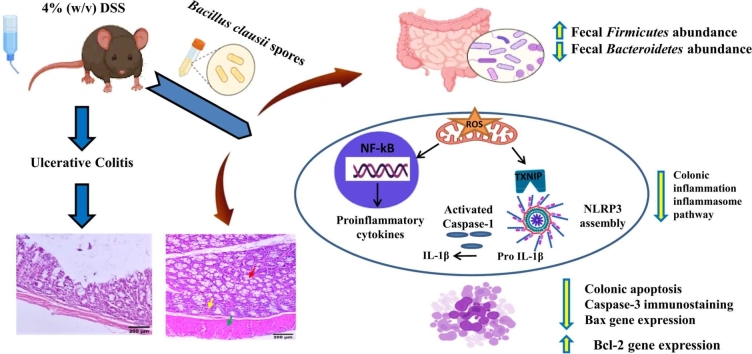
Abstract
Ulcerative colitis (UC), a persistent immune-mediated disorder lacking effective treatment, is distinguished by gut microbiota dysbiosis, abnormal activation of the NLRP3 inflammasome pathway, and apoptosis. Despite growing attention to these factors, understanding their significance in UC pathogenesis remains a challenge. The present study explores the potential therapeutic impact of Bacillus clausii (Bc) spores in a murine UC model induced by drinking 4 % (w/v) dextran sulfate sodium (DSS) in C57BL/6 mice. Subsequently, the DSS-induced mice were orally administered either Bc at varying concentrations (105 and 1010 Colony forming unit, CFU) or sulfasalazine (SSZ) at a dosage of 200 mg/kg for 7 days. The disease-specific activity index (DAI) was calculated daily utilizing parameters such as body weight, diarrhea, and bloody stool. Changes in fecal Firmicutes and Bacteroidetes abundance, colonic TXNIP and NLRP3 contents, as well as colonic caspase-1, IL-1β, Bax, and Bcl-2 expression, were investigated. Additionally, markers related to oxidative stress and inflammation, histopathological changes and caspase-3 immunohistochemistry testing were conducted. DSS-treated mice had significantly higher DAI scores compared to controls, indicating severe colitis. However, SSZ treatment or Bc (105 CFU) dramatically lowered DAI scores, with the highest Bc dosage (1010 CFU) producing the greatest improvement. Furthermore, Bc (1010 CFU) substantially (p < 0.05) boosted fecal Firmicutes while decreased Bacteroidetes, indicating reversal of gut dysbiosis. Bc effectively reduced colonic oxidative stress and inflammation by replenishing GSH and catalase and modulating the NF-κB, Nrf2/HO-1, and TXNIP/NLRP3 pathways. Additionally, Bc (1010 CFU) exhibited histologically almost normal mucosa, with maintained architecture and reduced apoptosis, as seen by normalization of Bcl2 and Bax with decreased caspase-3. Collectively, these findings point to the potential usefulness of Bc spores in preventing and treating DSS-induced colitis, positioning them as a promising candidate for UC management.
Keywords: Ulcerative colitis, Bacillus clausii, Firmicutes, Bacteroidetes, Inflammasome pathway, Apoptosis
Graphical Abstract
Highlights
-
•
Bc alleviates inflammation in colitis by inhibiting the TXNIP/NLPR3 cascade.
-
•
Bc reduces oxidative stress in colitis by boosting the Nrf2/HO-1 pathway.
-
•
Bc restores normal fecal Firmicutes and Bacteroidetes abundance in colitis.
-
•
Bc lowers colonic apoptosis by regulating caspase-3, Bax, and Bcl-2 levels.
-
•
Bc seems as a promising and safe choice for treatment/prevention of colitis.
1. Introduction
Inflammatory bowel disease (IBD) is considered as a growing global health challenge due to the ongoing rise in case numbers every year [1]. IBDs encompass distinct conditions, including Crohn's disease (CD) and ulcerative colitis (UC) [2]. UC, defined as persistent inflammation of colon mucosa, typically manifests in the third decade of life for both genders [3]. The currently treatment approaches for UC involve the use of steroids and immune suppressive agents [4]. However, these therapies work by counteracting inflammatory cytokines, yet they do not address the fundamental cause of UC [5]. Moreover, these treatments result in a range of potential side effects, including renal toxicity, hemolytic anemia, osteoporosis, insomnia, pancreatitis, bone marrow repression, non-Hodgkin lymphoma, hepatosplenic T-cell lymphoma, fatigue, diarrhea, nausea, and vomiting [6]. Long-term therapy for UC patients exposes them to additional risks such as opportunistic infections [7], cervical cancer [8], skin cancer [9], and reduced adherence to vaccination protocols [10], limiting future therapeutic applicability. To advance therapeutic impacts for UC, many therapeutic strategies were developed to enhance UC treatment outcomes. Among them, a variety of medicines have evolved, including alpha-4-integrin blocking drugs, anti-CD3 antibodies, probiotic bacterial therapy, epidermal growth factors, and others, with the promise of improved dosing and compliance with conventional compounds [11]. Natural substances have also been used to protect, cure, prevent, and minimize colonic inflammation [12]. These medicines provide comfort to 40 % of UC patients while reducing hazardous side effects and ensuring clinical remissions [13]. In addition, nanobiotechnology advancements have considerably improved IBD therapy, particularly for chronic conditions such as UC. Colon delivery, or specialized delivery of medication to the colon, has gained popularity as an alternative treatment approach that might be beneficial and enhance patients' quality of life [14]. Although these approaches have the potential to improve treatment outcomes and reduce side effects for patients suffering from this challenging autoimmune condition, further research is needed to create new viable therapies.
In the progression of UC, multiple apoptotic and oxidative stress signaling molecules are involved in regulating the process [15]. The generation of Bax and caspase-3 promotes apoptosis by upregulating the NF-κB signaling pathway. This leads to inflammatory processes and oxidative damage, compromising the intestinal mucosa's structural integrity [16]. Additionally, Bcl-2, initially identified in B-cell lymphoma, acts as a crucial suppressor gene involved in regulating apoptosis [17]. Nuclear erythroid-related factor 2 (Nrf-2) provides a key part in gene transcription that regulates cellular anti-inflammatory and antioxidant mechanisms [18]. Nrf2 activates reactive oxygen species (ROS)-scavenging enzymes that participate in active oxygen scavenging, and stress-responsive proteins, heme oxygenase-1 (HO-1) [19]. Increased oxidative stress in the absence of Nrf-2 induces NF-κB-mediated cytokine expression, as NF-κB is more readily generated in an oxidative environment [20]. These factors interact, leading to exaggerated inflammatory reactions, thereby contributing to colonic injury in UC [21]. Furthermore, the inflammasome, a complex of multiple proteins crucial to innate immunity, is implicated in triggering inflammatory responses observed in UC [22]. Among the various inflammasome subtypes, Nod-like receptor protein 3 (NLRP3) is an intricate protein complex that activates caspase-1, leading to the development and release of cytokines like interleukin (IL)-1β [23]. It is worth noting that TXNIP is an upstream partner of NLRP3, and their interaction is essential for downstream inflammasome activation [24].
Unfortunately, UC presents complex pathogenesis, with one prevailing theory attributing to an imbalance within the gut flora, leading to frequent inflammatory responses [25]. The balance between Firmicutes and Bacteroidetes, the most predominant gut microbiota phyla, is linked to maintaining physiological equilibrium, and alterations in this ratio can give rise to various pathological conditions. Notably, an elevation in certain Bacteroidetes species with reduction in Firmicutes phylum is linked to development of UC [5], [26]. The Firmicutes phylum comprises Gram-positive bacteria with rigid or semi-rigid cell walls, primarily from the genera Bacillus, Clostridium, Enterococcus, Lactobacillus, and Ruminococcus [27]. Conversely, the Bacteroidetes phylum encompasses approximately 7000 distinct species of Gram-negative bacteria, predominantly from the genera Bacteroides, Alistipes, Parabacteroides, and Prevotella [28]. The gut microbiota is crucial for regulating host homeostasis and immune function. Gut microbiota disruption is a prominent cause of inflammatory bowel illness, particularly UC [29]. Consequently, there is a pressing requirement for a safe therapeutic strategy with unique mechanisms that target inflammation and address disturbances in gut microbiome composition to effectively treat UC. Probiotic medication has been recommended to manage the gut flora in UC patients. Probiotics are well-tolerated and have little adverse effects, making them a promising therapy for UC patients [30]. However, further research is needed to fully understand the mechanisms of action of probiotics for UC. Bacillus, a Gram-positive bacterium known for its ability to form spores, is considered a crucial component of probiotics. Its capacity to efficiently secrete enzymes, antibiotics, and bioactive peptides, coupled with its adaptability to various fermentation conditions, has gained widespread recognition in biotechnology [31]. One notable characteristic of Bacillus is its spore-forming ability, which grants it high resistance and suitability for processing, storage, and survival in the gastrointestinal (GI) tract [32]. Bacillus clausii (Bc) has significant probiotic potential for treating gastrointestinal problems, promoting gut health, and boosting immune function. Bc supplementation, whether as a single or multi-strain probiotic, is typically well tolerated with little adverse effects, even at higher dosages [33]. Bc has been shown to reduce rotavirus/adenovirus excretion and stool frequency in children with acute diarrhea [34], as well as improve gut microbiota dysbiosis [35]. Furthermore, it has antimicrobial and immunomodulatory properties [36] and modulates gene expression linked to inflammatory processes, intestinal permeability, and cell differentiation [37]. However, there has been limited literature on Bc involvement in UC management, and its exact mechanisms are still unclear. Therefore, our aim was to explore the protective potential of Bc spores in a mouse model of DSS-induced colitis and identify the fundamental mechanism, encompassing oxidative stress, inflammation, microbiota modulation, and apoptosis. This study aims to establish an empirical basis for the investigation and utilization of Bacillus species in the treatment of colitis. Our research found that Bc treatment, particularly at high doses, protects against DSS-induced UC in C57BL/6 mice. This is accomplished by hindering the TXNIP/NLRP3 inflammasome activation-induced IL-1β generation and caspase-1 cleavage. Additionally, Bc reverses gut microbial dysbiosis while increasing colonic antioxidant functioning. Our findings suggest that gut dysbiosis and TXNIP/NLRP3 might be viable therapeutic targets for UC, and they provide theoretical support for the emergence of probiotic-based microbial products as a safe and effective supplementary therapy for UC.
