laboratory parameters.
The results indicated that factors like pre-eclampsia, diabetes, and infections did not significantly affect maternal or fetal outcomes in this study.
The Association Between Blood Component Therapy and Adverse Perinatal Outcomes
The relationship between blood component transfusion and adverse perinatal outcomes is illustrated in Tables 3, 4, and Figure 2. Among the 26 patients (17.8%, 26/146) who received prenatal transfusions, 42.3% (11/26) experienced adverse maternal outcomes, compared to 31.9% (29/91) during the delivery period. Although initial chi-square tests did not reveal significant associations (P=0.075 before and P=0.190 during delivery, P=0.238 overall), logistic regression identified a significant correlation between blood component transfusion and adverse maternal outcomes (P=0.044).
Table 3.
Blood Transfusion Association with Adverse Maternal Outcomes
| No. of Patients | No. of Events | No. of No Events | P* | |
|---|---|---|---|---|
| The association between blood component transfusion before delivery and maternal outcomes | ||||
| Total | 146 | 41 | 105 | |
| Blood component transfusion | 26 (17.8) | 11 (26.8) | 15 (14.3) | 0.075 |
| Only plasma transfusion | 9 (6.2) | 4 (9.8) | 5 (4.8) | 0.268 |
| Only cryoprecipitate transfusion | 2 (1.4) | 1 (2.4) | 1 (1.0) | 0.484 |
| Plasma and cryoprecipitate transfusion | 15 (10.3) | 6 (14.6) | 9 (8.6) | 0.362 |
| No blood component transfusion | 120 (82.2) | 30 (73.2) | 90(85.7) | 0.075 |
| The association between blood component transfusion at delivery and maternal outcomes | ||||
| Total | 146 | 41 | 105 | |
| Blood component transfusion | 91 (62.3) | 29 (70.7) | 62 (59.0) | 0.190 |
| Only plasma transfusion | 19 (13.0) | 7 (17.1) | 12 (11.4) | 0.362 |
| Only cryoprecipitate transfusion | 12 (8.2) | 3 (7.3) | 9 (8.6) | 1.000 |
| Plasma and cryoprecipitate transfusion | 60 (41.1) | 19 (46.3) | 41 (39.0) | 0.421 |
| No blood component transfusion | 55 (37.7) | 12 (29.3) | 43 (41.0) | 0.190 |
| The association between blood component transfusion and maternal outcomes | ||||
| Blood component transfusion | 96 (65.8) | 30 (73.2) | 66 (62.9) | 0.238 |
Notes: Bold font represents significant data. *The values are calculated by the chi-square test.
Table 4.
Blood Transfusion Association with Adverse Fetal Outcomes
| No. of Patients | No. of Events | No. of No Events | P* | |
|---|---|---|---|---|
| The association between blood component transfusion before delivery and fetal outcomes | ||||
| Total | 172 | 91 | 81 | |
| Blood component transfusion | 29 (16.9) | 17 (18.7) | 12 (14.8) | 0.499 |
| Only plasma transfusion | 10 (5.8) | 6 (6.6) | 4 (4.9) | 0.751 |
| Only cryoprecipitate transfusion | 2 (1.2) | 1 (1.1) | 1 (1.2) | 1.000 |
| Plasma and cryoprecipitate transfusion | 17 (9.9) | 10 (11.0) | 7 (8.6) | 0.607 |
| No blood component transfusion | 143 (83.1) | 74 (81.3) | 69 (85.2) | 0.499 |
| The association between blood component transfusion at delivery and fetal outcomes | ||||
| Total | 172 | 91 | 81 | |
| Blood component transfusion | 109 (63.4) | 71 (78.0) | 38 (46.9) | <0.001 |
| Only plasma transfusion | 20 (11.6) | 14 (15.4) | 6 (7.4) | 0.103 |
| Only cryoprecipitate transfusion | 13 (7.6) | 9 (9.9) | 4 (4.9) | 0.220 |
| Plasma and cryoprecipitate transfusion | 76 (44.2) | 48 (52.7) | 28 (34.6) | 0.017 |
| No blood component transfusion | 63 (36.6) | 20 (22.0) | 43 (53.1) | <0.001 |
| The association between blood component transfusion and fetal outcomes | ||||
| Blood component transfusion | 115 (66.9) | 72 (79.1) | 43 (53.1) | <0.001 |
Notes: Bold font represents significant data. *The values are calculated by the chi-square test. The study includes 124 singletons, 19 twins, 2 triplets and 1 quadruplet, totally 172 fetuses.
