Abstract
To contract, to deform, and remodel, the airway smooth muscle cell relies on dynamic changes in the structure of its mechanical force–bearing cytoskeleton. These alternate between a “fluid-like” (relaxed) state characterized by weak contractile protein–protein interactions within the cytoskeletal apparatus and a “solid-like” (contractile) state promoted by strong and highly organized molecular interactions. In this review, we discuss the roles for actin, myosin, factors promoting actin polymerization and depolymerization, adhesome complexes, and cell–cell junctions in these dynamic processes. We describe the relationship between these cytoskeletal factors, extracellular matrix components of bronchial tissue, and mechanical stretch and other changes within the airway wall in the context of the physical mechanisms of cytoskeletal fluidization–resolidification. We also highlight studies that emphasize the distinct processes of cell shortening and force transmission in airway smooth muscle and previously unrecognized roles for actin in cytoskeletal dynamics. Finally, we discuss the implications of these discoveries for understanding and treating airway obstruction in asthma.
Keywords: airway smooth muscle, airway hyper-responsiveness, asthma, actin, myosin
Airway smooth muscle (ASM) cells are grouped under the respiratory epithelial layer of the larger airways (trachea and bronchi as well as bronchioles) and less prominently within the lung parenchyma (Fig. 1) (1). Mechanical forces and contractility of ASM tissue are fundamentally responsible for airway narrowing and airway hyper-responsiveness (AHR), which is defined as exaggerated bronchoconstriction in response to contraction-inducing mediators (2). These mechanical forces result from constant variations in lung volume during breathing. ASM tension determines airway caliber throughout respiration and is modulated by breathing (especially deep inspirations) that exert periodic cycles of stretch-recoil on ASM cells (3). Soluble mediators of airway contraction, inflammatory factors, the quantity and composition of extracellular matrix (ECM), and the number and size of individual ASM cells modulate ASM tension. Underlying mechanisms involve both genomic and nongenomic regulation (3). ASM properties change in response to mechanical forces because of adaptive modifications in the organization of the cytoskeleton (4). Several cytoskeleton-related molecules regulate the cytoskeleton and the development of AHR in preclinical cellular and animal models.
Figure 1.
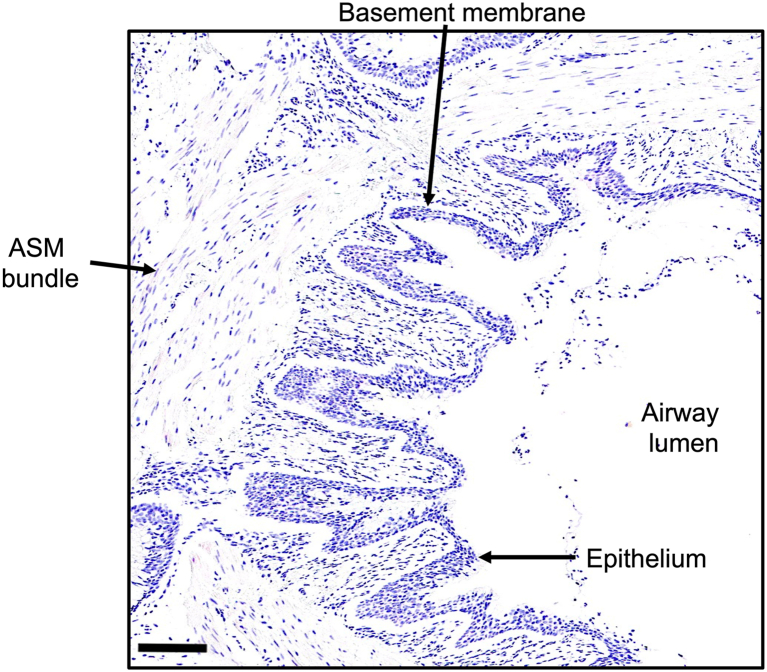
Lung histology. Human lung section stained with hematoxylin shows the airway lumen surrounded by a columnar epithelial layer and basement membrane. Beneath these layers lies the ASM bundle of the bronchiole. Scale bar represents 125 μm. ASM, airway smooth muscle.
The intrinsic mechanophenotype of the ASM cell and its contribution to AHR in asthma is a topic of intense investigation. Apart from specific G protein-coupled receptor (GPCR) antagonists (of muscarinic and leukotriene receptors), which may have clinical efficacy in selected patient populations, ASM-targeted treatments for asthma are primarily focused on increasing relaxation rather than preventing contraction (5). Unfortunately, the therapeutic effects of most relaxation-targeted treatments are acute and transient. Once overcome, the asthmatic airway recontracts. Accordingly, current investigation is focused on the development of drugs with the capacity to prevent ASM cells from hypercontracting in the first place. These treatments primarily target force-generating cytoskeletal pathways in ASM and their relationships to breathing and ECM interactions.
The concept of fluidization–resolidification rests on the premise that the ASM cell is continually in disequilibrium, a state driven by the activity of molecular motors (ATP-dependent fluctuations in protein conformation) and mechanical energy injected into the cell with each periodic stretch. These nonequilibrium dynamics confer three important physical properties to the ASM (1): structural malleability or the ability to switch between a “fluid-like” state that enables cellular migration, division, and deformation and a “solid-like” state that enables structural integrity by generating internal elastic stresses that counterbalance external mechanical forces (6, 7); (2): scale-free rheology, that is, the principle that dynamic cytoskeletal alterations do not occur over fixed relaxation times (8), implying that the ASM cell is close to a solid-like state at rest; (3): universality, that is, the cytoskeletal dynamics obey simple biophysical laws that are applicable over a wide range of molecular interventions, integrative scales (9, 10), and even cell types (11).
We discuss the physiological implications of the ASM fluidization–resolidification framework, describe its molecular origins including dynamically regulated intracellular effectors, mechanotransduction signaling pathways, and the role of mechanical stretch. Throughout, we highlight evidence for dysregulation of these mechanisms in asthma including the influence of asthma-related cytokines. Finally, we describe potential areas of future investigation and emerging therapeutic avenues to modify ASM cytoskeletal biomechanics to combat AHR.
Overview of ASM fluidization–resolidification as a unifying mechanism of contraction
The mechanical behavior of ASM cells bears strong resemblance to that of soft glass materials (12, 13, 14). In the absence of external mechanical perturbations, the ASM cell behaves like a solid with a Young’s modulus (the ratio of the stress [force per unit area] exerted on the object and the resulting axial strain [displacement or deformation] in the linear elastic region of the material) in the kPa range. However, when subjected to a single transient stretch like that imposed by breathing, the ASM cell promptly and acutely transitions to a fluid-like state. This state is characterized by cytoskeletal remodeling that increases by more than one order of magnitude and cytoskeletal softening and contractile force reduction by greater than 70% (11, 15). The fluidized ASM cell subsequently resolidifies by means of slowed structural rearrangements, cytoskeletal stiffening, and contractile force enhancement (11, 15). Stretch-induced fluidization–resolidification has been observed at the molecular level (6, 16), in the single isolated ASM cell (11), in ASM within intraparenchymal airways of human precision cut lung slices (PCLS) (17) and in isolated lung tissue (18). These dynamic changes correlate with changes in lung airway resistance following a deep inspiration (19).
Fluidization–resolidification of ASM can explain the long-standing observation of the salutary effects of a deep inspiration on airway caliber (20, 21, 22). It also provides a conceptual framework to explain ASM–airway dysfunction in asthma. The ASM cell fluidizes only when the applied strain exceeds a threshold value (11, 15). In the bronchial airway, the strain achieved in response to a breath varies inversely as a function of two physical factors: (i) stiffness of the noncontractile elements of other structural cells (e.g., epithelial cells, fibroblasts) and character of the ECM in the airway wall and (ii) stiffness and contractile force generated by the ASM cell itself.
In asthma, increased magnitude and velocity of ASM contraction (23), increased ASM cell number and mass (24, 25), abnormal airway-parenchymal interactions (26), and increased airway wall thickness because of excessive ECM deposition (27) all limit the actual strain imposed on the ASM cell by respiration (Fig. 2). The ASM cell becomes “frozen” in a stiffened/hypercontractile state (28, 29, 30, 31), effectively rendering it resistant to the beneficial effects of deep inspiration until either the contracting stimulus is removed or an ASM relaxant is delivered. In this review, we discuss fluidization—resolidification of the ASM cell and the failure of the ASM to fluidize in asthma as unifying mechanisms that underlie AHR. Advances in our understanding of these concepts have revealed previously unrecognized pathways for therapeutic intervention.
Figure 2.
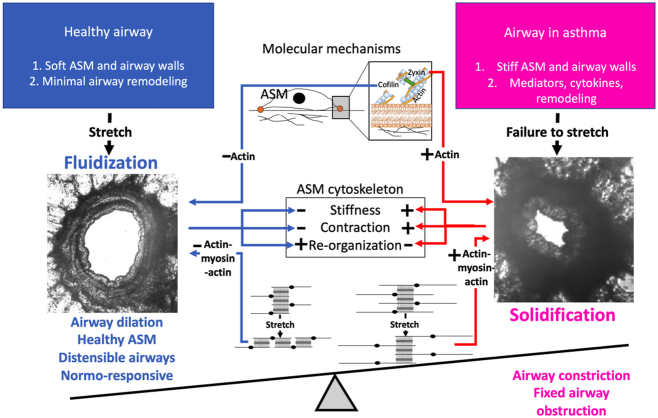
A proximate cause of bronchoconstriction in asthma is failure of the ASM to fluidize. The microenvironment of every cell resident within the airway wall possesses physical as well as humoral attributes. Inhaled corticosteroid (ICS)–insensitive humoral pathways (red) dispose toward airway remodeling and persistent asthma, whereas physical pathways (blue) are ordinarily sufficient to hold those humoral effects in check. These pathways include positive feedback loops that have potential to incite instability. Left, in the healthy airway, the microenvironment is soft and distensible, with minimal remodeling. With each deep breath, the actin in ASM dissociates, partly mediated by the actin severing protein cofilin and disruption of the actin (thin, black, short filaments) to myosin (thick, gray) connection. This leads to ASM fluidization, a physical state defined by ASM softening, force reduction, and the enhancement of molecular rearrangements. Right, in asthma, the airway is stiff, inflamed, contracted, remodeled, and with each deep breath, fails to stretch. The ASM solidifies, a physical state defined by stiffening, contraction, and molecular arrest. Molecular effectors include zyxin and enhanced actin (thin, black, long filaments) to myosin (thick, gray) connectivity. ASM, airway smooth muscle; FIR, fluctuation induced relengthening; latch, latch state.