2. Materials and methods
2.1. Chemicals and drugs
Bacillus clausii (Bc) spores (Enterogermina®, Sanofi S.P.A., Viale Luigi Bodio, 37/b 20158 Milan, Italy; batch number: 0I117) were utilized in the study. Sulfasalazine (SSZ, Colosalazine-EC®, The Arab Company for gelatin and pharmaceutical products, Alexandria, Egypt, batch number: 622400588113) was also employed. Dextran sulfate sodium (DSS) (CAS no. 26.4361710) was obtained from CHEM-LAB, Zedelgem, Belgium.
2.2. Experimental animals
Male C57BL/6 mice (weight, 20–25 g; age, 6–8 weeks) were procured from Theodor Bilharz Research Institute (TBRI)'s animal facility in Giza, Egypt. They were maintained in a pathogen-free environment with a temperature of 24–25 °C and an approximate humid of 50 %–60 %. All reasonable care was taken to ensure that the mice were treated humanely, that ethical standard was followed, and that animals were used as few times as possible to get scientifically relevant outcomes.
2.3. Induction of UC and experimental design
Thirty male mice (C57BL/6) were administered 4 % (w/v) DSS in their water to drink for a period of seven days [38], [39]. To assess the impact of Bc spores on colitis, mice were distributed at random to five groups of six each. These groups are: (I) Normal control mice, (II) DSS-intoxicated mice, (III) Mice with induced colitis were treated via oral gavage with SSZ (standard treatment) at a dosage of 200 mg/kg for 7 days [40], [41], and (IV and V) DSS-induced colitis mice orally treated with 105 and 1010 CFU of Bc spores, respectively, for 7 days.
2.4. Collection of samples
On the eighth day of the trial, feces were gathered under aseptic conditions. Mice were then weighed and euthanized using mild anesthesia induced by thiopental (50 mg/kg, i.p.). Following euthanasia, the colon was removed, and the degree of morphometric inflammation was assessed by measuring the length between the ileocecal canal junction and the anal boundary. The distal colon was preserved in 4 % formalin, immersed in paraffin, and sectioned into 5 μm-thick slices for histological and immunohistochemical examinations. To perform biochemical testing, ELISA, and RNA extraction, another section of the colon was taken, washed with ice-cold saline, dried, weighted, and homogenized to obtain a 10 % homogenate.
2.5. Evaluation of disease activity index (DAI)
The severity of intestinal disease was assessed by weight loss, stool consistency, and rectal hemorrhage. Throughout the experimental period, the mice were checked every day for weight changes, diarrhea, and rectal bleeding. The disease activity index (DAI) is based on three criteria: diarrhea (0, normal; 2, loose stools; 4, watery diarrhea); blood in the stool (0, no bleeding; 2, minor bleeding; 4, major bleeding); and body weight loss (0, ≤1 %; 1, 1–5 %; 2, 5–10 %; 3, 10–15 %; 4, >15 %) [42].
2.6. Measurement of colonic oxidative stress markers
The collected colon samples were washed using ice-cold saline. Next, 100 mg of colon tissues were homogenized in phosphate-buffered saline (PBS) on ice. Colon tissue homogenates were tested for reduced glutathione (GSH), catalase (CAT), malondialdehyde (MDA), and nitric oxide (NO) levels using commercial kits (Bio-diagnostic, Giza, Egypt) by employing the manufacturer's protocol.
2.7. Enzyme-linked immunosorbent assay (ELISA) for TXNIP and NLRP3
TXNIP and NLRP3 inflammatory proteins were measured in the colon tissue homogenates using ELISA kits from Sunlong Biotech Co., LTD (CAT no: SL0886Mo for TXNIP, CAT no: SL0887Mo for NLRP3) according to the manufacturer’s instructions. The results for TXNIP and NLRP3 markers were presented as pg/mg protein.
2.8. RNA extraction and quantitative real-time PCR (qRT-PCR) analysis
Total RNA was isolated from colon tissues in an RNase-free setting utilizing the RNeasy Mini kit (Qiagen) to examine the expression of Nrf2, HO-1, cleaved caspase-1, IL-1β, NF-kB, Bax, and Bcl-2. PCR reactions were performed using a StepOne™ Real-Time PCR System with 10 μl of 2 × PoweUp™ SYBR™ Green/ROX PCR Master Mix (Applied Biosystems, ThermoFisher Scientific, USA). To quantify the abundance of Firmicutes and Bacteroidetes in fecal samples, bacterial DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen Hidden, Germany). The primer sequences used are listed in Table 1. The comparative cycle threshold (Ct) (2−ΔΔCT) method [43] was employed to determine the relative expression. All Nrf2, HO-1, cleaved caspase-1, IL-1β, NF-kB, Bax, and Bcl-2 expression levels were normalized to the housekeeping β-actin gene. Meanwhile, the content of fecal Firmicutes and Bacteroidetes was normalized to the universal bacterium.
Table 1.
Primer sequences for quantitative real-time PCR analysis.
| Target gene(s) | Amplicon length (bp) | Primer sequence |
|---|---|---|
| Nrf2 | 225 | Forward primer: 5′-ATGATGGACTTGGAGCTGCC−3′ |
| Reverse primer: 5′-TTGTAACTGAGCGAAAAAGGCTTT−3′ | ||
| HO−1 | 127 | Forward primer: 5′-TTCAGAAGGGCCAGGTGACC−3′ |
| Reverse primer: 5′-AAGTAGACAGGGGCGAAGACTGG−3’ | ||
| Cleaved caspase−1 | 198 | Forward primer: 5′-GCGAAGCATACTTTCAGTTTC−3′ |
| Reverse primer: 5′-TCTCCTTCAGGACCTTGTCG−3′ | ||
| NF-kβ | 78 | Forward primer: 5′-CTGGTGGACACATACAGGAAGAC−3’ |
| Reverse primer: 5′-ATAGGCACTGTCTTCTTTCACCTC−3′ | ||
| IL−1β | 72 | Forward primer: 5′-GCTGCTACTCATTCACTGGCAA−3′ |
| Reverse primer: 5′-TGCTGCTGGTGATTCTCTTGTA−3′ | ||
| Bax | 109 | Forward primer: 5′-CCCGAGAGGTCTTTTTCC−3′ |
| Reverse primer: 5′-GCCTTGAGCACCAGTTTG−3′ | ||
| Bcl−2 | 84 | Forward primer: 5′-CCTGGCTGTCTCTGAAGACC−3′ |
| Reverse primer: 5′-CTCACTTGTGGCCCAGGTAT−3′ | ||
| Beta actin | 118 | Forward primer: 5′-GGGAATGGGTCAGAAGGACT−3′ |
| Reverse primer: 5′-CTTCTCCATGTCGTCCCAGT−3′ | ||
| Firmicutes | 126 | Forward primer: 5′- GAGYATGTGGTTTAATTCGAAGCA−3′ |
| Reverse primer: 5′- AGCTGACGACAACCATGCAC−3′ | ||
| Bacteroidetes | 136 | Forward primer: 5′-GAGAGGAAGGTCCCCCAC−3′ |
| Reverse primer: 5′-CGCTACTTGGCTGGTTCAG−3′ | ||
| Universal bacteria | 118 | Forward primer: 5′-GGGAATGGGTCAGAAGGACT−3′ |
| Reverse primer: 5′-CTTCTCCATGTCGTCCCAGT−3′ |
2.9. Macroscopic colonic damage scoring and histological analysis
Following the experiment, the colons were removed, placed on ice, and cleaned of fat and mesentery. The colons were inspected under a microscope (Axiovision version 4.8, Zeiss Germany) for signs of injury, such as inflammation, edema, and/or ulceration. The grading approach was as follows: 0 for normal, 1 for slight inflammation, 2 for mild inflammation along with edema, 3 for moderately inflammation with ulceration, and 4 for extreme inflammation with ulceration. For histological analysis, the distal and proximal colons were preserved in 4 % neutral buffered formalin for 24 h before being rinsed with tap water, dehydrated, cleaned in xylene, and immersed in paraffin. Five μm thick sections were used for hematoxylin and eosin (H&E) staining and light microscopy examination. Ten consecutive fields (x200 magnification) from each mouse were evaluated to identify inflammatory regions, crypt destruction, ulceration, and the presence of edema.
2.10. Immunohistochemical (IHC) analysis for caspase-3
The colon sections of the different groups were deparaffinized in xylene, washed in Tris-buffered saline and then treated with 3 % H2O2 in methanol for 5 min to block the endogenous peroxidase activity. Non-specific binding sites were blocked using non-serum protein block (DAKO, Carpinteria, CA) for 20 min. Following paraffin and endogenous peroxidase removal, antigen retrieval for monoclonal anti-caspase-3 staining (Santa Cruz Biotechnology, USA) was achieved by microwaving sections in citrate buffer (pH=6) for 15 min. After washing with PBS, a secondary antibody (Agilent Dako, CA, USA) was applied for 60 min. The reactivity was observed by visualizing immune-stained cells with 3,3′-diaminobenzidine chromogen (Agilent Dako, CA, USA). Subsequently, the slides were counterstained with hematoxylin, mounted and examined. The percentages of cytoplasmic caspase-3 positively stained cells in ten consecutive fields for each mouse (x200 magnification) were determined.
2.11. Statistical analysis
The data is shown as Mean ± SEM. To assess significant differences in mean values between the analyzed groups, the one-way ANOVA test was used, followed by Tukey's post hoc test (SPSS, version 16.0, Chicago, IL, USA). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Bc amended DAI, colon length, and colon macroscopic changes in DSS-induced colitis
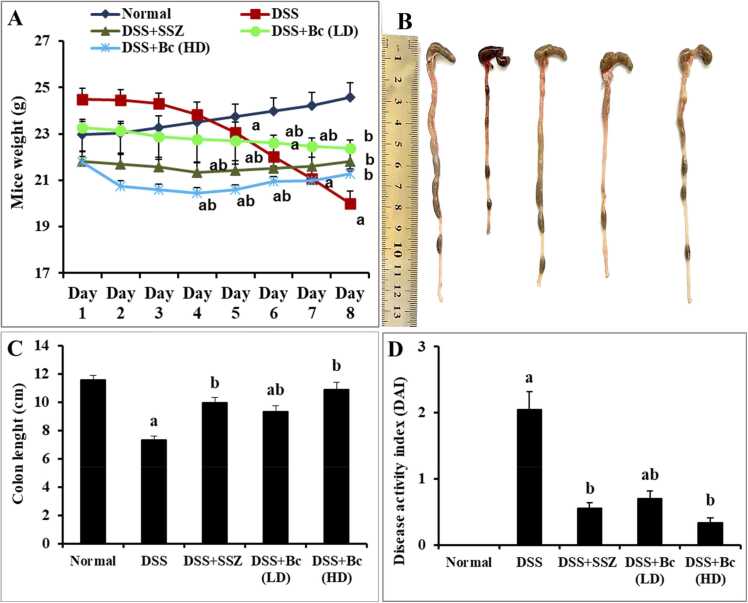
This investigation properly produced severe colitis in mice using DSS in drinking water, resulting in classic colitis symptoms such as bleeding diarrhea, ulcers, decreased weight, and colon shrinkage (Fig. 1A-D). Healthy mice gained weight consistently throughout the study, but DSS-intoxicated mice lost weight much more than normal control mice. Treatment with SSZ (200 mg/kg) or varying doses of Bc (105 CFU or 1010 CFU) led to notable recovery in body weight compared to the colitis group, with the highest dosage of Bc (1010 CFU) demonstrating the most significant improvement. DSS-intoxication caused evident colon shortening, suggesting colon inflammation; however, treatment with SSZ (200 mg/kg) or Bc (105 CFU or 1010 CFU) considerably alleviated this effect, with the highest concentration of Bc (1010 CFU) demonstrating the greatest recovery in colon length. Moreover, DSS-treated mice exhibited substantially elevated DAI scores compared to controls, indicative of severe colitis. However, SSZ administration or Bc (105 CFU) significantly reduced DAI scores, with the highest dose of Bc (1010 CFU) yielding the greatest improvement.
