Summary
Background
Given the chronic immune activation and inflammatory milieu associated with Long COVID and HIV, we assessed the prevalence of Long COVID in adults living with HIV; and investigated whether adults living with HIV were associated with increased chance of developing Long COVID compared to adults living without HIV
Methods
In this systematic review and meta-analysis, we searched Medline, EMBASE, CINHAL, PubMed and CENTRAL from inception until June 14th, 2024, for observational studies that measured the prevalence of Long COVID in adults living with HIV and the odds of developing Long COVID following a SARS-CoV-2 infection in people living with HIV compared to people living without HIV. Reviews, case reports, randomised control trials and editorials were excluded. The search was conducted without language restrictions. We performed meta-analysis of proportions to synthesise prevalence estimates using logit transformation and a sensitivity analysis using mixed-effects logistic regression. We used random-effects meta-analyses to summarize the odds ratio (OR) of developing Long COVID in adults living with HIV compared to adults living without HIV and conducted a sensitivity analysis including only studies with covariate-adjusted estimates that was planned a-priori. We used ROBINS-E for the risk of bias assessment and GRADE to rate the certainty of evidence. We identified statistical heterogeneity using Cochran's Q test and quantified it using the I2 statistic. For the Q test, a P < 0.10 was considered statistically significant. PROSPERO registration: CRD42024577616.
Findings
Our search returned 831 results, of which 8 studies (4489 participants) were deemed eligible for inclusion in the systematic review and meta-analysis. The prevalence of Long COVID in adults with HIV was 43% (95% CI: 32–54%, 8 studies; 1227 participants; low certainty, I2 < 0.0001). The association of HIV status with Long COVID was inconclusive, with wide confidence intervals (OR: 1.16, 95% CI: 0.58–2.29; 4 studies; 3556 participants, low certainty, I2 = 0.013). When the analysis was restricted to studies reporting covariate-adjusted estimates, adults living with HIV were associated with a higher odds of Long COVID than those not living with HIV (OR: 2.21, 95% CI: 1.12–4.36; 2 studies; 374 participants, low certainty, I2 = 0.51).
Interpretation
Current evidence indicates that the prevalence of Long COVID in adults living with HIV may be high, suggesting the need for increased awareness and education of healthcare providers and policy makers. Evidence on whether HIV positivity increases the risk of Long COVID is limited and inconclusive, highlighting a need for further research to clarify this potential association.
Funding
None.
Keywords: Long COVID, HIV, Prevalence, Odds ratio, Systematic review, meta-analysis
Research in context.
Evidence before this study
The pathophysiology of Long COVID is complex and multifactorial, involving persistent viral reservoirs, immune dysregulation, and inflammatory responses. Given the chronic immune activation and inflammatory milieu associated with HIV, it is plausible that adults living with HIV might be at an increased risk of Long COVID. Despite the biological plausibility, there is a paucity of data specifically examining the risk of Long COVID in adults living with HIV.
Added value of this study
The available evidence indicates that prevalence of Long COVID in adults living with HIV could be high (43%). There is limited and uncertain evidence indicating that people living with HIV could be at a higher risk of long-COVID than individuals not living with HIV.
Implications of all the available evidence
Additional research is needed to determine whether HIV positivity is a risk factor for Long COVID. Addressing this gap is critical for developing targeted interventions and management strategies for adults living with HIV affected by COVID-19.
Introduction
Long COVID is defined by the World Health Organisation (WHO), as having a history of probable or confirmed SARS-CoV-2 infection and experience symptoms for at least 12 weeks from the onset of COVID-19, lasting for a minimum of 2 months without an alternative diagnosis.1,2 Common symptoms include fatigue, post exertional malaise (PEM), shortness of breath, cognitive impairment, and various neurological manifestations,3,4 which generally have an impact on everyday functioning. Symptoms may be new onset, following initial recovery from an acute COVID-19 episode, or persist from the initial illness. Symptoms may also fluctuate or relapse over time.1,2 The prevalence of Long COVID varies widely. Prevalence estimates from nationwide UK studies5,6 range from 3.3 to 10%. Results of the Canadian COVID-19 Antibody and Health Survey in October 20227 suggest that 15% of those infected with SARS-CoV-2 may develop prolonged symptoms.
In 2022, an estimated 39.0 million people globally were living with HIV, 29.8 million people were accessing antiretroviral therapy (ART), 1.3 million people became newly infected with HIV and 630,000 people died from AIDS-related illnesses.8 Adults living with HIV represent a unique population with distinct vulnerabilities. Despite advances in ART that have transformed HIV into a manageable chronic condition,9, 10, 11 adults living with HIV often have higher rates of comorbidities, immune activation, and inflammation, which could potentially influence their susceptibility to and outcomes from COVID-19.12 The use of ART and being in a suppressed status have been associated with reduced risk of poor outcomes following SARS-CoV-2 infection compared to untreated individuals.13, 14, 15 However, the long-term implications of COVID-19 in adults living with HIV, and particularly the risk of developing Long COVID, remains underexplored.
The pathophysiology of Long COVID is complex and multifactorial, involving persistent viral reservoirs, immune dysregulation, and inflammatory responses.3,4,16,17 Given the chronic immune activation and inflammatory milieu associated with HIV,12 it is plausible that adults living with HIV might be at an increased risk of Long COVID.
Despite the biological plausibility, there is a paucity of data specifically examining the risk of Long COVID in adults living with HIV. Existing literature has primarily focused on acute COVID-19 outcomes,18,19 with limited attention to the long-term health impacts in this population. Addressing this gap is critical for developing targeted interventions and management strategies for adults living with HIV affected by COVID-19.
This study aims to synthesize the existing evidence on the prevalence of Long COVID in adults living with HIV, providing a comprehensive assessment of the long-term impacts of COVID-19 in this population. The first objective is to assess the prevalence of Long COVID in adults living with HIV. The second objective is to investigate whether adults living with HIV are associated with an increased risk of developing Long COVID compared to people without and HIV infection.
Methods
Study design
This systematic review with meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The protocol was registered with the PROSPERO number CRD42024577616.
