Abstract
The question we addressed in the current study is whether the mere prospect of monetary reward gain affects subjective time perception. To test this question, we collected trial-based confidence reports in a task where participants made categorical decisions about probe durations relative to the reference duration. When there was a potential to gain a monetary reward, the duration was perceived to be longer than in the neutral condition. Confidence, which reflects the perceived probability of being correct, was higher in the reward gain condition than in the neutral condition. We found that confidence influences the sense of time in different participants. Participants with high confidence reported perceiving the duration signaled by the monetary gain condition longer than participants with low confidence. Our results showed that only high confidence individuals overestimated the context of monetary gain. Finally, we found a negative relationship between confidence and time perception, and that confidence bias at the maximum uncertainty duration of 450 ms is predictive of time perception. Taken together, the current study demonstrates that subjective measures of the confidence profile caused an overestimation of time rather than the outcome valence of reward expectancy.
Keywords: Metacognition, Reward processing, Time perception
1. Introduction
Monetary reward is a well-known motivator for individuals to perform tasks or achieve goals (Adcock et al., 2006; Knutson et al., 2000). It has also been recognized as an extrinsic incentive that can influence decision making and time perception (Berridge, 2004; Wittmann & Paulus, 2008). Time perception refers to the subjective experience of time (Fontes et al., 2016), including its duration (Droit-Volet & Wearden, 2016), speed (Corbetta et al., 1991), and sense of time (Eagleman et al., 2005; Ivry & Schlerf, 2008; Wittmann, 2013). Confidence, on the other hand, relates to an individual-level of self-assurance (Kepecs & Mainen, 2012) in their abilities and decision making (Barthelmé & Mamassian, 2010; Kepecs et al., 2008; Navajas et al., 2017). It is a multifaceted construct that is influenced by a range of factors, including self-esteem, experience, and motivation (Frijda, 2010).
A number of studies have shown that monetary rewards can affect the perception of time (Apaydın et al., 2018; Delgado & Dickerson, 2012; Failing & Theeuwes, 2016; Wittmann & Paulus, 2008). For example, cognitive-behavioral findings in healthy human participants showed that the expectation of monetary reward could alter the subjective perception of duration in short time intervals. One piece of evidence showed that when a high amount of money was associated with an oddball disc, the perception of the oddball’s duration was overestimated compared to an oddball associated with a low amount of money or no money (Failing & Theeuwes, 2016). Notably, when a monetary reward was presented before the oddball and not by the oddball itself, the perception of duration remained unaffected. However, it has also been reported that when there is a potential to win money, the duration is perceived as longer than in loss or neutral conditions (Giersch & Coull, 2018). In other words, cuing a monetary reward prior to a duration judgment task distorts time, causing it to be overestimated compared to the reference duration (Giersch & Coull, 2018). Given the aforementioned different monetary reward conditions, a considerable body of literature generally associates attentional (Anderson, 2015, 2016; Chelazzi et al., 2013; Della Libera & Chelazzi, 2009; Failing & Theeuwes, 2018; Libera & Chelazzi, 2006) or intentional (Feldmann-Wüstefeld et al., 2016; Le Pelley et al., 2015, 2017; Makwana & Srinivasan, 2017) resources with monetary gain, which causes a longer perception of duration.
Another stream of literature has shown that monetary rewards can affect human confidence (Kiani & Shadlen, 2009; Lebreton et al., 2019; Mobbs et al., 2009; Pessoa, 2010). The expectation of a monetary reward can increase individuals’ confidence in their ability to perform a task, leading to better performance (Kragel et al., 2018; Murayama et al., 2010). For example, a study showed that confidence was behind the prospect of monetary gain in reinforcement- learning strategies (Lebreton et al., 2019). In this study, participants’ learning strategies differed between seeking gain and avoiding loss, with the former showing a higher confidence score. Neurophysiology studies also reported that monkeys did not select the sure target based on the difficulty of the stimulus but rather based on a sensation of uncertainty on each trial, indicating that the source of information about difficulty is not solely controlled by stimulus characteristics but also by internal variability that affects how reliable the evidence is to the decision-maker (Fleming & Dolan, 2012; Kiani & Shadlen, 2009). Also, neural recordings in rats proposed that a confidence estimate might be a basic and pervasive element of decision making, and the likelihood of a successful trial outcome may theoretically be calculated using a subjective indicator of decision-making confidence (Shuler & Bear, 2006).
Given the importance of confidence in decision making, however, the relationship between confidence and time perception is lacking in the literature (Bruno et al., 2023; Salem-Garcia et al., 2023). The current study investigated whether differences in confidence determine how participants perceive time in a monetary context. To estimate whether confidence distorts duration judgments, physically and mentally healthy individuals were tested. Verifying previous findings (Failing & Theeuwes, 2016; Giersch & Coull, 2018), we demonstrated that the perceived duration of the monetary gain condition was perceived as longer than the neutral condition and that confidence, as the perceived likelihood of being correct, was higher in a monetary gain scenario than in neutral and loss scenarios. We found that individual differences in confidence influenced duration judgments. For almost half of the participants, perceived duration was influenced by confidence but not for the other half. Participants with high-confidence perceived the monetary gain condition as longer than the neutral condition, whereas participants with low confidence did not. We also found a correlation between time perception and confidence level, where high confidence individuals were more engaged by the monetary gain contexts, which may lead to duration overestimation. Moreover, linear regression analysis revealed a stronger relationship between confidence of 450 ms and time perception and that confidence of maximum uncertainty at the duration of 450 ms is predictive of the perceived time in the monetary gain condition, and the more confident participants are in their longer responses at the 450 ms, the time will be more overestimated.
2. Material and Methods
Participants
Twenty-four healthy volunteers (18 females, aged 19–37 years, mean = 23.01, all right-handed, all with normal or corrected-to-normal vision) were recruited from the campus of the Heinrich-Heine-University Düsseldorf. None had a history of psychiatric or neurological disease, and none were taking any drugs or medication at the time of testing. The sample size was based on previous similar studies (Apaydın et al., 2018; Failing & Theeuwes, 2016; Lebreton et al., 2019). Written informed consent was obtained from all participants prior to participation in the study. Participants were tested in exchange for monetary compensation (€10 per hour) or course credits, and were additionally compensated based on their performance in a randomly selected phase. This study was approved by the local ethics committee of the Department of Psychology, Heinrich-Heine-University, Düsseldorf in accordance with the Declaration of Helsinki.
Stimuli
Adapted from the monetary incentive delay (MID) task (Knutson et al., 2000), the monetary incentive was an ecological picture of 50 cents (€0.5) outlined in blue, red, or gray colors signaled gain, loss, and no monetary outcome, respectively (Figure 1). For half of the participants, the gain cue was presented in blue and the loss in red; for the other half, the colors were reversed. The neutral cue was presented in gray for all participants. A white circle sized (1.93º) was used as both reference and probe stimulus. All stimuli were presented on screen (LCD, 27 inches, 240 Hz NVIDIA’s G-Sync, Acer XB272) with a resolution of 1920 × 1080 pixel and a refresh rate of 60 Hz. Stimuli were presented with Presentation V2.4. Each stimulus was centered on the screen with a homogeneous dark gray background (Figure 1).
Figure 1.