The molecular origins of stretch-induced fluidization–resolidification
Stretch-induced structural rearrangements are rapid. A transient stretch–unstretch maneuver occurs through fast F-actin disassembly and slow F-actin reassembly, with a magnitude and duration that mirrors the time course of fluidization–resolidification (32). Cofilin severs F-actin during stretch-initiated fluidization. Cofilin knockdown in ASM cells blunts fluidization compared with control cells without affecting resolidification (33). Further, cofilin-mediated actin depolymerization is limited to the unstretch phase and is preferentially stimulated by a decrease in tension (34, 35).
Studies of reconstituted actin–myosin networks in vitro have revealed that stretch-induced fluidization is mediated by disruption of myosin crosslinks (36) and actin–myosin crosslinks (37, 38). Forcible ASM stretch with short-actin filaments causes relative sliding of the filaments and a loss of the “overlap” zone comprising two filaments connected in tandem to a single myosin filament. The reorganization of actin filaments from a parallel to a “series” arrangement reduces their concerted contractile capacity. However, this mechanism alone cannot account fully for ASM cytoskeletal plasticity (7, 8), scale-free deformability (39), differential response to a homogenous versus a heterogeneous stretch (15), or the rapid F-actin disassembly followed by a gradual reassembly. Likewise, disruption of myosin crosslinks alone cannot explain the universality of the fluidization–resolidification response amongst muscle and nonmuscle (NM) cells (11).
The actin filament–restoring protein zyxin, which localizes to focal adhesions (force bearing, protein-rich hubs that connect the ASM cell membrane to the ECM), has a key function in the resolidification phase (40). Zyxin expression increases with acute stress fiber fragmentation, and zyxin knockdown in human ASM (HASM) or mouse PCLS does not affect fluidization but strongly reduces resolidification and associated recovery of force (40). Notably, there is increased accumulation of zyxin in lungs of patients with fatal asthma compared with nondiseased controls.
Strikingly, disruption of signaling cascades previously implicated in cellular responses to a prolonged stretch including PI3 kinase, Akt, Rho-associated coiled-coil forming kinase (ROCK), and mitogen-activated protein kinase kinase pathways has no noticeable effects on the acute fluidization–resolidification response in smooth muscle cells (32). Although transient stretch does not alter overall protein tyrosine phosphorylation patterns (32), generalized tyrosine phosphorylation induced by phenylarsine oxide, a membrane-permeable tyrosine phosphatase inhibitor, blunts the resolidification response (15). However, phenylarsine oxide’s effects might be due to decreased cellular ATP content, which is known to impede resolidification (11).
Molecular origins of contractile forces leading to ASM solidification—GPCR-evoked cell shortening
Two principal mechanisms operating independently of one another induce ASM solidification and increased contractile capacity. G protein signaling causes ASM cell shortening through actin and myosin filament crossbridging (41). In parallel, actin filament polymerization and adhesome complex formation increase cytoskeletal rigidity, which in turn exerts tension on cell membranes to transmit force to neighboring ASM cells and the underlying lung tissue via ECM proteins.
Among the most well-studied contractile mediators in the airways are acetylcholine (ACh), which is released from efferent vagal nerves, and histamine and leukotrienes, which are derived from lung resident mast cells. ACh, histamine, and leukotrienes and most other contractile agonists bind GPCRs, which activate heterotrimeric G proteins, typically those containing Gαq/11, through the exchange of GDP for GTP (Fig. 3) (42). Gαq–GTP stimulates phospholipase β to generate inositol 1,3,4 triphosphate and diacylglycerol (DAG) from phosphatidylinositol 4,5-bisphosphate within the membrane. Inositol 1,3,4 triphosphate activates cognate receptors on the sarcoplasmic reticulum (SR), which releases Ca2+ from intracellular stores. Ca2+ binds to calmodulin, which in turn elicits the phosphorylation and activation of myosin light chain kinase (MLCK) by Ca2+–calmodulin-dependent kinase. Muscarinic m3 receptors on ASM bind ACh and its analogs carbachol (CCh) and methacholine (MCh) also activate Gα12, which in turn activates several effectors including PI3K and RhoA. PI3K–RhoA-mediated activation of tROCK regulates MLC phosphorylation (43). Regulators of G protein signaling and GPCR-related kinases govern G protein activity. We have recently reviewed the extensive contributions of these two protein families to AHR and asthma elsewhere (44).
Figure 3.
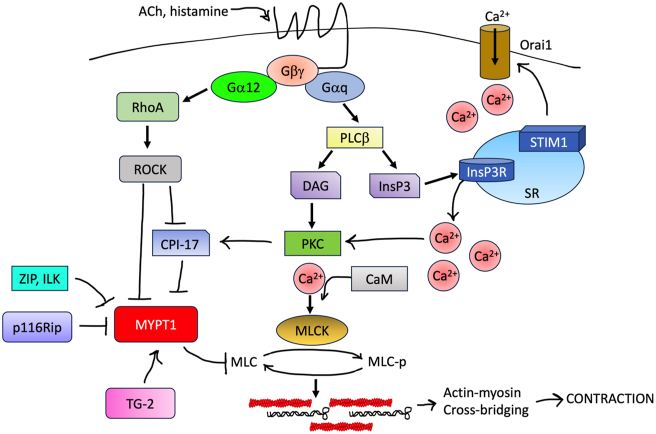
Solidification mediated by force generation pathways in ASM. Contraction-inducing mediators (CCh, histamine) activate GPCRs coupled to Gαq and/or Gα12. G protein activation stimulates activity of phospholipase Cβ (PLCβ), which generates an inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG). InsP3 induces release of Ca2+ from sarcoplasmic reticulum stores. Cytosolic Ca2+ binds calmodulin and activates myosin light chain kinase (MLCK). MLC phosphorylation on Ser19 by MLCK causes crossbridging of myosin filaments (black) with actin (red) and cell shortening along the longitudinal axis. Extracellular Ca2+ enters the cell through membrane-embedded channels (Orai1). In collaboration with the Ca2+ sensor STIM1, Orai1 mediates oscillations in intracellular Ca2+ levels that are required to sustain contraction. GPCRs also stimulate activity of guanine nucleotide exchange factors (GEFs) for RhoA. Activated RhoA stimulates ROCK-mediated inhibitory phosphorylation of MYPT1, which prevents MLC dephosphorylation and prolongs contraction. Other inhibitors of MYPT1 activity include CPI-17 and p116Rip. ASM, airway smooth muscle; CCh, carbachol; GPCR, G protein-coupled receptor.
Type II myosin, which is made up of two myosin heavy chains that intertwine to form a tail and bind actin at the head and neck, mediates cell shortening. This interaction is regulated by the association and phosphorylation of two associated light chain pairs (MLC17 and MLC20). MLCK phosphorylates MLC20 at Ser19, which promotes the interaction with actin filaments. Repetitive cycles of actin–myosin crossbridging along the cell’s longitudinal axis cause shortening and solidification through the development of tension (45).
Intracellular origins of contractile forces leading to ASM solidification—actin–myosin interactions
Fluctuating intracellular Ca2+ concentrations continuously regulate actin–myosin interactions. Sustained excitation–contraction coupling requires the repletion of intracellular Ca2+ stores, which occurs primarily through Ca2+-induced Ca2+ release (store-operated Ca2+ entry) (46). The influx of extracellular Ca2+ into the cytosol through plasma membrane (PM)–associated store operated Ca2+ channels (Orai1) in collaboration with the SR-associated Ca2+-sensing protein stromal interaction molecule 1 (STIM1) generates repetitive oscillations in intracellular Ca2+ levels (Fig. 3). Voltage-dependent L-type Ca2+ channels on the PM and ryanodine receptors on the SR also contribute to the maintenance of intracellular Ca2+ concentrations and contractility (47). Store-operated Ca2+ entry dysfunction may increase susceptibility to AHR. Airways in PCLS from Balb/c mice contract more effectively and have exhibited faster ASM Ca2+ oscillations in response to contraction-inducing mediators than those from C57BL/6 mice. These changes correspond with higher expression of STIM1 and Orai1 and increased AHR in asthma models (48). Type 2 cytokines IL-4 and IL-13 also induce Orai1 expression in HASM and in vivo in rodent asthma models (Table 1) (49, 50).
Table 1.
ASM cytoskeleton-related proteins dysregulated in asthma
| Protein | Function | Comment | References |
|---|---|---|---|
| Zyxin | Actin stabilization | Increased lung expression in fatal asthma | (40) |
| L-type Ca2+ channel (LDVCC) | Ca2+ oscillations | Upregulated in ASM by TNFα | (46) |
| STIM/Orai1 | SOCE/Ca2+ oscillations | Expression increased by type 2 cytokines | (49, 50) |
| ROCK | Inhibitory MYPT1 phosphorylation | Increased bronchial expression in asthma; ROCK inhibitors reduce AHR | (52) |
| RhoA | MLC phosphorylation | ASM expression increased by IL-13 | (57) |
| p116 (Rip) | RhoA-independent inhibition of MLC phosphorylation | Decreased expression in ASM cells in asthma | (58) |
| Nestin | Promotes actin polymerization | Increased in ASM in asthma | (87) |
| ARHGEF1 | RhoA activation | Upregulated in ASM from subjects with asthma and zyxin allergen challenged mice | (103) |
| IL-31 receptor | Potentiation of m3 muscarinic receptor mediated contraction | Upregulated by IL-4 plus IL-13 | (104) |
An extensive network of intracellular proteins dynamically controls myosin activation and actin crossbridging. Myosin light chain phosphatase, composed of a PP1c catalytic subunit and regulatory subunit (MYPT1), inhibits ASM contraction by binding myosin and dephosphorylating MLCK (51). Phosphorylation of MYPT1 on Ser507, Thr696, and Thr853 by ROCK and other kinases may inhibit myosin light chain phosphatase activity to prolong contraction. ROCK expression is increased in ASM from bronchi and bronchioles of lung tissue from patients with asthma compared with controls (52). Genetic deletion of RhoA or ROCK or the use of pharmacological inhibitors reduce AHR in mouse models of asthma (53, 54). However, mechanistic studies suggest that the RhoA pathway facilitates ASM solidification primarily by promoting actin polymerization rather regulating MYPT1 activity (55). The RhoA effector rhotekin induces NM myosin polymerization at the cell cortex (submembranous region) through an interaction with S100A4 (Fig. 4) (56). NM myosin filaments act as a platform for proteins required for the assembly of F-actin.