Search strategy and selection criteria
A comprehensive search was conducted in MEDLINE (Ovid), EMBASE (Ovid), CINHAL (EBSCO), PubMed and CENTRAL from the inception of the databases till June 14th, 2024. The search was conducted without language restrictions. Additionally, grey literature was searched using Google Scholar, preprint servers (e.g., medRxiv, bioRxiv), and conference proceedings. We also conducted a manual search on the reference lists of the included studies for additional investigations that met our inclusion criteria.
The search strategy was developed with consultation from a librarian, and included terms related to HIV, COVID-19, and Long COVID. Different combinations of keywords and Medical Subject Headings (MeSH) terms were used including but not limited to: “HIV Infections”, “HIV Seroprevalence” “HIV”, “HIV Antibodies”, “HIV Seronegativity”, “HIV Antigens”, “AIDS Serodiagnosis” “human immunodeficiency virus”, “human t lymphotropic virus type iii”, “acquired immune deficiency syndrome”, “Long COVID,” “post-acute sequelae of SARS-CoV-2 infection”, “Post-Acute COVID-19 Syndrome”, “post-covid”, “post sars cov∗”, “sequela∗ of sars cov∗”. The search strategy for Ovid Medline is available in eTable 1 in the Supplement.
Table 1.
Characteristics of the included studies.
| Study | Sample size | Sampling | Age mean (SD); years | % Females | SARS-COV2 diagnosis | Cut-off for long COVID diagnosis | Country | CD4 |
% Suppressed | Adjusted OR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean (SD) | % >200 | ||||||||||
| Barth 2024 | 254 | HIV surveillance cohort | 51 (14) | 24% | self-reported | >4 weeks | USA | – | 93.3 | 93 | n/a |
| Degli Antoni 2024 | 510 | HIV surveillance cohort | 53 (9) | 30% | Laboratory confirmed | >3 months | Italy | 775.11 (347.59) | – | – | n/a |
| Jassat 2022 | 2841 | National surveillance databases | 45 (16) | – | Confirmed (laboratory or rapid antigen) | >3 months | South Africa | – | – | – | no |
| Kamanzi 2023 | 94 | COVID surveillance cohort | 50 (11) | 57% | Laboratory confirmed | >3 months | Zambia | 541.05 (384.28) | – | 100 | yes; vaccination status, hospitalisation status and diabetes |
| Mazzitelli 2021 | 75 | HIV surveillance cohort | 50 (14) | 19% | Laboratory confirmed | >3 months | Italy | 590.66 (278.44) | – | 100 | n/a |
| Ogaz 2023 | 341 | Social networking sites and geospatial dating applications | 40 (15) | 0% | Confirmed (laboratory or rapid antigen) | >3 months | UK | – | – | – | no |
| Peluso 2023 | 280 | Long COVID surveillance cohort | 45 (16) | 44% | Unclear | >3 months | USA | 589.08 (290.19) | – | 90.7 | yes; sample collection >100 days, prior COVID-related hospitalization, age >50, sex, BMI >30, preexisting diabetes mellitus, hypertension, renal disease, autoimmune disease, CMV IgG seropositivity, EBNA IgG >600 U/mL, and EBV EA-D IgG positivity |
| Pujari 2021 | 94 | HIV surveillance cohort | – | 73% | Confirmed (laboratory or rapid antigen) | >4 weeks | India | – | 93.6 | 96.8 | n/a |
SD, Standard Deviation; OR, odds ratio; BMI, Body Mass Index; CD4, Cluster of Differentiation 4; CMV, Cytomegalovirus; EBNA, Epstein–Barr Nuclear Antigen; EBV EA-D, Epstein-Barr Virus Early Antigen-D.
We included studies involving adults living with HIV with a SARS-COV-2 infection (diagnosed or self-reported). Our comparator encompassed adults who are not infected with HIV, which hereafter will be referred to as “people living without HIV”, with a SARS-COV-2 infection (diagnosed or self-reported).
The primary outcome was the prevalence of Long COVID. The definitions for Long COVID have been constantly evolving in the past 4 years, as new evidence becomes available. According to a Delphi consensus guided by the World Health Organisation (WHO), post-covid condition (PCC) is defined as having a history of probable or confirmed SARS-CoV-2 infection and experience symptoms for at least 12 weeks from the onset of COVID-19, lasting for a minimum of 2 months without an alternative diagnosis.1,2 The National Institute for Health and Care Excellence (NICE) Guidance, refers to Long COVID as health issues that arise 4 weeks after a SARS-CoV-2 infection or the onset of acute COVID-19 symptoms.20 This term encompasses both ongoing symptomatic COVID-19 (from 4 to 12 weeks after COVID-19 onset) and post-COVID-19 condition (12 weeks or more after COVID-19 onset). The National Academies of Sciences, Engineering, and Medicine (NASEM) defined Long COVID as an infection-associated chronic condition which occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.21 Given the evolving nature of Long COVID, where definitions are updated as new evidence emerges, we aimed to ensure that no relevant studies were excluded based solely on a single definition. Therefore, we included studies with different definitions of Long COVID but pre-specified a minimum symptom duration of 4 weeks after COVID infection, provided other criteria used to characterize Long COVID were consistent with the guidelines available at the time the studies were conducted.
We included observational studies (cohort, case–control, cross-sectional.) that assessed the odds of developing Long COVID following a SARS-CoV-2 infection in people living with HIV compared to people living without HIV. We also included observational studies that assessed the prevalence of Long COVID in people living with HIV without a comparison group. Reviews, case reports, randomised control trials and editorials were excluded.
Procedures
All identified studies were exported to Covidence22 to remove duplicates. Two independent reviewers (DVP and PB) screened titles and abstracts for eligibility. Full texts of potentially relevant studies were assessed independently by the same reviewers. All discrepancies were resolved through consensus. To mitigate “double counting”, if two studies from the same cohort were identified, we included only largest in our analysis. In case of identical sample sizes, we used the most recent one. Google translate was used to assess any studies that were not written in English.
A standardized data extraction form was used to collect information from the included studies. One reviewer (DVP) conducted the data extraction, and a second reviewer (PB) cross verified all the extracted data. None of the included studies required translation.