Fig. 1.
Effect of Bc on clinical manifestations in mice with DSS-induced colitis. Daily body weight variations (A), representative images of colon length (B), quantitative analysis of colon length (C), and DAI recorded on the 8th day of the experiment (D). The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. a, b Significantly different from normal control and DSS-induced colitis groups at p < 0.05, respectively. DSS: dextran sulfate sodium; DAI: disease activity index; SSZ: sulfasalazine; Bc: Bacillus clausii; LD: low dose, FD: full dose.
3.2. Bc improved colonic oxidative stress/antioxidant-related markers in DSS-induced colitis
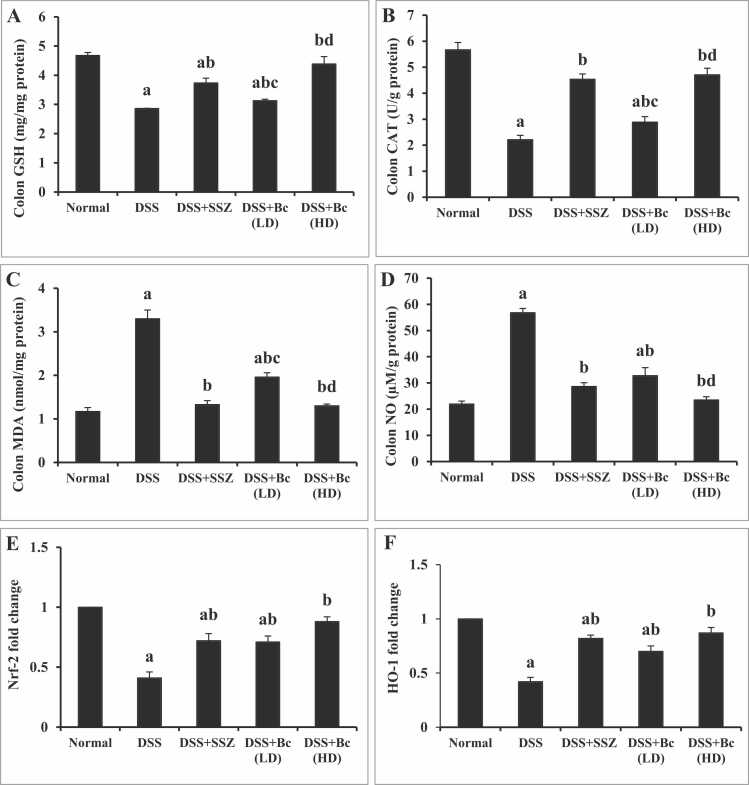
To look into the possible effect of Bc upon oxidative stress indicators and antioxidant-related biomarkers, this study examined the levels of MDA, NO, GSH, and CAT, as well as Nrf2/HO-1 gene expressions across the treated groups (Fig. 2). DSS-induced colitis mice exhibited a noteworthy (p < 0.05) reduction in colonic GSH (Fig. 2A) and CAT (Fig. 2B), coupled with diminished gene expressions of Nrf2 (Fig. 2E) and HO-1 (Fig. 2F), alongside a notable (p < 0.05) rise in colonic MDA (Fig. 2C) and nitrosative stress (NO) (Fig. 2D), indicating an impairment in antioxidant capacity and an elevation in oxidative damage. Treatment with SSZ led to substantial and significant restoration of colonic GSH and CAT contents, along with significant improvement in Nrf2 and HO-1 gene expressions compared to colitis group. Additionally, SSZ normalized (p < 0.05) colonic MDA and NO, suggesting a recovery in the ability to scavenge free radicals. Similarly, administration of Bc (105 CFU) resulted in significant recovery of colonic GSH and CAT contents, accompanied by improved Nrf2 and HO-1 gene expressions and a noticeable decrease (p < 0.05) in colonic MDA and NO contents in contrast to colitis group, pointing to enhanced free radical elimination. Notably, these effects were more pronounced with the higher dose of Bc (1010 CFU).
Fig. 2.
Effect of Bc on colonic GSH (A), CAT (B), MDA (C) and NO (D) contents, as well as Nrf2 (E) and HO-1 (F) expression in mice with DSS-induced colitis. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. a, b, c, d Significantly different from normal control, DSS-induced colitis, DSS+SSZ and DSS+Bc (LD) groups at p < 0.05, respectively. DSS: dextran sulfate sodium; SSZ: sulfasalazine; Bc: Bacillus clausii; LD: low dose, FD: full dose; GSH: reduced glutathione; CAT: catalase; MDA: malondialdehyde; NO: nitric oxide; Nrf2: Nuclear factor erythroid 2-related factor 2; HO-1: Heme oxygenase-1.
3.3. Bc suppressed the colonic inflammatory markers in DSS-induced colitis
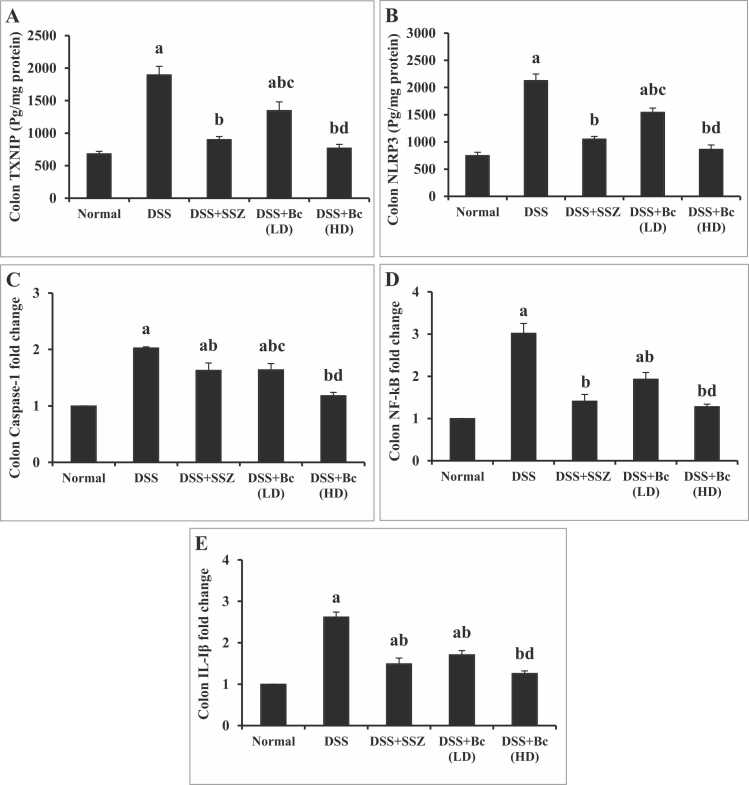
Colonic TXNIP and NLRP3 contents as well as colonic caspase-1, NF-кB, IL-β gene expressions were dramatically elevated (p < 0.05) in colitis group relative to normal control mice, indicating activation of inflammasome pathway. Treatment with either SSZ or Bc at a dose of (105 CFU) significantly diminished colonic TXNIP and NLRP3 contents, as well as caspase-1, NF-кB, and IL-β gene expression. Yet, treatment with Bc in its full dose normalized all inflammasome markers, reflecting better anti-inflammatory activity (Fig. 3).
Fig. 3.
Effect of Bc on colonic TXNIP (A) and NLRP3 (B) contents as well as caspase-1 (C), NF-кB (D), and IL-1β (E) gene expression in mice with DSS-induced colitis. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. a, b, c, d Significantly different from normal control, DSS-induced colitis, DSS+SSZ and DSS+Bc (LD) at p < 0.05, respectively. DSS: dextran sulfate sodium; SSZ: sulfasalazine; Bc: Bacillus clausii; LD: low dose, FD: full dose; TXNIP: thioredoxin-binding protein; NLRP3: pyrin domain containing 3; NF-кB: Nuclear factor kappa B; IL-1β: interleukin-1 beta.
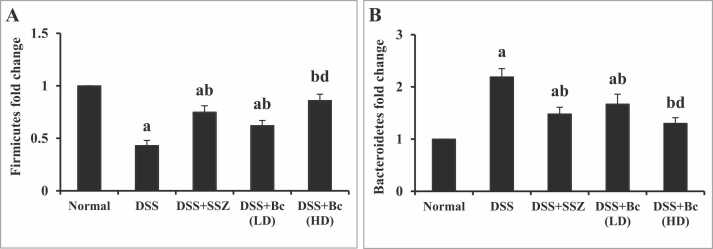
3.4. Bc modulated fecal Firmicutes and Bacteroidetes abundance in DSS-induced colitis
In DSS-induced colitis mice, there was a notable drop (p < 0.05) in the levels of fecal Firmicutes, accompanying an impressive increase in fecal Bacteroidetes abundance in contrast to the control group, indicating an imbalance in the gut microbiota (Fig. 4A & B). However, SZZ administration or Bc (105 CFU) led to a considerable improvement (p < 0.05) in fecal Firmicutes levels, while concurrently causing a considerable decrease in fecal Bacteroidetes abundance compared to the colitis group. Surprisingly, administering of Bc in a concentration of 1010 CFU demonstrated superior enhancement in fecal abundance of Firmicutes with pronounced reduction in fecal Bacteroidetes abundance relative to the lower dose (Fig. 4A-B).
Fig. 4.
Effect of Bc on fecal Firmicutes (A) and Bacteroidetes (B) abundance in mice with DSS-induced colitis. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. a, b, d Significantly different from normal control, DSS-induced colitis, and DSS+Bc (LD) at p < 0.05, respectively. DSS: dextran sulfate sodium; SSZ: sulfasalazine; Bc: Bacillus clausii; LD: low dose, FD: full dose.