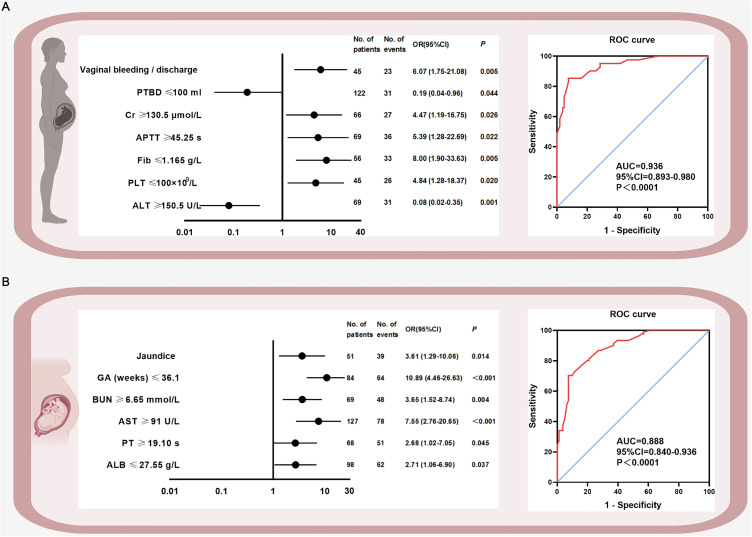
Figure 2.
High-risk factors of maternal and fetal adverse perinatal outcomes as well as ROC curves. Figure 2 is evaluated by multivariate logistic regression analysis. (A) shows the maternal high-risk factors and ROC curve, (B) shows the fetal high-risk factors and ROC curve. Bold font represents significant data.
Abbreviations: PTBD, plasma transfusion before delivery; Cr, creatinine; APTT, activated partial thromboplastin time; Fib, fibrinogen; PLT, platelet; ALT, alanine aminotransferase; GA, gestational age; BUN, blood urea nitrogen; AST, aspartate aminotransferase; PT, prothrombin time; ALB, albumin; ROC, receiver operating characteristic; OR, odds ratio; AUC, area under the receiver operating characteristic curve; CI, confidence interval.
For fetal outcomes, 58.6% (17/29) of infants whose mothers received prenatal transfusions experienced adverse outcomes, though this association was not statistically significant (P=0.499). However, 65.1% (71/109) of fetuses experienced adverse outcomes during delivery, with a significant association found between blood component transfusion and adverse fetal outcomes (P<0.001), particularly with plasma and cryoprecipitate transfusion (P=0.017).
High-Risk Factors Selection and Validation
Tables S3, S4, and Figure 2 present the logistic regression analysis results. Univariate analysis identified 19 maternal and 19 fetal predictors for adverse outcomes, which were further analyzed using multivariate logistic regression (Tables S3 and S4). Seven high-risk factors for maternal outcomes included vaginal bleeding/discharge, plasma transfusion before delivery, creatinine, APTT, fibrinogen, platelet count and alanine aminotransferase (ALT) (Figure 2A left). Six high-risk factors were selected for fetal outcomes: jaundice, GA, blood urea nitrogen (BUN), aspartate aminotransferase (AST), PT, and albumin (Figure 2B left). The AUCs for maternal and fetal outcomes were 0.936 (95% CI: 0.89–0.98, P<0.001) and 0.888 (95% CI: 0.84–0.94, P<0.001), respectively (Figure 2A and B right).
High-Risk Factors Training and Validation via ML
In Table S5, ML techniques validated the predictive accuracy of high-risk factors, with 5-fold cross-validation results showing maternal factor accuracy between 0.84 and 0.88 (AUC=0.88–0.92) and fetal factor accuracy between 0.75 and 0.78 (AUC=0.81–0.86). Maternal factor precision ranged from 0.86 to 0.93, with a negative predictive value of 0.73 to 0.83. Fetal factor precision ranged from 0.74 to 0.75, with a negative predictive value of 0.76 to 0.80. The diagnostic performance of the ML algorithms, shown in Figure S1, confirmed these findings.