Outcomes
Our main outcomes were the prevalence of Long COVID among adults living with HIV, and the odds ratio for Long COVID in adults living with HIV, compared to people without HIV. For the odds ratio, if both an adjusted and an unadjusted odds ratio was available, we extracted the adjusted. If the odds ratio was not reported, we extracted the number of Long COVID cases amongst people living with HIV and amongst people living without HIV and calculated the unadjusted odds ratio. We also extracted information on study characteristics (author, year, country, study design), participant characteristics (age, sex, gender, comorbidities, ART use), HIV status, and other relevant data, such as sample size, SARS-CoV-2 diagnosis (self-reported/confirmed) and severity, length of hospital stay, hospitalisation status (hospitalised, non-hospitalised, ICU), length of persistent symptoms (since SARS-CoV-2 infection) and Long COVID diagnosis (self-reported/diagnosed).
Statistics
The risk of bias was assessed using the Cochrane Risk Of Bias In Non-randomized Studies–of Exposure (ROBINS-E) tool.23 This tool provides a structured approach to assessing the risk of bias in observational epidemiological studies, by assessing the risk of bias in 7 distinct domains: bias due to confounding; bias arising from measurement of the exposure; selection bias; bias due to post-exposure interventions; bias due to missing data; bias in the measurement of the outcome; and selective reporting.23 Two reviewers (DVP, PB) independently assessed the risk of bias, and discrepancies were resolved through consensus.
We applied the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach to grade the certainty of the evidence. For each outcome, we rated the evidence as high, moderate, low, or very low certainty.24, 25, 26, 27, 28, 29, 30
We meta-analysed proportions using the logit transformation31 and reported summary results as proportions (95% confidence intervals, 95% CI). We used the inverse-variance (IV) random-effects (RE) model with the restricted maximum-likelihood (REML) estimator32 of the between-study variance to combine results in a two-stage approach. Given the potential variance instability of the logit transformation, we conducted a sensitivity analysis using a one-step approach, via a mixed-effects logistic regression model,33 using maximum-likelihood for estimation. To summarize odds ratios, we use the inverse-variance random-effects model with the REML estimator of the between-study variance, and reported summary ORs with a 95% CI.31,33 We identified statistical heterogeneity using Cochran's Q test and quantified it using the I2 statistic. For the Q test, a P < 0.10 was considered statistically significant.34 We also reported the 95% prediction interval for Long COVID prevalence among people living with HIV, showing the range of true prevalences to be estimated in future studies.35 We assessed small-study and publication bias graphically.36,37 We conducted an additional sensitivity analysis, based on the cut-off that was used for the Long COVID diagnosis, to only include studies that used the 12-week cut-off, and not the studies that used the 4-week cut-off. All sensitivity analyses were defined a priori in the protocol, with the exception of the cut-off point one. For associations, two-sided P values <0.05 were considered statistically significant. We conducted all analyses in Stata 18 (StataCorp, College Station, TX, EUA).
Role of the funding source
No funding was received for this study.
Results
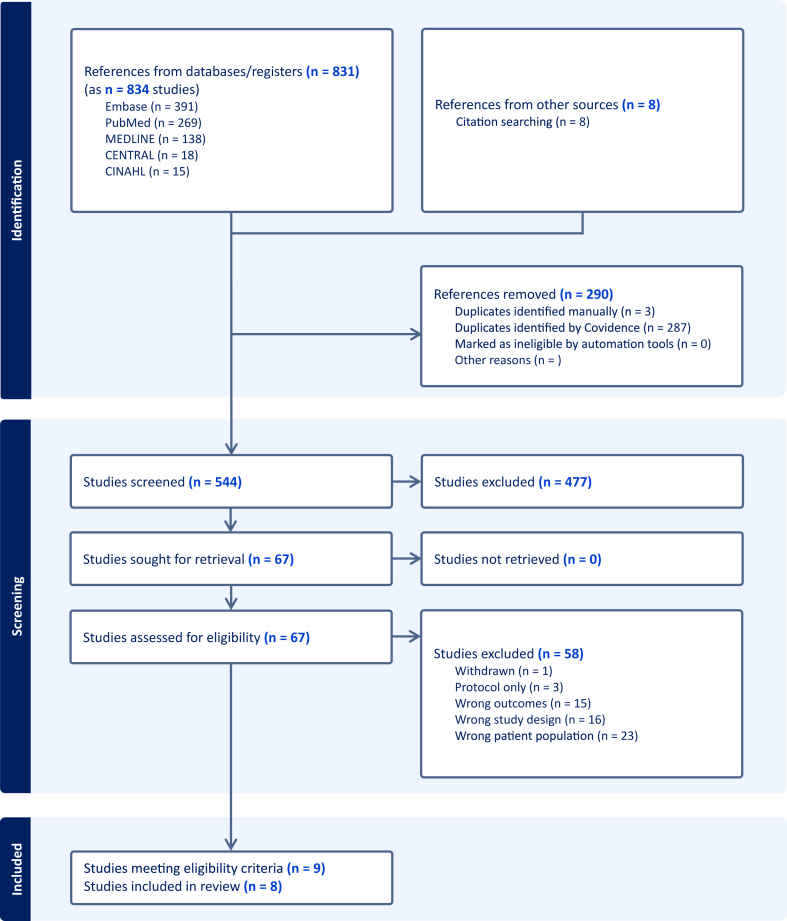
We identified 831 records. After removing the duplicates, we screened 544 studies in title and abstract level. A total of 63 studies underwent full review, and 9 studies (10 records) met our inclusion criteria (Fig. 1). The reason for exclusion at full text level are summarised in eTable 2 in the Supplement. Of the 9 initially included studies, two originated from the same pool of participants. Hence, a total of 8 studies (9 records) were included in our analysis, that included a total of 4489 participants.
Fig. 1.
Prisma flow chart. CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulated Index in Nursing and Allied Health Literature; n, number of studies.
Characteristics of the included studies and participants
The characteristics of the included studies are summarised in Table 1.
