Abstract
Fresh passion fruit is sensitive to chilling injury (CI) during storage at improper low temperature of 5 °C, which lowers the fruit quality and limits its shelf life. The present study aimed to determine the impacts of melatonin on CI development of passion fruit in relation to antioxidant ability and membrane lipid metabolism during refrigeration. In present study, passion fruit was treated with 0.50 mmol L−1 melatonin and distilled water (control) for 20 min, hereafter stockpiled at 5 °C. The results indicated that, in storage, melatonin-treated passion fruit showed the lower CI index and cell membrane permeability, lower superoxide anion production rate and malondialdehyde level, greater activities of catalase, superoxide dismutase and ascorbate peroxidase, higher levels of ascorbic acid and glutathione, and higher 1, 1-diphenyl-2-picrylhydrazyl radical scavenging capacity than control passion fruit. Besides, lower membrane lipid-degrading enzyme activities, lower contents of phosphatidic acid and saturated fatty acids (SFAs), higher levels of phosphatidylcholine, phosphatidylinositol and unsaturated fatty acids (USFAs), and greater ratio of USFAs to SFAs and index of USFAs were revealed in melatonin-treated passions than control passions. Thus, these results indicated that melatonin retained cell membrane structure via boosting antioxidant capacity and restricting membrane lipid degradation, accordingly increased the chilling resistance and delayed the CI development in fresh passion fruit.
Keywords: Passion fruit, Melatonin, Chilling injury, Antioxidant ability, Membrane lipid metabolism
Graphical abstract
Highlights
-
•
Melatonin rose chilling tolerance of cold-stored passion fruit.
-
•
Melatonin stabilized cell membrane integrity of cold-stored passion fruit.
-
•
Melatonin raised antioxidant ability and lowered ROS of cold-stored passion fruit.
-
•
Melatonin reduced level of membrane lipid metabolism of cold-stored passion fruit.
1. Introduction
Passion (Passiflora caerulea L.) fruit produces an egg-shaped fruit in subtropical and tropical areas (You et al., 2022). It has unique aroma and complex flavors, and contains nutrients such as phenolics, vitamins, and minerals (Rinaldi et al., 2019; Zhou et al., 2022; Wang et al., 2023a). However, after harvest, passion fruit is prone to rapid postharvest decay due to dehydration, shrinkage, or disease (You et al., 2022; Zhou et al., 2022). To prevent the fruit decay, low temperature storage is effective in preserving passion fruit quality (Sang and Hai, 2020). However, our previous observation revealed that an improper low temperature of 5 °C might cause chilling stress, and induce chilling injury (CI) symptoms, thus expediting quality deterioration in fresh passion fruit. Therefore, it is necessary to understand the mechanism of CI to retain the quality in fresh passion fruit.
The cell membrane affects the quality in fresh produce (López et al., 2021; Wang et al., 2020; Fan et al., 2022; Chen et al., 2024). An intact cell membrane is a key factor in increasing chilling tolerance to retard CI of harvested fruits (López-López et al., 2023; Song et al., 2022; Yi et al., 2024). The structure of the cell membrane is regulated by an antioxidant system (Chen et al., 2023; Zhang et al., 2023). The antioxidant enzymes, like ascorbate peroxidase (APX), superoxide dismutase (SOD) and catalase (CAT), as well as antioxidants, such as glutathione (GSH) and ascorbic acid (AsA), can affect antioxidant capacity and reactive oxygen species (ROS) elimination (Fan et al., 2024; Lin et al., 2024; Yi et al., 2024). Furthermore, the enhanced ROS and imbalanced ROS generation and scavenging can induce the oxidation reactions, and aggravate the low temperature injury. However, the treatments of γ-aminobutyric acid (GABA) (Fan et al., 2024), sodium hydrosulfide (Yi et al., 2024) or cold shock (Nian et al., 2022) might raise the activities of antioxidant enzymes, or increase the amounts of antioxidants, which enhance antioxidant ability but lower ROS level, thus slowing the CI of fresh produce. Indeed, the changed antioxidant levels acted as key role in CI development of fresh produce.
Additionally, membrane lipid metabolism affects cell membrane integrity in fresh produce (Hong et al., 2023; Li et al., 2023; Xu et al., 2023; Lin et al., 2024). The cell membrane can get damaged due to increased membrane lipid metabolism, namely, the disassembly of membrane lipid like phospholipids or unsaturated fatty acids (USFAs), which could result from the combined actions of membrane lipid-degrading enzymes (MLDEs), such as lipoxygenase (LOX), lipase or phospholipase D (PLD) (Lin et al., 2019; Wang et al., 2020; Li et al., 2023). Furthermore, under the chilling temperature, the lipid degradation and peroxidation occur, then affect the cell membrane integrity, causing the membrane damage (Li et al., 2023). However, the treatments of chitooligosaccharide (Han et al., 2023) or 24-epibrassinolide (Hu et al., 2022) lowered the membrane lipid metabolism, and then maintained the membrane lipid levels, and thus delayed the CI of fresh produce. Therefore, it was vital to evaluate the role of membrane lipid metabolism in regulating CI in fresh fruit. These findings elucidated that antioxidant level and membrane lipid metabolism could affect the CI development in fresh produce.
Melatonin is a naturally produced indole amine, which participates in some physiological processes, such as seed germination, fruit ripening, and senescence, etc. (Wang et al., 2020; Chen et al., 2022). Also, melatonin acts as a ROS scavenger for the removal of ROS, and then maintains the cells in a steady state (Wang et al., 2022). Furthermore, it had been indicated that melatonin might reinforce the plant defense responses to cold stress. Such as, melatonin might retain the levels of cell membrane lipid components, thus reducing the CI of bananas (Wang et al., 2022). Also, melatonin-enhanced pear fruit chilling tolerance might be associated with the regulations of AsA metabolism and antioxidant ability (Liu et al., 2024). Furthermore, Chen et al. (2022) reported that melatonin retained the cell membrane integrity, to enhance the defense response to low temperature stress, thus delaying the CI of guavas. So, we hypothesized that melatonin might have a potential capacity to delay passion fruit CI development by regulating the cell membrane structure and components, and antioxidant ability. So far, studies are limited on the impacts of melatonin in suppressing CI in fresh passion fruit by maintaining cell membrane structure through regulating antioxidant capacity and membrane lipid metabolism. Therefore, the present study aimed to evaluate the effectiveness of melatonin in mitigating CI in fresh passion fruit stored at an inappropriate low temperature. The metabolisms of antioxidant and membrane lipid were examined to identify mechanisms involved in stabilizing cell membrane, enhancing chilling tolerance, and reducing CI in passion fruit treated by melatonin.
2. Materials and methods
2.1. Passion fruit
Passion fruit cv. Qinmi NO. 9 (chromaticity levels of L∗, a∗ and b∗ respectively were 58.15 ± 1.64, −17.82 ± 0.59, and 41.56 ± 0.49) was obtained from passion orchard (Huian, Fujian, China) during the commercial maturity stage (Maturity was 90%). Fruits were transported to the laboratory and sorting was done to exclude wounded or diseased fruit. The passion fruit were then cleaned with distilled water and adopted for the next treatment.
2.2. The prior screening experiment
In the prior screening experiment, melatonin with different concentrations of 0 (control), 0.25, 0.50, 0.75 and 1.00 mmol L−1 were respectively adopted for treating fresh passions for 20 min, and hereafter chilled (5 °C). The results indicated that, during storage, the 0.50 mmol L−1 melatonin-treated group represented the optimal effectiveness in delaying the CI symptom of fresh passions than the control group and other melatonin-treated groups. On day 42, the order of CI index of passions in each group was 0.71 (0.50 mmol L−1) < 0.76 (0.75 mmol L−1) < 0.79 (1.00 mmol L−1) < 0.80 (0.25 mmol L−1) < 0.82 (control), respectively. So, this specific concentration of melatonin with 0.50 mmol L−1 was used in the current work.
2.3. The postharvest melatonin treatments
A total of thirty passion fruit were used to evaluate fruit attributes at the beginning of storage. The 360 passion fruit were separated evenly into two batches. One batch was soaked in 0.50 mmol L−1 melatonin for 20 min. The other batch was submerged in distilled water without melatonin for 20 min and was considered as a control group. After treatments, all passion fruit were air-dried for about 1 h, packed in polyethylene film bags with 0.02 mm thickness (ten fruit per bag). For the bag, its osmotic coefficients of O2, moisture and CO2 respectively were about 15.0 ± 0.2 L (m2·d·atm)−1, 250 ± 10 g (m2·d·atm) −1, and 50.0 ± 0.3 L (m2·d·atm) −1, respectively. Then, these fruits were stored at 5 °C with relative humidity 85-90% for forty-two days. Every seven days, the fruit (three bags) from each batch were removed to assess the CI and related metabolic indices of passion fruit. Furthermore, the equatorial surface of passion pericarp was sampled for the determination of following indicators.