Discussion
Main Findings
The key findings from this retrospective study are as follows: (1) patients in the PBCT group exhibited more severe symptoms; (2) blood component transfusion was identified as a marker of disease severity in both maternal (P=0.044) and fetal (P<0.001) outcomes; and (3) seven materal high-risk factors and six fetal high-risk factors were identified, with ML algorithms validating the predictive capabilities of these factors.
Interpretation
The Impact of Blood Component Transfusion in AFLP
The Impact of Blood Component Transfusion in Pregnancy-Related Liver Disease
As previously noted, AFLP remains one of the most life-threatening conditions for both mother and fetus.1 Despite advances in supportive obstetric care, the all-cause mortality associated with AFLP has improved but remains concerning.4 Early identification and targeted management are critical in enhancing survival rates.12
Patients with severe liver disease, including AFLP, are prone to coagulation disorders,3 often resulting in a heightened bleeding tendency.6 Blood transfusion therapy is frequently utilized to correct these coagulopathies, especially before invasive procedures.16–18 Despite the widespread use of blood component transfusions, particularly in non-pregnancy liver diseases, studies have questioned their efficacy due to associated risks, such as adverse reactions.19–21
The Role of Blood Component Transfusion in AFLP Patients
Our study demonstrated that blood transfusion guided by functional testing is commonly used in pregnancy-related liver diseases. Prophylactic transfusions were administered in most cases before cesarean delivery, showing positive effects on patient outcomes.5,22–24 Consistent with these researches, the mortality rate among AFLP patients in this study decreased compared to historical reports, and other adverse perinatal outcomes also showed a downward trend. We proved that blood component do have a significant effect on improve patients’ condition for the first time.
The Role of Blood Component Transfusion in Adverse Perinatal Outcomes
Despite these improvements, it is important to note that blood component transfusion is a marker of disease severity. Patients who received transfusions had worse clinical outcomes, as confirmed by logistic regression analysis. This underscores the dual role of transfusion: it helps manage coagulation disorders but also serves as an indicator of the underlying disease severity.
Optimizing Blood Transfusion Use in AFLP
To maximize the benefits of blood transfusion, clinicians must carefully balance the need for intervention with the following potential risks. Massive blood transfusions, particularly cryoprecipitate, may increase the risk of thrombotic events, especially in patients with liver disease who are already at higher risk for thrombosis.25 Pregnancy itself is a hypercoagulable state, with 80% of AFLP patients showing signs of DIC at delivery.26 As a result, it is essential to carefully manage blood component transfusion to reduce the risk of thrombosis while treating coagulation deficiencies. In addition, transfusions carry risks such as infection, immunosuppression, fluid overload, and portal hypertension.18,27–30 The limited availability of blood resources and the high cost of transfusion also present challenges in clinical settings,9,31,32 further emphasizing the need for scientific and judicious use of blood components in AFLP.
Prenatal High-Risk Factors for Adverse Perinatal Outcomes in AFLP
The clinical significance of AFLP can be seen through historical mortality rates, which reached as high as 80% from 1940 to 1970.22,33 With advances in supportive obstetric care, mortality rates have significantly declined. For instance, a study reported a maternal mortality rate of 7.4% between 1994 and 2005,34 while our research revealed a perinatal mortality rate of approximately 5.5%.
Though several risk factors for AFLP have been previously established (Table 5),1–4,22,35–44 high-risk factors specifically linked to adverse perinatal outcomes remain underexplored. Our systematic investigation identified several high-risk factors for both maternal and fetal outcomes. For maternal outcomes, key indicators include APTT, which reflects hepatic capacity for synthesizing coagulation factors,39 and fibrinogen levels, which are impacted by hepatocellular damage.45 Their connection highlights the significance of monitoring coagulation function. Hepatic aminotransferase levels generally increase from mild to moderate in AFLP patients.2,46,47 Elevated hepatic aminotransferase levels, were also confirmed as predictors of adverse perinatal outcomes. Even moderately elevated aminotransferase levels warrant close fetal monitoring, though clinicians should remain vigilant with mild or normal levels as well. Low platelet counts, likely due to reduced thrombopoietin production,48 and increased creatinine, an early marker of renal compromise,4,40 were additionally linked to adverse maternal outcomes. We noted that some of clinical manifestations were also associated with adverse maternal outcomes.
