Summary
Background
Limited studies have suggested an effect of dietary choline intake on uric acid levels. We aim to investigate the associations between choline intake and hyperuricemia (HUA), as well as the mediating role of kidney function in this relationship, among the Chinese population aged 6–17 years.
Methods
Participants were divided into quartiles according to residual energy-adjusted dietary choline intake in our cross-sectional study. Dietary choline intake was assessed using the 24-h dietary recalls method over three consecutive days, including two weekdays and one weekend day. The primary outcome was the HUA prevalence. Based on recommendation in Clinical Paediatric Nephrology (3rd ed), HUA is defined based on fasting serum uric acid levels, with cutoffs varying by age and sex. The associations between choline intake and HUA were analysed using weighted logistic regression models, restricted cubic spline models, and linear regression models. The mediated proportions of estimated glomerular filtration rate (eGFR) in the associations were estimated with mediation effect models. The data for this study were collected from the China National Nutrition and Health Surveillance of Children and Lactating Mothers (2016–2017) conducted between October 2016 and December 2018. Eligible participants were identified through a database search conducted from October to December 2023.
Findings
Among the 10749 participants, 3398 (31.6%) individuals were found to have HUA. A negative dose-dependent relationship was found between dietary choline intake and HUA. Compared to participants in the lowest intake quartile of total choline, phosphatidylcholine, and betaine, those in the 4th quartile had lower odds of HUA, with odds ratio (OR) of 0.75 (95% confidence interval [95% CI], 0.63–0.90), 0.75 (95% CI, 0.64–0.89), and 0.75 (95% CI, 0.59–0.94), respectively. The eGFR mediated 10.60%–14.58% of the associations. Participants in the 4th quartile of lipid-soluble dietary choline exhibited 24.00% reduced odds of HUA compared to those in the lowest intake quartile, with an OR of 0.76 (95% CI, 0.64–0.90).
Interpretation
Moderate to high intake of dietary choline (181.20–357.92 mg/d), particularly phosphatidylcholine (120.22–207.58 mg/d), and betaine (189.24–282.37 mg/d), may reduce the odds of HUA by improving glomerular filtration function. Further interventional studies are needed to establish causal relationships.
Funding
This work was supported by the National Natural Science Foundation of China (82003443, 42375180), the Natural Science Foundation of Guangdong Province of China (2024A1515012088), and the Construction of High-level University of Guangdong (G624330422).
Keywords: Choline, Betaine, HUA, eGFR, Children and adolescents
Research in context.
Evidence before this study
We searched PubMed for articles published in English using the keywords ‘choline’, ‘phosphatidylcholine’, ‘sphingomyelin’, ‘free choline’, ‘glycerophosphorylcholine’, ‘phosphorylcholine’, ‘betaine’, ‘dietary’, ‘kidney’, ‘eGFR’, ‘uric acid’, and ‘hyperuricemia’ from inception to 7 January 2024. To our knowledge, recent animal studies have found that dietary supplementation with choline reduces uric acid levels, but population evidence remains limited. The association of dietary choline intake with hyperuricemia (HUA) and the influence of kidney function in this process, especially in the paediatric population, is still unclear.
Added value of this study
We conducted an external, population-based validation of the association between dietary choline and HUA, elucidating that this association may be related to kidney function. The findings of our research, which is conducted among Chinese children and adolescents aged 6–17 years, indicated that a moderate to high intake of dietary choline (181.20–357.92 mg/d), particularly phosphatidylcholine (120.22–207.58 mg/d), and betaine (189.24–282.37 mg/d), may reduce the odds of HUA. The eGFR functioned as a mediator in the associations, with mediation proportions ranging from 10.60% to 14.58%. These findings address gaps in the existing literature and provide epidemiological evidence to support the dietary prevention and management strategies for HUA in children and adolescents.
Implications of all the available evidence
Choline may help reduce uric acid build-up, suggesting that moderate increases in dietary choline intake could be a viable option for better control of uric acid levels and prevention of HUA. Based on these findings, children and adolescents are encouraged to increase their intake of choline-rich foods, such as eggs, legumes, and whole grains, which may help lower uric acid levels and reduce the odds of HUA.
Introduction
Hyperuricemia (HUA), characterised by elevated serum uric acid levels, is a primary risk factor for gout and associated with hypertension, type 2 diabetes, and impairment of cardiovascular function.1,2 Alarmingly, the global prevalence of HUA has risen significantly in recent years, with particularly high rates observed among children and adolescents.3,4 Studies from China, the United States, and several European countries have reported that the overall prevalence of HUA among children and adolescents ranges from 0.6% to 50.4%.5, 6, 7 Consequently, global efforts to treat and prevent HUA in paediatric populations are increasing, with dietary and nutrient management identified as critical modifiable factors for reducing HUA risk.8
Choline is an essential nutrient involved in various physiological processes,9 which has been studied as an osmoregulatory agent to reduce serum homocysteine concentrations.10,11 Serum homocysteine levels are positively correlated with serum uric acid.12,13 Despite evidence of this effect in animal models such as broiler chickens and dogs, the association between choline and uric acid has not been confirmed in human populations.14,15 Uric acid is the final product of purine metabolism primarily derived from foods like red meat, organ meats, and seafood.16 Dietary guidelines recommend limiting foods high in purines to reduce the risk of HUA and gout attacks.17, 18, 19, 20 Given that many choline-rich foods overlap with purine-rich foods, the association between choline intake and uric acid is complex and warrants further investigation in large population studies.
Choline metabolism, as a methyl donor for creatine synthesis and methylation processes,21 can affect renal function through several pathways, including its involvement in phospholipid synthesis and the regulation of oxidative stress.22,23 Experimental studies further support these findings.14,24,25 Elevated uric acid levels may result from impaired renal function,26 as approximately 75% of uric acid is excreted through the kidneys.27 However, the relationship between choline intake and HUA and the role of renal function in this context remains unclear, leaving a significant gap in the literature. Moreover, most studies focused solely on total choline intake and minimal attention given to its various subtypes, including lipid-soluble compounds such as phosphatidylcholine (PtdCho) and sphingomyelin (Sphingo), as well as water-soluble compounds like free choline, glycerophosphorylcholine (GpCho), and phosphorylcholine (PCho).28
To address this gap, we conducted a cross-sectional study among Chinese children and adolescents aged 6–17 years to examine the relationship between dietary choline intake and HUA, and to assess the potential mediating role of kidney function in this association.
Methods
Study design and participants
A multi-stage cluster randomisation sampling method was used to collect the data from the China National Nutrition and Health Surveillance of Children and Lactating Mothers in 2016–2017 in 275 surveillance sites. The survey was conducted by the National Institute of Nutrition and Health, Chinese Center for Disease Control and Prevention (NINH, China CDC). County-level administrative units across 31 provinces (including autonomous regions and municipalities directly under the Central Government) in China were categorised into four groups: large cities, medium and small cities, general rural counties, and poor rural counties, resulting in 124 strata. After excluding missing data and adjusting for regional conditions, 109 strata were finalised. A total of 150 monitoring sites were selected, with 26 in large cities, 45 in medium and small cities, 46 in general rural areas, and 33 in poor rural areas, based on population distribution. The detailed sampling procedure is presented in Table S1. Participants completed a face-to-face interview, physical measurements, and provided biological samples, as described in detail elsewhere.29 Data for the survey were collected between October 2016 and December 2018.
Eligible participants were identified through a database search conducted from October to December 2023. Due to the unavailability of the entire national dataset, we utilised data from five provinces, including Inner Mongolia, Shandong, Jiangsu, Guangdong, and Guizhou, which across different regions of China: Northeast, North, Southwest, South, and East. These provinces capture a broad range of dietary patterns, socio-economic statuses, and lifestyle factors, offering sufficient variability in exposure variables to reflect the dietary habits of a substantial portion of Chinese children. We used uniform equipment and methods to conduct the surveys. We excluded participants without serum uric acid data, those with implausible energy intakes, defined as those in the highest or lowest 1% of the distribution of the ratio of energy intake to estimated energy requirement,30 and those with implausible height or with serum creatinine outliers identified by Tukey's methods as less than (Q1 − 1.5 × IQR) or more than (Q3 + 1.5 × IQR) in each year of age due to the age dependence of serum creatinine, where Q1 is the 25th percentile, Q3 is the 75th percentile31 (Figure S1).
