Abstract
Background
To determine whether accounting for posterior corneal surgically induced astigmatism (SIA) would improve toric intraocular lens power calculation prediction error.
Methods
A total of 189 eyes of 148 patients undergoing routine cataract surgery were included in the study. Standard and posterior keratometry were measured pre- and postoperatively. Centroid SIA with standard keratometry and posterior keratometry were calculated separately. Prediction errors for postoperative refractive astigmatism at 4 weeks postoperatively were compared for Barrett Toric with predicted posterior corneal astigmatism (PPCA); Barrett Toric with preoperative measured posterior corneal astigmatism (MPCA); Barrett Toric with postoperative MPCA, which accounts for posterior corneal SIA.
Results
There was a significant increase in PCA magnitude postoperatively (p < 0.001), although a change of >0.3D occurred in only 3% of eyes. There was a postoperative rotation in the steep meridian of >10° in 32% of eyes. The Barrett Toric formula with PPCA yielded a significantly smaller refractive astigmatism prediction error compared to when a postoperative MPCA value was used (p < 0.01). Postoperative MPCA had a lower proportion of eyes within 0.50, 0.75 and 1.00D of predicted refractive astigmatism than PPCA or preoperative MPCA, although this was not statistically significant.
Conclusion
This study demonstrated postoperative changes in posterior corneal astigmatism magnitude and the orientation of the steep meridian. However, accounting for posterior keratometric SIA in the Barrett Toric formula does not improve refractive astigmatism prediction accuracy.
Keywords: cataract, posterior corneal astigmatism, Toric IOL power calculations
Introduction
Accounting for the effect of posterior corneal astigmatism (PCA) has been a major advance in toric intraocular lens power calculation, improving accuracy significantly.1–3 PCA must be considered in order to avoid overcorrection in eyes with with-the-rule anterior corneal astigmatism and undercorrection in eyes with against-the-rule anterior corneal astigmatism.1 Nomograms and regressions have been devised in order to account for PCA,1–3 but these have largely been superseded by formulas incorporating more sophisticated predictive algorithms and direct measurement of the posterior corneal curvature using swept source optical coherence tomography.4–6
The estimation of surgically induced astigmatism (SIA) is a source of postoperative refractive astigmatism prediction error in toric intraocular lens (IOL) calculations.7 Previous studies have primarily examined SIA derived from standard keratometry values (kSIA) but neglected posterior keratometric surgically induced astigmatism (pkSIA). Kane et al found pkSIA to have a centroid close to zero and pkSIA for individual eyes to be unpredictable.8 The authors therefore did not recommend considering pkSIA in toric power calculations. In contrast, a large study by Wendelstein et al found that SIA measured using total keratometry, which incorporates measured posterior corneal astigmatism, was significantly different to that measured with standard keratometry and thus recommended that SIA using total keratometry should be used.9 However, this recommendation has not be tested to see if it yields superior predictive outcomes.
This study aims to ascertain whether using pkSIA with the Barrett Toric calculator will improve postoperative refractive astigmatism prediction accuracy, compared to predicted PCA or preoperative measured PCA.
Methods
Study Design and Procedures
Consecutive patients who underwent cataract surgery with implantation of a monofocal non-toric IOL from February 2022 to December 2023 in Cathedral Eye Clinic, Belfast, were included in this retrospective observational cohort study. All patients gave their written informed consent for their anonymised data to be submitted for audit and publication. The research adhered to the tenets of the Declaration of Helsinki. The study was approved by the Cathedral Eye Clinic Ethics Committee (study reference number CECREC18-08).
Preoperatively, all eyes underwent a full ophthalmic examination including biometry with swept-source optical coherence tomography (IOLMaster 700, Carl Zeiss Meditec AG, Jena, Germany). This device measures standard keratometry (K) and also posterior keratometry (PK). At the one-month postoperative visit, corrected distance visual acuity and manifest refraction were recorded. Keratometry was repeated using the same SS-OCT biometry device.