Three studies were conducted in Europe.38, 39, 40 Two studies in North America,41,42 two studies43,44 in Africa and one study45 was conducted in Asia. Four studies38,40,41,45 recruited people living with HIV from pre-existing HIV surveillance cohorts. Two studies42,43 recruited from pre-existing COVID surveillance cohorts. One study recruited from national surveillance databases,44 and one study recruited using social networking sites and geospatial dating applications.39 The median sample size was 267 participants (interquartile range [IQR]: 94–383). Four studies38,40,41,45 included only people living with HIV. Four studies39,42, 43, 44 included people living with HIV and a comparator group of people living without HIV. Of these, 2 studies39,44 reported the number of Long COVID cases for adults living with HIV and adults without HIV, which were used to calculate the unadjusted odds ratios; and 2 studies42,43 reported adjusted odds ratios. Specifically, Kamanzi et al.43 adjusted for vaccination status, hospitalisation status and diabetes; and Peluso et al.42 adjusted for timing of sample collection >100 days, prior COVID-related hospitalization, age >50 years, sex, BMI >30, preexisting diabetes mellitus, hypertension, renal disease, autoimmune disease, CMV IgG seropositivity, EBNA IgG >600 U/mL, and EBV EA-D IgG positivity.
The majority of the studies38, 39, 40,43, 44, 45 included participants with a diagnosed prior SARS-COV-2 infection (including laboratory confirmed or rapid-test). One study41 included both diagnosed and self-reported cases. One study42 did not specify. With regards to the severity of the prior SARS-COV2 infection, 1 study44 included only people that were hospitalised during the acute phase of SARS-CoV-2 infection, and one study40 included only people that were symptomatic during the acute phase of SARS-CoV-2 infection. The remaining studies38,39,41, 42, 43,45 did not report any restrictions. The mean average Cluster of Differentiation 4 (CD4) count in adults living with HIV was 624 (SD: 90). The mean percentage of adults living with HIV in suppressed status (viral load <200 copies/ml) was 96% (SD: 3.7)). Six studies38,40,42, 43, 44, 45 reported the sex of the participants, one study41 reported the gender, and one study39 reported both sex and gender. With regards to sex, the mean percentage of females was 35%. With regards to gender, no summary proportion can be estimated, due to the very low number of studies reporting gender. The median average age was 50 years (IQR: 45–51).
Risk of bias
The risk of bias assessment for each individual study is summarised in eFig. 1 in the Supplement. Two studies39,44 were rated to have high overall risk of bias and 6 studies38,40, 41, 42, 43,45 were rated as having some concerns. The most common sources of bias were bias due to confounding, selection bias and outcome measurement bias. The least common source of bias was bias due missing data, and selective reporting.
Prevalence of Long COVID in adults living with HIV
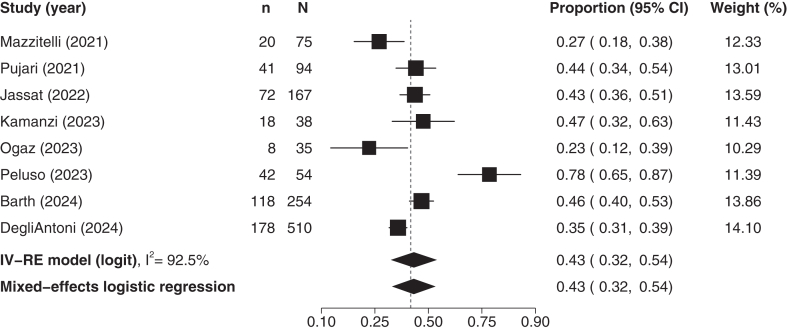
To quantify the burden of Long COVID in adults living with HIV, we synthesised the prevalence estimates of a total of 8 studies,38, 39, 40, 41, 42, 43, 44, 45 including 1227 participants. The median average (SD) age was 50 (IQR: 45–51)) years old and the mean percentage of females was 35% (SD: 23%). Six of the included studies38, 39, 40,42, 43, 44 used a 12-weeks cut-off for Long COVID diagnosis, and 2 studies41,45 used a 4 weeks cut-off. The pooled estimate suggested that the prevalence of Long COVID in adults living with HIV is 43% (95% CI: 32–54%; Fig. 2). Similar results were obtained in the sensitivity analysis using mixed-effects logistic regression (Fig. 2). The heterogeneity was deemed high (I2 = 92.5), with the 95% prediction interval extending from 12.3 to 79.7%. The certainty of evidence was rated as low, downgraded for high risk of bias and imprecision.
Fig. 2.
Prevalence of long COVID in HIV. The P value for heterogeneity was <0.0001. CI, Confidence Intervals; IV, Inverse Variance; RE, Random Effects; n, number of events; N, study population.
We conducted an additional sensitivity analysis including only studies using the 12-week cut-off for the Long COVID diagnosis. The pooled estimate was similar to the initial analysis (42% 95% CI: 27%–58%, 6 studies, 879 participants; eFig. 2).
Association between HIV status with the odds of long COVID
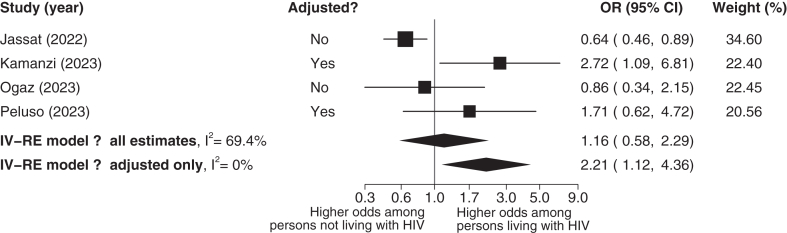
A total of 4 studies39,42, 43, 44 with 3556 participants were included in the analysis of the association between HIV status and the odds of Long COVID. The median average age was 45 years (IQR: 41–49), and the mean percentage of females was 34% (SD: 25%). All of the included studies used a 3-month cut-off for the diagnosis of Long COVID. In the main analysis, the association of developing Long COVID among adults living with HIV compared to those without HIV was inconclusive, with an estimated odds ratio of 1.16 (95% CI: 0.58–2.29), indicating substantial uncertainty around the true effect size. This difference was not statistically significant; P = 0.67, Fig. 3). The heterogeneity was deemed moderate (I2 = 69.4%). The certainty of evidence was rated as low, downgraded for high risk of bias and inconsistency. In a sensitivity analysis including only estimates adjusted for confounding factors (2 studies, 374 participants, median average age of 48 years (IQR: 45, 50), 51% female (SD: 7%)) adults living with HIV were associated with 2.21 times higher odds of developing Long COVID compared to people without HIV (OR: 2.21, 95% CI: 1.12–4.36, P = 0.02, Fig. 3). The heterogeneity was deemed low (I2 = 0%). The certainty of evidence was rated as low, downgraded for high risk of bias and imprecision.
