Abstract
Type 2 innate lymphoid cells (ILC2s) mainly reside in tissues with few lymphoid cells. How their tissue residency is regulated remains poorly understood. This study explores the inhibitory role of SLAM-family receptors (SFRs) on adaptive immune cells in ILC2 maintenance. We observed an increase in the population of ILC2s in Rag1-deficient mice. Homotypic engagement of SFRs between ILC2s and adaptive immune cells was identified as a potential mechanism. SFR deficiency led to an increase in ILC2s. Conditional deletion of SFRs on T and/or B cells led to an increased ILC2 abundance. Mechanistically, as ILC precursors differentiate into ILC2s, SFRs, primarily SLAMF3 and SLAMF5, are inhibitory, which impair IL-7–induced PI3K activation and enhance apoptosis via SHP-1. These findings reveal a mechanism by which adaptive immune cells negatively regulate the homeostasis of ILC2s and contribute to our understanding of the complex interplay between innate and adaptive immune cells in the regulation of immune responses.
SLAM-family receptors play a role in the intricate cross-talk between innate and adaptive immune cells.
INTRODUCTION
Type 2 innate lymphoid cells (ILC2s) play a critical role in type 2 immunity by inducing inflammation shortly after activation. Understanding the mechanisms that regulate the development and maintenance of ILC2s is of utmost importance. The initial stages of ILC development occur in the fetal liver and progress in the bone marrow (BM) and peripheral tissues (1–3). ILC2s are derived from hematopoietic stem cells through multiple developmental stages, including common lymphoid progenitor cells (CLPs), common progenitor to all helper-like ILCs (CHILPs), and ILC progenitor cells (ILCPs). Specific transcription factors, such as nuclear factor IL3 regulated (NFIL3), GATA binding protein 3 (GATA-3), thymocyte selection associated high mobility group box protein (TOX), and T cell factor 1 (TCF1) for CLPs; inhibitor of DNA binding 2 (ID2) and GATA-3 for CHILPs; promyelocytic leukemia zinc finger (PLZF), GATA-3, ID2, and TCF1 for ILCPs; and retinoic acid-related orphan receptor alpha (RORα), GATA-3, B cell leukemia 11b (BCL11b), and growth factor independence (GFI) for ILC2s, orchestrate these developmental processes (1, 4, 5). Cytokine signals, including interleukin-7 (IL-7), IL-33, and transforming growth factor–β (TGF-β), have also been found to facilitate ILC2 differentiation and maintenance (6). However, further investigation is needed to understand the negative regulatory mechanisms governing ILC2 differentiation and homeostasis.
The regulation of ILC2 development and maintenance is complex, especially within the regional immune microenvironment. Different tissues and organs have unique characteristics that contribute to the establishment of distinct regional features. Various factors, such as tissue-specific cytokines, metabolites, and neural signals, act as regulators of ILC2s (6). Cell-to-cell interactions also have a profound influence on ILC2 homeostasis and function within different microenvironments. For example, tissue-resident multipotent stromal cells (MSCs) support ILC2 proliferation through direct cell-to-cell interactions (7). In addition, receptors expressed on ILC2s, such as inducible co-stimulator (ICOS), programmed death-1 (PD-1), killer cell lectin-like receptor subfamily G member 1 (KLRG1), lymphocyte function-associated antigen 1 (LFA-1), and natural cytotoxicity triggering receptor 3 (NKp30), can regulate ILC2s through intercellular interactions (7–12). Notably, ILC2s prefer to reside in tissues with low densities of T and B cells, suggesting that the abundance of adaptive immune cells may disrupt the delicate homeostasis of ILC2s.
Signaling lymphocyte–activating molecule (SLAM) family receptors (SFRs) consists of seven members and act as self-ligands, except for SLAMF4 (2B4) that recognizes SLAMF2 (CD48). They transmit signals via their cytoplasmic immunoreceptor tyrosine switch motifs and primarily recruit SLAM-associated protein (SAP) (13, 14). SAP functions as an active signaling molecule that recruits Fyn kinase to regulate natural killer (NK) cell activation, T helper 2 differentiation, and NK-T cell development (15–23). The importance of SFR-SAP signaling is highlighted by the fact that the gene encoding SAP is mutated in X-linked lymphoproliferative (XLP) disease in humans (24). SAP also functions as a natural blocker molecule. Therefore, in the absence of SAP, SFRs recruit alternative molecules that contain the SH2 domain, such as phosphatases, SHP-1, SHP-2, and SHIP-1 (25). As a result, SAP-independent SFR signaling is implicated in the impaired activation of NK cells and humoral immunity in patients with XLP-1 (15, 20, 24). Notably, this signaling pathway also plays a role in the physiological regulation of immune cells lacking SAP expression. For instance, this signaling can suppress the phagocytosis of hematopoietic cells by macrophages (26). Now, whether this SAP-independent SFR signal has other immunoregulatory functions, especially in regulating immune events that are contact dependent among immune cells, still needs further research.
In this study, we present evidence to support the role of SAP-independent SFR signaling in maintenance of ILC2s. The deficiency of SFRs causes enhanced maintenance of ILC2s after the differentiation of ILCPs into ILC2s. In addition, the conditional deletion of SFRs on T and/or B cells results in an augmented population of ILC2s. Mechanistically, the loss of SAP expression during the differentiation of ILCPs into ILC2s makes SFR become inhibitory, which attenuates ILC2 maintenance signaling and IL-7–induced PI3K activation by recruiting SHP-1. These findings reveal an underappreciated mechanism through which adaptive immune cells regulate ILC2s via SFRs.
RESULTS
Adaptive immune cells are negatively associated with the abundance of ILC2s
Cell-to-cell interaction is known to play a crucial role in immune cell maintenance homeostasis and activation. To assess whether the homeostasis of ILC2s is influenced by neighboring immune cells in different tissues, such as BM, mesenteric lymph nodes (MLNs), small intestine (SI), large intestine (LI), lungs, and epididymal white adipose tissue (EWAT), flow cytometry was used to analyze ILC2s. Given the differences in the expression of specific makers on the ILC2s across different tissues (27), various markers were used to identify ILC2s. We confirmed that the gated ILC2s from all the tested tissues are GATA-3 positive (fig. S1, A to D). Consistent with previous reports, we found that, under naïve conditions, ILC2s were more abundant in the SI, LI, lungs, and EWAT compared to the other tissues (Fig. 1A). This observation was accompanied by a lower density of T and/or B cells (Fig. 1B). In addition, we observed a negative correlation between the number of ILC2s and the presence of adaptive immune cells (Fig. 1, C to E), indicating a potentially notable role for adaptive immune cells in the regulation of ILC2s during physiological status.
Fig. 1. Negative association between adaptive immune cells and the ILC2 population.
(A) Numbers of ILC2s in the bone marrow (BM), mesenteric lymph nodes (MLNs), small intestine (SI), large intestine (LI), lungs, and epididymal white adipose tissue (EWAT) of WT mice. n = 6 mice per group. (B) Density (cell number per gram) of CD4+ T cells (CD4+ T), CD8+ T cells (CD8+ T), and B cells (B) in the BM, MLN, SI, LI lungs, and EWAT of WT mice. n = 6 mice per group. (C to E) Correlation analysis between the number of ILC2s and CD4+ T cells (C), CD8+ T cells (D), or B cells (E) among BM, MLN, SI, LI, lungs, and EWAT of WT mice. n = 36. (F to K) Proportions and numbers of ILC2s in the BM (F), MLN (G), SI (H), LI (I), lungs (J), and EWAT (K) of WT and Rag1−/− mice. n = 3 to 8 mice per group. (L) Phosphate-buffered saline (PBS) control, T cells, B cells, or T + B cells from CD45.1 mice were transferred into CD45.2 Rag1−/− mice (as illustrated in fig. S1E). After 8 weeks, the number of CD45.2+ ILC2s was quantified. n = 4 mice per group. The data represent three independent experiments. All data are presented as means ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA) [(A), (B), and (L)], linear regression [(C) to (E)], or unpaired Student’s t test [(F) to (K)]. *P < 0.05, **P < 0.01, and ***P < 0.001. iv, intravenous.
Next, we investigated the influence of adaptive immune cells on the regulation of ILC2 quantity. Compared to wild-type (WT) control mice, Rag1-deficient (Rag1−/−) mice lacking T and B cells exhibited a significant increase in the proportion of ILC2s in the BM, MLN, SI, LI, and lungs (Fig. 1, F to K). In contrast, when T or B cells were transferred into Rag1−/− mice, the number of ILC2s in the BM, SI, LI, and lung declined (Fig. 1L and fig. S1E). To exclude the influence of NK cells in Rag1−/− mice (28), we used α-NK1.1 (PK136) antibodies to deplete NK cells. Our findings indicated that, in the absence of NK cells, Rag1 deficiency still led to an increase in ILC2 numbers (fig. S1, F and G). These findings suggest that T and B cells play a suppressive role in the regulation of ILC2 abundance.