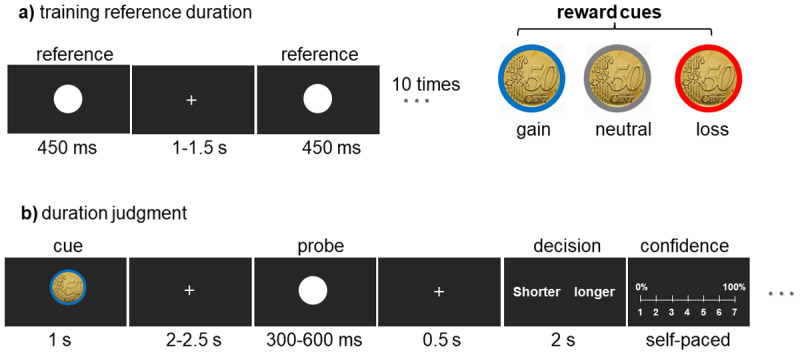
Experiment set-up. (a) Training reference duration (approximately 2 min). After a 5 s fixation cross, participants viewed a flashing white circle ten times with a random inter-trial interval (1–1.5 s) to learn the reference duration of (450 ms). (b) An example trial of the duration judgment task. The probe circle was selected from 300, 350, 400, 450, 500, 550, or 600 ms, followed by a monetary incentive cue of 1 s in either the blue, red, or gray color with a random fixation (2–2.5 s) in between. Participants responded to the decision probe within a 2 s time limit. They pressed a mouse button to indicate that the perceived probe duration was longer or shorter than the reference duration. Each trial was scored on a Likert scale from 1 (0% confident) to 7 (100% confident) to obtain a confidence score.
Experimental procedure and design
Instructions
Participants were given instructions for the entire experiment while sitting 70 cm away from the computer screen in a dimly lit room. To maintain participants’ motivation throughout the experiment, they were told that the reward outcome would be determined cumulatively according to their performance and that the final reward would be based on only one randomly selected phase. All participants completed a practice version of the task for approximately 2 min or until they demonstrated proficiency in the task before beginning the first phase.
Task Design
Participants performed a prospective duration judgment (Zakay & Block, 2004) experiment divided into five phases, all performed on a single day and separated by short rest intervals. In each phase, they first learned a reference duration (450 ms) presented by a flash of a white circle (Figure 1a) ten times with a variable interval (1–1.5 s). The training phase was performed before each main phase. Immediately after the training reference period, the main phase began, consisting of 63 trials (~10 min). During each trial, participants saw one of the three €0.5 monetary incentive cues (red, blue, or gray) for (1 s). After a random fixation delay of (2–2.5 s), the probe stimulus flashed for a variable duration drawn from 6 equiprobable durations (300–600 ms in 50 ms steps). After a (0.5 s) fixation delay, a decision screen appeared. Participants had (2 s) to respond whether they perceived the probe duration shorter or longer than the reference (two-alternative forced-choice task (Figure 1b). They registered their responses by clicking the left or right mouse button with their index and middle fingers, and the experiment continued as soon as participants pressed one of the two response buttons within the response time. A self-paced 1–7 Likert scale was added at the end of each trial, and participants were asked to indicate their decision confidence by moving the mouse from 1 (0%, not at all certain) to 7 (100%, definitely certain). Finally, the probe screen was replaced by a central fixation cross that jittered randomly in the range (3–7 s). They were also instructed to fixate the fixation point throughout the phases. Note that only the first trial in each phase started after a fixed (5 s) fixation delay. No feedback was provided for participants to avoid stress (Treadway et al., 2013) and learning effects on memory (Matthews & Meck, 2016). A single value was used for reward and punishment conditions to avoid the parametric effect of the motivational effect associated with the size of a potential reward (Pessiglione et al., 2007; Tom et al., 2007).
Supplementary information
The placement of probe texts (longer or shorter) was counterbalanced across trials. Onset times, response accuracy, and reaction times were recorded using Presentation V2.4. Reward outcomes were determined by task performance on each trial; participants gained 0.5 cents for a correct response in the gain condition and lost 0.5 cents for an incorrect or missed response in the loss condition. The neutral condition had no effect on reward outcomes. In the practice version, only the easy target durations (300 and 600 ms) were tested ten times (five times each) in a random order. In the main phases, all target durations were tested equally (9 times per phase). Participants were informed that only the target duration would vary in the display, while the incentives would be displayed for a fixed duration throughout the experiment. Each participant completed 315 trials. The duration of the experiment was approximately 60 min.
Analysis and psychometric function
We analyzed the proportion that participants reported the probe stimuli lasting longer than the standard. From this data, we estimated the psychometric curve for each reward condition separately implemented in quickpsy (Linares & López-Moliner, 2016) (http://dlinares.org/quickpsy.html). The point of subjective equality (PSE: the temporal duration at which participants felt equal to the reference temporal duration, i.e., the 50% probability to report the probe lasting longer) and slope were calculated for each condition.
The point of subjective equality (PSE) represents the specific perception at which participants perceive the probe stimulus as being equal to the standard stimulus. In this study, it is the point at which there is a 50% chance that the participant will judge the probe stimulus to be longer or shorter than the standard stimulus.”
Each participant’s PSE and slope for each reward condition (gain, loss, and neutral) were used for statistical significance testing using paired-samples t tests once normality was demonstrated (Shapiro–Wilk test). If the assumption of normality was violated, a nonparametric Wilcoxon signed-rank test was used. Note that in these cases, the median (Mdn) is reported instead of the mean and standard deviation. All statistics were calculated on the basis of 95% confidence interval (95% CI).
3. Results
Effect of reward on time perception
To establish a foundation for our subsequent investigation into the effect of confidence on time perception—the central hypothesis and primary research question—we need a foundational replication to examine how monetary reward gain alters the perception of time. To investigate whether reward gain influences time perception, we compared the Point of Subjective Equality (PSEs) between reward gain and neutral conditions. The PSE for gain had lower values for outcome (M = .462, SD = .03) than the PSE for neutral (M = .472, SD = .03). A paired-samples t test revealed that this difference was statistically significant; t(17) = –2.76, p = .0067 (one-tailed), CI [–.018, –.002], Cohen’s dav = .32 (Figure 2). Additionally, a paired-samples t test revealed no statistically significant differences between loss and neutral conditions; (M = .465 vs. M = .472), t(17) = –1.33, p = .201, CI [–.018, .004], Cohen’s dav = .24, as well as between gain and loss; (M = .462 vs. M = .465), t(17) = –.68, p = .505, CI [–.013, .007], Cohen’s dav = .11, suggesting that participants perceived only the monetary gain condition to last longer than the neutral condition. A Wilcoxon signed-rank test revealed no differences in the slope values between gain and neutral (Mdn = 106.195 vs. Mdn = 110.678), z = –1.33, p = .196, r = –.357, between gain and loss (Mdn = 106.195 vs. Mdn = 103.397), z = –1.023, p = .325, r = –.275, and between loss and neutral (Mdn = 103.397 vs. Mdn = 110.678), z = .631, p = .551, r = –.17, (Figure 2). This analysis confirms that the duration is overestimated exclusively in the gain condition when compared to the neutral condition. We introduced the loss condition to explore potential similarities in the impact of salience between attaining gain and avoiding loss. However, our results did not show significant differences between the loss and neutral conditions. The decision to use paired t test was deliberate, aimed at confirming the difference between the gain and neutral condition, which was a necessary step before addressing our main research question.
Figure 2.
