Abstract
Niclofolan is a commonly used veterinary drug worldwide. We reported a case of acute niclofolan intoxication in a pregnant woman. We recorded in detail her toxic symptoms, including nausea, vomiting, asthenia, blurred vision, and aberrant blood values. Notably stillbirth was recorded for the first time as indicating its human reproductive toxicity. We also found that an oral therapeutic dosage as described in the literature was likely to lead to stillbirth. Furthermore, we described the treatments received by the patient, including critical care, drug therapy, and plasma exchange, the outcomes of which were excellent. By studying this case, we aimed to enhance recognition and treatment of niclofolan intoxication and to raise concern regarding veterinary drug abuse and exposure risk.
Keywords: niclofolan intoxication, reproductive toxicity, intensive healthcare, case report
Introduction
Niclofolan, a yellow, water-insoluble, small molecule compound,1 also known as Bayer 9015 or Bilevon, is a commonly used veterinary drug worldwide. Its mechanism of action may involve inhibiting the activity of succinate dehydrogenase within the worm, thereby affecting its metabolic process and exhausting its energy supply. In recent decades, it was also used to treat metagonimiasis,2 clonorchis sinensis,3–5 paragonimiasis,6–9 and fascioliasis10–13 in humans, and many adverse effects were observed. Nowadays, it is rarely used in humans, but accidental poisoning cases are still reported frequently. Succinate dehydrogenase plays a crucial role in aerobic glycolysis and the tricarboxylic acid cycle in the human body, and niclofolan may also act on the cycle and inhibit mitochondrial energy metabolism, leading to pathological changes in metabolically active and liposoluble organs such as the nervous system, heart, liver, kidneys, muscles, and eyes. Recent research using optical coherence tomography revealed that niclofolan might harm the retina by inhibiting mitochondrial energy metabolism.14 Worldwide, studies of both its therapeutic use and accidental intake reveal that toxic symptoms mainly include gastrointestinal symptoms,4,5,9,10 nervous system symptoms,4–6,9,10 sweating,6,9,10 bodily pain,4–6,9,10 rash,9 cholestatic jaundice,12 aberrant blood values,9,10 optic symptoms,5,9,14 and in some cases of accidental ingestion, even fatal outcomes.15 As far as we know, its reproductive toxicity has only been reported in animals.10,16 Here, we describe the case of a pregnant woman who accidentally ingested niclofolan, which caused multiple toxic symptoms, as described above, notably including stillbirth. It is the first report of human reproductive toxicity. We recorded in detail the patient’s toxic symptoms and discussed dosage. In addition, we described the treatments she received and their excellent outcome. Furthermore, we aimed to raise concern regarding veterinary drug abuse and its exposure risk.
Case Presentation
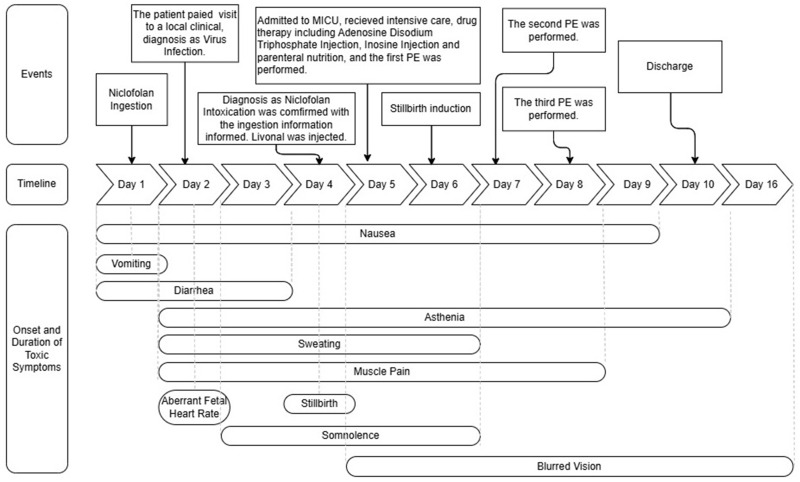
A 27-year-old local pregnant woman who was 25 weeks plus 1 day pregnant presented to the hospital with a confirmed diagnosis of niclofolan intoxication. Three days previously, the patient had mistakenly taken approximately 200 tablets of niclofolan (100 mg/tablet). The patient was admitted to the medical intensive care unit (MICU) immediately. Treatments undertaken during the hospitalization mainly included critical care, drug therapy, plasma exchange (PE), and stillbirth induction. On day 10 after ingestion our patient was discharged and her outcome was excellent. Three follow-up visits, one month apart, showed that no new symptoms occurred. The patient was in good health before ingesting niclofolan, but about 20 minutes after ingesting it developed symptoms including nausea, vomiting, diarrhea, asthenia, somnolence, blurred vision, aberrant fetal heart rate and, finally, stillbirth. A detailed timeline including the patient’s toxic symptoms, diagnosis, and treatment is shown in Figure 1. Aberrant blood values were also recorded, as shown in Table 1.
