Abstract
Imidazo based heterocyclic derivatives are considered as privileged scaffolds due to their presence in various pharmacologically active compounds and in marketed formulations. The present study reports toxicological evaluation of three imidazo based heterocyclic derivatives which are currently being investigated for their potential anticancer activity. Compounds IG-01–007, IG-01–008, and IG-01–009 were assessed for cytotoxicity, hemolysis, and DNA fragmentation activity. Acute oral toxicity studies were performed at doses of 300 mg/kg and 1000 mg/kg according to OECD guidelines, in both male and female Wistar rats. All test compounds at a concentration of 50 µM resulted in DNA fragmentation suggesting notable impact on DNA integrity. The in-vivo acute toxicity study indicated significant toxicity at doses of ≥ 1000 mg/kg, particularly for compounds IG-01–008 and IG-01–009, which caused hepatic damage and cholestasis in liver tissues. These results collectively suggest that imidazo based heterocyclic derivatives used in the present study exhibit cytotoxic potential.
Keywords: Toxicological evaluation, Cytotoxicity, OECD, Anticancer, Imidazo-pyrazine, Imidazo-pyridine, Heterocycles
Graphical Abstract
Highlights
-
•
Toxicological screening of new lead molecules is crucial in drug discovery to assess potential adverse effects.
-
•
Imidazo-pyridine/pyrazine heterocycles are found in number of pharmacologically active molecules.
-
•
Compounds IG-01–008 and IG-01–009 showed highest cytotoxicity as compared to IG-01–007.
-
•
Test compounds at 50 µM exhibited DNA fragmentation in HCT-116 and CT-26 cells.
-
•
At higher dose, test compounds showed signs of hepatotoxicity and spleen toxicity.
1. Introduction
Aromatic heterocyclic scaffolds, such as fused imidazole-based structures, are found in variety of natural products and play significant role in drug discovery and can be considerd as privileged structures [2], [1]. Combining the fused imidazole-based heterocycles with biphenyl structural moiety resulted in new scaffolds with enhanced physicochemical and pharmacological properties [3]. Imidazo-pyridine/pyrazine heterocycles are found in number of pharmacologically active molecules that display wide arrays of activities such as immune-stimulating properties against infectious skin conditions and have been reported to be effective against various cancers, including colon carcinoma, melanoma, lung carcinoma, bladder carcinoma, and breast carcinoma [4], [3]. These scaffolds are already present in several marketed formulations and bioactive compounds [6], [3], [5]. Additionally, these types of compounds have been reported to inhibit VEGF, arrest cell cycle and induce apoptosis in different studies [7]. Moreover, imidazo-based heterocyclic compounds have demonstrated DNA damage potential, contributing to their anticancer activity by interacting with DNA and generating reactive oxygen species, which can lead to genotoxicity and cell death [8].
Singh et al reported the synthesis of biphenyl linked fused imidazoles and evaluated the activity of these compounds against NCI-60 cell lines to identify potential hits with anticancer activity [3]. In the preliminary investigation, imidazo-pyridine/pyrazine heterocycles with a biphenyl substituent has shown promising results against various cancer cell lines, that includes of the breast cancer (MDA-MB-231, MDA-MB-468, MCF-7, and MCF-12A), colon cancer (HCT-15, HT29, and KM12), leukemia (CML), as well as ovarian cancer (OVCAR-3) cell lines. Interestingly, these compounds found to be non-toxic to several other cancer cell lines, and exhibited minimal toxicity towards normal cell lines such as HEK293 (human embryonic kidney cells) and MCF12A (human epithelial cell isolated from mammary glands) [3].
Various synthetic small molecules with anticancer potential are known, yet their utilization is restricted due to low effectiveness and challenges with solubility. The potential toxicity further limits the clinical evaluation of these new scaffolds. Consequently, the quest for innovative and promising anticancer drugs persists, indicating that the fight against cancer remains ongoing [9]. This emphasizes the importance of toxicological screening in evaluating the safety of new drug molecules, particularly in the context of anticancer agents, which often exhibit high toxicity [10], [11]. Consequently, toxicological screening of new drug molecules is crucial in drug discovery to assess potential adverse effects and determine safety profiles. Regulatory bodies like the FDA mandate such evaluations to ensure drugs are safe for use. Moreover, cytotoxic anticancer agents, known for high non-specific toxicity against other cells, require thorough evaluation through in-vitro and in-vivo studies to identify potential harm to genetic material or organs [12], [13], [14], [15], [16], [17]. Therefore, the present work focuses on evaluating the toxicological potential of three derivatives of imidazo-based heterocycles using both in-vitro and in-vivo studies. These synthesized compounds were tested in-vitro and in-vivo for their cytotoxic and toxicological evaluation in colorectal cancer cells (HCT-116 and CT-26) and in Wistar rats, respectively. Studies like cytotoxicity assay, DNA damage, apoptosis, cell migraiton assay and acute oral toxicity (as per OECD guidelines) followed by histopathological examination were carried out to establish the toxic potential of investigated compounds.
2. Materials and methods
2.1. Materials
Dulbecco’s Modified Eagle's Medium (DMEM), streptomycin, and penicillin were procured from Lonza, BioWhittaker (Walkersville, USA), RPMI-1640, Phosphate-buffered saline (PBS) and Fetal bovine serum (FBS) were procured from HiMedia Laboratories Pvt. Ltd. HCT-116 (human colorectal cancer cells) and CT-26 (murine colorectal cancer cells) were obtained from National Centre for Cell Science (NCCS), Pune, India. The cell lines were cultured in their respective media with 10 % FBS and 1 % penicillin-streptomycin and were maintained at 5 % CO2/95 % air humidified atmosphere at 37 °C in an incubator. The imidazo based heterocyclic derivatives 2-([1,1’-biphenyl]-4-yl)-N-(tert-butyl)imidazo[1,2-a]pyridine-3-amine (1, IG-01–007), 2-([1,1’-biphenyl]-4-yl)-N-(2,4,4-trimethylpentan-2-yl)imidazo[1,2-a]pyrazin-3-amine (2, IG-01–008), and 2(benzo[d][1,3]dioxol-5-yl)-N-(2,4,4-trimethylpenten-2-yl)imidazo[1,2-a]pyrazin-3-amine (3, IG-01–009) were synthesised in-house at Department of Chemistry, Panjab University, Chandigarh, (Fig. 1).
Fig. 1.
Chemical structures of the heterocyclic derivatives 1-3 investigated in the present study.
2.2. Animals
Study was conducted on fifty five healthy male Wistar rats (200–250 gm and 6–8 weeks old) provided by central animal house facility of Panjab University, Chandigarh, India. While Wistar rats of both sexes were approved, the distribution of rats was random and was done as per the availability of animals for the study. The protocol received approval from the Institutional Animal Ethical Committee (IAEC) of Panjab University, Chandigarh, India with protocol number PU/45/99/CPCSEA/IAEC/2021/559. The animals were housed in standard laboratory conditions at a temperature of 25 ± 2 °C, with relative humidity ranging from 30 % to 70 % and a photoperiod of 12 h. They were provided ad libitum access to pellet diet (Ashirwad Industries, Chandigarh, India) and water. Throughout the study, all animals received attentive and humane care.