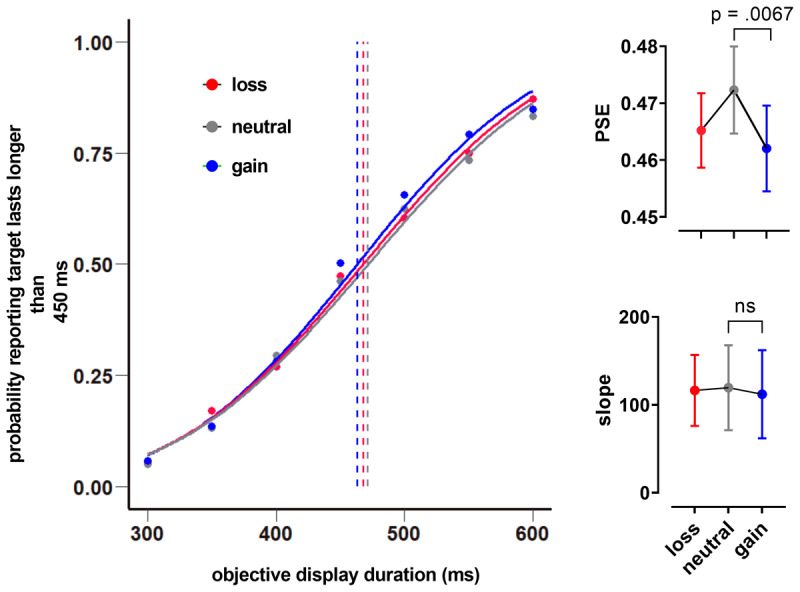
Result of reward influence on time perception. Probability of responses in which participants reported that the target duration lasted longer than the reference (450 ms) plotted as a function of the display duration for each reward condition, separately (gain, neutral, and loss). The points of subjective equality (PSE) are shifted to the left in the gain and loss conditions compared to the neutral condition. The PSE for reward conditions shows a significant difference between gain and neutral (top right). The slope values of the fitted psychometric function show a non-significant difference between gain and neutral (bottom right). (Note: bar graphs show mean and standard error).
Effect of reward on reaction time of perception (RTP)
We averaged reaction time scores of correct answers for each reward condition (gain, loss, and neutral) across six probe durations (300, 350, 400, 500, 550, 600 ms), (Figure 3a, left) and reaction time of answers that participants perceived to be longer on probe 450 ms, which is equal to reference duration (Figure 3a, right), separately. The mean RTP of correct responses was highest in the gain group (M = .604, SD = .17), followed by the neutral (M = .594, SD = .15) and loss (M = .589, SD = .15) conditions. A one-way analysis of variance (ANOVA) using the Welch F-ratio showed that this difference was not statistically significant, F(.596, 15055) = 0, p = 1, ή2p = 0. The median value for RTP of (450 ms) was highest in the loss group (Mdn = .593), followed by the neutral (Mdn = .580) and gain (Mdn = .577) groups. A Kruskal-Wallis test showed that this difference was not statistically significant, H = .01, p = .995, ηH = .04.
Figure 3.
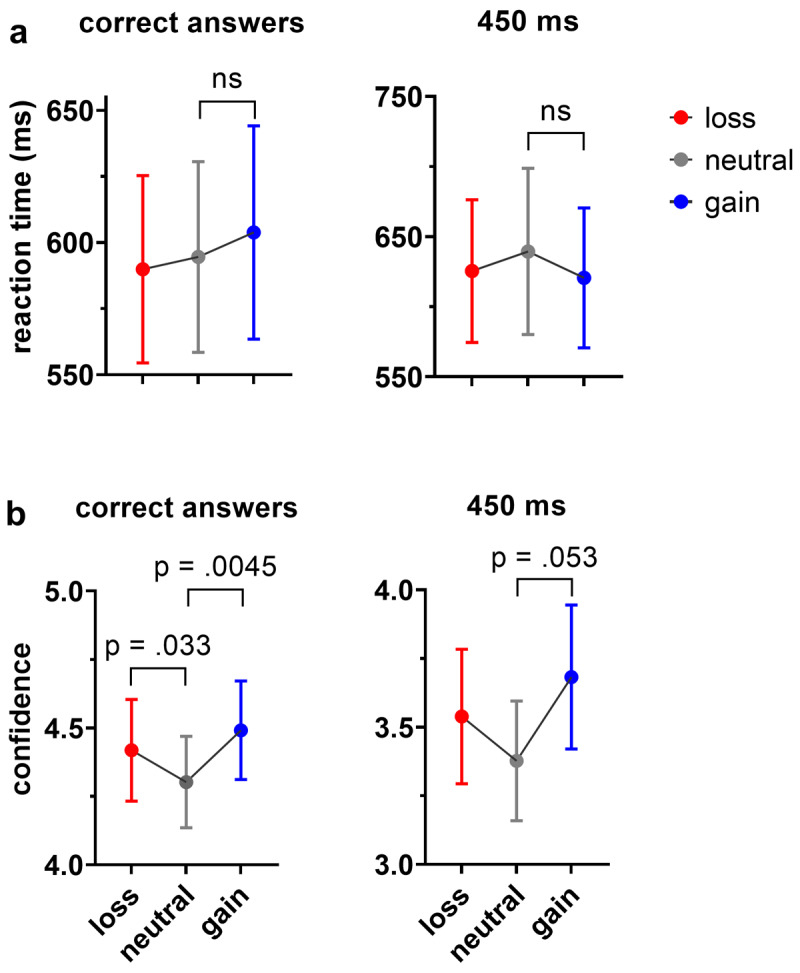
Reward influence on reaction time and confidence. (a) Mean values of reaction time across all probe durations, except for 450 ms, are shown on the left, while mean values of reaction time perceived as longer for 450 ms are shown on the right. (b) Mean confidence scores for all probe durations, except for 450 ms, are depicted on the left, and mean values of confidence ratings perceived as longer only for 450 ms are presented on the right. (The 450 ms is plotted separately since it is equal to the reference duration and no correct response is given for this duration).
Effect of reward on confidence
To examine the effect of the independent variable of reward on the dependent variable of confidence, we averaged the confidence scores of correct answers for each reward condition (gain, loss, and neutral) at six probe durations (300, 350, 400, 500, 550, and 600 ms), and the confidence scores of answers that participants perceived to be longer on probe 450 ms, which is equal to the fixed reference duration (Figure 3b, left). Note that the main comparison was between gain and neutral conditions as our foundational hypothesis. The median confidence score for correct answers was highest in the gain condition (Mdn = 4.51), followed by the loss (Mdn = 4.27) and neutral (Mdn = 4.09) conditions. A Wilcoxon signed-rank test showed that the difference between the gain and neutral conditions, z = 2.55, p = .0045 (one-tailed), r = –.42, and between loss and neutral conditions, z = 1.85, p = .033 (one-tailed), r = –.31 were statistically significant. The same test showed that the difference between gain and loss conditions was not statistically significant; z = 1.37, p = .182, r = –.23, (Figure 3b, left). Note that the comparison between the loss and neutral conditions was included for completeness. Although the Wilcoxon signed-rank test indicated a significant difference (p = .033, one-tailed), this significance may not hold under a two-tailed test, as the direction was not defined a priori. Switching from a one-tailed to a two-tailed test can double the p-value, potentially rendering it non-significant. However, this adjustment is not necessary for the gain versus neutral comparison. We included this information to further demonstrate the robustness of our hypothesis.”
Similarly, at the 450 ms, the median confidence for answers that were perceived longer was highest in the gain condition (Mdn = 3.79), followed by the loss (Mdn = 3.5) and neutral (Mdn = 3.29) conditions. A Wilcoxon signed-rank test showed that the difference between gain and neutral conditions was marginally significant; z = –1.63, p = .053 (one-tailed), r = –.27. The same test showed that the difference between gain and loss conditions, z = –.544, p = .3 (one-tailed), r = –.15, as well as between loss and neutral conditions z = .69, p = .25 (one-tailed), r = .2, were not statistically significant. This result indicates that the gain condition is perceived to last longer than the neutral condition with higher certainty. This pattern was even mirrored at the minimal perceptual (maximal uncertainty) level at a probe duration of 450 ms, although it reached a marginal level of significance (Figure 3b, right).
