Cerebral amyloid angiopathy (CAA) is a disease marked by recurrent intracranial hemorrhage (ICH) secondary to small-vessel deposition of amyloid-β. ICH in CAA is preferentially located in the cortex, especially the occipital lobes, due to preferential involvement of the cortical and leptomeningeal blood vessels. Symptomatic features of CAA depend on the location and severity of ICH and include cognitive impairment, transient focal neurological episodes, and ischemic stroke. We present a case of cerebral achromatopsia secondary to CAA. Based upon our review of the English ophthalmologic research, we believe this case to be novel.
A 69-year-old female with a history of hypertension and recurrent intracerebral hemorrhage presented to the clinic complaining of “visual impairment”. She suffered a right posterior temporal-occipital lobar hemorrhage extending to the right ventral occipitotemporal cortex 6 years prior, a left posterior temporal-occipital lobar hemorrhage extending to the left inferolateral occipital cortex 1 year prior, and a left anterolateral temporal lobar hemorrhage 2 weeks prior to presentation. At the time of each ICH, there was no evidence of trauma, neoplasm, arterio-venous malformations, aneurysms, heart arrythmia, or emboli. Additionally, her hypertension was well controlled on amlodipine and an echocardiogram was unrevealing.
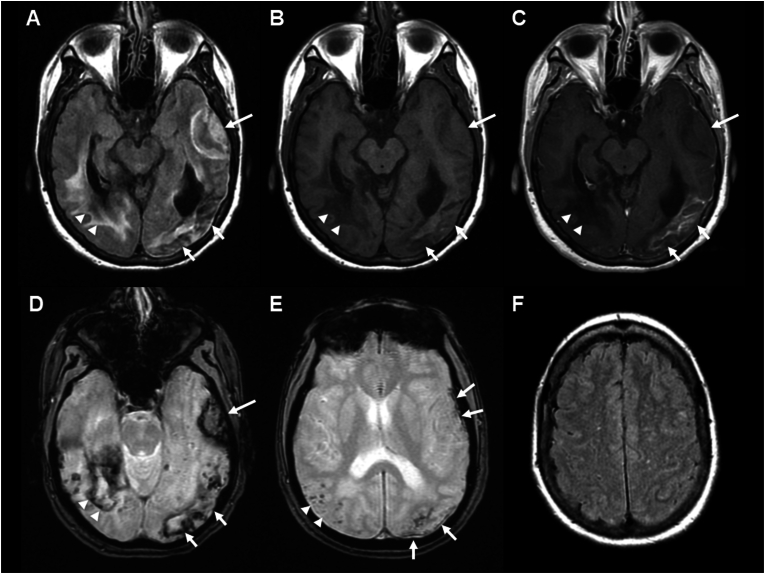
Magnetic resonance imaging (MRI) of the brain at onset of the most recent ICH (Fig. 1A–D) showed acute left anterolateral temporal lobar hemorrhage with intrinsic isointense T1 signal and mild local mass effect, late subacute left posterior temporal-occipital lobar hemorrhage with intrinsic hyperintense T1 signal and enhancement on postcontrast images, and chronic right posterior temporal-occipital lobar hemorrhage with intrinsic hypointense T1 and T2 signal with encephalomalacia and surrounding gliosis on T2 FLAIR (fluid attenuated inversion recovery). Additional areas of peripherally distributed microhemorrhages and cortical superficial siderosis are present on T2∗ GRE (gradient echo, Fig. 1E), and white matter hyperintense lesions in multi-spot pattern are present in bilateral centrum semiovale on T2 FLAIR (Fig. 1F). A probable diagnosis of CAA was made based on Boston criteria 2.0.1
Fig. 1.
Brain MRI (A) T2 FLAIR (fluid attenuated inversion recovery), (B) non-contrast T1 FSE (fast spin echo), (C) post-contrast T1 FSE, and (D) T2∗ GRE (gradient echo) sequences show multifocal intraparenchymal hemorrhages of varying ages, acute in left anterolateral temporal lobe (long arrow, isointense on T1), subacute in left posterior temporal-occipital lobe (short arrows, hyperintense on T1 with enhancement), and chronic in right posterior temporal-occipital lobe (arrow heads, hypointense on T1) with varying degree of surrounding vasogenic edema and gliosis on T2 FLAIR. Separate slice on T2∗ GRE (E) shows multiple microhemorrhages in right parieto-occipital juxtacortical distribution (arrow heads) as well as cortical superficial siderosis along the left anterolateral temporal and left parieto-occipital convexities (arrows). Findings meet probable CAA by Boston criteria 2.0: patient's age >50 years, at least two lobar hemorrhagic lesions (intraparenchymal hemorrhages, microhemorrhages, and cortical superficial siderosis), absence of deep hemorrhagic lesions, and presence of white matter hyperintense lesions in multispot pattern on T2 FLAIR (F).
