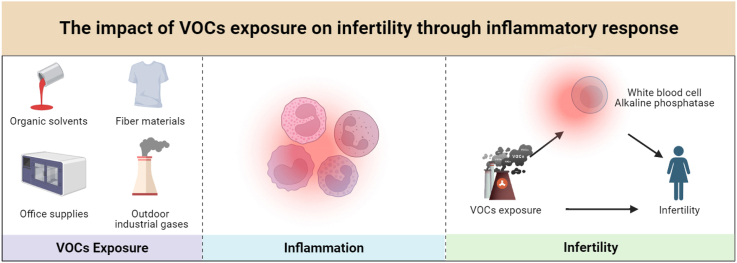
Abstact
Background
Growing evidence suggests that environmental pollutants exert a detrimental impact on female fertility. Among these pollutants, volatile organic compounds (VOCs), easily encountered in the environment, have garnered significant attention as prevalent airborne contaminants. Nevertheless, a definitive consensus regarding the association between VOCs and the incidence of infertility remains elusive.
Method
Conducted as a cross-sectional study, this research utilized data from three survey cycles of the NHANES program spanning from 2013 to 2018. The objective was to delve into the relationship between volatile organic compounds and the prevalence of infertility. The definition of infertility relied upon information derived from the reproductive health questionnaire. In order to comprehensively explore this relationship, various analytical models, including logistic regression, weighted quantile sum (WQS), and Bayesian kernel-machine regression (BKMR), were employed.
Result
A total of 1098 participants, 120 in the infertility group and 978 in the control group, were included. All 15 volatile organic compounds showed higher concentrations in the infertility group's urine. Multivariate regression revealed that the highest AMCC (N-acetyl-S-(N-methylcarbamoyl)-L-cysteine) and CYMA (2-hydroxyethyl mercapturic acid) quartiles associated with significant infertility increases, approximately 191 % and 128 %, respectively, versus the lowest quartile (OR for AMCC = 2.91; 95%CI: 1.33, 6.37; OR for CYMA = 2.28; 95%CI: 1.01, 5.15). This emphasizes AMCC and CYMA's role in infertility, supported by WQS and BKMR studies. Inflammation mediates AMCC's impact on infertility, enhancing our understanding of volatile organic compounds and reproductive health.
Conclusion
The study highlights the correlation between VOCs exposure, notably AMCC and CYMA, and infertility. It identifies inflammation as a mediating factor connecting AMCC to infertility.
Keywords: Environment pollution, Volatile organic chemicals, Inflammation, NHANES
Graphical abstract
1. Introduction
Infertility, a prevalent reproductive disorder, is characterized by the inability to achieve a clinical pregnancy after 12 months of regular unprotected sex [1]. Global studies by the World Health Organization indicate a current infertility prevalence of approximately 9 % [2]. In recent years, this prevalence has been on the rise, posing significant psychological, physical, and economic challenges to affected individuals and impacting societal stability [3]. Female factors contribute to 50 % of all infertility cases, while male factors account for 20–30 % [4]. Direct or indirect causes of female infertility encompass advanced age, endocrine issues, reproductive organ damage, ovarian insufficiency, endometriosis, polycystic ovary syndrome, or sexually transmitted diseases [5]. Previous research has linked infertility to factors such as obesity [6], smoking [7], alcohol consumption[8], and dietary habits [9]. Moreover, there is a growing body of evidence suggesting that environmental pollutants, including heavy metals, phthalates, and phenols [10], exert a negative influence on female fertility.
Amidst the accelerated pace of global urbanization and industrialization, volatile organic compounds (VOCs) are rapidly emerging as ubiquitous and intricate organic pollutants in the air. These compounds originate from diverse sources, including cigarette smoke, paints, industrial production, and automobile exhaust [11]. Unlike pollutants encountered through specific work environments or food, VOCs are primarily airborne, making them more easily accessible to the general population. Furthermore, human exposure to VOCs can be evaluated by monitoring their levels in blood, urine, breath, and sweat [12,13]. Previous research has linked VOCs to conditions such as asthma [14], pulmonary dysfunction [15], and cardiovascular disease [18]. However, uncertainties persist regarding the relationship between VOCs and the prevalence of infertility. Some studies have suggested the involvement of inflammation in infertility, with elevated inflammatory markers, such as leukocytes, potentially contributing to infertility development in non-obese women [16].
Building upon findings from related studies, our hypothesis posits that VOCs may constitute a novel environmental pollutant contributing to infertility. In this study, we employed logistic regression, weighted quantile sum (WQS), and Bayesian kernel machine regression (BKMR) models to shed light on the intricate relationship between VOCs and infertility prevalence. Additionally, mediation analysis was utilized to explore the potential association of inflammation as a mediator between VOCs and the prevalence of infertility.
2. Materials and methods
2.1. Data collection
All the data used in this cross-sectional study were derived from the National Health and Nutrition Examination Survey (NHANES) program. Conducted biennially since 1999, this survey requires participants to provide informed consent, furnish detailed information outlined in the consent form, and formally sign the consent document before joining the study. The dataset includes information on nutritional intake, physical health status, and relevant clinical indicators for the broader U.S. population.
Our study focused on findings from three cycles: 2013–2014 (n = 10,175), 2015–2016 (n = 9971), and 2017–2018 (n = 9254). Following a thorough collection and compilation of data on general clinical information and urine VOCs test results from a total of 29,400 individuals, we screened and ultimately selected 1098 participants for an in-depth analysis of the association between urinary VOCs and infertility. This selection process is illustrated in Fig. 1 through a detailed flowchart, delineating the inclusion/exclusion criteria.
Fig. 1.
Flowchart of participants included in this study. NHANES, National Health and Nutrition Examination Survey. VOCs, volatile organic compound metabolites.
2.2. Urine VOCs measurement
This method employs ultra-performance liquid chromatography coupled with electrospray tandem mass spectrometry (UPLC-ESI/MSMS) [17] as a quantitative procedure for measuring VOCs metabolites in human urine. The outcomes are expressed in ng/mL, representing the concentration of VOCs in urine. It's essential to highlight that 11 metabolites were excluded from the analysis due to partially similar reported values or having a lowest concentration exceeding one-third of the total population. Consequently, the final analysis focused on 15 VOC metabolites present in urine.
2.3. Assessment of infertility
The definition of infertility relies on responses collected through a comprehensive reproductive health survey questionnaire. Specifically, two key inquiries, RHQ074 and RHQ075, serve as the criteria for assessment. The first question probes whether individuals have actively endeavored to conceive for a minimum of one year without achieving pregnancy. Women responding affirmatively to this question and the second query, asking if they have sought medical assistance due to difficulties in conceiving, are categorized as individuals experiencing infertility. Conversely, those responding negatively to either of these questions are classified as within the normal range.