Effect of confidence on time perception in the reward context
Grouping individuals
In the previous sections, we found that time was perceived as lasting longer in the monetary gain condition than in the neutral condition, and that confidence was significantly higher in the monetary gain condition than in the neutral condition. However, based on this data alone, we cannot conclude that confidence influenced the perceived duration. To disentangle the role of confidence, we made confidence an independent variable by dividing participants into two groups: high confidence (HC) and low confidence (LC). We classified participants based on their overall mean confidence scores for the probe duration of maximum uncertainty (450 ms, where the probe equals the reference duration) for the neutral condition (no gain and no loss). Please note that there are no correct answers for this probe, so we calculated the overall confidence level for both shorter and longer answers for the classification purpose. It’s crucial to note that the purpose of Figure 4 is illustrative rather than serving as a representation of statistical tests. The following statistical tests and Figure 4 are designed to demonstrate the effectiveness of the grouping method employed in our study. To observe whether the participants’ classification was reliable, we compared the confidence level of the perception of 450 ms (longer hits; participants pressed longer choice) in the neutral condition. The HC group had higher confidence scores (M = 3.93, SD = .88) than the LC group (M = 2.82, SD = .6). An independent samples t test showed that this difference was statistically significant; t(14.07) = 3.12, p = .008 (Figure 4a, left). This pattern was repeated over other conditions as well, the HC group had higher confidence scores for gain (M = 4.52, SD = .76) than the LC group (M = 2.85, SD = .69). An independent samples t test showed that this difference was statistically significant, t(15.86) = 4.89, p = .0002, and the HC group had higher confidence scores for the loss condition (M = 4.17, SD = .59) than the LC group (M = 2.9, SD = 1.02). An independent samples t test showed that this difference was statistically significant; t(12.78) = 3.24, p = .007 (Figure 4a, left).
Figure 4.
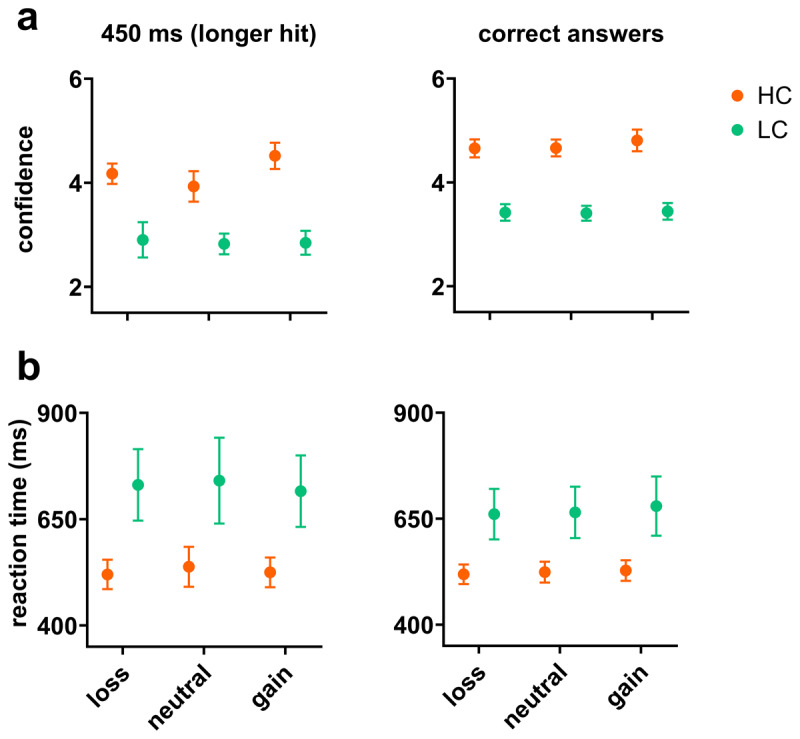
Comparing confidence scores and reaction times: High vs. Low Confidence Groups. (a) Group differences in confidence scores between LC and HC participants for 450 ms and correct answers. (b) Group differences in reaction times between LC and HC participants for 450 ms and correct answers. (Note: bar graphs are plotted based on the mean and standard error).
The overall pattern was also replicated at the confidence scores for correct answers. The HC group had higher scores than the LC group for gain (M = 5.1, SD = .64 vs. M = 3.88, SD = .64); t(16) = 4.06, p = .0001, for neutral (M = 4.93, SD = .57 vs. M = 3.67, SD = .43); t(14.92) = 5.26, p = .00018, and for loss (M = 5, SD = .66 vs. M = 3.84, SD = .6); t(15.86) = 3.91, p = .001 (Figure 4a, right).
We also compared the RTP of the two groups. The HC group responded faster than the LC group in all reward conditions and both correct answers and 450 ms (longer hits). The statistical test showed that all differences were significant, p < .001. This indicates that the individuals are well split based on their minimal perceptual level in neutral condition (Figure 4b) (Ais et al., 2016).
Effect of reward gain on time perception within HC and LC groups
The previous section confirmed that the two groups were satisfactorily classified. To investigate whether reward influences time perception, we compared the PSEs between reward conditions in each group separately. In the HC group, PSE neutral had higher scores for outcome (M = .463, SD = .024) than PSE gain (M = .447, SD = .031). A paired-samples t test showed that this difference was statistically significant; t(8) = 3.57, p = .004 (one-tailed), CI [–.026, –.006], Cohen’s dav = .58 (Figure 5, left). Comparisons between (gain vs. loss) and (loss vs. neutral) revealed no differences. The PSE loss had higher scores for outcome (M = .457, SD = .031) than the PSE gain (M = .447, SD = .031). A paired-samples t test showed that this difference was not statistically significant; t(8) = –1.58, p = .153, Cl [–.025, .005], Cohen’s dav = .33. Similarly, PSE neutral had higher scores for the outcome (M = .463, SD = .024) than the loss (M = .457, SD = .031). A paired-samples t test showed this difference was not statistically significant; t(8) = –.76, p = .466, Cl [–.023, .012], Cohen’s dav = .21. In the LC group, Wilcoxon signed-rank test showed that comparisons between reward conditions were not statistically significant; PSEs (neutral vs. gain); z = –.18, p = .196 (one-tailed), r = –.04, (gain vs. loss); z = –.53, p = .346, r = –.13, and (neutral vs. loss); z = –1.01, p = .240, r = –.24 (Figure 5, right). The overall pattern of the psychometric plot fitted to all participants is replicated and similar to the pattern of the psychometric plot of high-confident individuals. This pattern is not replicated for low-confident participants, meaning that the two groups perceived time differently and that only HC participants perceived the monetary gain condition as lasting longer than the neutral condition, not LC participants.
Figure 5.