2.3. Cytotoxicity assay
The cytotoxic effects of the compounds against HCT-116, and CT-26 were determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), via a rapid colorimetric assay with modifications [18]. Test compounds (1−3) were prepared by dissolving them in 100 % DMSO to create a 10 mM stock solution. This stock solution was then diluted with media to achieve final concentrations of 10 µM, 25 µM, 50 µM, and 100 µM. For the screening experiment, cells (7 × 103 cells/well) were seeded into 96-well culture plates containing 180 μL culture medium. The plates were then incubated for 24 h at 37 °C in a humidified incubator with 5 % CO2, 95 % air, and 100 % relative humidity. Test compound at the concentrations of 10 µM, 25 µM, 50 µM and 100 µM were added and incubated for another 24 h. Triplicates were maintained, and medium without the test compounds served as negative control. After 24 h, supernatant was replaced by media containing MTT (5 mg/mL) and the cells were incubated for 4 h. The medium with MTT was then discarded, and the resulting formazan crystals were dissolved in DMSO (100 µL) and absorbance was measured at 570 nm using microplate reader. Untreated cells were considered as 100 % viable and treated cells were compared with control to determine cell toxicity. The IC50 was determined using GraphPad Prism software Inc., CA, USA.
2.4. Hemolysis assay
The hemolysis assay was conducted to assess the toxicity of test compounds on red blood cells (RBCs), following the method suggested by Liaqat et al. [19]. Approximately 4 mL of whole rat blood was collected in EDTA tube to prevent coagulation. RBCs were isolated by centrifugation at 1500 rpm for 10 min at 4 °C and were then washed with ice-cold PBS to purify the cells. For the hemolysis assay, test compounds 1-3 were evaluated at concentrations 10 µM, 20 µM and 50 µM. The negative and positive controls were normal saline solution (0.9 % sodium chloride) and 0.1 % Triton X-100 in PBS, respectively. The samples were kept for incubation at 37 °C for 1 h, followed by centrifugation at 10,000 RPM for 3 minutes. The supernatant was collected and absorbance was measured at 540 nm using ELISA reader spectrophotometer (MPM 6, BioRad, USA), and percentage of hemolysis was calculated.
2.5. DNA fragmentation/damage
DNA fragmentation was evaluated using Hoechst staining, a visual technique used for detecting DNA breakage in individual mammalian cell [20]. HCT-116, and CT-26 cells (2 × 104 cells/well) were cultured onto a cover slip in a 6-well plate and incubated for 24 h at 37 °C in a humidified incubator with 5 % CO2, 95 % air, and 100 % relative humidity. The cells were then treated with test compounds 1-3 at concentration 50 μM, and 100 μM and incubated for additional 24 h. Following treatment, the washing was carried out three time for both untreated and treated cells using ice-cold PBS and then fixed with 4 % paraformaldehyde. The counterstaining of fixed cells were was done using 10 μg/mL solution of Hoechst dye and washed thrice using ice-cold PBS. The cells were then mounted and examined using a confocal microscope (Nikon C2 laser-scanning microscope) at excitation and emission wavelengths of 352 nm and 450 nm, respectively.
2.6. Cell migration assay
The in-vitro cell migration assay was carried out on HCT-116 and CT-26 cells to evaluate their migration ability [21]. HCT-116 and CT-26 cells (7 ×10 ³ cells/well) were seeded in 96-well plates containing 2 mL of culture medium and incubated for 24 h at 37 °C in a humidified incubator with 5 % CO₂. A scratch was made in the cell monolayer using a 10 µL pipette tip, and subsequently 1 mL of growth medium was used to remove the debris. The growth medium was then replaced with McCoy’s 5 A medium. The cells were treated with test compounds 1-3 at concentrations of 25 µM, and 50 µM, while control wells received fresh McCoy’s 5 A medium without the test compound. Immediately after treatment, images of the scratch were taken at 0 h and then at 24 h. The percentage of cell migration was calculated using ImageJ software by measuring the area between the migrated cells. The control area was set as 100 %, and this value was used to calculate the percentage of migration in the treated wells.
2.7. Acute toxicity study
OECD guidelines were followed to conduct the acute toxicity study, specifically test number TG 420 for assessing chemical substances [22]. The study initially began with a dose of 300 mg/kg, using one animal for each of three compounds (1-3). No toxicity sign/ mortality was observed till 14th day. Same procedure was repeated using a dose of 2000 mg/kg, and mortality of all the three animals was observed after 4 days of drug administration. Next, a middle range dose of 1000 mg/kg of three compounds was studied using another three animals and the animals were observed in the same manner. Toxicity sign such as tremors, and absence of alertness were appeared after 3–4 days as compared to control (treated with normal saline). Additionally, change in skin color and fur was also observed. Based on the above observations, acute toxicity studies were conducted using dose of 300 mg/kg and 1000 mg/kg for all three compounds, with group of 5 animals per dose. Following treatment, the animals were observed for 14 days. At the end of observation period, the animals were anesthetized with an intraperitoneal (i.p.) injection of ketamine hydrochloride (80 mg/kg) followed by xylazine (10 mg/kg), euthanized, and subjected to necropsy examination with recording of organ weights.
2.8. Body weight
Body weights of the rats were measured on day 0, 7, and 14 of the study. Changes in body weight were calculated using the formula given below:
2.9. Gross general behavior parameters
Body weight, water and food intake was observed at different time intervals for next 14 days. Behavioral parameters including changes in eye, skin and fur color, urination, ambulation, sniffing, rearing, tremors, convulsions, salivations, alertness, grooming, motor activity were observed at 30 minutes, 1 h, 4 h, 8 h and, then daily for next 14 days.
2.10. Biochemical parameters
2.10.1. Collection of blood sample
After completing the measurements of behavioral parameter on the 14th day, animals were anesthetized using an i.p. injection of ketamine hydrochloride (80 mg/kg) and xylazine (10 mg/kg). Blood samples were collected from the retro-orbital plexus, followed by centrifugation at 10,000 rpm for 10 minutes to separate serum and plasma, and stored at −20 °C until used for biochemical analysis.
2.10.2. Liver, kidney, and spleen tissue homogenate preparation
After collecting blood samples, mice were euthanized following IAEC guidelines. Organs were extracted for biochemical and histopathological analysis. Organs were rinsed with ice-cold saline (0.9 % sodium chloride), and homogenized in buffer containing 10 mM Tris HCl, 150 mM magnesium chloride, 1 mM EDTA, and 1 % Triton-X 100. The homogenate was centrifuged at 10,000 rpm for 20 minutes at 40 °C. The resulting supernatant was used for lipid peroxidation and superoxide dismutase assays.
2.10.3. Protein estimation
Protein concentration of each sample was measured using the Biuret method, with bovine serum albumin (BSA) as the standard. A working reagent was prepared by diluting stock solutions up to 100 mL with 0.2 N NaOH. Stock solution of Biuret solution was prepared by dissolving 4.5 gm of sodium potassium tartrate in 40 mL of 0.2 N NaOH, adding 1.5 gm of copper sulfate with constant stirring, followed by 0.5 gm of potassium iodide, and make up the volume up to 100 mL with 0.2 N NaOH. Protein standard was prepared by using 500 mg of BSA in 100 mL. Additionally, 0.1 mL of the homogenate was mixed with 2.9 mL of NaCl and 3 mL of the working Biuret reagent, thoroughly shaken and allowed to stand at room temperature for 10 min. The absorbance was then measured at 540 nm. Standard graphs were plotted using various concentrations of BSA for comparison.