Deficiency in SFR results in an increase in the number of ILC2s
Besides cytokine-mediated cell cross-talk, cellular communication heavily relies on the interaction between cellular receptors and their ligands through cellular contact. To explore the involvement of cellular contact in the suppression of ILC2s by adaptive immune cells, we followed a four-step approach to predict potential inhibitory receptors that are cellular contact dependent. First, we identified a gene set that potentially represents a pool of inhibitory receptors by overlapping three distinct categories from the gene ontology or UniProt database: “negative regulation of immune response,” “signal,” and “transmembrane” (Fig. 2A, left). Second, to investigate the role of cell contact in this process, we excluded cytokine receptor–related genes. Third, we analyzed the expression levels of these selected candidate receptors in ILC2s using RNA sequencing (RNA-seq) data analysis from the Gene Expression Omnibus (GEO) database (GSE117470), which revealed several receptors with high expression levels on ILC2s (Fig. 2A, middle). Last, we evaluated the expression of the corresponding ligands of these receptors in T and B cells using RNA-seq data from the GEO database (GSE184841) to determine whether these receptors could be triggered by their corresponding ligands expressed on adaptive immune cells. We found that SLAMF3 and SLAMF5 exhibited high levels of expression in ILC2s and adaptive immune cells (Fig. 2A, right).
Fig. 2. Increased ILC2 population in SFR deficient mice.
(A) Intersection analysis was conducted on three gene sets associated with negative regulation of immune response (GeneOntology database, GO0050777), transmembrane proteins (UniProt database, KW-0812), and signaling (UniProt database, KW-0732) (left). Their expression on ILC2s was then analyzed using the RNA sequencing (RNA-seq) dataset from the Gene Expression Omnibus (GEO) database (GSE117470), with cytokine receptor–related genes excluded (middle). The highly expressed genes on ILC2s in the middle panel were selected, and their ligand expression on adaptive immune cells was analyzed using the RNA-seq dataset from the GEO database (GSE184841, right). (B) Representative overlaid histograms showing the expression level of SFRs on ILC2s in the BM, MLN, SI, LI, lung, and EWAT from WT (blue) and SFR−/− (gray) mice. (C to H) Proportion and number of ILC2s in the BM (C), MLN (D), SI (E), LI (F), lungs (G), and EWAT (H) of WT and SFR−/−mice. n = 3 to 9 mice per group. (I) Number of DsRed+CD45.1−CD45.2+ ILC2s in the indicated organs from the mice (as illustrated in fig. S2C). n = 5 mice per group. The data [(B) to (I)] are representative of at least three independent experiments. All data are presented as means ± SEM. Statistical analysis was performed using an unpaired Student’s t test [(C) to (I)]. *P < 0.05, **P < 0.01. n.s. indicates not significant.
Considering that SLAMF3 and SLAMF5 are members of the SFR family, we performed flow cytometry analysis to evaluate the expression of all SFR members. The results confirmed the strong expression of SLAMF2, SLAMF3, and SLAMF5 in ILC2s from BM, MLN, SI, LI, lung, and EWAT (Fig. 2B). To investigate the regulatory function of SFRs on ILC2s, we compared SFR-knockout (SFR−/−) mice, which lack all seven SFR members, with their WT control. We found an increased number of ILC2s in the BM, MLN, and lungs of SFR−/− mice (Fig. 2, C to H). However, no significant differences were observed in the number of ILC1s and ILC3s between the WT and SFR−/− mice (fig. S2, A and B), suggesting the specific role of SFRs in regulating the ILC2 subset.
Next, we aimed to generate mice with a specific deletion of SFRs on ILCs using a mixed BM chimera assay (fig. S2C) (29). The resulting chimeras with Nfil3-deficient plus SFR-deficient BM exhibited almost 95% of ILC2s that did not express SFRs (fig. S2D). Furthermore, there was a significant increase in the number of ILC2s in the BM and MLN of the mixed chimeras with SFR deficiency (Fig. 2I). These pieces of evidence strongly support the crucial role of SFRs in the regulation of ILC2 abundance under normal physiological conditions.
To investigate the intrinsic role of SFRs in ILC2s, CD45.2+ DsRed+ WT and CD45.2+ SFR−/− BM were mixed and adoptively transferred into lethally irradiating CD45.1 mice (fig. S2E). ILC2s from CD45.2+ SFR−/− BM showed a significantly greater proportion in the BM and MLN (fig. S2F), suggesting that the inhibitory effect of SFRs on ILC2 maintenance is cell intrinsic.
SFRs specifically suppress ILC2 following differentiation from ILCPs
ILC2s originate from hematopoietic stem cells and undergo several developmental stages, starting with CLPs, CHILPs, and ILCPs. Our investigation revealed the dynamic expression of several SFRs at different stages of ILC2 development. Notably, as ILCPs transitioned into ILC2s, we observed a rapid loss of SLAMF4 expression and a significant increase in SLAMF3 and SLAMF5 expression (Fig. 3A), highlighting the crucial role of SFRs in regulating this developmental process. This prompted us to investigate the specific stages affected by SFR deletion in mice. While SFR deficiency resulted in an increased number of ILC2s, it did not significantly increase the number of CLPs and CHILPs, but SFR deficiency led to a decrease in ILCPs (Fig. 3, B and C). These findings indicate that SFRs play an inhibitory role in ILC2 stage following differentiation from ILCPs.
Fig. 3. Specific suppression of ILC2 stage by SFRs.
(A) Flow cytometry analysis was performed to evaluate the expression levels of SFR members on common lymphoid progenitor cells (CLPs), common progenitor to all helper-like ILCs (CHILPs), ILC progenitor cells (ILCPs), and ILC2s in the BM. The expression level was presented as the net mean fluorescence intensity (ΔMFI). n = 4 mice per group. (B and C) Representative flow cytometric profiles (B) and quantification (C) of CLPs, CHILPs, ILCPs, and ILC2s in the BM of WT and SFR−/− mice. n = 8 to 9 mice per group. The data are representative of three independent experiments. All data are presented as means ± SEM. Statistical analysis was conducted using one-way ANOVA (A) or unpaired Student’s t test (C). *P < 0.05, **P < 0.01, and ***P < 0.001. n.s. indicates not significant.
Adaptive immune cell–derived SFRs restrict ILC2 maintenance
Next, we sought to further validate the hypothesis regarding the suppressive role of adaptive immune cells on ILC2 homeostasis through the expression of SFR-mediated cell contact. Initially, we excluded the impact of SFR deficiency on the expression of IL-7 (fig. S3A), the abundance of adaptive immune cells (fig. S3, B and C), and the number and function of regulatory T cells (fig. S3, D and E). It is noteworthy that, in comparison to SI, LI, lung, and EWAT tissues, BM and MLN demonstrated higher densities of adaptive immune cells (Fig. 1B). In addition, we observed that the deficiency of SFRs led to a more pronounced alteration in the abundance of ILC2s in BM and MLN (Fig. 4A). Conversely, the lack of adaptive immune cells in Rag1-deficient mice led to a significant reduction in the magnitude of this alteration specifically in BM ILC2s (Fig. 4B). These findings imply a positive connection between the strength of SFR-mediated inhibition of ILC2s and the presence of adaptive immune cells.
Fig. 4. Restriction of ILC2 maintenance by SFRs derived from adaptive immune cells.
(A) Fold change (SFR−/−:WT) of ILC2s in the BM, MLN, SI, LI, lungs, and EWAT according to Fig. 2 (C to H). n = 3 to 8 mice per group. (B) Fold change of ILC2s in the BM of the indicated mice. n = 6 to 7 mice per group. (C) Number of CD45.1−CD45.2+DsRed− ILC2s in the indicated organs from the mice (as illustrated in fig. S3F). n = 6 mice per group. (D to I) Proportion and number of ILC2s in the BM (D), MLN (E), SI (F), LI (G), Lung (H), EWAT (I) of the indicated mice. n = 3 to 34 mice per group. The data are representative of at least two independent experiments. All data are presented as means ± SEM. Statistical analysis was conducted using one-way ANOVA [(A) and (D) to (I)] or an unpaired Student’s t test [(B) and (C)]. *P < 0.05, **P < 0.01, and ***P < 0.001. n.s. indicates not significant.
To assess the inhibitory effect of SFRs by adaptive immune cells on ILC2s in an in vivo model, we generated mixed chimeras by combining Rag1−/− BM with either WT or SFR−/− BM. This approach allowed us to obtain mice harboring WT or SFR−/− T and B cells (fig. S3, F and G). Notably, the lack of SFRs originating from adaptive immune cells resulted in an elevation of ILC2 abundance in BM, MLN, and lung (Fig. 4C). These findings suggest that SFRs derived from T and/or B cells may play a role in inhibiting ILC2 maintenance.
To investigate the specific contributions of bystander T and B cells in regulating the maintenance of ILC2s, we used conditional SFR knockout mice (SFRfl/fl) that were engineered with two LoxP sites into the Slam loci flanks (~400 kb). These mice were then bred with CD4-Cre and/or MB1-Cre mice to selectively eliminate SFR expression in T and/or B cells. The effectiveness of SFR deletion in T and B cells was confirmed by flow cytometric assessment (fig. S3, H to K).
Mice lacking SFRs specifically in B cells showed a significant increase in the number of ILC2s in the BM, similar to the phenotype observed in germline SFR knockout mice (Fig. 4D). Consistently, through immunofluorescence analysis of the distribution of B cells and ILC2s in the BM (fig. S4A), we observed the contacts between ILC2s and adjacent B cells (fig. S4B). However, the sole deletion of SFRs in T cells did not lead to an up-regulation of ILC2 counts and did not enhance ILC2 abundance when combined with the deletion of SFRs in B cells (Fig. 4D). Considering the predominance of B cells within the adaptive immune cell population in the BM (Fig. 1B), these results suggest a higher likelihood for B cells to interact with ILC2s in the BM environment and trigger homotypic engagement of SFRs to suppress ILC2 homeostasis.