Fig. 3.
Odds ratio among persons living with HIV vs persons not living with HIV. The P value for heterogeneity was 0.013 for all estimates and 0.51 for adjusted estimates only. OR, odds ratio; CI, Confidence Intervals; IV, Inverse Variance; RE, Random Effects; n, number of events; N, study population.
Small study effects and publication bias
Funnel plot assessments for the prevalence of Long COVID in adults living with HIV and the association between HIV status and the odds of Long COVID, are shown in eFig. 3 and eFig. 4, respectively. We could not detect any publication bias due to the small number of studies.
Discussion
In this systematic review with meta-analysis of 8 studies, including 4489 participants, we found a high prevalence of Long COVID in adults with HIV (43%). Similar results were obtained after considering only studies that used a 12-weeks cut-off, and not studies with a 4-week cut-off point for the Long COVID diagnosis (42%). However, caution is advised when interpreting these results due to the high risk of selection bias, bias in the measurement of the outcome and confounding, that could lead in an overestimation of the true prevalence and the high between study heterogeneity. The available evidence is still very limited as to whether individuals living with HIV could be at a higher risk of Long COVID compared to the general population. Based on only 4 studies, we detected suggestive, but imprecise evidence of an elevated risk for Long COVID in adults with HIV. Restricting our analyses to two studies (374 participants) that reported adjusted estimates, we found that people living with HIV might be associated with approximately twice the odds of developing Long COVID compared to those without HIV (OR: 2.21, 95% CI: 1.12–4.36).
Previous research has shown that HIV infection is associated with worse health outcomes and higher mortality rates during the acute phase of SARS-COV2 infection.19 Our review builds on this evidence and suggests that the risk for worse health outcomes may persist in the long-term after the acute SARS-COV2 infection stage. A previous systematic review46 without a meta-analysis, on risk factors for Long COVID in Africa suggested that HIV was not identified as a risk factor. However, these conclusions derived by only one cohort study, where the overall population of adults living with HIV was only 5% of the total study population. A recent systematic review with meta-analysis47 suggested that HIV infection was associated with an increased risk of Long COVID (odds ratio 2.20; 95% confidence interval 1.25–3.86). The reported estimates are considerably higher than the estimates reported in our initial analysis, but they are close to our analysis that was restricted to adjusted estimates only. However, there is a series of methodological shortcomings in this review. The authors did not assess the risk of bias of the included studies and the quality of evidence; hence, they failed to highlight the uncertainty of their OR estimate attributed to high risk of bias and imprecision. Moreover, the authors included two different studies from the same author that derive from the same cohort of participants. Hence, there is duplicate information in the data analysis that inflates the pooled estimate. In contrast, our paper identified this limitation and only included one of the two studies in the model, providing a more accurate estimate of the prevalence and the odds of developing Long COVID. In addition, the authors included in the analysis a study that only 10 out of 530 participants were living with HIV, hence we would argue that this study cannot provide an accurate estimate of the prevalence of Long COVID in people living with HIV and it should not have been included in the analysis. Besides, the authors entered the sample size for this study as 20 instead of 10 in the model, which further affects the accuracy of the results. Our review updates this evidence with an additional 4 studies contributing in the prevalence estimate and 2 studies contributing in the odds ratio estimate that were not identified in the previous review and offers a more robust estimate.
Still, the integrity of our results depends on the availability of data, and the methodological quality of the included studies. Due to the limited number of available studies, our results regarding the between-study heterogeneity may be underestimated. The most common source of bias was selection bias and bias in the measurement of the outcome. One factor that could introduce bias in the measurement of the outcome is the episodic nature of both HIV and Long COVID that could lead to misclassification; since it is possible that individuals that experience HIV-attributed symptoms were classified as having Long COVID, and vice-versa.
Another factor that could introduce bias in the measurement of the outcomes is the inconsistency in the definition of Long COVID, especially with regards to what is defined as “persistent”. Most of the studies included people that experienced symptoms for 3 or more months since the initial SARS-CoV-2 infection, which is in line with the most recent definition of Long COVID, but still, 2 of the studies included in the prevalence analysis included people that experienced symptoms for 4 or more weeks. Although the 4 weeks cut-off is in line with a previous Long COVID definition from NICE20 these studies could still include cases that could have experience a resolution in their symptoms by the 3 month cut-off. To address this issue, we conducted a sensitivity analysis including only studies that used the 3-month cut-off, further improving the robustness of our results. The 4-week cut-off limitation does not apply in our OR estimate, as all of the included studies used a 12-weeks cut-off.
Studies that use convenience sampling and recruit people from surveillance cohorts with direct access to care may be prone to selection bias. In our review, half of the studies that were included in the prevalence analysis recruited from HIV surveillance cohorts. Although HIV in some countries is a rare exposure and recruiting from the general population has the risk of not identifying enough cases, recruiting from a convenience sample such as an HIV surveillance cohort could introduce selection bias and, in our case, affect the measurement of the outcome. People living with HIV who are part of large HIV-surveillance cohorts, have easier access to line of care than the general population. That could potentially make them more likely to report and to get diagnosed with Long COVID than people in the general population. Hence, although our prevalence estimate is significantly higher than the Long COVID prevalence estimates for the general population from nationwide UK and Canadian studies5, 6, 7 (3–15%), it is possible that part of the difference could be attributed to different sampling methods and surveillance standards.
To address these biases and verify the integrity of our results we also assessed the odds of developing Long COVID in people living with HIV compared to people living without HIV. None of the included studies in the odds ratio estimate recruited people living with HIV from HIV surveillance cohorts. This can reduce the potential selection bias and bias in the measurement of the outcomes that was present in the prevalence analysis. To further improve the robustness of our results, we conducted a sensitivity analysis with the studies that reported adjusted OR estimates, to account for bias due to confounding. However, since only two studies reported adjusted estimates, caution is advised when interpreting the results of our sensitivity analysis.