2.11. Lipid peroxidation assay
The extent of lipid peroxidation was determined quantitatively by assessing thiobarbituric acid reactive substances following the method described by De Leon & Borges [23]. 0.5 mL of Tris-HCl was added to 0.5 mL of supernatant, and the mixture was incubated at 37 °C for 2 h. After incubation, 1 mL of trichloroacetic acid was introduced and centrifugation of mixture was done at 10,000 rpm for 10 minutes. Following centrifugation, 1 mL of 0.67 % thiobarbituric acid was added to the supernatant and tubes were kept in boiling water bath for 10 minutes. Thereafter the mixture was cooled down and to it 1 mL of double distilled water was added. The mixture was then used to measure the absorbance at 532 nm using Perkin Elmer UV/VIS spectrophotometer (Lamda 20, USA). To determine the amount of malondialdehyde molar extinction coefficient of 1.56 × 105 M−1 cm−1 was used and expressed as nanomoles (nmol) of malondialdehyde equivalents per milligram of protein.
2.12. Superoxide dismutase activity
Superoxide dismutase (SOD) activity was assessed following the method reported by Mesa-Herrera et al. [24]. SOD inhibits the reduction of nitro blue tetrazolium and its activity was determined for the different samples by measuring absorbance at 560 nm using a UV-Vis spectrophotometer (UV-1800 Shimadzu, Japan). Initiation of reaction was done by adding hydroxylamine hydrochloride to the assay mixture that contains nitro blue tetrazolium and test sample. Results were then expressed as units/mg of protein, with one unit of enzyme defined as the amount of enzyme that inhibits the reaction rate by 100 %.
2.13. Histopathological examination
After euthanasia, isolated organs (liver, kidney and spleen) from each rat were preserved in 10 % formalin solution for a period of 48 h. For histopathological assessment, tissue sections were fixed in paraffin, and 5 μM sections were cut using a rotary microtome. These sections were subsequently stained with haematoxylin and eosin, then examined under a light microscope. Comparisons were made between the sections of treated animals and those of control rats. Representative images were taken with the Nikon microscope aided with the imaging software (NIS Element BR 3.0; Japan).
2.14. Statistical analysis
Data were analysed using two-way ANOVA followed by Tukey’s test for multiple comparisons. All values were presented as Mean ± SEM ap < 0.05 as compared to the control group. Additionally, data were expressed as mean ± standard deviation (SD). Statistical comparisons were made using GraphPad Prism (GraphPad Software Inc., CA, USA) and p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of imidazo based heterocyclic derivatives on viability of HCT-116 and CT-26 cells
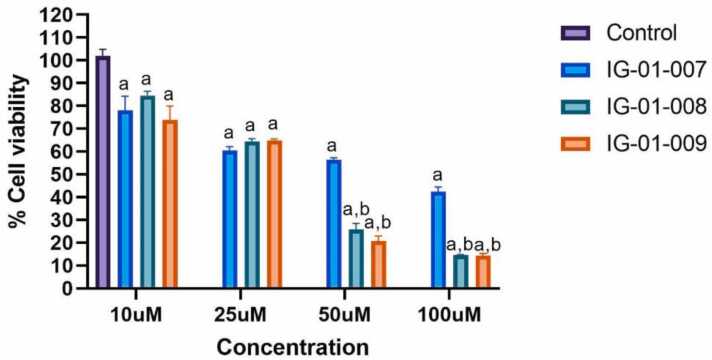
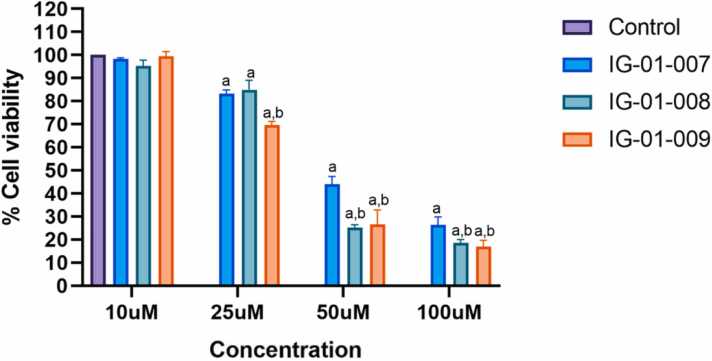
The in-vitro cytotoxicity potential of three synthesized compounds (IG-01–007, IG-01–008, and IG-01–009) was examined on HCT-116 and CT-26 cell lines using MTT assay at concentrations of 10 μM, 25 μM, 50 μM, and 100 μM. In the HCT-116 cell line, no significant reduction in cell viability was observed at 10 μM and 25 μM for all three compounds. However, at concentrations of 50 μM and 100 μM, IG-01–008 and IG-01–009 exhibited higher cytotoxicity compared to IG-01–007. The IC50 values for HCT-116 were 30.09 ± 1.58 μM for IG-01–007, 30.90 ± 10.6 μM for IG-01–008, and 27.5 ± 11 μM for IG-01–009 (Fig. 2a). In contrast, for the CT-26 cell line, the compounds showed dose-responsive inhibition with IC50 values of 61.19 ± 21.16 μM for IG-01–007, 48.08 ± 22.61 μM for IG-01–008, and 40.86 ± 17.05 μM for IG-01–009 (Fig. 2b). These findings, analysed using GraphPad Prism, demonstrate that the compounds are more cytotoxic towards the HCT-116 cancer cell line compared to the CT-26 cell line, suggesting enhanced toxicity towards human derived colon cancer cells. Overall, the synthesized compounds exhibit dose-dependent anti-proliferative potential against cancer cell lines.
Fig. 2a.
% Cell viability of HCT-116 cells treated with Imidazo based heterocycle derivatives. Data were analysed using two-way ANOVA followed by Tukey’s multiple comparison test. All values are expressed as mean ± SEM, ap < 0.05 compared to control, and bp < 0.05 compared to IG-01–007.
Fig. 2b.
% Cell viability of CT-26 cells treated with Imidazo based heterocycle derivatives. Data were analysed using two-way ANOVA followed by Tukey’s multiple comparison test. All values are expressed as mean ± SEM, ap < 0.05 compared to control, and bp < 0.05 compared to IG-01–007.
3.2. Effect of imidazo based heterocycle derivatives on % hemolysis of RBCs suspension
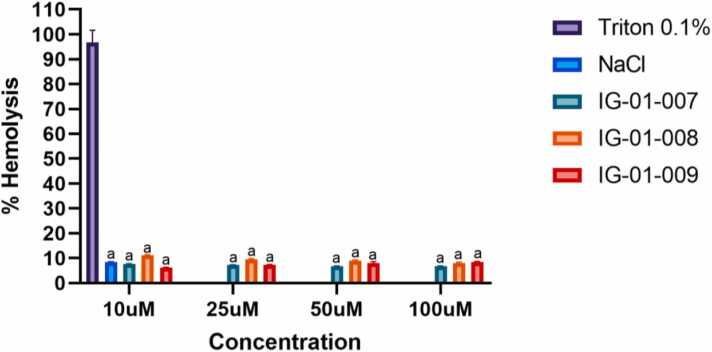
The hemolytic potential of synthesized compounds (IG-01–007, IG-01–008, and IG-01–009) was evaluated on fresh whole blood samples of healthy rat at concentration of 10 μM, 25 μM, 50 μM, and 100 μM. All the test compounds did not show any hemolytic activity (p < 0.001) as compared to positive control (Fig. 3). These findings suggest, all the synthesized compounds were non-hemolytic and safe for administration.
Fig. 3.
% Hemolysis of RBCs suspension on treatment with imidazo based heterocycle derivatives. Data were analysed using two-way ANOVA followed by Tukey’s multiple comparison test. All values are expressed as Mean ± SEM. ap < 0.05 compared to control.