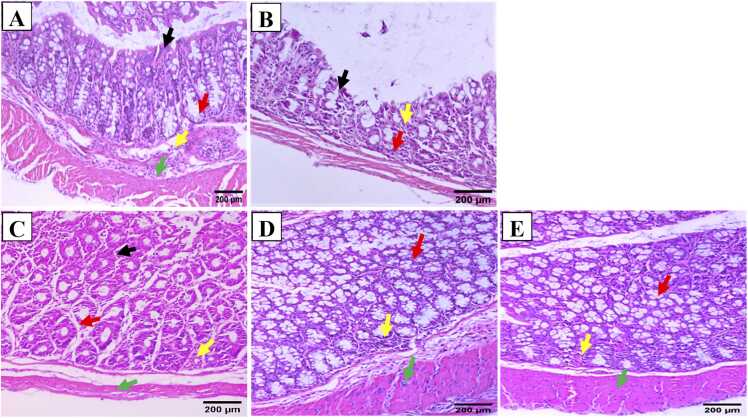
3.5. Bc reversed the histopathological changes in DSS-induced colitis
Colonic sections from normal mucosa exhibited intact architecture characterized by regular-profiled glands, preserved goblet cells, and maintained glandular/crypts ratio, as well as intact lamina propria and muscle layer (Fig. 5A). Conversely, colonic sections from mice with DSS-induced colitis displayed epithelial atrophy characterized by the absence of glands and mucosal flattening. In addition, the mucosa was infiltrated with acute and chronic inflammatory cells, and the glands in the lamina propria were replaced by collagen and inflammatory cells (Fig. 5B). On the other hand, colonic sections from the group treated with SSZ showed maintained architecture of the colonic mucosa, featuring glands with a regular profile and arrangement, along with intact crypts. Additionally, there was a mild inflammatory infiltrate observed in the lamina propria (Fig. 5C). Similarly, colonic sections from mice given a low dosage of Bc spores (105 CFU) displayed nearly normal mucosa with maintained architecture (Fig. 5D). Fortunately, colonic sections from mice administered a higher dosage of Bc spores (1010 CFU) exhibited nearly normal mucosa, with preserved architecture (Fig. 5E). Glands displayed a regular profile and arrangement, maintaining the glands/crypts ratio. Additionally, both the lamina propria and muscle layer remained intact without any significant abnormalities (Fig. 5E).
Fig. 5.
Effect of Bc on colon histological alterations (H & E; x200) in normal control (A), 4 % (w/v) DSS-induced colitis (B), DSS-induced colitis treated with SSZ (200 mg/kg) (C), DSS-induced colitis treated with Bc at doses of 105 CFU (D), and 1010 CFU (E). Black arrows indicated mucosal epithelial lining cells, red arrows indicated linear crypts, yellow arrows indicated lamina propria, and green arrows indicated muscle layer. DSS: dextran sulfate sodium; SSZ: sulfasalazine; Bc: Bacillus clausii; HD: half dose, FD: full dose.
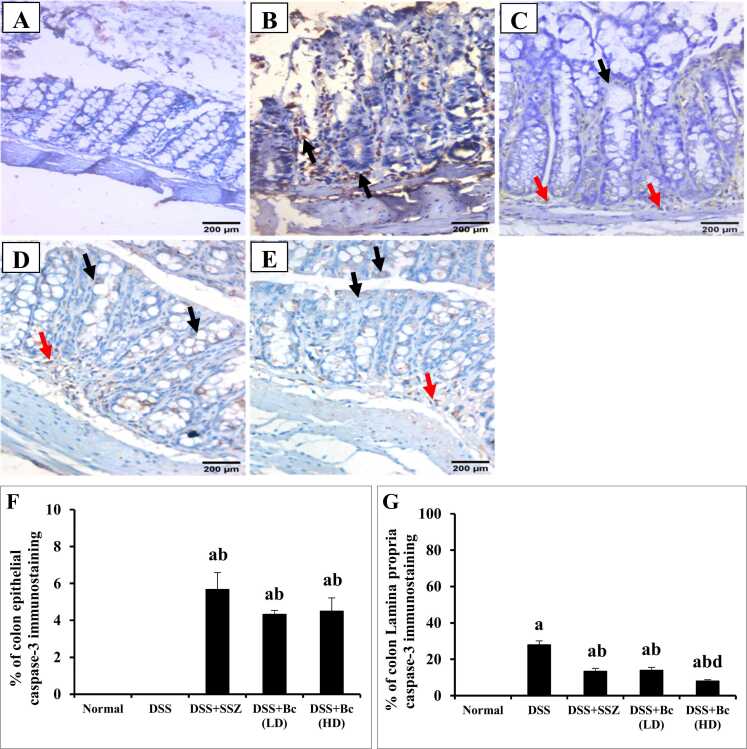
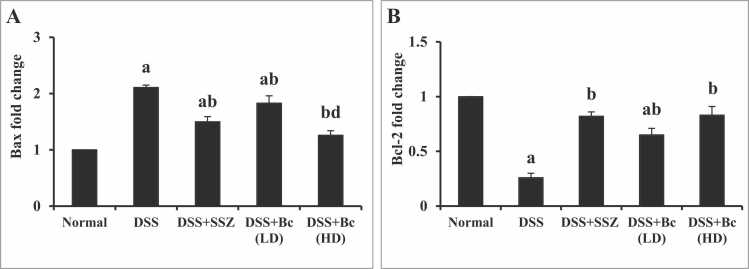
3.6. Bc diminished apoptosis in DSS-induced colitis
Sections of normal colonic mucosa exhibited negative expression of caspase-3 neither in the intestinal epithelial cells nor in the lamina propria (Fig. 6A). While the colonic mucosa of animals with colitis revealed no expression of caspase-3 in the mucosal epithelial lining cells, and cytoplasmic brownish moderate number of positively stained cells (28 %) in lamina propria mononuclear cells (Fig. 6B & F-G). Additionally, in DSS-induced colitis, significant increases in colonic Bax gene expression by 2.11-fold, coupled with a reduction in colonic Bcl-2 expression by 3.85-fold, were observed compared with the normal control group (Fig. 7). Colonic sections from SSZ-treated group showed mild expression of caspase-3 (5.67 %) in the intestinal epithelial cells and moderate number of positively stained cells (13.33 %) in the lamina propria mononuclear cells (Fig. 6C & F-G). Furthermore, treatment with SSZ dramatically decreased colonic Bax gene expression, while an increase in colonic Bcl-2 expression was observed compared to the colitis group (Fig. 7). Colonic sections from the group treated with Bc spores at a low dose of 105 CFU showed mild expression of caspase-3 (4.33 %) in the intestinal epithelial cells and moderate expression (14 %) in the lamina propria mononuclear cells (Fig. 6D & F-G). Colonic sections from the group treated with Bc spores at a high dose of 1010 CFU showed mild expression of caspase-3 (4.5 %) in the intestinal epithelial cells and mild expression (8 %) in the lamina propria mononuclear cells (Fig. 6E-G). Notably, treatment with Bc at a high dose resulted in the normalization of colonic Bax and Bcl-2 gene expression (Fig. 7).
Fig. 6.
Effect of Bc spores on colonic caspase-3 immunostaining changes (DAB, IHC, x200) in normal control (A), 4 % (w/v) DSS-induced colitis (B), DSS-induced colitis treated with SSZ (200 mg/kg) (C), DSS-induced colitis treated with low dose of Bc spores (105 CFU) (D), Bc spores 1010 CFU (E), and % of caspase-3 epithelial (F) and Lamina propria immunostaining (G). Black arrows indicated mucosal epithelial lining cells and red arrows indicated number of lamina propria. DSS: dextran sulfate sodium; SSZ: sulfasalazine; Bc: Bacillus clausii. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. a, b, d Significantly different from normal, DSS-induced colitis, or DSS+Bc (LD) groups at p < 0.05, respectively.
Fig. 7.
Effect of Bc on colonic Bax (A) and Bcl-2 (B) gene expression in mice with DSS-induced colitis. The values are presented as means of 6 mice ± SEM. One-way ANOVA was used for statistical analysis, followed by Tukey's post hoc test. a, b, d Significantly different from normal control, DSS-induced colitis, and DSS+Bc (LD) at p < 0.05, respectively. DSS: dextran sulfate sodium; SSZ: sulfasalazine; Bc: Bacillus clausii; LD: low dose, FD: full dose.
4. Discussion
UC is a widespread persistent inflammatory illness that affects the digestive system. It is distinguished by recurrent and extensive mucosal inflammation that often originates in the rectum and continues to the proximal portions of the colon [44]. Despite its high prevalence, UC poses significant challenges for treatment due to its high resistance to drugs and propensity for recurrence [45]. Moreover, prolonged inflammation and increased disease severity may elevate the risk of colon cancer in certain patients [46]. Several drugs such as 5-Aminosalicylic acid (5-ASA) remains the primary treatment for UC; however, its mechanism of action is not fully understood. It appears to work locally, exerting anti-inflammatory effects by inhibiting the synthesis of prostaglandins and leukotrienes in the colonic mucosa [47]. Despite this, the relief from colonic inflammation is temporary, and symptom recurrence is common [48]. Other treatments, such as tofacitinib, immunosuppressants, and biologics, have shown some clinical efficacy but are limited by their long-term effectiveness and associated side effects [49]. Since the gut microbiota is crucial for maintaining host health and energy conservation, gut dysbiosis can contribute to the onset and progression of colitis [50]. Consequently, there is a pressing need for new UC treatments that both restore gut microbiota balance and provide sustained inflammation suppression.