Furthermore, because T and B cells are the main subsets of adaptive immune cells in MLN (Fig. 1B), we observed cell contact between ILC2s and these cell types (fig. S4, C and D), and the absence of SFRs in either T or B cells individually increased the number of ILC2s (Fig. 4E). However, the concurrent loss of SFRs in both T and B cells, as well as germline SFR knockout, led to a more pronounced augmentation in ILC2 counts in MLN (Fig. 4E). Similarly, the concurrent absence of SFRs in T and B cells significantly elevated the number of ILC2s in the lungs, whereas the ILC2 count resulting from the individual absence of SFRs in T or B cells remained comparable in the lung (Fig. 4H). Notably, the loss of SFRs in T and/or B cells did not affect the number of ILC2s in the SI, LI, or EWAT (Fig. 4, F, G, and I), suggesting that the inhibitory effect of SFR on ILC2 homeostasis may rely on the abundance of adaptive immune cells in these tissues. These genetic insights highlight the crucial inhibitory function exerted by SFRs expressed in T and B cells in suppressing the maintenance of ILC2s within specific tissue immune microenvironments.
SLAMF3 and SLAMF5 are responsible for suppressing the maintenance of ILC2s
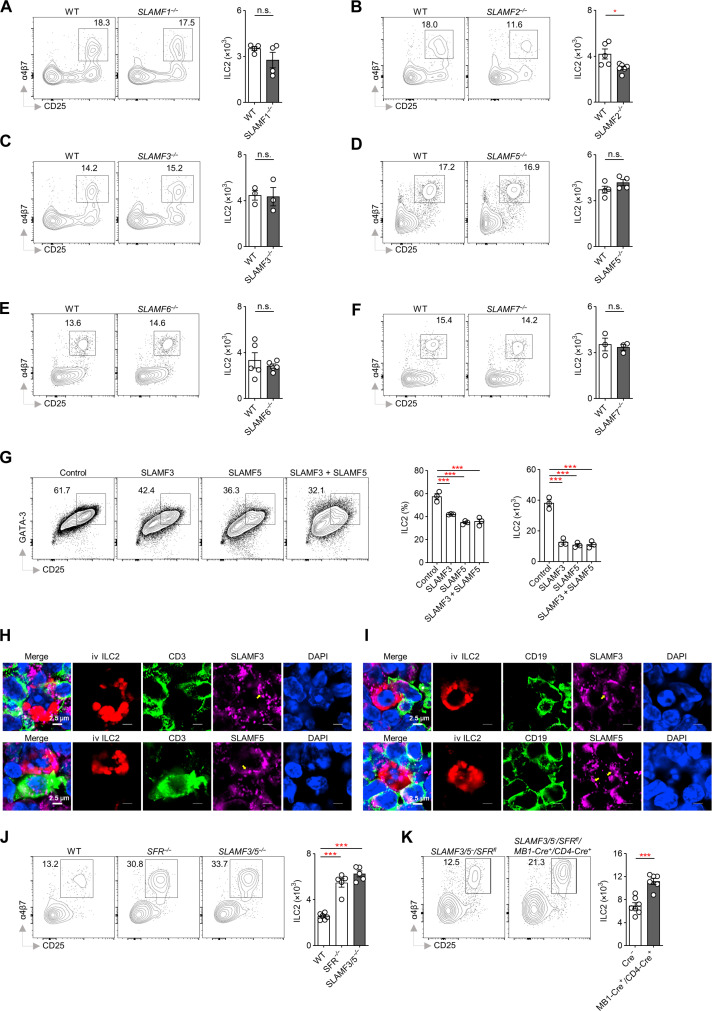
Through experiments using mice lacking individual members of SFRs, we found that the absence of any single SLAM member did not lead to a significant increase in ILC2 levels (Fig. 5, A to F), indicating redundancy within the SFRs (15, 20, 26, 30–32). However, only three receptors—SLAMF2, SLAMF3, and SLAMF5—were detected to be expressed on ILC2s (Fig. 2B), suggesting their potential involvement in this regulatory context. Because SLAMF2 lacks signaling function (33, 34), we focused on SLAMF3 and SLAMF5.
Fig. 5. Suppression of ILC2 population by specific SFR members, namely SLAMF3 and SLAMF5.
(A to F) Proportion and number of ILC2s in the BM of WT and SLAMF1−/− (A), SLAMF2−/− (B), SLAMF3−/− (C), SLAMF5−/− (D), SLAMF6−/− (E), and SLAMF7−/− (F) mice. n = 3 to 6 mice per group. (G) Flow cytometric profiles of cells derived from sorted CHILPs of WT mice cultured on OP9-DL1 cells ectopically expressing with or without SLAMF3 and/or SLAMF5 in the presence of IL-7 and IL-33 for 18 days, and analyzed for the percentage and number of ILC2s. n = 3 per group. (H and I) Confocal microscopic analysis of the polarization of SLAMF3 or SLAMF5 (pink) on the contact surface (yellow arrow) between ILC2s (DsRed, red) and their bystander T [(H), CD3, green] or B [(I), CD19, green] cells within the MLN from Nfil3−/− mice, 7 days after transfer of DsRed+ ILC2s. (J) Proportion and number of ILC2s in the BM of the indicated mice. n = 5 to 6 mice per group. (K) Proportion and number of ILC2s in the BM of the indicated mice. n = 6 to 7 mice per group. The data are representative of at least two independent experiments. All data are presented as means ± SEM. Statistical analysis was conducted using an unpaired Student’s t test [(A) to (F) and (K)] or one-way ANOVA [(G) and (J)]. *P < 0.05 and ***P < 0.001. n.s. indicates not significant.
To investigate the role of SLAMF3 and SLAMF5, we overexpressed these receptors as ligands on SFR-null OP9-DL1 cell lines and cocultured them with WT CHILPs under conditions that promote ILC2 differentiation. We found that either SLAMF3 or SLAMF5 effectively inhibited ILC2 number (Fig. 5G). The immunofluorescence analysis revealed the polarization of SLAMF3 or SLAMF5 at the contact surfaces between ILC2s and their bystander T and B cells (Fig. 5, H and I), indicating a potential role for these receptors in triggering signaling between these immune cell types. Subsequently, we used SLAMF3/5 double-knockout (SLAMF3/5−/−) mice to examine the compensatory effects mediated by these receptors. The simultaneous loss of both receptors significantly increased ILC2 levels, similar to the outcome observed in germline SFR knockout mice (Fig. 5J). Hence, it can be concluded that SLAMF3 and SLAMF5, as the primary members of SFRs, play a crucial role in constraining ILC2 maintenance.
To investigate the potential role of SLAMF3 and SLAMF5 expression on adaptive immune cells in controlling ILC2 homeostasis, we conducted crossbreeding experiments between SLAMF3/5−/− mice and SFRfl/fl mice. This approach resulted in offspring with a half deficiency of SLAMF3/5 and LoxP-inserted SFR loci flanking sites that could be excised by Cre recombinase. By using CD4 and MB1-driven Cre expression, we successfully eliminated SLAMF3 and SLAMF5 expression in T or B cells (fig. S5A), which led to a significant increase in the number of ILC2s in the BM (Fig. 5K). These findings suggest that adaptive immune cells have the ability to suppress ILC2s through the expression of SLAMF3 and SLAMF5.
While SFR deficiency resulted in increased ILC2 numbers, the absence of SLAMF2 showed a significant decrease in ILC2 counts (Fig. 5B). As SLAMF2 serves as a ligand for SLAMF4 signaling, we observed that the expression of SLAMF4 occurs only before the ILC2 stage (Fig. 3A), indicating that SLAMF4 likely plays a promoting role in early ILC2 development. Previous studies have demonstrated that the interaction between SLAMF2 and SLAMF4 signaling promotes the differentiation of ILCPs in humans (35). Consistently, we found that SLAMF2 deficiency resulted in a decrease in the number of ILCPs in mice (fig. S5B), while BM chimeric mice that ectopically expressed SLAMF4 in hematopoietic stem cells showed a significant increase in the proportion of ILCPs (fig. S5, C to E). To gain further insight into the specific function of SLAMF4, we used a mouse model with deficiencies in all SFR members except for SLAMF2 and SLAMF4 (SLAMF1/3/5/6/7−/−). In this model, we observed an increase in ILC2 numbers compared to complete SFR gene deletion (fig. S5F), highlighting the positive influence exerted by SLAMF4. However, the exclusive presence of SLAMF4 in the context of all SFR member deficiencies did not produce a further increase in ILC2 abundance (fig. S5F), suggesting that SLAMF2-independent activation of SLAMF4 is not possible.
In addition, our results indicated that CD2, which can interact with SLAMF2 was not expressed on ILCPs and ILC2s (fig. S5G). Moreover, the genetic removal of CD2 had no significant impact on ILC2 levels (fig. S5H). Therefore, it can be concluded that the signaling mediated by SLAMF2 acting upon SLAMF4 plays a positive role specifically in the early stages of ILC2 development, before their maturation into fully formed ILC2s.