3.3. DNA fragmentation/damage
As evident form the cytotoxicity assay, the test compounds were cytotoxic at higher concentration of 50 µM and 100 µM, the DNA fragmentation assay was carried out for three test compounds at concentration of 50 µM. The HCT-116, and CT-26 cells were exposed to 50 µM concentrations of test compounds for duration of 24 h. Following the treatment, cells were stained with Hoechst 33342, a dye that specifically binds to DNA highlighting the minor grooves. Prior to treatment, the untreated cells displayed a typical morphology, predominantly spindle-shaped with some round cells, forming a cohesive layer at approximately 60–70 % confluency (bright field microscopy). However, upon exposure to the test compounds there was a noticeable reduction in cell growth accompanied by apoptotic characteristics such as cellular rounding, shrinkage, nuclear condensation, and DNA fragmentation, as indicated by arrows (Fig. 4a, and Fig. 4b). One of the reason for cytotoxicity of these compounds can be associated with DNA fragmentation.
Fig. 4a.
Confocal microscopy images of HCT-116 cells stained with Hoechst dye, where the arrow indicates DNA fragmentation. (a) Control, and (b) IG-01–007, (c) IG-01–008, (d) IG-01–009 are tested at concentration 50 µM.
Fig. 4b.
Confocal microscopy images of CT-26 cells stained with Hoechst dye, where the arrow indicates DNA fragmentation. (a) Control, and (b) IG-01–007, (c) IG-01–008, (d) IG-01–009 are tested at concentration 50 µM.
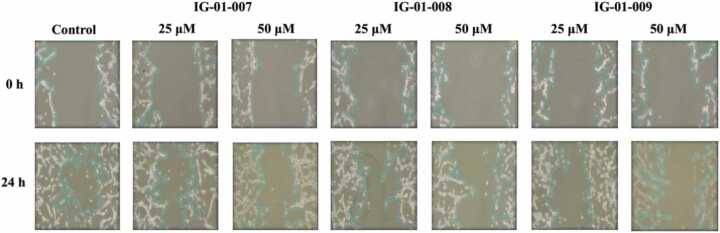
3.4. Effect of imidazo based heterocycle derivatives on migration of HCT-116, and CT-26 cells
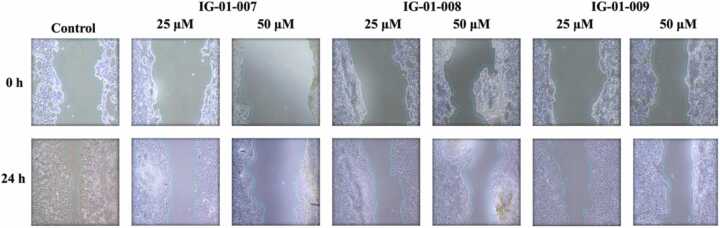
Cell migration assay was conducted to determine the effect of imidazo based heterocycle derivatives on migration capability of HCT 116, and CT-26 cells. The cells were seeded in 6-well plate and treated with a fixed concentration of compounds IG-01–007, IG-01–008 and IG-01–009 and a control well for 24 h. The cells migrate to form monolayer in growth medium, the % migration of cells was measured under inverted phase contrast microscope. After 24 h of incubation, significant growth of cells was observed in control well reflecting normal proliferation nature of cancer cells whereas in the treated wells the cell migration was inhibited by test compounds. In HCT-116 cells, at concentrations of 25 μM and 50 μM, compounds IG-01–007, IG-01–008, and IG-01–009 % migration of cell was 41.75 %, 36.33 %, 34.01 %, and 35.07 %, 20.76 %, 20.65 %, respectively. Meanwhile, in CT-26 cells, at the same concentrations of 25 μM and 50 μM, the migration percentages for compounds IG-01–007, IG-01–008, and IG-01–009 were 45.61 %, 35.65 %, 44.4 %, and 30.62 %, 39.17 %, 28.83 %, respectively. The cell migration of control and treated cells showed in (Fig. 5a, Fig. 5b, and Fig. 5c).
Fig. 5a.
Cell migration assay on HCT-116 cells.
Fig. 5b.
Cell migration assay on CT-26 cells.
Fig. 5c.
% Cell migration of (a) HCT-116, and (b) CT-26 cells.
3.5. In-vivo acute oral toxicity
3.5.1. Effect of imidazo based heterocycle derivatives on gross behavior of animals on acute oral administration
The acute toxicity effect of test compounds IG-01–007, IG-01–008, IG-01–009 was determined with dose 300 mg/kg, 1000 mg/kg and 2000 mg/kg, as per the OECD guideline 420 (fixed dose procedure). Mortality was observed at a dose of 2000 mg/kg and toxicity signs were seen at 1000 mg/kg. The general behavior of the treated and control animals were observed on 0, 7th, and 14th day of the study. At dose 300 mg/kg and 1000 mg/kg for compound IG-01–007 and IG-01–008 rat showed absence of alertness, locomotor activity, ambulation, grooming and sniffing on 14th day, whereas, compound IG-01–009 showed slightly more signs of toxicity. Additionally, compound IG-01–009 showed same behavior at dose 1000 mg/kg on day 7 and 14. However, tremors, change in eye and skin color was observed in all the three compounds at dose level of 1000 mg/kg. As mortality was observed at a dose of 2000 mg/kg, the LD50 of all three compounds is considered to be less than 2000 mg/kg, in accordance with the OECD acute toxicity guidelines. The parameters observed for acute oral toxicity study after the administration of the compounds compared with control (normal saline) and 1 % DMSO group are presented in Table 1.
Table 1.
Effect on gross behavior of animals on oral administration of imidazo based heterocycle derivatives at different dose
| Sr. no. | Parameters |
IG-01–007 |
IG-01–008 |
IG-01–009 |
|||
|---|---|---|---|---|---|---|---|
| 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | ||
| 1. | Change in eye color | N | N | O | O | O | O |
| 2. | Urination | N | N | N | N | N | N |
| 3. | Sniffing | N | N | A | A | A | A |
| 4. | Tremors | N | O | N | O | N | O |
| 5. | Convulsions | N | N | N | N | N | N |
| 6. | Rearing | N | N | N | A | A | A |
| 7. | Alertness | A | A | A | A | N | A |
| 8. | Salivation | N | N | N | N | N | N |
| 9. | Locomotor activity | A | N | A | A | A | A |
| 10. | Change in skin color | N | N | N | O | O | O |
| 11. | Grooming | N | A | A | N | N | A |
| 12. | Ambulation | A | A | A | A | N | A |
| 13. | Mortality | N | N | N | N | N | N |
For compounds IG-01–007, IG-01–008, and IG-01–009 gross general behavior of animals on acute oral toxicity study. N = Normal, O=Observed, A=Absent.
3.5.2. Effect of imidazo based heterocycle derivatives on food intake, body weight, and organs weight of animal on acute oral administration
3.5.2.1. Food intake
On 7th day, animals treated with compounds IG-01–008 and IG-01–009 at dose 300 mg/kg and 1000 mg/kg exhibited a significant decrease in food consumption compared to the control group. Furthermore, compound IG-01–009 demonstrated a significant reduction in food intake compared to 1 % DMSO treated animals. On the14th day, animals treated with compounds IG-01–008 and IG-01–009 at dose 300 mg/kg and IG-01–007, IG-01–008 and IG-01–009 at dose 1000 mg/kg, exhibited a significant decrease in food intake compared to the control group. However, specifically, IG-01–008 and IG-01–009 at doses of 300 mg/kg and 1000 mg/kg showed a significant reduction in food intake compared to the group treated with 1 % DMSO (Table 2).
Table 2.