During UC progresses, immune responses become dysregulated, and abnormal inflammatory signals can disrupt the epithelial barrier and lead to dysbiosis of the intestinal flora [51]. Using probiotics to preserve microbial balance can help prevent and treat disorders such as IBD. Consequently, there is growing interest in modifying the dysbiotic gut microbiota with probiotics as a potential strategy for reducing inflammation and alleviating UC symptoms. Certain probiotics, such as Lactobacilli, Bifidobacteria, and Bacillus strains, have been shown to have positive benefits [52]. Notably, the spore-forming characteristic of Bacillus confers strong resilience and makes them well suited for processing, storage, and survival in the digestive system, proving their potential as probiotics [32]. Moreover, Bc spores are of particular interest due to their ability to withstand the low pH environment of the stomach, biliary acids and varying temperatures while passing through the human GI system, reducing permeability, and promoting a local immunological response [53], offering them an extra benefit over other probiotics. Bacillus species can prevent and cure gastrointestinal problems [54]. Bacillus probiotic Enterogermina, including Bc, has been shown to help cure gastrointestinal illnesses [55]. Therefore, the goal of this study is to ascertain the efficiency of Bc spores in ameliorating the DSS-induced colitis and to understand the underlying mechanisms. In this study, colitis was induced in mice by providing 4 % (w/v) DSS in drinking water. This successfully replicated the pathological alterations observed in patients with UC, including symptoms such as severe diarrhea, loose stools, evident fecal blood, and substantial weight loss [56]. Additionally, mice exposed to DSS exhibited a significant reduction in colon length, indicative of severe inflammation, swelling, and ulceration in the colon. The colonic mucosa showed numerous signs of injury, including degeneration, erosion, congestion, inflammatory cell infiltration, necrosis, and enhanced goblet cell activity. However, orally administering Bc reversed the clinical symptoms induced by DSS-induced colitis. This reversal was characterized by decreased weight loss, improved stool uniformity, and less bleeding from the rectal area. Moreover, histological analysis of colon tissue showed that Bc treatment restored the integrity of colon tissues by reducing inflammation, diminishing epithelial erosion, and increasing colon length. These findings show that Bc dramatically reduces colonic inflammation and protects mice from DSS-induced colitis. These findings align with a previous study [57], which reported significant remission of UC symptoms and improvements in psychological indices following Bc treatment. Similarly, another study demonstrated that Bacillus spp. mitigated DSS-induced colitis in mice by reducing weight loss and bleeding while ameliorating colon histological changes and preserving colon length [58].
Both oxidative stress and inflammation are pivotal variables associated with the pathogenesis of UC, contributing to tissue damage and dysbiosis of the intestinal microbiota [59]. The human defense system's ability to combat oxidative damage is linked to inflammation. During inflammatory processes, immune cells that invade the gut generate and release of ROS. The resulting oxidative stress damages the intestinal mucosa, particularly due to the excessive production of ROS overwhelming the tissue's antioxidant defenses, thus perpetuating or worsening mucosal inflammation. Thus, restoring the balance between antioxidants and ROS is a natural way to develop new drugs for colitis treatment. The Nrf2, a multifunctional regulator, is pivotal in mitigating oxidative stress and inflammation. Nrf2 acts as a protective mediator by controlling the activity of genes encoding antioxidant, anti-inflammatory, and detoxifying proteins, thereby playing a crucial role in cellular defense mechanisms [60]. Moreover, HO-1 has positive benefits via guarding against cellular oxidative damage, controlling apoptosis, and moderating inflammation [60], [61]. Nrf2 boosts the expression in various genes, including detoxification enzymes such as GSH and CAT, which are involved in drug metabolism and the scavenging of ROS. Increased expression of these detoxification enzymes enhances the cell's defense against ROS, thereby shielding against oxidative injury, inflammatory processes, and the death of cells [62]. In the colitis model studied here, increased oxidative stress was observed, supported by raised MDA levels and decreased GSH content and CAT activity in colon tissues. These results are similar with a previous report by Shahid et al. [63], who emphasized the importance of oxidative stress in UC etiology. Administration of Bc reduces oxidative stress as evidenced by the avoidance of GSH, CAT, Nrf2, and HO-1 depletion, along with a notable reduction in colonic MDA and NO contents. The recovery of antioxidant defense capacity, alongside the suppression of inflammatory cytokines, strongly suggests the anti-UC efficacy of Bc. Similarly, prior research stated that Bacillus intake substantially led to the improvement of antioxidant enzymes and diminution of oxidative stress and inflammatory indicators in UC rats by raising Nrf2 and HO-1 levels [64].
Excessive ROS can activate the inflammasome pathway, particularly through the TXNIP/NLRP3 axis [65], [66]. TXNIP is an ubiquitously expressed protein that interacts with thioredoxin and adversely controls its production and activity [67]. A previous research used blood and colon samples from individuals with IBD to show that TXNIP can distinguish between IBD and non-IBD patients [68]. NLRP3 is a critical sensor of microbial and danger signals, affecting the mucosal immune response by boosting the maturation of pro-inflammatory cytokines, including IL-1β [69]. The NLRP3 inflammasome is a critical component of the inflammatory process that is activated by a variety of stimuli, including ATP, microbial agonists, and pore-forming toxins [70]. It has been further highlighted that the role of the NLRP3 inflammasome is not only as an important mediator of host defense but also as an effective regulator of homeostasis in the gut by controlling the integrity of intestinal epithelial cells, thus regulating the gut's immune response to microbiota [62]. NLRP3 is also required to prevent commensal bacterial growth, which can damage the intestinal epithelium and exacerbate the microbial inflammatory response in colitis [71]. Consequently, a better understanding of molecular mechanisms and interactions between gut microbiota and NLRP3 inflammasome activation may facilitate future exploration of potential therapeutic targets for UC. The NLRP3 inflammasome multiprotein aggregate comprises NLRP3 and the effector protein caspase-1. Caspase-1 transforms IL-1β and IL-18 into their active variants. Furthermore, caspase-1 induces pyroptosis, which causes cell lysis and the release of cytosol components towards the surrounding environment [72]. This study demonstrated that the inflammasome pathway was activated, as shown by increased colonic TXNIP and NLRP3 levels, along with elevated gene expression of caspase-1 and IL-1β. This activation, however, was significantly reversed with Bc treatment, particularly at higher doses. Notably, the inhibition or deficiency of the NLRP3 inflammasome complex which includes caspase-1, IL-1β, and NLRP3, has been shown to mitigate inflammation and reduce the pathophysiology of DSS-induced UC [73]. Furthermore, previous research highlights a connection between TXNIP, NLRP3 expression, and NF-κB activity [74], [75], [76]. Activated NF-κB translocates into the nucleus, enhancing the production of pro-IL-1β, pro-IL-18, and inactive NLRP3 [76], which in turn activates caspase-1. Active caspase-1 then proteolytically cleaves the pro-forms of the cytokines, enabling release of the mature cytokines to foster inflammation. Moreover, NF-κB serves as a crucial controller of inflammatory conditions, natural immunity, and tissue stability [77]. Excessive NF-κB activation may contribute to the initiation and progression of colitis in animal models and IBD patients [78]. The current study revealed significant activation of NF-κB in DSS-induced colitis in mice. Treatment with a low dose of SSZ or Bc reduced NF-κB gene expression in the colon, while full-dose Bc therapy restored colonic NF-κB gene expression. These results suggest that Bc exerts anti-inflammatory effects in DSS-induced colitis by inhibiting the NF-κB and TXNIP/NLRP3 inflammasome pathways. This aligns with studies demonstrating that Bacillus species alleviate colitis symptoms in mice and exhibit anti-inflammatory properties via regulating the immune system and suppressing the generation of pro-inflammatory cytokines [79], [80].
The human gut microbiota plays a critical role in immune regulation, with long-term implications for overall health. Zhang et al. [81], had highlighted the significant impact of microbiome dysregulation on the development of colitis. Both colitis patients and mouse models exhibit alterations in the diversity and composition of the intestinal microbiome. When the intestinal microbiota is out of balance, the body's immunity is weakened, the intestinal defense and immunoregulatory systems are compromised, and the relative pathogenic factors are elevated, which can lead to intestinal mucosal invasion or worsen existing conditions [54]. Consequently, modulating the gut microbiota's composition has gained attention as a promising therapeutic strategy for preventing and managing UC. Utilizing probiotics to address dysbiosis presents an alternative to conventional pharmacological interventions [82]. In the current research, DSS disrupts colonic integrity by increasing gut permeability and altering the resident microbiota's composition, which results in a decrease in fecal Firmicutes abundance and an increase in Bacteroidetes abundance. Firmicutes with anti-inflammatory properties have been shown to slow the course of IBD. On the other hand, pro-inflammatory Bacteroidetes can exacerbate IBD by affecting the synthesis of cytokines. This might be because some delicate families of microbes are eliminated from the intestinal canal when inflammatory reactions are irritated, which promotes the survival of the species that modifies the inflammatory course [83]. Previous research has shown that DSS-treated mice often display dysbiosis in their gut microbiota, characterized by a decrease in symbiotic and commensal bacteria and/or an increase in pathogenic bacteria [84]. These findings align with earlier studies demonstrating that DSS induction leads to increased abundance of colitogenic strains such as Bacteroidaceae [85], and that these strains are highly prevalent in individuals with UC [86]. Notably, treatment with Bc reversed the DSS-induced alterations in the relative abundances of Firmicutes and Bacteroidetes. This reversal of gut dysbiosis may be considered the primary mechanism underlying the amelioration of colitis in mice. Previous studies have confirmed the role of Bacillus spp. in improving gut dysbiosis. For instance, Bacillus cereus has been shown to enhance intestinal barrier function and modify the gut microbiota to alleviate colitis symptoms in rats [87]. Bacillus subtilis and Bc spores reduce inflammation while also promoting gut barrier function and overall gastrointestinal health [88]. In mice, Bc improved mild DSS-induced colitis, lowering colonic inflammation scores and altering the gut microbiota composition [89]. Bacillus subtilis may alleviate the severity of colitis by regulating inflammatory cytokines, enhancing tight junction proteins, and reshaping the gut microbiota [90].
The process of apoptosis in intestinal epithelial cells has been recognized as an early event in the onset of UC, which playing a pivotal role in disease progression. Targeted apoptosis has emerged as a potential novel strategy for UC treatment [16]. Various apoptotic signaling molecules are implicated in UC development. Bcl-2, initially identified in B-cell lymphoma, acts as a crucial anti-apoptotic gene [91]. Upon stimulation by apoptotic signals, pro-apoptotic proteins like Bax induce alterations in the permeability of the external mitochondrial barrier, resulting in the expulsion of cytochrome c from mitochondria, thereby activating the effector protein; caspase-3 to initiate downstream apoptotic signals and induce cell apoptosis [92]. Moreover, it was demonstrated that inhibition of the NF-κB pathway in UC rats could mitigate cell apoptosis, reduce inflammatory infiltration, and effectively alleviate colonic inflammatory damage [93]. In the present study, apoptosis was activated in DSS-induced colitis mice, as evidenced by enhanced intestinal mucosa caspase-3 immunostaining, along with elevated colonic expressions of Bax and NF-κB genes, and reduced expression of the anti-apoptotic colonic Bcl-2 gene. These findings were consistent with Giriş et al. [17] who revealed heightened Bax expression and reduced Bcl-2 expression in UC model rats. Treatment with Bc at low dose reduced colon apoptosis, as indicated by decreased caspase-3 immunostaining, Bax and NF-κB gene expressions, and increased Bcl-2 gene expression. Remarkably, a high dosage of Bc can reverse apoptosis by restoring normal gene expression for Bcl-2, Bax, and NF-κB, as well as lowering colonic caspase-3 immunostaining.