SAP-independent SFR signaling inhibits ILC2 homeostasis
SFRs are known to recruit SAP-related adaptors or SAP-independent phosphatases, playing a critical role in intercellular communication (14, 25). We revealed that ILC2s did not express SAP or its homologs like Ewing’s sarcoma–associated transcript 2 (EAT-2) (Fig. 6A and fig. S6A). Moreover, the absence of SAP does not significantly increase the number of ILC2s (Fig. 6B). To exclude the redundant role of SAP-family adaptors, we used mice lacking both SAP and EAT-2, in which ILC2 number was also not affected (Fig. 6C). These data indicate that the regulation of ILC2 homeostasis mediated by SFRs is independent of SAP-related adaptors.
Fig. 6. SAP-independent SLAMF3/5 inhibitory signaling hinders after ILCP differentiation into ILC2s.
(A) Representative overlaid histograms and quantification of net MFI of SAP expression in the indicated cells from the BM. n = 5 mice per group. (B and C) Proportion and number of ILC2s in the BM of WT and SAP−/− (B) and SAP−/−EAT-2−/− (C) mice. n = 3 mice per group. (D) BM chimera mice were generated by reconstituting lethally irradiated CD45.1 WT recipients with WT or SLAMF3/5-deficient BM cells that were infected with MSCV retrovirus encoding GFP control (MSCV-control) or SAP (MSCV-SAP). After 16 weeks, the proportion and the number of ILC2s in the BM of the indicated mice were analyzed. n = 5 to 8 mice per group. The data are representative of at least two independent experiments. All data are presented as means ± SEM. Statistical analysis was conducted using one-way ANOVA [(A) and (D)] or an unpaired Student’s t test [(B) and (C)]. **P < 0.01. n.s. indicates not significant.
SAP binding obstructs the interaction between SFRs and phosphatases, with its absence enabling SFRs to effectively recruit phosphatases that hinder immune cell functions (25). The inhibitory effects of SFRs on ILC2 homeostasis only become evident after ILCPs differentiate into ILC2s (Fig. 3C). During this process, there is a spontaneous decrease in SAP protein expression (Fig. 6A), suggesting that, as SAP expression decreases, SFRs shift their recruitment to phosphatases, leading to the manifestation of inhibitory effects.
To confirm this hypothesis, BM chimera mice were generated by lethally irradiating CD45.1 WT recipients and reconstituting them with WT or SLAMF3/5-deficient BM cells ectopically expressing SAP. Ectopic expression of SAP in hematopoietic stem cells resulted in a significant increase in the number of ILC2s in WT mice (Fig. 6D and fig. S6B). Furthermore, in the presence of exogenous SAP expression, the absence of SLAMF3 and SLAMF5 did not further increase the number of ILC2s (Fig. 6D). These findings indicate that the gradual loss of SAP expression by ILC2s after differentiation from ILCPs may enable SLAMF3 and SLAMF5 to exert their inhibitory functions.
SLAMF3 and SLAMF5 restrain ILC2 homeostasis through the action of SHP-1
When SAP is absent in immune cells, SFRs can recruit phosphatases such as SHP-1, SHP-2, and SHIP-1, which impede the function of immune cells (14, 25). Among these phosphatases, only the phosphorylation of SHP-1 showed a significant decrease in SLAMF3/5−/− mice compared to their WT counterparts, particularly during ILC2 stage (Fig. 7, A to H). This indicates that SHP-1 may specifically mediate the inhibitory effects of SLAMF3 and SLAMF5 during ILC2 stage.
Fig. 7. Inhibition of the IL-7 activated PI3K pathway by SLAMF3 and SLAMF5 through SHP-1.
(A and B) The expression levels of SHP-1, SHP-2, and SHIP-1 in BM ILC2s of the indicated mice. n = 5 mice per group. (C to H) The phosphorylation levels of SHP-1 [(C) and (D)], SHP-2 [(E) and (F)], and SHIP-1 [(G) and (H)] in the indicated cells from the indicated mice. n = 6 to 7 mice per group. (I) The proportion and number of ILC2s in the BM of the indicated mice. n = 4 to 6 mice per group. (J) ILC2s from SHP-1fl/fl and SHP-1fl/fl/CD122-Cre+ mice were cultured with IL-7 in the indicated OP9-DL1 cells for 7 days and subsequently analyzed for ILC2 numbers. n = 3 per group. (K) PI3K phosphorylation in freshly isolated or IL-7–stimulated BM ILC2s from the indicated mice. n = 10 to 11 mice per group. (L to N) BCL-2 expression (L), annexin V+DAPI− population (M), and caspase activity (N) in BM ILC2s from the indicated mice. n = 4 to 6 mice per group. (O to Q) PI3K phosphorylation (O), BCL-2 expression (P), and annexin V+DAPI− population (Q) in BM ILC2s from the indicated mice. n = 3 to 8 mice per group. The data represent at least two independent experiments and are presented as means ± SEM. Statistical analysis was performed using an unpaired Student’s t test [(B), (D), (F), (H), (I), and (L) to (Q)] or one-way ANOVA [(J) and (K)]. *P < 0.05, **P < 0.01, and ***P < 0.001; n.s. indicates not significant.
To further elucidate the inhibitory role of SHP-1 in regulating the level of ILC2s, we used a breeding strategy by crossing SHP-1fl/fl mice with CD122-Cre+ mice. CD122, also known as IL-2Rβ, is a critical subunit of the receptor complex responsible for signaling pathways induced by both IL-2 and IL-15 (36). Using fate-mapping techniques, where cells with Cre recombinase activity were marked by yellow fluorescent protein expression, we observed that ~80% of ILC2s exhibited efficient deletion driven by CD122-Cre (fig. S7A). Intriguingly, we observed a significant expansion of ILC2s in the BM of SHP-1fl/fl/CD122-Cre+ mice compared to that of SHP-1fl/fl mice (Fig. 7I), indicating the involvement of SHP-1 in modulating the inhibitory mechanisms that regulate ILC2 maintenance. On the basis of the normal number of ILC2s in WT, CD122Cre+, and SHP-1fl/fl mice, CD45.2+ DsRed+ WT BM was selected as control to mix with CD45.2+ SHP-1fl/fl/CD122-Cre+ BM to generate mixed BM chimeras in CD45.1 recipients for investigating the intrinsic role of SHP-1 in ILC2s (fig. S7B). ILC2s derived from CD45.2+ SHP-1fl/fl/CD122-Cre+ BM exhibited a significantly higher proportion in the BM (fig. S7C), suggesting that the inhibitory effect of SHP-1 on ILC2 maintenance is cell intrinsic.
To investigate the role of SHP-1 in SLAMF3- and SLAMF5-mediated inhibition, ILC2s from SHP-1fl/fl and SHP-1fl/fl/CD122-Cre+ mice were cultured on OP9-DL1 cells ectopically expressing with or without SLAMF3 and SLAMF5. We found that SLAMF3 and SLAMF5 significantly inhibited the number of ILC2s (Fig. 7J). However, the activation of SLAMF3 and SLAMF5 in the absence of SHP-1 did not lead to a decrease in the number of ILC2s (Fig. 7J), suggesting that SHP-1 is involved in the inhibition of ILC2s by SLAMF3 and SLAMF5.
SLAMF3 and SLAMF5 inhibit ILC2 cell survival by attenuating IL-7/PI3K signaling
To further investigate the mechanism by which SLAMF3 and SLAMF5 inhibit ILC2 development, we examined whether SFR/SHP-1 signaling regulates the expression of IL-2, IL-33, and IL-7 receptors. We found no significant differences in the expression levels of these receptors between WT and SLAMF3/5−/− BM ILC2s (fig. S7, D to F). In addition, we evaluated the expression levels of key transcription factors involved in ILC2 development, such as NFIL3, TCF-1, TOX, ID2, PLZF, GATA-3, RORα, BCL11b, EST1, and GFI1, and found no evidence to suggest that SLAMF3 and SLAMF5 regulate these transcription factors (fig. S7, G to M).
Considering the important role of IL-7 in promoting ILC2 maintenance (37–39), as well as the inhibitory ability of SHP-1 in dephosphorylating PI3K (40–42), which is a downstream effector of the IL-7 signal (43, 44), we aimed to investigate whether SLAMF3 and SLAMF5 could inhibit the PI3K signaling pathway downstream of IL-7. Under physiological conditions, we observed an increase in the phosphorylation level of PI3K in BM ILC2s lacking SLAMF3 and SLAMF5 (Fig. 7K). A similar tendency was observed in the WT:SFR−/− mixed BM chimera (figs. S2E and S7N), suggesting the intrinsic role of SFRs in regulating PI3K phosphorylation. Furthermore, upon stimulation with IL-7 in vitro, the phosphorylation of PI3K in BM ILC2s significantly increased, and this effect was further enhanced in the absence of SLAMF3 and SLAMF5 (Fig. 7K). Therefore, it can be inferred that SLAMF3 and SLAMF5 inhibit the activation of the PI3K pathway by IL-7.
Consistent with previous studies supporting the essential role of IL-7 in inducing BCL-2 expression (38), BCL-2 is an antiapoptotic protein involved in the downstream cascade of PI3K signaling (45). Our findings indicate that the loss of SLAMF3 and SLAMF5 enhances the expression of BCL-2 in ILC2s (Fig. 7L), thereby significantly reducing cell apoptosis (Fig. 7, M and N). The WT:SFR−/− mixed BM chimera assays also showed an intrinsic reduce of cell apoptosis in SFR-deficient ILC2s (figs. S2E and S7O). In conclusion, these results suggest that the inhibitory effects of SLAMF3 and SLAMF5 on IL-7 signaling contribute to the accelerated apoptosis and decreased maintenance of ILC2s.