Effect of imidazo based heterocycle derivatives on food consumed by animals. Data values are presented as mean ± S.E.M, with n = 5.
| Days |
Groups (weight in gm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | 1 % DMSO |
IG-01–007 |
IG-01–008 |
IG-01–009 |
||||
| 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | |||
| 0 day | 94.4 ± 0.8124 | 92.5 ± 1.5 | 93.8 ± 1.1575 | 94.8 ± 0.80 | 92.2 ± 0.374 | 92.4 ± 1.16619 | 91 ± 0.707 | 91.4 ± 1.964 |
| 7thday | 94.2 ± 0.663 | 91.5 ± 1.5 | 90.4 ± 1.288 | 88.6 ± 1.363 | 86.2 ± 0.969 | 83.2 ± 0.86 | 83.4 ± 1.029 | 79.2 ± 2.083 |
| 14thday | 94.6 ± 1.568 | 90 ± 2 | 89.2 ± 2.009 | 82.4 ± 1.939 | 81.2 ± 0.916 | 76 ± 1.702 | 77.8 ± 1.067 | 74.4 ± 1.691 |
3.5.2.2. Body weight
Body weight of each rat was taken on day 0, 7th, and 14th. On day 14th, body weights of treated animals with compound IG-01–008 and IG-01–009 at dose 1000 mg/kg were found to be significantly lowered compared to control, whereas in comparison with 1 % DMSO, compound IG-01–009 also showed significant reduction in body weight. There was gradual decrease in body weight of rats on 7th and 14th day for compound IG-01–009 at 300 mg/kg and IG-01–007, IG-01–008, IG-01–009 at 1000 mg/kg (Table 3).
Table 3.
Effect of imidazo based heterocycle derivatives on change in body weight (gm) of animals. Data values are presented as mean ± S.E.M, with n = 5.
| Days |
Groups (change in weight in gm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | 1 % DMSO |
IG-01–007 |
IG-01–008 |
IG-01–009 |
||||
| 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | |||
| 0 day | 235 ± 5.477 | 232.5 ± 2.5 | 233 ± 2 | 244 ± 15.6844 | 242 ± 5.1478 | 249 ± 8.124 | 245 ± 5 | 246 ± 15.033 |
| 7th day | 253 ± 5.385 | 250 ± 5 | 248 ± 3.391 | 238 ± 16.17 | 254 ± 6.595 | 236 ± 8.124 | 247 ± 5.147 | 230 ± 14.142 |
| 14th day | 271 ± 5.099 | 257.5 ± 2.5 | 253 ± 8 | 231 ± 15.033 | 257 ± 6.04 | 225 ± 8.366 | 243 ± 4.636 | 213 ± 16.552 |
3.5.2.3. Wet organ weight
There was no significant change observed between the wet organ weight of kidney and spleen of treated and control group animals but there was marked decrease in weight of liver compared to control and 1 % DMSO (Table 4).
Table 4.
Effect of imidazo based heterocycle derivatives on change in organ weight of animals. Data values are presented as mean ± S.E.M, with n = 5.
| Organ |
Groups (change in weight in gm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | 1 % DMSO |
IG-01–007 |
IG-01–008 |
IG-01–009 |
||||
| 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | |||
| Liver | 8.036 ± 0.257 | 8.34 ± 0.0871 | 6.58 ± 0.535 | 6.204 ± 0.2716 | 6.21 ± 0.15 | 6.74 ± 0.332 | 6.652 ± 0.1819 | 6.622 ± 0.108 |
| Kidney | 2.205 ± 0.1108 | 2.38 ± 0.024 | 1.6568 ± 0.110 | 1.854 ± 0.11788 | 2.058 ± 0.123 | 1.954 ± 0.110 | 1.966 ± 0.1258 | 1.8067 ± 0.0677 |
| Spleen | 0.8066 ± 0.1501 | 1.023 ± 0.0433 | 0.654 ± 0.075 | 0.716 ± 0.0508 | 0.83 ± 0.0763 | 0.747 ± 0.1135 | 0.825 ± 0.0265 | 0.696 ± 0.0632 |
3.5.3. Effect of imidazo based heterocycle derivatives on change in serum biochemistry
All the test compounds showed a dose dependent increase in the level of serum biochemistry i.e., serum glutamic-pyruvic transaminase (ALT), serum glutamic-oxaloacetic transaminase (AST), albumin, globulin, A/G ratio, alkaline phosphatase (ALP) and triglycerides level at dose 300 mg/kg and 1000 mg/kg (Table 5).
Table 5.
Effect of imidazo based heterocycle derivative in change in serum biochemistry at dose 300 mg/kg and 1000 mg/kg. Data values are expressed as mean ± S.E.M, n = 3.
| Parameters | Normal range | Units | Control | 1 % DMSO |
IG-01–007 |
IG-01–008 |
IG-01–009 |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | 300 mg/kg | 1000 mg/kg | |||||
| SGPT (ALT) | 18–45 | U/L | 48 ± 2.645 | 42 ± 3.214 | 47.66 ± 2.728 | 75.33 ± 4.05 | 60.33 ± 3.756 | 79.0 ± 1.732 | 63 ± 3.785 | 147.33 ± 8.87 |
| SGOT (AST) | 74–183 | U/L | 149 ± 2.081 | 191.66 ± 4.977 | 193 ± 12.74 | 242 ± 3.785 | 255 ± 5.291 | 302.33 ± 4.096 | 283.66 ± 6.33 | 353 ± 7.371 |
| Total protein | 5.2–7.1 | g/dL | 6.30 ± 0.3214 | 6.66 ± 0.296 | 7.03 ± 0.491 | 7.40 ± 0.324 | 7.266 ± 0.523 | 7.60 ± 0.3183 | 7.266 ± 0.491 | 7.866 ± 0.12 |
| Albumin | 3.4–4.8 | g/dL | 4.066 ± 0.5783 | 3.33 ± 0.355 | 3.33 ± 0.437 | 3.683 ± 0.261 | 3.166 ± 0.2905 | 3.67 ± 0.2055 | 2.78 ± 0.3189 | 3.77 ± 0.18950 |
| Globulin | 1.5–2.5 | g/dL | 3.366 ± 0.4255 | 3.866 ± 0.1763 | 3.80 ± 0.404 | 4.006 ± 0.1034 | 4.30 ± 0.529 | 4.35 ± 0.144 | 4.113 ± 0.2623 | 4.286 ± 0.3319 |
| A/G ratio | 1.58–2.67 | - | 2.366 ± 0.348 | 0.92 ± 0.0529 | 0.69 ± 0.0608 | 0.6966 ± 0.0317 | 0.736 ± 0.0523 | 0.923 ± 0.0648 | 0.79 ± 0.032 | 0.8466 ± 0.0317 |
| ALP | 62–230 | U/L | 204 ± 7.81 | 289 ± 8.386 | 307.33 ± 7.218 | 343.0 ± 10.785 | 391.33 ± 9.261 | 501.66 ± 13.64 | 403.33 ± 12.5 | 602.66 ± 8.41 |
| Triglycerides | 20–114 | mg% | 111 ± 3.785 | 114 ± 4.61 | - | 115 ± 4.055 | - | 115.33 ± 5.487 | - | 151.33 ± 5.81 |
3.5.4. Effect of imidazo based heterocycle derivatives on oxidative stress in vital organs of rats
3.5.4.1. Effect on lipid peroxidation
-
(a)
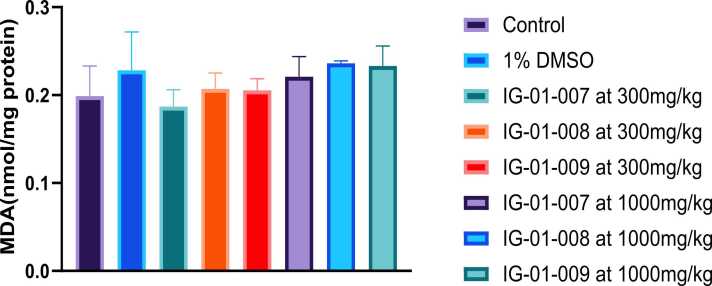
Liver: There was insignificant difference seen in MDA levels between control and 1 % DMSO. For compound IG-01–009 at dose of 300 mg/kg significant increase in MDA levels were seen compared to control. However, at dose 1000 mg/kg, MDA levels were significantly higher compare to control for compounds IG-01–007, IG-01–008, and IG-01–009. Overall all the compounds showed dose dependent increase in MDA level (Fig. 6, Fig. 7)
-
(b)
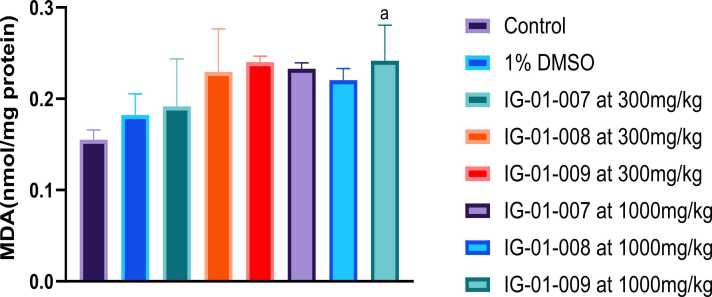
Spleen: There was insignificant difference seen in MDA levels between control and 1 % DMSO. Only for compound IG-01–009 at the dose of 1000 mg/kg, it induced oxidative stress and significant increase the levels of MDA were observed and compared with control (Fig. 8).