Table 5.
High-Risk Factors in Previously Published Literature
| Research Outcome | Research Object | Patients Number | High-Risk Factors | Reference |
|---|---|---|---|---|
| Incidence of AFLP | AFLP patients | 57 patients | Multiple pregnancy | [3] |
| Incidence of AFLP | AFLP patients | Review | Multiple pregnancy, long-chain 3-hydroxyacyl coenzyme A dehydrogenase defect | [36] |
| Incidence of AFLP | AFLP patients | 3 patients | Multiple pregnancy | [37] |
| Incidence of AFLP | AFLP patients | Review | Defects in fatty acid metabolism | [1] |
| Incidence of AFLP | AFLP patients | Review | Multifetal gestation, nulliparity, male fetus, and fetal fatty acid oxidation disorders | [22] |
| Maternal death | AFLP patients; | 32 patients | Multifetal gestation and nulliparity for maternal death | [2] |
| Fetal death | Fetuses | 41 fetuses | Male fetus, and delivery mode for fetal death | [2] |
| Postpartum recovery time | AFLP patients | 44 patients | Platelets, total protein and total bilirubin level | [4] |
| Duration of recovery | AFLP patients | 43 patients | Total serum bilirubin, prothrombin time, plasma fibrinogen and platelet counts shortly before delivery | [38] |
| Incidence of AFLP | AFLP patients | 11 patients | Multiple pregnancies, male fetus, and preeclampsia | [39] |
| Maternal complications | AFLP patients; | 56 patients | Prothrombin time and international normalized ratio | [40] |
| Maternal and fetal complications | Fetuses | 61 fetuses | Gestational age at delivery, direct bilirubin and fibrin degradation products | [40] |
| Incidence of AFLP | AFLP patients | Review | First pregnancy, multifetal pregnancy, male fetus, low body mass index, and fatty acid oxidation disorder | [41] |
| Incidence of AFLP | AFLP patients | 133 patients | Fatty acid oxidation in mitochondria, hepatic oxidative stress and elevated serum arachidonic acid | [42] |
| Maternal mortality | AFLP patients | 133 patients | History of abortion, total bilirubin, serum creatinine | [42] |
| Predicting mortality of AFLP | AFLP patients | 78 patients | International normalized ratio, total bilirubin, serum creatinine and platelet | [43] |
Notes: Bold font represents significant data.
For fetal outcomes, our study identified BUN levels ≥6.65mmol/L and the presence of jaundice as significant risk factors. GA and PT, both established predictors of fetal morbidity,41 were also highlighted. GA was positively associated with fetal mortality, reinforcing the need for prompt delivery when AFLP is diagnosed or strongly suspected. Continued gestation can exacerbate disease progression and worsen fetal outcomes. Hypoproteinemia, a common complication of AFLP,49 was found to correlate with poor fetal outcomes in this study.
Study Strengths and Limitations
This study has several notable strengths. First, it is the first to analyze the association between blood component transfusion and adverse perinatal outcomes in AFLP patients. Second, it is the largest single-center clinical study on this topic, providing a comprehensive and reliable selection of indicators. Lastly, ML was employed to validate the predictive abilities of high-risk factors.
However, limitations exist. Notably, no patients underwent liver biopsy, the gold standard for diagnosing AFLP, relying instead on non-invasive methods with their own limitations. Additionally, the diagnosis of DIC was based primarily on clinical manifestations and evaluated retrospectively, which may have influenced diagnostic accuracy.
Conclusion
This study highlights that blood component transfusion plays a significant role in improving adverse perinatal outcomes and serves as a marker of overall disease severity in AFLP patients. Key prenatal high-risk factors, including coagulation abnormalities, hepatorenal function, and clinical manifestations, are critical for assessing maternal and fetal risks. To optimize outcomes, it is essential to apply blood transfusion therapies scientifically and identify high-risk patients promptly, ensuring timely and appropriate interventions to reduce complications and improve prognosis for both mother and fetus.