2.4. Assessment of covariates
The study gathered a spectrum of demographic information from participants, encompassing factors like age, gender, ethnicity, literacy, and poverty-to-income ratios (PIR). Additionally, physical indicators such as BMI, lifestyle behaviors (alcohol consumption, smoking status), comorbidities (hypertension, diabetes mellitus), and renal function data (urinary albumin, urinary creatinine) were systematically analyzed as covariates. Ethnicity categories included Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other Race. Educational attainment was classified into categories like less than high school, high school graduate, college or above, and not recorded. Household economic status was stratified based on PIR, with less than 1.3 denoted as low income, 1.3 to 3.5 as middle income, and ≥3.5 as high income.
Drinking status was characterized by three states (lifetime abstainer, former drinker, current drinker) derived from questionnaire responses. Similarly, smoking status was classified into three states (never, former, and current smoker). Diabetes mellitus was defined through questionnaire results, current use of insulin or antidiabetic medication, or meeting specific criteria: fasting blood glucose level ≥126 mg/dL, 2-h postprandial blood glucose level ≥200 mg/dL, or glycosylated hemoglobin ≥6.5 %[18]. Hypertension determination relied on self-reported diagnosis, ongoing use of antihypertensive medication, or meeting criteria for systolic/diastolic blood pressure ≥140/90 mmHg. Laboratory data included urinary albumin and creatine concentrations. In assessing chronic inflammation, white blood cell count and serum alkaline phosphatase were chosen as indicators based on prior research [19,20].
2.5. Statistical analyses
Descriptive statistics were conducted on the baseline characteristics of both the infertile and control groups. Research suggests that weighting may introduce over-adjustment bias, prompting the recommendation for unweighting [19,20]. Categorical data underwent chi-square testing, while quantitative data were subjected to the Kruskal-Wallis test. Subsequently, the Kruskal-Wallis test was employed to analyze the comparison of VOCs in urine across different subgroups. The correlation between distinct urinary VOCs and infertility was explored using interquartile logistic regression. Four interquartile logistic regression models were formulated, designating the first quartile (Q1) as the control group. In the analysis, an initial non-adjusted model was constructed to directly scrutinize the association between urinary VOCs and infertility. Two additional models, each incorporating different covariates, were subsequently developed for in-depth analysis. Model 1 incorporated adjustments for age, ethnicity, education level, and PIR, while Model 2 further included variables such as smoking status, alcohol consumption, diabetes, hypertension, BMI, UALB, and UCR. To better comprehend the relationship between the 15 urinary VOCs and infertility, a smoothed curve fitting method was employed for illustration. Pearson correlation was utilized to ascertain the correlation between the concentrations of these 15 urinary VOCs, and heat maps were generated. Furthermore, the relationship between mixtures of VOCs and infertility was evaluated through both WQS regression analysis and BKMR. In WQS analyses, the combined and individual effects of urinary VOCs on infertility prevalence were assessed. This involved calculating weighted linear indices and assigning corresponding weights. WQS indices were constructed in both positive and negative directions through 1000 iterations of bootstrapping, with the threshold set at 1/15. The overall impact of the mixture of urinary VOCs on infertility prevalence was explored using a Bayesian variable selection framework. Each VOC's effect on infertility prevalence was assessed through the calculation of posterior inclusion probabilities (PIP), ranging from the 25th to the 75th percentile. This model engaged in 25,000 iterations utilizing the Markov Chain Monte Carlo algorithm.A PIP threshold of 0.5 was employed to define significance for the results. The relationship between urinary VOCs and inflammation (leukocytes and alkaline phosphatase) was probed via linear regression analysis. Direct and indirect relationships, along with the extent of mediating effects, were explored through mediation analysis. Results were adjusted for all covariates. All analyses, encompassing WQS, BKMR, and mediation analyses, were executed using R (version 4.2.3). Differences with P < 0.05 were deemed statistically significant.
3. Result
3.1. The baseline characteristics of participants
During the 2013–2018 NHANES cycle, a total of 29,400 participants were surveyed. Based on our exclusion criteria, 28,302 participants were excluded from the analysis. The final sample included 1089 women aged 18 to 50 who had completed the reproductive health questionnaire, provided a history of infertility, and underwent urine volatile organic compound (VOC) testing. The basic characteristics of both the included and excluded participants are detailed in Supplementary Table 1. The baseline characteristics of the infertility group (120 cases) and the control group (978 cases) were outlined in Table 1. Significant differences were observed between the two groups regarding age, education level, PIR, pregnancy history, BMI, diabetes, and urine creatinine. However, no significant differences were observed in terms of ethnicity, smoking status, alcoholic status, hypertension, and urinary albumin.
Table 1.
Characteristics of participants.