Consistent with the results observed in SLAMF3/5−/− mice, we found that ILC2s with SHP-1 deficiency displayed increased phosphorylation of PI3K and up-regulated expression of BCL-2 (Fig. 7, O and P), resulting in reduced apoptosis (Fig. 7Q). Further experiments using WT:SHP-1–deficient mixed BM chimera assays also confirmed that the effects in the SHP-1 deficient mice were ILC2 intrinsic (fig. S7P). These findings provide further evidence supporting the regulatory role of SHP-1 in mediating the inhibitory effects of SLAMF3 and SLAMF5 on ILC2s.
Because IL-7 is crucial for cytokine production and proliferation in ILC2s (38, 46), we also found that in vitro stimulation with IL-7 significantly up-regulates cytokine production and Ki-67 expression in SLAMF3- and SLAMF5-deficient ILC2s (fig. S7, Q and R), suggesting that SLAMF3 and SLAMF5 may also be involved in regulating IL-7–dependent cytokine production and proliferation in ILC2s.
In summary, our data demonstrate that bystander adaptive immune cells hinder ILC2 homeostasis through the interaction of SLAMF3 and SLAMF5. Following ILCP differentiation into ILC2s, there is a spontaneous loss of SAP protein expression, which allows SLAMF3 and SLAMF5 to inhibit the IL-7–driven PI3K signaling cascade via SHP-1, thereby preventing cell survival (fig. S8).
DISCUSSION
ILC2s are known to preferentially reside in tissues with low densities of T and B cells, such as the lungs, intestines, and adipose tissues. This observation raises the possibility that the presence of adaptive immune cells in these tissues could affect the homeostasis of ILC2 populations. In this study, we demonstrate that adaptive immune cells have an inhibitory impact on ILC2s within a physiological context. The interaction between SFRs on ILC2s and adaptive immune cells hinders the maintenance of ILC2s. Mechanistically, ILC2s lose expression of SAP after differentiating from ILCPs, which allow SFRs to suppress PI3K signaling downstream of IL-7 through the phosphatase SHP-1. Therefore, our study reveals a mechanism by which adaptive immune cells limit the population of ILC2s.
Diverse tissues and organs exhibit distinct structural, physiological, and cellular features that intricately construct regional microenvironments, influencing local cell homeostasis and disease onset. Notably, ILC2s appear to heavily depend on these regional microenvironments, as evidenced by prior investigation revealing distinct transcriptional profiles across various tissues, even in the absence of IL-25, IL-33, and thymic stromal lymphopoietin (TLSP) signaling cascades (47). On the basis of the tissue-resident nature of ILC2s, we validated the inhibitory influence of adaptive immune cells on ILC2 homeostasis through SFRs. Despite the presence of adaptive immune cells among organs, the strength of this inhibitory effect correlates with the abundance of adaptive immune cells within microenvironments. Furthermore, the composition of T and B cells within specific tissue immune microenvironments also determines their suppression on ILC2s maintenance through SFRs. Consequently, our findings not only enhance our understanding of how the regional adaptive immune microenvironment regulates ILC2 homeostasis but also provide an explanation for the tissue-resident behavior of ILC2s.
Adaptive immune cells regulate ILC2s through various mechanisms across different tissues. Although we have found that T and/or B cells can inhibit ILC2s via SRFs, the phenotypic differences seen in SFR−/− and Rag1−/− mice across different tissues suggest the existence of SRF-independent regulatory mechanisms. Cytokines are an important means by which adaptive immune cells regulate ILC2s. For example, T cells can compete for IL-7 binding to limit ILC2 homeostasis (48), while cytokines secreted by T cells, such as IL-10 (49), TGF-β (50), interferon-γ (51–53), and IL-22 (54), may play a regulatory role in ILC2s. In addition, there are regulatory interactions between adaptive immune cells and ILC2s that depend on ligand-receptor interactions, such as ICOS/ICOS ligand (ICOSL) (8), PD-1/programmed Death-Ligand 1 (PD-L1) (12), and OX40 (CD134)/OX40L (CD252) (29). As our study highlights the importance of the microenvironment in facilitating cell-cell cross-talk, the regulatory effects of adaptive immune cells on ILC2s across different tissues and their physiological significance deserve further investigation.
Our study introduces a paradigm for exploring the mechanisms governing the interaction between ILC2s and neighboring cells. Cell-to-cell communication through direct contact heavily relies on the interplay between ligands and receptors. On the basis of this understanding, we conducted bioinformatics analyses to examine the expression profiles of ligands and receptors on adaptive immune cells and ILC2s. In addition to the discovery of SLAMF3 and SLAMF5, this approach also predicted a potential interaction between DPP4 and PTPRC. This prediction was supported by recent research regarding PTPRC’s suppressive role in ILC2 development and activity (55), validating the accuracy of our analytical approach. Given the adjacency of ILC2s with various cellular types (7, 53, 56–58), including innate immune cells, stromal cells, and neurocytes, our study opens up important avenues for exploring undiscovered cellular interactions between ILC2s and their surrounding cells.
It is crucial to consider the regulatory effects of each surface receptor member during different developmental phases, as the expression levels of these receptors dynamically change during ILC2 development. For example, the loss of SLAMF4 signaling leads to an increase in the population of ILC2s, despite the fact that ILC2s themselves do not express SLAMF4. This suggests that SLAMF4 may have a functional role before the developmental phase of ILC2s. We and others have demonstrated that the interaction between SLAMF2 and SLAMF4 signaling promotes the differentiation of ILCPs in mice and humans (35), respectively. Similarly, the presence of SAP protein in ILCPs, but not in ILC2s, allows SFRs to specifically exert inhibitory effects on ILC2s rather than ILCPs. Furthermore, the increased expression levels of SLAMF3 and SLAMF5 during the differentiation of ILCPs into ILC2s indicate an enhanced inhibitory capacity during the ILC2 stage. Therefore, changes in the expression profiles of surface receptors and their adaptors may play important roles in shaping the functional outcomes mediated by these receptors. However, further investigations are required to understand the factors driving these changes in expression levels.
The specific inhibition of ILC2 stage through SFRs is closely linked to the gradual loss of SAP expression. SAP binding hinders the interaction between SFRs and phosphatases, and its absence allows SFRs to effectively recruit phosphatases that impede immune cell functions (25). Therefore, the transient nature of SAP enables it to dynamically regulate the function of SFRs by modulating its expression levels. As ILCPs differentiate into ILC2s, there is a decline in SAP expression, which enables SFRs to recruit SHP-1 and exert inhibitory effects. This might explain why we observed the inhibitory function of SFRs only after ILCPs have differentiated into ILC2s. However, further research is necessary to fully understand the underlying mechanisms that drive the spontaneous decline in SAP expression during this process.
The protein tyrosine phosphatase SHP-1 plays a pivotal role in regulating cellular signal transduction that governs activation control and apoptotic processes. In T cells, SHP-1 exerts its regulatory influence by interacting with key substrates such as zeta-chain associated protein kinase 70 (ZAP70) (59), lymphocyte-specific protein tyrosine kinase (LCK) (60), PI3K (61), and Vav guanine nucleotide exchange factor (VAV) (62). As a result, it plays crucial roles in TCR signaling, effector function, and T helper cell differentiation (63). Notably, SHP-1 is ubiquitously expressed across all mature hematopoietic lineages. Although previous studies have elucidated an inverse correlation between SHP-1 expression and the PI3K/BCL2 pathway in ILC2s (42), there is still a lack of genetic evidence to substantiate SHP-1’s regulatory role in ILC2s. In this study, we used the SHP-1–deficient mouse model to demonstrate the negative impact of SHP-1 on ILC2 quantity and its regulation of the PI3K/BCL2 pathway and antiapoptotic processes.
MATERIALS AND METHODS
Mice
Mice lacking SLAM family members (SFR−/−), SLAMF1-deficient (SLAMF1−/−), SLAMF2-deficient (SLAMF2−/−), SLAMF3-deficient (SLAMF3−/−), SLAMF5-deficient (SLAMF5−/−), SLAMF6-deficient (SLAMF6−/−), SLAMF7-deficient (SLAMF7−/−), SLAMF1/2/3/5/6/7-deficient (SLAMF1/2/3/5/6/7−/−), SLAMF1/3/5/6/7-deficient (SLAMF1/3/5/6/7−/−), SLAMF3 and SLAMF5–double-deficient (SLAMF3/5−/−), SAP-deficient (SAP−/−), SAP and EAT-2–double-deficient (SAP−/−EAT-2−/−), CD2-deficient (CD2−/−), CD122-cre, and SFRfl/fl mice were generated using CRISPR-Cas9–based genome editing in our laboratory (15, 26, 64). CD45.1 (no. 002014), Rag1−/− (no. 002216), C57BL/6 (no. 000664), and SHP1fl/fl (no. 008336) mice were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA). Mice were euthanized by carbon dioxide inhalation. All experimental/control animals were housed under the same conditions. All mice were maintained in specific pathogen–free animal facilities at Tsinghua University. All experiments involving animals were conducted with the explicit permission granted by the Animal Ethics Committee of Tsinghua University, in accordance with their official guidelines and regulations (THU-02-2024-0353A).