Acknowledgments
We would like to acknowledge the patients and their fetuses who participated in the study for their contribution of data. Meanwhile, we want to express gratitude to the doctors and nurses as well as the study participators in our study for help treating the patients carefully and preparing all the data meticulously.
Abbreviations
AFLP, acute fatty liver of pregnancy; PBCT, prenatal blood component transfusion; GA, gestational age; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; PT, prothrombin time; APTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation; PPH, postpartum hemorrhage; ROC, receiver operating characteristic; YI, Youden index; AUC, area under the receiver operating characteristic curve; OR, odds ratio; CI, confidence interval; ML, machine learning.
Data Sharing Statement
In order to regulate the personal data more sensitive and cautious, the datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethical Committee of Qilu Hospital of Shandong University (protocol number 2019020; 2019; Approval date, March 26, 2019). The need for written informed consent was waived because of the retrospective nature of the study. Before the analysis, the privacy of each patient was hidden.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Liu J, Ghaziani TT, Wolf JL. Acute fatty liver disease of pregnancy: updates in pathogenesis, diagnosis, and management. Am J Gastroenterol. 2017;112(6):838–846. doi: 10.1038/ajg.2017.54 [DOI] [PubMed] [Google Scholar]
- 2.Cheng N, Xiang T, Wu X, Li M, Xie Y, Zhang L. Acute fatty liver of pregnancy: a retrospective study of 32 cases in South China. J Matern Fetal Neonatal Med. 2014;27(16):1693–1697. doi: 10.3109/14767058.2013.871704 [DOI] [PubMed] [Google Scholar]
- 3.Knight M, Nelson-Piercy C, Kurinczuk JJ, Spark P, Brocklehurst P, System UKOS. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951–956. doi: 10.1136/gut.2008.148676 [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Huang K, Ji B, et al. Acute fatty liver of pregnancy in a Chinese Tertiary Care Center: a retrospective study. Arch Gynecol Obstet. 2019;300(4):897–901. doi: 10.1007/s00404-019-05259-w [DOI] [PubMed] [Google Scholar]
- 5.Lisman T, Bernal W. Hemostatic issues in pregnancy-induced liver disease. Thromb Res. 2017;151(Suppl 1):S78–S81. doi: 10.1016/S0049-3848(17)30073-7 [DOI] [PubMed] [Google Scholar]
- 6.Bianchini M, De Pietri L, Villa E. Coagulopathy in liver diseases: complication or therapy? Dig Dis. 2014;32(5):609–614. doi: 10.1159/000360514 [DOI] [PubMed] [Google Scholar]
- 7.Forkin KT, Colquhoun DA, Nemergut EC, Huffmyer JL. The coagulation profile of end-stage liver disease and considerations for intraoperative management. Anesth Analg. 2018;126(1):46–61. doi: 10.1213/ANE.0000000000002394 [DOI] [PubMed] [Google Scholar]
- 8.Moncharmont P, Barday G, Meyer F. les correspondants d’Hemovigilance et de securite transfusionnelle Rhone A. Adverse transfusion reactions in patients with liver disease. Transfus Med. 2018;28(4):331–332. doi: 10.1111/tme.12514 [DOI] [PubMed] [Google Scholar]
- 9.Lisman T, Porte RJ. Value of preoperative hemostasis testing in patients with liver disease for perioperative hemostatic management. Anesthesiology. 2017;126(2):338–344. doi: 10.1097/ALN.0000000000001467 [DOI] [PubMed] [Google Scholar]
- 10.Roberts LN, Bernal W. Management of bleeding and thrombosis in critically ill patients with liver disease. Semin Thromb Hemost. 2015;41(5):520–526. doi: 10.1055/s-0035-1550431 [DOI] [PubMed] [Google Scholar]
- 11.Ch’ng CL, Morgan M, Hainsworth I, Kingham JG. Prospective study of liver dysfunction in pregnancy in Southwest Wales. Gut. 2002;51(6):876–880. doi: 10.1136/gut.51.6.876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vigil-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143–1146. doi: 10.3109/14767058.2010.531325 [DOI] [PubMed] [Google Scholar]