Ethics
All participants and their guardian had given informed consent in writing to participate in the study. The Ethics Review Board of NINH, China CDC approved the protocol (No. 201614).
Procedures
Dietary data for the main analyses were collected using the 24-h dietary recall method. Trained enumerators conducted face-to-face interviews to gather detailed information on meals consumed over three consecutive days (two school days and one rest day) at home, school, and outside. The recall forms recorded food names, portion sizes, and intake of breakfast, lunch, dinner, snacks, beverages, and dietary supplements. Additionally, cooking oil and condiments used at home or in school canteens were weighed over three days to track intake of key items like oil, salt, and monosodium glutamate. We also used the Food Frequency Questionnaires (FFQs) to collect dietary information as a complementary analysis to increase the stability of the results. The standardised questionnaire, curated by experts from the NINH and China CDC, has been validated and applied in other large-scale dietary surveys of children in China.32, 33, 34, 35 The survey covered 11 food categories and 59 sub-categories, representing the most common foods consumed by Chinese residents. Investigators, equipped with tablets, paper questionnaires, and dietary maps, were trained on the questionnaire's structure and content. Before the survey, they explained its purpose to participants and enquired about each food item listed in the FFQ, including consumption in the past month, frequency, and portion size. For younger participants, their guardians assisted in completing the survey. Nutrient intake was estimated based on the China Food Composition Tables, Standard Edition (6th ed).36 Since there is no information on choline and betaine levels in Chinese foods, dietary values for choline and betaine are based on the United States Department of Agriculture (USDA) database.37,38 Total choline is formed from PtdCho, Sphingo, free choline, GpCho, and PCho. We calculated energy-adjusted choline intake using the residual method.39
The primary outcome of this study was the prevalence of HUA. Fasting venous blood samples were collected from participants in the morning. Serum uric acid was measured using colourimetry (Roche C702 automagical analyser). Based on recommendation in Clinical Paediatric Nephrology (3rd ed) and previous studies,40, 41, 42 participants were classified as having HUA if their serum uric acid levels exceeded the following thresholds: 320 μmol/L for those aged 6–10 years, 470 μmol/L in boys and 350 μmol/L in girls aged 11–15 years, and 420 μmol/L in boys and 360 μmol/L in girls aged 15 years and older. Those below these levels were assigned to the normal group.
Glomerular filtration rate (GFR) reflects the efficiency of the glomeruli to filter blood and remove waste products, which is often used to indicate changes in kidney function. Estimated GFR (eGFR) was calculated from height and serum creatinine using the Schwartz formula,43 different coefficients would be used for different populations, including K of 0.55 for participants aged 6–12 years, and 0.77 for males and 0.55 for females for those older than 12 years.44,45
The basic information of the participants (including age, sex, nationality, etc.) was collected using standardised questionnaires. Anthropometric measurements were obtained using a unified method and equipment and fasting venous blood samples were collected.46,47
Based on previous studies,48, 49, 50, 51 we considered various confounding factors that could influence dietary choline intake or serum uric acid, including sex, age, nationality, educational level of the primary caregiver, active physical activity, smoking or exposure to second-hand smoke, alcohol consumption, healthy dietary index, dietary energy, percentage of energy from protein, livestock and poultry meat, fishery products, legumes, total cereals, eggs, and sugar-sweetened beverages. Family history of diabetes or hypertension, fasting blood glucose, body mass index (BMI), hypertriglyceridemia, and hypertension were also accounted for. Active physical activity was defined as self-reported time spent on activities causing shortness of breath or sweating which averaging more than 1 h per day.52 Smoking, second-hand smoke exposure, and alcohol consumption were based on self-reports. BMI was calculated using standardised equipment, with participants categorised as normal weight, overweight or obesity according to criteria specific to sex and age.53 Hypertriglyceridaemia was determined based on guideline utilising four indicators: total cholesterol, triglyceride, low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C).54 Definitions of healthy dietary index are presented in Table S2.55,56 Hypertension was defined according to criteria based on sex, age, and height.57
Statistics
Details of missing covariates were presented in Table S3. Given the rigorous sampling design of this study, we consider the missing data to be random.58 We applied multiple imputation by chained equations (MICE) to address the missing data, creating 10 datasets to leverage its ability to manage both continuous and categorical variables while accommodating complex interrelationships. MICE was chosen based on the missing at random (MAR) assumption, supported by our assessment of missing data patterns.59 All variables were included in the multiple imputation model to ensure comprehensive imputation of missing values. The characteristics of participants were presented as the mean (standard deviation [SD]) for continuous variables and the number (percentage [%]) for categorical variables. Based on the results of the Shapiro–Wilk test and the Kolmogorov–Smirnov test, the differences in characteristics of participants across HUA and normal groups were assessed by the Chi-square test, Student's t-test, or Wilcoxon rank sum test, as appropriate.
Participants were divided into quartiles according to residual energy-adjusted dietary choline intake. We examined the odds ratios (ORs) and 95% confidence intervals (95% CIs) of HUA according to quartiles of residual energy-adjusted choline intake using weighted logistic regression analysis. This analysis accounted for stratification and clustering (primary sampling units). The trend test was carried out by taking the median of each residual energy-adjusted choline intake quartile as a continuous variable in the model. Similar calculations were conducted to assess the associations of choline-contributing compounds and betaine with HUA.
Based on past studies,14,15 we hypothesised that dietary choline intake may affect uric acid levels by improving renal filtration. We also performed generalised linear models to examine the associations of residual energy-adjusted choline intake with eGFR, eGFR with uric acid and HUA prevalence (Tables S4 and S5). Further, we adopted the model-based mediation analysis framework proposed by Tingley et al. to study the mediating effects of eGFR and constructed two models.60 The first model was the mediation model, a linear regression model, which was used to test the hypothesised relationship between eGFR and choline intake, as well as the covariates. The second model was the outcome model, a logistic regression of HUA by energy-adjusted choline intake, covariates, and the hypothesised eGFR. The two models were subsequently merged to determine the mediation proportion, which was calculated as the mean ratio of each participant's indirect effect to the total effect. This was achieved by dividing the average causal mediation effect by the sum of the average causal mediation effect and the average direct effect. A quasi-Bayesian estimation approach with 1000 iterations was employed to estimate the mediating effect. Mediation triangles visualised the direct and indirect effects. Furthermore, we divided choline-contributing compounds into lipid-soluble choline (including PtdCho and Sphingo) and water-soluble choline (including free choline, PCho, and GpCho), then analysed repeatedly as above.
The potential non-linear association between choline intake and HUA was explored through nonparametrically restricted cubic spline regression, with knots positioned at the 25th, 50th, and 75th percentiles. Additionally, multiple linear regression models were employed to investigate the relationship between choline intake and serum uric acid. Moreover, we performed interaction analyses to examine the association between choline intake and HUA among populations in different age groups and sex.
In the analysis, several covariates were adjusted. To reduce the impact of multicollinearity on the regression results, all selected covariates were screened using the least absolute shrinkage and selection operator (LASSO) as detailed in Figure S2. Further, we also calculated their variance inflation factors, which were all less than 2. In model 1, adjustments were made for sex (male or female), age (years, continuous), and energy (kcal/d, continuous). Model 2 included further adjustments for nationality (Han or others), educational level of the primary caregiver (illiteracy, primary school, junior high school, or high school, and above), active physical activity (yes or no), smoking or exposure to second-hand smoke (everyday, 4–6 days/week, 1–3 days/week, less than 1 day/week, or never), alcohol consumption (current, former, or never), healthy dietary index (continuous), percentage of energy from protein (%, continuous), livestock and poultry meat (g/d, continuous), fishery products (g/d, continuous), legumes (g/d, continuous), total cereals (g/d, continuous), eggs (g/d, continuous), and sugar-sweetened beverages (ml/d, continuous). Model 3 further adjusted for family history of diabetes (yes or no), family history of hypertension (yes or no), fasting blood glucose (mmol/L, continuous), BMI (normal, overweight, or obesity), hypertriglyceridemia (yes or no), and hypertension (yes or no).