Unfortunately, there is little clinical research on the therapeutic effects of probiotics in UC, with the majority conducted on animals. Validating animal experimental findings for clinical use takes more time [94]. The efficacy of Bc and probiotics in general varies according on bacterial species, dose, and form. There is an urgent need, therefore, for mechanistic research employing cutting-edge technologies to investigate the properties, modes of action, and influence on the general composition and diversity of the gut microbiome in GI illnesses before clinical therapy may be adopted. In addition, the combination of genetic engineering with probiotics for the treatment of UC has a bright future in terms of increasing probiotic efficacy [94]. Optimizing the therapeutic use of Bc-containing probiotics will need a deeper understanding of the processes that underpin reported health effects.
5. Conclusion
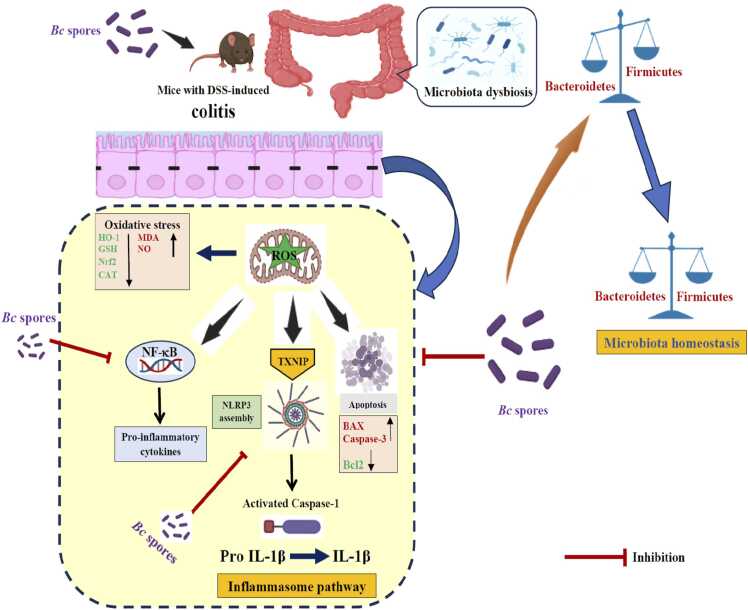
To summarize, Bc has been shown to diminish colon inflammation and associated pathological symptoms in DSS-induced UC. Bc suppresses NF-κB, apoptosis and TXNIP/NLRP3 inflammasome signaling pathways, resulting in reduced caspase-1 cleavage and IL-1β production. It also maintains the antioxidant/oxidant balance, which helps to safeguard the structural integrity of the intestinal mucosa. Crucially, Bc was shown to correct DSS-induced dysbiosis as well as the relative abundances of Firmicutes and Bacteroidetes, highlighting that restoring gut microbiota balance may be the key mechanism driving colitis amelioration in mice (Fig. 8).
Fig. 8.
A schematic diagram demonstrating Bc's mechanism of action in protecting against DSS-induced colitis.
These findings offer new insights into Bc's anti-UC action and inspire scientists to conduct future research into Bc's influence on the gut microbiota in health and disease models in order to explore other therapeutic uses for this probiotic. Furthermore, the extent to which Bc reduces the unfavorable effects of current therapy regimens, as well as its synergistic benefits, should be explored. However, one of our study's limitations is that we only used two dosages of Bc, which may be insufficient for clinical trials or dose-response analysis. Furthermore, more research is needed to understand how NLRP3 inflammasome pathway inhibition efficiently heals and manages UC, as well as how Bc regulates the NLRP3 inflammasome-related proteins, intestinal mucosal integrity, and gut microbiota homeostasis. Due to a paucity of relevant clinical research, more clinical studies on B. clausii are required to better identify the ideal dosage and duration of treatment, as well as the optimal use of Bc. Overall, the findings of this study suggest that Bc treatment could serve as a feasible alternative therapeutic avenue for treating UC and potentially mitigating associated symptoms.
Ethics statement
The experimental procedures complied with the ethical guidelines of the TBRI Ethics Committee for the Care and Use of Laboratory Animals (ethical approval number: PT 682).
Funding
The authors didn’t receive any financial support.
Author statement
We certify that all authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
CRediT authorship contribution statement
Maha B. Salem: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Naglaa M. El-Lakkany: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Formal analysis, Conceptualization. Olfat A. Hammam: Investigation, Formal analysis. Sayed H. Seif el-Din: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Chen X., Xiang X., Xia W., Li X., Wang S., Ye S., Tian L., Zhao L., Ai F., Shen Z., Nie K., Deng M., Wang X. Evolving trends and burden of inflammatory bowel disease in Asia, 1990-2019: a comprehensive analysis based on the global burden of disease study. J. Epidemiol. Glob. Health. 2023;13:725–739. doi: 10.1007/S44197-023-00145-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saber S., Abd El-Kader E.M. Novel complementary coloprotective effects of metformin and MCC950 by modulating HSP90/NLRP3 interaction and inducing autophagy in rats. Inflammopharmacology. 2021;29:237–251. doi: 10.1007/S10787-020-00730-6. [DOI] [PubMed] [Google Scholar]
- 3.Kayal M., Shah S. Ulcerative colitis: current and emerging treatment strategies. J. Clin. Med. 2019;9 doi: 10.3390/JCM9010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estevinho M.M., Rocha C., Correia L., Lago P., Ministro P., Portela F., Trindade E., Afonso J., Peyrin-Biroulet L., Magro F. Features of fecal and colon microbiomes associate with responses to biologic therapies for inflammatory bowel diseases: a systematic review. Clin. Gastroenterol. Hepatol. 2020;18:1054–1069. doi: 10.1016/J.CGH.2019.08.063. [DOI] [PubMed] [Google Scholar]
- 5.Shen Z.H., Zhu C.X., Quan Y.S., Yang Z.Y., Wu S., Luo W.W., Tan B., Wang X.Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018;24:5–14. doi: 10.3748/WJG.V24.I1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra R., Dhawan P., Srivastava A.S., Singh A.B. Inflammatory bowel disease: Therapeutic limitations and prospective of the stem cell therapy. World J. Stem Cells. 2020;12:1050–1066. doi: 10.4252/WJSC.V12.I10.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feuerstein J.D., Moss A.C., Farraye F.A. Ulcerative colitis. Mayo Clin. Proc. 2019;94:1357–1373. doi: 10.1016/J.MAYOCP.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Allegretti J.R., Barnes E.L., Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflamm. Bowel Dis. 2015;21:1089–1097. doi: 10.1097/MIB.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long M.D., Martin C.F., Pipkin C.A., Herfarth H.H., Sandler R.S., Kappelman M.D. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399.e1. doi: 10.1053/J.GASTRO.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wasan S.K., Coukos J.A., Farraye F.A. Vaccinating the inflammatory bowel disease patient: deficiencies in gastroenterologists knowledge. Inflamm. Bowel Dis. 2011;17:2536–2540. doi: 10.1002/IBD.21667. [DOI] [PubMed] [Google Scholar]
- 11.Sebastian S.A., Kaiwan O., Co E.L., Mehendale M., Mohan B.P. Current pharmacologic options and emerging therapeutic approaches for the management of ulcerative colitis: a narrative review. Spartan Med. Res. J. 2024;9 doi: 10.51894/001C.123397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subudhi R.N., Poonia N., Singh D., Arora V. Natural approaches for the management of ulcerative colitis: evidence of preclinical and clinical investigations. Nat. Prod. Bioprospect. 2024;14 doi: 10.1007/S13659-024-00463-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue J.C., Yuan S., Meng H., Hou X.T., Li J., Zhang H.M., Chen L.L., Zhang C.H., Zhang Q.G. The role and mechanism of flavonoid herbal natural products in ulcerative colitis. Biomed. Pharmacother. 2023;158 doi: 10.1016/J.BIOPHA.2022.114086. [DOI] [PubMed] [Google Scholar]
- 14.da Silva A.S., Balbé F.P., Fontana T., da L., Fernandes S., Rech V.C. Nanotechnology applications in Ulcerative colitis: recent developments and future directions. Discip. Sci. | Nat. e Tecnol. ógicas. 2024;24:21–29. doi: 10.37779/nt.v24i3.4777. [DOI] [Google Scholar]
- 15.Chen B., Dong X., Zhang J.L., Sun X., Zhou L., Zhao K., Deng H., Sun Z. Natural compounds target programmed cell death (PCD) signaling mechanism to treat ulcerative colitis: a review. Front. Pharmacol. 2024;15 doi: 10.3389/FPHAR.2024.1333657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M., Xu B., Liu L., Wang D. Oridonin attenuates dextran sulfate sodium‑induced ulcerative colitis in mice via the Sirt1/NF‑κB/p53 pathway. Mol. Med. Rep. 2022;26 doi: 10.3892/MMR.2022.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giriş M., Depboylu B., Doǧru-Abbasoǧlu S., Erbil Y., Olgaç V., Aliş H., Aykaç-Toker G., Uysal M. Effect of taurine on oxidative stress and apoptosis-related protein expression in trinitrobenzene sulphonic acid-induced colitis. Clin. Exp. Immunol. 2008;152:102–110. doi: 10.1111/J.1365-2249.2008.03599.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/ANNUREV-PHARMTOX-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H., Johnston L.J., Wang F., Ma X. Triggers for the Nrf2/ARE Signaling Pathway and Its Nutritional Regulation: Potential Therapeutic Applications of Ulcerative Colitis. Int. J. Mol. Sci. 2021;22 doi: 10.3390/IJMS222111411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganesh Yerra V., Negi G., Sharma S.S., Kumar A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013;1:394–397. doi: 10.1016/J.REDOX.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surh Y.J., Chun K.S., Cha H.H., Han S.S., Keum Y.S., Park K.K., Lee S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res. - Fundam. Mol. Mech. Mutagen. 2001;480–481:243–268. doi: 10.1016/S0027-5107(01)00183-X. [DOI] [PubMed] [Google Scholar]
- 22.Tourkochristou E., Aggeletopoulou I., Konstantakis C., Triantos C. Role of NLRP3 inflammasome in inflammatory bowel diseases. World J. Gastroenterol. 2019;25:4796–4804. doi: 10.3748/WJG.V25.I33.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue J.C., Yuan S., Hou X.T., Meng H., Liu B.H., Cheng W.W., Zhao M., Li H.Ben, Guo X.F., Di C., Li M.J., Zhang Q.G. Natural products modulate NLRP3 in ulcerative colitis. Front. Pharmacol. 2023;14 doi: 10.3389/FPHAR.2023.1265825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagatsuma K., Nakase H. Contradictory Effects of NLRP3 Inflammasome Regulatory Mechanisms in Colitis. Int. J. Mol. Sci. 2020;21:1–24. doi: 10.3390/IJMS21218145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartor R.B. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006;3:390–407. doi: 10.1038/NCPGASTHEP0528. [DOI] [PubMed] [Google Scholar]