Figure 1.
Case Timeline.
Table 1.
Laboratory Values After Niclofolan Ingestion
| Day 2 | Day 4 | Day 5 | Day 6 | Day 8 | Day 10 | Day 16 | |
|---|---|---|---|---|---|---|---|
| WBC(10^9/L) | 13.84 | 12.38 | 17.87 | 10.03 | 8.41 | 7.42 | |
| Neu(10^9/L) | 9.66 | 15.96 | 7.54 | 5.57 | 4.96 | ||
| AST(U/L) | 591 | 657 | 242 | 133 | 96 | 27 | |
| ALT(U/L) | 94 | 89 | 39 | 32 | 39 | 23 | |
| CK(U/L) | 5653 | 3571 | 1270 | 615 | |||
| CK-MB(U/L) | 190 | 81 | 34 | 19 | |||
| α-HBDH(U/L) | 489 | 281 | 200 | 198 |
Notes: Laboratory values after niclofolan ingestion: white cell count (WBC, 10^9/L; normal range 3.5–9.5); neutrophil (Neu, 10^9/L; normal range 1.8–6.3); aspartate aminotransferase (AST, U/L; normal range 13–35); alanine aminotransferase (ALT, U/L; normal range 7–40); creatine kinas (CK, U/L; normal range 20–195); creatine kinase, MB form (CK-MB, U/L; normal range <25); α-Hydroxybutyrate Dehydrogenase (α-HBDH, U/L; normal range 72–182).
Discussion
Part 1: Toxicity Effects
The toxic symptoms of our patient were similar to those described in relevant literature. (1) Niclofolan directly stimulates toxic gastrointestinal reactions and can cause persistent gastrointestinal toxic symptoms. Gastrointestinal toxic symptoms have been observed before, including nausea4,9,10 and vomiting.5 Our patient, who had not experienced vomiting during this pregnancy, about 20 minutes after ingestion quickly developed nausea and spontaneous vomiting, as the earliest toxic symptoms, followed by diarrhea for 3 days. (2) Niclofolan’s lipid solubility leads to its significant neurotoxicity, including symptoms like severe headache,4,5 dizziness,5,9 nausea,4,9 and vomiting.5 In our patient, nausea and asthenia were observed. Nausea began on day 1 of the accidental ingestion and persisted until day 9; asthenia occurred on day 2 and became increasingly evident and lasted during the whole hospitalization period. (3) Sweating6,9,10 and muscle and joint pain4,5,9,10 are widely mentioned in the literature. One study reported that, after using niclofolan, all 26 individuals involved experienced sweating and 22 complained of muscle pain, making these the most common adverse reactions.9 On day 2, our patient developed sweating and muscle pain, which gradually disappeared during the hospitalization period. (4) Optic toxic symptoms have often been recorded as more severe, such as blurred vision, papilledema, and retinal damage.5,9,14 In our patient, blurred vision occurred on day 5 and did not return to normal until day 16; this was the longest lasting of the toxic symptoms. (5) Aberrant blood values, as reported in previous cases,9,10 were also present in our case, including an increased white cell count, increased myocardial enzymes and increased liver enzymes; these counts gradually returned to normal. These effects indicated that the drug could have toxic effects on the patient’s liver and heart. However, it was not clear in this case whether the hematological aberration was related to niclofolan or the recent stillborn, or both.