Figure 4.
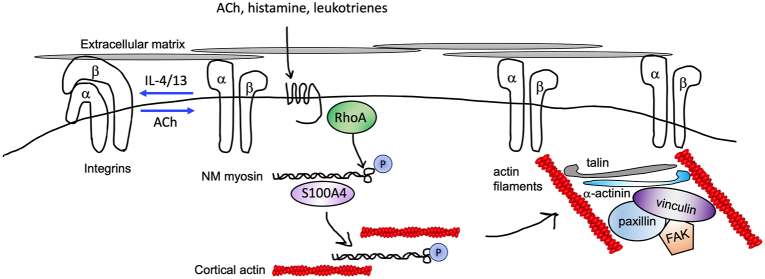
Integrin-mediated mechanosensing initiates cytoskeletal remodeling. ASM cells adhere to extracellular matrix through discrete attachments called focal adhesions (or adhesomes) enriched in proteins that regulate cytoskeletal dynamics. In resting cells, integrins exist in a closed (inactive) dimeric conformation. Contractile mediators (acetylcholine [ACh]) promote adoption of an open, active integrin conformation and RhoA-dependent phosphorylation of type 2 myosin and association with accessory proteins such as S100A4, which facilitates myosin polymerization and crosslinking to cortical (peripheral) actin. Peripheral actin filaments provide a scaffold for integrin linkage to intracellular actin filaments and coupling with adhesome proteins including talin, α-actinin, vinculin, and paxillin. Type 2 cytokines (IL-4/13) prevent talin binding to integrins and formation of the open conformation. ASM, airway smooth muscle.
Additional mechanisms exist to limit actin–myosin-mediated solidification independent of RhoA. Several kinases (ZIP kinase, integrin-linked kinase [ILK]) inhibit MYPT1 activity directly or indirectly by activating the phosphatase CPI-17 (57). p116(Rip), whose expression in increased in ASM from patients with asthma, interacts with F-actin, myosin, and MYPT1 (58). Transgelin 2, a transmembrane protein activated by endogenous proteases such as metallothionein 2 -attenuates bronchoconstriction ex vivo (59). Administration of metallothionein 2 or TG12 agonists reduces AHR in allergen-challenged mice with minimal effects on allergic lung inflammation (Table 2). TG-2 agonists inhibit contractile signaling through several mechanisms including MYPT1 dephosphorylation and inhibitory RhoA and ezrin phosphorylation (60).
Table 2.
Therapeutic interventions targeting cytoskeletal mechanics in asthma—Implications for fluidization and solidification
| Drug/compound | Target | Fluidization–solidification mechanism(s) | Comments | References |
|---|---|---|---|---|
| Y16 | ARHGEF12 | Actin polymerization | Attenuated IL-17 induced bronchial ring hypercontraction | (53) |
| Fasudil/hyroxyfasudil | ROCK | Reduced MYPT1 phosphorylation | Decreased AHR in mouse models and PCLS; anti-inflammatory | (54) |
| TSG12/TSG1180 | Transgelin-2 | MYPT1 dephosphorylation; RhoA, ezrin phosphorylation | Elicit bronchodilation and reduce AHR in mouse models | (59, 60) |
| Volasertib (BI6727) | Plk1 | Actin polymerization | Relaxation of mouse bronchial rings; reduced HDM associated AHR; Phase III clinical trials for cancer | (77) |
| Imatinib | c-Abl | Actin polymerization | Reduced AHR in short term RDBPC trial of patients with severe asthma | (81) |
| FR900359 | Gαq | Ca2+ flux, MLC phosphorylation | Decreased AHR in several animal models of asthma | (107) |
| R59949 | diacylglycerol kinase (DGKζ) | Ca2+ flux (PLCβ) | Decreased AHR in mouse models and PCLS; anti-inflammatory | (108, 109) |
| Pitavastatin, atorvastatin | RhoA | Inhibits MLC phosphorylation, actin polymerization | Reduces AHR in mice and PCLS contraction; lower risk of exacerbations in people treated with statins for dyslipidemia | (116, 117) |
Contractile force transmission leading to ASM solidification—adhesome complexes
Membrane–cytoskeletal and membrane–ECM interactions have a pivotal role in ASM solidification. ASM cells transmit force to neighboring cells or the underlying lung tissue upon sensing mechanical strain–stress through integrin receptors. Integrin activation leads to the recruitment of cytoskeletal and signaling proteins at the cytosolic face of the PM in adhesome complexes (1). Adhesomes transmit tension generated by cell shortening to outside the cell membrane while simultaneously transmitting external mechanical forces to the ASM cell interior.
Integrins are single-pass integral cell membrane proteins consisting of alpha and beta subunits, which form various heterodimers that bind to various ECM components, including collagen, laminin, and fibrin through their extracellular domains (Fig. 5A) (61). HASM primarily express α1-, α3-, β1-, β3-, and β5 integrins (1). Integrins have different affinities for ECM ligands based on their ability to adopt distinct conformations. Integrin ligand-binding recruits intracellular adhesome complexes of talin, α-actinin, ILK, and focal adhesion kinase (FAK) to its cytosolic domain, a process termed “outside–in” signaling. ACh recruits talin to the integrin cytosolic domain, causing also them to adopt a high affinity “open” conformation—termed “inside–out” signaling (62).
Figure 5.
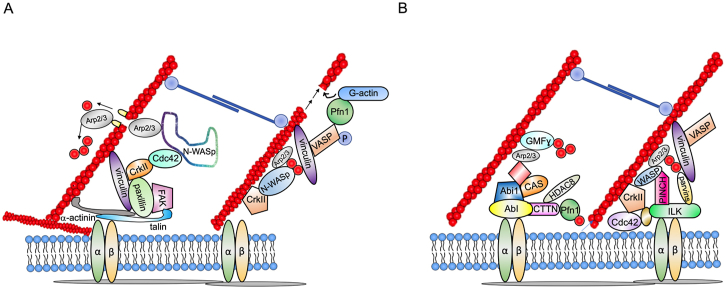
Mechanisms of actin polymerization and regulation.A, phosphorylation of paxillin by focal adhesion kinase (FAK) recruits N-WASp to the adhesome complex through interactions with the adapter protein CrkII. Paxillin phosphorylation recruits GEFs for cdc42 (G protein receptor kinase interacting tyrosine phosphorylated [GIT and Pak-interacting exchange factor (PIX)]) to the complex. WASp binds to activated (GTP-bound) cdc42 and activates Arp2/3, a protein complex structurally related to G-actin that binds existing actin filaments and promotes branching. Tetramers of VASP bind vinculin and elicit actin filament elongation by recruiting profilin (Pfn-1) bound to G-actin. B, actin polymerization–depolymerization is regulated by kinases including the IPP (integrin-linked kinase [ILK], particularly interesting new cysteine–histidine-rich protein [PINCH], parvin) complex, c-Abl (linked to the adhesome by the adapters CAS and Abi1), and by nonkinase regulators including glia maturation factor γ (GMFγ), histone deacetylase 8 (HDAC8), and cortactin (CTTN).
Airway inflammation and remodeling associated with established asthma (63), including increased deposition of ECM proteins, may alter integrin functions (64). IL-4 or IL-13 promotes formation of a low-affinity “bent” conformation and proteolytic inactivation of talin (65, 66). Disruption of α5β1 or α2β1 integrin binding to collagen I or fibronectin, respectively, inhibits IL-13-induced enhanced contraction of human bronchial rings in response to MCh and reduces airway resistance in allergen-challenged mice (67), indicating that integrin–ECM interactions contribute to AHR pathogenesis and may represent an effective therapeutic target.
Contractile force transmission leading to ASM solidification—focal adhesions and actin polymerization
Integrin activation and assembly of adhesome complexes occur at FAs, where various regulators of actin polymerization and depolymerization assemble and disassemble in a dynamic and highly regulated fashion to mediate solidification or fluidization, respectively.
Talin and α-actinin form heterodimers that bridge actin filaments to activated integrins and facilitate binding of vinculin, paxillin and kinases including ILK and FAK (Fig. 4). Blockade of the talin-α actinin interaction reduces tension development in canine tracheal rings without affecting MLC phosphorylation or actin polymerization (68). ACh induces RhoA- and phosphorylation-dependent conformational changes in vinculin (complexed with paxillin), which exposes binding sites for talin, α-actinin, and actin filaments (69, 70). In turn, FAK dependent phosphorylation of paxillin recruits neuronal Wiskott–Aldrich syndrome protein (N-WASp) indirectly through the SH2 domains of the adapter protein CRKII (70). N-WASp-CRKII complexes bind guanine nucleotide exchange factors (GEFs) for the small GTPase cdc42 including DOCK180 and PIX, which leads to activation of Cdc42 and N-WASp coupling to the Arp2/3 complex (Fig. 5A) (4, 71). Arp2/3 are structurally similar to G-actin and provide a foundation for the formation of side branches on pre-existing actin filaments (41). F-actin filament elongation is mediated by vasodilator-stimulated phosphoprotein (VASP), which is anchored to the adhesome by activated vinculin. VASP tetramers catalyze incorporation of G-actin at expanding actin filament tips in coordination with profilin-1 (Pfn-1). ACh stimulation induces VASP phosphorylation on Ser157, which is required for the regulation of ASM actin dynamics and contraction in a vinculin-dependent manner (72).