To evaluate the robustness of our findings, we conducted several sensitivity analyses. Firstly, we explored the relationship between residual energy-adjusted choline intake and HUA using data collected from FFQs, and model-based mediation analyses were utilised to explore the mediating effect of eGFR. We then repeated these analyses four times: once assessing the associations between energy-unadjusted choline intake and HUA, again using data without interpolation, then including sample weights as covariates and finally additionally adjusting for the province where participants located. Considering unmeasured errors in our study, we also calculated the E-values to account for the errors.
Analyses were performed using R statistical software (version 4.3.2 for Windows). Statistical tests were two-sided, and P values less than 0.05 were considered statistically significant.
Role of the funding source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Characteristics of participants
After excluding participants without serum uric acid data (n = 3763), with implausible energy intakes (n = 782), and those with implausible height (n = 5) or with outliers of serum creatinine (n = 118), a total of 10749 participants aged 6–17 years were eligible for inclusion in the study (Figure S1). Table 1 showed the characteristics of participants who were 7062 (65.7%) female and 9230 (85.9%) Han nationality. A total of 3398 children and adolescents were found to have HUA with a prevalence of 3398 (31.6%). The prevalence of HUA was 1339 (36.3%) in boys and 2059 (29.2%) in girls. Individuals in the HUA group were more likely to be smokers, former or current alcohol consumers, and higher consumers of sugar-sweetened beverages. Additionally, these participants were more likely to have a family history of diabetes or to be overweight, obesity or have hypertriglyceridemia. The mean (SD) intake of total choline and betaine were 232.2 (142.1) and 120.8 (116.5) mg/d, respectively. The mean (SD) intake of choline-contributing compounds including PtdCho, Sphingo, free choline, GpCho, and PCho were 147.4 (116.5) mg/d, 12.0 (8.7) mg/d, 46.6 (29.1) mg/d, 21.2 (14.9) mg/d, and 6.3 (5.2) mg/d, respectively.
Table 1.
Characteristics of participants.
| Characteristic | Total | Normal | Hyperuricemia | P-value |
|---|---|---|---|---|
| Participants, No. (%) | 10749 (100.0) | 7351 (68.4) | 3398 (31.6) | |
| Female, No. (%) | 7062 (65.7) | 5003 (68.1) | 2059 (60.6) | <0.001 |
| Age, mean (SD), years | 12.3 (3.2) | 12.2 (3.2) | 12.4 (3.2) | 0.057 |
| 6∼11 years, No. (%) | 3967 (36.9) | 2639 (35.9) | 1328 (39.1) | |
| 12∼17 years, No. (%) | 6782 (63.1) | 4712 (64.1) | 2070 (60.9) | |
| Nationality, No. (%) | <0.001 | |||
| Han | 9230 (85.9) | 6230 (84.8) | 3000 (88.3) | |
| Others | 1519 (14.1) | 1121 (15.2) | 398 (11.7) | |
| Province where participants located, No. (%) | <0.001 | |||
| Inner Mongolia | 1327 (12.3) | 993 (13.5) | 334 (9.8) | |
| Shandong | 2195 (20.4) | 1688 (23.0) | 507 (14.9) | |
| Jiangsu | 2178 (20.3) | 1653 (22.5) | 525 (15.5) | |
| Guangdong | 3351 (31.2) | 1748 (23.8) | 1603 (47.2) | |
| Guizhou | 1698 (15.8) | 1269 (17.3) | 429 (12.6) | |
| Educational level of the primary caregiver, No. (%) | 0.002 | |||
| Illiteracy | 604 (5.6) | 452 (6.1) | 152 (4.5) | |
| Primary school | 2756 (25.6) | 1902 (25.9) | 854 (25.1) | |
| Junior high school | 3998 (37.2) | 2718 (37.0) | 1280 (37.7) | |
| High school and above | 3391 (31.5) | 2279 (31.0) | 1112 (32.7) | |
| Active physical activity, No. (%) | 4346 (40.4) | 2867 (39.0) | 1479 (43.5) | <0.001 |
| Smoking or exposure to second-hand smoke, No. (%) | 0.001 | |||
| Everyday | 1126 (10.5) | 778 (10.6) | 348 (10.2) | |
| 4–6 days/week | 407 (3.8) | 268 (3.6) | 139 (4.1) | |
| 1–3 days/week | 1340 (12.5) | 853 (11.6) | 487 (14.3) | |
| Less than 1 day/week | 1611 (15.0) | 1097 (14.9) | 514 (15.1) | |
| Never | 6265 (58.3) | 4355 (59.2) | 1910 (56.2) | |
| Alcohol consumption, No. (%) | <0.001 | |||
| Current | 445 (4.1) | 273 (3.7) | 172 (5.1) | |
| Former | 1021 (9.5) | 650 (8.8) | 371 (10.9) | |
| Never | 9283 (86.4) | 6428 (87.4) | 2855 (84.0) | |
| Healthy dietary index, mean (SD) | 60.1 (5.8) | 60.1 (5.8) | 60.1 (5.9) | 0.769 |
| Dietary energy, mean (SD), kcal/d | 1379.6 (635.3) | 1376.8 (632.4) | 1385.9 (641.7) | 0.488 |
| Percentage of energy from protein, mean (SD), % | 16.0 (4.5) | 15.8 (4.3) | 16.5 (4.9) | <0.001 |
| Livestock and poultry meat, mean (SD), g/d | 92.3 (127.1) | 91.3 (123.5) | 94.7 (134.5) | 0.196 |
| Fishery products, mean (SD), g/d | 52.2 (197.2) | 46.5 (183.4) | 64.4 (223.7) | <0.001 |
| Legumes, mean (SD), g/d | 7.2 (19.3) | 7.3 (19.7) | 7.1 (18.4) | 0.663 |
| Sugar-sweetened beverages, mean (SD), ml/d | 83.4 (147.4) | 80.5 (149.7) | 89.8 (142.0) | 0.002 |
| Total cereals, mean (SD), g/d | 152.9 (120.0) | 151.2 (119.7) | 156.7 (120.5) | 0.026 |
| Eggs, mean (SD), g/d | 53.7 (139.2) | 54.2 (141.6) | 52.6 (133.8) | 0.589 |
| Family history, No. (%) | ||||
| Diabetes | 1345 (12.5) | 872 (11.9) | 473 (13.9) | 0.003 |
| Hypertension | 3619 (33.7) | 2433 (33.1) | 1186 (34.9) | 0.069 |
| Fasting blood glucose, mean (SD), mmol/L | 5.2 (0.6) | 5.1 (0.6) | 5.2 (0.6) | <0.001 |
| BMI, mean (SD), kg/m2 | 18.6 (3.8) | 18.2 (3.5) | 19.4 (4.4) | <0.001 |
| Normal, No. (%) | 8806 (81.9) | 6300 (85.7) | 2506 (73.7) | |
| Overweight, No. (%) | 1108 (10.3) | 689 (9.4) | 419 (12.3) | |
| Obesity, No. (%) | 835 (7.8) | 362 (4.9) | 473 (13.9) | |
| Hypertriglyceridemia, No. (%) | 2186 (20.3) | 1333 (18.1) | 853 (25.1) | <0.001 |
| Hypertension, No. (%) | 472 (4.4) | 325 (4.4) | 147 (4.3) | 0.863 |
| eGFR, mean (SD), ml/(min 1.73 m2) | 149.7 (26.9) | 152.3 (27.8) | 144.1 (23.8) | <0.001 |