Niclofolan is teratogenic and embryotoxic in small animals such as rats,10,16 however, its human reproductive toxicity has not been reported before. Our patient was a 27-year-old gravida 2, para1 female at 25 weeks plus 1 day of pregnancy, with no history of miscarriage or any previous abnormal history of pregnancy. She had previously given birth to a healthy child who grew up well and is now 4 years of age. The patient had no significant underlying diseases or a family history of major genetic diseases. During this pregnancy, the patient had received regular prenatal examinations including physical, blood and ultrasound tests, all of which suggested the fetus had been developing well. Throughout this pregnancy, the patient did not experience any discomfort like abdominal pain, vaginal bleeding, or abnormal vaginal discharge. The patient denied any toxic or radiational contact history before this ingestion. On this occasion, after eight hours of the accidental ingestion, an ultrasound showed the aberrant fetal heart rate. And on day 4, stillbirth occurred. The patient was diagnosed with niclofolan intoxication, and we believed that there was a clear and strong causal relationship between the niclofolan ingestion and stillbirth, indicating its human reproductive toxicity. No deformities were detected in the appearance of the fetus. The membranes and placenta were incomplete, while the placental pathology examination revealed acute chorioamnionitis and necrosis of the umbilical vessels, suggesting that niclofolan might have toxic effects on the placenta. Nevertheless, we cannot rule out the possibility that a Livanol injection or other factors might have contributed to these findings. The reproductive toxic effects could be the result of niclofolan’s metabolic process-blocking mechanism and exhausting of the energy supply to the placenta and fetus, leading to placenta damage and stillbirth. However, it remains unclear if niclofolan directly crossed the placenta to affect the fetus or caused placental damage that affected the fetus indirectly, or whether both mechanisms were involved. Furthermore, an aberrant fetal heart rate developed during the first 2 days, and stillbirth occurred on day 4 in this case, suggesting its quick human reproductive toxicity.
Part 2: Toxicity Dosage
In the relevant literature, therapeutic dosages of niclofolan in humans ranged from 2 mg/kg body weight to 11 mg/kg body weight in total, and adverse reactions were widely observed. One report described niclofolan as having a therapeutic effect on Clonorchis infection, and side effects were observed in most cases,17 which suggested its low safety margin. There appeared to be a correlation between adverse symptoms and drug dosages. It mentioned that niclofolan given orally in a single dose of 2 mg/kg body weight showed mild and transient side effects.8 Another report mentioned that mild and transient side effects were recorded in all groups but more severe symptoms were observed in the higher dosage group.4 Another report mentioned that severe visual impairments were observed only in the higher dosage group; for example, blurred vision, papilledema, retinitis, and a case of permanent vision loss were observed in a group receiving a total dosage of 6 mg/kg body weight.9 Additionally, a case involving a total dosage of 11 mg/kg body weight reported severe adverse effects, including blurred vision, papilledema, and some symptoms such as vomiting and sweating, which lasted for 4 months.5 Furthermore, a recent report described three children, 4–6 years of age, who died within one week of accidental niclofolan poisoning of total dosages ranging from 400–1000 mg.15
Following oral administration, niclofolan reached peak blood concentration after an average of 33 hours in sheep18 and 24–48 hours in humans.9 Our patient’s vomiting of yellow gastric substances occurred 20 minutes after accidental ingestion, therefore the exact dosage absorbed could not be determined. Although our patient had taken 200 tablets, at a total dose of 20000 mg, given that the patient’s symptoms were similar to the therapeutic cases reported and she recovered quickly, it was hypothesized that most of the ingested drug was expelled through vomiting and very little was absorbed. Consequently, it could not be clear which dosage led to stillbirth in this case. However, the therapeutic dosages of niclofolan described in the relevant literature caused numerous adverse effects that were similar to our patient’s symptoms, so it could be inferred that an oral therapeutic dosage of niclofolan could potentially lead to stillbirth. Meanwhile, studies have shown that niclofolan has been found in soil19 and used in animal-based food production systems,20 so we express concern regarding whether it could affect the human fetus following exposure to a even a tiny dose. This issue requires more attention and further research.
Part 3: Intensive Treatment
The intensive treatments detailed in Figure 1 resulted in the rapid recovery of our patient and an excellent outcome. The main points emphasized were as follows. (1) The patient’s spontaneous vomiting soon after ingestion, which led to minimal absorption, suggest that methods like immediate emesis induction, gastric lavage, and catharsis, performed as soon as possible to minimize toxin absorption, were crucial. (2) During hospitalization, drugs including adenosine disodium triphosphate injection, inosine injection, and parenteral nutrition were administered to enhance energy supply and minimize organ damage. (3) Niclofolan is known to be insoluble in water, making it impervious to removal via hemodialysis, while plasma exchange (PE) directly removes the toxin-containing plasma and replaces it with fresh frozen plasma, offering more efficiency. In our patient, PE was performed three times, with demonstrable improvements in symptoms and blood values after each treatment, as shown in Figure 1 and Table 1.