- 26.Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V., Reyes J.A., Shah S.A., LeLeiko N., Snapper S.B., Bousvaros A., Korzenik J., Sands B.E., Xavier R.J., Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13 doi: 10.1186/GB-2012-13-9-R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7 doi: 10.3390/MICROORGANISMS7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibiino G., Lopetuso L.R., Scaldaferri F., Rizzatti G., Binda C., Gasbarrini A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018;50:635–639. doi: 10.1016/J.DLD.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Bhosle A., Bae S., McIver L.J., Pishchany G., Accorsi E.K., Thompson K.N., Arze C., Wang Y., Subramanian A., Kearney S.M., Pawluk A., Plichta D.R., Rahnavard A., Shafquat A., Xavier R.J., Vlamakis H., Garrett W.S., Krueger A., Huttenhower C., Franzosa E.A. Discovery of bioactive microbial gene products in inflammatory bowel disease. Nature. 2022;606:754–760. doi: 10.1038/S41586-022-04648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvamani S., Mehta V., Ali El Enshasy H., Thevarajoo S., El Adawi H., Zeini I., Pham K., Varzakas T., Abomoelak B. Efficacy of probiotics-based interventions as therapy for inflammatory bowel disease: a recent update. Saudi J. Biol. Sci. 2022;29:3546. doi: 10.1016/J.SJBS.2022.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todorov S.D., Ivanova I.V., Popov I., Weeks R., Chikindas M.L. Bacillus spore-forming probiotics: benefits with concerns? Crit. Rev. Microbiol. 2022;48:513–530. doi: 10.1080/1040841X.2021.1983517. [DOI] [PubMed] [Google Scholar]
- 32.Elshaghabee F.M.F., Rokana N., Gulhane R.D., Sharma C., Panwar H. Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 2017;8 doi: 10.3389/FMICB.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee L.-H., Loo K.-Y., Law J.W.-F., Tan L.T.-H., Ratnasingam V., Thurairajasingam S., Chan K.-G., Letchumanan V. Bacillus causii: potential probiotic effects beyond gastrointestinal diseases. Gut. 2024;73:A206–A207. doi: 10.1136/GUTJNL-2024-IDDF.132. [DOI] [Google Scholar]
- 34.Ianiro G., Rizzatti G., Plomer M., Lopetuso L., Scaldaferri F., Franceschi F., Cammarota G., Gasbarrini A. Bacillus clausii for the treatment of acute diarrhea in children: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10 doi: 10.3390/NU10081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pradhan B., Guha D., Naik A.K., Banerjee A., Tambat S., Chawla S., Senapati S., Aich P. Probiotics L. acidophilus and B. clausii Modulate Gut Microbiota in Th1- and Th2-Biased Mice to Ameliorate Salmonella Typhimurium-Induced Diarrhea. Probiotics Antimicrob. Proteins. 2019;11:887–904. doi: 10.1007/S12602-018-9436-5. [DOI] [PubMed] [Google Scholar]
- 36.Dar H.Y., Pal S., Shukla P., Mishra P.K., Tomar G.B., Chattopadhyay N., Srivastava R.K. Bacillus clausii inhibits bone loss by skewing Treg-Th17 cell equilibrium in postmenopausal osteoporotic mice model. Nutrition. 2018;54:118–128. doi: 10.1016/J.NUT.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Caro S.Di, Tao H., Grillo A., Franceschi F., Elia C., Zocco M.A., Gasbarrini G., Sepulveda A.R., Gasbarrini A. Bacillus clausii effect on gene expression pattern in small bowel mucosa using DNA microarray analysis. Eur. J. Gastroenterol. Hepatol. 2005;17:951–960. doi: 10.1097/00042737-200509000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Salem M.B., Elzallat M., Mostafa Mohammed D., Hammam O.A., Abdel-Wareth M.Tamim A., Hassan M. Helix pomatia mucin alleviates DSS-induced colitis in mice: Unraveling the cross talk between microbiota and intestinal chemokine. Heliyon. 2024;10 doi: 10.1016/J.HELIYON.2024.E37362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salem M.B., El-Lakkany N.M., Seif el-Din S.H., Hammam O.A., Samir S. Diosmin alleviates ulcerative colitis in mice by increasing Akkermansia muciniphila abundance, improving intestinal barrier function, and modulating the NF-κB and Nrf2 pathways. Heliyon. 2024;10 doi: 10.1016/J.HELIYON.2024.E27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Y.F., Zhou J.T., Qu C., Dou Y.X., Huang Q.H., Lin Z.X., Xian Y.F., Xie J.H., Xie Y.L., Lai X.P., Su Z.R. Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NF-κB activation on dextran sulfate sodium-induced ulcerative colitis in mice. J. Ethnopharmacol. 2017;198:389–398. doi: 10.1016/J.JEP.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 41.Salem M.B., Elzallat M., Mohammed D.M., Samir S., Hammam O.A., Abdel-Wareth M.T.A. Cornu aspersum mucin attenuates indomethacins-induced gastric ulcers in mice via alleviating oxidative stress and inflammation. Heliyon. 2023;9 doi: 10.1016/J.HELIYON.2023.E15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo W., Sun Y., Liu W., Wu X., Guo L., Cai P., Wu X., Wu X., Shen Y., Shu Y., Gu Y., Xu Q. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy. 2014;10:972–985. doi: 10.4161/AUTO.28374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao X., Huang X., Zhou Z., Lin X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 2013;3:71. /pmc/articles/PMC4280562/ (accessed September 20, 2023) [PMC free article] [PubMed] [Google Scholar]
- 44.Ungaro R., Mehandru S., Allen P.B., Peyrin-Biroulet L., Colombel J.F. Ulcerative colitis. Lancet (Lond., Engl. ) 2017;389:1756. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dragoni G., Innocenti T., Amiot A., Castiglione F., Melotti L., Festa S., Savarino E.V., Truyens M., Argyriou K., Noviello D., Molnar T., Bouillon V., Bezzio C., Eder P., Fernandes S., Kagramanova A., Armuzzi A., Oliveira R., Viola A., Ribaldone D.G., Drygiannakis I., Viganò C., Calella F., Gravina A.G., Pugliese D., Chaparro M., Ellul P., Vieujean S., Milla M., Caprioli F., Aratari A., Belli F., Botto I., Bretto E., Cremer A., Buono A.Dal, Giorgi D.A., Gisbert J.P., Kapsoritakis A., Koutroubakis I., Laffusa A., Lobaton T., Nardone O.M., Neves J., Oleg K., Pellegrino R., Poggioli G., Rottoli M., Saibeni S. Rates of adverse events in patients with ulcerative colitis undergoing colectomy during treatment with tofacitinib vs biologics: a multicenter observational study. Am. J. Gastroenterol. 2024;119:1525–1535. doi: 10.14309/AJG.0000000000002676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaghari-Tabari M., Targhazeh N., Moein S., Qujeq D., Alemi F., Majidina M., Younesi S., Asemi Z., Yousefi B. From inflammatory bowel disease to colorectal cancer: what’s the role of miRNAs? Cancer Cell Int. 2022;221(22):1–21. doi: 10.1186/S12935-022-02557-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Datta P., Rewers-Felkins K., Kallem R.R., Baker T., Hale T.W. Determination of mesalamine levels in human milk as a function of dose. Breastfeed. Med. 2019;14:98–101. doi: 10.1089/BFM.2018.0111. [DOI] [PubMed] [Google Scholar]
- 48.Jin J.J., Ko I.G., Hwang L., Kim S.H., Jee Y.S., Jeon H., Park S.B., Jeon J.W. Simultaneous treatment of 5-aminosalicylic acid and treadmill exercise more effectively improves ulcerative colitis in mice. Int. J. Mol. Sci. 2024;25 doi: 10.3390/IJMS25105076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonovas S., Lytras T., Nikolopoulos G., Peyrin-Biroulet L., Danese S. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment. Pharmacol. Ther. 2018;47:454–465. doi: 10.1111/APT.14449. [DOI] [PubMed] [Google Scholar]
- 50.Dong W.R., Li Y.Y., Liu T.T., Zhou G., Chen Y.X. Ethyl acetate extract of Terminalia chebula alleviates DSS-induced ulcerative colitis in C57BL/6 mice. Front. Pharmacol. 2023;14 doi: 10.3389/FPHAR.2023.1229772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zundler S., Becker E., Schulze L.Lou, Neurath M.F. Immune cell trafficking and retention in inflammatory bowel disease: mechanistic insights and therapeutic advances. Gut. 2019;68:1688–1700. doi: 10.1136/GUTJNL-2018-317977. [DOI] [PubMed] [Google Scholar]
- 52.Andrade J.C., Almeida D., Domingos M., Seabra C.L., Machado D., Freitas A.C., Gomes A.M. Commensal obligate anaerobic bacteria and health: production, storage, and delivery strategies. Front. Bioeng. Biotechnol. 2020;8:550. doi: 10.3389/FBIOE.2020.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isolauri E., Sütas Y., Kankaanpää P., Arvilommi H., Salminen S. Probiotics: effects on immunity. Am. J. Clin. Nutr. 2001;73 doi: 10.1093/AJCN/73.2.444S. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y.J., Li S., Gan R.Y., Zhou T., Xu D.P., Bin Li H. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015;16:7493–7519. doi: 10.3390/IJMS16047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urdaci M.C., Bressollier P., Pinchuk I. Bacillus clausii probiotic strains: antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004;38 doi: 10.1097/01.MCG.0000128925.06662.69. [DOI] [PubMed] [Google Scholar]
- 56.Zhang S., Cao Y., Huang Y., Zhang S., Wang G., Fang X., Bao W. Aqueous M. oleifera leaf extract alleviates DSS-induced colitis in mice through suppression of inflammation. J. Ethnopharmacol. 2024;318 doi: 10.1016/J.JEP.2023.116929. [DOI] [PubMed] [Google Scholar]
- 57.Bamola V.D., Dubey D., Samanta P., Kedia S., Ahuja V., Madempudi R.S., Neelamraju J., Chaudhry R. Role of a probiotic strain in the modulation of gut microbiota and cytokines in inflammatory bowel disease. Anaerobe. 2022;78 doi: 10.1016/J.ANAEROBE.2022.102652. [DOI] [PubMed] [Google Scholar]