| Dietary choline, mean (SD), mg/d | ||||
| Total choline | 232.2 (142.1) | 232.2 (145.5) | 232.3 (134.4) | 0.981 |
| Phosphatidylcholine | 147.4 (116.5) | 148.2 (121.1) | 145.9 (105.9) | 0.346 |
| Sphingomyelin | 12.0 (8.7) | 11.9 (8.8) | 12.4 (8.6) | 0.008 |
| Free choline | 46.6 (29.1) | 46.5 (29.0) | 46.8 (29.5) | 0.551 |
| Glycerophosphocholine | 21.2 (14.9) | 20.9 (14.6) | 22.0 (15.6) | <0.001 |
| Phosphocholine | 6.3 (5.2) | 6.3 (5.2) | 6.5 (5.3) | 0.065 |
| Betaine | 120.8 (116.5) | 124.9 (121.4) | 112.1 (104.7) | <0.001 |
Association of choline intake with HUA and mediating effects of eGFR
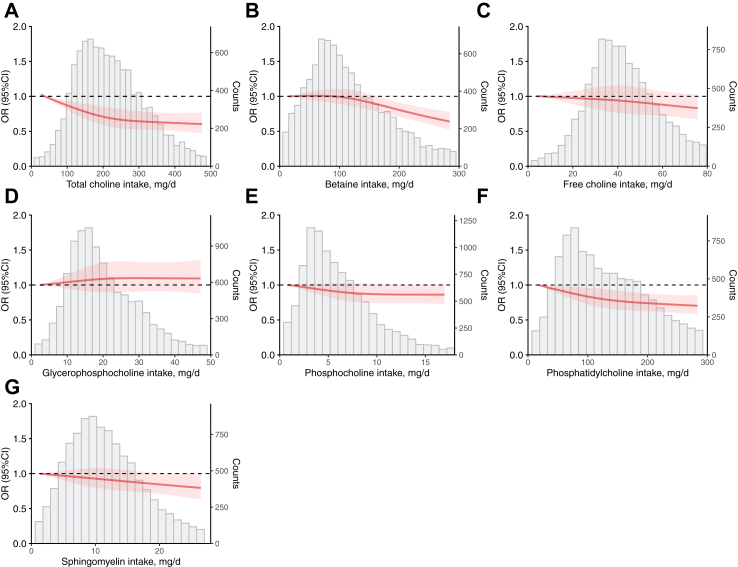
A negative dose-dependent relationship between choline intake and HUA is shown in Fig. 1. As shown in Table 2, after adjustment for potential confounders (model 3), compared with those in the lowest quartile of choline intake, participants in the 2nd to 4th quartiles had a lower prevalence of HUA, with ORs of 0.81 (95% CI, 0.72–0.92), 0.81 (95% CI, 0.71–0.93), and 0.75 (95% CI, 0.63–0.90), respectively. For each quartile increment, the odds of HUA decreased by 8.00% (OR, 0.92; 95% CI, 0.87–0.97; P for trend = 0.006). Similarly, the prevalence of HUA in the 4th quartiles was 25.00% lower for PtdCho (OR, 0.75; 95% CI, 0.64–0.89; P for trend = 0.002), and 25.00% lower for betaine (OR, 0.75; 95% CI, 0.59–0.94; P for trend = 0.016) in model 3. The results of the multiple linear regression analysis showed an inverse association between choline intake and serum uric acid in model 3, as indicated by the total choline, betaine, PtdCho, and Sphingo (Table S6).
Fig. 1.
Multiple adjusted restricted cubic spline models for the associations of residual energy-adjusted choline intake with HUA. The models were adjusted for sex (male or female), age (years, continuous), energy (kcal, continuous), nationality (Han or others), educational level of the primary caregiver (illiteracy, primary school, junior high school or high school, and above), active physical activity (yes or no), smoking or exposure to second-hand smoke (everyday, 4–6 days/week, 1–3 days/week, less than 1 day/week or never), alcohol consumption (current, former, never), healthy dietary index (continuous), percentage of energy from protein (%, continuous), livestock and poultry meat (g/d, continuous), fishery products (g/d, continuous), legumes (g/d, continuous), total cereals (g/d, continuous), eggs (g/d, continuous), sugar-sweetened beverages (ml/d, continuous), family history of diabetes (yes or no), family history of hypertension (yes or no), fasting blood glucose (mmol/L, continuous), BMI (normal, overweight, or obesity), hypertriglyceridemia (yes or no), and hypertension (yes or no). (A) for total choline; (B) for betaine; (C) for free choline; (D) for GpCho; (E) for PCho; (F) for PtdCho; and (G)for Sphingo.
Table 2.
Odds ratios (95% CIs) of residual energy-adjusted choline intake with HUA.
| Dietary choline | Residual energy-adjusted intake quartiles, ORs (95% CI) |
P-trend | Per quartile increment | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Total choline | ||||||
| Median intake (IQR), mg/d | 117.02 (89.59, 134.89) | 181.20 (165.27, 196.48) | 249.14 (230.95, 268.74) | 357.92 (321.25, 422.32) | ||
| Events, n (%) | 861 (8.01) | 803 (7.47) | 856 (7.96) | 878 (8.17) | ||
| Model 1 | 1 (reference) | 0.90 (0.80, 1.01) | 0.99 (0.87, 1.13) | 1.02 (0.89, 1.18) | 0.497 | 1.02 (0.97, 1.06) |
| Model 2 | 1 (reference) | 0.81 (0.72, 0.92) | 0.83 (0.73, 0.95) | 0.77 (0.65, 0.91) | 0.010 | 0.93 (0.88, 0.98) |
| Model 3 | 1 (reference) | 0.81 (0.72, 0.92) | 0.81 (0.71, 0.93) | 0.75 (0.63, 0.90) | 0.006 | 0.92 (0.87, 0.97) |
| Phosphatidylcholine | ||||||
| Median level (IQR), mg/d | 34.80 (24.97, 43.66) | 71.91 (61.57, 83.03) | 120.22 (105.77, 136.13) | 207.58 (176.34, 263.27) | ||
| Events, n (%) | 831 (7.73) | 836 (7.78) | 877 (8.16) | 854 (7.94) | ||
| Model 1 | 1 (reference) | 0.99 (0.87, 1.13) | 1.03 (0.90, 1.18) | 1.01 (0.87, 1.16) | 0.812 | 1.01 (0.96, 1.05) |
| Model 2 | 1 (reference) | 0.88 (0.77, 1.00) | 0.87 (0.76, 0.99) | 0.77 (0.66, 0.91) | 0.005 | 0.93 (0.88, 0.97) |
| Model 3 | 1 (reference) | 0.89 (0.77, 1.01) | 0.85 (0.74, 0.98) | 0.75 (0.64, 0.89) | 0.002 | 0.92 (0.87, 0.97) |
| Sphingomyelin | ||||||
| Median intake (IQR), mg/d | 3.20 (2.02, 4.19) | 6.63 (5.86, 7.39) | 10.00 (9.10, 11.11) | 15.70 (13.75, 18.94) | ||
| Events, n (%) | 797 (7.41) | 836 (7.78) | 835 (7.77) | 930 (8.65) | ||
| Model 1 | 1 (reference) | 1.02 (0.89, 1.16) | 1.15 (0.98, 1.34) | 1.16 (1.00, 1.34) | 0.032 | 1.06 (1.01, 1.11) |
| Model 2 | 1 (reference) | 0.96 (0.85, 1.09) | 0.99 (0.87, 1.13) | 0.88 (0.76, 1.02) | 0.144 | 0.97 (0.92, 1.01) |
| Model 3 | 1 (reference) | 0.98 (0.86, 1.12) | 0.98 (0.85, 1.13) | 0.86 (0.74, 1.00) | 0.085 | 0.96 (0.91, 1.01) |
| Free choline | ||||||
| Median intake (IQR), mg/d | 18.15 (14.36, 20.87) | 27.33 (25.25, 29.44) | 36.61 (34.04, 39.54) | 54.00 (47.42, 66.06) | ||
| Events, n (%) | 849 (7.90) | 860 (8.00) | 833 (7.75) | 856 (7.96) | ||
| Model 1 | 1 (reference) | 1.05 (0.92, 1.20) | 1.00 (0.87, 1.15) | 1.01 (0.85, 1.19) | 0.924 | 1.00 (0.94, 1.05) |
| Model 2 | 1 (reference) | 1.01 (0.88, 1.16) | 0.95 (0.82, 1.10) | 0.93 (0.79, 1.09) | 0.311 | 0.97 (0.92, 1.03) |
| Model 3 | 1 (reference) | 1.01 (0.87, 1.18) | 0.95 (0.81, 1.10) | 0.93 (0.79, 1.11) | 0.355 | 0.97 (0.92, 1.03) |
| Glycerophosphocholine | ||||||
| Median intake (IQR), mg/d | 7.01 (5.77, 8.09) | 11.34 (10.29, 12.49) | 16.55 (15.06, 18.37) | 26.88 (23.11, 32.71) | ||
| Events, n (%) | 797 (7.41) | 816 (7.59) | 869 (8.08) | 916 (8.52) | ||