- 13.Di Nisio M, Baudo F, Cosmi B, et al. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian society for haemostasis and thrombosis (SISET). Thromb Res. 2012;129(5):e177–84. doi: 10.1016/j.thromres.2011.08.028 [DOI] [PubMed] [Google Scholar]
- 14.Bantis LE, Nakas CT, Reiser B. Construction of confidence intervals for the maximum of the Youden index and the corresponding cutoff point of a continuous biomarker. Biom J. 2019;61(1):138–156. doi: 10.1002/bimj.201700107 [DOI] [PubMed] [Google Scholar]
- 15.Janssens A, Martens FK. Reflection on modern methods: revisiting the area under the ROC Curve. Int J Epidemiol. 2020;49(4):1397–1403. doi: 10.1093/ije/dyz274 [DOI] [PubMed] [Google Scholar]
- 16.Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol. 1987;67(3):365–368. doi: 10.1111/j.1365-2141.1987.tb02359.x [DOI] [PubMed] [Google Scholar]
- 17.Harrison MF. The misunderstood coagulopathy of liver disease: a review for the acute setting. West J Emerg Med. 2018;19(5):863–871. doi: 10.5811/westjem.2018.7.37893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinmouth A. Evidence for a rationale use of frozen plasma for the treatment and prevention of bleeding. Transfus Apher Sci. 2012;46(3):293–298. doi: 10.1016/j.transci.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 19.Benson AB, Burton JR Jr, Austin GL, et al. Differential effects of plasma and red blood cell transfusions on acute lung injury and infection risk following liver transplantation. Liver Transpl. 2011;17(2):149–158. doi: 10.1002/lt.22212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weeder PD, Porte RJ, Lisman T. Hemostasis in liver disease: implications of new concepts for perioperative management. Transfus Med Rev. 2014;28(3):107–113. doi: 10.1016/j.tmrv.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 21.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406–3417. doi: 10.1182/blood-2008-10-167643 [DOI] [PubMed] [Google Scholar]
- 22.Nelson DB, Byrne JJ, Cunningham FG. Acute fatty liver of pregnancy. Obstet Gynecol. 2021;137(3):535–546. doi: 10.1097/AOG.0000000000004289 [DOI] [PubMed] [Google Scholar]
- 23.Li L, Huang D, Xu J, et al. The assessment in patients with acute fatty liver of pregnancy (AFLP) treated with plasma exchange: a cohort study of 298 patients. BMC Pregnancy Childbirth. 2023;23(1):171. doi: 10.1186/s12884-023-05503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham FG, Nelson DB. Disseminated intravascular coagulation syndromes in obstetrics. Obstet Gynecol. 2015;126(5):999–1011. doi: 10.1097/AOG.0000000000001110 [DOI] [PubMed] [Google Scholar]
- 25.Tripodi A, Anstee QM, Sogaard KK, Primignani M, Valla DC. Hypercoagulability in cirrhosis: causes and consequences. J Thromb Haemost. 2011;9(9):1713–1723. doi: 10.1111/j.1538-7836.2011.04429.x [DOI] [PubMed] [Google Scholar]
- 26.Nelson DB, Yost NP, Cunningham FG. Hemostatic dysfunction with acute fatty liver of pregnancy. Obstet Gynecol. 2014;124(1):40–46. doi: 10.1097/AOG.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 27.Sorensen B, Bevan D. A critical evaluation of cryoprecipitate for replacement of fibrinogen. Br J Haematol. 2010;149(6):834–843. doi: 10.1111/j.1365-2141.2010.08208.x [DOI] [PubMed] [Google Scholar]
- 28.Franchini M, Lippi G. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfus. 2012;10(1):23–27. doi: 10.2450/2011.0015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crochemore T, de Toledo Piza FM, Silva E, Correa TD. Thromboelastometry-guided hemostatic therapy: an efficacious approach to manage bleeding risk in acute fatty liver of pregnancy: a case report. J Med Case Rep. 2015;9:202. doi: 10.1186/s13256-015-0690-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannucci PM, Tripodi A. Liver disease, coagulopathies and transfusion therapy. Blood Transfus. 2013;11(1):32–36. doi: 10.2450/2012.0151-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bevan DH. Cryoprecipitate: no longer the best therapeutic choice in congenital fibrinogen disorders? Thromb Res. 2009;124(Suppl 2):S12–6. doi: 10.1016/S0049-3848(09)70159-8 [DOI] [PubMed] [Google Scholar]