- 58.Jing Y., Liu H., Xu W., Yang Q. Amelioration of the DSS-induced colitis in mice by pretreatment with 4,4′-diaponeurosporene-producing Bacillus subtilis. Exp. Ther. Med. 2017;14:6069. doi: 10.3892/ETM.2017.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X.Y., Meng L., Shen L., Ji H.F. Regulation of gut microbiota by vitamin C, vitamin E and β-carotene. Food Res. Int. 2023;169 doi: 10.1016/J.FOODRES.2023.112749. [DOI] [PubMed] [Google Scholar]
- 60.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/S00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2 doi: 10.1038/SIGTRANS.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L., Peng P., Ding N., Jia W., Huang C., Tang Y. Oxidative stress, inflammation, gut dysbiosis: what can polyphenols do in inflammatory bowel disease? Antioxid. (Basel, Switz. ) 2023;12 doi: 10.3390/ANTIOX12040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shahid M., Raish M., Ahmad A., Bin Jardan Y.A., Ansari M.A., Ahad A., Alkharfy K.M., Alaofi A.L., Al-Jenoobi F.I. Sinapic acid ameliorates acetic acid-induced ulcerative colitis in rats by suppressing inflammation, oxidative stress, and apoptosis. Molecules. 2022;27 doi: 10.3390/MOLECULES27134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y., Wang B., Tang L., Zhou Y., Wang Q., Gong L., Ni J., Li W. Probiotic bacillus alleviates oxidative stress-induced liver injury by modulating gut-liver axis in a rat model. Antioxid. (Basel, Switz. ) 2022;11 doi: 10.3390/ANTIOX11020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saber S., Youssef M.E., Sharaf H., Amin N.A., El-Shedody R., Aboutouk F.H., El-Galeel Y.A., El-Hefnawy A., Shabaka D., Khalifa A., Saleh R.A., Osama D., El-Zoghby G., Gobba N.A. BBG enhances OLT1177-induced NLRP3 inflammasome inactivation by targeting P2X7R/NLRP3 and MyD88/NF-κB signaling in DSS-induced colitis in rats. Life Sci. 2021;270 doi: 10.1016/J.LFS.2021.119123. [DOI] [PubMed] [Google Scholar]
- 66.Salem M.B., Morsi E.A., El-Wakil E.A., El-Lakkany N.M., Abou-shousha T., Abdel-Hady H. HPLC fingerprinting/GC-MS analysis, and efficacy of Hypericum perforatum against cisplatin-induced hepato-renal toxicity in mice with insights into the TXNIP/NLRP3 pathway. J. Appl. Pharm. Sci. 2023;13:037–047. doi: 10.7324/JAPS.2023.6683. [DOI] [Google Scholar]
- 67.Alhawiti N.M., Al Mahri S., Aziz M.A., Malik S.S., Mohammad S. TXNIP in metabolic regulation: physiological role and therapeutic outlook. Curr. Drug Targets. 2017;18 doi: 10.2174/1389450118666170130145514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Requena T., Martínez-Cuesta M.C., Peláez C. Diet and microbiota linked in health and disease. Food Funct. 2018;9:688–704. doi: 10.1039/C7FO01820G. [DOI] [PubMed] [Google Scholar]
- 69.Zhen Y., Zhang H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019;10 doi: 10.3389/FIMMU.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y., Tan L., Li C., Wu H., Ran D., Zhang Z. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Express. 2020;10 doi: 10.1186/S13568-020-01053-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang D., Wang Z., Chen Y., Guo Q., Dong Y. Interactions between gut microbes and NLRP3 inflammasome in the gut-brain axis. Comput. Struct. Biotechnol. J. 2023;21:2215. doi: 10.1016/J.CSBJ.2023.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miao E.A., Rajan J.V., Aderem A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011;243:206–214. doi: 10.1111/J.1600-065X.2011.01044.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hua L., Liang S., Zhou Y., Wu X., Cai H., Liu Z., Ou Y., Chen Y., Chen X., Yan Y., Wu D., Sun P., Hu W., Yang Z. Artemisinin-derived artemisitene blocks ROS-mediated NLRP3 inflammasome and alleviates ulcerative colitis. Int. Immunopharmacol. 2022;113 doi: 10.1016/J.INTIMP.2022.109431. [DOI] [PubMed] [Google Scholar]
- 74.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K.A., Hornung V., Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/JIMMUNOL.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pellegrini C., Antonioli L., Lopez-Castejon G., Blandizzi C., Fornai M. Canonical and non-canonical activation of NLRP3 inflammasome at the crossroad between immune tolerance and intestinal inflammation. Front. Immunol. 2017;8 doi: 10.3389/FIMMU.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Q., Zuo R., Wang K., fei Nong F., Fu Y. jun, Huang S. wei, Pan Z. feng, Zhang Y., Luo X., Deng X. liang, Zhang X. xue, Zhou L., Chen Y. Oroxindin inhibits macrophage NLRP3 inflammasome activation in DSS-induced ulcerative colitis in mice via suppressing TXNIP-dependent NF-κB pathway. Acta Pharmacol. Sin. 2020;416(41):771–781. doi: 10.1038/s41401-019-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banoth B., Chatterjee B., Vijayaragavan B., Prasad M.V.R., Roy P., Basak S. Stimulus-selective crosstalk via the NF-κB signaling system reinforces innate immune response to alleviate gut infection. Elife. 2015;4:1–56. doi: 10.7554/ELIFE.05648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atreya I., Atreya R., Neurath M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008;263:591–596. doi: 10.1111/J.1365-2796.2008.01953.X. [DOI] [PubMed] [Google Scholar]
- 79.Khalifa A., Sheikh A., Ibrahim H.I.M. Bacillus amyloliquefaciens Enriched Camel Milk Attenuated Colitis Symptoms in Mice Model. Nutrients. 2022;14 doi: 10.3390/NU14091967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang X., Ai F., Ji C., Tu P., Gao Y., Wu Y., Yan F., Yu T. A Rapid Screening Method of Candidate Probiotics for Inflammatory Bowel Diseases and the Anti-inflammatory Effect of the Selected Strain Bacillus smithii XY1. Front. Microbiol. 2021;12 doi: 10.3389/FMICB.2021.760385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang T., Zhong H., Lin L., Zhang Z., Xue K., He F., Luo Y., Wang P., Zhao Z., Cong L., Pang P., Li X., Shan H., Yan Z. Core microbiome-associated proteins associated with ulcerative colitis interact with cytokines for synergistic or antagonistic effects on gut bacteria. ISME J. 2024;18 doi: 10.1093/ISMEJO/WRAE146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong-Chew R.M., de Castro J.A.A., Morelli L., Perez M., Ozen M. Gut immune homeostasis: the immunomodulatory role of Bacillus clausii, from basic to clinical evidence. Expert Rev. Clin. Immunol. 2022;18:717–729. doi: 10.1080/1744666X.2022.2085559. [DOI] [PubMed] [Google Scholar]
- 83.Li C., Deng L., Pu M., Ye X., Lu Q. Coptisine alleviates colitis through modulating gut microbiota and inhibiting TXNIP/NLRP3 inflammasome. J. Ethnopharmacol. 2024;335 doi: 10.1016/J.JEP.2024.118680. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Q., Wu Y., Wang J., Wu G., Long W., Xue Z., Wang L., Zhang X., Pang X., Zhao Y., Zhao L., Zhang C. Accelerated dysbiosis of gut microbiota during aggravation of DSS-induced colitis by a butyrate-producing bacterium. Sci. Rep. 2016;6 doi: 10.1038/SREP27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohkusa T., Koido S. Intestinal microbiota and ulcerative colitis. J. Infect. Chemother. 2015;21:761–768. doi: 10.1016/J.JIAC.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 86.Walker A.W., Sanderson J.D., Churcher C., Parkes G.C., Hudspith B.N., Rayment N., Brostoff J., Parkhill J., Dougan G., Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11 doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheng K., Xu Y., Kong X., Wang J., Zha X., Wang Y. Probiotic Bacillus cereus alleviates dextran sulfate sodium-induced colitis in mice through improvement of the intestinal barrier function, anti-inflammation, and gut microbiota modulation. J. Agric. Food Chem. 2021;69:14810–14823. doi: 10.1021/ACS.JAFC.1C03375. [DOI] [PubMed] [Google Scholar]
- 88.Vittoria M., Horwell E., Bastoni D., Saggese A., Baccigalupi L., Cutting S.M., Ricca E. Bacillus subtilis SF106 and Bacillus clausii SF174 spores reduce the inflammation and modulate the gut microbiota in a colitis model. Benef. Microbes. 2024;15:343–355. doi: 10.1163/18762891-BJA00016. [DOI] [PubMed] [Google Scholar]
- 89.M. Scaldaferri, F., Graziani, C., Mora, V., Petito, V., Lopetuso, L.R., Puca, P., Ianiro, G., Napolitano, D., Quaranta, G., Masucci, L. and Sanguinetti, Bacillus clausii (O/C, SIN, N/R, T) Improves Acute Mild Colitis in Mice while In-Vivo Modulating Gut Microbiota, (n.d.). 〈https://meddocsonline.org/annals-of-gastroenterology-and-the-digestive-system/bacillus-clausii-oc-sin-nr-t-improves-acute-mild-colitis-in-mice-while-in-vivo-modulating-gut-microbiota.html〉 (accessed November 3, 2024).
- 90.Luo R., Zhang J., Zhang X., Zhou Z., Zhang W., Zhu Z., Liu H., Wang L., Zhong Z., Fu H., Jing B., Peng G. Bacillus subtilis HH2 ameliorates TNBS-induced colitis by modulating gut microbiota composition and improving intestinal barrier function in rabbit model. J. Funct. Foods. 2020;74 doi: 10.1016/J.JFF.2020.104167. [DOI] [Google Scholar]
- 91.Alam M., Ali S., Mohammad T., Hasan G.M., Yadav D.K., Hassan M.I. B cell lymphoma 2: a potential therapeutic target for cancer therapy. Int. J. Mol. Sci. 2021;22 doi: 10.3390/IJMS221910442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vringer E., Tait S.W.G. Mitochondria and cell death-associated inflammation. Cell Death Differ. 2022;302(30):304–312. doi: 10.1038/s41418-022-01094-w. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arab H.H., Al-Shorbagy M.Y., Saad M.A. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem. Biol. Interact. 2021;335 doi: 10.1016/J.CBI.2021.109368. [DOI] [PubMed] [Google Scholar]
- 94.Huang C., Hao W., Wang X., Zhou R., Lin Q. Probiotics for the treatment of ulcerative colitis: a review of experimental research from 2018 to 2022. Front. Microbiol. 2023;14 doi: 10.3389/FMICB.2023.1211271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.