Based on the steep meridian of standard keratometry values, eyes were classified as with-the-rule (WTR; steep meridian of 60 to 120 degrees), against-the-rule (ATR; steep meridian of 0 to 30 degrees or 150 to 180 degrees) or oblique (OBL, meridians outside the limits of WTR or ATR astigmatism). Instead of using the terms WTR and ATR for posterior corneal astigmatism, to avoid confusion caused by the posterior cornea acting as a negative lens, eyes were classified based on the steep meridian of posterior keratometry values as vertical (steep meridian of 60 to 120 degrees), horizontal (steep meridian of 0 to 30 degrees or 150 to 180 degrees) or oblique (meridians outside the limits of vertical or horizontal PCA).
Eyes were included if there was ≥0.50 D corneal astigmatism preoperatively on standard keratometry and if the clear corneal incision was centred on a meridian of 90 to 120°. The location of the corneal incision was verified intraoperatively using an intraoperative alignment system (Callisto, Carl Zeiss Meditec AG, Jena, Germany) or postoperatively on slit-lamp examination.
Exclusion criteria were postoperative best-corrected distance visual acuity of <6/12, previous keratorefractive or other corneal surgery, incomplete keratometry data and irregular anterior corneal astigmatism.
Surgical Technique
Phacoemulsification was performed through a superior 2.4mm or 2.5mm clear corneal incision followed by capsular bag implantation of the RayOne Aspheric RAO800C IOL (Rayner Intraocular Lenses Limited, Worthing, UK) which is a single-piece hydrophobic acrylic monofocal non-toric intraocular lens.
Residual Refractive Astigmatism Prediction Error
Standard surgically induced astigmatism and posterior corneal SIA were calculated for each eye by calculating the vectoral difference between postoperative and preoperative keratometry for K and PK. Centroid kSIA and pkSIA values were calculated for right and left eyes using vector analysis.10 Toric IOL calculations were performed using the Barrett Toric calculator v2.0 (https://calc.apacrs.org/toric_calculator20/Toric%20Calculator.aspx). In order to control for any error in the toricity ratio, the IOL constant was optimised for each eye individually using a modification of a recently published web scraping method.11 The predicted refractive astigmatism for an IOL with 0 cylinder power (ie, non-toric) was calculated using postoperative standard keratometry values and (1) predicted posterior corneal astigmatism (PPCA), (2) preoperative measured PCA (preoperative MPCA) and (3) postoperative measured PCA (postoperative MPCA). For the measured posterior corneal astigmatism calculations, posterior keratometry values were entered in the “Measured Posterior Cornea/IOLMaster 700 TK” fields on the online Barrett Toric calculator. Inputting the postoperative MPCA values equates to incorporating the achieved or measured pkSIA.
Refractive astigmatism prediction error was calculated as the vectoral difference between the predicted refractive cylinder and the achieved postoperative refractive cylinder.10
Change in Meridian and Magnitude of Corneal Astigmatism
Surgically induced astigmatism is a vector composed of magnitude (corneal astigmatism in dioptres) and direction (meridian or axis in degrees). In order to present these components separately, we present a simple algebraic difference between the preoperative and postoperative steep meridian, and the preoperative and postoperative corneal astigmatism magnitude. The absolute difference in meridian is presented, rather than classifying as clockwise or anticlockwise. It is assumed that the greatest rotation that can occur is 90°, ie, preoperative steep meridian of 10° and postoperative steep meridian of 170° would represent a torque of 20o rather than 160°. The change in magnitude of corneal astigmatism again is presented as the algebraic difference between preoperative and postoperative magnitude.