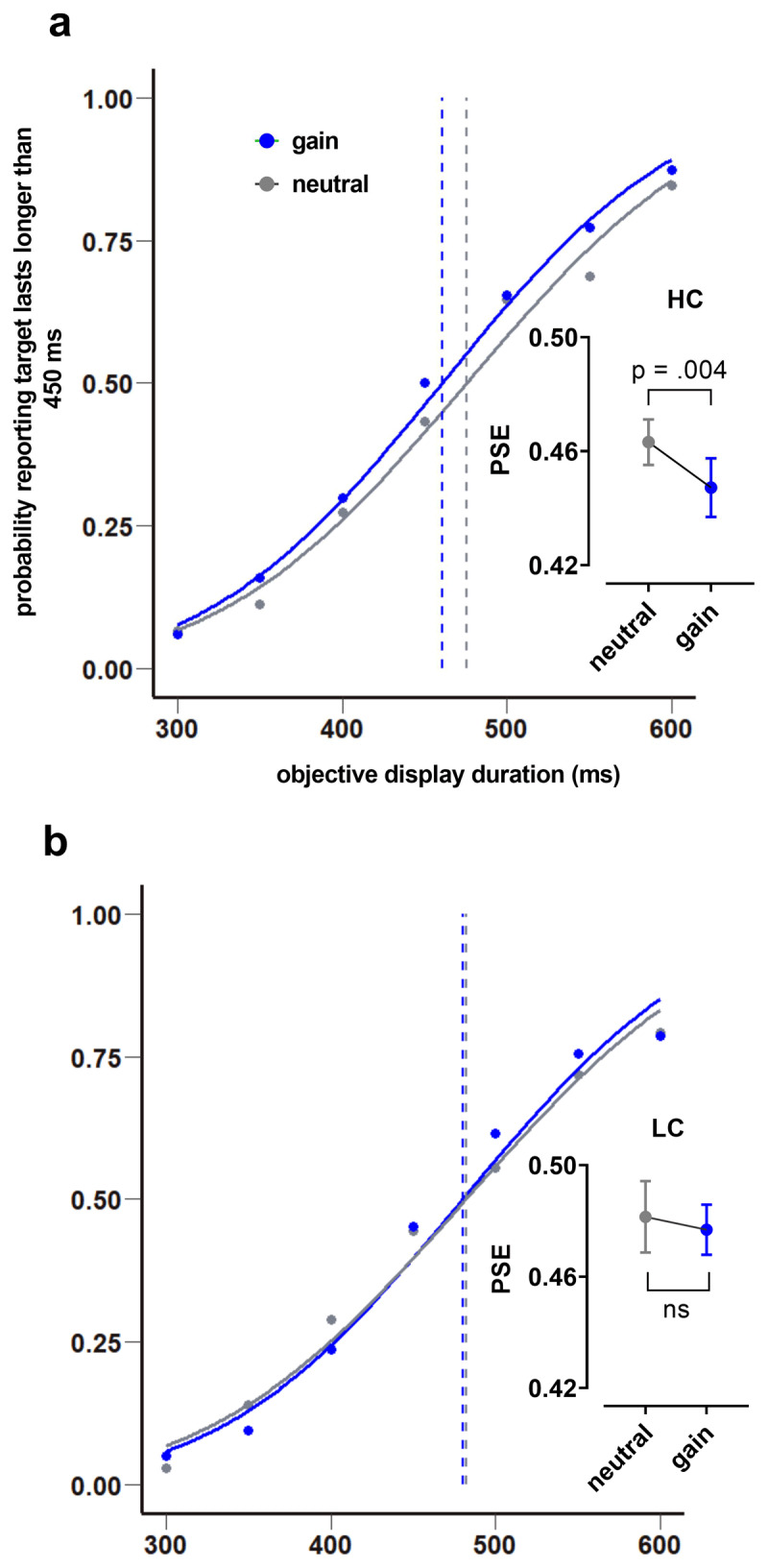
Effect of reward gain on time perception within HC and LC groups. Comparison of PSEs between gain and neutral conditions within HC (a), and LC (b) groups are plotted with corresponding psychometric functions. The PSE is shifted to the left in the gain compared to neutral condition only in HC group, not in LC group. Comparison of the mean of PSEs between gain and neutral shows significant difference in HC group (a). (Note: bar graphs are plotted based on the mean and standard error).
Effect of reward gain on time perception between HC and LC groups
The LC group had higher scores for PSE gain (M = .48, SD = .03) than the HC group (M = .45, SD = .03). An independent sample t test showed this difference was statistically significant; t(15.71) = –2.17, p = .023 (one-tailed), CI [–.059, –.001] (Figure 6). The LC group had higher scores for PSE neutral (M = .48, SD = .04) than the HC group (M = .46, SD = .02). An independent sample t test showed that this difference was not statistically significant; t(13.41) = –1.21, p = .123 (one-tailed), CI [–.051, .014]. The LC group had higher scores for PSE loss (M = .473, SD = .02) than the HC group (M = .457, SD = .03). An independent sample t test showed that this difference was not statistically significant; t(14.77) = –1.2, p = .124 (one-tailed), CI [–.043, .012]. These results indicate that group with high confidence level perceived the duration of gain condition longer than group with low confidence (Figure 6).
Figure 6.
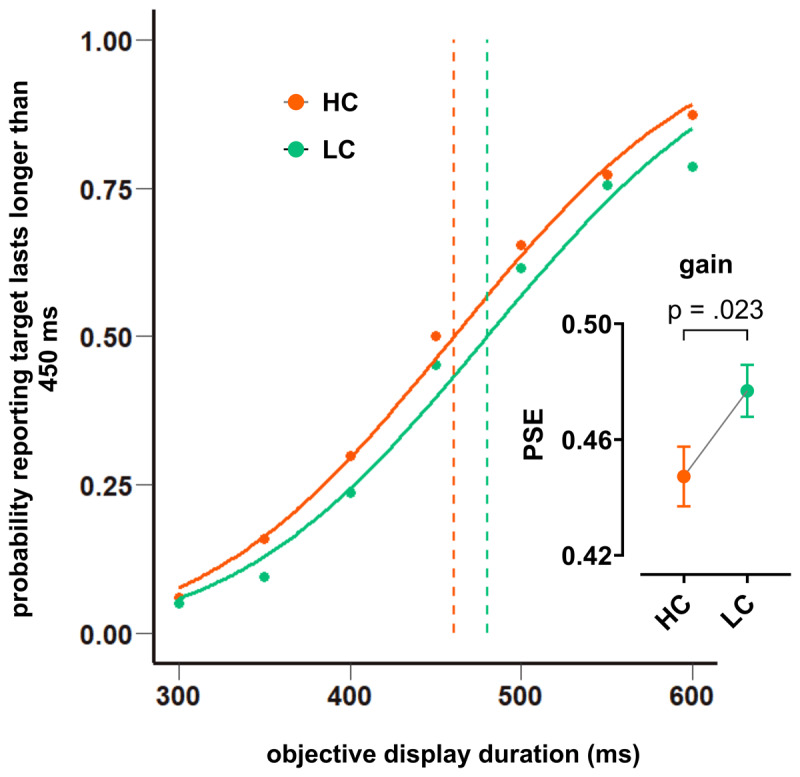
Effect of reward gain on time perception between HC and LC groups. Comparison of PSEs between the HC and LC groups in the gain condition are plotted with corresponding psychometric functions. The PSE is shifted to the left in the HC compared to LC group. The comparison of the means of PSEs between the HC and LC groups indicates significant difference in between groups. (Note: bar graph is plotted based on the mean and standard error).
Correlation and regression analyses
To examine the relationship between confidence and time perception in the monetary gain condition (condition of interest), Pearson’s R correlations (negative correlation) were performed between the PSE and confidence at the maximum uncertainty level (450 ms) and between the PSE and confidence of correct answers. The relationship between the PSE and confidence (450 ms) variables was found to be statistically significant; r(16) = –.57, 95% Cl [–.82, –.143], p = .0067 (Figure 7a), as well as between the PSE and confidence at correct answers; r(16) = –.44, CI[–.752, .036], p = .034 (Figure 7b). Nonsignificant results were found between the PSE and confidence variables in other conditions of rewards. Additionally, a linear regression analysis was conducted to examine whether the confidence variable predicted PSE at the gain condition. The PSE gain condition was significantly predicted by confidence at the maximum uncertainty 450 ms (longer hits). The results of the regression model indicated that the confidence level of 450 ms explained 32.68% of the variance, and an overall collective significant effect was found; R2 = .33, F(1, 16) = 7.77, p = .013. The PSE gain condition was not significantly predicted by the confidence of correct answers.; R2 = .19, F(1,16) = 3.81, p = .069. Moreover, further negative correlations were performed between RTP and confidence. The relationship between the RTP and confidence (450 ms, longer hits) variables was found to be statistically significant; r(16) = –.595, p = .015 (corrected for multiple comparisons). No significant results were found between other conditions (p > .05). This means that HC group was faster and more confident than LC in their responses.
Figure 7.