Long COVID is an unprecedented clinical condition with a lot of uncertainty on the underlying pathophysiological mechanisms and is characterized by a variety of physical, mental and cognitive symptoms that may be multi-dimensional, episodic, and unpredictable in nature.48 The shortage on available diagnostic tests at the early stage of the pandemic left a lot of people with an indefinite diagnosis, making studying and understanding the prevalence and pathogenesis of Long COVID in people without a confirmed infection is even more challenging.
When designing health services, it is crucial to take into consideration the needs of the population they are designed for. The high prevalence of Long COVID in a population like adults living with HIV that is already susceptible to infections, inflammation, and chronic diseases like cancer and cardiovascular diseases12; is an urgent matter of public health that requires action. Managing two chronic conditions requires comprehensive and coordinated healthcare. Specifically, persons living with Long COVID may experience mental and cognitive health related consequences of the conditions, such as implications, including anxiety, depression, poor memory and cognitive dysfunction.3 Adults living with HIV have a higher chance of developing mood, anxiety, and cognitive disorders,49 and depression50 is one of the most common mental health conditions faced by people with HIV. Research suggests that the mental health of people living with HIV was further aggravated during the pandemic, due to the lack of social support, financial hardship and economic vulnerability.51 Hence, adults living with HIV and Long COVID might face challenges in accessing specialized care, managing multiple medications, and attending regular medical appointments. The overlapping burden of managing two chronic conditions could increase the risk of mental health issues, and it can impact daily functioning, quality of life, and adherence to HIV treatment regimens.
Ensuring continuity of care and integrated healthcare services is essential. Hence, it is crucial to ensure that the available Long COVID programs and healthcare providers following people living with HIV are equipped to handle the needs of people living with both conditions. For instance, health professionals who are following people with Long COVID may find it difficult to assess patients with both conditions, with regards to which assessment tools they should use, the presence of multiple symptoms that overlap, and the episodic and multi-dimensional nature of the symptoms and this can create a gap in clinical care and misclassification errors. Health care providers who are following people living with HIV also need proper guidance, as they are usually required to not only managing ART treatment but also to navigating the complex health challenges and services needed for those experiencing Long COVID. Continuity of care should also be considered in the context of healthcare service availability. Different countries have established distinct systems for managing HIV and Long COVID, and even within the same country, healthcare infrastructure can vary greatly across regions. These differences impact not only access to care but also the quality and consistency of services provided. Ensuring that individuals receive the necessary treatment, while accounting for the existing systems and their limitations, presents a complex challenge. Factors such as resource allocation, healthcare workforce availability, and the level of integration between primary, secondary, and specialized care all influence how well continuity of care can be maintained. In some areas, particularly those with limited healthcare resources or strained systems, access to essential treatments may be delayed or unevenly distributed, further complicating care continuity. Addressing these challenges requires cross-sector collaboration among healthcare providers, policymakers, and community organizations to enable balancing the specific needs of the population with the realities of the local healthcare landscape, ensuring that all individuals have equitable access to the care they require.
The episodic nature of Long COVID symptoms may be similar to the episodic nature of HIV, where some health challenges can fluctuate daily or over longer periods of time.52,53 Hence, there might be some value in using a conceptual framework of disability that was originally derived from the perspectives of persons living with HIV52 which may be applicable to describe the multidimensional and episodic nature of the context of Long COVID to improve and inform Long COVID care for this population.54 Having a conceptual framework of disability for Long COVID may facilitate the therapeutic pathways for both adults living with HIV and people without HIV. For people living with both conditions, having clear assessment tools that would allow differential diagnosis between the two conditions with regards to which conditions the episodic symptoms are attributed is crucial, since each underlying condition may require a different therapeutic approach. Even more, in situations where the symptoms are interrelated or attributed to both conditions, adults living with HIV may not respond to a single-condition therapeutic model and may need a more integrative approach.
The demographics of the population of the included studies limits the generalisability of our results. A high percentage of adults living with HIV in the included studies were part of surveillance cohorts and the vast majority was virally suppressed, something that is not surprising as most countries have adopted the UNAIDS 95-95-95 global targets for epidemic control,55 which aim to ensure by 2030 that 95% of HIV-positive people know their HIV status, 95% of people diagnosed with HIV receive sustained antiretroviral therapy (ART), and 95% of people on ART have viral suppression. However, the suppression status in the included studies was ranging from 91 to 100%, which is significantly higher than the suppression status reported in 2020 in Canada (83%)56 and in 2021 in the US (66%).57 Although both countries are working towards achieving the 95-95-95 UNAID goal, there is still a large portion of adults living in countries who do not have access to ART. Long COVID may also occur in adults living with HIV unaware of their HIV status. Hence, more research is required with sample sizes enriched with adults living with HIV in a non-suppressed status, to better understand the risk and the implications of HIV in the development of Long COVID in this population.
Similarly, the mean values of CD4 counts in adults living with HIV in the included studies were well above the healthy cut-off, and in similar levels with the counts in people without HIV. This did not allow us to conduct any disaggregated analysis, to further examine potential implications of low CD4 counts in the development of Long COVID. However, since there is a link between low CD4 counts and worse health outcomes in the acute stage of SARS-COV-2 infection,58,59 exploring the link between low CD4 counts and Long COVID in adults living with HIV could be a potential target of future research.
Last but not least, extracting sex and gender was one of the greatest challenges in this review. Some studies reported that they measured gender but presented the proportions under “male” and “female” labels, which suggests sex, not gender. The only study that reported both sex and gender had female sex listed in the exclusion criteria, and only reported two categories, men and gender diverse people. Hence, no estimates could be calculated with regards to the representation of women or gender diverse people among the included studies. Still, the representation of females in the included studies was lower than the representation of males. That could be of major concern, especially since the latest update on 95-95-95 identified adolescent girls, young women and transgendered people as key populations that are still at high risk of new HIV infections due to social stigma, violence, discrimination and social exclusion.60 Hence, there is an urgent need for more inclusive research.
The available evidence indicates that prevalence of Long COVID in adults living with HIV could be high (43%). There is limited and uncertain evidence indicating that people living with HIV could be at a higher risk of long-COVID than individuals not living with HIV. Additional research is needed to determine whether HIV positivity is a risk factor for Long COVID and to develop coordinated care pathways and targeted therapies to provide more holistic, patient-centred care, for the management of Long COVID in people living with HIV.