Conclusions
We described a case of acute niclofolan intoxication in a pregnant woman. We recorded in detail her symptoms, including stillbirth, indicating its human reproductive toxicity. Additionally, we discussed the dosages and toxic symptoms described in the literature alongside those of our patient and concluded that therapeutic dosages in the former probably led to stillbirth. We also described treatments including critical care, drug therapy, and PE, the outcomes of which were excellent.
This case report served to enhance recognition and treatment of niclofolan intoxication in humans, and to fill a gap in the record of its human reproductive toxicity. It also underscored the need for governments to implement stricter control of veterinary drugs, thereby reducing the incidence of human poisoning, and served to raise concern about their exposure risks.
Take-Away Lessons
As niclofolan is widely used as a veterinary drug worldwide, when encountering patients from areas where the drug can be easily obtained, with symptoms as mentioned in our report, we should be highly aware of drug intoxication.
Proper treatment for niclofolan intoxication should be initiated as soon as possible to minimize its damage to organs.
More attention must be paid to veterinary drug administration by the public and the government regarding its abuse and possible exposure risk, especially in terms of its reproductive toxicity.
Patient’s Perspective
I took the pills accidentally, and minutes later I experienced symptoms like nausea, vomiting, and diarrhea. Then I went to see a doctor without revealing this ingestion until the stillbirth was found. I never imagined it could cause such a loss. Then I was admitted to MICU and received good care. With the ongoing treatments I felt my condition improved quickly then I met my hospital discharge very soon. In my hometown (the pastoral area), we can buy niclofolan freely as a veterinary drug, and we are used to storing it at home. Now I realize how dangerous it is.
Acknowledgments
The authors thank Fei Wang for his valuable assistance with revisions.
Funding Statement
This work was supported by the Health Commission of the Chengdu Research Fund (grant no. 2023292), and partly by the GENERTEC MEDICAL Research Fund (grant no. TYYLKYJJ-2023-017).
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Ethics Approval and Informed Consent
The ethical committee of the institution, the Sichuan Provincial Women's and Children’s Hospital/The Affiliated Women’s and Children’s Hospital of Chengdu Medical College, has confirmed that no ethical approval is required. Written informed consent has been obtained from the patient to publish this article.
Disclosure
The authors declare no conflicts of interest.
References
- 1.National Center for Biotechnology Information. PubChem compound summary for CID 5284586, niclofolan. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Niclofolan. Accessed July 30, 2024.
- 2.Rim HJ, Chu DS, Lee JS, Joo KH, Won CY. Anthelmintic effects of various drugs against metagonimiasis. Kisaengchunghak Chapchi. 1978;16(2):117–122. doi: 10.3347/kjp.1978.16.2.117 [DOI] [PubMed] [Google Scholar]
- 3.Ambroise-Thomas P, Wegner DH, Goullier A, Desgeorges PT, Billiault X. Essais de traitement des opistorchiases humaines par le niclofolan (Bay 9015). A propos de 51 cas [Clinical trial of niclofolan (Bay 9015) in the treatment of human opisthorchiasis. A propos of 51 cases]. Bull Soc Pathol Exot Filiales. 1980;73(3):293–301. [PubMed] [Google Scholar]
- 4.Bunnag D, Harinasuta T, Desakorn V. Studies on the chemotherapy of human opisthorchiasis: II. Clinical trial of niclofolan. Southeast Asian J Trop Med Public Health. 1981;12(1):107–109. [PubMed] [Google Scholar]