2.4. Evaluation of CI index
The 10 passion fruit were adopted to appraise the fruit CI index, which was assessed visually based on fruit surface percentage that exhibited browning or pitting with the scale from 0 to 4: 0 (none), 1 (1–10 %), 2 (11–25 %), 3 (26–50 %), and 4 (≥51 %), according to Chen et al. (2022) and Han et al. (2023). The index of CI was computed as Σ (CI scale × fruit number in each scale)/(4 × total fruit number).
2.5. Assay of cell membrane permeability (CMP)
Based on the procedure of Chen et al. (2022), thirty round pericarp pieces (0.2 cm2) from five fruit pericarp in equatorial position were employed for determining the relative leakage rate that estimated a CMP level. The unit was denoted as a percentage (%).
2.6. Determination of superoxide anion (O2−.) production rate and malondialdehyde (MDA) content
Based on the procedure of Lin et al. (2020), 1 g of pericarp tissue from five passions was ground by the 5 mL of phosphate buffered saline (PBS) (50 mM, pH 7.8) including 1 mM ethylene diamine tetraacetic acid (EDTA). After centrifuged, the obtained supernatant was used to assay O2−. production rate, with a unit of μmol g−1 min−1 based on fresh weight. Furthermore, pericarp tissue (1 g) from 5 passions was ground via the 10 mL of trichloroacetic acid (TCA), then centrifuged. The MDA content was assayed by obtained supernatant, with a unit of nmol g−1 based on fresh weight.
2.7. Assay of antioxidant enzyme and MLDE activities
Based on the literature of Lin et al. (2020), 1 g of pericarp tissue from 5 passion fruit was used to acquire supernatant by grinding with 10 mL of 50 mM PBS (pH 7.0). The above supernatant was adopted for the assay of antioxidant enzyme activities (CAT, APX and SOD).
Furthermore, the activities of MLDEs (e.g., lipase, LOX and PLD) were determined by referring to literatures of Zhang et al. (2018) and Kuang et al. (2023). Pericarp tissue (1 g) from five passions was ground by 10 mL of acetic acid buffer (0.1 M, pH 5.6). After centrifugation, the supernatant was taken for a detection of PLD level. In addition, pericarp tissue (1 g) from five fruit was taken to gain supernatant via grinding with 10 mL of 0.2 M sodium phosphate buffer (pH 7.8), then adopted to an assay of lipase activity. Furthermore, pericarp tissue (1 g) from 5 passions were ground by 10 mL of sodium phosphate buffer (0.1 M, pH 6.8), hereafter centrifuged. LOX activity was assayed by collected supernatant.
Based on the Bradford (1976), the protein content of the supernatant was analyzed. The above results were shown as U mg−1 protein based on fresh weight.
2.8. Determination of antioxidant contents
Following the methodology of Lin et al. (2020), 1 g of pericarp tissue from five passions was dissolved in 10 mL of 5% TCA, and the supernatant was obtained to determine AsA amount after centrifugation. Moreover, 1 g of pericarp tissue from 5 passions was dissolved in 5% TCA containing 5 mM EDTA, and the supernatant was obtained to determine GSH amount after centrifugation. The results were mentioned as mg g−1 based on fresh weight.
2.9. Determination of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging ability
Following the methodology of Lin et al. (2020), the pericarp tissue (1 g) from 5 passion fruit was dissolved in 80% ethanol to gain supernatant, then taken to conduct DPPH radical scavenging ability. Its result was denoted in %.
2.10. Determination of phospholipid amounts of cell membrane
For contents of phospholipids, including phosphatidylcholine (PC), phosphatidic acid (PA) and phosphatidylinositol (PI), the procedures of Zhang et al. (2018) and Kuang et al. (2023) were applied for the measurement based on 5 g of pericarp tissue from 5 fruit. The above tissue was mixed with 15 mL of chloroform: methanol (2:1, v/v). After centrifugation, the chloroform phase of above admixture was gained, mixed with the acetone, and shaken. Then, the admixture was dried with N2, then mixed with chloroform: methanol (2:1, v/v) for dissolution. Finally, this solution was taken to conduct phospholipid contents. Their results were presented as mg g−1 based on fresh weight.
2.11. Assay of fatty acid amounts of cell membrane
According to the procedures of Zhang et al. (2018) and Kuang et al. (2023), 5 g of pericarp tissue from five passions were taken to dry to constant weight. The dry tissue (0.5 g) was dissolved in 2 mL of KOH (0.4 M). The admixture was mixed by 8 mL of distilled water, and 2 mL of benzene: petroleum ether (1:1, v/v), afterwards shaken. The supernatant was collected to assay amounts of fatty acids like saturated fatty acids (SFAs) and USFAs, and their results were represented by %. Moreover, index of USFAs (IUFA) and ratio of USFAs to SFAs (U/S) were also computed for evaluating the unsaturated level of cell membrane.
2.12. Statistic analyses
In present study, all indicators were determined three times. In the figure, each data was represented as the mean ± standard error (n = 3). SPSS software with version 21.0 was adopted for analyzing the gained data base on one-way ANOVA and Duncan's test. The P < 0.05 or P < 0.01 expressed the marked differences.
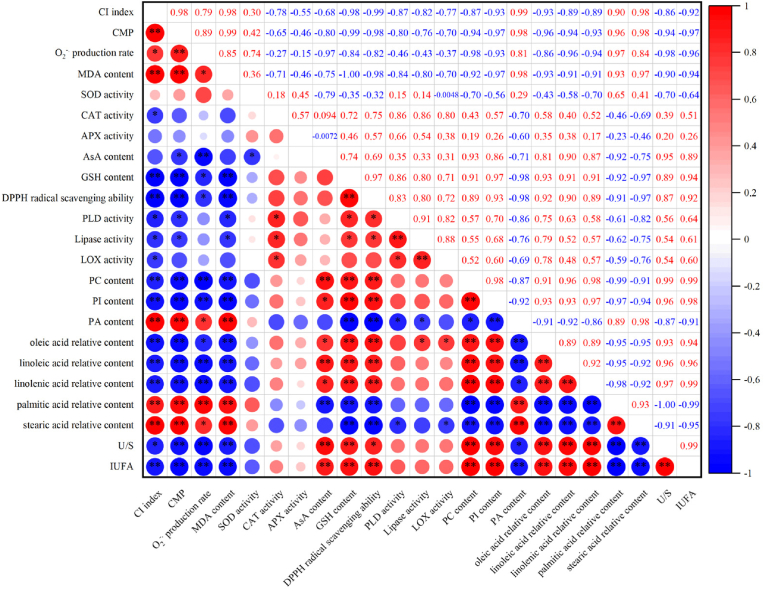
Furthermore, the correlation coefficient (r) was calculated from the average by three replicates. The Origin software with version 2022 was adopted for drawing the correlation plot. The asterisk ∗ or ∗∗ in r value indicated a correlation at P < 0.05 or P < 0.01, respectively.
3. Results
3.1. Changes in CI index and CMP
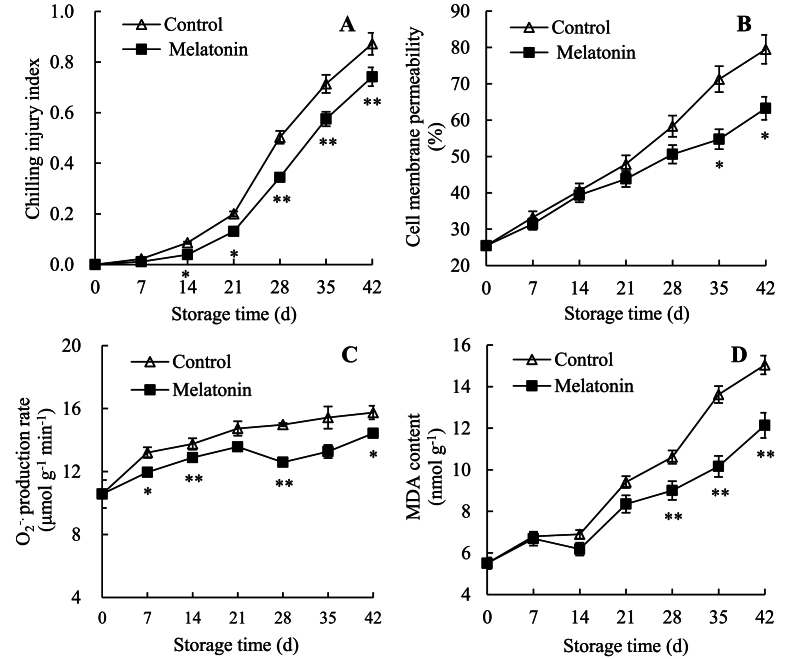
In storage, fresh passion fruit showed a rapid increase in CI index (Fig. 1A) and CMP (Fig. 1B). The statistical analyses revealed that, compared to control fruit, melatonin-treated fruit showed notably lower CI index on days 14–42, and clearly lower CMP on days 35–42. For example, on day 42, CI index in melatonin-treated group was 85.04% of control group, but CMP of melatonin-treated group was 79.59% of control group. Therefore, melatonin application could delay CI development and lower CMP in fresh passions.
Fig. 1.