Fig. 6.
Effect of imidazo-based heterocycle derivatives on MDA levels (nmol/mg protein) in the liver of Wistar rats of both sexes. Data were analysed by one-way ANOVA followed by Tukey’s test for multiple comparison. All values are expressed as Mean ± SEM. ap < 0.05 compared to control, bp < 0.05 compared to 1 % DMSO, cp < 0.05 compared to IG-01–007 at 300 mg/kg, and dp < 0.05 compared to IG-01–008 at 300 mg/kg.
Fig. 7.
Effect of imidazo based heterocycle derivatives on MDA (nmol/mg protein) in kidney of male and female Wistar rats. Data were analysed using one-way ANOVA followed by Tukey’s multiple comparison test. All values were expressed as Mean ± SEM. *p < 0.05 as compared to control.
Fig. 8.
Effect of imidazo based heterocycle derivatives on MDA (nmol/mg protein) in spleen of male and female Wistar rats. Data were analysed by one-way ANOVA followed by Tukey’s test multiple comparison. All values were expressed as mean ± SEM. ap < 0.05 as compared to control.
3.5.4.2. Effect on superoxide dismutase (SOD)
-
(a)
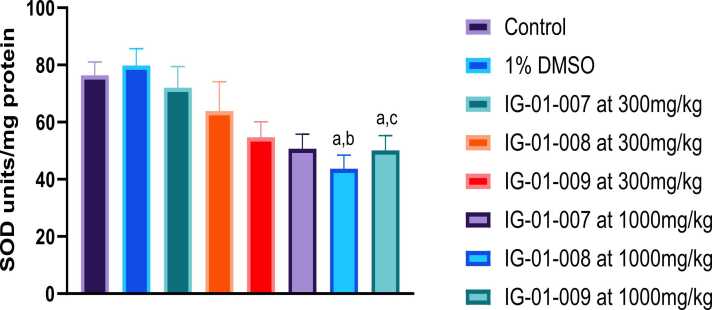
Liver: There was insignificant change seen in SOD levels in between control and 1 % DMSO. For compound IG-01–009 at the dose of 1000 mg/kg, significantly depletion in SOD level was observed in liver compare with control. However, a significant reduction in SOD level was observed in animals treated with IG-01–009 at dose 300 mg/kg compared to those treated with IG-01007. Overall compounds showed dose dependent decrease in SOD level (Fig. 9).
-
(b)
Kidney: There was insignificant change in SOD levels in control but for compound IG-01–008 and IG-01–009 at 1000 mg/kg SOD levels significantly decreased in comparison with 1 % DMSO. For compound IG-01–008 at 1000 mg/kg SOD levels were significantly decreased when compared with IG-01–007 at 300 mg/kg. However, for compound IG-01–009 at 1000 mg/kg SOD levels found decreased significantly in comparison with IG-01–008 at 1000 mg/kg. Overall, all the test compounds showed dose dependent reduction in SOD level (Fig. 10). compared to 1 % DMSO, bp < 0.05 compared to IG-01–007 at 300 mg/kg, and cp < 0.05 compared to IG-01–008 at 1000 mg/kg.
-
(c)
Spleen: There was insignificant difference of SOD levels in control and 1 % DMSO. For compound IG-01–008, and IG-01–009 at the dose of 300 mg/kg and compounds IG-01–007, IG-01–008, and IG-01–009 at 1000 mg/kg there was significant decrease seen in SOD level compared with control and 1 % DMSO. However, compounds IG-01–007, IG-01–008, and IG-01–009 at 1000 mg/kg showed significant reduction of SOD level when compared with IG-01–007 and IG-01–008 at 300 mg/kg (Fig. 11).
Fig. 9.
Effect of imidazo based heterocycles derivatives on SOD level in liver of male and female Wistar rats. Data were analysed using one-way ANOVA followed by Tukey’s multiple comparison test. All values are expressed as Mean ± SEM. ap < 0.05 as compared to control, bp < 0.05 as compared to IG-01–007 at 300 mg/kg.
Fig. 10.
Effect of imidazo based heterocycle derivatives on antioxidant enzyme in kidney of male and female Wistar rats. Data were analysed using one-way ANOVA followed by Tukey’s multiple comparison test. All values are expressed as Mean ± SEM. ap < 0.05 compared to 1 % DMSO, bp < 0.05 compared to IG-01–007 at 300 mg/kg, and cp < 0.05 compared to IG-01–008 at 1000 mg/kg.
Fig. 11.
Effect of imidazo based heterocycle derivatives on antioxidant enzyme in spleen of male and female Wistar rats. Data were analysed by one-way ANOVA followed by Tukey’s test multiple comparison. All values were expressed as Mean ± SEM. ap < 0.05 as compared to control, bp < 0.05 as compared to 1 % DMSO, cp < 0.05 as compared to IG-01–007 at 300 mg/kg, dp < 0.05 as compared to IG-01–008 at 300 mg/kg.
3.5.5. Effect of imidazo based heterocycle derivatives on tissues sections of vital organs
-
(a)
Liver: The liver sections of the control group showed typical hepatic architecture (Fig. 12: 1a and 1b). Liver sections (Fig. 12: 2a and 2b) of compound IG-01–007 at dose 300 mg/kg were found to be healthy with normal hepatocytes while some sections were found to have non-uniform cell structure (which represents regeneration and are marked with arrows). Liver sections (Fig. 12: 3a and 3b) of compound IG-01–008 at dose 300 mg/kg showed marked pathological changes characterized by mild steatosis and presence of micro and macro vesicles (represented by arrow and mild inflammation indicated by circle). Compound IG-01–009 treated liver sections (Fig. 12: 4a and 4b) at dose 300 mg/kg showed mild to moderate steatosis indicated by circle whereas weak cell wall integrity of hepatocytes are indicated by arrow (Fig. 12). Liver sections (Fig. 13: 5a and 5b) of group treated with 1 % DMSO showed normal hepatic structure with mild fatty liver changes. Liver sections (Fig. 13: 6a and 6b) of compound IG-01–007 at dose 1000 mg/kg were found to be healthy with mild activation of kupffer cells indicated by circle and moderate steatosis indicated by arrow. Compound IG-01–008 at dose 1000 mg/kg showed moderate to severe fatty liver change with presence of sinusoidal spaces indicated by arrow and multi necrotic foci filled with mild hemorrhage indicated by circle (Fig. 13: 7a and 7b). Liver sections (Fig. 13: 8a and 8b) of compound IG-01–009 at dose 1000 mg/kg showed multi necrotic foci filled with hemorrhage and presence of infiltrative cells indicated by circle and showing severe steatosis with micro vesicular fatty change indicated by arrow (Fig. 13).