Mechanotransduction pathways leading to ASM solidification—kinase regulators of adhesome functions
Several non-receptor kinases and related proteins regulate actin remodeling-dependent solidification in adhesomes dynamically. Contractile stimulation recruits the cytosolic IPP (ILK–PINCH–Parvin) complex consisting of ILK, the adapter PINCH (Particularly Interesting New Cysteine-Histidine rich protein), and α-parvin, an actin-binding protein, to integrin-linked adhesion sites to promote solidification (Fig. 5B) (73). Although ILK phosphorylates paxillin in other smooth muscle types, the specific substrates of ILK in ASM have not been firmly established (74). p21-activated kinases (PAKs) constitute a family of ROCK activated Ser/Thr kinases that elicit actin polymerization by means of paxillin phosphorylation on Ser273, recruitment of cdc42 GEFs GIT1 and βPIX, and cdc42 activation (55).
The PAK-related kinase, Ste20-like kinase (SLK) also promotes actin-dependent solidification by means of paxillin phosphorylation on Ser272 by Polo-like kinase (Plk), which elicits N-WASp activation (75, 76). Plk1 antagonism shows therapeutic promise; a specific inhibitor (Volasertib, BI6727) impairs bronchial ring contraction and the development of AHR induced by house dust mite (HDM) sensitization and challenge in mice (77). Moreover, treatment of MCh-precontracted mouse tracheal rings with volasertib induces relaxation, suggesting its application as a bronchodilator. Plk1 and PAK1 may also have a role in fluidization through phosphorylation of the intermediate filament protein vimentin, which leads to disassembly of the WASp-Arp2/3 complex (73, 77).
The Src family kinase c-Abl regulates solidification through interactions with Abelson interactor 1 (Abi1) and CAS, which is essential for N-WASp-mediated F-actin formation in ASM (73). Expression of both c-Abl and Abi1 is increased in mouse asthma models and ASM cells from patients with severe asthma (78, 79). Smooth muscle-specific c-Abl knockout or administration of c-Abl inhibitors imatinib or GNF-5 reduces bronchoconstriction in models of chronic asthma in mice. Moreover, Imatinib and GNF5 exert additive effects on β-agonist-evoked bronchodilation, suggesting that they also affect fluidization (80). Notably, in a short term, randomized, double blind, placebo-controlled study of patients with severe asthma, imatinib reduced AHR and lung mast cell counts over a 24-week period (81).
Mechanotransduction pathways of ASM solidification—inducers of actin filament polymerization
Actin polymerization is a complex, dynamically coordinated process. Profilin-1 (Pfn-1) catalyzes the exchange of ADP for ATP on monomeric G-actin, releasing actin monomers from thymosin-β4, an actin sequestration protein (82) for incorporation into the barbed (fast growing) ends of actin filaments through interactions with VASP. Pfn-1 also promotes solidification by preventing spontaneous Arp2/3-mediated actin filament branching (83). Pfn-1 interactions with cortactin are also critical for solidification. Treatment with a cell permeable decoy peptide (CTTN-1) inhibits actin polymerization in HASM and contractile force development by tracheal rings by inhibiting cortactin-Pfn-1 interactions and F-actin formation independent of MLC phosphorylation (82). Cortactin is highly regulated by HDAC8-mediated deacetylation (84) and c-abl mediated phosphorylation on Tyr412, and interaction with the adapter protein CAS (85). Human genome-wide association studies (GWASs) have uncovered an intronic SNP in CTTN (rs3802780) that confers increased risk of severe asthma (odds ratio: 1.71).
Nestin is a type VI intermediate filament protein that mediates solidification (86). ACh elicits Plk1-mediated nestin phosphorylation on Thr315, which in turn leads to the formation of a nestin–Plk1–vimentin complex and actin polymerization through the recruitment of Pfn-1 and cortactin to the PM. Nestin expression is increased in ASM cells from subjects with asthma compared with controls. Smooth muscle–specific nestin knockout in mice reduces AHR in a model of chronic asthma induced by long-term HDM challenge in part because of reduced ASM hyperplasia and Th2 inflammation (87).
Mechanotransduction pathways of ASM fluidization—regulators of actin depolymerization/filament stability
Cofilin, implicated in fluidization elicited by ASM stretch, is a member of a family of actin-depolymerization factor proteins that severe F-actin when dephosphorylated on Ser-3 by calcineurin in a Ca2+-dependent fashion. Cofilin also mediates actin filament debranching by removing the Arp2/3 complex (88). Cofilin activation is required for ACh-induced tension development and maintenance of tone in tracheal smooth muscle (89, 90). Coronins constitute another family of proteins that bind F-actin and Arp2/3 in yeast and can promote actin oligomerization but have not yet been studied in ASM (88). GMFγ (glia maturation factor γ) is an actin debranching factor that is highly expressed in ASM and localizes to FAs (91). At homeostasis, GMFγ binds tonically to the Arp2/3 complex, eliciting F-actin disassembly and inhibiting nucleation of new actin filaments. ACh stimulation of HASM induces c-abl-mediated GMFγ phosphorylation and dissociation from the Arp2/3 complex. GMFγ knockdown by siRNA reduces contraction of human bronchial rings ex vivo and actin polymerization in HASM (92).
β-catenin forms a complex with membrane-associated cadherins (N-cadherin in ASM) and regulates F-actin filament stability through actin-binding proteins including α-catenin, α-actinin, and p120 catenin (93). Knockdown of β-catenin or a pharmacological inhibitor of β-catenin–N-cadherin interactions inhibits ASM contraction through unclear mechanisms that are independent of actin polymerization or MLC phosphorylation (94, 95).
Effects of asthma-related cytokines on ASM solidification
Several type 2 cytokines increase the severity of AHR by augmenting solidification pathways. IL-4 and IL-13 enhance contraction of human bronchial rings to histamine and leukotriene D4 by upregulating expression of mRNA cognate receptors (HRH1 and CYSLTR1, respectively) (96). Accordingly, dupilumab, a therapeutic antibody antagonist of IL-4Rα, blocks this response (96). IL-13 also increases the expression of RhoA and CPI-17 in human and mouse ASM (57), suggesting that it also promotes solidification by regulating actin–myosin interactions and actin polymerization.
Other asthma-related cytokines may alter solidification mechanisms. IL-17, which has been implicated in the pathogenesis of severe, steroid-resistant asthma, enhances RhoA activation in ASM by means of the RhoGEF ARHGEF12 (97, 98). Treatment of mice with a RhoGEF inhibitor (Y16) weakens IL-17A-induced tracheal ring hypercontraction, and Arhgef12−/− mice have decreased allergen-induced AHR compared with controls (53). Tumor necrosis factor alpha, a cytokine frequently elevated in the airways of patients with type 2 low asthma (99), upregulates voltage-dependent L-type Ca2+ channel expression in mouse ASM (47). Other studies suggest that tumor necrosis factor alpha increases force generation in porcine tracheal smooth muscle by upregulating actin expression and polymerization with little effect on MLC phosphorylation (100). Transforming growth factor beta (TGFβ) is upregulated in airways of patients with established asthma and contributes to remodeling (101). TGFβ pretreatment of HASM enhances CCh-induced Ca2+ flux, MLC and MYTP1 phosphorylation in an ROCK-dependent but RhoA-independent fashion (102). A separate study found increased expression of the RhoGEF ARHGEF1 in ASM cells from subjects with asthma, TGFβ-treated ASM cells from healthy donors, and allergen-challenged mice. ARHGEF1 knockdown suppresses TGFβ-induced hypercontraction of bradykinin-stimulated rat bronchioles (103). The IL-31 receptor (IL-31R) is expressed on human and mouse ASM cells and is upregulated by IL-4/13 and in allergen-challenged mice. While IL-31 itself has no direct impact on contractility, IL-31R forms a complex with m3 muscarinic receptors to stabilize m3R expression. IL-31R binds m3R and enhances agonist-induced Ca2+ flux and MLC phosphorylation in ASM (104).
Cytokine-induced changes in ASM contractile responses can inform GWAS of asthma. Specifically, the results of ASM contraction measurements in cells from the same donors used for transcriptomic and epigenetic studies revealed three significant outcomes: (1) IL-13 and IL-17A altered expression of ASM genes that were among the most prevalent variants in asthma GWAS studies; (2) IL-17A-induced changes in contractility were highly correlated with changes to the transcriptional landscape; (3) molecular quantitative trail loci for ASM gene expression and cellular quantitative trait loci for ASM contraction were enriched in asthma GWAS SNPs (105). Thus, cytokine-mediated transcriptional regulation of ASM contractile responses has a profound impact on the asthma phenotype.
Therapeutic opportunities to reduce ASM solidification and/or enhance ASM fluidization in asthma
Studies of cytoskeletal mechanisms in ASM have uncovered new therapeutic targets for the treatment of AHR by either enhancing fluidization or reducing resolidification (Table 2). At the receptor level, for example, α-adrenergic receptor expression has been documented in HASM (106). Epinephrine, an α- and β-adrenergic agonist used for severe asthma exacerbations, induces contraction of β-agonist desensitized HASM cells in a α1-adrenergic receptor–dependent fashion. This phenotypic switch may partially explain why prolonged β-agonist and β-antagonist therapy often worsens bronchospasm and suggests a role for α-adrenergic receptor antagonists to partially fluidize ASM cells that are in a refractory solidified state during acute asthma exacerbations.