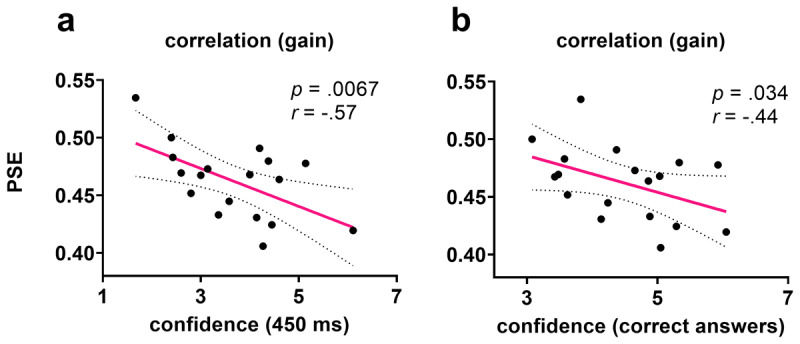
Correlation between confidence and time perception. (a) Pearson correlation (one-tail) between confidence (450 ms) and PSE in the gain condition. (b) Pearson correlation (one-tailed) between confidence (correct answers) and PSE in the gain condition.
Early-late trials no effect on confidence
To determine whether there was a primacy or recency effect in the early trials on confidence scores, we split the data into two halves and compared confidence levels between the early and late halves. There were no significant results (p > .05) between the two halves of the data when all probe durations were considered (300–600, step = 50 ms), even with more stringent probe durations (400, 450, and 500 ms), (p > .05). This confirms that participants performed the task well without fading the reference duration in their minds. In other words, if there was a significant difference between the confidence levels of early and late trials, a second experiment should have been designed and conducted in a different way, such as testing the reference and probes on a trial-by-trial basis, or by making the phases shorter (Mo, 1974).
Discussion
The primary aim of this study is to investigate whether the overestimation of time duration is attributable to the reward gain condition or the metacognitive profile (confidence) of the participants in the gain condition. We found a relationship between confidence, monetary reward, and time perception. The higher the degree of confidence, the longer time was perceived in a situation of financial gain compared to neutral.
First, we replicated findings from previous studies showing that monetary reward has an effect on perceived time: the gain condition was perceived as lasting longer than the neutral condition. Evidently, the monetary gain condition had a smaller PSE than the neutral condition. This result is consistent with related studies that emphasized that modulations in time perception are due to the increased attentional deployment caused by the monetary gain condition (Anderson, 2016; Chelazzi et al., 2013). They argued that high-money condition recruits subjective salience (Hickey et al., 2010) and therefore attracts more attention than low- or no-money. The greater increase in attentional deployment causes time to be perceived as extended. Arguably, in our study, the money loss condition had lower PSEs than the neutral condition, although they did not reach a significant level. This is inconsistent with studies that have found a positive relationship between arousal level and duration overestimation (Chib et al., 2012; Gil & Droit-Volet, 2012; Lake et al., 2016) because reward processing studies have shown that anticipation of monetary loss, which facilitates avoidance behavior, involves the same arousal level as anticipation of monetary reward, which facilitates approach behavior (Knutson et al., 2000). The current results can be discussed as that monetary gain and loss (Chib et al., 2012) conditions attract more attention from the valence gate than the arousal gate (robust reward processing studies, introduced that monetary incentives arising from the two orthogonal components, namely, arousal (from calm to excited) and valence (from pleasurable to aversive)), subject to the basic asymmetry in attentional choice between saving and earning money (Hu et al., 2018). This suggests that altered time perception is based on specific mechanism of reward valence as one of the main components of Monetary Incentive Delay task, which may play an important role in the regulation of gaining behavior and decision making.
Next, we showed that the monetary reward influenced confidence. The monetary gain condition received a significantly higher confidence score compared to the neutral condition for correct answers and even for the probe 450 ms, albeit being marginally significant (p = .053). This finding is consistent with a study showing that participants were more confident in their decisions when learning to seek monetary gains than when learning to avoid monetary losses, despite equal difficulty and performance between these two contexts (Lebreton et al., 2019). Neuroimaging studies (Diekhof et al., 2012; Kable & Glimcher, 2007) have demonstrated that confidence is biased toward gain and seems to be beneficial for monetary payouts (Fleming, Whiteley, et al., 2010). For example, a study demonstrated that confidence and difference in value are separate behavioral manifestations of the same underlying decision variable (Bartra et al., 2013; Rouault & Fleming, 2020). Also, a neurophysiology study found that decision certainty is encoded by the same neurons that reflect decision formation (Kiani & Shadlen, 2009), and in our study participants were more confident while making decisions about the duration judgment of the gain condition compared to other conditions.
Third, we took a step forward and revealed how confidence levels affect time perception. We grouped participants into high and low confidence individuals, and the findings remained unaffected for the high-confidence (HC) group. HC participants overestimated gain condition compared with the neutral condition. Previous studies showed that the correlation between confidence and objective performance varies for different people and is related to individual differences in brain structure (Fleming, Weil, et al., 2010) and connectivity (Allen et al., 2018), and to individual differences in mental calculation confidence (Navajas et al., 2017). Individual differences in confidence may also be related to differences in brain activity and neurochemistry. For example, studies have shown that high confidence individuals show increased activity in brain regions associated with reward and motivation, such as the ventral striatum (Diekhof et al., 2012). In contrast, individuals with low levels of confidence may exhibit reduced activity in these brain regions. Another possible explanation can be the regulatory focus theory that explains differences in strategic tendencies between individuals in their sensitivity to gains and losses, which result in variations in how they address problems (Higgins, 1997). Promotion-focused individuals have strong sensitivity to positive outcomes—gains and non-gains. In contrast, prevention-focused individuals have a high sensitivity to negative outcomes—non-losses and losses. To make an analogy, in our study HC group might be considered as individuals with a promotion focus and LC group as prevention focus, and this difference in their strategic tendencies causes a fundamental difference in their confidence level and subsequently the perceived time. This finding can be discussed by a very recent study that found a distinct pattern of inter-individual variations between individuals who used only perceptual differences to score their confidence and people who additionally used information that had no bearing on their discriminating judgments (Knutson et al., 2000). It is also possible to emphasize that our data imply that HC participants made all decisions in goods space, and in value comparisons HC group valued more gain trials than other conditions (De Martino et al., 2013; Wunderlich et al., 2010).
Finally, we found a negative correlation between confidence of correct answers and time perception and a regression between confidence of maximum uncertainty at 450 ms and time perception on gain condition across all participants. This means that the more the participants are confident in their correct answers, the longer the perceived time. Similarly, the more the participants are confident in their errors, the longer the time perceived. Notably, the confidence in error trials that are perceived longer is predictive of time perception. This may be indicative of the metacognitive ability, and that in lower confidence is associated with higher metacognitive ability about knowing their errors (Mazancieux et al., 2020; Rouault et al., 2018).
In conclusion, individual differences in confidence levels can significantly influence how individuals perceive time in a monetary context. Understanding these differences is essential for developing effective strategies for motivating and engaging individuals in a range of settings, such as education, healthcare, and management. By identifying the factors that contribute to individual differences in confidence levels, we can develop tailored interventions that can enhance an individual’s motivation, focus, and ability to complete tasks successfully.
Data Accessibility Statement
https://osf.io/zjvdk/files/osfstorage/65f2312a852037104c880c0c.
Funding Statement
This research was supported by the European Research Council (project moreSense grant agreement n. 757184).
Ethics and Consent
All experiments were in accordance with the Declaration of Helsinki and were approved by the local ethics committee of the Faculty of Mathematics and Natural Sciences of Heinrich Heine University, Düsseldorf (Identification No. 757184).
Competing Interests
The authors have no competing interests to declare.
Author Contributions
E.Z. Funding acquisition, Project administration, Supervision.