| Characteristics | Total (N = 1098) | Infertility |
P-value | |
|---|---|---|---|---|
| No (N = 978) | Yes (N = 120) | |||
| Age (years) | 33.74 ± 9.69 | 33.34 ± 9.79 | 37.03 ± 8.10 | <0.01 |
| Ethnicity [n (%)] | 0.52 | |||
| Mexican American | 189 (17.21) | 170 (17.38) | 19 (15.83) | |
| Other Hispanic | 122 (11.11) | 112 (11.45) | 10 (8.33) | |
| Non-Hispanic White | 344 (31.33) | 305 (31.19) | 39 (32.50) | |
| Non-Hispanic Black | 235 (21.40) | 203 (20.76) | 32 (26.67) | |
| Other Race | 208 (18.94) | 188 (19.22) | 20 (16.67) | |
| Education level [n (%)] | <0.01 | |||
| Less than high school | 154 (14.03) | 136 (13.91) | 18 (15.00) | |
| High school or equivalent | 187 (17.03) | 171 (17.48) | 16 (13.33) | |
| College or above | 645 (58.74) | 561 (57.36) | 84 (70.00) | |
| Not recorded | 112 (10.20) | 110 (11.25) | 2 (1.67) | |
| PIR [n (%)] | 0.04 | |||
| <1.3 | 444 (40.44) | 405 (41.41) | 39 (32.50) | |
| 1.3 to < 3.5 | 368 (33.52) | 329 (33.64) | 39 (32.50) | |
| ≥3.5 | 286 (26.05) | 244 (24.95) | 42 (35.00) | |
| Smoking status [n (%)] | 0.09 | |||
| Never | 790 (71.95) | 712 (72.80) | 78 (65.00) | |
| Former | 122 (11.11) | 102 (10.43) | 20 (16.67) | |
| Current | 186 (16.94) | 164 (16.77) | 22 (18.33) | |
| Alcoholic status [n (%)] | 0.77 | |||
| Lifetime abstainer | 180 (18.22) | 161 (18.55) | 19 (15.83) | |
| Former drink | 129 (13.06) | 113 (13.02) | 16 (13.33) | |
| Current drink | 679 (68.72) | 594 (68.43) | 85 (70.83) | |
| Pregnancy History [n (%)] | <0.01 | |||
| Has been pregnant before | 672 (61.20) | 568 (58.08) | 104 (86.67) | |
| Has never been pregnant before | 212 (19.31) | 198 (20.25) | 14 (11.67) | |
| Not recorded | 214 (19.49) | 212 (21.68) | 2 (1.67) | |
| Hypertension | 0.08 | |||
| No | 881 (80.24) | 792 (80.98) | 89 (74.17) | |
| Yes | 217 (19.76) | 186 (19.02) | 31 (25.83) | |
| Diabetes | 0.02 | |||
| No | 1019 (92.81) | 914 (93.46) | 105 (87.50) | |
| Yes | 79 (7.19) | 64 (6.54) | 15 (12.50) | |
| BMI (kg/m2) | 29.39 ± 8.10 | 29.18 ± 8.08 | 31.11 ± 8.08 | 0.04 |
| Urinary Albumin (mg/dL) | 8.33 (7.76,8.94) | 8.16 (7.56,8.80) | 9.89 (8.21,11.92) | 0.46 |
| Urine Creatinine (mg/dL) | 89.29(85.37,93.39) | 87.34(83.28,91.59) | 106.89(93.72,121.90) | <0.01 |
All values were presented as mean ± SE, or median (IQR), or counts (proportion). BMI, body mass index. PIR, family poverty income ratio.
Univariate regression modeling results in Supplementary Table 2 revealed a significant correlation between PIR and the prevalence of infertility. Table 2 presented the findings from the analysis of urinary VOCs in both the control and infertility groups. The abbreviations for these 15 urinary VOCs, corresponding to their full names, were detailed in Supplementary Table 3. It was evident that the concentration of all 15 VOCs in the participants' urine was higher than that of the non-infertile group. With the exceptions of ATCA, SBMA, and 2HPMA, where differences were not significant, significant differences were observed for the remaining 12 VOCs.
Table 2.
The concentrations of VOCs in urine of control and infertility subgroups.
| VOCs (urine, ng/ml) | Infertility |
P-value | |
|---|---|---|---|
| No (N = 978) | Yes (N = 120) | ||
| 2MHA | 24.53 (22.70,26.50) | 32.63 (26.08,40.82) | 0.02 |
| 3,4–MHA | 148.20 (136.57,160.81) | 203.55 (160.26,258.53) | 0.01 |
| AAMA | 49.63 (46.35,53.15) | 61.52 (50.85,74.43) | 0.03 |
| AMCC | 117.49 (109.65,125.90) | 174.73 (144.79,210.84) | <0.01 |
| ATCA | 149.97 (140.87,159.67) | 171.57 (143.79,204.72) | 0.17 |
| SBMA | 6.74 (6.30,7.22) | 7.870 (6.43,9.63) | 0.14 |
| CEMA | 78.45 (73.46,83.78) | 94.55 (78.15,114.39) | 0.03 |
| CYMA | 2.88 (2.53,3.29) | 4.82 (3.25,7.13) | <0.01 |
| DHBMA | 267.57 (254.41,281.42) | 334.25 (292.32,382.18) | <0.01 |
| 2HPMA | 29.21 (27.23,31.33) | 34.51 (28.33,42.04) | 0.06 |
| 3HPMA | 233.69 (217.66,250.89) | 299.12 (244.29,366.25) | 0.01 |
| MA | 120.80 (113.93,128.08) | 154.35 (131.02,181.84) | <0.01 |
| MHBMA3 | 4.56 (4.20,4.94) | 6.27 (4.99,7.88) | 0.01 |
| PGA | 178.42 (169.17,188.18) | 227.38 (194.62,265.65) | <0.01 |
| HMPMA | 220.74 (205.88,236.67) | 281.53 (229.57,345.24) | 0.02 |
3.2. Exploring the link between urinary VOCs and infertility
To explore potential associations between quartiles of urinary VOCs concentrations and the prevalence of infertility, multivariate regression models were employed. The concentrations of various VOCs were categorized into four quartiles, ensuring an equal distribution of participants in each quartile, with quartile 1 (Q1) serving as the reference. The results in Table 3 revealed that 10 VOCs metabolites (3,4-MHA, AMCC, CYMA, DHBMA, 2HPMA, 3HPMA, MA, MHBMA3, PGA, and HMPMA) were associated with a higher prevalence of infertility in both the unadjusted model and Model 1. In Model 2, only 2 VOCs metabolites (AMCC and CYMA) maintained an association with a higher prevalence of infertility. The highest quartile for AMCC and CYMA was linked to an increase in infertility prevalence of approximately 191 % and 128 %, respectively, compared to the lowest quartile (OR for AMCC = 2.91; 95%CI: 1.33, 6.37; OR for CYMA = 2.28; 95%CI: 1.01, 5.15). Linear trend analysis demonstrated significant associations between AMCC and CYMA concentrations and infertility prevalence in both multivariate regression models (P = 0.01 for both trends).
Table 3.
Multivariate logistic regression analysis of the prevalence of infertility using urine VOCs.