An inhibitor of Gαq (FR900359) nearly abolishes HASM solidification by inhibiting Ca2+ flux and airway contraction in PCLS. Inhalation of FR900359 strongly prevents MCh-induced bronchoconstriction in allergen-challenged mice with little to no effects on airway inflammation or systemic side effects (107). Further downstream of Gαq, DAG is degraded to phosphatidic acid by DAG kinase ζ (DGKζ) in ASM. Administration of a DGK inhibitor (R59949) to mice reduces AHR and CCh-evoked PCLS airway constriction (108). DGK inhibition in HASM results in an increased DAG–phosphatidic acid ratio, which in turn prevents Gαq-mediated PLCβ activation in a feedback loop (109). Notably, administration of R59949 to mice also impairs cytokine production by T helper type 2 cells (108), thereby reducing their effects on ASM solidification pathways.
Various PI3K isoforms are also required for airway contraction. A selective inhibitor of PI3Kγ weakens ACh-induced mouse PCLS contraction by inhibiting Ca2+ oscillations (110). Another study demonstrated that siRNA-mediated PI3Kδ knockdown or pharmacological inhibitors reduces CCh-induced contraction of human PCLS and elicits relaxation even in β-agonist desensitized airways (111). These interventions impaired CCh-induced solidification mediated by MLC phosphorylation.
RhoA–ROCK expression is increased in Th2 cytokine (IL4/13)-treated HASM and allergen-challenged mice. Inhalation of ROCK inhibitors such as Y27632 and fasudil inhibits the development of allergen-induced AHR by reducing ASM contraction and airway inflammation (112). ROCK inhibition can also synergistically enhance stretch-induced ASM fluidization (113). Newer generation ROCK inhibitors such as netarsudil are used safely in patients with glaucoma to treat intraocular hypertension (114), suggesting that inhaled versions of these drugs might be developed for asthma treatment.
Statins including pitavastatin and atorvastatin, which are widely used to reduce serum cholesterol levels, have emerged as potential therapy for asthma (115). By inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A reductase, the rate-limiting enzyme of sterol biosynthesis in the mevalonate pathway, statins deplete membrane pools of the metabolite geranylgeranyl pyrophosphate. This process reduces geranylgeranylation-mediated localization of RhoA at the PM (116). Treatment of PCLS with pitavastatin weakens contraction in unstimulated and agonist (histamine and MCh)-stimulated airways ex vivo. Pitavastatin counteracts solidification by means of reduced MLC phosphorylation and F-actin formation. Under a dynamic stretch environment, pitavastatin also promotes ASM fluidization (116). Inhalation of atorvastatin impairs development of allergen-induced AHR in mice through multiple mechanisms including reduced inflammation and remodeling (117). A retrospective study of patients visiting Ajou University Medical Center in Korea found that taking statins for dislipidemia deceased the risk for severe and nonsevere asthma exacerbations (118).
Other compounds that potentiate stretch-induced ASM fluidization or inhibit stretch-induced ASM resolidification by affecting actin polymerization might be developed, including those targeting zyxin, cofilin, or Abl, or actin–myosin connectivity (119). These drugs may complement conventional therapies such as β-agonists, which do not affect this process (116). For example, activation of the bitter taste receptor AS2R14 elicits airway relaxation by promoting cofilin-mediated destabilization of F-actin (120). Overall, ASM cytoskeleton–targeted drugs demonstrate promise for the treatment of AHR.
Conclusions
The concept of ASM hypercontraction as an essential cause of airway obstruction in all asthma endotypes is supported by the rapid and sustained clinical improvement associated with ASM ablation strategies (bronchial thermoplasty) (121). Recent investigations have uncovered previously unrecognized extrinsic factors that augment ASM contraction, including ECM stiffness and cell–cell coupling (122), obesity (123, 124), and epithelial crosstalk (125). Conversely, these studies also revealed that persistent bronchospasm may itself propagate inflammation by eliciting epithelial extrusion from the airway and lung damage (126).
To understand these emerging concepts, it is imperative to view ASM contraction not as a static state but as a continuously adapting dynamic process that proceeds over a time scale of seconds to minutes and is inescapable, ever-present, and dominant in the living breathing lung (127). This dynamic process conforms to the phenomenological description of fluidization and resolidification in inorganic materials, with supportive evidence gleaned from multiple levels—contractile proteins, the ASM cell in culture, the muscle strip, the ASM within the bronchial airway, and the lung. We emphasize that this physical description is interdependent with molecular effectors and modulators of ASM contraction, including both GPCR-mediated and actin-mediated contractile and relaxation pathways. Neither does it minimize the effects of mediators, cytokines, and genetic contributions. It does, however, set strict physical expectations to biochemical changes. Unravelling this complexity will advance our understanding of contractile mechanisms.
The evidence that ASM cells cultured from patients with asthma solidify more effectively in response to contractile mediators is conflicting (128). The contribution of autonomous defects in cytoskeletal protein expression and/or function to ASM hypercontraction in chronic established asthma also requires further investigation. Recent studies of the biomechanical properties of ASM cells cultured from subjects with asthma (23) and a proteomic study of intrapulmonary airways (129) revealed hypercontractility in diseased samples. The phenotype was associated with upregulation of zyxin and the ASM-specific protein smoothelin, among others. Further proteogenomic and functional examination of the smaller airways from patients with asthma in their native environment (e.g., PCLS or organoids) will advance our understanding of contractile mechanisms and no doubt uncover previously unrecognized therapeutic targets.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Author contributions
N.F., R.K., I.V.J., N.L.P., and M.M. conceptualization; K.M.D., N.F., R.K., I.V.J., N.L.P., and M.M. investigation; K.M.D., N.F., R.K., I.V.J., N.L.P., and M.M. writing–original draft; K.M.D., N.F., R.K., I.V.J., N.L.P., and M.M. writing–review & editing.
Funding and additional information
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases/National Institutes of Health (grant AI001083-14; to K.M.D.) and by National Institutes of Health (grant R21 AI151695-01; to R.K.).
Reviewed by members of the JBC Editorial Board. Edited by Enrique De La Cruz
References
- 1.Zhang W., Wu Y., S J.G. Membrane adhesion junctions regulate airway smooth muscle phenotype and function. Physiol. Rev. 2023;103:2321–2347. doi: 10.1152/physrev.00020.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong D.J.P., Martin J.G., Lauzon A.M. Airway smooth muscle function in asthma. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.993406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasuda Y., Wang L., Chitano P., Seow C.Y. Critical roles of airway smooth muscle in mediating deep-inspiration-induced bronchodilation: a big stretch? Respir. Res. 2023;24:250. doi: 10.1186/s12931-023-02538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang D.D. Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling. Respir. Res. 2015;16:134. doi: 10.1186/s12931-015-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salciccioli J.D., Israel E. As-needed inhaled corticosteroids as add-on therapy versus SMART therapy: an evolving understanding of the two approaches in the management of moderate-to-severe asthma. Curr. Opin. Pulm. Med. 2023;29:209–214. doi: 10.1097/MCP.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 6.Bursac P., Lenormand G., Fabry B., Oliver M., Weitz D.A., Viasnoff V., et al. Cytoskeletal remodelling and slow dynamics in the living cell. Nat. Mater. 2005;4:557–561. doi: 10.1038/nmat1404. [DOI] [PubMed] [Google Scholar]
- 7.Gunst S.J., Fredberg J.J. The first three minutes: smooth muscle contraction, cytoskeletal events, and soft glasses. J. Appl. Physiol. (1985) 2003;95:413–425. doi: 10.1152/japplphysiol.00277.2003. [DOI] [PubMed] [Google Scholar]
- 8.Fabry B., Maksym G.N., Butler J.P., Glogauer M., Navajas D., Fredberg J.J. Scaling the microrheology of living cells. Phys. Rev. Lett. 2001;87 doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 9.Lavoie T.L., Krishnan R., Siegel H.R., Maston E.D., Fredberg J.J., Solway J., et al. Dilatation of the constricted human airway by tidal expansion of lung parenchyma. Am. J. Respir. Crit. Care Med. 2012;186:225–232. doi: 10.1164/rccm.201202-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan R., Canovic E.P., Iordan A.L., Rajendran K., Manomohan G., Pirentis A.P., et al. Fluidization, resolidification, and reorientation of the endothelial cell in response to slow tidal stretches. Am. J. Physiol. Cell Physiol. 2012;303:C368–C375. doi: 10.1152/ajpcell.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trepat X., Deng L., An S.S., Navajas D., Tschumperlin D.J., Gerthoffer W.T., et al. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cloitre M., Borrega R., Leibler L. Rheological aging and rejuvenation in microgel pastes. Phys. Rev. Lett. 2000;85:4819–4822. doi: 10.1103/PhysRevLett.85.4819. [DOI] [PubMed] [Google Scholar]
- 13.Weitz D.A. Condensed matter. Memories of paste. Nature. 2001;410:32–33. doi: 10.1038/35065199. [DOI] [PubMed] [Google Scholar]