| Model 1 | 1 (reference) | 1.09 (0.93, 1.28) | 1.12 (0.95, 1.32) | 1.23 (1.04, 1.45) | 0.022 | 1.07 (1.01, 1.12) |
| Model 2 | 1 (reference) | 1.08 (0.92, 1.27) | 1.08 (0.92, 1.27) | 1.09 (0.92, 1.28) | 0.344 | 1.03 (0.97, 1.08) |
| Model 3 | 1 (reference) | 1.11 (0.94, 1.30) | 1.13 (0.96, 1.33) | 1.11 (0.94, 1.31) | 0.210 | 1.03 (0.98, 1.09) |
| Phosphocholine | ||||||
| Median intake (IQR), mg/d | 1.49 (1.04, 1.88) | 3.06 (2.65, 3.50) | 5.00 (4.46, 5.62) | 8.67 (7.34, 10.89) | ||
| Events, n (%) | 831 (7.73) | 806 (7.50) | 854 (7.94) | 907 (8.44) | ||
| Model 1 | 1 (reference) | 0.92 (0.81, 1.04) | 1.06 (0.92, 1.24) | 1.10 (0.90, 1.34) | 0.194 | 1.04 (0.98, 1.11) |
| Model 2 | 1 (reference) | 0.85 (0.75, 0.96) | 0.94 (0.81, 1.08) | 0.90 (0.75, 1.09) | 0.522 | 0.98 (0.92, 1.04) |
| Model 3 | 1 (reference) | 0.85 (0.75, 0.97) | 0.92 (0.79, 1.07) | 0.87 (0.72, 1.05) | 0.280 | 0.97 (0.91, 1.03) |
| Betaine | ||||||
| Median intake (IQR), mg/d | 29.84 (1.60, 46.62) | 81.09 (71.16, 91.18) | 128.79 (114.76, 146.1) | 222.15 (189.24, 282.37) | ||
| Events, n (%) | 894 (8.32) | 928 (8.63) | 843 (7.84) | 733 (6.82) | ||
| Model 1 | 1 (reference) | 1.09 (0.96, 1.25) | 0.95 (0.79, 1.15) | 0.78 (0.62, 0.99) | 0.040 | 0.92 (0.85, 0.99) |
| Model 2 | 1 (reference) | 1.05 (0.92, 1.19) | 0.93 (0.78, 1.11) | 0.79 (0.64, 0.99) | 0.041 | 0.92 (0.86, 0.99) |
| Model 3 | 1 (reference) | 1.04 (0.91, 1.19) | 0.91 (0.75, 1.09) | 0.75 (0.59, 0.94) | 0.016 | 0.90 (0.84, 0.98) |
Model 1 was adjusted for sex (male or female), age (years, continuous), and energy (kcal/d, continuous).
Model 2 additionally adjusted nationality (Han or others), educational level of the primary caregiver (illiteracy, primary school, junior high school, or high school, and above), active physical activity (yes or no), smoking or exposure to second-hand smoke (everyday, 4–6 days/week, 1–3 days/week, less than 1 day/week, or never), alcohol consumption (current, former, or never), healthy dietary index (continuous), percentage of energy from protein (%, continuous), livestock and poultry meat (g/d, continuous), fishery products (g/d, continuous), legumes (g/d, continuous), total cereals (g/d, continuous), eggs (g/d, continuous), and sugar-sweetened beverages (ml/d, continuous).
Model 3 additionally adjusted family history of diabetes (yes or no), family history of hypertension (yes or no), fasting blood glucose (mmol/L, continuous), BMI (normal, overweight, or obesity), hypertriglyceridemia (yes or no), and hypertension (yes or no).
We then examined the association of choline intake from lipid- and water-soluble sources with the prevalence of HUA. After controlling for the potential effects of confounding variables (model 3), participants in the 4th intake quartile of lipid-soluble dietary choline had a lower prevalence of HUA compared to those in the lowest intake quartile, with an OR of 0.76 (95% CI, 0.64–0.90) for HUA. For each quartile increment, the odds of HUA decreased by 9.00% (OR, 0.91; 95% CI, 0.87–0.96) (Table 3).
Table 3.
Odds ratios (95% CIs) of residual energy-adjusted choline intake from lipid- and water-soluble sources with HUA.
| Dietary choline | Residual energy-adjusted intake quartiles, ORs (95% CI) |
P-trend | Per quartile increment | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Lipid-soluble dietary cholinea | ||||||
| Median intake (IQR), mg/d | 39.11 (27.94, 48.97) | 79.13 (68.15, 91.04) | 130.00 (115.13, 146.49) | 222.74 (189.38, 279.62) | ||
| Events, n (%) | 828 (7.70) | 837 (7.79) | 875 (8.14) | 858 (7.98) | ||
| Model 1 | 1 (reference) | 1.01 (0.88, 1.17) | 1.03 (0.90, 1.18) | 1.02 (0.89, 1.18) | 0.708 | 1.01 (0.96, 1.05) |
| Model 2 | 1 (reference) | 0.90 (0.78, 1.04) | 0.87 (0.76, 0.99) | 0.78 (0.67, 0.91) | 0.003 | 0.93 (0.88, 0.97) |
| Model 3 | 1 (reference) | 0.91 (0.79, 1.05) | 0.85 (0.74, 0.97) | 0.76 (0.64, 0.90) | 0.002 | 0.91 (0.87, 0.96) |
| Water-soluble dietary cholinea | ||||||
| Median intake (IQR), mg/d | 30.93 (25.66, 35.19) | 45.27 (42.06, 48.61) | 59.71 (55.75, 63.68) | 83.53 (74.78, 98.58) | ||
| Events, n (%) | 832 (7.74) | 825 (7.68) | 857 (7.97) | 884 (8.22) | ||
| Model 1 | 1 (reference) | 0.96 (0.85, 1.09) | 1.06 (0.92, 1.22) | 1.10 (0.93, 1.31) | 0.204 | 1.04 (0.98, 1.10) |
| Model 2 | 1 (reference) | 0.92 (0.81, 1.06) | 0.99 (0.85, 1.15) | 0.96 (0.81, 1.15) | 0.897 | 1.00 (0.94, 1.06) |
| Model 3 | 1 (reference) | 0.94 (0.82, 1.07) | 1.00 (0.86, 1.16) | 0.98 (0.82, 1.17) | 0.994 | 1.00 (0.94, 1.06) |
Model 1 was adjusted for sex (male or female), age (years, continuous), and energy (kcal/d, continuous).
Model 2 additionally adjusted nationality (Han or others), educational level of the primary caregiver (illiteracy, primary school, junior high school, or high school, and above), active physical activity (yes or no), smoking or exposure to second-hand smoke (everyday, 4–6 days/week, 1–3 days/week, less than 1 day/week, or never), alcohol consumption (current, former, or never), healthy dietary index (continuous), percentage of energy from protein (%, continuous), livestock and poultry meat (g/d, continuous), fishery products (g/d, continuous), legumes (g/d, continuous), total cereals (g/d, continuous), eggs (g/d, continuous), and sugar-sweetened beverages (ml/d, continuous).
Model 3 additionally adjusted family history of diabetes (yes or no), family history of hypertension (yes or no), fasting blood glucose (mmol/L, continuous), BMI (normal, overweight, or obesity), hypertriglyceridemia (yes or no), and hypertension (yes or no).
Lipid-soluble dietary choline was categorised as phosphatidylcholine and sphingomyelin, and water-soluble dietary choline comprised free choline, glycerophosphorylcholine, and phosphorylcholine.