| Non-adjusted |
Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |
| 2MHA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.00 (0.55, 1.80) | 0.99 | 1.00 (0.55, 1.84) | 0.99 | 0.848 (0.455, 1.580) | 0.60 |
| Q3 | 1.63 (0.94, 2.80) | 0.08 | 1.72 (0.98, 3.02) | 0.06 | 1.331 (0.727, 2.435) | 0.35 |
| Q4 | 1.52 (0.88, 2.63) | 0.14 | 1.52 (0.85, 2.71) | 0.15 | 1.175 (0.596, 2.319) | 0.64 |
| P for trend | 0.04 | 0.04 | 0.27 | |||
| 3,4–MHA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.28 (0.69, 2.36) | 0.43 | 1.39 (0.74, 2.59) | 0.31 | 1.202 (0.630, 2.291) | 0.58 |
| Q3 | 2.05 (1.16, 3.63) | 0.01 | 2.15 (1.19, 3.86) | 0.01 | 1.721 (0.906, 3.271) | 0.10 |
| Q4 | 1.98 (1.12, 3.51) | 0.02 | 2.22 (1.21, 4.05) | 0.01 | 1.893 (0.926, 3.873) | 0.08 |
| P for trend | 0.01 | 0.01 | 0.04 | |||
| AAMA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.36 (0.76, 2.44) | 0.30 | 1.30 (0.72, 2.36) | 0.38 | 1.065 (0.575, 1.973) | 0.84 |
| Q3 | 1.69 (0.96, 2.97) | 0.07 | 1.77 (1.00, 3.16) | <0.05 | 1.310 (0.692, 2.479) | 0.41 |
| Q4 | 1.59 (0.90, 2.80) | 0.11 | 1.65 (0.92, 2.98) | 0.09 | 0.987 (0.464, 2.098) | 0.97 |
| P for trend | 0.08 | 0.06 | 0.71 | |||
| AMCC | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.57 (0.81, 3.02) | 0.18 | 1.58 (0.81, 3.09) | 0.18 | 1.558 (0.785, 3.090) | 0.20 |
| Q3 | 2.25 (1.21, 4.17) | 0.01 | 2.26 (1.20, 4.25) | 0.01 | 2.057 (1.042, 4.060) | 0.04 |
| Q4 | 3.24 (1.78, 5.88) | 0.01 | 3.21 (1.72, 6.01) | 0.01 | 2.910 (1.329, 6.372) | 0.01 |
| P for trend | 0.01 | 0.01 | 0.01 | |||
| ATCA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.14 (0.65, 2.01) | 0.65 | 1.19 (0.67, 2.11) | 0.56 | 1.110 (0.618, 1.994) | 0.73 |
| Q3 | 1.37 (0.79, 2.38) | 0.26 | 1.51 (0.85, 2.68) | 0.16 | 1.176 (0.641, 2.157) | 0.60 |
| Q4 | 1.40 (0.81, 2.42) | 0.22 | 1.49 (0.84, 2.64) | 0.17 | 0.966 (0.501, 1.864) | 0.92 |
| P for trend | 0.17 | 0.12 | 0.89 | |||
| SBMA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.34 (0.76, 2.35) | 0.31 | 1.44 (0.81, 2.56) | 0.21 | 1.190 (0.654, 2.165) | 0.57 |
| Q3 | 1.43 (0.82, 2.48) | 0.21 | 1.46 (0.82, 2.57) | 0.20 | 1.018 (0.547, 1.894) | 0.95 |
| Q4 | 1.38 (0.79, 2.41) | 0.26 | 1.35 (0.75, 2.41) | 0.32 | 0.830 (0.420, 1.640) | 0.59 |
| P for trend | 0.27 | 0.36 | 0.59 | |||
| CEMA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.92 (0.51, 1.66) | 0.77 | 0.92 (0.50, 1.67) | 0.78 | 0.743 (0.397, 1.391) | 0.35 |
| Q3 | 1.52 (0.88, 2.61) | 0.13 | 1.53 (0.88, 2.68) | 0.13 | 1.109 (0.596, 2.064) | 0.74 |
| Q4 | 1.50 (0.87, 2.57) | 0.14 | 1.50 (0.85, 2.67) | 0.16 | 0.913 (0.439, 1.897) | 0.81 |
| P for trend | 0.04 | 0.07 | 0.92 | |||
| CYMA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.70 (0.92, 3.15) | 0.09 | 1.62 (0.87, 3.02) | 0.13 | 1.494 (0.784, 2.847) | 0.22 |
| Q3 | 2.21 (1.23, 3.99) | 0.01 | 2.20 (1.20, 4.02) | 0.01 | 1.750 (0.900, 3.404) | 0.10 |
| Q4 | 2.15 (1.19, 3.89) | 0.01 | 2.34 (1.25, 4.38) | 0.01 | 2.278 (1.007, 5.153) | 0.04 |
| P for trend | 0.01 | 0.01 | 0.01 | |||
| DHBMA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.90 (0.47, 1.72) | 0.76 | 0.91 (0.47, 1.74) | 0.77 | 0.865 (0.440, 1.701) | 0.67 |
| Q3 | 2.01 (1.18, 3.48) | 0.01 | 2.15 (1.21, 3.81) | 0.01 | 1.773 (0.905, 3.476) | 0.10 |
| Q4 | 2.06 (1.18, 3.59) | 0.01 | 2.22 (1.24, 3.99) | 0.01 | 1.644 (0.681, 3.966) | 0.27 |
| P for trend | 0.01 | 0.01 | 0.06 | |||
| 2HPMA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.67 (0.93, 2.99) | 0.09 | 1.74 (0.95, 3.17) | 0.07 | 1.455 (0.783, 2.705) | 0.24 |
| Q3 | 1.61 (0.89, 2.90) | 0.11 | 1.66 (0.91, 3.05) | 0.10 | 1.232 (0.631, 2.405) | 0.54 |
| Q4 | 1.97 (1.11, 3.48) | 0.02 | 2.08 (1.15, 3.74) | 0.02 | 1.522 (0.758, 3.057) | 0.24 |
| P for trend | 0.03 | 0.03 | 0.20 | |||
| 3HPMA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.39 (0.76, 2.55) | 0.28 | 1.44 (0.78, 2.67) | 0.24 | 1.175 (0.620, 2.228) | 0.62 |
| Q3 | 1.81 (1.01, 3.23) | 0.04 | 1.81 (1.00, 3.28) | 0.04 | 1.426 (0.745, 2.730) | 0.28 |
| Q4 | 2.11 (1.19, 3.72) | 0.01 | 2.21 (1.23, 3.97) | 0.01 | 1.634 (0.788, 3.389) | 0.19 |
| P for trend | 0.01 | 0.01 | 0.19 | |||
| MA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.26 (0.68, 2.34) | 0.47 | 1.29 (0.69, 2.41) | 0.43 | 1.162 (0.610, 2.216) | 0.65 |
| Q3 | 1.88 (1.06, 3.34) | 0.03 | 1.95 (1.08, 3.51) | 0.03 | 1.550 (0.806, 2.979) | 0.19 |
| Q4 | 2.16 (1.23, 3.80) | 0.01 | 2.21 (1.23, 3.97) | 0.01 | 1.473 (0.659, 3.295) | 0.35 |
| P for trend | 0.01 | 0.01 | 0.17 | |||
| MHBMA3 | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.00 (0.55, 1.84) | 0.99 | 1.02 (0.55, 1.89) | 0.94 | 0.873 (0.461, 1.653) | 0.68 |
| Q3 | 1.48 (0.85, 2.60) | 0.17 | 1.44 (0.81, 2.56) | 0.22 | 1.150 (0.605, 2.185) | 0.67 |
| Q4 | 1.91 (1.11, 3.28) | 0.02 | 1.98 (1.12, 3.50) | 0.02 | 1.650 (0.788, 3.452) | 0.18 |
| P for trend | 0.01 | 0.01 | 0.09 | |||
| PGA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.99 (0.54, 1.81) | 0.97 | 0.99 (0.54, 1.83) | 0.98 | 0.921 (0.489, 1.737) | 0.80 |
| Q3 | 1.24 (0.69, 2.21) | 0.47 | 1.33 (0.73, 2.42) | 0.35 | 1.164 (0.592, 2.289) | 0.66 |
| Q4 | 2.18 (1.28, 3.72) | 0.01 | 2.29 (1.31, 4.01) | 0.01 | 1.854 (0.839, 4.093) | 0.13 |
| P for trend | 0.01 | 0.01 | 0.06 | |||
| HMPMA | ||||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.92 (0.51, 1.65) | 0.78 | 0.97 (0.54, 1.76) | 0.92 | 0.798 (0.430, 1.483) | 0.48 |
| Q3 | 1.13 (0.65, 1.98) | 0.66 | 1.15 (0.65, 2.04) | 0.63 | 0.800 (0.415, 1.545) | 0.51 |
| Q4 | 1.68 (0.99, 2.83) | 0.04 | 1.75 (1.01, 3.02) | 0.04 | 1.227 (0.587, 2.568) | 0.59 |
| P for trend | 0.03 | 0.03 | 0.44 | |||
Non-adjusted: model without any covariates; Model 1: model adjusted by age, ethnicity, education level and PIR; Model 2: model age, ethnicity, education level, PIR, BMI, alcohol drinking, smoking, hypertension, diabetes, urine albumin and creatine. OR, odds ratio. CI, confidence interval. VOCs, volatile organic compound metabolites.