Statistical Analysis
Statistical analyses were completed using Microsoft Excel and the Eyetemis analysis tool.12 The Eyetemis tool uses the robust t-test and the robust Hotelling T2 test for univariate and bivariate analysis, in which the data in the distribution tails are trimmed to reduce the effect of outliers. Accuracy of refractive astigmatism prediction is assessed by comparing the trimmed mean values of the astigmatic prediction error magnitude using the robust t-test. Trueness refers to the closeness of agreement between the centroid value of test results and a reference centroid value and is evaluated by comparing the trimmed centroid astigmatic prediction errors using the robust Hotelling T2 test.13 Precision refers to the closeness of agreement between test results, or the spread of the error, and is assessed by comparing the trimmed mean values of the refractive astigmatism prediction error and trimmed centroid refractive prediction error for each method using the robust t-test.13 Cochran’s Q test was used to compare the proportions of eyes within 0.25/0.50/0.75/1.00 D of achieved postoperative refractive astigmatism. Normality was tested with a Shapiro–Wilk test. Correlation for non-normal data was assessed with Spearman’s test. Difference in medians of non-normal paired data was tested with the Wilcoxon signed rank test. A chi-squared test was used to compare unpaired groups with different sample sizes. A p value less than 0.05 was considered statistically significant.
Results
One hundred and eighty-nine eyes of 148 patients were included in the study. Preoperative biometric characteristics are summarised in Table 1. The mean optimised IOL constant was 118.48.
Table 1.
Baseline Characteristics
| Parameter | Values |
|---|---|
| Right eye, n (%), Left eye, n (%) | 99 (52.4%), 90 (47.6%) |
| Mean axial length (mm) ± SD, range | 24.06 ± 1.47, 20.49–28.05 |
| Mean IOL power (D) ± SD, range | 19.84 ± 4.16, 9.0–29.0 |
| Mean keratometry (D) ± SD, range | 43.58 ± 1.58, 38.77–50.37 |
| Mean standard corneal astigmatism magnitude (D) ± SD, range | 1.10 ± 0.57, 0.50–3.46 |
| Mean posterior corneal astigmatism magnitude (D) ± SD, range | −0.28 ± 0.14, −0.81–0.00 |
| Standard corneal astigmatism orientation, % (n) | |
| WTR | 59.3% (112) |
| Oblique | 11.6% (22) |
| ATR | 29.1% (55) |
| Posterior corneal astigmatism orientation, % (n) | |
| Vertical | 95.8% (181) |
| Oblique | 1.6% (3) |
| Horizontal | 2.6% (5) |
| Mean postoperative spherical equivalent (D) ± SD, range | −0.38 ± 0.47, −1.88–0.50 |
| Mean postoperative cylinder power (D) ± SD, range | 0.85 ± 0.71, 0.00–3.25 |
Abbreviations: WTR, With-the-rule; ATR, Against-the-rule.
Preoperative and postoperative corneal astigmatism are summarised in Table 2. Standard SIA and posterior corneal SIA are summarised in Table 3.
Table 2.
Centroid Preoperative and Postoperative Standard Corneal Astigmatism (SCA) and Posterior Corneal Astigmatism (PCA)
| Preoperative | Postoperative | |||
|---|---|---|---|---|
| Right | Left | Right | Left | |
| Centroid SCA (D @ o) | 0.42 @ 96° | 0.39 @ 101° | 0.31 @ 55° | 0.08 @ 112° |
| Mean absolute SCA magnitude (D) ± SD | 1.12 ± 0.62 | 1.07 ± 0.52 | 1.05 ± 0.59 | 0.88 ± 0.54 |
| Centroid PCA (D @ o) | −0.26D @ 97° | −0.25D @ 89° | −0.28D @ 96° | −0.30D @ 90° |
| Mean absolute PCA magnitude (D) ± SD | 0.29 ± 0.14D | 0.27 ± 0.13D | 0.31 ± 0.17D | 0.33 ± 0.17D |
Abbreviations: SCA, Standard corneal astigmatism; PCA, Posterior corneal astigmatism.
Table 3.