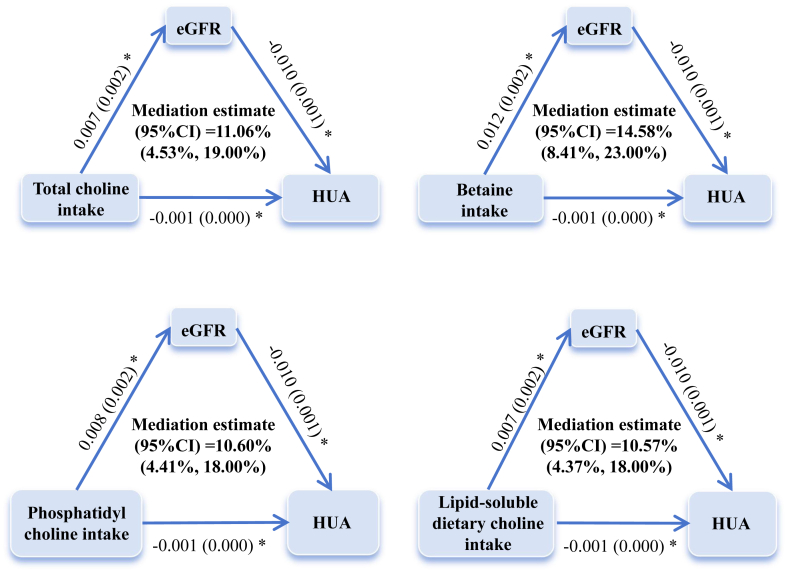
Mediation analyses revealed that eGFR mediated 10.60% (95% CI, 4.41%–18.00%) to 14.58% (95% CI, 8.41%–23.00%) of the association between choline intake and HUA. Fig. 2 presented the direct and indirect effect estimates for intakes of total choline, PtdCho, betaine, and lipid-soluble dietary choline as mediated effect triangles, with P-values less than 0.05 for the indirect effects.
Fig. 2.
Mediating effects of eGFR on the associations of residual energy-adjusted choline intake with HUA. Numbers represent β estimates (standard error) of the associations between each pair of variables. The models were adjusted for sex (male or female), age (years, continuous), energy (kcal, continuous), nationality (Han or others), educational level of the primary caregiver (illiteracy, primary school, junior high school or high school, and above), active physical activity (yes or no), smoking or exposure to second-hand smoke (everyday, 4–6 days/week, 1–3 days/week, less than 1 day/week or never), alcohol consumption (current, former, never), healthy dietary index (continuous), percentage of energy from protein (%, continuous), livestock and poultry meat (g/d, continuous), fishery products (g/d, continuous), legumes (g/d, continuous), total cereals (g/d, continuous), eggs (g/d, continuous), sugar-sweetened beverages (ml/d, continuous), family history of diabetes (yes or no), family history of hypertension (yes or no), fasting blood glucose (mmol/L, continuous), BMI (normal, overweight, or obesity), hypertriglyceridemia (yes or no), and hypertension (yes or no). Lipid-soluble dietary choline was summarised as phosphatidylcholine and sphingomyelin, and water-soluble dietary choline included free choline, glycerophosphorylcholine, and phosphorylcholine. ∗ Indicates P < 0.05.
Subgroups and sensitivity analyses
Table S7 presented the associations of residual energy-adjusted choline intake with HUA stratified by age groups and sex, which were in alignment with those reported in the main results. Tables S8–S12 and Figures S3–S7 presented the results of sensitivity analyses. We found that total choline, PtdCho, and betaine presented a stable negative association with HUA and mediating effect in sensitivity analyses. However, when we analysed using data from FFQs, only a negative correlation and mediating effect of betaine with HUA were observed. Furthermore, the E-values were 2.00 (1.46), 2.00 (1.50), and 2.00 (1.32) for total choline, PtdCho, and betaine intakes, respectively, making it unlikely that there are unmeasured confounders with effects that exceed choline (Table S13).
Discussion
In this cross-sectional study of Chinese children and adolescents, we found that a moderate to high increase in choline intake was positively associated with lower odds of HUA. Analysis of choline subtypes revealed that moderate to high intake of betaine and PtdCho was associated with reduced odds of HUA. Additionally, our findings indicated that higher intake of lipid-soluble choline had a lower prevalence of HUA. The eGFR functioned as a mediator in these associations, with mediation proportions ranging from 10.60% to 14.58%. These results provided epidemiologic evidence supporting dietary prevention and management of HUA in children and adolescents, which is of significant public health importance.
Serum uric acid levels in children and adolescents vary with age, rising significantly after 9–10 years in both sex, likely due to increased muscle mass, metabolic changes, and puberty.4,61 Recent surveys in China report that the prevalence of paediatric HUA ranges from 10.1% to 55.1%, depending on regional and population characteristics.7,49,62 A meta-analysis of 11 population-based studies estimated the prevalence of HUA in children and adolescents aged 6–19 years at 26.3%,7 consistent with our findings. Variations in prevalence across studies may be attributed to the absence of standardised diagnostic criteria for HUA in this population,61 as well as differences in dietary habits, lifestyle, and metabolic factors.8 At present, only animal studies on broiler chickens and dogs have investigated the relationship between choline and uric acid.14,15 Although these randomised controlled trials confirmed the effect of choline supplementation on reducing uric acid levels, further studies are needed in populations. We conducted an external population-based validation of the association between dietary choline and HUA and elucidated that the mechanism of this association may be related to renal function. It would be valuable to clarify the preventive effect of choline in the diet on elevated levels of uric acid.
Choline and betaine play critical roles in the methylation cycle, with betaine providing methyl groups for the conversion of homocysteine to methionine.63 This methylation cycle is interconnected with purine metabolism,64 which produces uric acid as a final product. Methylation disturbance may affect purine catabolism, leading to increased uric acid production.65 Furthermore, choline serves as a precursor for PtdCho, a key component of cell membranes, which can reduce oxidative stress. Oxidative stress may influence purine metabolism, potentially increasing uric acid production.66 Oxidative stress can also impair renal function, reducing uric acid excretion and contributing to HUA.1 Thus, choline's impact on renal function is vital for uric acid regulation. Given choline's role in maintaining membrane integrity and preventing oxidative damage, it may protect renal cells from injury.67,68 Any impairment in kidney function may reduce uric acid clearance and contribute to HUA.
Therefore, children and adolescents are advised to increase their intake of choline-rich foods, such as eggs, legumes and whole grains, which may help lower uric acid levels and reduce the odds of HUA. We propose that future intervention studies explore the effects of choline supplements in this population.
To our knowledge, we are the first study to explore the association between dietary choline and odds of HUA, as well as to assess the potential mediating role of kidney function among Chinese children and adolescents aged 6–17 years. Our research included participants from diverse geographic and economic backgrounds. We used residual energy-adjusted choline intake for a more accurate dietary assessment and defined uric acid outcomes with age- and sex-specific cut-off points. We also controlled for bias by adjusting for lifestyle factors, food intakes and chronic diseases.
Nevertheless, there are several limitations in this study. Firstly, although the participants represent a wide range of dietary patterns, socio-economic statuses, and lifestyle factors, the findings from five provinces may not fully capture the nutritional practices, environmental exposures, and genetic diversity of the entire Chinese population. Secondly, variations in laboratory practices may introduce some degree of measurement bias. We recommend that future multi-centre studies utilise a central laboratory for uric acid testing to ensure uniformity in methods and instrumentation. Furthermore, recall bias is difficult to avoid. To minimise this, we used both FFQs and 3-day 24-h dietary recalls data for the analysis of choline intake and HUA. Finally, cross-sectional studies are limited in their ability to establish causal associations and temporal relationships, and the possibility of residual confounding from unmeasured or unknown factors cannot be fully ruled out. Future prospective cohort studies are necessary.
In conclusion, moderate to high intake of dietary choline (181.20–357.92 mg/d), particularly phosphatidylcholine (120.22–207.58 mg/d), and betaine (189.24–282.37 mg/d), may reduce the odds of HUA by improving glomerular filtration function.
Contributors
Conceptualization, D.L., T.L., and Y.L.; methodology, C.L., Z.D., and D.L.; software, C.L., J.L., and Z.D.; formal analysis, C.L., J.L., and S.Y.; investigation, Y.L., L.Y., Q.Z., and T.L.; data curation, D.L., T.L., and X.D.; resources, Y.L., T.L., and D.L.; writing—original draft, C.L. and Z.D.; writing—review and editing, C.L., J.L., D.L., and L.C.; visualization, C.L. and Z.D.; supervision, D.L., T.L., and Y.L.; project administration, T.L. and D.L.; funding acquisition, T.L. and D.L. All authors have read and agreed to the published version of the manuscript. C.L., J.L., Z.D., L.C., S.Y., Y.L., Q.Z., X.D., L.Y., T.L., and D.L. have verified the underlying data. Y.L., T.L., and D.L. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
In accordance with the policy of the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention, the data related to this research cannot be publicly disclosed. However, interested parties can contact the corresponding authors to request access to the data or source code of this study.