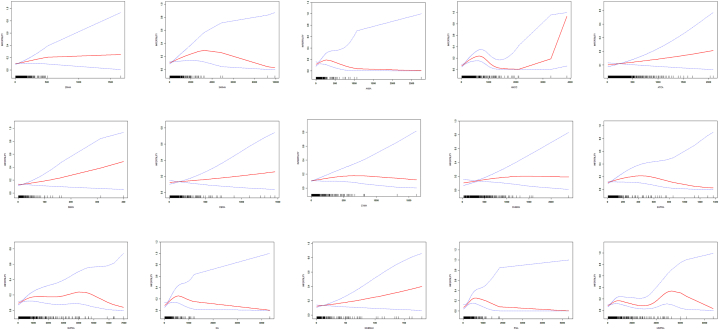
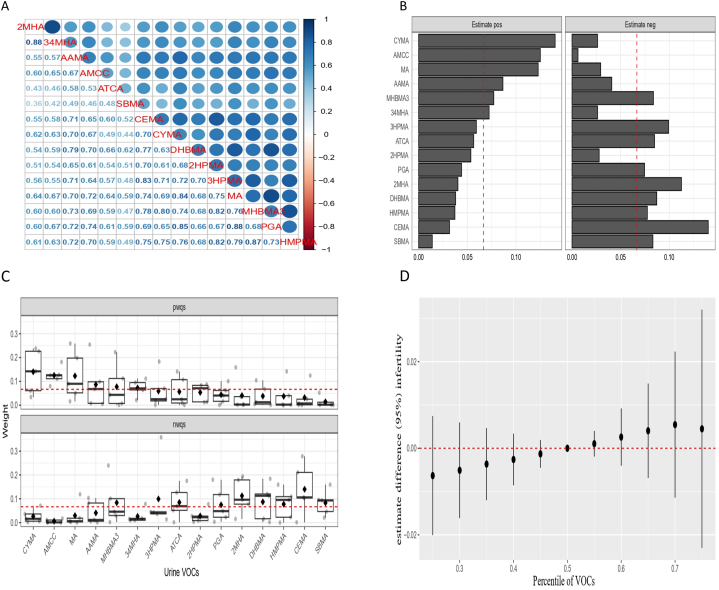
The smoothed curve fits illustrating the relationship between these 15 urinary VOCs and infertility (Fig. 2). Fig. 3A displayed Pearson correlation coefficients between the concentrations of urinary VOCs, revealing a significant correlation among these 15 VOCs. The r-values between AMCC and DHBMA, MA, PGA, and HMPMA were 0.70, 0.72, 0.74, and 0.70, respectively, while r-values between CYMA and CEMA, AAMA, 3HPMA, and MHBMA3 were 0.70, 0.70, 0.71, 0.80, and 0.75, respectively. These findings suggested a synergistic trend in the concentration changes of these VOCs in urine.
Fig. 2.
The association between urine VOCs and infertility. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95 % confidence interval from the fit.
Fig. 3.
(A) The thermogram of urinary VOCs mixture displays positive correlations in blue. (B-C) Weight map for WQS regression index of urine VOCs mixture. (D) The comprehensive impact of urine VOCs mixture on infertility in the BKMR model.
3.3. Urine VOCs exposure and infertility risk in WQS model
Utilizing the WQS model, we explored the intricate relationship between the cumulative effects of fifteen urinary VOCs and the prevalence of infertility. As detailed in Supplementary Table 4, the WQS index revealed a positive correlation between urinary VOCs and infertility prevalence (OR = 1.63, 95 % CI 1.07, 1.96, P = 0.03). Correspondingly, the negative WQS regression unveiled an association between urinary VOCs and the prevalence of infertility (OR = 1.58, 95 % CI 1.03, 1.88, P = 0.04). Upon meticulous adjustment for all covariates, the positive WQS regression outcomes underscored that CYMA held the highest weight in infertility at 0.14, closely followed by AMCC at 0.13. Moreover, MA, AAMA, MHBMA3, and 3,4-MH4 all exceeded one-fifteenth. Conversely, the negative WQS regression outcomes highlighted that CEMA commanded the highest weight in infertility at 0.14, with SBMA, HMPMA, DHBMA, 2MHA, PGA, ATCA, 3HPMA, and MHBMA3 also surpassing one in fifteen (Fig. 3B and C).
3.4. The relationship between exposure to VOCs and the risk of infertility using the BKMR model
In the BKMR model, a discernible upward trajectory was observed in the relationship between a combination of 15 urinary VOCs metabolites and infertility. As the co-exposure exceeded the 50th percentile, a statistically significant upward trend manifested, indicating an elevated risk of infertility associated with the cumulative exposure to diverse urinary VOCs metabolites (Fig. 3D). Supplementary Table 5 succinctly outlines the PIP results obtained from the BKMR model. AMCC exhibited a PIP result of 0.90, while CYMA demonstrated a PIP result of 0.79. Additionally, compounds surpassing the 0.5 threshold include AAMA (0.54), ATCA (0.71), SBMA (0.79), and DHBMA (0.69).