Centroid Surgically Induced Astigmatism Using Standard and Posterior Keratometry
| Right | Left | |
|---|---|---|
| Centroid kSIA (D @ o) | 0.48 @ 115° | 0.32 @ 98° |
| Mean absolute kSIA magnitude (D) ± SD, range | 0.65 ± 0.38, 0.00–1.91 | 0.59 ± 0.40, 0.00–2.45 |
| Centroid pkSIA (D @ o) | 0.02 @ 89 | 0.05 @ 98° |
| Mean absolute pkSIA magnitude (D) ± SD, range | 0.12 ± 0.09, 0.00–0.78 | 0.15 ± 0.09, 0.00–0.41 |
Abbreviations: kSIA, Standard keratometry SIA; pkSIA, Posterior keratometry SIA.
There was a statistically significant positive correlation between preoperative standard corneal astigmatism and posterior corneal astigmatism magnitude (ρ = 0.308, p < 0.001) and between preoperative standard corneal astigmatism and pkSIA magnitude (ρ = 0.362, p < 0.001). There was a statistically significant increase in posterior corneal astigmatism magnitude postoperatively (p < 0.001).
Mean Absolute Error for Prediction of Postoperative Refractive Astigmatism
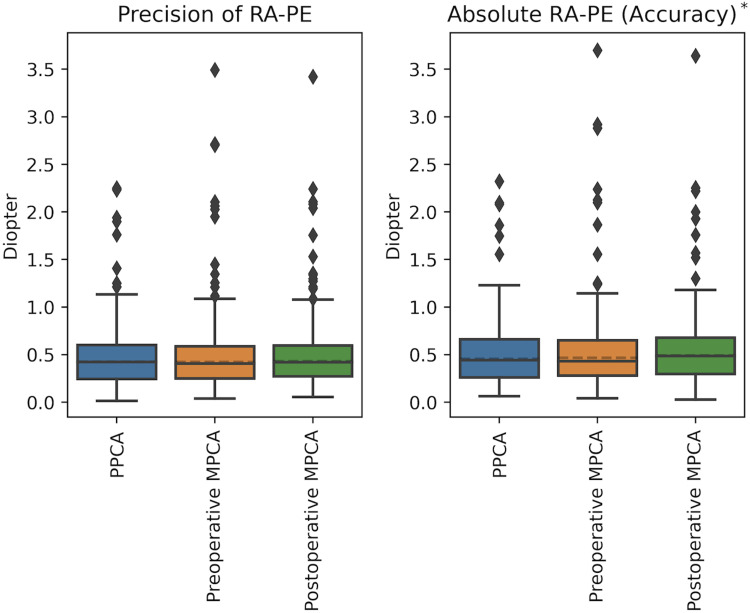
Refractive prediction errors are summarised in Table 4 and Figure 1. With respect to the mean refractive astigmatism prediction error, the Barrett Toric formula with PPCA was significantly more accurate than when postoperative MPCA was used (p < 0.01). Preoperative MPCA was more accurate than postoperative MPCA (p = 0.04). There was no statistically significant difference in precision between the groups.
Table 4.