Effects of melatonin on fruit chilling injury index (A), and cell membrane permeability (B), O2−. production rate (C) and MDA content (D) in pericarp of passions. Value in the figure indicated as mean ± standard error (n = 3). On same storage day, compared with control passions, the clear discrepancies in melatonin-treated passions are respectively displayed via ∗ (P < 0.05) or ∗∗ (P < 0.01). △, control group; ■, melatonin-treated group.
3.2. Changes in O2−. production rate and MDA content
Fig. 1C revealed that, in control passion fruit, O2−. production rate increased from 10.57 μmol g−1 min−1 on day 0 to 15.74 μmol g−1 min−1 on day 42. However, in melatonin-treated fruit, O2−. production rate increased from 10.57 μmol g−1 min−1 (day 0) to 14.43 μmol g−1 min−1 (day 42). Furthermore, compared to control passion fruit, melatonin-treated passion fruit retained prominently lower level on days 7–14, 28 and 42.
Fig. 1D exhibited that, in control passion fruit, MDA level increased from 5.50 nmol g−1 on day 0 to 15.04 nmol g−1 on day 42. In contrast, in melatonin-treated passion fruit, MDA level increased from 5.50 nmol g−1 (day 0) to 12.14 nmol g−1 (day 42). Furthermore, MDA level was significantly lower in the melatonin-treated passions than in the control passions on days 28–42.
Therefore, melatonin application could reduce O2−. and MDA levels in fresh passion fruit.
3.3. Changes in antioxidant enzyme activities and antioxidant substance contents
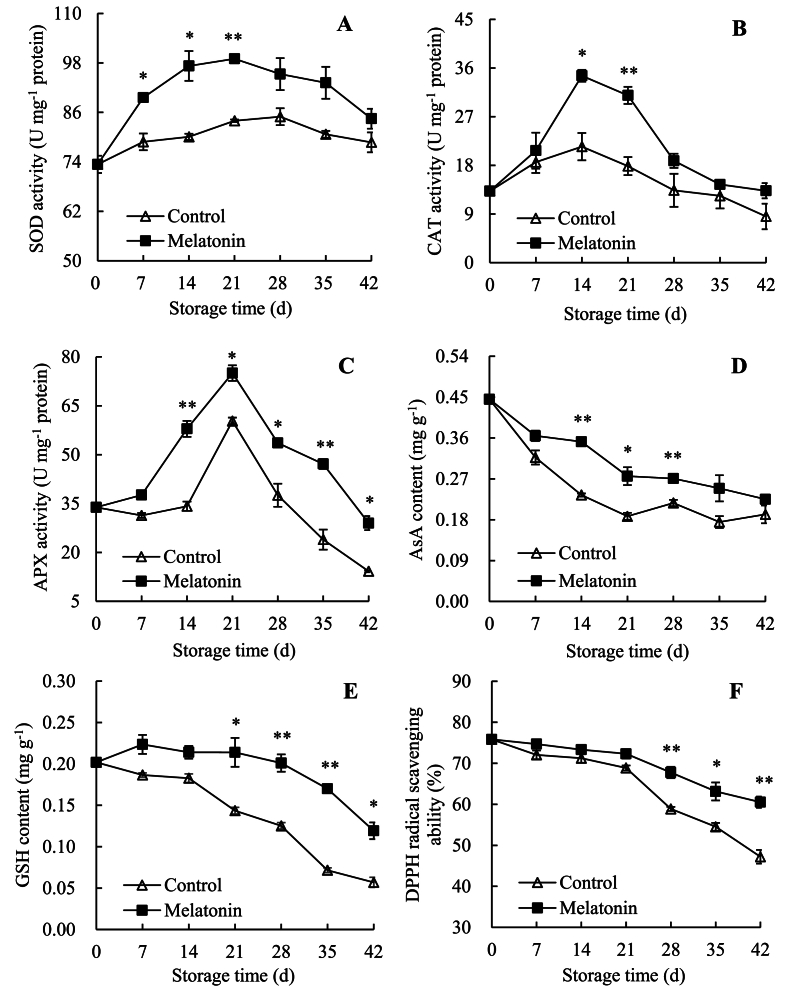
Results were revealed in Fig. 2A, in control passion fruit, SOD activity increased rapidly from day 0 and peaked on day 28 (84.96 U mg−1 protein), but decreased rapidly till day 42. However, in melatonin-treated passion fruit, SOD activity elevated from 73.40 U mg−1 protein on day 0 to 99.01 U mg−1 protein on day 21, but decreased to 84.48 U mg−1 protein on day 42. On days 7–21, SOD activity was conspicuously greater in the melatonin-treated fruit than in the control group.
Fig. 2.
Effects of AEW on activities of SOD (A), CAT (B) and APX (C), contents of AsA (D) and GSH (E), and DPPH radical scavenging ability (F) in pericarp of passions. Value in the figure indicated as mean ± standard error (n = 3). On same storage day, compared with control passions, the clear discrepancies in melatonin-treated passions are respectively displayed via ∗ (P < 0.05) or ∗∗ (P < 0.01). △, control group; ■, melatonin-treated group.
Fig. 2B exhibited that, in control passion fruit, CAT activity ascended sharply on days 0–14 but decreased rapidly after 14 d. In contrast, melatonin-treated passion fruit revealed higher CAT activity, with obvious differences on days 14–21.
Fig. 2C showed that, in control fruit, APX activity decreased slowly on days 0–7, increased slowly on days 7–14, increased sharply until day 21, and decreased rapidly after 21 d. However, in melatonin-treated fruit, APX activity increased rapidly on days 0–21, but decreased sharply on days 21–42. On days 14–42, the melatonin-treated group kept significantly higher level than the control group. Particularly, on day 42, melatonin-treated passion fruit had 1.04-fold higher activity than control passion fruit.
Fig. 2D and E revealed that the overall downtrend of AsA and GSH levels were shown in passion fruit within storage. Regarding AsA content, control group and melatonin-treated group reduced from 0.45 mg g−1 on day 0 to 0.19 mg g−1 and 0.22 mg g−1 on day 42, respectively. By contrast, melatonin-treated passion fruit represented higher level than control passion fruit, with high discrepancies on days 14–28. Regarding GSH amount, these two groups descended from 0.20 mg g−1 on day 0 to 0.06 mg g−1 and 0.12 mg g−1 on day 42, respectively. Additionally, the melatonin-treated group had markedly higher value than the control group on days 21–42.
Therefore, melatonin treatment increased the activities of antioxidant enzymes (SOD, APX, and CAT) and levels of antioxidant substances (AsA and GSH) of fresh passion fruit.
3.4. Changes in DPPH radical scavenging ability
Fig. 2F exhibited that DPPH radical scavenging ability of control fruit and melatonin-treated fruit decreased from 75.83% on day 0 to 47.21% and 60.53% on day 42, respectively. Compared to control fruit, melatonin-treated fruit exhibited higher level, with obvious differences on days 28–42. Therefore, melatonin treatment elevated the DPPH radical scavenging ability of fresh passion fruit.
3.5. Changes in MLDE activities
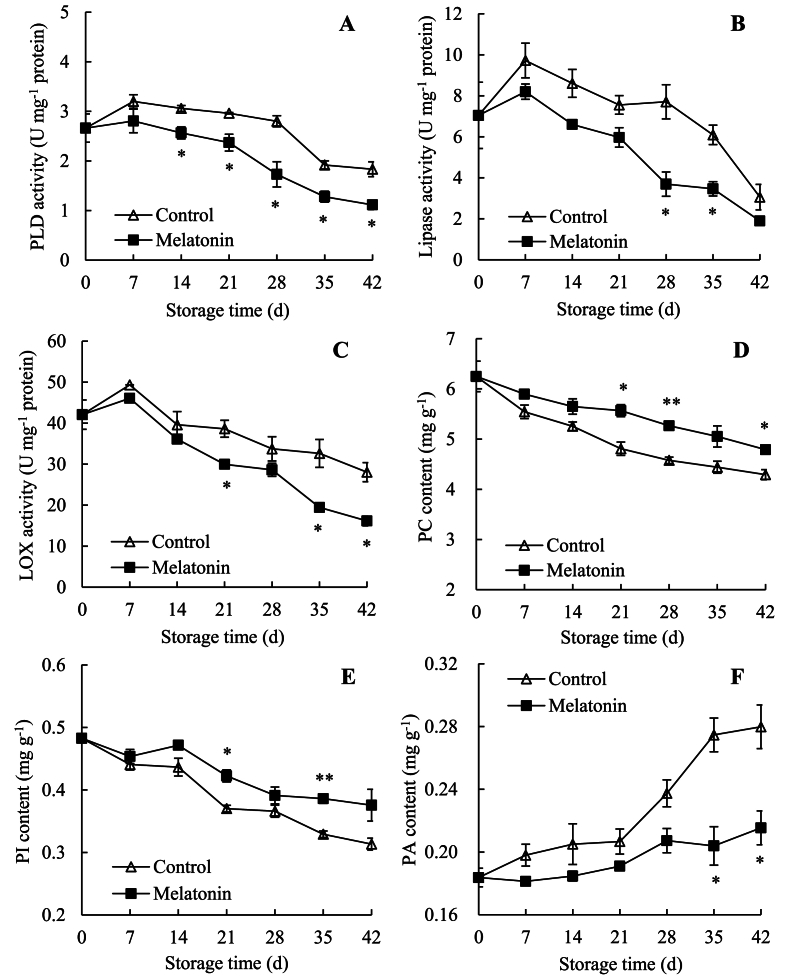
Fig. 3A indicated that PLD activity of control passion fruit elevated swiftly until it reached a peak of 3.20 U mg−1 protein on day 7 and descended to 1.83 U mg−1 protein on day 42. However, in melatonin-treated passion fruit, PLD activity increased slowly until it reached a peak of 2.81 U mg−1 protein on day 7 and descended to 1.11 U mg−1 protein on day 42. Compared with control fruit, melatonin-treated fruit displayed lower value, with marked discrepancies on days 14–42.