- 32.Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. JAMA. 1994;271(10):777–781. [PubMed] [Google Scholar]
- 33.Sherlock S. Acute fatty liver of pregnancy and the microvesicular fat diseases. Gut. 1983;24(4):265–269. doi: 10.1136/gut.24.4.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibai BM. Imitators of severe preeclampsia. Obstet Gynecol. 2007;109(4):956–966. doi: 10.1097/01.AOG.0000258281.22296.de [DOI] [PubMed] [Google Scholar]
- 35.Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067–1076. doi: 10.1002/hep.22130 [DOI] [PubMed] [Google Scholar]
- 36.Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897–906. doi: 10.3748/wjg.15.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182–193. doi: 10.1016/j.clinre.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 38.Davidson KM, Simpson LL, Knox TA, D’Alton ME. Acute fatty liver of pregnancy in triplet gestation. Obstet Gynecol. 1998;91(5 Pt 2):806–808. doi: 10.1016/s0029-7844(97)00477-8 [DOI] [PubMed] [Google Scholar]
- 39.Meng J, Wang S, Gu Y, Lv H, Jiang J, Wang X. Prenatal predictors in postpartum recovery for acute fatty liver of pregnancy: experiences at a tertiary referral center. Arch Gynecol Obstet. 2016;293(6):1185–1191. doi: 10.1007/s00404-015-3941-5 [DOI] [PubMed] [Google Scholar]
- 40.Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751–756. doi: 10.1111/j.1447-0756.2010.01242.x [DOI] [PubMed] [Google Scholar]
- 41.Zhang YP, Kong WQ, Zhou SP, Gong YH, Zhou R. Acute fatty liver of pregnancy: a retrospective analysis of 56 cases. Chin Med J. 2016;129(10):1208–1214. doi: 10.4103/0366-6999.181963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terrault NA, Williamson C. Pregnancy-associated liver diseases. Gastroenterology. 2022;163(1):97–117e1. doi: 10.1053/j.gastro.2022.01.060 [DOI] [PubMed] [Google Scholar]
- 43.Gao Q, Ma Y, Qu X, Zheng X. Risk factors in patients with acute fatty liver of pregnancy: the role of abortion, total bilirubin and serum creatinine. Arch Gynecol Obstet. 2024;310(1):153–159. doi: 10.1007/s00404-023-07234-y [DOI] [PubMed] [Google Scholar]
- 44.Peng Q, Zhu T, Huang J, Liu Y, Huang J, Zhang W. Factors and a model to predict three-month mortality in patients with acute fatty liver of pregnancy from two medical centers. BMC Pregnancy Childbirth. 2024;24(1):27. doi: 10.1186/s12884-023-06233-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lisman T, Bakhtiari K, Adelmeijer J, Meijers JC, Porte RJ, Stravitz RT. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemost. 2012;10(7):1312–1319. doi: 10.1111/j.1538-7836.2012.04770.x [DOI] [PubMed] [Google Scholar]
- 46.Mjahed K, Charra B, Hamoudi D, Noun M, Barrou L. Acute fatty liver of pregnancy. Arch Gynecol Obstet. 2006;274(6):349–353. doi: 10.1007/s00404-006-0203-6 [DOI] [PubMed] [Google Scholar]
- 47.Nelson DB, Yost NP, Cunningham FG. Acute fatty liver of pregnancy: clinical outcomes and expected duration of recovery. Am J Obstet Gynecol. 2013;209(5):456e1–7. doi: 10.1016/j.ajog.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 48.Allison MG, Shanholtz CB, Sachdeva A. Hematological issues in liver disease. Crit Care Clin. 2016;32(3):385–396. doi: 10.1016/j.ccc.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 49.Gao Q, Qu X, Chen X, et al. Outcomes and risk factors of patients with acute fatty liver of pregnancy: a multicentre retrospective study. Singapore Med J. 2018;59(8):425–430. doi: 10.11622/smedj.2018001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In order to regulate the personal data more sensitive and cautious, the datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.