3.5. Inflammation mediates the association between VOCs and infertility risk
A significant positive correlation surfaced between AMCC and the inflammatory biomarker alkaline phosphatase (Supplementary Table 6). Further insights from Model 1 (OR = 0.99, 95 % CI 0.97–1.00, P < 0.05) and Model 2 (OR = 0.99, 95 % CI 0.98–1.00, P < 0.04) in Supplementary Table 7 revealed that alkaline phosphatase levels were linked to the risk of infertility. The concentration of alkaline phosphatase played a mediating role in the positive correlation between AMCC concentration and the prevalence of infertility, accounting for 5.10 % (P = 0.04) (Table 4).
Table 4.
Mediating role and proportion of inflammatory biomarkers in the relationship between VOCs exposure and infertility incidence.
| Pathways | Indirect |
95 % CI | Mediation |
95 % CI | P- value |
|---|---|---|---|---|---|
| effect | proportions | ||||
| AMCC→ WBC →infertility | −2.92e-03 | −1.25e-02,0.00 | 1.11 % | −0.18,0.04 | 0.48 |
| AMCC→ ALP →infertility | 1.06e-02 | 4.82e-04,0.03 | 5.10 % | 0.01,0.20 | 0.04 |
| CYMA→ WBC →infertility | −1.61e-06 | −1.45e-05,0.00 | 0.88 % | −0.22,0.19 | 0.74 |
| CYMA→ ALP →infertility | 2.19e-05 | −6.28e-05,0.00 | 13.90 % | −0.09,1.29 | 0.06 |
This model Adjust all covariates. CI, confidence interval. WBC, white blood cell. ALP, alkaline phosphatase. VOCs, volatile organic compound metabolites.
4. Discussion
In the context of rapid global urbanization and industrialization, VOCs which are pervasive and complex organic pollutants in the air, are on the rise, exacerbating environmental pollution challenges. Extensive research has consistently demonstrated a strong association between environmental pollutants such as heavy metals, phthalates, and phenols, and female infertility [21,22]. The identification of these environmental contributors to infertility has not only deepened our understanding of its etiology but also laid the groundwork for timely and targeted interventions to mitigate infertility. While some pollutants linked to infertility have been identified, VOCs, as widespread and easily accessible pollutants, are now being investigated for their significant associations with various health conditions, including liver damage, diabetes mellitus, and arthritis [[23], [24], [25]]. Nevertheless, the complex relationship between VOC exposure and infertility remains largely unexplored and requires further investigation.
Currently, data on multiple VOCs in the urine of both adults and children are available [26], but there is limited information specifically concerning individuals with infertility. As a result, to draw comparisons with previous studies, we have been constrained to comparing our findings with available data on pregnant women. Boyle et al. measured the levels of 28 VOCs in the urine of pregnant women during late gestation in the United States [27]. Our findings indicate that the concentrations of most measured VOCs in the urine of these pregnant women were more comparable to those observed in our non-infertile group.
When evaluating the impact of environmental pollutants on human health, exposure assessments typically involve measuring pollutant concentrations in blood, urine, breath, and sweat [28]. Due to the volatility of VOCs, it is challenging to accurately detect VOC concentrations directly in breath and blood. Likewise, the accuracy of VOC measurements in urine can be compromised by losses during collection [29]. As VOC metabolites, which are more stable than their parent compounds, exhibit lower volatility, urine samples offer increased stability and longer biological half-lives compared to blood [27]. Therefore, in our study, urine was selected as the preferred biomarker for assessing VOC exposure due to its inherent stability. By collecting demographic and clinical data, we conducted a comprehensive analysis of both the infertility and control cohorts. Using a range of statistical methods, including logistic regression, WQS and BKMR, we successfully identified AMCC and CYMA as key factors associated with infertility.
The impact of inflammation on female fertility is extensive and complex, influencing various physiological processes, including hormone regulation, ovarian function, uterine health, and embryo implantation [30]. A prime example of this is endometriosis, where chronic inflammation is a key contributor to infertility in affected patients [31]. Similarly, individuals with polycystic ovary syndrome (PCOS) often experience a low-grade chronic inflammatory state, which can lead to adverse outcomes such as ovarian dysfunction and, ultimately, infertility [32]. While the primary pathologies in these conditions are linked to infertility, broader research has revealed a direct connection between inflammation and infertility in the general female population of the United States [33].
White blood cells are commonly used as markers of inflammation, while serum alkaline phosphatase, a novel and more predictive inflammatory marker, has recently gained recognition for its role in assessing inflammatory responses [34,35]. Given the common inflammatory pathways involved, it is reasonable to explore whether VOC exposure contributes to infertility through inflammatory mechanisms, although research in this area is limited. In our study, mediation analysis suggests that alkaline phosphatase mediates a positive correlation between AMCC concentration and infertility incidence, accounting for 5.10 % of the effect. This finding implies that systemic inflammation could be a potential mechanism by which AMCC exposure leads to infertility.
AMCC serves as a key biomarker for evaluating exposure to dimethylformamide (DMF), with its primary metabolic pathway involving α-hydroxylation via CYP450 enzymes, ultimately converting DMF to AMCC. AMCC has a strong association with DMF toxicity and exhibits a relatively long half-life in the human body [36]. Animal studies have shown that long-term DMF exposure can result in reproductive system issues, including reduced fertility and abnormal fetal development [37,38]. High doses of DMF present potential toxicity risks to the reproductive system, potentially leading to reproductive dysfunction or fetal developmental abnormalities. These compounds may induce various pathological changes during development. Both in vivo and in vitro studies indicate that DMF exposure can elevate various inflammatory factors, including reactive oxygen species, and promote an increase in inflammatory cells such as white blood cells [39,40]. In addition, a survey conducted in China has suggested that AMCC may affect pulmonary function by modulating inflammatory responses [41]. Therefore, it is plausible that AMCC contributes to infertility through similar inflammatory pathways, further supporting the role of inflammation in VOC-related infertility.