Fig. 3.
Effects of AEW on activities of PLD (A), lipase (B) and LOX (C), and contents of PC (D), PI (E) and PA (F) in pericarp of passions. Value in the figure indicated as mean ± standard error (n = 3). On same storage day, compared with control passions, the clear discrepancies in melatonin-treated passions are respectively displayed via ∗ (P < 0.05) or ∗∗ (P < 0.01). △, control group; ■, melatonin-treated group.
Fig. 3B revealed that lipase activity of control fruit raised rapidly within days 0–7, lowered rapidly on days 7–21, increased slowly until day 28, and decreased sharply after 28 d. While lipase activity in melatonin-treated fruit enhanced swiftly within days 0–7, but reduced sharply until 42 d. Moreover, melatonin-treated fruit revealed lower level than control fruit within storage, with marked differences on days 28–35. For example, on day 42, lipase activity of melatonin-treated passion fruit was 37.92% lower than control passion fruit.
Fig. 3C indicated that LOX activity of control fruit raised rapidly until day 7, but declined swiftly after day 7. By contrast, melatonin-treated fruit revealed lower LOX level than control passions, with clear differences on days 21 and 35–42. For instance, on day 42, LOX activity of the melatonin-treated fruit was 42.39% lower than the control fruit.
Hence, melatonin application might reduce the activities of MLDEs, such as PLD, LOX, and lipase, in fresh passion fruit.
3.6. Changes in phospholipid contents of cell membrane
The decrease in PC content was measured in passion fruit on days 0–42 (Fig. 3D). On day 42, PC content of control passion fruit was 32.11% lower than on day 0, but PC content of melatonin-treated passion fruit was 23.34% lower than on day 0. Besides, melatonin-treated group represented a higher value than control group, with marked differences on days 21–28 and day 42.
Fig. 3E indicated that, a reduction of PI content was measured in control passion fruit on days 0–42. However, PI content in melatonin-treated passion fruit decreased rapidly on days 0–7, increased rapidly within days 7–14, and decreased on days 14–42. Compared to control fruit, PI content was clearly higher in melatonin-treated fruit on days 21 and 35. On day 42, melatonin-treated group was 1.23 times of control fruit.
Fig. 3F showed that PA content in control passion fruit increased rapidly during storage. On day 42, it was 34.35% higher than on day 0. Nevertheless, in melatonin-treated passion fruit, PA content decreased slowly on days 0–7, increased rapidly until day 28, decreased rapidly until day 35, and increased rapidly after 35 d. On day 42, it was 17.26% higher than on day 0. Besides, melatonin-treated fruit had a highly lower value than control fruit on days 35–42.
Therefore, melatonin treatment suppressed the decreased PC and PI levels, and delayed the increase in PA level in fresh passion fruit.
3.7. Changes in fatty acid contents and unsaturated level of cell membrane
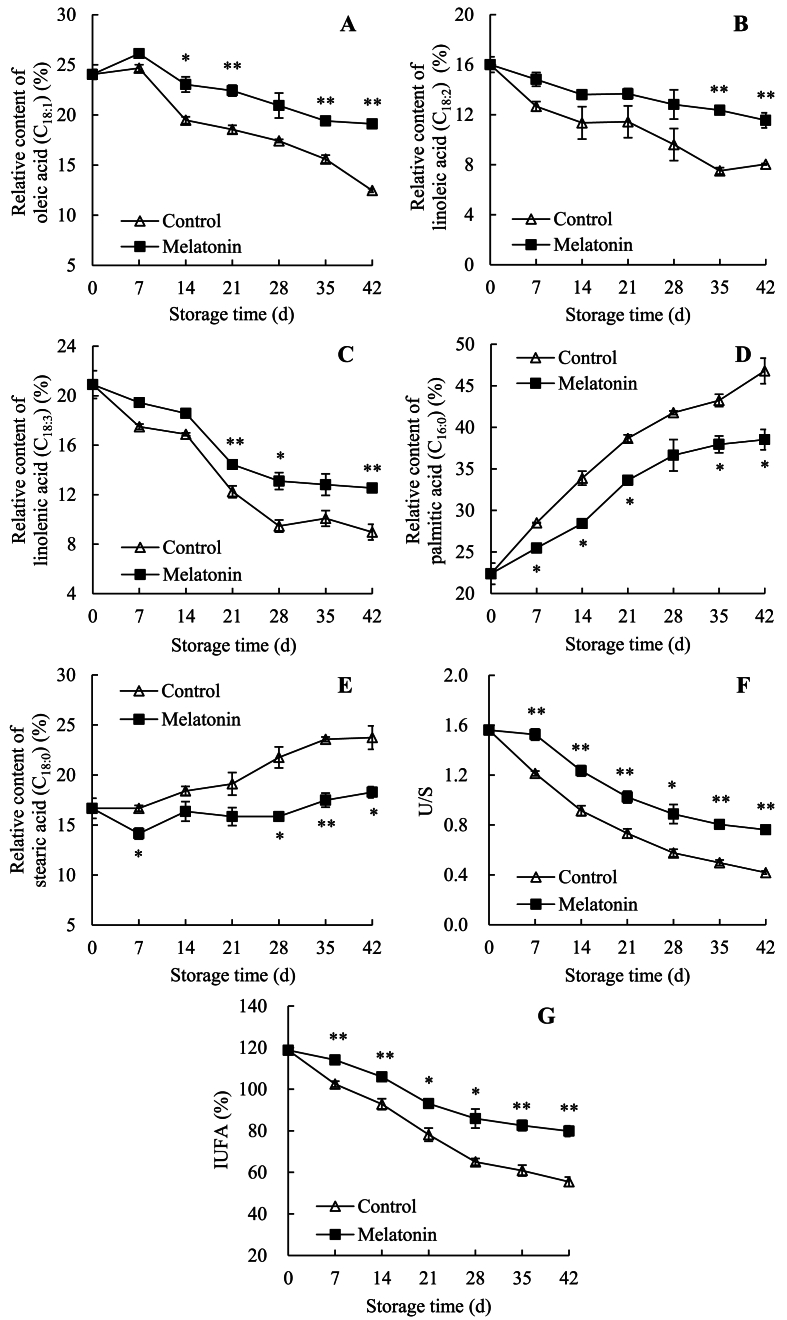
Fig. 4A showed that the oleic acid (C18:1) relative content of passion fruit expressed a rising trend within days 0–7 and a decreasing trend during days 7–42. In contrast, melatonin-treated fruit showed a greater level than control fruit within storage, with marked differences on days 14–21 and 35–42. For example, on day 42, the melatonin-treated group was 1.53 times of non-melatonin-treated fruit.
Fig. 4.
Effects of AEW on relative contents of fatty acids (A–E), and levels of U/S (F) and IUFA (G) in pericarp of passion fruit. Value in the figure indicated as mean ± standard error (n = 3). On same storage day, compared with control passions, the clear discrepancies in melatonin-treated passions are respectively displayed via ∗ (P < 0.05) or ∗∗ (P < 0.01). △, control group; ■, melatonin-treated group.
Fig. 4B showed that the linoleic acid (C18:2) relative content of control passion fruit dropped swiftly throughout days 0–14, increased slightly until day 21, decreased rapidly on days 21–35, and finally raised slowly until day 42. However, for melatonin-treated passion fruit, linoleic acid relative content displayed a similar trend on days 0–35, but decreased on days 35–42. The melatonin-treated group revealed a markedly higher value than control fruit on days 35–42. For example, on day 42, the melatonin-treated group had a 0.44-fold higher value than control fruit.
Fig. 4C revealed that the relative level of linolenic acid (C18:3) in control passion fruit descended from 20.90% on day 0 to 8.97% on day 42. However, in melatonin-treated passion fruit, linolenic acid relative content decreased from 20.90% on day 0 to 12.54% on day 42. Besides, melatonin-treated fruit displayed an obviously higher value than control fruit on days 21–28 and day 42.
Fig. 4D exhibited that, in control passion fruit, palmitic acid (C16:0) relative amount increased sharply on days 0–42. Whereas, palmitic acid relative content was lower in melatonin-treated passion fruit than in control passion fruit, with marked differences on days 7–21 and 35–42. For example, on day 42, relative amount of palmitic acid in melatonin-treated passion fruit was 82.33% of control passion fruit.