- 5.Hong ST, Lee SH, Ahn HS, Yun CK. A case of niclofolan (Bilevon(R)) intoxication. Kisaengchunghak Chapchi. 1982;20(1):49–52. doi: 10.3347/kjp.1982.20.1.49 [DOI] [PubMed] [Google Scholar]
- 6.Nwokolo C, Volkmer KJ. Single dose therapy of paragonimiasis with menichlopholan. Am J Trop Med Hyg. 1977;26(4):688–692. doi: 10.4269/ajtmh.1977.26.688 [DOI] [PubMed] [Google Scholar]
- 7.Fauveau V. Pathologie des migrants: la paragonimose (ou distomatose pulmonaire) [Pathology of migrants: paragonimiasis (or pulmonary distomatosis)]. Bull Soc Pathol Exot Filiales. 1981;74(1):84–91. [PubMed] [Google Scholar]
- 8.Ripert C, Carrie J, Ambroise-Thomas P, Baecher R, Kum NP, Same-Ekobo A. Etude épidémiologique et clinique de la paragonimose au Cameroun. Résultats du traitement par le niclofolan [Epidemiologic and clinical study of paragonimosis in Cameroon. Results of niclofolan treatment]. Bull Soc Pathol Exot Filiales. 1981;74(3):319–331. [PubMed] [Google Scholar]
- 9.Cao WJ. [Preliminary observation on the treatment of paragonimiasis westermani (thoracic type) with domestic niclofolan in 26 cases (author’s transl)]. Zhonghua Nei Ke Za Zhi. 1980;19(3):226–229. [PubMed] [Google Scholar]
- 10.Eckhardt T, Heckers H. Treatment of human fascioliasis with niclofolan. Gastroenterology. 1981;81(4):795–798. doi: 10.1016/0016-5085(81)90510-2 [DOI] [PubMed] [Google Scholar]
- 11.Kum PN, Nchinda TC. Pulmonary paragonimiasis in Cameroon. Trans R Soc Trop Med Hyg. 1982;76(6):768–772. doi: 10.1016/0035-9203(82)90102-x [DOI] [PubMed] [Google Scholar]
- 12.Reshef R, Lok AS, Sherlock S. Cholestatic jaundice in fascioliasis treated with niclofolan. Br Med J. 1982;285(6350):1243–1244. doi: 10.1136/bmj.285.6350.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanijanonta S, Bunnag D, Harinasuta T. Paragonimus heterotremus and other Paragonimus spp. in Thailand: pathogenesis, clinic and treatment. Arzneimittelforschung. 1984;34(9B):1186–1188. [PubMed] [Google Scholar]
- 14.Guan WX, Zhao XF, Yu WH, Peng XY. OPTICAL COHERENCE TOMOGRAPHY FINDINGS IN PRESUMED VETERINARY ANTHELMINTIC DRUG-INDUCED RETINAL TOXICITY: a Glimpse into Underlying Mechanism. Retina. 2024;44(8):1456–1462. doi: 10.1097/IAE.0000000000004128 [DOI] [PubMed] [Google Scholar]
- 15.Zhangyan G, Yipei L, Yi W. Three children who died from accidental nitrochlorophenol poisoning. Chin J Prim Med Pharm. 2023;30(10):1567–1569. doi: 10.3760/cma.j.cn341190-20221124-00951 [DOI] [Google Scholar]
- 16.Juszkiewicz T, Rakalska Z, Dzierzawski A. Effet embryopathique du 3,3’-dichloro-5,5’-dinitro-0,0’-biphénol (Bayer 9015) chez le hamster doré [Embryopathic effect of 3,3’-dichloro-5,5’-dinitro-0,0’-biphenol (Bayer 9015) in the golden hamster]. Eur J Toxicol. 1971;4(6):525–528. [PubMed] [Google Scholar]
- 17.Rim HJ. Therapy of fluke infections in the past. Rev Arzneimittelforschung. 1984;34(9B):1127–1129. [PubMed] [Google Scholar]
- 18.Ali BH, El Sheikh HA, McKellar QA. Pharmacokinetics of niclofolan in desert sheep. J Vet Pharmacol Ther. 1990;13(2):217–219. doi: 10.1111/j.1365-2885.1990.tb00771.x [DOI] [PubMed] [Google Scholar]
- 19.Chiaia-Hernández AC, Scheringer M, Müller A, et al. Target and suspect screening analysis reveals persistent emerging organic contaminants in soils and sediments. Sci Total Environ. 2020;740:140181. doi: 10.1016/j.scitotenv.2020.140181 [DOI] [PubMed] [Google Scholar]
- 20.Tuck S, Furey A, Danaher M. Analysis of anthelmintic and anticoccidial drug residues in animal-derived foods. Chem Anal Non-Antimicrob Vet Drug Residues Food. 2016;2016: 245–309. doi: 10.1002/9781118696781.ch5 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.