Declaration of interests
We declare no competing interests.
Acknowledgements
We would like to thank all the participants who took part in the China National Nutrition and Health Surveillance of Children and Lactating Mothers in 2016–2017, and the staff members who carried out this study. We would also like to acknowledge the support of the National Natural Science Foundation of China (82003443, 42375180), the Natural Science Foundation of Guangdong Province of China (2024A1515012088), and the Construction of High-level University of Guangdong (G624330422).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.103012.
Contributor Information
Yiya Liu, Email: 1463211395@qq.com.
Tao Liu, Email: gztt_2002@163.com.
Dan Liu, Email: liudan0717@smu.edu.cn.
Appendix ASupplementary data
References
- 1.Gherghina M.E., Peride I., Tiglis M., Neagu T.P., Niculae A., Checherita I.A. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int J Mol Sci. 2022;23(6) doi: 10.3390/ijms23063188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawley W.T., Jungels C.G., Stenmark K.R., Fini M.A. U-shaped association of uric acid to overall-cause mortality and its impact on clinical management of hyperuricemia. Redox Biol. 2022;51 doi: 10.1016/j.redox.2022.102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen-Xu M., Yokose C., Rai S.K., Pillinger M.H., Choi H.K. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey, 2007-2016. Arthritis Rheumatol. 2019;71(6):991–999. doi: 10.1002/art.40807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo Y.J., Shim Y.S., Lee H.S., Hwang J.S. Association of serum uric acid levels with metabolic syndromes in Korean adolescents. Front Endocrinol (Lausanne) 2023;14 doi: 10.3389/fendo.2023.1159248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford E.S., Li C., Cook S., Choi H.K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115(19):2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 6.Luciano R., Shashaj B., Spreghini M., et al. Percentiles of serum uric acid and cardiometabolic abnormalities in obese Italian children and adolescents. Ital J Pediatr. 2017;43(1):3. doi: 10.1186/s13052-016-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao J., Ye P., Lu J., et al. Prevalence and related factors of hyperuricaemia in Chinese children and adolescents: a pooled analysis of 11 population-based studies. Ann Med. 2022;54(1):1608–1615. doi: 10.1080/07853890.2022.2083670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Major T.J., Topless R.K., Dalbeth N., Merriman T.R. Evaluation of the diet wide contribution to serum urate levels: meta-analysis of population based cohorts. BMJ. 2018;363 doi: 10.1136/bmj.k3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leermakers E.T., Moreira E.M., Kiefte-de Jong J.C., et al. Effects of choline on health across the life course: a systematic review. Nutr Rev. 2015;73(8):500–522. doi: 10.1093/nutrit/nuv010. [DOI] [PubMed] [Google Scholar]
- 10.Ueland P.M. Choline and betaine in health and disease. J Inherit Metab Dis. 2011;34(1):3–15. doi: 10.1007/s10545-010-9088-4. [DOI] [PubMed] [Google Scholar]
- 11.Chiuve S.E., Giovannucci E.L., Hankinson S.E., et al. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am J Clin Nutr. 2007;86(4):1073–1081. doi: 10.1093/ajcn/86.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J., Zhou J., Shao Z., Chen X., Yu Z., Zhao W. Association between serum uric acid and homocysteine levels among adults in the United States: a cross-sectional study. BMC Cardiovasc Disord. 2023;23(1):599. doi: 10.1186/s12872-023-03586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y., Wu Z., Wu J., Chen Z., Li P. Serum homocysteine level is positively correlated with serum uric acid level in U.S. adolescents: a cross sectional study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.818836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmoudi M., Azarfar A., Khosravinia H. Partial replacement of dietary methionine with betaine and choline in heat-stressed broiler chickens. J Poultry Sci. 2018;55(1):28–37. doi: 10.2141/jpsa.0170087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilcol Y.O., Yilmaz Z., Ulus I.H. Endotoxin alters serum-free choline and phospholipid-bound choline concentrations, and choline administration attenuates endotoxin-induced organ injury in dogs. Shock. 2005;24(3):288–293. doi: 10.1097/01.shk.0000174018.02688.4b. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Chen S., Yuan M., Xu Y., Xu H. Gout and diet: a comprehensive review of mechanisms and management. Nutrients. 2022;14(17) doi: 10.3390/nu14173525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto A., Harris H.R., Vitonis A.F., Chavarro J.E., Missmer S.A. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol. 2018;219(2):178.e1–178.e10. doi: 10.1016/j.ajog.2018.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y., Cao D., Chen Z., et al. Red and processed meat consumption and cancer outcomes: umbrella review. Food Chem. 2021;356 doi: 10.1016/j.foodchem.2021.129697. [DOI] [PubMed] [Google Scholar]
- 19.Li R., Yu K., Li C. Dietary factors and risk of gout and hyperuricemia: a meta-analysis and systematic review. Asia Pac J Clin Nutr. 2018;27(6):1344–1356. doi: 10.6133/apjcn.201811_27(6).0022. [DOI] [PubMed] [Google Scholar]
- 20.Dietary Guide for Hyperuricemia and Gout Patients (WS/T 560-2017) Biomed Environ Sci. 2023;36(9):897–898. doi: 10.3967/bes2023.118. [DOI] [PubMed] [Google Scholar]
- 21.Shronts E.P. Essential nature of choline with implications for total parenteral nutrition. J Am Diet Assoc. 1997;97(6):639–646. doi: 10.1016/S0002-8223(97)00161-2. 649; quiz 647-8. [DOI] [PubMed] [Google Scholar]
- 22.Zeisel S.H., Niculescu M.D. Perinatal choline influences brain structure and function. Nutr Rev. 2006;64(4):197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo F., Dai Q., Zeng X., et al. Renal function is associated with plasma trimethylamine-N-oxide, choline, L-carnitine and betaine: a pilot study. Int Urol Nephrol. 2021;53(3):539–551. doi: 10.1007/s11255-020-02632-6. [DOI] [PubMed] [Google Scholar]
- 24.Baris E., Simsek O., Arici M.A., Tosun M. Choline and citicoline ameliorate oxidative stress in acute kidney injury in rats. Bratisl Lek Listy. 2023;124(1):47–52. doi: 10.4149/BLL_2023_007. [DOI] [PubMed] [Google Scholar]
- 25.Hasson D.C., Watanabe-Chailland M., Romick-Rosendale L., et al. Choline supplementation attenuates experimental sepsis-associated acute kidney injury. Am J Physiol Ren Physiol. 2022;323(3):F255–F271. doi: 10.1152/ajprenal.00033.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipkowitz M.S. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep. 2012;14(2):179–188. doi: 10.1007/s11926-012-0240-z. [DOI] [PubMed] [Google Scholar]
- 27.Ponticelli C., Podestà M.A., Moroni G. Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. 2020;98(5):1149–1159. doi: 10.1016/j.kint.2020.05.056. [DOI] [PubMed] [Google Scholar]
- 28.Wiedeman A.M., Barr S.I., Green T.J., Xu Z., Innis S.M., Kitts D.D. Dietary choline intake: current state of knowledge across the life cycle. Nutrients. 2018;10(10) doi: 10.3390/nu10101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu D., Zhao L., Zhang J., et al. China nutrition and health surveys (1982-2017) China CDC Wkly. 2021;3(9):193–195. doi: 10.46234/ccdcw2021.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D., Li Z.H., Shen D., et al. Association of sugar-sweetened, artificially sweetened, and unsweetened coffee consumption with all-cause and cause-specific mortality: a large prospective cohort study. Ann Intern Med. 2022;175(7):909–917. doi: 10.7326/M21-2977. [DOI] [PubMed] [Google Scholar]
- 31.Yan R., Zhang C., Wang C., Sun Z., Peng X. Evaluation of glomerular filtration rate estimation equations based on serum creatinine in healthy Chinese children and adolescents: a nationwide cross-sectional study. BMJ Paediatr Open. 2023;7(1) doi: 10.1136/bmjpo-2023-002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fung T.T., Stampfer M.J., Manson J.E., Rexrode K.M., Willett W.C., Hu F.B. Prospective study of major dietary patterns and stroke risk in women. Stroke. 2004;35(9):2014–2019. doi: 10.1161/01.STR.0000135762.89154.92. [DOI] [PubMed] [Google Scholar]