Refraction Astigmatism Prediction Error
| PPCA | Preoperative MPCA | Postoperative MPCA | |
|---|---|---|---|
| MRA-PE (D) ± SD | 0.50 ± 0.36 | 0.55 ± 0.44 | 0.54 ± 0.49 |
| tr-Centroid Error (D @ degrees) | 0.16 @ 99 | 0.21 @ 89 | 0.24 @ 91 |
| % of eyes within vector prediction error magnitude | |||
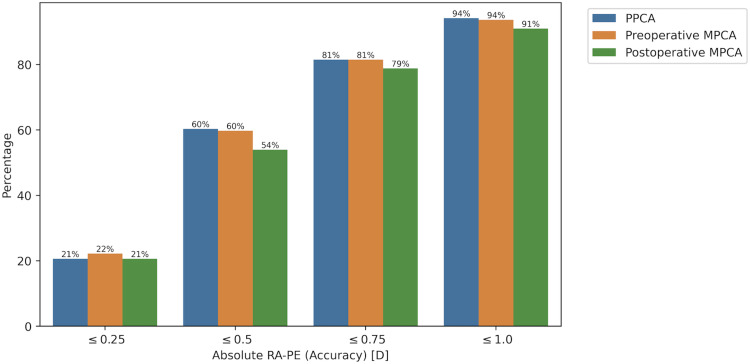
| ≤0.25 | 21% | 22% | 21% |
| ≤0.50 | 60% | 60% | 54% |
| ≤0.75 | 81% | 81% | 79% |
| ≤1.00 | 94% | 94% | 91% |
Abbreviations: MRA-PE, Mean refractive astigmatism prediction error; tr-Centroid Error, Trimmed refractive astigmatism prediction error; PPCA, Predicted posterior corneal astigmatism; MPCA, Measured posterior corneal astigmatism.
Figure 1.
Precision and accuracy of the refractive astigmatism prediction error (RA-PE) when using PPCA, preoperative MPCA or postoperative MPCA. * = Groups are statistically different.
Percentage of Eyes Within 0.25/0.50/0.75/1.00D of Predicted Postoperative Refractive Astigmatism
The proportion of eyes within a given dioptric prediction error range is displayed in Figure 2. For refractive astigmatism prediction errors within 0.25D, the Barrett Toric formula with preoperative MPCA performed slightly better than with PPCA or postoperative MPCA. For errors within 0.50, 0.75 and 1.00D, PPCA and preoperative MPCA performed equally, while postoperative MPCA had a consistently lower proportion of eyes within each error range. However, none of these differences were statistically significant.
Figure 2.
Percentage of eyes with absolute refractive astigmatism prediction error (RA-PE) within 0.25/0.50/0.75/1.00 D for Barrett Toric formula using PPCA, preoperative MPCA or postoperative MPCA.
Centroid Error for Prediction of Postoperative Refractive Astigmatism
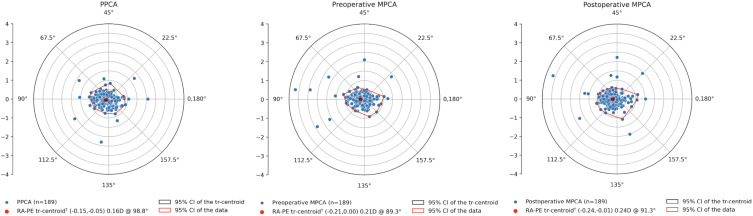
All centroid errors demonstrated a WTR bias (Table 4 and Figure 3). The centroid error when using PPCA was significantly different to postoperative MPCA (p < 0.01). The difference between centroid errors for PPCA and preoperative MPCA was of borderline significance (p = 0.05). There was no significant difference between the centroid errors for preoperative MPCA and postoperative MPCA (p = 0.06).
Figure 3.
Double angle plots of the refractive astigmatism prediction error for (A) PPCA, (B) preoperative MPCA and (C) postoperative MPCA. † = Statistically different from zero.
Change in Magnitude and Rotation of Steep Meridian of Posterior Corneal Astigmatism
Postoperative change in PCA magnitude and rotation of the steep keratometry meridian is displayed in Table 5. A change in magnitude of >0.1D occurred in a third of eyes, but only 3% had a change of >0.3D. There was no significant difference in magnitude change between eyes with higher (>0.3D) or lower (≤0.3D) preoperative PCA.
Table 5.