- 14.Viasnoff V., Jurine S., Lequeux F. How are colloidal suspensions that age rejuvenated by strain application? Faraday Discuss. 2003;123:253–266. doi: 10.1039/b204377g. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan R., Park C.Y., Lin Y.C., Mead J., Jaspers R.T., Trepat X., et al. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenormand G., Bursac P., Butler J.P., Fredberg J.J. Out-of-equilibrium dynamics in the cytoskeleton of the living cell. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2007;76 doi: 10.1103/PhysRevE.76.041901. [DOI] [PubMed] [Google Scholar]
- 17.Mondonedo J.R., Bartolak-Suki E., Bou Jawde S., Nelson K., Cao K., Sonnenberg A., et al. A high-throughput system for cyclic stretching of precision-cut lung slices during acute cigarette smoke extract exposure. Front. Physiol. 2020;11:566. doi: 10.3389/fphys.2020.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredberg J.J., Inouye D., Miller B., Nathan M., Jafari S., Raboudi S.H., et al. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am. J. Respir. Crit. Care Med. 1997;156:1752–1759. doi: 10.1164/ajrccm.156.6.9611016. [DOI] [PubMed] [Google Scholar]
- 19.Thorpe C.W., Salome C.M., Berend N., King G.G. Modeling airway resistance dynamics after tidal and deep inspirations. J. Appl. Physiol. (1985) 2004;97:1643–1653. doi: 10.1152/japplphysiol.01300.2003. [DOI] [PubMed] [Google Scholar]
- 20.Nadel J.A., Tierney D.F. Effect of a previous deep inspiration on airway resistance in man. J. Appl. Physiol. 1961;16:717–719. doi: 10.1152/jappl.1961.16.4.717. [DOI] [PubMed] [Google Scholar]
- 21.Moore B.J., Verburgt L.M., King G.G., Pare P.D. The effect of deep inspiration on methacholine dose-response curves in normal subjects. Am. J. Respir. Crit. Care Med. 1997;156:1278–1281. doi: 10.1164/ajrccm.156.4.96-11082. [DOI] [PubMed] [Google Scholar]
- 22.Gump A., Haughney L., Fredberg J. Relaxation of activated airway smooth muscle: relative potency of isoproterenol vs. tidal stretch. J. Appl. Physiol. (1985) 2001;90:2306–2310. doi: 10.1152/jappl.2001.90.6.2306. [DOI] [PubMed] [Google Scholar]
- 23.An S.S., Mitzner W., Tang W.Y., Ahn K., Yoon A.R., Huang J., et al. An inflammation-independent contraction mechanophenotype of airway smooth muscle in asthma. J. Allergy Clin. Immunol. 2016;138:294–297.e294. doi: 10.1016/j.jaci.2015.12.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chetty A., Nielsen H.C. Targeting airway smooth muscle hypertrophy in asthma: an approach whose time has come. J. Asthma Allergy. 2021;14:539–556. doi: 10.2147/JAA.S280247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodruff P.G., Dolganov G.M., Ferrando R.E., Donnelly S., Hays S.R., Solberg O.D., et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am. J. Respir. Crit. Care Med. 2004;169:1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 26.Ding D.J., Martin J.G., Macklem P.T. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J. Appl. Physiol. (1985) 1987;62:1324–1330. doi: 10.1152/jappl.1987.62.3.1324. [DOI] [PubMed] [Google Scholar]
- 27.Bai T.R., Knight D.A. Structural changes in the airways in asthma: observations and consequences. Clin. Sci. (Lond) 2005;108:463–477. doi: 10.1042/CS20040342. [DOI] [PubMed] [Google Scholar]
- 28.Winkler T., Venegas J.G. Self-organized patterns of airway narrowing. J. Appl. Physiol. (1985) 2011;110:1482–1486. doi: 10.1152/japplphysiol.01163.2010. [DOI] [PubMed] [Google Scholar]
- 29.Venegas J.G., Winkler T., Musch G., Vidal Melo M.F., Layfield D., Tgavalekos N., et al. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature. 2005;434:777–782. doi: 10.1038/nature03490. [DOI] [PubMed] [Google Scholar]
- 30.Oliver M.N., Fabry B., Marinkovic A., Mijailovich S.M., Butler J.P., Fredberg J.J. Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason? Am. J. Respir. Cell Mol. Biol. 2007;37:264–272. doi: 10.1165/rcmb.2006-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fredberg J.J., Jones K.A., Nathan M., Raboudi S., Prakash Y.S., Shore S.A., et al. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J. Appl. Physiol. (1985) 1996;81:2703–2712. doi: 10.1152/jappl.1996.81.6.2703. [DOI] [PubMed] [Google Scholar]
- 32.Chen C., Krishnan R., Zhou E., Ramachandran A., Tambe D., Rajendran K., et al. Fluidization and resolidification of the human bladder smooth muscle cell in response to transient stretch. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan B., Krishnan R., Park C.Y., Watanabe R.A., Panganiban R., Butler J.P., et al. Transient stretch induces cytoskeletal fluidization through the severing action of cofilin. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314:L799–L807. doi: 10.1152/ajplung.00326.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brieher W. Mechanisms of actin disassembly. Mol. Biol. Cell. 2013;24:2299–2302. doi: 10.1091/mbc.E12-09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayakawa K., Tatsumi H., Sokabe M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J. Cell Biol. 2011;195:721–727. doi: 10.1083/jcb.201102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith D., Ziebert F., Humphrey D., Duggan C., Steinbeck M., Zimmermann W., et al. Molecular motor-induced instabilities and cross linkers determine biopolymer organization. Biophys. J. 2007;93:4445–4452. doi: 10.1529/biophysj.106.095919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mijailovich S.M., Butler J.P., Fredberg J.J. Perturbed equilibria of myosin binding in airway smooth muscle: bond-length distributions, mechanics, and ATP metabolism. Biophys. J. 2000;79:2667–2681. doi: 10.1016/S0006-3495(00)76505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo K.H., Wang L., Pare P.D., Ford L.E., Seow C.Y. Myosin thick filament lability induced by mechanical strain in airway smooth muscle. J. Appl. Physiol. (1985) 2001;90:1811–1816. doi: 10.1152/jappl.2001.90.5.1811. [DOI] [PubMed] [Google Scholar]
- 39.Fabry B., Maksym G.N., Butler J.P., Glogauer M., Navajas D., Taback N.A., et al. Time scale and other invariants of integrative mechanical behavior in living cells. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2003;68 doi: 10.1103/PhysRevE.68.041914. [DOI] [PubMed] [Google Scholar]
- 40.Rosner S.R., Pascoe C.D., Blankman E., Jensen C.C., Krishnan R., James A.L., et al. The actin regulator zyxin reinforces airway smooth muscle and accumulates in airways of fatal asthmatics. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W., Gunst S.J. Molecular mechanisms for the mechanical modulation of airway responsiveness. J. Eng. Sci. Med. Diagn. Ther. 2019;2:0108051–0108058. doi: 10.1115/1.4042775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pera T., Penn R.B. Bronchoprotection and bronchorelaxation in asthma: new targets, and new ways to target the old ones. Pharmacol. Ther. 2016;164:82–96. doi: 10.1016/j.pharmthera.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo E.J., Cao G., Koziol-White C.J., Ojiaku C.A., Sunder K., Jude J.A., et al. Galpha(12) facilitates shortening in human airway smooth muscle by modulating phosphoinositide 3-kinase-mediated activation in a RhoA-dependent manner. Br. J. Pharmacol. 2017;174:4383–4395. doi: 10.1111/bph.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuentes N., McCullough M., Panettieri R.A., Jr., Druey K.M. RGS proteins, GRKs, and beta-arrestins modulate G protein-mediated signaling pathways in asthma. Pharmacol. Ther. 2021;223 doi: 10.1016/j.pharmthera.2021.107818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito M., Okamoto R., Ito H., Zhe Y., Dohi K. Regulation of myosin light-chain phosphorylation and its roles in cardiovascular physiology and pathophysiology. Hypertens. Res. 2022;45:40–52. doi: 10.1038/s41440-021-00733-y. [DOI] [PubMed] [Google Scholar]
- 46.Dwivedi R., Drumm B.T., Griffin C.S., Dudem S., Bradley E., Alkawadri T., et al. Excitatory cholinergic responses in mouse primary bronchial smooth muscle require both Ca(2+) entry via l-type Ca(2+) channels and store operated Ca(2+) entry via Orai channels. Cell Calcium. 2023;112 doi: 10.1016/j.ceca.2023.102721. [DOI] [PubMed] [Google Scholar]
- 47.Ding S., Zhang J., Yin S., Lu J., Hu M., Du J., et al. Inflammatory cytokines tumour necrosis factor-alpha and interleukin-8 enhance airway smooth muscle contraction by increasing L-type Ca(2+) channel expression Clin. Exp. Pharmacol. Physiol. 2019;46:56–64. doi: 10.1111/1440-1681.13030. [DOI] [PubMed] [Google Scholar]
- 48.Zeng Z., Cheng M., Li M., Wang T., Wen F., Sanderson M.J., et al. Inherent differences of small airway contraction and Ca(2+) oscillations in airway smooth muscle cells between BALB/c and C57BL/6 mouse strains. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1202573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiang L.L., Wan Q.Q., Wang Y.M., He S.J., Xu W.J., Ding M., et al. IL-13 regulates Orai1 expression in human bronchial smooth muscle cells and airway remodeling in asthma mice model via LncRNA H19. J. Asthma Allergy. 2022;15:1245–1261. doi: 10.2147/JAA.S360381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J.H., Gao H.W., Gao D.D., Yang W.Y., Zhao M.K., Shen B., et al. Exercise reduces airway smooth muscle contraction in asthmatic rats via inhibition of IL-4 secretion and store-operated Ca(2+) entry pathway Allergy. Asthma Immunol. Res. 2023;15:361–373. doi: 10.4168/aair.2023.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvarez-Santos M.D., Alvarez-Gonzalez M., Estrada-Soto S., Bazan-Perkins B. Regulation of myosin light-chain phosphatase activity to generate airway smooth muscle hypercontractility. Front. Physiol. 2020;11:701. doi: 10.3389/fphys.2020.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L., Chitano P., Pare P.D., Seow C.Y. Upregulation of smooth muscle Rho-kinase protein expression in human asthma. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.01785-2019. [DOI] [PubMed] [Google Scholar]