Contributors
PB and DVP conceived the idea for the review. PB, DVP designed, undertook the literature search, coordinated the study. PB, DVP, and NB acquired data, screened records, extracted data, and assessed risk of bias. PB, TVP and DVP conducted the data analysis. DVP and PB wrote the first draft of the manuscript. EM, JCM, KKO, KQ, MM, FR, AMC, TVP and SS gave crucial intellectual input and provided critical revisions in the manuscript. DVP and PB have verified the underlying data and had the final responsibility for the decision to submit for publication. All authors had full access to all the data in the study and approved the final version of the manuscript.
Data sharing statement
The guarantor (PB) is willing to examine all requests for the full dataset after two years from the date of this publication.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
PB was supported by Canadian Institutes of Health Research (FRN 191927). TVP is funded by the Chevening Scholarship Program (Foreign and Commonwealth Office, United Kingdom). DVP was supported by the Arthritis Society Doctoral Award, the Canadian Behavioural Trials and Interventions Network, the Ontario Graduate Student Award and the Bone and Joint Institute Transdisciplinary Award. JCM was supported by the Canada Research Chair in Musculoskeletal Health Outcomes and Knowledge Translation, and the Dr. James Roth Research Chair in Musculoskeletal Measurement and Knowledge Translation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102993.
Appendix A. Supplementary data
References
- 1.WHO A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [cited 2022 Nov 9]; Available from: [DOI] [PMC free article] [PubMed]
- 2.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2. https://www.nature.com/articles/s41579-022-00846-2 [cited 2023 Mar 3]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altmann D.M., Whettlock E.M., Liu S., Arachchillage D.J., Boyton R.J. The immunology of long COVID. Nat Rev Immunol. 2023;23(10):618–634. doi: 10.1038/s41577-023-00904-7. [DOI] [PubMed] [Google Scholar]
- 5.Hastie C.E., Lowe D.J., McAuley A., et al. True prevalence of long-COVID in a nationwide, population cohort study. Nat Commun. 2023;14(1):7892. doi: 10.1038/s41467-023-43661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Self-reported coronavirus (COVID-19) infections and associated symptoms, England and Scotland - Office for national statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/selfreportedcoronaviruscovid19infectionsandassociatedsymptomsenglandandscotland/november2023tomarch2024#cite-this-article [cited 2024 Jul 22]; Available from:
- 7.Canada PHA of Post-COVID-19 condition (long COVID) 2021. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/symptoms/post-covid-19-condition.html [cited 2024 Jul 22]; Available from:
- 8.New report from UNAIDS shows that AIDS can be ended by 2030 and outlines the path to get there. https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2023/july/unaids-global-aids-update [cited 2024 Aug 8]; Available from:
- 9.Trickey A., Sabin C.A., Burkholder G., et al. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: a collaborative analysis of cohort studies. Lancet HIV. 2023;10(5):e295–e307. doi: 10.1016/S2352-3018(23)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNAIDS welcomes new research on ‘opt-out’ HIV testing in England. https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2023/november/20231129_new-research-on-opt-out-hiv-testing-england [cited 2024 Jul 22]; Available from:
- 11.Global AIDS strategy 2021-2026. https://www.unaids.org/en/Global-AIDS-Strategy-2021-2026 [cited 2024 Jul 22]; Available from:
- 12.Deeks S.G., Overbaugh J., Phillips A., Buchbinder S. HIV infection. Nat Rev Dis Prim. 2015;1(1) doi: 10.1038/nrdp.2015.35. [DOI] [PubMed] [Google Scholar]
- 13.Höft M.A., Burgers W.A., Riou C. The immune response to SARS-CoV-2 in people with HIV. Cell Mol Immunol. 2024;21(2):184–196. doi: 10.1038/s41423-023-01087-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertagnolio S., Thwin S.S., Silva R., et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV. 2022;9(7):e486–e495. doi: 10.1016/S2352-3018(22)00097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risk factors for coronavirus disease 2019 COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2021;73(7):e2005–e2015. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein J., Wood J., Jaycox J.R., et al. Distinguishing features of long COVID identified through immune profiling. Nature. 2023;623(7985):139–148. doi: 10.1038/s41586-023-06651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danwang C., Noubiap J.J., Robert A., Yombi J.C. Outcomes of patients with HIV and COVID-19 co-infection: a systematic review and meta-analysis. AIDS Res Ther. 2022;19(1):3. doi: 10.1186/s12981-021-00427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambrosioni J., Blanco J.L., Reyes-Urueña J.M., et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. 2021;8(5):e294–e305. doi: 10.1016/S2352-3018(21)00070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overview|COVID-19 rapid guideline: managing the long-term effects of COVID-19|Guidance|NICE. 2020. https://www.nice.org.uk/guidance/ng188 [cited 2024 Aug 8]; Available from: [Google Scholar]
- 21.National Academies of Sciences E and Medicine . 2024. A Long COVID Definition: A Chronic, Systemic disease state with profound consequences. [PubMed] [Google Scholar]
- 22.Covidence systematic review software, veritas heath inovation. www.covidence,org Melbourne, Australia, Available from:
- 23.Higgins J.P., Morgan R.L., Rooney A.A., et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E) Environ Int. 2024;186 doi: 10.1016/j.envint.2024.108602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt G., Oxman A.D., Akl E.A., et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Balshem H., Helfand M., Schünemann H.J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt G.H., Oxman A.D., Vist G., et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64(4):407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt G.H., Oxman A.D., Montori V., et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64(12):1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt G.H., Oxman A.D., Kunz R., et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64(12):1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt G.H., Oxman A.D., Kunz R., et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt G.H., Oxman A.D., Kunz R., et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 32.Langan D., Higgins J.P., Jackson D., et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10(1):83–98. doi: 10.1002/jrsm.1316. [DOI] [PubMed] [Google Scholar]