-
(b)
Kidney: Kidney sections of control (normal saline) and 1 % DMSO group showed normal renal cortex and glomerular tuft (Fig. 14: Sections 1a and 2). All the treated animal kidney sections were found normal. Compound IG-01–009 at dose 1000 mg/kg showed mild diminished and distorted glomeruli indicated by circle with dilated tubules indicated by arrows in Section 8 (Fig. 14).
-
(c)
Spleen: Sections of control spleen and 1 % DMSO showed normal splenic architecture with normal lymphoid follicles and sinuses (Fig. 15: 1, 2). Spleen sections of compound IG-01–007 at dose 300 mg/kg and 1000 mg/kg showed diffuse white pulp and distorted lymphoid architecture (Fig. 15: 3, 4). Sections of compound IG-01–008 at dose 300 mg/kg showed ill-defined spleen section additionally 1000 mg/kg group showed disrupt white pulp (Fig. 15: Sections 5 and 6). Compound IG-01–009 at dose 300 mg/kg and 1000 mg/kg showed (Fig. 15: Sections 7 and 8) excess in red pulp, disorientation of white pulp and no clearly visible lymphoid follicles (Fig. 15).
Fig. 12.
Light micrographs of the liver sections from different treatment groups and control with H&E staining. (1a to 4a= 10x, 100μm and 1b to 4b = 40x magnification, 50 μm), (Fig. 11: 1a and1b represent control, 2a and 2b represent IG-01–007 at 300 mg/kg, 3a and 3b represent IG-01–008 at 300 mg/kg, 4a and 4b represent IG-01–009 at 300 mg/kg).
Fig. 13.
Light micrographs of the liver sections from different treatment groups and control with H & E staining. (5a to 8a= 10x, 100μm and 5b to 8b = 40x magnification, 50μm), (Fig. 12: 5a and 5b represent 1 % DMSO, 6a and 6b represent IG-01–007 at 1000 mg/kg, 7a and 7b represent IG-01–008 at 1000 mg/kg, 8a and 8b represent IG-01–009 at 1000 mg/kg).
Fig. 14.
Light micrographs of the kidney sections from different treatment groups and control with H & E staining (1a to 8 = 40x magnification, 50 μm). (1a) control, (2) 1 % DMSO, (3) IG-01–007 (300 mg/kg), (4) IG-01–007 (1000 mg/kg), (5) IG-01–008 (300 mg/kg), (6) IG-01–008 (1000 mg/kg), (7) IG-01–009 300 mg/kg, (8) IG-01–009 (1000 mg/kg).
Fig. 15.
Light micrographs of the spleen sections from different treatment groups and control with H & E staining (1–8 = 10 x magnification, 100 μm). (1) control, (2) 1 % DMSO, (3) IG-01–007 (300 mg/kg), (4) IG-01–007 (1000 mg/kg), (5) IG-01–008 (300 mg/kg), (6) IG-01–008 (1000 mg/kg), (7) IG-01–009 (300 mg/kg), (8) IG-01–009(1000 mg/kg).
4. Conclusion
The imidazo-based pyridine/pyrazine compounds hold significant promise in pharmacology, particularly in oncology. Their aromatic heterocyclic structure often lends them favorable pharmacokinetic properties and biological activity. Their anti-cancer potential has been widely explored due to their ability to interact with various cellular targets implicated in cancer progression. However, toxicity is a critical consideration in the development of anti-cancer drugs. Even though these compounds show promise in combating cancer, their effects on normal cells and tissues must be thoroughly evaluated to ensure their safety profile. Toxicological assessments are crucial in this regard, helping researchers understand the potential adverse effects these compounds may have on living organisms. These assessments typically involve a range of studies, including acute toxicity studies, sub-chronic toxicity studies, genotoxicity studies, and carcinogenicity studies. These studies aim to determine the dose-dependent toxic effects, potential for DNA fragmentation, and carcinogenic potential of the compounds. Additionally, researchers often investigate the effects of compounds on specific organs or systems, such as the liver, and kidneys. By conducting comprehensive toxicological assessments, researchers can better understand the safety profile of imidazo-based pyridine/pyrazine compounds and make informed decisions regarding their development as anti-cancer drugs. This process is crucial for ensuring that potential therapeutic benefits outweigh any associated risks, ultimately contributing to the development of safer and more effective treatments for cancer.
In the present study, the series of imidazo based heterocycle derivatives reported to have anticancer potential were considered for toxicological assessment. In the cytotoxicity assay, the samples of IG-01–007, IG-01–008, and IG-01–009 were tested at concentrations 10 μM, 25 μM, 50 μM and 100 μM and showed significant toxicity. Compounds IG-01–008 and IG-01–009 showed highest cytotoxicity as compared to IG-01–007 and showed continuous decrease in cell viability with the increase of the concentration of test compounds. Moreover, all the test compounds also showed DNA fragmentation at concentration 50 µM. In cell migration assay, at 25 μM and 50 μM concentrations, the test compounds showed significant inhibition of migration of cell as compared to control. In the in-vivo studies, IG-01–007, IG-01–008, and IG-01–009 at dose 300 mg/kg and 1000 mg/kg showed toxicity which was assessed by change in gross behavior, decrease in food intake, change in body weight and organ weight, elevation in LOD, reduction in SOD levels and higher levels of serum biochemistry markers AST, ALT, and ALP. At higher dose, these compounds showed signs of hepatotoxicity and spleen toxicity which was seen in histopathology study. The findings of the present study indicate that imidazo-based pyridine and pyrazine heterocycles exhibit significant toxicological effects, which are critical to understand in the development of anticancer drugs.
Abbreviations
DMEM: Dulbecco’s modified eagle’s medium; PBS: Phosphate-buffered saline; FBS: Fetal bovine serum; RBC: Red blood cells; IAEC: Institutional Animal Ethical Committee; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; RBCs: red blood cells; i.p: Intraperitoneal; BSA: Bovine serum albumin; SOD: Superoxide dismutase; SD: Standard deviation; N: Normal; O: Observed; A: Absent; ALP: Alkaline phosphatase.
Ethical approval
All the animals used in this study were maintained in accordance with the guidelines set forth by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The protocol received approval from the Institutional Animal Ethical Committee (IAEC) of Panjab University, Chandigarh, India with protocol number PU/45/99/CPCSEA/IAEC/2021/559.
CRediT authorship contribution statement
Manpreet Kaur: Resources, Methodology. Manish Jain: Writing – review & editing, Formal analysis, Data curation. Deepak B. Salunke: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization. Ishika Gupta: Resources, Methodology. Sunil Kumar: Writing – original draft, Methodology. Pankaj Kumar: Writing – review & editing, Data curation. Ajay Singhal: Writing – original draft, Methodology. Sandip V. Pawar: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
SVP and DBS are thankful to Anusandhan National Research Foundation (ANRF), Govt. of India for research funding [Core Research Grant, File No. CRG/2022/003884] to carry out this research work. The authors acknowledge the SAIF facility of DST at Panjab University, Chandigarh, India and DST-FIST and UGC-CAS funded facility of UIPS used for analysis and characterization.