On physical exam, her visual acuity was 20/50 in both eyes (OU). Humphrey visual field (HVF) 24-2 showed a mean deviation (MD) of −23.03 dB (dB) in the right eye (OD) and −26.69 dB in the left eye (OS) (Fig. 2). Her visual field defect was consistent with a complete right homonymous hemianopsia juxtaposed on a left superior homonymous quadrantanopia. Optical coherence tomography (OCT) showed a global retinal nerve fiber layer (RNFL) thickness of 95 μm OD and 85 μm OS. Intraocular pressure was 11 mmHg OD and 10 mmHg OS. Fundoscopy revealed no hemorrhage or emboli. There was no anisocoria, ptosis, or relative afferent pupillary defect (RAPD). The patient reported that her vision was completely black and white OU. She identified zero of 14, Ishihara color plates OU. Further neurological examination demonstrated alexia without agraphia, likely secondary to her left posterior temporal-occipital lobar hemorrhage. There was no prosopagnosia, anosognosia, or cognitive impairment. There was no family or personal history of Alzheimer disease.
Fig. 2.
Humphrey visual field (HVF) demonstrating concomitant right homonymous hemianopsia and left-superior homonymous quadrantanopia secondary to cerebral amyloid angiopathy (CAA)-related intracranial hemorrhages.
At follow-up 8 months later, the patient reported partial restoration of her color vision and states she retaught herself how to read using techniques for teaching children with learning disabilities. On last exam, her visual acuity improved to 20/25 OU, and Ishihara color plates test improved to 7/14 OD and 4/14 OS.
Cerebral achromatopsia is a rare condition characterized by loss of color vision due to a cerebral etiology and is generally accepted to result from significant bilateral occipitotemporal damage, particularly the ventral occipital cortex close to areas V4v, V4α, and V8.2 Cerebral achromatopsia with concomitant alexia without agraphia has previously been described associated with left medial occipital ± lateral occipital damage affecting the periventricular white matter, inferior optic radiation, and inferior visual association cortex, consistent with this patient's presentation.3
Cerebral achromatopsia has previously been reported secondary to ischemic stroke of the posterior cerebral artery (PCA) with improvement in color vision deficit over time.4 This patient's partial recovery of color vision over 8 months is in keeping with the previous longitudinal report and may be due to neuroplasticity.4
Cerebral achromatopsia is often the consequence of bilateral ventral occipital lesions and frequently associated with prosopagnosia (from right ventral occipitotemporal lesion) and pure alexia/alexia without agraphia (from left ventral occipitotemporal lesion).2,3 In this case, the patient had bilateral ventral occipital lesions and associated alexia without agraphia but no evidence of prosopagnosia. Additionally, visuospatial function deficits have previously been described in CAA and correlated with an increase in white matter hyperintensities and cerebral microbleeds; however, cerebral achromatopsia has not previously been described in CAA.5 Clinicians should be aware that visual manifestations of cerebral damage caused by CAA may include cerebral achromatopsia.
CRediT authorship contribution statement
Michael D. Woods: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Pamela Davila Siliezar: Data curation, Formal analysis, Writing – review & editing. Noor Laylani: Data curation, Formal analysis, Writing – review & editing. Elliott R. Friedman: Formal analysis, Writing – review & editing. Steve H. Fung: Data curation, Formal analysis, Validation, Writing – review & editing. Andrew G. Lee: Conceptualization, Formal analysis, Project administration, Supervision, Validation, Writing – review & editing.
CRediT authorship contribution statement
Michael D. Woods: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Pamela Davila Siliezar: Data curation, Formal analysis, Writing – review & editing. Noor Laylani: Data curation, Formal analysis, Writing – review & editing. Elliott R. Friedman: Formal analysis, Writing – review & editing. Steve H. Fung: Data curation, Formal analysis, Validation, Writing – review & editing. Andrew G. Lee: Conceptualization, Formal analysis, Project administration, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors have no conflict of interest.
References
- 1.Charidimou A., Boulouis G., Frosch M.P., et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. Aug 2022;21(8):714–725. doi: 10.1016/S1474-4422(22)00208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvier S.E., Engel S.A. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cerebr Cortex. Feb 2006;16(2):183–191. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- 3.Damasio A.R., Damasio H. Anatomic basis of pure alexia. Neurology. 1983;33(12):1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- 4.von Arx S.W., Muri R.M., Heinemann D., Hess C.W., Nyffeler T. Anosognosia for cerebral achromatopsia--a longitudinal case study. Neuropsychologia. Mar 2010;48(4):970–977. doi: 10.1016/j.neuropsychologia.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Valenti R., Charidimou A., Xiong L., et al. Visuospatial functioning in cerebral amyloid angiopathy: a pilot study. J Alzheimers Dis. 2017;56(4):1223–1227. doi: 10.3233/JAD-160927. [DOI] [PubMed] [Google Scholar]