Postoperative Change in Astigmatism Magnitude and Rotation of the Steep Meridian
| Change in Magnitude | Rotation of Steep Meridian | |||||
|---|---|---|---|---|---|---|
| >0.1D | >0.2D | >0.3D | >5o | >10° | >20° | |
| All eyes (n = 189) | 33.3% | 7.9% | 3.2% | 58.2% | 32.3% | 13.8% |
| Pre-op posterior corneal astigmatism magnitude (D) | ||||||
| 0.00–0.30 (n = 105) | 35.2% | 7.6% | 3.8% | 67.6% | 46.7% | 21.9% |
| >0.30 (n = 84) | 31.0% | 8.3% | 2.4% | 46.4% | 14.3% | 3.6% |
| 0.469 | 0.891 | 0.560 | 0.005 | <0.001 | <0.001 | |
There was a postoperative rotation in the steep meridian of >5° in 58% of eyes and a rotation of >20° in 14% of eyes. Eyes with lower preoperative PCA magnitude demonstrated a larger postoperative rotation than those with higher preoperative PCA magnitude.
Discussion
This study demonstrated that incorporating posterior corneal SIA into the Barrett Toric formula did not improve its performance. Furthermore, using a predicted PCA value, rather than accounting for pkSIA by using a postoperative measured PCA value, yielded a smaller mean absolute astigmatism prediction error and a smaller centroid prediction error.
We found that 86% of eyes had a postoperative rotation in the steep meridian of PCA of ≤20° and 92% of eyes had an absolute change in astigmatism magnitude of ≤0.2D. Overall, pkSIA is small, with a centroid pkSIA of close to zero in right and left eyes, which is similar to that reported in two previous studies.8,14 We found a maximum pkSIA magnitude of 0.78D, which is higher than that reported by Kane et al,8 although this was a single outlying value and the next largest pkSIA magnitude was 0.41D, the same as found in Kane’s study. A study by Nemeth et al found a higher mean pkSIA magnitude and a maximum magnitude of 1.39D, although this was measured with a Scheimpflug imaging device and so values are not directly comparable.15
Our study identified a positive correlation between preoperative corneal astigmatism magnitude and pkSIA magnitude, with a similar correlation coefficient reported by Nemeth et al.15 The study by Kane et al found no such correlation.8
A postoperative increase in PCA magnitude was observed. A previous study found an increase in PCA magnitude in the first postoperative week, with subsequent flattening which did not change significantly beyond the third postoperative week.16 Another study comparing nasal and temporal 2.4mm incisions found a similar effect, with no significant changes found at 4 weeks postoperatively compared to baseline.17 The increase in PCA is likely to be related to focal steepening around the main corneal incision and its subsequent resolution.17
Other authors have suggested methods for accounting for the effect of PCA when considering SIA. Wendelstein et al studied SIA using standard keratometry and total keratometry (TK) values and found that TK-SIA differed significantly from conventional SIA and therefore suggested using TK-SIA.9 Holladay has proposed using postoperative refractive outcomes to devise total SIA, which will incorporate the effect of PCA, IOL tilt and IOL decentration.18 The Barrett Toric formula is not published, and we therefore do not know what algorithm is used to account for PCA. There is typically a nasal outward tilt of the IOL in the pseudophakic eye, with respect to the visual axis.19 This will induce ATR astigmatism, which adds to the ATR effect of PCA in most eyes.20 The Barrett Toric algorithm may include correcting the ATR effect that is attributable to IOL tilt and PCA.
All centroid prediction errors in this study are WTR. The Barrett Toric formula with PPCA has a smaller WTR centroid error compared to Barrett Toric with MPCA. This may be due to the inherent incorporation of posterior corneal SIA within the Barrett Toric algorithm as all toric algorithms are developed from postoperative manifest refraction. Using the measured MPCA may then cause a double correction of the posterior corneal SIA and therefore a WTR overshoot.