Reagents
Antibodies against CD3 [17A2; flow cytometry (F), 1:500; immunofluorescence (IF), 1:200], CD19 (eBio1D3; F, 1:500), NK1.1 (PK136; F, 1:500), CD11b (M1/70; F, 1:500), ST2 (RMST2-2; F, 1:500), CD48 (HM48-1; F, 1:500), 2B4 (eBio244F4; F, 1:500), CD45.1 (A20; F, 1:500), CD45.2 (104; F, 1:500), CD4 (GK1.5; F, 1:500), CD8 (53-6.7; F, 1:500), Sca-1 (D7; F, 1:500), Gr-1 (RB6-8C5; F, 1:500), KLRG1 (2F1; F, 1:500), CD90.2 (53-2.1; F, 1:500), GATA-3 (TWAJ; F, 1:100), CD127 (A7R34; F, 1:500), ID2 (ILCID2; F, 1:250), Nfil3 (S2M-E19; F, 1:250), PLZF (Mags.21F7; F, 1:250), TOX (TXRX10; F, 1:250), CD25 (PC61.5; F, 1:500), rat immunoglobulin G (IgG; H+L) (Thermo Fisher Scientific, no. A11006; IF, 1:200); rabbit IgG (H+L) (Thermo Fisher Scientific, no. A11008; IF, 1:200), and streptavidin–allophycocyanin (APC) (Thermo Fisher Scientific, no. SA1005; IF, 1:200) were obtained from Thermo Fisher Scientific. Antibodies against SLAM (TC15-12F12.2; F, 1:500), Ly9 (ly9ab3; F, 1:500; IF, 1:200), CD84 (CD84.7; F, 1:500; IF, 1:200), Ly108 (330-AJ; F, 1:500), and SLAMF7 (4G2; F, 1:500) were obtained from BioLegend. Antibodies against SAP (1A9; F, 1:250) were obtained from BD Biosciences. Lineage markers include CD3, CD19, CD11b, NK1.1, and Gr1. Antibodies against CD19 (no. 3574; IF, 1:200), p-SHP-1 (Y564; F, 1:200), p-SHP-2 (Y542; F, 1:200), p-SHIP-1 (Tyr1020; F, 1:200), Alexa Fluor 647–conjugated p-PI3K p85 (Tyr458)/p55 (Tyr199) (E3U1H; F, 1:100), APC-conjugated TCF1/TCF7 (C63D9; F, 1:200), SHP-1 (E1U6R; F, 1:200), SHP-2 (D50F2; F, 1:200), and SHIP-1 (E8M5D; F, 1:200) antibodies were obtained from Cell Signaling Technology. Collagenase D and deoxyribonuclease I (DNase I) were obtained from Roche.
In vitro ILC2 differentiation assay
OP9-DL1 cells were cultured in minimum essential medium–α (MEM-α) medium supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (100 IU/ml) at 37°C in a 5% CO2 and normal O2 atmosphere. For the in vitro ILC2 differentiation assays, Lin−CD127+Flt3−α4β7+CD25− CHILP cells from the BM of the indicated mice were sorted and cocultured with mitomycin C–treated OP9-DL1 cells in 96-well plates. The culture medium used was MEM-α complete medium containing 20% FBS, penicillin/streptomycin (100 IU/ml), IL-7 (15 ng/ml), and IL-33 (15 ng/ml). The coculture was maintained for 18 days.
In vitro stimulation of ILC2s
For the analysis of ILC2 cytokine production and proliferation, WT and SLAMF3/5−/− mice were used to sort lung ILC2s, which were then cultured in RPMI 1640 complete medium [containing penicillin-streptomycin (100 U/ml) and 10% FBS] supplemented with IL-7 (15 ng/ml) in 96-well cell culture plate (U bottom) for 72 hours. Subsequently, the expression of Ki-67 by ILC2s was analyzed using intracellular staining. Following a 3-hour restimulation with phorbol 12-myristate 13-acetate plus ionomycin, the expression of IL-5 and IL-13 by ILC2s was analyzed using intracellular staining. For the analysis of PI3K phosphorylation levels, BM ILC2s were stimulated with or without IL-7 (15 ng/ml) for 30 min. For in vitro activation of SLAMF3 and SLAMF5, ILC2s were cultured on OP9-DL1 cells ectopically expressing with SLAMF3 and SLAMF5 in the presence of IL-7 (15 ng/ml) for 7 days.
Tissue preparation and immune cell isolation
To collect cells from the lungs or EWAT, the tissues were minced and digested using a solution of collagenase D (2 mg/ml; Roche) and DNase I (20 μg/ml; Roche) in RPMI 1640 medium with 5% FBS. The digestion was carried out on a shaker at 180 rpm for 45 min at 37°C. Afterward, the digested tissue was passed through 70-mm cell strainers to obtain a single-cell suspension, and, then, the red blood cells were lysed. To isolate cells from the SI and LI, intestinal tissues were opened longitudinally, cut into 1-cm pieces after eliminating Peyer’s patches, and washed with Hanks’ balanced salt solution medium containing 5 mM EDTA and 1 mM dithiothreitol for 30 min at 37°C to remove epithelial cells. Then, intestinal tissues were digested with collagenase D (0.5 mg/ml) and DNase I (20 μg/ml) at 180 rpm for 45 min at 37°C. Afterward, the digested tissue was passed through 70-mm cell strainers and enriched with 40% Percoll gradient before red blood cells were lysed. For the isolation of cells from the MLN, the tissues were manually homogenized using 70-μm cell strainers. BM cells were collected by flushing out the marrow from the femurs and tibias using a syringe with phosphate-buffered saline (PBS).
Flow cytometry
Cell surface markers were stained with antibodies. To detect transcription factors, the ILC2s were fixed, permeabilized, and then stained with appropriate antibodies. For the detection of phosphorylated signaling proteins, ILC2s were fixed using Phosflow Lyse/Fix buffer, followed by permeabilization with Phosflow Perm buffer III (BD Biosciences), and staining with the indicated antibodies from Cell Signaling Technology. Flow cytometry data were collected using an LSRFortessa instrument (BD Biosciences) and analyzed using FlowJo software.
Histology
For immunofluorescence staining, BM or MLN was fixed in 4% paraformaldehyde, embedded in Optimal Cutting Temperature (O.C.T.) compound, and sliced into 15-μm frozen sections. The slides were fixed with 1% paraformaldehyde and then blocked/permeabilized by PBS with 5% bovine serum albumin (BSA) and 0.5% Triton X-100 (Sigma-Aldrich). Following this, the slides were incubated for 1 hour at room temperature with specific primary antibodies in permeabilization buffer (PBS with 5% BSA and 0.2% Triton X-100), washed twice, and incubated overnight at 4°C with the fluorescently labeled secondary antibody in permeabilization buffer. Last, the slides were stained with 4′,6-diamidino-2-phenylindole (DAPI). Confocal microscopy was performed with Nikon AX. Images were processed with the NIS-Elements software. Anti-CD19 (Cell Signaling Technology, no. 3574; IF, 1:200), anti-CD3 (Thermo Fisher Scientific, no. 16-0032-82; IF, 1:200), anti-rat IgG (H+L) (Thermo Fisher Scientific, no. A11006; IF, 1:200), anti-rabbit IgG (H+L) (Thermo Fisher Scientific, no. A11008; IF, 1:200), streptavidin-APC (Thermo Fisher Scientific, no. SA1005; IF, 1:200), anti-SLAMF3 (BioLegend, no. 122903; IF, 1:200), and anti-SLAMF5 (BioLegend, no. 122803; IF, 1:200) were used for immunofluorescence staining.
Real-time PCR
Total RNA from BM cells or fluorescence-activated cell sorting–sorted ILC2s was extracted using the TRIzol Kit (Invitrogen) and reverse transcribed using the reverse transcription system (Promega, A3500). Quantitative polymerase chain reaction (PCR) was performed using SYBR Green–based detection. The expression levels of the genes of interest were normalized to the expression of β-actin.
Generation of BM chimeras
The indicated genotypes of mixed BM chimeras were generated by reconstituting lethally irradiated CD45.1 WT recipients with 6 × 105 mixed BM cells (mixed 1:1). After 12 weeks of reconstitution, the mice were subjected to analysis. For exogenous expression of SAP or SLAMF4 protein, the indicated mice were treated with 5-fluorouracil for 4 days, and BM cells were collected for spin infection with murine stem cell virus (MSCV) retrovirus encoding control green fluorescent protein (GFP) or SAP or SLAMF4. The MSCV-SAP–encoding or MSCV-SLAMF4–encoding viral vector also encodes GFP. Retrovirally transduced BM cells (1 × 106) were transplanted into sublethally irradiated CD45.1 WT recipients. Reconstitution of recipients was assessed by flow cytometry at 12 or 16 weeks after transplantation.
Transfer of T and B cells
PBS control or 1 × 106 T cells or 1 × 106 B cells from CD45.1 mouse were transferred into CD45.2 Rag1−/− mice. The recipients were assessed by flow cytometry at 8 weeks after transplantation.