M.TS. designing research, performing research, analyzing data, and writing the paper.
References
- 1.Adcock, R. A., Thangavel, A., Whitfield-Gabrieli, S., Knutson, B., & Gabrieli, J. D. E. (2006). Reward-Motivated Learning: Mesolimbic Activation Precedes Memory Formation. Neuron, 50(3), 507–517. 10.1016/j.neuron.2006.03.036 [DOI] [PubMed] [Google Scholar]
- 2.Ais, J., Zylberberg, A., Barttfeld, P., & Sigman, M. (2016). Individual consistency in the accuracy and distribution of confidence judgments. Cognition, 146, 377–386. 10.1016/j.cognition.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 3.Allen, E. A., Damaraju, E., Eichele, T., Wu, L., & Calhoun, V. D. (2018). EEG Signatures of Dynamic Functional Network Connectivity States. Brain Topography, 31(1), 101–116. 10.1007/s10548-017-0546-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, B. A. (2015). Value-driven attentional priority is context specific. Psychonomic Bulletin & Review, 22(3), 750–756. 10.3758/s13423-014-0724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson, B. A. (2016). The attention habit: How reward learning shapes attentional selection: The attention habit. Annals of the New York Academy of Sciences, 1369(1), 24–39. 10.1111/nyas.12957 [DOI] [PubMed] [Google Scholar]
- 6.Apaydın, N., Üstün, S., Kale, E. H., Çelikağ, İ., Özgüven, H. D., Baskak, B., & Çiçek, M. (2018). Neural Mechanisms Underlying Time Perception and Reward Anticipation. Frontiers in Human Neuroscience, 12, 115. 10.3389/fnhum.2018.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barthelmé, S., & Mamassian, P. (2010). Flexible mechanisms underlie the evaluation of visual confidence. Proceedings of the National Academy of Sciences, 107(48), 20834–20839. 10.1073/pnas.1007704107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartra, O., McGuire, J. T., & Kable, J. W. (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge, K. C. (2004). Motivation concepts in behavioral neuroscience. Physiology & Behavior, 81(2), 179–209. 10.1016/j.physbeh.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 10.Bruno, A., Sudkamp, J., & Souto, D. (2023). A metacognitive approach to the study of motion-induced duration biases reveals inter-individual differences in forming confidence judgments. Journal of Vision, 23(3), 15. 10.1167/jov.23.3.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chelazzi, L., Perlato, A., Santandrea, E., & Della Libera, C. (2013). Rewards teach visual selective attention. Vision Research, 85, 58–72. 10.1016/j.visres.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 12.Chib, V. S., De Martino, B., Shimojo, S., & O’Doherty, J. P. (2012). Neural Mechanisms Underlying Paradoxical Performance for Monetary Incentives Are Driven by Loss Aversion. Neuron, 74(3), 582–594. 10.1016/j.neuron.2012.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbetta, M., Miezin, F., Dobmeyer, S., Shulman, G., & Petersen, S. (1991). Selective and divided attention during visual discriminations of shape, color, and speed: Functional anatomy by positron emission tomography. The Journal of Neuroscience, 11(8), 2383–2402. 10.1523/JNEUROSCI.11-08-02383.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel, L. & López-Moliner, Joan. (n.d.). quickpsy: An R Package to Fit Psychometric Functions for Multiple Groups (Version Aug-2016) [Computer software]. 10.32614/CRAN.package.quickpsy [DOI] [Google Scholar]
- 15.De Martino, B., Fleming, S. M., Garrett, N., & Dolan, R. J. (2013). Confidence in value-based choice. Nature Neuroscience, 16(1), 105–110. 10.1038/nn.3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgado, M. R., & Dickerson, K. C. (2012). Reward-Related Learning via Multiple Memory Systems. Biological Psychiatry, 72(2), 134–141. 10.1016/j.biopsych.2012.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della Libera, C., & Chelazzi, L. (2009). Learning to Attend and to Ignore Is a Matter of Gains and Losses. Psychological Science, 20(6), 778–784. 10.1111/j.1467-9280.2009.02360.x [DOI] [PubMed] [Google Scholar]
- 18.Diekhof, E. K., Kaps, L., Falkai, P., & Gruber, O. (2012). The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude – An activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia, 50(7), 1252–1266. 10.1016/j.neuropsychologia.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 19.Droit-Volet, S., & Wearden, J. (2016). Passage of Time Judgments Are Not Duration Judgments: Evidence from a Study Using Experience Sampling Methodology. Frontiers in Psychology, 7. 10.3389/fpsyg.2016.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eagleman, D. M., Tse, P. U., Buonomano, D., Janssen, P., Nobre, A. C., & Holcombe, A. O. (2005). Time and the Brain: How Subjective Time Relates to Neural Time. The Journal of Neuroscience, 25(45), 10369–10371. 10.1523/JNEUROSCI.3487-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Failing, M., & Theeuwes, J. (2016). Reward alters the perception of time. In Cognition (Vol. 148, pp. 19–26). 10.1016/j.cognition.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 22.Failing, M., & Theeuwes, J. (2018). Selection history: How reward modulates selectivity of visual attention. Psychonomic Bulletin & Review, 25(2), 514–538. 10.3758/s13423-017-1380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldmann-Wüstefeld, T., Brandhofer, R., & Schubö, A. (2016). Rewarded visual items capture attention only in heterogeneous contexts. Psychophysiology, 53(7), 1063–1073. 10.1111/psyp.12641 [DOI] [PubMed] [Google Scholar]
- 24.Fleming, S. M., & Dolan, R. J. (2012). The neural basis of metacognitive ability. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1594), 1338–1349. 10.1098/rstb.2011.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming, S. M., Weil, R. S., Nagy, Z., Dolan, R. J., & Rees, G. (2010). Relating Introspective Accuracy to Individual Differences in Brain Structure. Science, 329(5998), 1541–1543. 10.1126/science.1191883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleming, S. M., Whiteley, L., Hulme, O. J., Sahani, M., & Dolan, R. J. (2010). Effects of Category-Specific Costs on Neural Systems for Perceptual Decision-Making. Journal of Neurophysiology, 103(6), 3238–3247. 10.1152/jn.01084.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontes, R., Ribeiro, J., Gupta, D. S., Machado, D., Lopes-Júnior, F., Magalhães, F., Bastos, V. H., Rocha, K., Marinho, V., Lima, G., Velasques, B., Ribeiro, P., Orsini, M., Pessoa, B., Araujo Leite, M. A., & Teixeira, S. (2016). Time perception mechanisms at central nervous system. Neurology International, 8(1). 10.4081/ni.2016.5939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frijda, N. H. (2010). Impulsive action and motivation. Biological Psychology, 84(3), 570–579. 10.1016/j.biopsycho.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 29.Giersch, A., & Coull, J. T. (2018). TRF1: It Was the Best of Time (s). Timing & Time Perception, 6, 317–318. 10.1163/22134468-00603001 [DOI] [Google Scholar]
- 30.Gil, S., & Droit-Volet, S. (2012). Emotional time distortions: The fundamental role of arousal. Cognition & Emotion, 26(5), 847–862. 10.1080/02699931.2011.625401 [DOI] [PubMed] [Google Scholar]
- 31.Hickey, C., Chelazzi, L., & Theeuwes, J. (2010). Reward Changes Salience in Human Vision via the Anterior Cingulate. The Journal of Neuroscience, 30(33), 11096–11103. 10.1523/JNEUROSCI.1026-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins, E. T. (1997). Beyond pleasure and pain. American Psychologist, 52(12), 1280–1300. 10.1037/0003-066X.52.12.1280 [DOI] [PubMed] [Google Scholar]