Data availability
Data will be made available on request.
References
- 1.Hu F., Zhang L., Nandakumar K.S., Cheng K. Imidazole scaffold based compounds in the development of therapeutic drugs. Curr. Top. Med. Chem. 2021;21(28):2514–2528. doi: 10.2174/1568026621666210527103225. [DOI] [PubMed] [Google Scholar]
- 2.Martins P., Jesus J., Santos S., Raposo L.R., Roma-Rodrigues C., Baptista P.V., Fernandes A.R. Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine's tool box. Molecules. 2015;20(9):16852–16891. doi: 10.3390/molecules200916852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh R., Pandrala M., Malhotra S.V., Pawar G.P., Chaudhari V.D., Salunke D.B. Design, synthesis, anti-cancer screening and structure activity relationship studies of biphenyl linked fused imidazoles. J. Indian Chem. Soc. 2020;97(8):1237–1244. [Google Scholar]
- 4.Krishnamoorthy R., Anaikutti P. Iodine catalyzed synthesis of imidazo [1, 2-a] pyrazine and imidazo [1, 2-a] pyridine derivatives and their anticancer activity. RSC Adv. 2023;13(51):36439–36454. doi: 10.1039/D3RA07842F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajduk P.J., Bures M., Praestgaard J., Fesik S.W. Privileged molecules for protein binding identified from NMR-based screening. J. Med. Chem. 2000;43(18):3443–3447. doi: 10.1021/jm000164q. [DOI] [PubMed] [Google Scholar]
- 6.Sanghai N., Jain V., Preet R., Kandekar S., Das S., Trivedi N., Mohapatra P., Priyadarshani G., Kashyap M., Das D., Satapathy S.R. Combretastatin A-4 inspired novel 2-aryl-3-arylamino-imidazo-pyridines/pyrazines as tubulin polymerization inhibitors, antimitotic and anticancer agents. MedChemComm. 2014;5(6):766–782. doi: 10.1039/C3MD00357D. [DOI] [Google Scholar]
- 7.Kamal A., Ramakrishna G., Raju P., Rao A.S., Viswanath A., Nayak V.L., Ramakrishna S. Synthesis and anticancer activity of oxindole derived imidazo [1, 5-a] pyrazines. Eur. J. Med. Chem. 2011;46(6):2427–2435. doi: 10.1016/j.ejmech.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Jie L.I., Shuyuan Z.H., Hairong X.I., Weiming C.H., Zhang Y., Zhang W., Zhihong G.U., Wenjie M.E. Imidazole-based phenanthroline derivatives induce DNA damage-mediated apoptosis to suppress hepatocellular carcinoma. J. Holist. Integr. Pharm. 2022;3(2):177–189. doi: 10.1016/S2707-3688(23)00057-2. [DOI] [Google Scholar]
- 9.Ali I., Lone M.N., Aboul-Enein H.Y. Imidazoles as potential anticancer agents. MedChemComm. 2017;8(9):1742–1773. doi: 10.1039/c7md00067g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szymański P., Markowicz M., Mikiciuk-Olasik E. Adaptation of high-throughput screening in drug discovery—toxicological screening tests. Int. J. Mol. Sci. 2011;13(1):427–452. doi: 10.3390/ijms13010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toolaram A.P., Kümmerer K., Schneider M. Environmental risk assessment of anti-cancer drugs and their transformation products: a focus on their genotoxicity characterization-state of knowledge and short comings. Mutat. Res./Rev. Mutat. Res. 2014;760:18–35. doi: 10.1016/j.mrrev.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 12.21 CFR Part 314 -- Applications for FDA approval to market a new drug. (2024). https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314?toc= 1.
- 13.Atkins J.T., George G.C., Hess K., Marcelo-Lewis K.L., Yuan Y., Borthakur G., Khozin S., LoRusso P., Hong D.S. Pre-clinical animal models are poor predictors of human toxicities in phase 1 oncology clinical trials. Br. J. Cancer. 2020;123(10):1496–1501. doi: 10.1038/s41416-020-01033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain A.K., Singh D., Dubey K., Maurya R., Mittal S., Pandey A.K. Vitro Toxicology. Academic Press; 2018. Models and methods for in vitro toxicity; pp. 45–65. [DOI] [Google Scholar]
- 15.Krewski D., Acosta D., Andersen M., Anderson H., Bailar J.C., Boekelheide K., Brent R., Charnley G., Cheung V.G., Green S., Kelsey K.T., Kerkvliet N.I., Li A.A., McCray L., Meyer O., Patterson R.D., Pennie W., Scala R.A., Solomon G.M., Stephens M., Yager J., Zeise L. Toxicity testing in the 21st century: a vision and a strategy. J. Toxicol. Environ. Health B Crit. Rev. 2010;13(2-4):51–138. doi: 10.1080/10937404.2010.483176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martín-Banderas L., Muñoz-Rubio I., Prados J., Álvarez-Fuentes J., Calderón-Montaño J.M., López-Lázaro M., Arias J.L., Leiva M.C., Holgado M.A., Fernández-Arévalo M. In vitro and in vivo evaluation of Δ⁹-tetrahidrocannabinol/PLGA nanoparticles for cancer chemotherapy. Int J. Pharm. 2015;487(1-2):205–212. doi: 10.1016/j.ijpharm.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 17.Parasuraman S. Toxicological screening. J. Pharmacol. Pharmacother. 2011;2(2):74–79. doi: 10.4103/0976-500X.81895. 〈https://doi.org/10.4103/2F0976-500X.81895〉 (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Sheddi E.S., Farshori N.N., Al-Oqail M.M., Musarrat J., Al-Khedhairy A.A., Siddiqui M.A. Portulaca oleracea seed oil exerts cytotoxic effects on human liver cancer (HepG2) and human lung cancer (A-549) cell lines. Asian Pac. J. Cancer Prev. 2015;16(8):3383–3387. doi: 10.7314/apjcp.2015.16.8.3383. [DOI] [PubMed] [Google Scholar]
- 19.Liaqat N., Jahan N., Anwar T., Qureshi H. Green synthesized silver nanoparticles: optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.952006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam M.J., Das A.K., Muntaha S. Cytometry investigation of myo-inositol-induced growth inhibition, apoptosis induction and cell cycle arrest in the human prostate cancer cell line (DU-145) Bangladesh J. Med. Sci. 2024;23(3):655–665. [Google Scholar]
- 21.Bobadilla A.V., Arévalo J., Sarró E., Byrne H.M., Maini P.K., Carraro T., Balocco S., Meseguer A., Alarcón T. In vitro cell migration quantification method for scratch assays. J. R. Soc. Interface. 2019;16(151):20180709. doi: 10.1098/rsif.2018.0709. (28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OECD . OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing; Paris: 2002. Test No. 420: Acute oral toxicity - fixed dose procedure. [DOI] [Google Scholar]
- 23.De-Leon J.A., Borges C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. JoVE (J. Vis. Exp. ) 2020;12(159) doi: 10.3791/61122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mesa-Herrera F., Quinto-Alemany D., Díaz M. A sensitive, accurate, and versatile method for the quantification of superoxide dismutase activities in biological preparations. React. Oxyg. Species. 2019;7(19):10–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.