- 53.Fong V., Hsu A., Wu E., Looney A.P., Ganesan P., Ren X., et al. Arhgef12 drives IL17A-induced airway contractility and airway hyperresponsiveness in mice. JCI Insight. 2018;3 doi: 10.1172/jci.insight.123578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franova S., Molitorisova M., Kalmanova L., Palencarova J., Joskova M., Smiesko L., et al. The anti-asthmatic potential of Rho-kinase inhibitor hydroxyfasudil in the model of experimentally induced allergic airway inflammation. Eur. J. Pharmacol. 2023;938 doi: 10.1016/j.ejphar.2022.175450. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W., Bhetwal B.P., Gunst S.J. Rho kinase collaborates with p21-activated kinase to regulate actin polymerization and contraction in airway smooth muscle. J. Physiol. 2018;596:3617–3635. doi: 10.1113/JP275751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang W., Gunst S.J. S100A4 is activated by RhoA and catalyses the polymerization of non-muscle myosin, adhesion complex assembly and contraction in airway smooth muscle. J. Physiol. 2020;598:4573–4590. doi: 10.1113/JP280111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakai H., Suto W., Kai Y., Chiba Y. Mechanisms underlying the pathogenesis of hyper-contractility of bronchial smooth muscle in allergic asthma. J. Smooth Muscle Res. 2017;53:37–47. doi: 10.1540/jsmr.53.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komatsu S., Wang L., Seow C.Y., Ikebe M. p116(Rip) promotes myosin phosphatase activity in airway smooth muscle cells. J. Cell Physiol. 2020;235:114–127. doi: 10.1002/jcp.28949. [DOI] [PubMed] [Google Scholar]
- 59.Yin L.M., Xu Y.D., Peng L.L., Duan T.T., Liu J.Y., Xu Z., et al. Transgelin-2 as a therapeutic target for asthmatic pulmonary resistance. Sci. Transl Med. 2018;10 doi: 10.1126/scitranslmed.aam8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan H.K., Li B., Wu L., Wang X.L., Lv Z.Y., Liu Z., et al. Discovery of zolinium TSG1180 as a novel agonist of transgelin-2 for treating asthma. Biomed. Pharmacother. 2023;167 doi: 10.1016/j.biopha.2023.115556. [DOI] [PubMed] [Google Scholar]
- 61.Li S., Sampson C., Liu C., Piao H.L., Liu H.X. Integrin signaling in cancer: bidirectional mechanisms and therapeutic opportunities. Cell Commun Signal. 2023;21:266. doi: 10.1186/s12964-023-01264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klapholz B., Brown N.H. Talin - the master of integrin adhesions. J. Cell Sci. 2017;130:2435–2446. doi: 10.1242/jcs.190991. [DOI] [PubMed] [Google Scholar]
- 63.Varricchi G., Brightling C.E., Grainge C., Lambrecht B.N., Chanez P. Airway remodelling in asthma and the epithelium: on the edge of a new era. Eur. Respir. J. 2024;63 doi: 10.1183/13993003.01619-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito J.T., Lourenco J.D., Righetti R.F., Tiberio I., Prado C.M., Lopes F. Extracellular matrix component remodeling in respiratory Diseases: what has been found in clinical and experimental studies? Cells. 2019;8:342. doi: 10.3390/cells8040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y., Huang Y., Zhang W., Gunst S.J. The proprotein convertase furin inhibits IL-13-induced inflammation in airway smooth muscle by regulating integrin-associated signaling complexes. Am. J. Physiol. Lung Cell Mol. Physiol. 2021;321:L102–L115. doi: 10.1152/ajplung.00618.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lockett A.D., Wu Y., Gunst S.J. Elastase alters contractility and promotes an inflammatory synthetic phenotype in airway smooth muscle tissues. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314:L626–L634. doi: 10.1152/ajplung.00334.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu S., Ngo U., Tang X.Z., Ren X., Qiu W., Huang X., et al. Integrin alpha2beta1 regulates collagen I tethering to modulate hyperresponsiveness in reactive airway disease models. J. Clin. Invest. 2021;131 doi: 10.1172/JCI138140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang W., Gunst S.J. Dynamic association between alpha-actinin and beta-integrin regulates contraction of canine tracheal smooth muscle. J. Physiol. 2006;572:659–676. doi: 10.1113/jphysiol.2006.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang Y., Day R.N., Gunst S.J. Vinculin phosphorylation at Tyr1065 regulates vinculin conformation and tension development in airway smooth muscle tissues. J. Biol. Chem. 2014;289:3677–3688. doi: 10.1074/jbc.M113.508077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W., Huang Y., Wu Y., Gunst S.J. A novel role for RhoA GTPase in the regulation of airway smooth muscle contraction Can. J. Physiol. Pharmacol. 2015;93:129–136. doi: 10.1139/cjpp-2014-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang D.D., Zhang W., Gunst S.J. The adapter protein CrkII regulates neuronal Wiskott-Aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. J. Biol. Chem. 2005;280:23380–23389. doi: 10.1074/jbc.M413390200. [DOI] [PubMed] [Google Scholar]
- 72.Wu Y., Gunst S.J. Vasodilator-stimulated phosphoprotein (VASP) regulates actin polymerization and contraction in airway smooth muscle by a vinculin-dependent mechanism. J. Biol. Chem. 2015;290:11403–11416. doi: 10.1074/jbc.M115.645788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang D.D. The dynamic actin cytoskeleton in smooth muscle. Adv. Pharmacol. 2018;81:1–38. doi: 10.1016/bs.apha.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 74.Mahavadi S., Grider J.R., Murthy K.S. Muscarinic m2 receptor-mediated actin polymerization via PI3 kinase gamma and integrin-linked kinase in gastric smooth muscle. Neurogastroenterol Motil. 2019;31 doi: 10.1111/nmo.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang W., Huang Y., Gunst S.J. p21-Activated kinase (Pak) regulates airway smooth muscle contraction by regulating paxillin complexes that mediate actin polymerization. J. Physiol. 2016;594:4879–4900. doi: 10.1113/JP272132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y., Wang R., Tang D.D. Ste20-like kinase-mediated control of actin polymerization is a new mechanism for thin filament-associated regulation of airway smooth muscle contraction. Am. J. Respir. Cell Mol. Biol. 2020;62:645–656. doi: 10.1165/rcmb.2019-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li J., Wang R., Gannon O.J., Rezey A.C., Jiang S., Gerlach B.D., et al. Polo-like kinase 1 regulates vimentin phosphorylation at ser-56 and contraction in smooth muscle. J. Biol. Chem. 2016;291:23693–23703. doi: 10.1074/jbc.M116.749341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cleary R.A., Wang R., Wang T., Tang D.D. Role of Abl in airway hyperresponsiveness and airway remodeling. Respir. Res. 2013;14:105. doi: 10.1186/1465-9921-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang R., Wang Y., Liao G., Chen B., Panettieri R.A., Jr., Penn R.B., et al. Abi1 mediates airway smooth muscle cell proliferation and airway remodeling via Jak2/STAT3 signaling. iScience. 2022;25 doi: 10.1016/j.isci.2022.103833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nayak A.P., Lim J.M., Arbel E., Wang R., Villalba D.R., Nguyen T.L., et al. Cooperativity between beta-agonists and c-Abl inhibitors in regulating airway smooth muscle relaxation. FASEB J. 2021;35 doi: 10.1096/fj.202100154R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cahill K.N., Katz H.R., Cui J., Lai J., Kazani S., Crosby-Thompson A., et al. KIT inhibition by imatinib in patients with severe refractory asthma. N. Engl. J. Med. 2017;376:1911–1920. doi: 10.1056/NEJMoa1613125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang R., Cleary R.A., Wang T., Li J., Tang D.D. The association of cortactin with profilin-1 is critical for smooth muscle contraction. J. Biol. Chem. 2014;289:14157–14169. doi: 10.1074/jbc.M114.548099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skruber K., Warp P.V., Shklyarov R., Thomas J.D., Swanson M.S., Henty-Ridilla J.L., et al. Arp2/3 and mena/VASP require profilin 1 for actin network assembly at the leading edge. Curr. Biol. 2020;30:2651–2664.e2655. doi: 10.1016/j.cub.2020.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li J., Chen S., Cleary R.A., Wang R., Gannon O.J., Seto E., et al. Histone deacetylase 8 regulates cortactin deacetylation and contraction in smooth muscle tissues. Am. J. Physiol. Cell Physiol. 2014;307:C288–C295. doi: 10.1152/ajpcell.00102.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y., Rezey A.C., Wang R., Tang D.D. Role and regulation of Abelson tyrosine kinase in Crk-associated substrate/profilin-1 interaction and airway smooth muscle contraction. Respir. Res. 2018;19:4. doi: 10.1186/s12931-017-0709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y., Liao G., Wu Y., Wang R., Tang D.D. The intermediate filament protein nestin serves as a molecular hub for smooth muscle cytoskeletal signaling. Respir. Res. 2023;24:157. doi: 10.1186/s12931-023-02473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liao G., Wang R., Wu Y., Maheshwari N.K., Penn R.B., Tang D.D. Nestin drives allergen-induced airway smooth muscle hyperplasia and airway remodeling. Allergy. 2024;79:744–746. doi: 10.1111/all.15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goode B.L., Eskin J., Shekhar S. Mechanisms of actin disassembly and turnover. J. Cell Biol. 2023;222 doi: 10.1083/jcb.202309021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao R., Du L., Huang Y., Wu Y., Gunst S.J. Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J. Biol. Chem. 2008;283:36522–36531. doi: 10.1074/jbc.M805294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gazzola M., Henry C., Lortie K., Khadangi F., Park C.Y., Fredberg J.J., et al. Airway smooth muscle tone increases actin filamentogenesis and contractile capacity. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318:L442–L451. doi: 10.1152/ajplung.00205.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerlach B.D., Tubbesing K., Liao G., Rezey A.C., Wang R., Barroso M., et al. Phosphorylation of GMFgamma by c-abl coordinates lamellipodial and focal adhesion dynamics to regulate airway smooth muscle cell migration. Am. J. Respir. Cell Mol. Biol. 2019;61:219–231. doi: 10.1165/rcmb.2018-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang T., Cleary R.A., Wang R., Tang D.D. Glia maturation factor-gamma phosphorylation at Tyr-104 regulates actin dynamics and contraction in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 2014;51:652–659. doi: 10.1165/rcmb.2014-0125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blaschuk O.W. Potential therapeutic applications of N-cadherin antagonists and agonists. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jansen S.R., Van Ziel A.M., Baarsma H.A., Gosens R. beta-Catenin regulates airway smooth muscle contraction. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299:L204–L214. doi: 10.1152/ajplung.00020.2010. [DOI] [PubMed] [Google Scholar]