NK cell depletion
To deplete NK cells, mice received intraperitoneal injections of 50 μg of anti-NK1.1 antibody (clone PK136) three times a week for 8 weeks.
Gene-expression analysis
In Fig. 2A (left), three gene sets involving the regulation of immune response signal (GeneOntology database, GO0050777), transmembrane (UniProt database, KW-0812), and signal (UniProt database, KW-0732) were overlapped. Cytokine receptor–related genes were excluded for further analysis. The RNA-seq dataset of ILC2s within different tissues in Fig. 2A (middle) and fig. S6A was obtained from the GEO database [GSE117470; (47)] and processed using R. Only the RNA-seq dataset of WT mice (GSM3301507 to GSM3301509, GSM3301513 to GSM3301518, GSM3301523 to GSM3301528, GSM3301532 to GSM3301536, and GSM3301541 to GSM3301546) was used. In Fig. 2A (right), RNA-seq data from the GEO database [GSE184841; (65)] regarding naïve mesentery ILC2s (GSM5598070 and GSM5598071), spleen CD4+ T cells (GSM5598074 and GSM5598075), spleen CD8+T cells (GSM5598076 and GSM5598077), and spleen B cells (GSM5598078 and GSM5598079) were used.
Statistical analyses
Prism 8 software was used for performing one-way analysis of variance (ANOVA), unpaired two-tailed Student’s t tests, and linear regression analyses. P values were calculated using two-tailed Student’s t tests when comparing one group and one-way ANOVA when comparing more than one group. All data are presented as means ± SEM. Differences with a P value below 0.05 were considered statistically significant.
Acknowledgments
Funding: The research presented in this publication was financially supported by the Natural Science Foundation of China (to Z.D., grant numbers 32330034 and 31821003; to S.C., grant number 82371734; and to D.L., grant number 82103327), the National Key Research and Developmental Program of China (grant number 2022YFF0710602), Anhui Provincial Department of Education (grant number 2023AH010085 to Z.D. and grant number 2022AH030114 to S.C.), and Tsinghua University Initiative Scientific Research Program of Precision Medicine (2023ZLA002).
Authors contributions: Writing—original draft: Z.D., S.C., and Y.W. Conceptualization: Z.D., S.C., D.L., and Y.W. Investigation: S.C., D.L., and Y.W. Writing—review and editing: Z.D., S.C., D.L., and Y.W. Methodology: Z.D., Y.L., D.L., and Y.W. Resources: Z.D., Y.L., S.C., and Y.W. Data curation: Y.W. Funding acquisition: Z.D. and S.C. Validation: Z.D., S.C., D.L., and Y.W. Supervision: Z.D. and S.C. Formal analysis: Y.W. Project administration: Z.D., S.C., and Y.W. Visualization: Z.D., S.C., D.L., and Y.W.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The previously published RNA-seq data of GSE117470 (47) (specifically GSM3301507 to GSM3301509, GSM3301513 to GSM3301518, GSM3301523 to GSM3301528, GSM3301532 to GSM3301536, and GSM3301541 to GSM3301546; www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE117470) and GSE131996 (65) (specifically GSM5598070, GSM5598071, GSM5598074, GSM5598075, GSM5598076, GSM5598077, GSM5598078, and GSM5598079; www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE131996) from Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) database were used here. The remaining data are available within the article. This study did not generate new unique reagents.
Supplementary Materials
This PDF file includes:
Figs. S1 to S8
REFERENCES AND NOTES
- 1.Ghaedi M., Takei F., Innate lymphoid cell development. J. Allergy Clin. Immunol. 147, 1549–1560 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Yang Q., Li F., Harly C., Xing S., Ye L., Xia X., Wang H., Wang X., Yu S., Zhou X., Cam M., Xue H.-H., Bhandoola A., TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat. Immunol. 16, 1044–1050 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Constantinides M. G., McDonald B. D., Verhoef P. A., Bendelac A., A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zook E. C., Kee B. L., Development of innate lymphoid cells. Nat. Immunol. 17, 775–782 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Serafini N., Vosshenrich C. A. J., Di Santo J. P., Transcriptional regulation of innate lymphoid cell fate. Nat. Rev. Immunol. 15, 415–428 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Kabata H., Moro K., Koyasu S., The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 286, 37–52 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Rana B. M. J., Jou E., Barlow J. L., Rodriguez-Rodriguez N., Walker J. A., Knox C., Jolin H. E., Hardman C. S., Sivasubramaniam M., Szeto A., Cohen E. S., Scott I. C., Sleeman M. A., Chidomere C. I., Cruz Migoni S., Caamano J., Jorgensen H. F., Carobbio S., Vidal-Puig A., McKenzie A. N. J., A stromal cell niche sustains ILC2-mediated type-2 conditioning in adipose tissue. J. Exp. Med. 216, 1999–2009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maazi H., Patel N., Sankaranarayanan I., Suzuki Y., Rigas D., Soroosh P., Freeman G. J., Sharpe A. H., Akbari O., ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 42, 538–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salimi M., Xue L., Jolin H., Hardman C., Cousins D. J., McKenzie A. N., Ogg G. S., Group 2 innate lymphoid cells express functional NKp30 receptor inducing type 2 cytokine production. J. Immunol. 196, 45–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trabanelli S., Chevalier M. F., Martinez-Usatorre A., Gomez-Cadena A., Salome B., Lecciso M., Salvestrini V., Verdeil G., Racle J., Papayannidis C., Morita H., Pizzitola I., Grandclement C., Bohner P., Bruni E., Girotra M., Pallavi R., Falvo P., Leibundgut E. O., Baerlocher G. M., Carlo-Stella C., Taurino D., Santoro A., Spinelli O., Rambaldi A., Giarin E., Basso G., Tresoldi C., Ciceri F., Gfeller D., Akdis C. A., Mazzarella L., Minucci S., Pelicci P. G., Marcenaro E., McKenzie A. N. J., Vanhecke D., Coukos G., Mavilio D., Curti A., Derre L., Jandus C., Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 8, 593 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salimi M., Barlow J. L., Saunders S. P., Xue L., Gutowska-Owsiak D., Wang X., Huang L.-C., Johnson D., Scanlon S. T., McKenzie A. N. J., Fallon P. G., Ogg G. S., A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 210, 2939–2950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor S., Huang Y., Mallett G., Stathopoulou C., Felizardo T. C., Sun M.-A., Martin E. L., Zhu N., Woodward E. L., Elias M. S., Scott J., Reynolds N. J., Paul W. E., Fowler D. H., Amarnath S., PD-1 regulates KLRG1+ group 2 innate lymphoid cells. J. Exp. Med. 214, 1663–1678 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma C. S., Nichols K. E., Tangye S. G., Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu. Rev. Immunol. 25, 337–379 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Cannons J. L., Tangye S. G., Schwartzberg P. L., SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 29, 665–705 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Chen S., Yang M., Du J., Li D., Li Z., Cai C., Ma Y., Zhang L., Tian Z., Dong Z., The self-specific activation receptor SLAM family Is critical for NK cell education. Immunity 45, 292–304 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Dong Z., Davidson D., Perez-Quintero L. A., Kurosaki T., Swat W., Veillette A., The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity 36, 974–985 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Dong Z., Cruz-Munoz M.-E., Zhong M.-C., Chen R., Latour S., Veillette A., Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat. Immunol. 10, 973–980 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Bendelac A., Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182, 2091–2096 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei D. G., Lee H., Park S.-H., Beaudoin L., Teyton L., Lehuen A., Bendelac A., Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J. Exp. Med. 202, 239–248 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S., Cai C., Li Z., Liu G., Wang Y., Blonska M., Li D., Du J., Lin X., Yang M., Dong Z., Dissection of SAP-dependent and SAP-independent SLAM family signaling in NKT cell development and humoral immunity. J. Exp. Med. 214, 475–489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannons J. L., Yu L. J., Hill B., Mijares L. A., Dombroski D., Nichols K. E., Antonellis A., Koretzky G. A., Gardner K., Schwartzberg P. L., SAP regulates TH2 differentiation and PKC-θ-mediated activation of NF-kappaB1. Immunity 21, 693–706 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Davidson D., Shi X., Zhang S., Wang H., Nemer M., Ono N., Ohno S., Yanagi Y., Veillette A., Genetic evidence linking SAP, the X-linked lymphoproliferative gene product, to Src-related kinase FynT in TH2 cytokine regulation. Immunity 21, 707–717 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Wu C., Nguyen K. B., Pien G. C., Wang N., Gullo C., Howie D., Sosa M. R., Edwards M. J., Borrow P., Satoskar A. R., Sharpe A. H., Biron C. A., Terhorst C., SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat. Immunol. 2, 410–414 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Rigaud S., Fondaneche M.-C., Lambert N., Pasquier B., Mateo V., Soulas P., Galicier L., Le Deist F., Rieux-Laucat F., Revy P., Fischer A., de Saint Basile G., Latour S., XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 444, 110–114 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Dong Z., Veillette A., How do SAP family deficiencies compromise immunity? Trends Immunol. 31, 295–302 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Li D., Xiong W., Wang Y., Feng J., He Y., Du J., Wang J., Yang M., Zeng H., Yang Y.-G., Wu N., Chen S., Dong Z., SLAMF3 and SLAMF4 are immune checkpoints that constrain macrophage phagocytosis of hematopoietic tumors. Sci. Immunol. 7, eabj5501 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Meininger I., Carrasco A., Rao A., Soini T., Kokkinou E., Mjösberg J., Tissue-specific features of innate lymphoid cells. Trends Immunol. 41, 902–917 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Karo J. M., Schatz D. G., Sun J. C., The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell 159, 94–107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halim T. Y. F., Rana B. M. J., Walker J. A., Kerscher B., Knolle M. D., Jolin H. E., Serrao E. M., Haim-Vilmovsky L., Teichmann S. A., Rodewald H.-R., Botto M., Vyse T. J., Fallon P. G., Li Z., Withers D. R., McKenzie A. N. J., Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity 48, 1195–1207.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K.-M., McNerney M. E., Stepp S. E., Mathew P. A., Schatzle J. D., Bennett M., Kumar V., 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J. Exp. Med. 199, 1245–1254 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson L. A., Vaidya S. V., Goldfarb R. H., Mathew P. A., 2B4(CD244)-mediated activation of NK cells reduces metastases of B16F10 melanoma in mice. Anticancer Res 23, 3651–3655 (2003). [PubMed] [Google Scholar]