- 33.Hu, K., De Rosa, E., & Anderson, A. K. (2018). Differential temporal salience of earning and saving. Nature Communications, 9(1), 2843. 10.1038/s41467-018-05201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivry, R. B., & Schlerf, J. E. (2008). Dedicated and intrinsic models of time perception. Trends in Cognitive Sciences, 12(7), 273–280. 10.1016/j.tics.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kable, J. W., & Glimcher, P. W. (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience, 10(12), 1625–1633. 10.1038/nn2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kepecs, A., & Mainen, Z. F. (2012). A computational framework for the study of confidence in humans and animals. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1594), 1322–1337. 10.1098/rstb.2012.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kepecs, A., Uchida, N., Zariwala, H. A., & Mainen, Z. F. (2008). Neural correlates, computation and behavioural impact of decision confidence. Nature, 455(7210), 227–231. 10.1038/nature07200 [DOI] [PubMed] [Google Scholar]
- 38.Kiani, R., & Shadlen, M. N. (2009). Representation of Confidence Associated with a Decision by Neurons in the Parietal Cortex. Science, 324(5928), 759–764. 10.1126/science.1169405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knutson, B., Westdorp, A., Kaiser, E., & Hommer, D. (2000). FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. NeuroImage, 12(1), 20–27. 10.1006/nimg.2000.0593 [DOI] [PubMed] [Google Scholar]
- 40.Kragel, P. A., Kano, M., Van Oudenhove, L., Ly, H. G., Dupont, P., Rubio, A., Delon-Martin, C., Bonaz, B. L., Manuck, S. B., Gianaros, P. J., Ceko, M., Reynolds Losin, E. A., Woo, C.-W., Nichols, T. E., & Wager, T. D. (2018). Generalizable representations of pain, cognitive control, and negative emotion in medial frontal cortex. Nature Neuroscience, 21(2), 283–289. 10.1038/s41593-017-0051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lake, J. I., LaBar, K. S., & Meck, W. H. (2016). Emotional modulation of interval timing and time perception. Neuroscience & Biobehavioral Reviews, 64, 403–420. 10.1016/j.neubiorev.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Pelley, M. E., Pearson, D., Griffiths, O., & Beesley, T. (2015). When goals conflict with values: Counterproductive attentional and oculomotor capture by reward-related stimuli. Journal of Experimental Psychology: General, 144(1), 158–171. 10.1037/xge0000037 [DOI] [PubMed] [Google Scholar]
- 43.Le Pelley, M. E., Seabrooke, T., Kennedy, B. L., Pearson, D., & Most, S. B. (2017). Miss it and miss out: Counterproductive nonspatial attentional capture by task-irrelevant, value-related stimuli. Attention, Perception, & Psychophysics, 79(6), 1628–1642. 10.3758/s13414-017-1346-1 [DOI] [PubMed] [Google Scholar]
- 44.Lebreton, M., Bacily, K., Palminteri, S., & Engelmann, J. B. (2019). Contextual influence on confidence judgments in human reinforcement learning. PLOS Computational Biology, 15(4), e1006973. 10.1371/journal.pcbi.1006973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libera, C. D., & Chelazzi, L. (2006). Visual Selective Attention and the Effects of Monetary Rewards. Psychological Science, 17(3), 222–227. 10.1111/j.1467-9280.2006.01689.x [DOI] [PubMed] [Google Scholar]
- 46.Makwana, M., & Srinivasan, N. (2017). Intended outcome expands in time. Scientific Reports, 7(1), 6305. 10.1038/s41598-017-05803-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matthews, W. J., & Meck, W. H. (2016). Temporal cognition: Connecting subjective time to perception, attention, and memory. Psychological Bulletin, 142(8), 865–907. 10.1037/bul0000045 [DOI] [PubMed] [Google Scholar]
- 48.Mazancieux, A., Fleming, S. M., Souchay, C., & Moulin, C. J. A. (2020). Is there a G factor for metacognition? Correlations in retrospective metacognitive sensitivity across tasks. Journal of Experimental Psychology: General, 149(9), 1788–1799. 10.1037/xge0000746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mo, S. S. (1974). Comparative judgment of temporal duration as a function of numerosity. Bulletin of the Psychonomic Society, 3(5), 377–379. 10.3758/BF03333504 [DOI] [Google Scholar]
- 50.Mobbs, D., Yu, R., Meyer, M., Passamonti, L., Seymour, B., Calder, A. J., Schweizer, S., Frith, C. D., & Dalgleish, T. (2009). A Key Role for Similarity in Vicarious Reward. Science, 324(5929), 900–900. 10.1126/science.1170539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murayama, K., Matsumoto, M., Izuma, K., & Matsumoto, K. (2010). Neural basis of the undermining effect of monetary reward on intrinsic motivation. Proceedings of the National Academy of Sciences, 107(49), 20911–20916. 10.1073/pnas.1013305107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navajas, J., Hindocha, C., Foda, H., Keramati, M., Latham, P. E., & Bahrami, B. (2017). The idiosyncratic nature of confidence. Nature Human Behaviour, 1(11), 810–818. 10.1038/s41562-017-0215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pessiglione, M., Schmidt, L., Draganski, B., Kalisch, R., Lau, H., Dolan, R. J., & Frith, C. D. (2007). How the Brain Translates Money into Force: A Neuroimaging Study of Subliminal Motivation. Science, 316(5826), 904–906. 10.1126/science.1140459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pessoa, L. (2010). Embedding reward signals into perception and cognition. Frontiers in Neuroscience, 4. 10.3389/fnins.2010.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rouault, M., & Fleming, S. M. (2020). Formation of global self-beliefs in the human brain. Proceedings of the National Academy of Sciences, 117(44), 27268–27276. 10.1073/pnas.2003094117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rouault, M., McWilliams, A., Allen, M. G., & Fleming, S. M. (2018). Human Metacognition Across Domains: Insights from Individual Differences and Neuroimaging. Personality Neuroscience, 1, e17. 10.1017/pen.2018.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salem-Garcia, N., Palminteri, S., & Lebreton, M. (2023). Linking confidence biases to reinforcement-learning processes. Psychological Review, 130(4), 1017–1043. 10.1037/rev0000424 [DOI] [PubMed] [Google Scholar]
- 58.Shuler, M. G., & Bear, M. F. (2006). Reward Timing in the Primary Visual Cortex. Science, 311(5767), 1606–1609. 10.1126/science.1123513 [DOI] [PubMed] [Google Scholar]
- 59.Tom, S. M., Fox, C. R., Trepel, C., & Poldrack, R. A. (2007). The Neural Basis of Loss Aversion in Decision-Making Under Risk. Science, 315(5811), 515–518. 10.1126/science.1134239 [DOI] [PubMed] [Google Scholar]
- 60.Treadway, M. T., Buckholtz, J. W., & Zald, D. H. (2013). Perceived stress predicts altered reward and loss feedback processing in medial prefrontal cortex. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wittmann, M. (2013). The inner sense of time: How the brain creates a representation of duration. Nature Reviews Neuroscience, 14(3), 217–223. 10.1038/nrn3452 [DOI] [PubMed] [Google Scholar]
- 62.Wittmann, M., & Paulus, M. P. (2008). Decision making, impulsivity and time perception. Trends in Cognitive Sciences, 12(1), 7–12. 10.1016/j.tics.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 63.Wunderlich, K., Rangel, A., & O’Doherty, J. P. (2010). Economic choices can be made using only stimulus values. Proceedings of the National Academy of Sciences, 107(34), 15005–15010. 10.1073/pnas.1002258107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zakay, D., & Block, R. (2004). Prospective and retrospective duration judgments: An executive-control perspective. Acta Neurobiologiae Experimentalis, 64(3), 319–328. 10.55782/ane-2004-1516 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
https://osf.io/zjvdk/files/osfstorage/65f2312a852037104c880c0c.