- 33.Liu D., Zhao L.Y., Yu D.M., et al. Dietary patterns and association with obesity of children aged 6-17 years in medium and small cities in China: findings from the CNHS 2010-2012. Nutrients. 2018;11(1) doi: 10.3390/nu11010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D., Ju H., Yang Z.Y., et al. Food frequency questionnaire for Chinese children aged 12-17 years: validity and reliability. Biomed Environ Sci. 2019;32(7):486–495. doi: 10.3967/bes2019.066. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., Wang H., Wang Y., et al. Dietary patterns and their associations with childhood obesity in China. Br J Nutr. 2015;113(12):1978–1984. doi: 10.1017/S0007114515001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y.X. Peking University Medical Press; Beijing, China: 2018. Chinese Food Composition Table. [Google Scholar]
- 37.Patterson K., Bhagwat S., Williams J., Howe J., Holden J. USDA database for the choline content of common foods 2008. http://www.ars.usda.gov/SP2UserFiles/Place/80400525/Data/Choline/Choln02.pdf
- 38.Zeisel S.H., Mar M.H., Howe J.C., Holden J.M. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133(5):1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 39.Xia P.F., Zhang Y.B., Liu G., Pan A. The application of energy adjustment models in nutritional epidemiology. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(2):228–232. doi: 10.3760/cma.j.issn.0253-9624.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Noone D.G., Marks S.D. Hyperuricemia is associated with hypertension, obesity, and albuminuria in children with chronic kidney disease. J Pediatr. 2013;162(1):128–132. doi: 10.1016/j.jpeds.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Kher K., Schnaper H.W., Greenbaum L.A. Clinical Pediatric Nephrology. 3rd ed. CRC Press; Boca Raton: 2003. Reference data for paediatric nephrology; pp. 493–509. [Google Scholar]
- 42.Wang S.H., Zhu H.J., Duan L., et al. Serum uric acid level and its influencing factors in patients with diabetes insipidus. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2023;45(1):44–49. doi: 10.3881/j.issn.1000-503X.15039. [DOI] [PubMed] [Google Scholar]
- 43.Pierce C.B., Muñoz A., Ng D.K., Warady B.A., Furth S.L., Schwartz G.J. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99(4):948–956. doi: 10.1016/j.kint.2020.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subspecialty Group of Nephrology; the Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics Clinical practice guideline for early screening of pediatric chronic kidney disease in China (version 2021) Zhonghua Er Ke Za Zhi. 2022;60(9):858–868. doi: 10.3760/cma.j.cn112140-20220714-00647. [DOI] [PubMed] [Google Scholar]
- 45.Subspecialty Group of Nephrology, the Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics Evidence-based guideline on the diagnosis and management of growth retardation in pediatric chronic kidney disease. Zhonghua Er Ke Za Zhi. 2022;60(9):869–876. doi: 10.3760/cma.j.cn112140-20220715-00648. [DOI] [PubMed] [Google Scholar]
- 46.National Health Commission of the People's Republic of China . China Union Medical University Press; Beijing, China: 2018. China Health Statistics Yearbook in 2018. [Google Scholar]
- 47.Yu D., Zhao L., Zhang J., et al. China Nutrition and Health Surveys (1982-2017) China CDC Wkly. 2021;3(9):193–195. doi: 10.46234/ccdcw2021.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Real J., Forné C., Roso-Llorach A., Martínez-Sánchez J.M. Quality reporting of multivariable regression models in observational studies: review of a representative sample of articles published in biomedical journals. Medicine (Baltimore) 2016;95(20):e3653. doi: 10.1097/MD.0000000000003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong B., Liu F., Zhang S., et al. Associations between dietary patterns and serum uric acid concentrations in children and adolescents: a cross-sectional study. Food Funct. 2023;14(21):9803–9814. doi: 10.1039/d3fo03043a. [DOI] [PubMed] [Google Scholar]
- 50.Louck L., Cara K., Klatt K., Wallace T., Chung M. The relationship of circulating choline and choline-related metabolite levels with health outcomes: a scoping review of genome-wide association studies and Mendelian randomization studies. Adv Nutr. 2024;15(2) doi: 10.1016/j.advnut.2023.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng S., Shan L., You Z., et al. Dietary patterns, uric acid levels, and hyperuricemia: a systematic review and meta-analysis. Food Funct. 2023;14(17):7853–7868. doi: 10.1039/d3fo02004e. [DOI] [PubMed] [Google Scholar]
- 52.Composing and editorial board of physical activity guidelines for Chinese. Physical activity guidelines for Chinese (2021) Zhonghua Yufang Yixue Zazhi. 2022;56(1):7–8. doi: 10.3760/cma.j.cn112150-20211119-01070. [DOI] [PubMed] [Google Scholar]
- 53.National Health Commission of the People's Republic of China Screening for overweight and obesity among school-age children and adolescents. 2018. http://www.nhc.gov.cn/ewebeditor/uploadfile/2018/03/20180330094031236.pdf
- 54.Subspecialty Group of Rare Diseases, the Society of Pediatrics, Chinese Medical Association Expert consensus on diagnosis and management of dyslipidemia in children. Zhonghua Er Ke Za Zhi. 2022;60(7):633–639. doi: 10.3760/cma.j.cn112140-20211108-00936. [DOI] [PubMed] [Google Scholar]
- 55.The State Council of the People's Republic of China Healthy China action plan (2019-2030) 2019. http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=e9275fb95d5b4295be8308415d4cd1b2
- 56.Li Y., Schoufour J., Wang D.D., et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. 2020;368 doi: 10.1136/bmj.l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Health Commission of the People’s Republic of China Reference of screening for elevated blood pressure among children and adolescents aged 7~18 years. 2018. http://www.nhc.gov.cn/ewebeditor/uploadfile/2018/07/20180705095101600.pdf
- 58.Shah A.D., Bartlett J.W., Carpenter J., Nicholas O., Hemingway H. Comparison of random forest and parametric imputation models for imputing missing data using MICE: a CALIBER study. Am J Epidemiol. 2014;179(6):764–774. doi: 10.1093/aje/kwt312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Buuren S., Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Software. 2011;45 doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 60.Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. Mediation: R package for causal mediation analysis. J Stat Software. 2014;59(5):1–38. [Google Scholar]
- 61.Dai C., Wang C., Xia F., et al. Age and gender-specific reference intervals for uric acid level in children aged 5-14 years in Southeast Zhejiang Province of China: hyperuricemia in children may need redefinition. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.560720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo X., Xiao N., Jing X., Zhu Z., Zhang H. Analysis of the prevalence and influencing factors of hyperuricemia in children and adolescents aged 6-17 years in northeastern Sichuan Province. J Pediatr (Rio J) 2023;99(6):604–609. doi: 10.1016/j.jped.2023.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 64.Ulrey C.L., Liu L., Andrews L.G., Tollefsbol T.O. The impact of metabolism on DNA methylation. Hum Mol Genet. 2005;14(Spec No 1):R139–R147. doi: 10.1093/hmg/ddi100. [DOI] [PubMed] [Google Scholar]
- 65.Kadayifci F.Z., Zheng S., Pan Y.X. Molecular mechanisms underlying the link between diet and DNA methylation. Int J Mol Sci. 2018;19(12):4055. doi: 10.3390/ijms19124055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ossani G., Dalghi M., Repetto M. Oxidative damage lipid peroxidation in the kidney of choline-deficient rats. Front Biosci. 2007;12:1174–1183. doi: 10.2741/2135. [DOI] [PubMed] [Google Scholar]
- 67.Caffrey E.B., Sonnenburg J.L., Devkota S. Our extended microbiome: the human-relevant metabolites and biology of fermented foods. Cell Metabol. 2024;36(4):684–701. doi: 10.1016/j.cmet.2024.03.007. [DOI] [PubMed] [Google Scholar]
- 68.Bekdash R.A. Neuroprotective effects of choline and other methyl donors. Nutrients. 2019;11(12) doi: 10.3390/nu11122995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.