- 32.Cruz-Munoz M.-E., Dong Z., Shi X., Zhang S., Veillette A., Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat. Immunol. 10, 297–305 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Chen S., Li D., Wang Y., Li Q., Dong Z., Regulation of MHC class I-independent NK cell education by SLAM family receptors. Adv. Immunol. 145, 159–185 (2020). [DOI] [PubMed] [Google Scholar]
- 34.McArdel S. L., Terhorst C., Sharpe A. H., Roles of CD48 in regulating immunity and tolerance. Clin. Immunol. 164, 10–20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tufa D. M., Yingst A. M., Trahan G. D., Shank T., Jones D., Shim S., Lake J., Winkler K., Cobb L., Woods R., Jones K., Verneris M. R., Human innate lymphoid cell precursors express CD48 that modulates ILC differentiation through 2B4 signaling. Sci. Immunol. 5, eaay4218 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao W., Lin J.-X., Leonard W. J., Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., Furusawa J.-i., Ohtani M., Fujii H., Koyasu S., Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Takami D., Abe S., Shimba A., Asahi T., Cui G., Tani-Ichi S., Hara T., Miyata K., Ikutani M., Takatsu K., Oike Y., Ikuta K., Lung group 2 innate lymphoid cells differentially depend on local IL-7 for their distribution, activation, and maintenance in innate and adaptive immunity-mediated airway inflammation. Int. Immunol. 35, 513–530 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Robinette M. L., Bando J. K., Song W., Ulland T. K., Gilfillan S., Colonna M., IL-15 sustains IL-7R-independent ILC2 and ILC3 development. Nat. Commun. 8, 14601 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi L., Bian Z., Kidder K., Liang H., Liu Y., Non-lyn Src family kinases activate SIRPalpha-SHP-1 to inhibit PI3K-Akt2 and dampen proinflammatory macrophage polarization. J. Immunol. 207, 1419–1427 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raymond A.-A., Javary J., Breig O., Neaud V., Rosenbaum J., Reptin regulates insulin-stimulated Akt phosphorylation in hepatocellular carcinoma via the regulation of SHP-1/PTPN6. Cell Biochem. Funct. 35, 289–295 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Helou D. G., Shafiei-Jahani P., Hurrell B. P., Painter J. D., Quach C., Howard E., Akbari O., LAIR-1 acts as an immune checkpoint on activated ILC2s and regulates the induction of airway hyperreactivity. J. Allergy Clin. Immunol. 149, 223–236.e6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winer H., Rodrigues G. O. L., Hixon J. A., Aiello F. B., Hsu T. C., Wachter B. T., Li W., Durum S. K., IL-7: Comprehensive review. Cytokine 160, 156049 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Jiang Q., Li W. Q., Aiello F. B., Mazzucchelli R., Asefa B., Khaled A. R., Durum S. K., Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 16, 513–533 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Cao Y., Wen H., Leng C., Feng S., MiR-29a mediates the apoptotic effects of TNF-alpha on endothelial cells through inhibiting PI3K/AKT/BCL-2 axis. J. Biochem. Mol. Toxicol. 38, e23598 (2024). [DOI] [PubMed] [Google Scholar]
- 46.Sheikh A., Lu J., Melese E., Seo J. H., Abraham N., IL-7 induces type 2 cytokine response in lung ILC2s and regulates GATA3 and CD25 expression. J. Leukoc. Biol. 112, 1105–1113 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricardo-Gonzalez R. R., Van Dyken S. J., Schneider C., Lee J., Nussbaum J. C., Liang H.-E., Vaka D., Eckalbar W. L., Molofsky A. B., Erle D. J., Locksley R. M., Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 19, 1093–1099 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin C. E., Spasova D. S., Frimpong-Boateng K., Kim H.-O., Lee M., Kim K. S., Surh C. D., Interleukin-7 availability is maintained by a hematopoietic cytokine sink comprising innate lymphoid cells and T cells. Immunity 47, 171–182.e4 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Bonne-Annee S., Bush M. C., Nutman T. B., Differential modulation of human innate lymphoid cell (ILC) subsets by IL-10 and TGF-β. Sci. Rep. 9, 14305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L., Tang J., Yang X., Zanvit P., Cui K., Ku W. L., Jin W., Zhang D., Goldberg N., Cain A., Ni B., Zhao K., Wu Y., Chen W., TGF-β induces ST2 and programs ILC2 development. Nat. Commun. 11, 35 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duerr C. U., McCarthy C. D. A., Mindt B. C., Rubio M., Meli A. P., Pothlichet J., Eva M. M., Gauchat J.-F., Qureshi S. T., Mazer B. D., Mossman K. L., Malo D., Gamero A. M., Vidal S. M., King I. L., Sarfati M., Fritz J. H., Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat. Immunol. 17, 65–75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moro K., Kabata H., Tanabe M., Koga S., Takeno N., Mochizuki M., Fukunaga K., Asano K., Betsuyaku T., Koyasu S., Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat. Immunol. 17, 76–86 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Cautivo K. M., Matatia P. R., Lizama C. O., Mroz N. M., Dahlgren M. W., Yu X., Sbierski-Kind J., Taruselli M. T., Brooks J. F., Wade-Vallance A., Caryotakis S. E., Chang A. A., Liang H.-E., Zikherman J., Locksley R. M., Molofsky A. B., Interferon gamma constrains type 2 lymphocyte niche boundaries during mixed inflammation. Immunity 55, 254–271.e7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korn L. L., Thomas H. L., Hubbeling H. G., Spencer S. P., Sinha R., Simkins H. M. A., Salzman N. H., Bushman F. D., Laufer T. M., Conventional CD4+ T cells regulate IL-22-producing intestinal innate lymphoid cells. Mucosal Immunol. 7, 1045–1057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui G., Shimba A., Jin J., Hojo N., Asahi T., Abe S., Ejima A., Okada S., Ohira K., Kato R., Tani-Ichi S., Yamada R., Ebihara T., Shiroguchi K., Ikuta K., CD45 alleviates airway inflammation and lung fibrosis by limiting expansion and activation of ILC2s. Proc. Natl. Acad. Sci. U.S.A. 120, e2215941120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahlgren M. W., Jones S. W., Cautivo K. M., Dubinin A., Ortiz-Carpena J. F., Farhat S., Yu K. S., Lee K., Wang C., Molofsky A. V., Tward A. D., Krummel M. F., Peng T., Molofsky A. B., Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity 50, 707–722.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klose C. S. N., Mahlakoiv T., Moeller J. B., Rankin L. C., Flamar A.-L., Kabata H., Monticelli L. A., Moriyama S., Putzel G. G., Rakhilin N., Shen X., Kostenis E., Konig G. M., Senda T., Carpenter D., Farber D. L., Artis D., The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardoso V., Chesne J., Ribeiro H., Garcia-Cassani B., Carvalho T., Bouchery T., Shah K., Barbosa-Morais N. L., Harris N., Veiga-Fernandes H., Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plas D. R., Johnson R., Pingel J. T., Matthews R. J., Dalton M., Roy G., Chan A. C., Thomas M. L., Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science 272, 1173–1176 (1996). [DOI] [PubMed] [Google Scholar]
- 60.Chiang G. G., Sefton B. M., Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J. Biol. Chem. 276, 23173–23178 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Cuevas B., Lu Y., Watt S., Kumar R., Zhang J., Siminovitch K. A., Mills G. B., SHP-1 regulates Lck-induced phosphatidylinositol 3-kinase phosphorylation and activity. J. Biol. Chem. 274, 27583–27589 (1999). [DOI] [PubMed] [Google Scholar]
- 62.Stebbins C. C., Watzl C., Billadeau D. D., Leibson P. J., Burshtyn D. N., Long E. O., Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol. Cell. Biol. 23, 6291–6299 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lorenz U., SHP-1 and SHP-2 in T cells: Two phosphatases functioning at many levels. Immunol. Rev. 228, 342–359 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y., Quan Y., He J., Chen S., Dong Z., SLAM-family receptors promote resolution of ILC2-mediated inflammation. Nat. Commun. 15, 5056 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hikichi Y., Motomura Y., Takeuchi O., Moro K., Posttranscriptional regulation of ILC2 homeostatic function via tristetraprolin. J. Exp. Med. 218, e20210181 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S8