Acrolein, a highly reactive unsaturated aldehyde, can be produced endogenously through lipid peroxidation and exogenously via combustion, chemical synthesis, cigarette smoke, and e-cigarette vapor exposure [42]. Acrylonitrile, once metabolized and excreted in urine, is converted into CYMA, 2-Hydroxyethyl Methacrylate, and N-acetyl-S-(1-cyano-2-hydroxyethyl)-l-cysteine through the mercapturic acid pathway [43]. Epidemiological studies in China have documented reproductive and developmental effects among workers exposed to acrolein, including infertility, birth defects, and miscarriages [44]. Prior research has demonstrated that long-term exposure to acrolein triggers inflammation in animal models [45]. Consistent with these findings, our study suggests a link between CYMA and the onset and progression of infertility. However, unlike previous animal studies which indicated that CYMA exposure might be associated with inflammation, our results suggest that alkaline phosphatase does not mediate the relationship between CYMA concentration and infertility. This discrepancy may indicate the need for a larger sample size in future infertility studies and a more targeted selection of inflammatory markers.
This study represents an initial investigation into the association between VOC exposure and infertility. The data were derived from a robust, population-based database that followed strict quality control measures. We employed a variety of statistical techniques and controlled for potential confounders to strengthen the reliability of our findings. However, the cross-sectional design of the study limits our ability to infer causality between VOC exposure and infertility. Additionally, the NHANES database lacks data on certain variables, such as cosmetic use and industrial wastewater exposure, which could influence our results. Moreover, we were unable to exclude women currently using contraceptive pills, which may have impacted participant selection.
In conclusion, our study provides preliminary evidence linking VOC exposure to infertility, identifying AMCC and CYMA as key contributing factors. We highlighted the role of inflammation as a potential mediating mechanism between AMCC exposure and infertility. Nevertheless, further prospective studies and experimental research are necessary to fully elucidate the pathogenic mechanisms underlying VOC overexposure and infertility.
5. Conclusion
The study highlights the correlation between VOCs exposure, notably AMCC and CYMA, and infertility. It identifies inflammation as a mediating factor connecting AMCC to infertility.
CRediT authorship contribution statement
Wen Zhang: Writing – original draft, Resources, Project administration, Methodology, Investigation. Jiarong He: Writing – original draft, Software, Resources, Project administration, Methodology. Fang Zhao: Writing – original draft. Jing Pan: Writing – original draft. Jiefu Wen: Writing – original draft. Lijun Jiang: Writing – original draft. Mingming Zhang: Investigation, Funding acquisition, Formal analysis, Data curation.
Ethics approval and consent to participate
The NHANES is a public-use dataset available through the website. The NHANES protocol was approved by the institutional review board of the Centers for Disease Control and Prevention. NHANES has obtained written informed consent from all participants.
Data availability
Data will be made available on request.
Funding
This work was funded by the Natural Science Foundation of Hunan Province, China (Grant No. 2022JJ70059) and the Fundamental Research Funds for the Central Universities of Central South University (Grant No. 2022ZZTS0952)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40902.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tabong P.T., Adongo P.B. Infertility and childlessness: a qualitative study of the experiences of infertile couples in northern Ghana. BMC Pregnancy Childbirth. 2013;13:72. doi: 10.1186/1471-2393-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boivin J., Bunting L., Collins J.A., Nygren K.G. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum. Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 3.Inhorn M.C., Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update. 2015;21:411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A., Mulgund A., Hamada A., Chyatte M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan X., Chen Z., Xia C., Zhong R., Liu L., Long L. Increased levels of urine volatile organic compounds are associated with diabetes risk and impaired glucose homeostasis. J. Clin. Endocrinol. Metab. 2024;109:e531–e542. doi: 10.1210/clinem/dgad584. [DOI] [PubMed] [Google Scholar]
- 6.Broughton D.E., Moley K.H. Obesity and female infertility: potential mediators of obesity's impact. Fertil. Steril. 2017;107:840–847. doi: 10.1016/j.fertnstert.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Practice Committee of American Society for Reproductive M Smoking and infertility. Fertil. Steril. 2008;90:S254–S259. doi: 10.1016/j.fertnstert.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 8.La Vignera S., Condorelli R.A., Balercia G., Vicari E., Calogero A.E. Does alcohol have any effect on male reproductive function? A review of literature. Asian J. Androl. 2013;15:221–225. doi: 10.1038/aja.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu Y.H., Chavarro J.E., Souter I. Diet and female fertility: doctor, what should i eat? Fertil. Steril. 2018;110:560–569. doi: 10.1016/j.fertnstert.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Canipari R., De Santis L., Cecconi S. Female fertility and environmental pollution. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17238802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace L.A., Pellizzari E.D., Hartwell T.D., Davis V., Michael L.C., Whitmore R.W. The influence of personal activities on exposure to volatile organic compounds. Environ. Res. 1989;50:37–55. doi: 10.1016/s0013-9351(89)80047-7. [DOI] [PubMed] [Google Scholar]
- 12.McFarlan E.M., Mozdia K.E., Daulton E., Arasaradnam R., Covington J., Nwokolo C. Pre-analytical and analytical variables that influence urinary volatile organic compound measurements. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei C., Chen Y., Yang Y., Ni D., Huang Y., Wang M., et al. Assessing volatile organic compounds exposure and prostate-specific antigen: national health and nutrition examination survey, 2001-2010. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.957069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paciencia I., Cavaleiro Rufo J., Silva D., Martins C., Mendes F., Farraia M., et al. Exposure to indoor endocrine-disrupting chemicals and childhood asthma and obesity. Allergy. 2019;74:1277–1291. doi: 10.1111/all.13740. [DOI] [PubMed] [Google Scholar]
- 15.Yoon H.I., Hong Y.C., Cho S.H., Kim H., Kim Y.H., Sohn J.R., et al. Exposure to volatile organic compounds and loss of pulmonary function in the elderly. Eur. Respir. J. 2010;36:1270–1276. doi: 10.1183/09031936.00153509. [DOI] [PubMed] [Google Scholar]