Our study aimed to control for other sources of error in toric IOL power calculations, thereby isolating the effect of pkSIA. Postoperative standard keratometry values were used in order to account for kSIA. A non-toric IOL model was used in order to eliminate the error arising from postoperative measurement of toric intraocular lens axis.5 The IOL constant was optimised for each eye individually,21 addressing the error related to the toricity ratio which is used to vertex cylinder power from the IOL plane to the corneal plane. A single intraocular lens injector type was used to avoid this variation in SIA attributable to injectors with different designs.22
A limitation is that the postoperative keratometry and manifest refraction were taken at a relatively early time point. The one-month postoperative visit is clinically relevant because manifest refraction is typically measured and assumed to be stable at this point. Studies have shown that there is a small change in standard SIA beyond one month and therefore using a later time point may be preferable.9,23 Previous studies have suggested that changes in the posterior corneal astigmatism stabilise by 3–4 weeks postoperatively.16,17 However, longer follow-up would confirm this and therefore may be preferable in future studies. In addition, our findings are relevant to eyes undergoing cataract surgery with a superior corneal incision, and these results should be validated for surgery involving a temporal corneal incision.
Conclusion
This study found that there is a small but statistically significant increase in PCA magnitude postoperatively. The steep meridian of PCA rotates by more than 10° in a third of eyes. However, despite these observed postoperative changes in PCA, accounting for posterior keratometric SIA in the Barrett Toric formula does not improve prediction accuracy. The current approach of using an SIA value based only on standard keratometry is therefore recommended when using the Barrett Toric formula.
Funding Statement
No funding was received for this study.
Disclosure
The authors have no conflict of interests to declare.
References
- 1.Koch DD, Jenkins RB, Weikert MP, Yeu E, Wang L. Correcting astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J Cataract Refract Surg. 2013;39(12):1803–1809. doi: 10.1016/j.jcrs.2013.06.027 [DOI] [PubMed] [Google Scholar]
- 2.Goggin M, Zamora-Alejo K, Esterman A, van ZL. Adjustment of anterior corneal astigmatism values to incorporate the likely effect of posterior corneal curvature for toric intraocular lens calculation. J Refract Surg. 2015;31(2):98–102. doi: 10.3928/1081597X-20150122-04 [DOI] [PubMed] [Google Scholar]
- 3.Abulafia A, Koch DD, Wang L, et al. New regression formula for toric intraocular lens calculations. J Cataract Refract Surg. 2016;42(5):663–671. doi: 10.1016/j.jcrs.2016.02.038 [DOI] [PubMed] [Google Scholar]
- 4.LaHood BR, Goggin M. Measurement of posterior corneal astigmatism by the IOLMaster 700. J Refract Surg. 2018;34(5):331–336. doi: 10.3928/1081597X-20180214-02 [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Koch DD. Comparison of accuracy of a toric calculator with predicted vs measured posterior corneal astigmatism. J Cataract Refract Surg. 2023;49(1):29–33. doi: 10.1097/j.jcrs.0000000000001025 [DOI] [PubMed] [Google Scholar]
- 6.Stewart S, Yeo TK, Moutari S, McNeely R, Moore JE. Accuracy of toric intraocular lens formulas with measured posterior corneal astigmatism of different orientations. Am J Ophthalmol. 2024;266:26–36. doi: 10.1016/j.ajo.2024.04.029 [DOI] [PubMed] [Google Scholar]
- 7.Hirnschall N, Findl O, Bayer N, et al. Sources of error in toric intraocular lens power calculation. J Refract Surg. 2020;36(10):646–652. doi: 10.3928/1081597X-20200729-03 [DOI] [PubMed] [Google Scholar]
- 8.Kane JX, LaHood BR, Goggin M. Analysis of posterior corneal surgically induced astigmatism following cataract surgery with a 1.8-mm temporal clear corneal incision. J Refract Surg. 2023;39(6):381–386. doi: 10.3928/1081597X-20230426-01 [DOI] [PubMed] [Google Scholar]
- 9.Wendelstein J, Casazza M, Riaz KM, et al. Characteristics of surgically induced astigmatism after standardized microincisional cataract surgery with a superior limbal incision. J Cataract Refract Surg. 2023;49(10):1025–1035. doi: 10.1097/j.jcrs.0000000000001271 [DOI] [PubMed] [Google Scholar]
- 10.Holladay JT, Moran JR, Kezirian GM. Analysis of aggregate surgically induced refractive change, prediction error, and intraocular astigmatism. J Cataract Refract Surg. 2001;27(1):61–79. doi: 10.1016/S0886-3350(00)00796-3 [DOI] [PubMed] [Google Scholar]
- 11.Lupardi E, Hoffer KJ, Fontana L, Savini G. Method to analyze the refractive outcomes of online intraocular lens power formulas. J Cataract Refract Surg. 2023;49(3):321–322. doi: 10.1097/j.jcrs.0000000000001122 [DOI] [PubMed] [Google Scholar]
- 12.Eyetemis - Eye procedures analysis tool. [Accessed 25, Jun 2024]. Available from: https://www.eyetemis.com/.