Fig. 4E exhibited that stearic acid (C18:0) relative level of control passion fruit changed a little on days 0–7, increased on days 7–35, and rose slightly on days 35–42. However, stearic acid relative content of the melatonin-treated group decreased rapidly on days 0–7, increased rapidly within days 7–14, decreased slowly until day 28, and finally increased rapidly on days 28–42. In contrast, the melatonin-treated group retained lower stearic acid relative content than the control group, with remarkable differences on days 7 and 28–42. Especially, on day 42, stearic acid relative content in the melatonin-treated group was 77.00% of the control group.
The sharp decline in U/S (Fig. 4F) and IUFA (Fig. 4G) were measured in control passion fruit during storage. Whereas, these two indices of melatonin-treated group revealed the higher levels than the control group on days 0–42, with marked differences on days 7–42. On day 42, the U/S of melatonin-treated passions was 82.30% higher than control fruit, but IUFA in melatonin-treated passions was 43.98% higher than control fruit.
Therefore, melatonin treatment slowed the reduced USFA levels (C18:1, C18:2, and C18:3), and the increased SFA levels (C16:0, and C18:0), as well as retained the unsaturated level of cell membrane in fresh passion fruit.
4. Discussion
4.1. Melatonin delayed CI development in fresh passion fruit and its relationship with cell membrane structure
The cell membrane serves as a critical barrier for the physiological function and activity of plant cells (Wang et al., 2020; Hu et al., 2022). The integrated cellular membrane acts as a key for the maintenance of membrane stability, and the response to environmental stress, such as CI (Hu et al., 2022; Xu et al., 2023). Cell membrane integrity is reflected in the level of CMP, which may be measured by the change in relative leakage rate (Wang et al., 2023b; Zhang et al., 2023). The lower CMP level was associated with the integrated cell membrane and increased chilling resistance of fresh produce. For example, the treatment with GABA (Fan et al., 2022) or cold shock (Nian et al., 2022) reduced CMP level to maintain the membrane integrity, thereby increasing cold-stress tolerance of fresh produce.
In this study, control passion fruit represented the increasing CI index (Fig. 1A) and CMP (Fig. 1B) throughout storage. By correlation analysis, CI index exhibited a positive correlation with CMP level during storage (r = 0.98∗∗) (Fig. 5). Thus, passion fruit CI development was related to damaged cell membrane. Whereas, in contrast to the control passion fruit, the melatonin-treated group exhibited lower CI index (Fig. 1A) and CMP (Fig. 1B) on days 0–42. So, these data indicated that melatonin alleviated the cell membrane damage and stabilized its structure, thereby conferring the chilling resistance, but alleviating the CI occurrence in fresh passion fruit. Similarly, Huang et al. (2024) reported that MeJA might delay the mango fruit CI development though reducing the cell membrane damages. Likewise, Yi et al. (2024) displayed that sodium hydrosulfide-reduced CMP was positively correlated with the CI attenuation in zucchini fruit.
Fig. 5.
Correlation plot of parameters involve in antioxidant ability and membrane lipid metabolism of control passions during storage. The asterisk ∗ or ∗∗ respectively reveal the correlation at level P < 0.05 or P < 0.01 between all parameters. Blue or red respectively denote the negative or positive correlation.
4.2. Melatonin delayed CI development in fresh passion fruit and its relationship with antioxidant level
Antioxidant system is disordered due to abnormal ROS production, to break the equilibrium of ROS production-elimination, thus damaging cell membranes and accelerating decay symptoms in fresh produce (Tang et al., 2021; Zhang et al., 2023). ROS, such as O2−., destabilizes the cell membrane and induces severe membrane lipid peroxidation, leading to accumulated MDA, and ultimately disrupting the normal function of plant cells (Lin et al., 2020; Lin et al., 2023). Consequently, O2−. or MDA levels are the key indicators for evaluating ROS level or membrane lipid peroxidation, respectively. The study showed that cold stress could induce excess ROS accumulation, involving lipid peroxidation and loss of cell membrane integrity, thereby resulting in CI of fresh produce (Song et al., 2022). Whereas, the lower ROS accumulation and reduced membrane lipid peroxidation could stabilize the cell membranes, and delayed the CI development in fresh produce (Ali et al., 2023; Fan et al., 2024; Song et al., 2022). Therefore, the levels of ROS and MDA could affect cell membrane integrity, which regulated CI development in fresh produce.
In this study, O2−. production rate (Fig. 1C) and MDA level (Fig. 1D) increased in control passion fruit on days 0–42. Likewise, the CI index (Fig. 1A) and CMP (Fig. 1B) in control fruit showed an increasing trend on days 0–42. Correlation analyses exhibited that, during storage, the correlations of CI index vs. O2−. production rate (r = 0.79∗), CI index vs. MDA level (r = 0.98∗∗), CMP vs. O2−. production rate (r = 0.89∗∗) and CMP vs. MDA level (r = 0.99∗∗) were positive in control passion fruit (Fig. 5). Thus, the severe CI of passion fruit correlated well with cell membrane damage due to increased ROS concentration and MDA level. On the contrary, during storage, melatonin-treated passion fruit had lower levels of O2−. (Fig. 1C) and MDA (Fig. 1D), lower CI index (Fig. 1A), and lower CMP (Fig. 1B) than control passion fruit. Therefore, we hypothesized that melatonin promoted chilling resistance and mitigated CI symptom in passion fruit, which resulted from melatonin-retained cell membrane structure through decreasing ROS content and reducing membrane lipid peroxidation. Similarly, sodium hydrosulfide could lower the O2−. and MDA contents, to alleviate the cell membrane damage, accordingly suppressing the CI development of zucchini fruit (Yi et al., 2024). Besides, melatonin might show a powerful ability for decreasing ROS level, thereby alleviating CI occurrence of eggplant fruit (Song et al., 2022).
Furthermore, to avoid oxidative stresses due to excess ROS, plants induce the adaptive mechanisms, including the antioxidant system. Enhancing antioxidant ability can overcome the oxidative damage from ROS, and mitigate CI in fresh fruit under chilling stress (Islam et al., 2022). In the antioxidant system, antioxidant enzymes, including APX, SOD, and CAT, are vital for lowering ROS accumulation (Li et al., 2024; Lv et al., 2022; Lin et al., 2022). SOD performs dismutation for O2−. scavenging (Han et al., 2023; Huang et al., 2024; Yi et al., 2024). APX and CAT scavenge H2O2 (Zhang et al., 2023; Fan et al., 2024). Additionally, nonenzymatic antioxidants, such as AsA and GSH, are also important components for lowering ROS content and mitigating oxidative injury to the cell membranes (Zhang et al., 2023). AsA can increase APX activity to eliminate H2O2, but GSH scavenges H2O2 and other form of ROS (Lin et al., 2022). Also, DPPH radical scavenging activity acts as a key indicator to express the antioxidant activity of fresh produce (Lin et al., 2022; Zhang et al., 2023). Moreover, chilling stress might induce the increases of antioxidant enzyme activities to activate antioxidant ability, and then clear ROS at the early stage of storage. However, the gradual reduction in antioxidant enzyme activities after their maxima might lower the antioxidant ability but exacerbate the ROS-induced oxidative stress (Huang et al., 2024).
In present study, the rising trend of activities of SOD, CAT and APX (Fig. 2A–C) were measured in control fruit on days 0–28, 0–14 and 0–21, respectively, which could be a result of increased ROS under chilling stress. These findings suggested that, in the early and middle stages of storage, the increased antioxidant enzyme activities could eliminate the excrescent ROS. Additionally, in the middle and late stages of storage, control fruit showed decreased activities of antioxidant enzymes including SOD, CAT and APX (Fig. 2A–C). Besides, during storage, control passion fruit had lower levels of AsA, GSH (Fig. 2D and E), and DPPH radical scavenging activity (Fig. 2F), but higher values of ROS (Fig. 1C), MDA (Fig. 1D), CI (Fig. 1A), and CMP (Fig. 1B). Thus, the CI development of passion fruit was related to a decreased antioxidant ability, to induce ROS production and membrane lipid peroxidation, thereby damaging cell membrane. Nevertheless, compared to control passion fruit, melatonin-treated passion fruit had higher antioxidant enzyme activities (Fig. 2A–C), antioxidants (Fig. 2D and E), and DPPH radical scavenging activity (Fig. 2F), whereas the lower values of O2−. (Fig. 1C), MDA (Fig. 1D), CI index (Fig. 1A), and CMP (Fig. 1B) during storage. Therefore, melatonin might retain higher antioxidant level of fruit to reduce ROS generation and delay membrane lipid peroxidation, then stabilize cell membranes, thereby improving chilling resistance and suppressing CI development in fresh passions. These findings accorded with previous studies displaying that the cold shock-treated papaya fruit showed the alleviated CI symptom, which was resulted from higher antioxidant level for reducing levels of ROS and MDA (Nian et al., 2022). Moreover, GABA might slow down Chinese olive CI development via reducing oxidative damage to cell membrane by raising antioxidant enzyme activities or antioxidant contents (Fan et al., 2024).