- 95.Wang T., Wang R., Cleary R.A., Gannon O.J., Tang D.D. Recruitment of beta-catenin to N-cadherin is necessary for smooth muscle contraction. J. Biol. Chem. 2015;290:8913–8924. doi: 10.1074/jbc.M114.621003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manson M.L., Safholm J., James A., Johnsson A.K., Bergman P., Al-Ameri M., et al. IL-13 and IL-4, but not IL-5 nor IL-17A, induce hyperresponsiveness in isolated human small airways. J. Allergy Clin. Immunol. 2020;145:808–817.e802. doi: 10.1016/j.jaci.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 97.Ramakrishnan R.K., Al Heialy S., Hamid Q. Role of IL-17 in asthma pathogenesis and its implications for the clinic. Expert Rev. Respir. Med. 2019;13:1057–1068. doi: 10.1080/17476348.2019.1666002. [DOI] [PubMed] [Google Scholar]
- 98.Kudo M., Melton A.C., Chen C., Engler M.B., Huang K.E., Ren X., et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Habib N., Pasha M.A., Tang D.D. Current understanding of asthma pathogenesis and biomarkers. Cells. 2022;11:2764. doi: 10.3390/cells11172764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sieck G.C., Dogan M., Young-Soo H., Osorio Valencia S., Delmotte P. Mechanisms underlying TNFalpha-induced enhancement of force generation in airway smooth muscle. Physiol. Rep. 2019;7 doi: 10.14814/phy2.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu G., Philp A.M., Corte T., Travis M.A., Schilter H., Hansbro N.G., et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol. Ther. 2021;225 doi: 10.1016/j.pharmthera.2021.107839. [DOI] [PubMed] [Google Scholar]
- 102.Ojiaku C.A., Cao G., Zhu W., Yoo E.J., Shumyatcher M., Himes B.E., et al. TGF-beta1 evokes human airway smooth muscle cell shortening and hyperresponsiveness via Smad3. Am. J. Respir. Cell Mol. Biol. 2018;58:575–584. doi: 10.1165/rcmb.2017-0247OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaifta Y., MacKay C.E., Irechukwu N., O'Brien K.A., Wright D.B., Ward J.P.T., et al. Transforming growth factor-beta enhances Rho-kinase activity and contraction in airway smooth muscle via the nucleotide exchange factor ARHGEF1. J. Physiol. 2018;596:47–66. doi: 10.1113/JP275033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akkenepally S.V., Yombo D.J.K., Yerubandi S., Reddy G.B., Deshpande D.A., McCormack F.X., et al. Interleukin 31 receptor alpha promotes smooth muscle cell contraction and airway hyperresponsiveness in asthma. Nat. Commun. 2023;14:8207. doi: 10.1038/s41467-023-44040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thompson E.E., Dang Q., Mitchell-Handley B., Rajendran K., Ram-Mohan S., Solway J., et al. Cytokine-induced molecular responses in airway smooth muscle cells inform genome-wide association studies of asthma. Genome Med. 2020;12:64. doi: 10.1186/s13073-020-00759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deeney B.T., Cao G., Orfanos S., Lee J., Kan M., Himes B.E., et al. Epinephrine evokes shortening of human airway smooth muscle cells following beta(2) adrenergic receptor desensitization. Am. J. Physiol. Lung Cell Mol. Physiol. 2022;323:L142–L151. doi: 10.1152/ajplung.00444.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matthey M., Roberts R., Seidinger A., Simon A., Schroder R., Kuschak M., et al. Targeted inhibition of G(q) signaling induces airway relaxation in mouse models of asthma. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aag2288. [DOI] [PubMed] [Google Scholar]
- 108.Singh B.K., Lu W., Schmidt Paustian A.M., Ge M.Q., Koziol-White C.J., Flayer C.H., et al. Diacylglycerol kinase zeta promotes allergic airway inflammation and airway hyperresponsiveness through distinct mechanisms. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aax3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma P., Yadav S.K., Shah S.D., Javed E., Lim J.M., Pan S., et al. Diacylglycerol kinase inhibition reduces airway contraction by negative feedback regulation of Gq-signaling. Am. J. Respir. Cell Mol. Biol. 2021;65:658–671. doi: 10.1165/rcmb.2021-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang H., Abel P.W., Toews M.L., Deng C., Casale T.B., Xie Y., et al. Phosphoinositide 3-kinase gamma regulates airway smooth muscle contraction by modulating calcium oscillations. J. Pharmacol. Exp. Ther. 2010;334:703–709. doi: 10.1124/jpet.110.168518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koziol-White C.J., Yoo E.J., Cao G., Zhang J., Papanikolaou E., Pushkarsky I., et al. Inhibition of PI3K promotes dilation of human small airways in a rho kinase-dependent manner. Br. J. Pharmacol. 2016;173:2726–2738. doi: 10.1111/bph.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L., Chitano P., Seow C.Y. Mechanopharmacology of Rho-kinase antagonism in airway smooth muscle and potential new therapy for asthma. Pharmacol. Res. 2020;159 doi: 10.1016/j.phrs.2020.104995. [DOI] [PubMed] [Google Scholar]
- 113.Yasuda Y., Wang L., Chitano P., Seow C.Y. Rho-kinase inhibition of active force and passive tension in airway smooth muscle: a strategy for treating airway hyperresponsiveness in asthma. Biology (Basel) 2024;13:115. doi: 10.3390/biology13020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benitez B., Anter A.M., Arcuri J., Bhattacharya S.K. Currently available prostanoids for the treatment of glaucoma and ocular hypertension. A. Review Curr. Opin. Pharmacol. 2024;74 doi: 10.1016/j.coph.2023.102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y., Saradna A., Ratan R., Ke X., Tu W., Do D.C., et al. RhoA/Rho-kinases in asthma: from pathogenesis to therapeutic targets. Clin. Transl Immunol. 2020;9 doi: 10.1002/cti2.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu R.A., Zeki A.A., Ram-Mohan S., Nguyen N., Bai Y., Chmiel K., et al. Inhibiting airway smooth muscle contraction using pitavastatin: a role for the mevalonate pathway in regulating. Cytoskeletal Proteins Front. Pharmacol. 2020;11:469. doi: 10.3389/fphar.2020.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu M.W., Liu R., Wu H.Y., Chen M., Dong M.N., Huang Y.Q., et al. Atorvastatin has a protective effect in a mouse model of bronchial asthma through regulating tissue transglutaminase and triggering receptor expressed on myeloid cells-1 expression. Exp. Ther. Med. 2017;14:917–930. doi: 10.3892/etm.2017.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park C., Jang J.H., Kim C., Lee Y., Lee E., Yang H.M., et al. Real-world effectiveness of statin therapy in adult asthma. J. Allergy Clin. Immunol. Pract. 2024;12:399–408.e396. doi: 10.1016/j.jaip.2023.10.029. [DOI] [PubMed] [Google Scholar]
- 119.Lavoie T.L., Dowell M.L., Lakser O.J., Gerthoffer W.T., Fredberg J.J., Seow C.Y., et al. Disrupting actin-myosin-actin connectivity in airway smooth muscle as a treatment for asthma? Proc. Am. Thorac. Soc. 2009;6:295–300. doi: 10.1513/pats.200808-078RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Woo J.A., Castano M., Kee T.R., Lee J., Koziol-White C.J., An S.S., et al. A Par3/LIM kinase/cofilin pathway mediates human airway smooth muscle relaxation by TAS2R14. Am. J. Respir. Cell Mol. Biol. 2023;68:417–429. doi: 10.1165/rcmb.2022-0303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Akaba T., Jo T., Iwagami M., Hashimoto Y., Matsui H., Fushimi K., et al. Reduced asthma exacerbations in adult patients treated with bronchial. Thermoplasty J. Allergy Clin. Immunol. Pract. 2023;11:3076–3083.e3073. doi: 10.1016/j.jaip.2023.04.036. [DOI] [PubMed] [Google Scholar]
- 122.Polio S.R., Stasiak S.E., Jamieson R.R., Balestrini J.L., Krishnan R., Parameswaran H. Extracellular matrix stiffness regulates human airway smooth muscle contraction by altering the cell-cell coupling. Sci. Rep. 2019;9:9564. doi: 10.1038/s41598-019-45716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mizuta K., Matoba A., Shibata S., Masaki E., Emala C.W., Sr. Obesity-induced asthma: role of free fatty acid receptors. Jpn. Dent. Sci. Rev. 2019;55:103–107. doi: 10.1016/j.jdsr.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Panganiban R.A.M., Yang Z., Sun M., Park C.Y., Kasahara D.I., Schaible N., et al. Antagonizing cholecystokinin A receptor in the lung attenuates obesity-induced airway hyperresponsiveness. Nat. Commun. 2023;14:47. doi: 10.1038/s41467-022-35739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kilic O., Yoon A., Shah S.R., Yong H.M., Ruiz-Valls A., Chang H., et al. A microphysiological model of the bronchial airways reveals the interplay of mechanical and biochemical signals in bronchospasm. Nat. Biomed. Eng. 2019;3:532–544. doi: 10.1038/s41551-019-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bagley D.C., Russell T., Ortiz-Zapater E., Stinson S., Fox K., Redd P.F., et al. Bronchoconstriction damages airway epithelia by crowding-induced excess cell extrusion. Science. 2024;384:66–73. doi: 10.1126/science.adk2758. [DOI] [PubMed] [Google Scholar]
- 127.Atia L., Fredberg J.J. A life off the beaten track in biomechanics: imperfect elasticity, cytoskeletal glassiness, and epithelial unjamming. Biophys. Rev. (Melville) 2023;4 doi: 10.1063/5.0179719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khalfaoui L., Pabelick C.M. Airway smooth muscle in contractility and remodeling of asthma: potential drug target mechanisms. Expert Opin. Ther. Targets. 2023;27:19–29. doi: 10.1080/14728222.2023.2177533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ijpma G., Kachmar L., Panariti A., Matusovsky O.S., Torgerson D., Benedetti A., et al. Intrapulmonary airway smooth muscle is hyperreactive with a distinct proteome in asthma. Eur. Respir. J. 2020;56:1902178. doi: 10.1183/13993003.02178-2019. [DOI] [PubMed] [Google Scholar]