- 33.Lin L., Chu H. Meta-analysis of proportions using generalized linear mixed models. Epidemiology. 2020;31(5):713–717. doi: 10.1097/EDE.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira T.V., Patsopoulos N.A., Salanti G., Ioannidis J.P. Critical interpretation of Cochran's Q test depends on power and prior assumptions about heterogeneity. Res Synth Methods. 2010;1(2):149–161. doi: 10.1002/jrsm.13. [DOI] [PubMed] [Google Scholar]
- 35.IntHout J., Ioannidis J.P.A., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7) doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67(8):897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Degli Antoni M., Maifredi G., Storti S., et al. Long-term symptoms after SARS-CoV-2 infection in a cohort of people living with HIV. Infection. 2024 doi: 10.1007/s15010-024-02288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogaz D., Allen H., Reid D., et al. COVID-19 infection and vaccination uptake in men and gender-diverse people who have sex with men in the UK: analyses of a large, online community cross-sectional survey (RiiSH-COVID) undertaken November-December 2021. BMC Public Health. 2023;23(1):829. doi: 10.1186/s12889-023-15779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzitelli M., Trunfio M., Sasset L., et al. Factors associated with severe COVID-19 and post-acute COVID-19 syndrome in a cohort of people living with HIV on antiretroviral treatment and with undetectable HIV RNA. Viruses. 2022;14(3):493. doi: 10.3390/v14030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barth S., Kulie P., Monroe A., Horberg M., Castel A., Cohort D.C. Prevalence and risk factors for post-COVID conditions of COVID-19 among persons with HIV in Washington, DC. AIDS Care. 2024;36(9):1358–1368. doi: 10.1080/09540121.2024.2357811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peluso M.J., Deveau T.-M., Munter S.E., et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest. 2023;133(3) doi: 10.1172/JCI163669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamanzi P., Mulundu G., Mutale K., Mumba C., Ngalamika O. HIV and inflammatory markers are associated with persistent COVID-19 symptoms. Immun Inflamm Dis. 2023;11(5) doi: 10.1002/iid3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jassat W., Mudara C., Vika C., et al. A cohort study of post-COVID-19 condition across the Beta, Delta, and Omicron waves in South Africa: 6-month follow-up of hospitalized and nonhospitalized participants. Int J Infect Dis. 2023;128(c3r, 9610933):102–111. doi: 10.1016/j.ijid.2022.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pujari S., Gaikwad S., Chitalikar A., Dabhade D., Joshi K., Bele V. Long-coronavirus disease among people living with HIV in western India: an observational study. Immun Inflamm Dis. 2021;9(3):1037–1043. doi: 10.1002/iid3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müller S.A., Isaaka L., Mumm R., et al. Prevalence and risk factors for long COVID and post-COVID-19 condition in Africa: a systematic review. Lancet Global Health. 2023;11(11):e1713–e1724. doi: 10.1016/S2214-109X(23)00384-4. [DOI] [PubMed] [Google Scholar]
- 47.Yang X., Shi F., Zhang H., et al. Long COVID among people with HIV: a systematic review and meta-analysis. HIV Med. 2024 doi: 10.1111/hiv.13708. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown D.A., O'Brien K.K. Conceptualising long COVID as an episodic health condition. BMJ Glob Health. 2021;6(9) doi: 10.1136/bmjgh-2021-007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Liu M., Lu Q., et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology. 2020;95(19):e2610–e2621. doi: 10.1212/WNL.0000000000010752. [DOI] [PubMed] [Google Scholar]
- 50.Mudra Rakshasa-Loots A., Whalley H.C., Vera J.H., Cox S.R. Neuroinflammation in HIV-associated depression: evidence and future perspectives. Mol Psychiatry. 2022;27(9):3619–3632. doi: 10.1038/s41380-022-01619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong C., Queiroz A., Hoskin J. The impact of the COVID-19 pandemic on mental health, associated factors and coping strategies in people living with HIV: a scoping review. J Int AIDS Soc. 2023;26(3) doi: 10.1002/jia2.26060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Brien K.K., Bayoumi A.M., Strike C., Young N.L., Davis A.M. Exploring disability from the perspective of adults living with HIV/AIDS: development of a conceptual framework. Health Qual Life Outcome. 2008;6(1):76. doi: 10.1186/1477-7525-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Brien K.K., Davis A.M., Strike C., Young N.L., Bayoumi A.M. Putting episodic disability into context: a qualitative study exploring factors that influence disability experienced by adults living with HIV/AIDS. J Int AIDS Soc. 2009;12:1–11. doi: 10.1186/1758-2652-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Brien K.K., Brown D.A., McDuff K., et al. Conceptual framework of episodic disability in the context of Long COVID: findings from a community-engaged international qualitative study. medRxiv. 2024 doi: 10.1101/2024.05.28.24308048. [DOI] [Google Scholar]
- 55.Understanding fast-track | UNAIDS. https://www.unaids.org/en/resources/documents/2015/201506_JC2743_Understanding_FastTrack [cited 2024 Aug 8];Available from:
- 56.Canada takes action by endorsing global declaration on Undetectable = Untransmittable (U=U) - Canada.ca. https://www.canada.ca/en/public-health/news/2022/07/canada-takes-action-by-endorsing-global-declaration-on-undetectable--untransmittable-uu.html [cited 2024 Aug 8]; Available from:
- 57.Ending the HIV epidemic in the US goals | EHE initiative | CDC. https://www.cdc.gov/ehe/php/about/goals.html [cited 2024 Aug 8]; Available from:
- 58.Yang X., Sun J., Patel R.C., et al. Associations between HIV infection and clinical spectrum of COVID-19: a population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV. 2021;8(11):e690–e700. doi: 10.1016/S2352-3018(21)00239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tesoriero J.M., Swain C.-A.E., Pierce J.L., et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open. 2021;4(2):e2037069. doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reductions in new HIV infections in several Global HIV prevention coalition countries, but global progress needs to be accelerated | UNAIDS. https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2024/march/20240313_global-hiv-prevention-coalition?_gl=1∗12plsg7∗_gcl_au∗MTg5MTQ1Mjg5LjE3MjE2NjM4NjI.∗_ga∗MTU1MTQ4OTk0MS4xNzIxNjYzODYy∗_ga_T7FBEZEXNC∗MTcyMzA1NzY0MC41LjAuMTc [cited 2024 Aug 8];Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.