4.3. Melatonin delayed CI development in fresh passion fruit and its relationship with membrane lipid metabolism
Some key degrading enzymes in membrane lipid metabolism, including lipase, LOX, or PLD, are considered to cause the structural alterations in cellular membranes (Hu et al., 2022; Lin et al., 2023; Li et al., 2024). PLD can trigger severe phospholipid catabolism (Hu et al., 2022), which may catalyze the hydrolysis of PC or PI, thereby producing PA (Kuang et al., 2023; Lin et al., 2024). Lipase may induce the generation of free fatty acids, such as SFAs and USFAs, from PA (Lin et al., 2024). LOX may result in the formation of hydroperoxide and MDA through the oxidation of USFAs, leading to lipid peroxidation and destabilization of the cell membranes (Lin et al., 2023). Moreover, chilling stress could increase the MLDE activities and disorder the cell membrane, and thus, induce the CI in fresh produce (Kuang et al., 2023; Li et al., 2023). However, the decreased MLDE activities could retain cell membrane structure, and delay CI in fresh produce (Chen et al., 2024). Hence, MLDE activities were related to cellular membrane alteration and CI development in fresh produce. In this study, compared with control passions, melatonin-treated passion fruit had lower levels of MLDEs, such as PLD (Fig. 3A), lipase (Fig. 3B), and LOX (Fig. 3C) on days 0–42. Besides, the melatonin-treated group had lower CI index (Fig. 1A) and CMP level (Fig. 1B) than the control group in storage. Taken together, these findings implied that melatonin heightened chilling resistance and reduced CI development in fresh passion fruit by retaining the cell membrane structure through lowering the MLDE activities. The inhibited MLDE levels had also been exhibited in 24-epibrassinolide-treated peaches (Hu et al., 2022) and melatonin-treated bananas (Wang et al., 2022). This inhibition was related to delayed CI development.
The phospholipids, as main constituents of cell membranes, may influence the stabilization of cellular membranes, as well as regulate the cellular metabolism (Kuang et al., 2023; Wang et al., 2023b). Their presence is conducive to coping with various abiotic stresses, especially cold stress. Additionally, phospholipids are mainly presented as PA, PC, and PI (Han et al., 2023; Lin et al., 2023). PI and PC are the two major phospholipids that retain the cellular membrane structure (Kuang et al., 2023; Lin et al., 2023). Although PA acts as a product of PC or PI through the hydrolysis of PLD (Han et al., 2023; Chen et al., 2024), whereas PA can be further resolved into free fatty acids through the degradative action of lipase (Wang et al., 2020). Furthermore, fatty acids are also key members of membrane lipid, and play vital roles in maintaining cell membrane integrity (Han et al., 2023; Lin et al., 2023). Based on the saturation, fatty acids are divided into two major types: SFAs and USFAs (Lin et al., 2024). High levels of USFAs help stabilize cellular membrane fluidity and stability, thereby enhancing the ability to cope with stress, such as CI (Han et al., 2023). Additionally, U/S and IUFA are influenced by the changed values of SFAs and USFAs (Lin et al., 2023), which are used as indicators for evaluating cell membrane unsaturation (Hong et al., 2023).
In present study, control passion fruit exhibited the downward trend of levels of PC (Fig. 3D), PI (Fig. 3E) and USFAs, such as oleic acid (Fig. 4A), linoleic acid (Fig. 4B), and linolenic acid (Fig. 4C), and the decreasing levels of U/S (Fig. 4F) and IUFA (Fig. 4G), and increasing levels of PA (Fig. 3F) and SFAs, including palmitic acid (Fig. 4D) and stearic acid (Fig. 4E), during storage. Besides, in storage, control fruit had an increase in CI index (Fig. 1A) and CMP level (Fig. 1B). For control fruit, the CI index revealed negative correlations with the levels of PC, PI, oleic acid, linoleic acid, linolenic acid, U/S, and IUFA, with r values of −0.87∗∗, −0.93∗∗, −0.93∗∗, −0.89∗∗, −0.89∗∗, −0.86∗, and −0.92∗∗, respectively (Fig. 5). However, CI index was positively correlated with the values of PA, palmitic acid, and stearic acid, with r values of 0.99∗∗, 0.90∗∗, and 0.98∗∗, respectively (Fig. 5). Furthermore, CMP was inversely correlated with the levels of PC, PI, oleic acid, linoleic acid, linolenic acid, U/S, and IUFA, with r values of −0.94∗∗, −0.97∗∗, −0.96∗∗, −0.94∗∗, −0.93∗∗, −0.94∗∗, and −0.97∗∗, respectively (Fig. 5). Nevertheless, CMP revealed positive correlations with values of PA, palmitic acid, and stearic acid, with r values of 0.98∗∗, 0.96∗∗, and 0.98∗∗, respectively (Fig. 5). Therefore, the CI development of fresh passion fruit was due to structural damage of the cellular membrane, which correlated with membrane lipid decomposition, such as the decrease in PC, PI and USFAs, but accumulation of PA and SFAs. Similarly, the decomposition of USFAs, PC and PI had also been shown in the bananas (Li et al., 2023) or Chinese olives (Kuang et al., 2023) treated with the chilling temperature. This decomposition was related to accelerated CI occurrence.
Moreover, in contrast to control passion fruit, melatonin-treated fruit had greater levels of PC (Fig. 3D), PI (Fig. 3E), USFAs (Fig. 4A–C), U/S (Fig. 4F), and IUFA (Fig. 4G), as well as lower values of PA (Fig. 3F), and SFAs (Fig. 4D–E) in storage. Besides, on days 0–42, melatonin-treated fruit represented lower activities of PLD (Fig. 3A) and LOX (Fig. 3C), CI index (Fig. 1A), and CMP level (Fig. 1B) than control fruit. These effects were likely due to melatonin's higher levels of PC and PI, as well as its lower content of PA, achieved by inhibiting PLD activity. Melatonin also stimulated the desaturation of SFAs and increased the accumulation of USFAs by reducing LOX activity. This resulted in the higher stabilization and unsaturation of the cell membrane, thereby strengthening chilling resistance and delaying CI development in fresh passion fruit. Similarity, Hu et al. (2022) reported that 24-epibrassinolide might reduce activities of MLDEs, like PLD, LOX and lipase, to inhibit PI and PC hydrolysis and stabilize USFA contents, thereby alleviating the membrane damages and enhancing chilling tolerance in peaches. Furthermore, Han et al. (2023) revealed that chitooligosaccharide alleviated muskmelon fruit CI development by mitigating the degradation of USFAs, PC or PI.
5. Conclusions
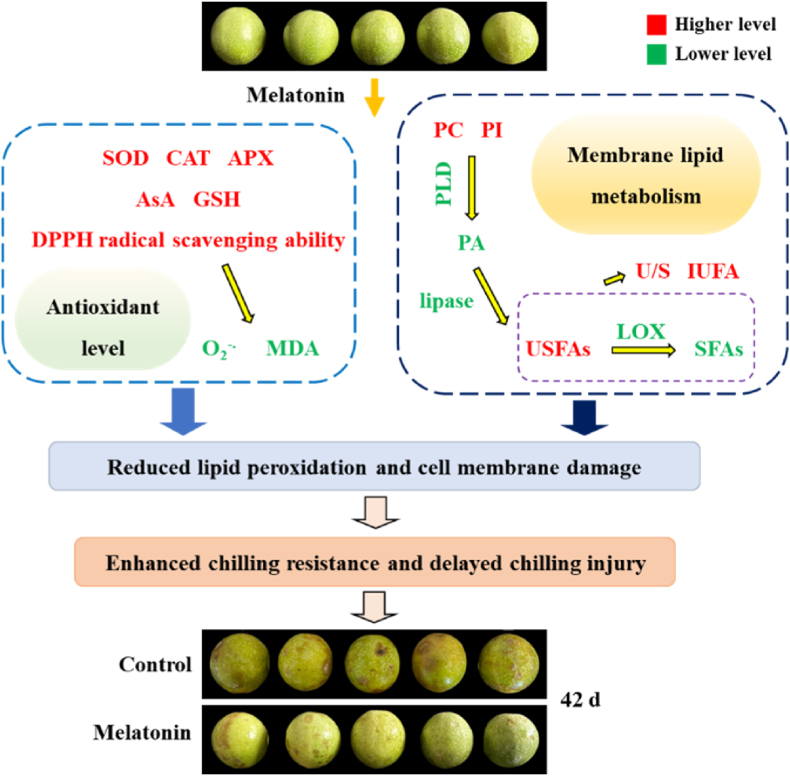
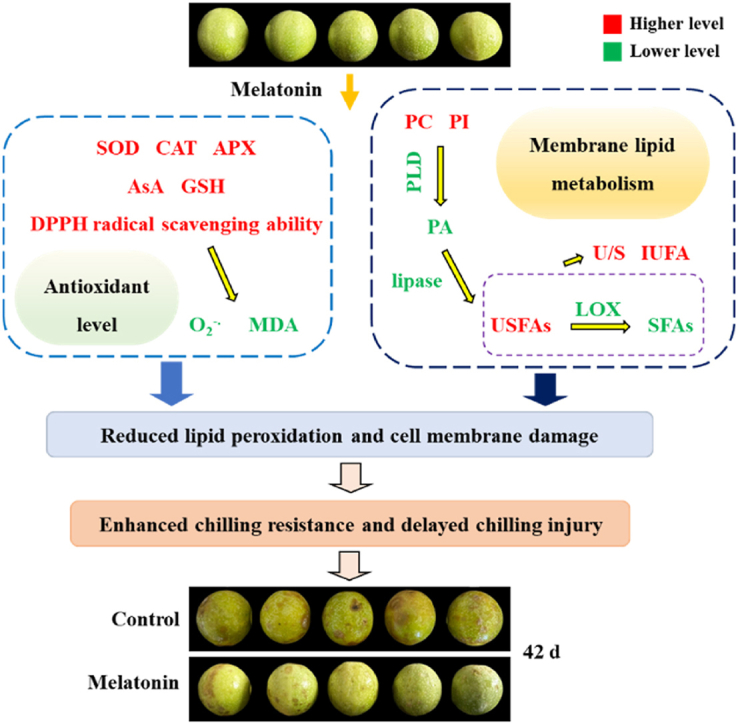
Therefore, the present study expounded that melatonin might delay CI development in fresh passion fruit by modulating antioxidant ability and membrane lipid metabolism. On one hand, melatonin decreased ROS and MDA levels, resulting from the increased antioxidant enzyme activities, antioxidant contents, and DPPH radical scavenging ability. On the other hand, melatonin raised PI, USFA, and PC levels, and retained cell membrane unsaturation via lowering MLDE activities. Overall, the results revealed that melatonin might stabilize the cell membrane by boosting antioxidant activity and reducing membrane lipid degradation, which enhance chilling resistance and mitigate CI development in fresh passion fruit. A model of how melatonin alleviated CI in fresh passion fruit by stabilizing the cell membrane through the modulation of antioxidant ability and membrane lipid metabolism was shown in Fig. 6. So, in the future, the above technique may be employed to retard the CI, and preserve the quality of fresh passions via stabilizing cell membrane structure.