- 13.ISO 5725-1:1994(en), Accuracy (trueness and precision) of measurement methods and results — part 1: general principles and definitions. [Accessed 7, Dec 2023.]. Available from: https://www.iso.org/obp/ui/#iso:std:iso:5725:-1:ed-1:v1:en.
- 14.Langenbucher A, Szentmáry N, Cayless A, et al. Surgically induced astigmatism after cataract surgery - a vector analysis. Curr Eye Res. 2022;47(9):1279–1287. doi: 10.1080/02713683.2022.2052108 [DOI] [PubMed] [Google Scholar]
- 15.Nemeth G, Berta A, Szalai E, Hassan Z, Modis L. Analysis of surgically induced astigmatism on the posterior surface of the cornea. J Refract Surg. 2014;30(9):604–608. doi: 10.3928/1081597X-20140723-01 [DOI] [PubMed] [Google Scholar]
- 16.Sheoran K, Arya SK, Bansal RK, Jinagal J, Jha UP. Surgically induced astigmatism and posterior corneal curvature changes following phacoemulsification. Indian J Ophthalmol. 2022;70(2):406–412. doi: 10.4103/ijo.IJO_882_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi K, Sato T, Yoshida M, Yoshimura K. Corneal shape changes of the total and posterior cornea after temporal versus nasal clear corneal incision cataract surgery. Br J Ophthalmol. 2019;103(2):181–185. doi: 10.1136/bjophthalmol-2017-311710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272–283. doi: 10.1016/j.jcrs.2018.09.028 [DOI] [PubMed] [Google Scholar]
- 19.Hirnschall N, Buehren T, Bajramovic F, Trost M, Teuber T, Findl O. Prediction of postoperative intraocular lens tilt using swept-source optical coherence tomography. J Cataract Refract Surg. 2017;43(6):732–736. doi: 10.1016/j.jcrs.2017.01.026 [DOI] [PubMed] [Google Scholar]
- 20.Weikert MP, Golla A, Wang L. Astigmatism induced by intraocular lens tilt evaluated via ray tracing. J Cataract Refract Surg. 2018;44(6):745–749. doi: 10.1016/j.jcrs.2018.04.035 [DOI] [PubMed] [Google Scholar]
- 21.Hoffer KJ, Aramberri J, Haigis W, et al. Protocols for studies of intraocular lens formula accuracy. Am J Ophthalmol. 2015;160(3):403–405.e1. doi: 10.1016/j.ajo.2015.05.029 [DOI] [PubMed] [Google Scholar]
- 22.Khoramnia R, Baur ID, Łabuz G, et al. Enlargement of the main corneal incision: a clinical intraindividual comparison of two preloaded intraocular lens injectors. J Cataract Refract Surg. 2022;49(2):165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borasio E, Mehta JS, Maurino V. Surgically induced astigmatism after phacoemulsification in eyes with mild to moderate corneal astigmatism: temporal versus on-axis clear corneal incisions. J Cataract Refract Surg. 2006;32(4):565–572. doi: 10.1016/j.jcrs.2005.12.104 [DOI] [PubMed] [Google Scholar]