- 16.Lin L.Y., Chuang H.C., Liu I.J., Chen H.W., Chuang K.J. Reducing indoor air pollution by air conditioning is associated with improvements in cardiovascular health among the general population. Sci. Total Environ. 2013;463–464:176–181. doi: 10.1016/j.scitotenv.2013.05.093. [DOI] [PubMed] [Google Scholar]
- 17.Alwis K.U., Blount B.C., Britt A.S., Patel D., Ashley D.L. Simultaneous analysis of 28 urinary voc metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (uplc-esi/msms) Anal. Chim. Acta. 2012;750:152–160. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes A. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson K.K., Loch-Caruso R., Meeker J.D. Exploration of oxidative stress and inflammatory markers in relation to urinary phthalate metabolites: nhanes 1999-2006. Environ. Sci. Technol. 2012;46:477–485. doi: 10.1021/es202340b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zang X., Qin W., Xiong Y., Xu A., Huang H., Fang T., et al. Using three statistical methods to analyze the association between aldehyde exposure and markers of inflammation and oxidative stress. Environ. Sci. Pollut. Res. Int. 2023;30:79437–79450. doi: 10.1007/s11356-023-27717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel S., Zhou C., Rattan S., Flaws J.A. Effects of endocrine-disrupting chemicals on the ovary. Biol. Reprod. 2015;93:20. doi: 10.1095/biolreprod.115.130336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sifakis S., Androutsopoulos V.P., Tsatsakis A.M., Spandidos D.A. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017;51:56–70. doi: 10.1016/j.etap.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen H.D., Oh H., Hoang N.H.M., Jo W.H., Kim M.S. Environmental science and pollution research role of heavy metal concentrations and vitamin intake from food in depression: a national cross-sectional study (2009-2017) Environ. Sci. Pollut. Res. Int. 2022;29:4574–4586. doi: 10.1007/s11356-021-15986-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen P., Leray V., Diez M., Serisier S., Le Bloc'h J., Siliart B., et al. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008;92:272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 25.Shirasu M., Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011;150:257–266. doi: 10.1093/jb/mvr090. [DOI] [PubMed] [Google Scholar]
- 26.Kuang H., Li Z., Lv X., Wu P., Tan J., Wu Q., et al. Exposure to volatile organic compounds may be associated with oxidative DNA damage-mediated childhood asthma. Ecotoxicol. Environ. Saf. 2021;210 doi: 10.1016/j.ecoenv.2020.111864. [DOI] [PubMed] [Google Scholar]
- 27.Boyle E.B., Viet S.M., Wright D.J., Merrill L.S., Alwis K.U., Blount B.C., et al. Assessment of exposure to vocs among pregnant women in the national children's study. Int. J. Environ. Res. Publ. Health. 2016;13:376. doi: 10.3390/ijerph13040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y.S., Blount B.C., Valentin-Blasini L., Applewhite H.S., Xia Y., Watson C.H., et al. Simultaneous determination of six mercapturic acid metabolites of volatile organic compounds in human urine. Chem. Res. Toxicol. 2009;22:1018–1025. doi: 10.1021/tx800468w. [DOI] [PubMed] [Google Scholar]
- 29.Xu X., Weisel C.P. Human respiratory uptake of chloroform and haloketones during showering. J. Expo. Anal. Environ. Epidemiol. 2005;15:6–16. doi: 10.1038/sj.jea.7500374. [DOI] [PubMed] [Google Scholar]
- 30.Ehsani M., Mohammadnia-Afrouzi M., Mirzakhani M., Esmaeilzadeh S., Shahbazi M. Female unexplained infertility: a disease with imbalanced adaptive immunity. J. Hum. Reprod. Sci. 2019;12:274–282. doi: 10.4103/jhrs.JHRS_30_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsay S.F., Luciano D.E., Luciano A.A. Emerging therapy for endometriosis. Expert Opin Emerg Drugs. 2015;20:449–461. doi: 10.1517/14728214.2015.1051966. [DOI] [PubMed] [Google Scholar]
- 32.Patel S. Polycystic ovary syndrome (pcos), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018;182:27–36. doi: 10.1016/j.jsbmb.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Xu H., Yan J., Wen Q., Ma M., Xu N., et al. Inflammatory markers are associated with infertility prevalence: a cross-sectional analysis of the nhanes 2013-2020. BMC Publ. Health. 2024;24:221. doi: 10.1186/s12889-024-17699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Q., Wan J., Nan W., Li S., He B., Peng Z. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in us adults from nhanes 2011-2018. J. Hazard Mater. 2024;464 doi: 10.1016/j.jhazmat.2023.133005. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.H., Cho A.R., Lee Y.J. Relationship between serum alkaline phosphatase and low muscle mass index among Korean adults: a nationwide population-based study. Biomolecules. 2021;11 doi: 10.3390/biom11060842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kafferlein H.U., Mraz J., Ferstl C., Angerer J. Analysis of metabolites of n,n-dimethylformamide in urine samples. Int. Arch. Occup. Environ. Health. 2004;77:427–432. doi: 10.1007/s00420-004-0538-x. [DOI] [PubMed] [Google Scholar]
- 37.Fail P.A., George J.D., Grizzle T.B., Heindel J.J. Formamide and dimethylformamide: reproductive assessment by continuous breeding in mice. Reprod. Toxicol. 1998;12:317–332. doi: 10.1016/s0890-6238(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy G.L. Toxicology of dimethyl and monomethyl derivatives of acetamide and formamide: a second update. Crit. Rev. Toxicol. 2012;42:793–826. doi: 10.3109/10408444.2012.725028. [DOI] [PubMed] [Google Scholar]
- 39.Wang C., Yang J., Lu D., Fan Y., Zhao M., Li Z. Oxidative stress-related DNA damage and homologous recombination repairing induced by n,n-dimethylformamide. J. Appl. Toxicol. 2016;36:936–945. doi: 10.1002/jat.3226. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M., Zheng M., Wu Z., Wang L., Guan M., Zhao W., et al. [effects of n, n-dimethylformamide on hepatic antioxidant capacity and liver ppars mrna levels in rats] Wei Sheng Yan Jiu. 2018;47:352–357. [PubMed] [Google Scholar]
- 41.Wang B., Yang S., Guo Y., Wan Y., Qiu W., Cheng M., et al. Association of urinary dimethylformamide metabolite with lung function decline: the potential mediating role of systematic inflammation estimated by c-reactive protein. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138604. [DOI] [PubMed] [Google Scholar]
- 42.Henning R.J., Johnson G.T., Coyle J.P., Harbison R.D. Acrolein can cause cardiovascular disease: a review. Cardiovasc. Toxicol. 2017;17:227–236. doi: 10.1007/s12012-016-9396-5. [DOI] [PubMed] [Google Scholar]
- 43.Wu C.F., Uang S.N., Chiang S.Y., Shih W.C., Huang Y.F., Wu K.Y. Simultaneous quantitation of urinary cotinine and acrylonitrile-derived mercapturic acids with ultraperformance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2012;402:2113–2120. doi: 10.1007/s00216-011-5661-4. [DOI] [PubMed] [Google Scholar]
- 44.Neal B.H., Collins J.J., Strother D.E., Lamb J.C. Weight-of-the-evidence review of acrylonitrile reproductive and developmental toxicity studies. Crit. Rev. Toxicol. 2009;39:589–612. doi: 10.1080/10408440903052855. [DOI] [PubMed] [Google Scholar]
- 45.Cui Y., Xie X., Jia F., He J., Li Z., Fu M., et al. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell. Physiol. Biochem. 2015;35:353–363. doi: 10.1159/000369701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.