Fig. 6.
The possible mechanism of melatonin delaying chilling injury of fresh passion fruit by stabilizing cell membrane though acting on antioxidant ability and membrane lipid metabolism. In figure, compared to control passions, green fonts denoted that melatonin-treated passions had the lower values of indicators, but red fonts denoted that melatonin-treated passions had the higher values of indicators.
CRediT authorship contribution statement
Yuzhao Lin: Investigation, Experimental work, Data curation, Writing – original draft. Hongbin Chen: Conceptualization, Writing – review & editing. Yazhen Chen: Experimental work, Data curation. Bowen Tan: Investigation, Experimental work. Xuanjing Jiang: Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Natural Science Foundation of Fujian Province of China (Grant Nos. 2023J01902, 2024J08069, and 2021J01976) and the Research Start-up Project of Introduced Talent of Quanzhou Normal University of Fujian Province of China (Grant No. H23026).
Handling Editor: Professor A.G. Marangoni
Data availability
Data will be made available on request.
References
- Ali S., Nawaz A., Naz S., Ali M., Ejaz S., Azam M., Razzaq K. Exogenous melatonin mitigates chilling injury in zucchini fruit by enhancing antioxidant system activity, promoting endogenous proline and GABA accumulation, and preserving cell wall stability. Postharvest Biol. Technol. 2023;204 doi: 10.1016/j.postharvbio.2023.112445. [DOI] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen H.B., Lin H.T., Jiang X.J., Lin M.S., Fan Z.Q. Amelioration of chilling injury and enhancement of quality maintenance in cold-stored guava fruit by melatonin treatment. Food Chem. X. 2022;14 doi: 10.1016/j.fochx.2022.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.Z., Lin H.T., Zhang H.L., Chen Y., Lin M.S., Zheng Y., Fan Z.Q., Wang H., Chen Y.H., Lin Y.F. Dicyclohexylcarbodiimide and disodium succinate regulate the browning development in fresh longan pericarp by modulating the antioxidant system and the metabolisms of membrane lipids and phenolics. Postharvest Biol. Technol. 2023;203 doi: 10.1016/j.postharvbio.2023.112388. [DOI] [Google Scholar]
- Chen Y.M., Lin B., Lin Y.F., Sang Y.Y., Lin M.S., Fan Z.Q., Chen Y.H., Wang H., Lin H.T. Involvements of membrane lipid and phenolic metabolism in reducing browning and chilling injury of cold-stored Chinese olive by γ-aminobutyric acid treatment. Postharvest Biol. Technol. 2024;209 doi: 10.1016/j.postharvbio.2023.112664. [DOI] [Google Scholar]
- Fan Z.Q., Lin B., Lin H.T., Lin M.S., Chen J.Y., Lin Y.F. γ-Aminobutyric acid treatment reduces chilling injury and improves quality maintenance of cold-stored Chinese olive fruit. Food Chem. X. 2022;13 doi: 10.1016/j.fochx.2022.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z.Q., Lin B., Lin Y.F., Chen Y.Z., Chen G., Chen J.Y., Wang H., Chen Y.H., Lin H.T. Alleviation of chilling injury in cold-stored Chinese olive (Canarium album Lour.) fruit by γ-aminobutyric acid treatment in relation to ROS metabolism. Sci. Hortic. 2024;327 doi: 10.1016/j.scienta.2024.112851. [DOI] [Google Scholar]
- Han Z.H., Li B.J., Gong D., Xie P.D., Yu L.R., Wang Y., Han Y., Li Y.C., Prusky D., Romanazzi G., Bi Y. Preharvest chitooligosaccharide spray alleviates chilling injury in harvested muskmelon fruit by regulating membrane lipid metabolism and activating antioxidant enzyme activity. Postharvest Biol. Technol. 2023;204 doi: 10.1016/j.postharvbio.2023.112452. [DOI] [Google Scholar]
- Hong K.Q., Yao Q.S., Golding J.B., Pristijono P., Zhang X.M., Hou X.W., Yuan D.B., Li Y.X., Chen Y., Song K.H., Chen J. Low temperature storage alleviates internal browning of ‘Comte de Paris’ winter pineapple fruit by reducing phospholipid degradation, phosphatidic acid accumulation and membrane lipid peroxidation processes. Food Chem. 2023;404 doi: 10.1016/j.foodchem.2022.134656. [DOI] [PubMed] [Google Scholar]
- Hu S.Q., Ma Y.Q., Xie B., Hou Y.Y., Jia Z.Y., Zhao L.Y., Zheng Y.H. 24-Epibrassinolide improves chilling tolerance by regulating PpCBF5-mediated membrane lipid metabolism in peach fruit. Postharvest Biol. Technol. 2022;186 doi: 10.1016/j.postharvbio.2022.111844. [DOI] [Google Scholar]
- Huang T., Liu G.S., Zhu L.S., Liu J.L., Xiang Y., Xu X.B. Mitigation of chilling injury in mango fruit by methyl jasmonate is associated with regulation of antioxidant capacity and energy homeostasis. Postharvest Biol. Technol. 2024;211 doi: 10.1016/j.postharvbio.2024.112801. [DOI] [Google Scholar]
- Islam M., Ali S., Nawaz A., Naz S., Ejaz S., Shah A.A., Razzaq K. Postharvest 24-epibrassinolide treatment alleviates pomegranate fruit chilling injury by regulating proline metabolism and antioxidant activities. Postharvest Biol. Technol. 2022;188 doi: 10.1016/j.postharvbio.2022.111906. [DOI] [Google Scholar]
- Kuang X.Y., Chen Y.Z., Lin H.T., Lin H., Chen G., Lin Y.F., Chen Y.H., Wang H., Fan Z.Q. Comprehensive analyses of membrane lipids and phenolics metabolisms reveal the developments of chilling injury and browning in Chinese olives during cold storage. Food Chem. 2023;416 doi: 10.1016/j.foodchem.2023.135754. [DOI] [PubMed] [Google Scholar]
- Li Q., Lin H., Lin H.T., Lin M.S., Wang H., Wei W., Chen J.Y., Lu W.J., Shao X.F., Fan Z.Q. The metabolism of membrane lipid participates in the occurrence of chilling injury in cold-stored banana fruit. Food Res. Int. 2023;173 doi: 10.1016/j.foodres.2023.113415. [DOI] [PubMed] [Google Scholar]
- Li M.L., Lin H.T., Wang C., Chen Y.Z., Lin M.S., Hung H.C., Lin Y.F., Fan Z.Q., Wang H., Chen Y.H. Acidic electrolyzed-oxidizing water treatment mitigated the disease progression in Phomopsis longanae Chi-infected longans by modulating ROS and membrane lipid metabolism. Food Chem. 2024;449 doi: 10.1016/j.foodchem.2024.139175. [DOI] [PubMed] [Google Scholar]
- Lin Y.X., Lin H.T., Chen Y.H., Wang H., Ritenour M.A., Lin Y.F. Hydrogen peroxide-induced changes in activities of membrane lipids-degrading enzymes and contents of membrane lipids composition in relation to pulp breakdown of longan fruit during storage. Food Chem. 2019;297 doi: 10.1016/j.foodchem.2019.124955. [DOI] [PubMed] [Google Scholar]
- Lin Y.Z., Lin H.T., Zeng L.Z., Zheng Y., Chen Y.Z., Fan Z.Q., Lin Y.F. DNP and ATP regulate the pulp breakdown development in Phomopsis longanae Chi-infected longan fruit through modulating the ROS metabolism. Food Chem. X. 2022;14 doi: 10.1016/j.fochx.2022.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.X., Lin H.T., Fan Z.Q., Wang H., Lin M.S., Chen Y.H., Hung Y.C., Lin Y.F. Inhibitory effect of propyl gallate on pulp breakdown of longan fruit and its relationship with ROS metabolism. Postharvest Biol. Technol. 2020;168 doi: 10.1016/j.postharvbio.2020.111272. [DOI] [Google Scholar]
- Lin Y.Z., Lin H.T., Zeng L.Z., Lin M.S., Chen Y.H., Fan Z.Q., Wang H., Lin Y.F. DNP and ATP regulate the breakdown occurrence in the pulp of Phomopsis longanae Chi-infected longan fruit through modulating the metabolism of membrane lipid. Food Chem. 2023;409 doi: 10.1016/j.foodchem.2022.135330. [DOI] [PubMed] [Google Scholar]
- Lin L.J., Lin H.T., Zheng S.J., Chen Y.Z., Zhang H.L., Lin M.S., Fan Z.Q., Wang H., Chen Y.H., Lin Y.F. Metabolisms of ROS and membrane lipid participate in Pestalotiopsis microspora-induced disease occurrence of harvested Chinese olives. Postharvest Biol. Technol. 2024;210 doi: 10.1016/j.postharvbio.2023.112720. [DOI] [Google Scholar]
- Liu L., Huang A.Q., Wang B., Zhang H., Zheng Y.H., Wang L. Melatonin mobilizes the metabolism of sugars, ascorbic acid and amino acids to cope with chilling injury in postharvest pear fruit. Sci. Hortic. 2024;323 doi: 10.1016/j.scienta.2023.112548. [DOI] [Google Scholar]
- López A.G., López V.J., Vega G.M., Bojorquez A.W., Delgado V.F., Ayón R.L., López L.M. Antioxidant enzymatic changes in bell pepper fruit associated with chilling injury tolerance induced by hot water. J. Food Biochem. 2021;45(11) doi: 10.1111/jfbc.13966. [DOI] [PubMed] [Google Scholar]
- López-López M.E., Ramírez-Perales M.F., Ayón-Reyna L.E., Delgado-Vargas F., Cruz-Mendivil A., Vega-García M.O. Impact of two hot water treatments applied sequentially on oxidative metabolism related to chilling injury tolerance in mango fruit. Rev. Chapingo Ser. Hortic. 2023;29(1):19–38. doi: 10.5154/r.rchsh.2022.05.009. [DOI] [Google Scholar]
- Lv Y.M., Elnur E., Wang W., Thakur K., Du J., Li H.N., Ma W.P., Liu Y.Q., Ni Z.J., Wei Z.J. Hydrogen sulfide treatment increases the antioxidant capacity of fresh Lingwu Long Jujube (Ziziphus jujuba cv. Mill) fruit during storage. Curr. Res. Food Sci. 2022;5:949–957. doi: 10.1016/j.crfs.2022.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nian Y.W., Wang N., Li R., Shao Y.Z., Li W. Cold shock treatment alleviates chilling injury in papaya fruit during storage by improving antioxidant capacity and related gene expression. Sci. Hortic. 2022;294 doi: 10.1016/j.scienta.2021.110784. [DOI] [Google Scholar]
- Rinaldi M.M., de Campos Dianese A., Costa A.M., de Oliveira da Silva Assis D.F., de Oliveira T.A.R., de Oliveira Assis S.F. Post-harvest conservation of Passiflora alata fruits under ambient and refrigerated condition. Food Sci. Technol. 2019;39:889–896. doi: 10.1590/fst.14018. [DOI] [Google Scholar]
- Sang N., Hai L.H. Effect of ratio of bees wax and carnauba wax in mixed wax on respiration rate, weight loss, fruit decay and chemical quality of Vietnamese passion fruits during low temperature storage. Pakistan J. Biotechnol. 2020;17:63–70. doi: 10.34016/pjbt.2020.17.2.63. [DOI] [Google Scholar]
- Song L.J., Zhang W.W., Li Q., Jiang Z.X., Wang Y.H., Xuan S.X., Zhao J.J., Luo S.X., Shen S.X., Chen X.P. Melatonin alleviates chilling injury and maintains postharvest quality by enhancing antioxidant capacity and inhibiting cell wall degradation in cold-stored eggplant fruit. Postharvest Biol. Technol. 2022;194 doi: 10.1016/j.postharvbio.2022.112092. [DOI] [Google Scholar]
- Tang J.Y., Chen H.B., Lin H.T., Hung Y.C., Xie H.L., Chen Y.H. Acidic electrolyzed water treatment delayed fruit disease development of harvested longans through inducing the disease resistance and maintaining the ROS metabolism systems. Postharvest Biol. Technol. 2021;171 doi: 10.1016/j.postharvbio.2020.111349. [DOI] [Google Scholar]
- Wang H.L., Chen H.B., Lin Y., Li M.L., Liu Q.Q., Lin Y.Z., Jiang X.J., Chen Y.H. Insights into the Isolation, Identification, and biological characterization analysis of and novel control strategies for Diaporthe passiflorae in postharvest passion fruit. J. Fungi. 2023;9:1034. doi: 10.3390/jof9101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Hu M.J., Yuan D.B., Yun Z., Gao Z.Y., Su Z.H., Zhang Z.K. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020;160 doi: 10.1016/j.postharvbio.2019.111066. [DOI] [Google Scholar]
- Wang Z.Q., Zhang L., Duan W.H., Li W., Wang Q., Li J.K., Song H.M., Xu X.B. Melatonin maintained higher contents of unsaturated fatty acid and cell membrane structure integrity in banana peel and alleviated postharvest chilling injury. Food Chem. 2022;397 doi: 10.1016/j.foodchem.2022.133836. [DOI] [PubMed] [Google Scholar]
- Wang K., Zhu G., Li Y.L., Chen S.Q., Rashid A., Wang X.T., Wu X.Y. Non-thermal effects of microwave irradiation alleviates postharvest chilling injury of peach fruit by retarding phenolic accumulation and enhancing membrane stability. Food Chem. 2023;411 doi: 10.1016/j.foodchem.2023.135448. [DOI] [PubMed] [Google Scholar]
- Xu P., Huber D.J., Gong D., Yun Z., Pan Y.G., Jiang Y.M., Zhang Z.K. Amelioration of chilling injury in ‘Guifei’ mango fruit by melatonin is associated with regulation of lipid metabolic enzymes and remodeling of lipidome. Postharvest Biol. Technol. 2023;198 doi: 10.1016/j.postharvbio.2022.112233. [DOI] [Google Scholar]
- Yi B.H., Liu Y., Wu Z.G., Zheng Y.H., Chen H.J., Jin P. Hydrogen sulfide alleviates chilling injury of zucchini fruit by regulating antioxidant capacity, endogenous hydrogen sulfide, proline, and polyamine metabolism. Postharvest Biol. Technol. 2024;208 doi: 10.1016/j.postharvbio.2023.112638. [DOI] [Google Scholar]
- You M., Duan X.Y., Li X., Luo L.J., Zhao Y., Pan H.H., Gong W.L., Yang L.R., Xiang Z., Li G.F. Effect of 1-Methylcyclopropene combined with chitosan-coated film on storage quality of passion fruit. Sustain. Chem. Pharm. 2022;27 doi: 10.1016/j.scp.2022.100679. [DOI] [Google Scholar]
- Zhang S., Lin Y.Z., Lin H.T., Lin Y.X., Chen Y.H., Wang H., Shi J., Lin Y.F. Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-induced disease development and pericarp browning of harvested longan fruit in association with membrane lipids metabolism. Food Chem. 2018;244:93–101. doi: 10.1016/j.foodchem.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Zhang H.L., Shan T.T., Chen Y., Lin M.S., Chen Y.Z., Lin L.J., Chen Y.H., Wang H., Fan Z.Q., Lin H.T., Lin Y.F. Salicylic acid treatment delayed the browning development in the pericarp of fresh longan by regulating the metabolisms of ROS and membrane lipid. Sci. Hortic. 2023;318 doi: 10.1016/j.scienta.2023.112073. [DOI] [Google Scholar]
- Zhou Y.F., Zhong Y.X., Li L., Jiang K., Gao J., Zhong K., Pan M.F., Yan B. A multifunctional chitosan-derived conformal coating for the preservation of passion fruit. LWT--Food Sci. Technol. 2022;163 doi: 10.1016/j.lwt.2022.113584. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.